- Department of Radiation Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China
Esophageal cancer (EC) is one of the most common cancers with poor survival in the world. Nowadays, a generous number of clinical trials are underway on the use of immunotherapy in EC patients, especially the programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors. However, only a few patients could benefit from single-agent therapy. Others need combination therapies to enhance the response rate and survival. In this review, we focus on PD-1/PD-L1 inhibitors and its combination options in EC patients. We also summarized the potential predictive biomarkers for PD-1/PD-L1 inhibitors treatment.
Introduction
Esophageal cancer (EC) is the seven most common cancer and ranks second in the cause of cancer-related death worldwide (1). It comprises esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), with different pathogenesis and distribution. ESCC accounts for ~90% of EC in Asia and has a close relationship with smoking, having hot food or water and alcohol consumption (2). Whereas, EAC is the dominant type in western countries and is usually caused by chronic gastroesophageal reflux disease (GORD), obesity and Barrett's esophagus (3). Esophagogastric junction (EGJ) adenocarcinoma is often grouped with EAC because of their similar etiology.
Traditional therapies for EC patients include surgery, chemotherapy, radiotherapy (RT), and targeted therapy. However, most patients relapse quickly after the initial therapy. Meanwhile, EC patients always have a lack of oncogenic driver mutations (4), and the addition of the targeted drugs can only prolong survival for a few months (5). Hence, novel drugs with definitive efficacy to improve overall survival (OS) are expected.
In recent clinical trials of EC, interest is very high in immunotherapies, which involved immune checkpoint inhibitors (ICIs), adoptive T-cell therapy, cancer vaccines, and oncolytic viruses. Immunotherapies act on different steps of the anti-tumor immunity to enhance the host's immunity and strengthen anti-tumor responses. A series of events occurred in steps to eliminate cancer cells and were identified as the cancer-immunity cycle (6). First, tumor-specific antigens are specifically recognized by dendritic cells (DCs) or antigen-presenting cells (APCs) (step 1). After that, antigens are presented to T cells (step 2) and participate in the priming and activation of effector T cells (step 3). Next, activated effector T cells traffic to (step 4) and infiltrate to cancer cells (step 5). Then, effector T cells recognize (step 6) and finally kill the cancer cells (step 7). The death of cancer cells further results in more release of tumor-specific antigens (step 1 again) and strengthens the cancer-immunity cycle. However, cancer cells could evade immune surveillance through various mechanisms, including faulty recognition of neoantigens, inhibition of T-cell infiltration and suppression of effector T cells (7).
ICIs, especially programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors, have demonstrated clinical benefit in multiple cancers and generated tremendous interest. Immune checkpoints are immunosuppressive proteins and help to maintain immunologic homeostasis in hosts (8). Interaction of ICI with checkpoint breaks immunosuppression and enhances anti-tumor immunity (9). PD-1/PD-L1 inhibitors are the most well-known immune checkpoints. The PD-L1 on the surface of tumor cells binds to PD-L1 on cancer cells, and reduce the function of cytotoxic T lymphocyte (CTL), inhibit the anti-tumor function of T cells, and lead to immune escape in the effector phase of the cancer-immunity cycle (10).
We summarize the clinical studies of PD-1/PD-L1 inhibitors and the combination options for EC in this review.
PD-1/PD-L1 Inhibitors in EC
PD-L1 overexpression has been reported in around 40% of EC patients and is related to worse OS (11). Various methods could be used to detect PD-L1 expression in different cancers, including immunohistochemistry (IHC) staining, enzyme-linked immunosorbent assay (ELISA), immunofluorescence (IF), flow cytometry (FC) and so on. IHC is the most common and convenient one. Now, four diagnostic kits are commercially available: the 22C3 and 28-8 clones of Dako Autostainer Link 48 platform1, 2; the SP263 and SP142 clones of Ventana BenchMark ULTRA platform3, 4. The PD-L1 antibody used in IHC staining is the Dako 22C3 pharmDx (12). Comparison studies of different PD-L1 antibodies are urgently needed.
Furthermore, combined positive score (CPS) is used to define the PD-L1 status instead of the tumor proportion score (TPS) for EC patients. CPS is defined as the ratio of the combining number of PD-L1 positive tumor cells and immune cells (lymphocytes, macrophages) by IHC staining to the total number of tumor cells. The maximum score is 100, and a higher score showing greater likelihood of response to PD-1/PD-L1 inhibitors (12).
The most studied PD-1/PD-L1 inhibitors were Nivolumab (OPDIVO, Bristol-Myers Squibb Co.), Pembrolizumab (KEYTRUDA, Merck Sharp & Dohme Co., Inc.), Avelumab (BAVENCIO, EMD Serono Inc.), Atezolizumab (TECENTRIQ, Genentech Inc.), and Durvalumab (IMFINZI, AstraZeneca Inc.). Plenty of new PD-1/PD-L1 inhibitors are being investigated now, including PD-1 inhibitors: Camrelizumab (SHR-1210, Jiangsu HengRui Medicine Co., Ltd.), Sintilimab [IBI308, Innovent Biologics (Suzhou) Co. Ltd.], Spartalizumab (PDR001, Novartis Pharmaceuticals), Tislelizumab (BGB-A317, BeiGene), Toripalimab (triprizumab, teripalimab, JS001, Shanghai Junshi Biosciences Co., Ltd), HLX-10 (Shanghai Henlius Biotech, Inc.) and PD-L1 inhibitor: SHR-1316 (Jiangsu HengRui Medicine Co., Ltd.) and CS1001 (CStone Pharmaceuticals Co., Ltd.).
The Ongoing Clinical Trials in EC
ICIs have shown considerable objective response rates (ORR), durable toxicities and even prolong the OS in several cancers, including advanced EC. However, the effective rates of single-agent were 31% in melanoma and only 17% in lung cancer, respectively (13). And the response rate of ICI alone in EC patients varied from 9.9 to 33.3% in the reported studies (14). The combination of PD-1/PD-L1 inhibitors with other therapies, such as chemoradiotherapy (CRT), other ICIs, cancer vaccines, and target drugs, was supposed to make the tumor more immunogenic, produce a synergistic effect, and gather stronger clinical benefit.
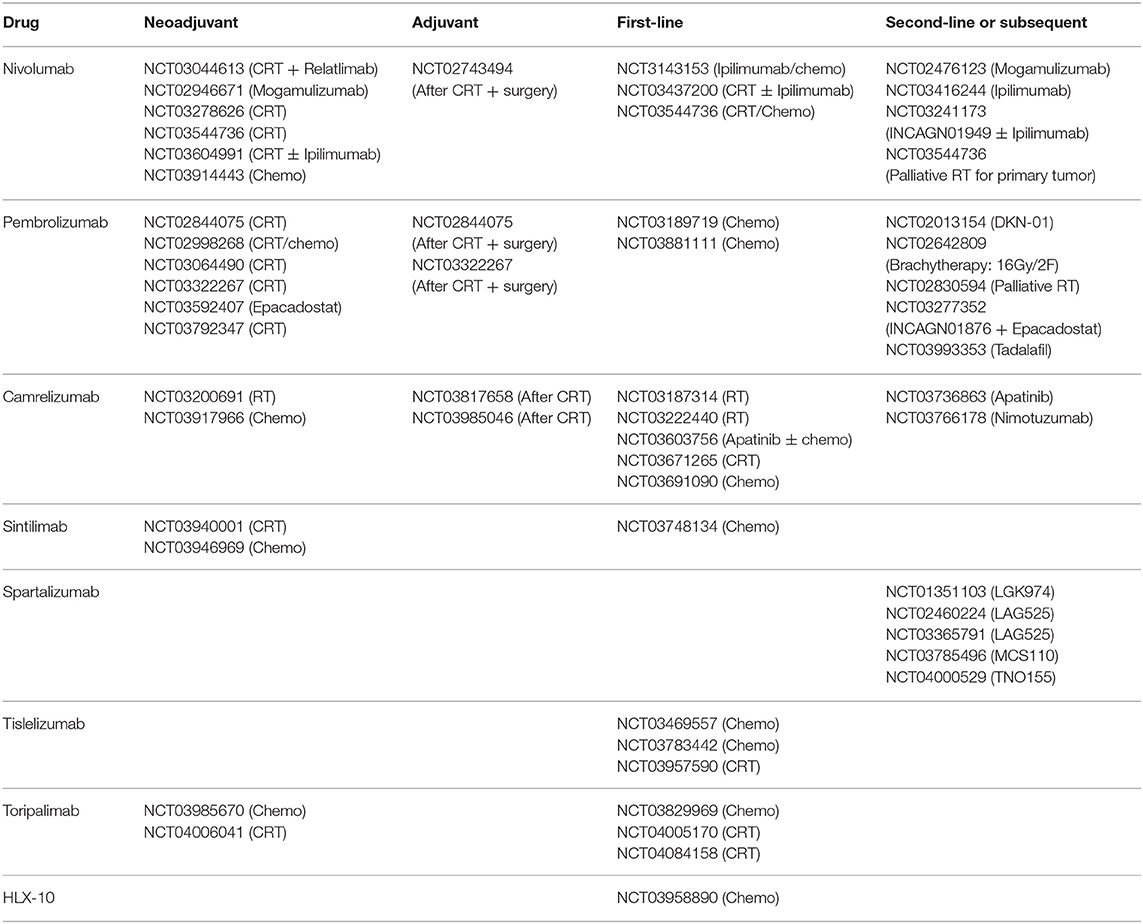
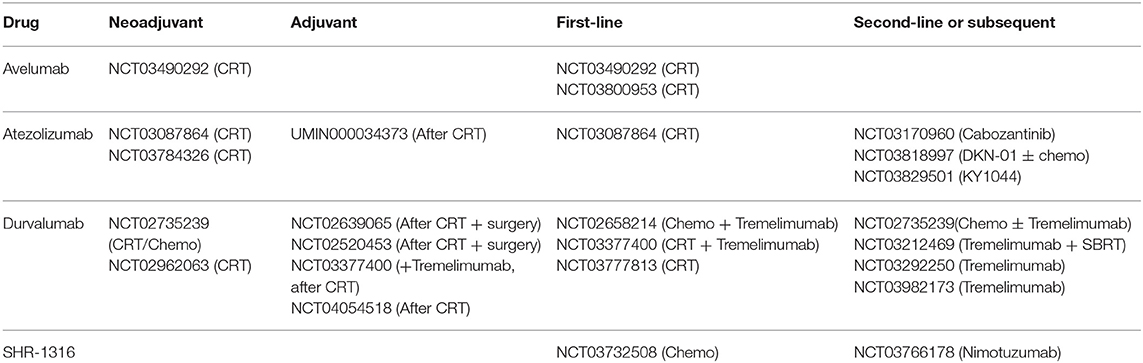
We searched the ClinicalTrials.gov database with the search terms “immunotherapy,” “esophageal cancer,” “PD-1,” “PD-L1,” and their variants. Then we screened the search results and recorded the clinical trial numbers. With the numbers, we obtained relevant articles from PubMed, Embase, American Society of Clinical Oncology (ASCO), ASCO Gastrointestinal Cancers Symposium, European Society for Medical Oncology (ESMO) and World Organization for Specialized Studies on Diseases of the Esophagus (OESO) databases. All the trials of PD-1/PD-L1 inhibitors in EC patients with different stages were included (Tables 1, 2), and we focused on trials with published results in this review. The detailed information of published trials were shown in Table S1.
As Neoadjuvant Treatment
The CROSS study showed that neoadjuvant concurrent CRT induced 23% pathologic complete response (pCR) and prolong median overall survival (mOS) (49 vs. 24 months; hazard ratio (HR) = 0.657, 95% confidence interval (CI): 0.495–0.871, p = 0.003) without extra toxicities compared with surgery alone (15). Now, neoadjuvant CRT followed by surgery is the standard treatment for resectable locally advanced EC patients. However, up to 50% of patients relapsed in one year after surgery and the 5-year survival is only 43% (16). Alternative treatments are needed to further improve the survival outcomes with durable toxicity.
According to preclinical studies of EC, RT could induce immunogenic cell death (ICD), consequently release neoantigens, alter tumor microenvironment (TME), and finally activate the immune response (17). Besides, the expression of PD-L1 and CD8+ CTLs in TME could be upregulated by prior CRT. In turn, ICIs also provide synergistic effect to RT through targeting and modulating various T cells population. A growing number of clinical trials are preforming now to evaluate the safety and efficacy of combining CRT with PD-1/PD-L1 inhibitors before surgery (Table 3).
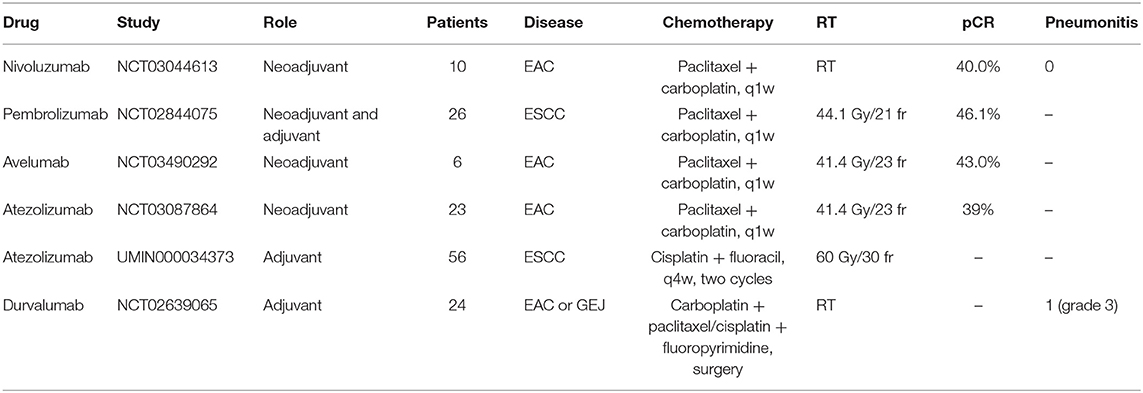
Nivolumab is a humanized IgG4 monoclonal PD-1 antibody. Its neoadjuvant role was assessed in the trial NCT03044613. This study recruited 16 EC patients to receive two cycles of induction nivolumab before CRT and three additional cycles of nivolumab concurrently with CRT (18). The esophagectomy was performed 6–10 weeks after the last nivolumab. To date, ten EAC patients have had surgery, and the pCR was 40% (4/10). Combination therapy has acceptable toxicity and did not delay the surgery. Toxicities of note include steroid-responsive grade 3 dermatitis (1/16) and grade 3 hepatitis (1/16).
Pembrolizumab is another humanized IgG4 monoclonal PD-1 antibody. The trial NCT02844075 enrolled 28 ESCC patients to receive neoadjuvant CRT plus pembrolizumab (19). Twenty-six patients performed esophagectomy in 5 weeks after the neoadjuvant treatment completed. Two patients died after surgery because of acute lung injury. After surgery, patients were treated with pembrolizumab for up to 24 months or until progression, death or unacceptable toxicity. The pCR in the primary tumor was 46.1% (12/26, 95% CI: 28.8–64.6) and the 1-year survival rate was 80.8% (mOS has not been reached). The common treatment-related adverse events (trAEs) were neutropenia (50.0%) and increased liver enzyme (30.8%). There was a tendency toward better disease-free survival (DFS) in patients reached pCR in the primary tumor (HR = 0.33, p = 0.1).
As for PD-L1 inhibitors, the trial NCT03490292 tested the safety and efficacy of avelumab with CRT in esophageal or EGJ adenocarcinoma patients (20). Avelumab was given after the last dose of chemotherapy on day 29. Surgery was performed 8 weeks after CRT completion and patients received eight cycles of avelumab after resection. One EAC, three Siewert I EGJ and two Siewert II EGJ cancer patients were enrolled. Five patients underwent R0 resection and one patient had R1 resection because tumor had invaded to the adventitial surface. In this trial, pCR was 43% and no dose-limited toxicity (DLT) or grade ≥ 3 immune-related adverse events (irAEs).
In another phase II study, PERFRCT trial (NCT03087864), resectable EAC patients were enrolled to receive five cycles of concurrent CRT and atezolizumab before surgery (21). So far, 39 patients were recruited and 23 patients have had R0 resection. The pCR was 39% (9/23). Grade 3–4 adverse events (AEs) occurred in 48.4% (15/31) patients and were all manageable. There was no report of surgery delay.
As Adjuvant Treatment
The value of postoperative chemotherapy in resectable esophageal and EGJ cancers remains uncertain in the previous trials (22–24). After R0 resection, observation is advised for ESCC patients by National Comprehensive Cancer Network (NCCN) guidelines (25). However, NCCN guidelines recommend either chemotherapy or observation for EAC patients who received preoperative CRT and surgery.
For unresectable locally advanced ESCC, the definitive CRT and observation after that is the standard treatment. However, the complete response (CR) rate is only 11 to 25%, 1-year relapse-free survival (RFS) rate is 50.0% and mOS is only 9–10 months (26). The increase of radiation dose or addition of any adjuvant treatment could not improve the local control rate or provide a survival benefit (27, 28). Based on the result of PACIFIC trial (NCT02125467), durvalumab made an 11-month advantage in progression-free survival (PFS) over placebo (16.8 vs. 5.6 months; HR = 0.52, 95% CI: 0.42–0.65) and better OS (HR = 0.69, 95% CI: 0.55–0.86) as adjuvant treatment after definitive CRT in stage III non-small cell lung cancer (NSCLC) (29, 30).
The adjuvant role of PD-1/PD-L1 inhibitors in EC have been reported in the 2019 ASCO meeting. A phase II trial, NCT02639065 was designed for patients with resected locally advanced esophageal or EGJ adenocarcinoma who had a viable tumor in the surgical specimen after neoadjuvant CRT and R0 resection (31). Enrolled 24 patients received durvalumab for up to 12 months after CRT and surgery. The median number of adjuvant durvalumab cycles was 12.5 (range: 2–13). Three patients developed grade 3 irAEs, one each with pneumonitis, hepatitis, and colitis. At data cutoff, seven (29%) patients relapsed, six (25%) patients had a distant relapse (lung, brain, bone, cervical lymph nodes) and one (0.4%) patient had a locoregional relapse. The 1-year RFS rate, 1-year survival rate, and 2-year survival rate were 79.2, 95.5, and 59.2%, respectively. Median survival time after relapse was 11.1 months (95% CI: 0.1–11.3 months).
In the TENERGY trial (UMIN000034373), unresectable locally advanced ESCC patients without distant metastasis were enrolled and treated with atezolizumab for up to 12 months within 4 weeks after two cycles definitive CRT (32). So far, 50 patients have been enrolled to evaluate the adjuvant role of atezolizumab.
Based on the published data, the addition of PD-1/PD-L1inhibitors as neoadjuvant or adjuvant treatment demonstrated promising efficacy with acceptable toxicity. The trials are ongoing with camrelizumab, sintilimab, and toripalimab as showed in Table 1. Further studies are awaited to identify the most beneficial patients according to PD-L1 status and so on.
As First-Line Treatment
First-line platinum-based doublet chemotherapy provides a limited survival benefit in advanced ESCC patients. To gain better survival, an effective combination with other therapy is urgently required.
Combination With RT
Several studies evaluated the efficacy of PD-1/PD-L1 inhibitors plus RT as first-line treatment in advanced EC patients. As mentioned before, RT could enhance the anti-tumor immunity and induce a synergistic effect with PD-1/PD-L1 inhibitors.
The combination of RT with camrelizumab has been tested in a phase II, single-arm study for patients with locally advanced EC intolerant to or refused CRT (NCT03187314) (33). Sixteen patients were treated with camrelizumab (5 cycles) and RT (60 Gy/30 fr) as first-line treatment. One (7.1%) patient had CR and 13 (92.9%) patients had a partial response (PR). At the data cut off, two patients had metastasis, and median survival had not been reached.
Another phase Ib trial NCT03222440 evaluated camrelizumab with RT as first-line therapy in 20 ESCC patients and observed two (11.1%) patients had CR and 13 (72.2%) patients with PR (34). Patients were treated with RT (60 Gy/30 fr) and concurrent camrelizumab (from the start of RT, up to 16 cycles). Two (11.1%) patients had CR, 13 (72.2%) patients had PR, and three (16.7%) patients had stable disease (SD).
Combination With Chemotherapy
Chemotherapy could also facilitate the anti-tumor response. When combined with immunotherapy, chemotherapy could promote the presentation of tumor antigen, enhance the filtration of CTL and improve the efficacy of checkpoint inhibition (35, 36).
The phase II trial, NCT03469557 evaluated the tolerability of tislelizumab combined with chemotherapy (cisplatin and fluorouracil) as first-line treatment in Chinese patients with inoperable, locally advanced ESCC (37). A total of 15 patients were enrolled and grade ≥ 3 AEs occurred in eight patients, including one grade 5 hepatic dysfunction. Four patients discontinued treatment because of AEs, including grade 3 tracheal fistula, grade 3 lung infection, grade 2 pneumonitis, and grade 3 aspartate aminotransferase (AST) increasing. The efficacy data remains unmatured.
The phase III study KEYNOTE-590 (NCT03189719, MK-3475-590) of pembrolizumab plus chemotherapy (cisplatin and fluorouracil) as first-line therapy for advanced or metastatic EC is ongoing. Its China Extension study, NCT03881111 is also underway now.
Combination With ICIs
Since the different mechanisms of ICIs, the combination of PD-1/PD-L1 inhibitors with other ICIs may be significant. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) on T cells interact with peripheral membrane B7 on APCs and impede the activation of T cells (38). So, CTLA-4 inhibitors (ipilimumab or tremelimumab) act on the antigen presentation phase to induce CTL and produce a synergistic effect with PD-1/PD-L1 inhibitors. CTLA-4 inhibitors are the most frequent combination option and yielded enhanced responses with a manageable safety profile in multiple cancers.
The phase I trial of NCT02658214 evaluated the safety of durvalumab and tremelimumab in combination with chemotherapy (cisplatin and fluorouracil) as first-line treatment for advanced ESCC patients (39). Six patients were recruited to receive four cycles of combination treatment. Four patients had recurrent disease and two were newly diagnosed with advanced ESCC. Three patients had grade ≥ 3 chemotherapy-related AEs (two grade 2 neutropenia and one grade 4 neutropenia). Two patients had irAEs and were grade 1 or 2 diarrhea, pruritus, rash, and increased AST. There were no trAEs-related discontinuation or death. Two of the six patients had a confirmed PR at data cutoff.
The trial CheckMate 648 (NCT03143153) was a randomized, phase III study to compare nivolumab plus ipilimumab or nivolumab combining with chemotherapy (cisplatin and fluorouracil) vs. chemotherapy alone as first-line treatment in unresectable, recurrent, or metastatic ESCC patients. Future results will provide more safety and efficacy data on the combination of PD-1/PD-L1 inhibitors and CTLA-4 inhibitors.
Combination With Target Drugs
NCT03603756 was a phase II study of camrelizumab combined with apatinib [a vascular endothelial growth factor receptor 2 (VEGFR2) inhibitor] and chemotherapy (liposomal paclitaxel and nedaplatin) as the first-line treatment for advanced ESCC (40). 30 patients received six to nine cycles of combination treatment, followed by maintenance therapy (camrelizumab, apatinib, or both). The ORR was 80.0% (24/30) and the disease control rate (DCR) was 96.7% (29/30). PFS and OS data had not matured. The most common grade 3–4 AEs were leucopenia (83.3%) and neutropenia (60.0%). The incidence of capillary hemangiomas was dramatically decreased because of the inhibition effect of apatinib to VEGFR2, which made their combination more reasonable.
As Second-Line or Subsequent Treatment
For patients who failed in the treatment of standard platinum-based doublet chemotherapy, second-line, and subsequent treatment options are limited. The 5-year survival rate was ~20% in all stages and only 5% in advanced patients (41). The mOS is always shorter than one year in metastatic patients (42). The safety and efficacy of PD-1/PD-L1 inhibitors were assessed in tremendous clinical trials (Table 4).
PD-1/PD-L1 Inhibitors Alone
Nivolumab
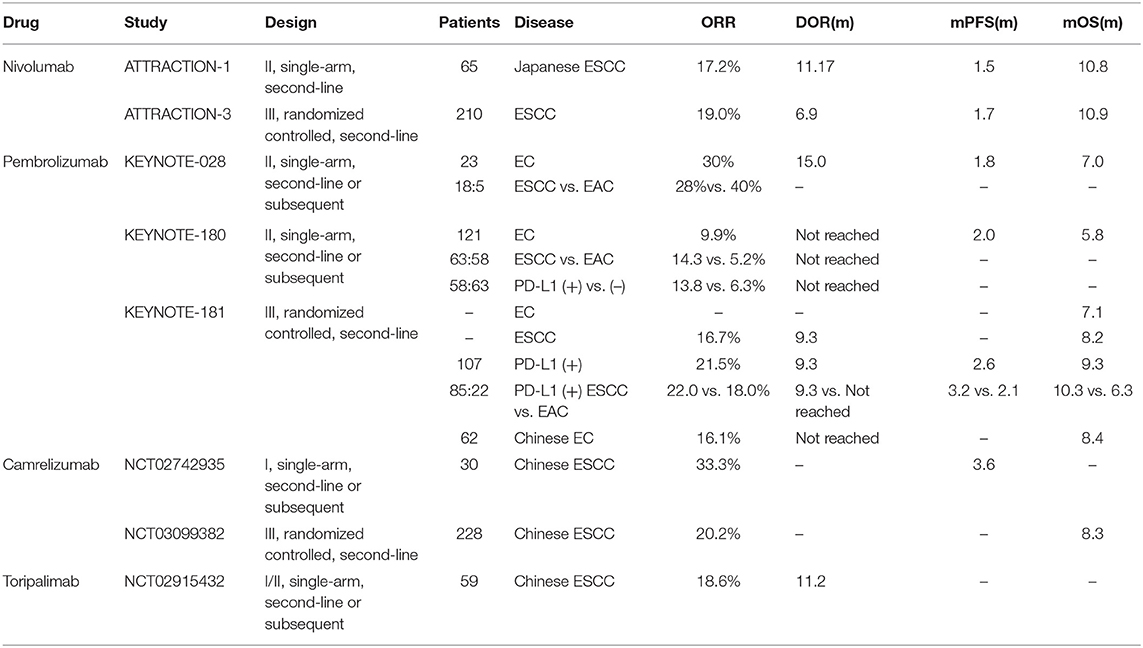
ATTRACTION-1 (ONO-4538-07/JapicCTI-No.142422) study was a single-arm, phase II study enrolled 65 Japanese ESCC patients with unresectable or recurrent EC and were refractory to or intolerant to standard chemotherapy (fluoropyrimidine and platinum) (43). In this trial, patients were unselected with PD-L1 expression and received second-line nivolumab. ORR was 17.2% (95% CI: 9.9–28.2%) with three CR and eight PR. The reported median duration of response (mDOR) was 11.17 months (95% CI: 3.02-not reached). The median progression-free survival (mPFS) was 1.5 months (95% CI: 1.4–2.8 months) and mOS was 10.8 months (95% CI: 7.4–13.3 months). Thirty-nine patients (60%) had trAEs, and grade 3 or 4 AEs happened in 14 and 3% of patients, respectively. Diarrhea (14%) and decreased appetite (9%) were the most common ones. Treatment discontinuation occurred in seven (11%) patients due to lung infection, decreased appetite, interstitial lung disease, hepatic function abnormal, hyponatremia, dyspnoea, and eosinophilic pneumonia. There was no treatment-related death.
Furthermore, the randomized, phase III trial, ATTRACTION-3 (NCT02569242, ONO-4538-24/CA209-473) is performing to compare nivolumab with chemotherapy (docetaxel or paclitaxel) as second-line therapy in unresectable or recurrent ESCC patients with PD-L1-unselected (44). So far, 419 patients were randomized and 96% (401/419) were Asian patients. ORR was 19% (95% CI: 14–26.0%) and mDOR was 6.9 months (95% CI: 5.4–11.1 months) in the nivolumab group (210 patients). Compared with chemotherapy group, the nivolumab group showed better mOS (10.9 vs. 8.4 months; HR = 0.77, 95% CI: 0.62–0.96; p = 0.02) (45). The mPFS didn't display a statistically significant difference (1.7 vs. 3.4 months; HR = 1.08, 95% CI: 0.87–1.34). Fewer trAEs were reported in the nivolumab group (any grade, 66 vs. 95%; grade 3–4, 18 vs. 63%).
Pembrolizumab
KEYNOTE-028 (NCT02054806) was a single-arm, phase Ib trial that enrolled PD-L1 positive (CPS > 1) locally advanced or metastatic EC patients to receive pembrolizumab (12). Among the 23 enrolled patients, 18 (78%) patients were ESCC, and others were EAC. ORR was 30% (95% CI: 13–53%) in all patients, 28% in ESCC patients and 40% in EAC patients. And the mDOR was 15 months (95% CI: 6–26 months). Overall, mPFS was 1.8 months (95% CI: 1.7–2.9 months) and mOS was 7.0 months (95% CI: 4.3–17.7 months). Only nine patients (39%) experienced trAEs, and grade 3 trAEs occurred in four patients (17%). There was no grade 4 trAE, death or discontinuation in this trial. KEYNOTE-028 firstly demonstrated the manageable toxicity and durable anti-tumor activity of pembrolizumab in EC.
KEYNOTE-180 (NCT02559687) was a single-arm, phase II study which enrolled patients with locally advanced or metastatic ESCC and EAC (including Siewert type I EGJ adenocarcinoma) who refractory to at least two prior systemic treatments (46). One hundred twenty-one patients were enrolled with unselected PD-L1 expression. 63 (52.1%) patients were ESCC and 58 (47.9%) patients had PD-L1 positive (CPS ≥ 10). ORR was 9.9% (two CR and ten PR) and mDOR was not reached (1.9–14.4 months). The mPFS was 2.0 months (95% CI: 1.9–2.1 months) and mOS was 5.8 months (95% CI: 4.5–7.2 months) in all patients. ORR was higher in ESCC subgroup (14.3 vs. 5.2%), and better in PD-L1 positive subgroup (13.8 vs. 6.3%). In the 35 PD-L1 positive ESCC patients, ORR was 20.0% (95% CI: 8.0–37.0) and duration of response (DOR) varied from 4.2 to 25.1+ months, with 14.3% (5/35) patients being effective for over 6 months and 8.6% (3/35) patients having responses more than 12 months. Overall, 19 (15.7%) patients had grade 3–5 trAEs. Only seven patients discontinued due to AEs and one patient died of pneumonitis.
Since pembrolizumab was certified as an effective and safe third-line treatment in the trial KEYNOTE-028 and KEYNOTE-180, the trial KEYNOTE-181 (NCT02564263) evaluated its upfront use as second-line treatment. The trial enrolled 628 patients with locally advanced or metastatic EC who progressed on or after the standard chemotherapy (47). Patients were randomized to receive either pembrolizumab or chemotherapy: paclitaxel, docetaxel or irinotecan. In all the patients, the pembrolizumab group did not display better OS (7.1 vs. 7.1 months; HR = 0.89, 95% CI: 0.75–1.05, p = 0.0560). However, in the 222 (35.4%) patients with PD-L1 positive (CPS ≥ 10), ORR was higher (21.5 vs. 6.1%, p = 0.0006) and DOR was longer (9.3 vs. 7.7 months) in pembrolizumab as compared with chemotherapy. Hence, pembrolizumab could meaningful improve the OS as the second-line therapy compared with chemotherapy in PD-L1 positive (CPS ≥ 10) patients (mOS: 9.3 vs. 6.7 months; HR = 0.69, 95% CI: 0.52–0.93, p = 0.0074). For the 401 ESCC patients, ORR (16.7 vs. 7.4%, p = 0.0022) was better in the pembrolizumab group and the mOS was 8.2 months (95% CI: 6.7–10.0 months) in the pembrolizumab group and 7.1 months (95% CI: 6.1–8.2) in chemotherapy group (HR: 0.78, 95% CI: 0.63–0.96, p = 0.0095). For the PD-L1 positive ESCC patients, ORR (22.0 vs 7.0%), DOR (9.3 vs. 7.7 months), mPFS (3.2 vs. 2.3 months; HR = 0.66, 95% CI: 0.48–0.92), and mOS (10.3 vs. 6.7, HR = 0.62, 95% CI: 0.46–0.90) were better in the pembrolizumab group compared with chemotherapy group. Importantly, the incidence of trAEs in the pembrolizumab group was lower than the chemotherapy group (Any grade, 64.3 vs. 86.1%; grade 3–5, 18.2 vs. 40.9%). And there were five treatment-related deaths in each group.
Camrelizumab
Camrelizumab is a novel humanized high-affinity IgG4-kappa PD-1 monoclonal antibody that independently developed by Chinese biopharma. In the phase I study, NCT02742935, 30 ESCC patients failed to at least one systemic treatment were enrolled (48). Twenty-one (70.0%) patients had received two or more previous chemotherapy, 19 (63.3%) patients had radiation and 14 (46.7%) patients had esophagectomy. ORR was 33.3% (11/30), and mPFS was 3.6 months (95% CI: 0–7.2 months). The trAEs occurred in 25 (83.3%) patients and reactive capillary hemangiomas was the most frequent trAE (76.7%, 23/30), which was likely caused by activating the vascular endothelial growth factor (VEGF)/VEGF receptor pathway. Three (10.0%) grade 3 trAEs were reported: two (6.7%) pneumonitis and one (3.3%) increased cardiac troponin I. No grade 4–5 trAEs and no discontinuation because of trAEs in this trial.
The phase III trial, ESCORT study (NCT03099382), compared camrelizumab and chemotherapy (docetaxel or irinotecan) as the second-line treatment for advanced ESCC. The latest results were reported at the 15th OESO World Conference as an oral presentation (49). In the 448 enrolled patients, 228 patients were randomized to the camrelizumab group and reached an ORR of 20.2% and a 12-month survival rate of 33.7%. The mOS of camrelizumab group was better than the chemotherapy group (8.3 vs. 6.2 months; HR = 0.71, 95% CI: 0.57–0.87, p = 0.001).
Toripalimab
Toripalimab is another humanized PD-1 monoclonal antibody developed by Chinese biopharma. The latest data of a phase Ib/II trial, NCT02915432, was presented on the 2019 ASCO meeting (50). In this study, 59 advanced chemo-refractory ESCC patients were treated with toripalimab and the ORR was 18.6% (one CR and ten PR), mDOR was 11.2 months. Grade 3–5 trAEs occurred in 30.5% (18/59) of patients.
Based on these studies, pembrolizumab has been approved by the Food and Drug Administration (FDA) as the second-line treatment for recurrent, locally advanced or metastatic ESCC with PD-L1 positive (CPS ≥ 10) (51). It has also been approved as the third-line or subsequent therapy option for esophageal and EGJ adenocarcinomas with PD-L1 positive (CPS ≥ 1) before. Generous clinical trials are investigating the role of PD-1/PD-L1 inhibitors in advanced EC as subsequent treatment (durvalumab: NCT01938612, NCT02639065; pembrolizumab: NCT02971956, NCT02998268; CS1001: NCT03312842, NCT03744403) or as second-line treatment (sintilimab: NCT03116152; tislelizumab: NCT03430843, toripalimab: NCT03474640; pembrolizumab: NCT03933449). With previous studies showing its efficacy in ESCC patients, nivolumab has yet to secure FDA approval.
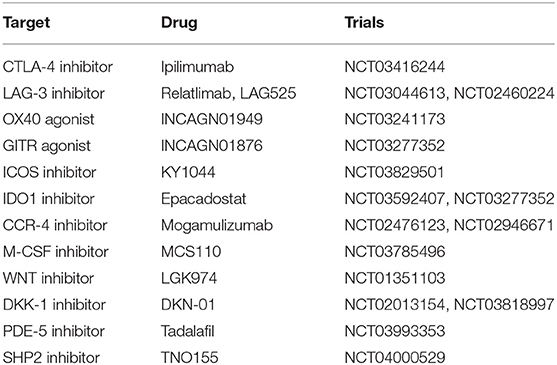
Combination With Immunoregulatory Factors
To boost the efficacy of PD-1/PD-L1 inhibitors in EC patients, we concentrate on its combination with different immunoregulatory factors to activate anti-tumor immunity (Table 5).
LAG-3
Lymphocyte activation gene-3 (LAG-3, CD223) is regularly expressed on activated T cells and natural killer (NK) cells. The expression and upregulation of LAG-3 and PD-1 on tumor-infiltrating lymphocytes (TILs) leading to the inactivate of effector T cells and cause tumor growth (52). Relatlimab (BMS-986016) and LAG525 are humanized monoclonal anti-LAG-3 antibodies. LAG-3 inhibitors hinder the interaction of LAG-3 with MHC class II and then repair the activity of effector T cells to kill tumor cells. The combination of PD-1/PD-L1 inhibitors and anti-LAG-3 antibody is under investigation now (NCT03044613, NCT02460224) and is expected to produce a synergistic effect.
OX40
OX40 (CD134) is a T cell co-stimulatory receptor and mainly expressed on activated T cells, and regulatory T (Treg) cells (53). The binding of OX40 to OX40L provokes the activation and proliferation of T cells, enhances effector T-cell differentiation and decreases the immunosuppressive function of Treg cells. INCAGN01949 is a humanized immunoglobulin G1 agonistic monoclonal antibody of OX40 and is being judged in EC patients (NCT03241173).
IDO1
Indoleamine 2.3-dioxygenase-1 (IDO1) is an intracellular enzyme and overexpressed in various cancers. As a metabolic mediator, IDO1 enzyme transfer tryptophan to tryptophan catabolites (54). This conversion lessens the activity of effector T cells and promotes the differentiation and function of Treg through upregulating FoxP3. Epacadostat is an oral IDO inhibitor and is being investigated with PD-1/PD-L1 inhibitors (NCT03592407, NCT03277352).
There are also novel multi-target antibodies, including SL-279252 (PD-1 and OX40L inhibitor) and INBRX-105 [PD-L1 and 41BB (CD137) inhibitor]. The safety and efficacy of these agents may be reported in forthcoming studies.
Prospect: Identify Beneficial Patients
Considering the low effective rate of ICI in EC, predictive biomarkers are needed to decide patients more likely to react to ICI and identify patients resistant to ICI and need a combination or alter treatment. Predictive biomarkers identified in NSCLC including PD-L1 status, tumor mutation burden (TMB), mismatch repair deficiency (MMR), and microsatellite instability (MSI). Whether they make a similar role in EC is uncertain.
Pathological Types and Ethnic Difference
As mentioned before, ESCC is more widespread in Asia, and clinical trials reveal higher ORRs in ESCC patients with PD-1/PD-L1 inhibitors compared with EAC. ATTRACTION-1 reported an ORR of 17% (95% CI: 10–28%) in 65 advanced Japanese ESCC patients treated with nivolumab (43). KEYNOTE-028 enrolled 23 EC patients and found that ORR was 28% in ESCC and 40% in EAC patients with pembrolizumab treatment, respectively (12). However, the large sample trial KEYNOTE-180 showed that the ORR for patients with pembrolizumab was 14.3% (95% CI: 6.7–25.4%) in ESCC and 5.2% (95% CI: 1.1–14.4%) in EAC patients (46). Furthermore, the KEYNOTE-181 trial revealed that in all PD-L1 positive patients, ESCC ones might have better ORR (22.0 vs. 18.0%), mPFS (3.2 vs. 2.1 months), and mOS (10.3 vs. 6.3 months) than EAC ones (47). There was no direct comparison between ESCC and EAC patients in these studies.
Regarding the better response to PD-1/PD-L1 in ESCC, Asian patients may be the more beneficial population. The latest results of Chinese patients in KEYNOTE-181 were presented on the 2019 ESMO meeting (55). Among the 123 enrolled advanced EC patients, 119 had ESCC and 54 had PD-L1 positive. In the 62 patients treated with pembrolizumab, ORR was 16.1% and mDOR was not reached (4.4+ to 14.6+ months). The mOS was longer in the pembrolizumab group in all the Chinese patients (8.4 vs. 5.6 months; HR = 0.55, 95% CI: 0.36–0.82), in the ESCC (8.4 vs. 5.6 months; HR = 0.55, 95% CI: 0.37–0.83), and in the PD-L1 positive patients (12.0 vs. 5.3 months; HR = 0.34, 95% CI: 0.17–0.69). Chinese patients perhaps have better mOS, but the comparison with other patients was lacked.
Thus, Asian and non-Asian patients may have different efficacy even within ESCC. Further analysis is needed to help draw an accurate conclusion.
PD-L1 Status and TILs
The predictive role of PD-L1 is still controversial in EC. Some studies believe that PD-L1 expression in tumor and immune cells is associated with the efficacy of PD-1/PD-L1 inhibitors (56). In the KEYNOTE-180 trial, patients with PD-L1 positive (CPS ≥ 10) had better ORR (13.8 vs. 6.35%) when treating with pembrolizumab (57). This was also supported by the trial KEYNOTE-181, which showed that pembrolizumab could significantly improve OS compared with chemotherapy in PD-L1 positive (CPS ≥ 10) EC patients (mOS: 9.3 vs. 6.7 months; HR = 0.69, 95% CI: 0.52–0.93, p = 0.0074) (47). However, PD-L1 status was not significantly related to ORR and DCR in the trial NCT02742935 of camrelizumab in Chinese ESCC (48). Besides, in another clinical trial of toripalimab (NCT02915432), the PD-L1 status was also not a predictive biomarker for clinical benefit in Chinese ESCC patients (58).
In some PD-L1 positive patients, the efficacy of PD-1/PD-L1 inhibitors is still low. Besides the heterogeneity of PD-L1 expression, TILs may be the possible reason. TILs consist of a group of heterogenous lymphocytes that infiltrate the tumor and participated in anti-tumor response. The high level of TILs in TME is correlated to better survival in patients with EC (59), NSCLC (60), breast cancer (61), and so on. Furthermore, TILs were also associated with the clinical benefit from PD-1/PD-L1 inhibitors in melanoma (62). In KEYNOTE-001 (NCT01295827) study, patients of melanoma were treated with pembrolizumab. CD8+ T-cell densities were higher in the pretreatment tumor samples of responding patients (63). In another study of patients with melanoma, CD8+, CD3+, and CD45RO+ T-cell densities in pretreatment samples were associated with response to PD-1 inhibitor (64). However, the predictive value of TILs and the most important cells in TILs are still unknown for EC so far.
The relationship of PD-L1 status and the efficacy of PD-1/PD-L1 inhibitors remains uncertain, and the function of other factors in TME still needed to be considered and provide more evidence in the future.
TMB, MSI, and dMMR
TMB is the number of non-synonymous somatic gene mutations (Mb) of sequenced DNA and higher TMB tumors are likely to produce more neoantigens, induce a specific T cell response, and further enhance the anti-tumor immunity. High TMB correlates with clinical benefit from ICIs in patients with melanoma and NSCLC (65, 66).
The number of TMB varies in different cancers. TMB is low in EC according to the reports of patients from western countries. (67). However, the analysis in Chinese EC patients showed higher TMB (68). The expression of TMB in EC is unclear, and the proper cutoff value of high TMB is also undecided. In phase Ib/II trial, NCT02915432, chemo-refractory ESSC patients received toripalimab and 11 (23.4%) patients with high TMB (≥12 Mutations/Mb) showed no significant advantage in ORR or OS (50). More studies are required to judge the role of TMB in EC patients and the proper cutoff value of high TMB.
Mismatch repair genes are genes that replace nucleotides incorrectly incorporated during DNA replication. Deficient DNA mismatch repair (dMMR) means a lack of these genes and produce a lot of short repeated sequences in the DNA (microsatellite) and more tumor-specific mutation (higher TMB) (69). MSI can be divided into high (MSI-H), low (MSI-L) and stable (MS-S). So far, NCCN guidelines have recommended pembrolizumab as the second-line or subsequent treatment for MSI-H or dMMR solid cancers, including EC (25). Although dMMR or MSI-H are predictive biomarkers for PD-1/PD-L1 inhibitors, this appears to be more of a gastric cancer phenomenon and only occurs in about 8% of EC patients (69).
Other Predictive Biomarkers
Despite the potential biomarkers mentioned above, many different predictive biomarkers are also studied now. The trial NCT02915432 analyzed the amplification of the chromosome 11q13 region in ESSC patients received toripalimab. Forty-eight percentage (24/50) patients had 11q13 amplification, which resulted in elevated mRNA expression of corresponding genes, including Cyclin D1 (CCND1) and fibroblast growth factor family members (FGF3/4/19) (50). Patients without 11q13 amplification, had considerably better ORR (30.8 vs. 4.2%, p = 0.024) and mPFS (3.7 vs. 2.0 months; HR = 0.47, 95% CI: 0.24–0.91, p = 0.025).
In another trial about camrelizumab, ESCC patients with an increased baseline lactate dehydrogenase (LDH) had lower ORR (p = 0.02) and shorter PFS (p = 0.002) and OS (p < 0.0001) than patients with normal LDH (NCT02742935) (70). Meanwhile, the increase of LDH during treatment was related to disease progression. Multivariate Cox analysis shown that LDH (HR = 0.18), C-reactive protein (CRP) (HR = 0.27), the number of organs involved (HR = 0.31), absolute monocyte count (HR =0.33), and Eastern Cooperative Oncology Group (ECOG) performance status (HR = 0.36) are independent prognostic factors in this trial.
Currently, the predictive role of a single biomarker is limited, combined prediction models of multiple biomarkers may be available in the future.
Conclusion
Based on the previous results, PD-1/PD-L1 inhibitors were durable and effective in EC, though many questions remain unanswered. Firstly, most trials are single-arm designed. More randomized controlled trials are demanded to compare the efficacy of PD-1/PD-L1 inhibitors and control treatment. Secondly, the control treatment in the current studies was chemotherapy alone rather than other more effective therapies, such as CRT. Thirdly, the response rates of PD-1/PD-L1 inhibitors alone were limited. Its combination with chemotherapy, RT, targeted drugs or other immune modulates may improve the anti-tumor activity. It is extremely important to identify patients who most likely gain clinical benefit from PD-1/PD-L1 inhibitors. More predictive biomarkers are investigated to refine the optimal patient for single-agent treatment and those require combination therapies. We also include the AEs of PD-1/PD-L1 inhibitor alone or combined with others, especially the incidence of pneumonitis.
Author Contributions
HY and LY designed the study. HY collected data of clinical trials and drafted the manuscript together with KW. TW, ML, BL, and SL coordinated, edited, and completed the drafting of the manuscript. LY revised and edited the final version of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by the Science and Technology Department, Henan Province (grant numbers: 152300410164, 192102310048, and SB201901113).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00300/full#supplementary-material
Footnotes
1. ^https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-22c3-pharmdx/pd-l1-ihc-22c3-pharmdx-for-autostainer-link-48-94448
2. ^https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-28-8-pharmdx/pd-l1-ihc-28-8-pharmdx-for-autostainer-link-48-76917
3. ^https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-_sp142-assay1.html
4. ^https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-_sp263-assay1.html
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.215512
2. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. (2013) 19:5598–606. doi: 10.3748/wjg.v19.i34.5598
3. Dong J, Levine DM, Buas MF, Zhang R, Onstad L, Fitzgerald RC, et al. Interactions between genetic variants and environmental factors affect risk of esophageal adenocarcinoma and barrett's esophagus. Clin Gastroenterol Hepatol. (2018) 16:1598–606. doi: 10.1016/j.cgh.2018.03.007
4. Kim J, Bowlby R, Mungall AJ, Robertson AG, Odze RD, Cherniack AD, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. (2017) 541:169–75. doi: 10.1038/nature20805
5. Dutton SJ, Ferry DR, Blazeby JM, Abbas H, Dahle-Smith A, Mansoor W, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. (2014) 15:894–904. doi: 10.1016/S1470-2045(14)70024-5
6. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. (2013) 39:1–8. doi: 10.1016/j.immuni.2013.07.012
7. Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. (2013) 39:61–73. doi: 10.1016/j.immuni.2013.07.005
8. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. (2015) 348:56–61. doi: 10.1126/science.aaa8172
9. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
10. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. (2017) 18:153. doi: 10.1038/nri.2017.108
11. Vrána D, Matzenauer M, Neoral C, Aujeský R, Vrba R, Melichar B, et al. From tumor immunology to immunotherapy in gastric and esophageal cancer. Int J Mol Sci. (2019) 20:13. doi: 10.3390/ijms20010013
12. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Lunceford M, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. (2018) 36:61–67. doi: 10.1200/JCO.2017.74.9846
13. Liu M, Guo F. Recent updates on cancer immunotherapy. Precision Clin Med. (2018) 1:65–74. doi: 10.1093/pcmedi/pby011
14. Alsina M, Moehler M, Lorenzen S. Immunotherapy of esophageal cancer: current status, many trials and innovative strategies. Oncol Res Treat. (2018) 41:266–71. doi: 10.1159/000488120
15. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
16. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. (2015) 1:1325–32. doi: 10.1001/jamaoncol.2015.2756
17. Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: from concept to the clinic. Cancer. (2016) 122:1659–71. doi: 10.1002/cncr.29889
18. Kelly RJ, Smith KN, Anagnostou V, Thompson E, Hales RK, Battafarano RJJ, et al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J Clin Oncol. (2019) 37 (Suppl. 4):142. doi: 10.1200/JCO.2019.37.4_suppl.142
19. Lee S, Ahn BC, Park SY, Kim DJ, Lee CG, Cho J, et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). Ann Oncol. (2019) 30(Suppl. 5):mdz266.018. doi: 10.1093/annonc/mdz266.018
20. Uboha NV, Maloney JD, McCarthy D, Deming DA, LoConte NK, Matkowskyj K, et al. Safety of neoadjuvant chemoradiation (CRT) in combination with avelumab (A) in the treatment of resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J Clin Oncol. (2019) 37 (Suppl. 15):4041. doi: 10.1200/JCO.2019.37.15_suppl.4041
21. Ende T, Clerq NC, Henegouwen MIB, Gisbertz SS, Meijer SL, Schokker S, et al. A phase II feasibility trial of neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: The PERFECT trial. J Clin Oncol. (2019) 37 (Suppl. 15):4045. doi: 10.1200/JCO.2019.37.15_suppl.4045
22. Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. (2011) 11:181. doi: 10.1186/1471-2407-11-181
23. Zahoor H, Luketich JD, Levy RM, Awais O, Winger DG, Gibson MK, et al. A propensity-matched analysis comparing survival after primary minimally invasive esophagectomy followed by adjuvant therapy to neoadjuvant therapy for esophagogastric adenocarcinoma. J Thorac Cardiovasc Surg. (2015) 149:538–47. doi: 10.1016/j.jtcvs.2014.10.044
24. Drake J, Tauer K, Portnoy D, Weksler B. Adjuvant chemotherapy is associated with improved survival in patients with nodal metastases after neoadjuvant therapy and esophagectomy. J Thorac Dis. (2019) 11:2546–54. doi: 10.21037/jtd.2019.05.66
25. NCCN Guidelines Version 2. Esophageal and Esophagogastric Junction Cancers. (2019). Available online at: https://jnccn.org/view/journals/jnccn/17/7/article-p855.xml
26. Welsh J, Settle SH, Amini A, Xiao L, Suzuki A, Hayashi Y, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. (2012) 118:2632–40. doi: 10.1002/cncr.26586
27. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. (2002) 20:1167–74. doi: 10.1200/JCO.2002.20.5.1167
28. Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. (2013) 14:627–37. doi: 10.1016/S1470-2045(13)70136-0
29. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. (2018) 379:2342–50. doi: 10.1056/NEJMoa1809697
30. Gray JE, Villegas AE, Daniel DB, Vicente D, Murakami S, Hui R, et al. Three-year overall survival update from the PACIFIC trial. J Clin Oncol. (2019) 37 (Suppl. 15):8526. doi: 10.1200/JCO.2019.37.15_suppl.8526
31. Mamdani H, Schneider BJ, Abushahin LI, Birdas TJ, Kesler K, Burney H, et al. Safety and efficacy of durvalumab following multimodality therapy for locally advanced esophageal and GEJ adenocarcinoma: results from Big Ten Cancer Research Consortium study. J Clin Oncol. (2019) 37 (Suppl. 15):4058. doi: 10.1200/JCO.2019.37.15_suppl.4058
32. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, et al. TENERGY: multicenter phase II study of atezolizumab monotherapy following definitive chemoradiotherapy with 5-FU plus cisplatin in patients with locally advanced esophageal squamous cell carcinoma. J Clin Oncol. (2019) 37 (Suppl. 15):TPS4141. doi: 10.1200/JCO.2019.37.15_suppl.TPS4141
33. Jing Z, Du D, Zhang N, Dai H, Wang X, Hua Y, et al. Combination of radiation therapy and anti-PD-1 antibody SHR-1210 in treating patients with Esophageal Squamous Cell Cancer. Int J Radiat Oncol Biol Phys. (2018) 102 (Suppl. 3):e31. doi: 10.1016/j.ijrobp.2018.07.520
34. Pang Q, Li X, Zhang W, Qian B, Zhang X, Chen X, et al. Safety and effect of radiation therapy combined with anti-PD-1 antibody SHR-1210 as first- line treatment on patients with intolerable concurrent chemora- diotherapy esophageal cancer: a phase 1b clinical trial. Int J Radiat Oncol Biol Phys. (2018) 102 (Suppl. 3):e39. doi: 10.1016/j.ijrobp.2018.07.538
35. Wanderley CW, Colón DF, Luiz JPM, Oliverira FF, Viacava PR, Leite CA, et al. Paclitaxel reduces tumor growth by reprogramming tumor- associated macrophages to an M1 profile in a TLR4- dependent manner. Cancer Res. (2018) 78:5891–900. doi: 10.1158/0008-5472.CAN-17-3480
36. Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. (2015) 4:e1008866. doi: 10.1080/2162402X.2015.1008866
37. Xu N, Yuan XL, Wang BH, Bai YX, Li EX, Li YY, et al. Tislelizumab in combination with chemotherapy for the treatment of Chinese patients (pts) with esophageal squamous cell carcinoma (ESCC): results from one cohort of an ongoing phase 2 study. J Clin Oncol. (2019) 37 (Suppl. 4):14. doi: 10.1200/JCO.2019.37.4_suppl.14
38. Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. (2009) 229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x
39. Lee DH, Kim HR, Keam B, Kato K, Kuboki Y, Vlahovic G, et al. Evaluation of safety and tolerability of durvalumab (D) and tremelimumab (T) in combination with first-line chemotherapy in patients (pts) with esophageal squamous-cell carcinoma. (ESCC). J Clin Oncol. (2019) 37 (Suppl. 4):146. doi: 10.1200/JCO.2019.37.4_suppl.146
40. Zhang B, Qi L, Wang X, Jiang J, Zhang X, Liu Y, et al. Phase 2 study of camrelizumab (anti-PD-1 antibody) combined with apatinib and chemotherapy for the first-line treatment of advanced esophageal squamous cell carcinoma. J Clin Oncol. (2019) 37 (Suppl. 15):4033. doi: 10.1200/JCO.2019.37.15_suppl.4033
41. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. (2003) 349:2241–52. doi: 10.1056/NEJMra035010
42. Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. (2015) 33:1760–9. doi: 10.1200/JCO.2014.60.1799
43. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. (2017) 18:631–9. doi: 10.1016/S1470-2045(17)30181-X
44. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
45. Cho BC, Kato K, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in advanced esophageal squamous cell carcinoma (ESCC): the phase III ATTRACTION-3 study. Ann Oncol. (2019) 30 (Suppl. 5):851–934. doi: 10.1093/annonc/mdz394.028
46. Kato K, Kojima T, Hochhauser D, Bennouna J, Hollebecque A, Lordick F, et al. Pembrolizumab in previously treated metastatic esophageal cancer: longer term follow-up from the phase 2 KEYNOTE-180 Study. J Clin Oncol. (2019) 37 (Suppl. 15):4032. doi: 10.1200/JCO.2019.37.15_suppl.4032
47. Shah MA, Adenis A, Enzinger PC, Kojima T, Muro K, Bennouna J, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J Clin Oncol. (2019) 37 (Suppl. 15):4010. doi: 10.1200/JCO.2019.37.15_suppl.4010
48. Huang J, Xu BH, Mo HN, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. (2018) 24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439
49. Huang J, Xu JM, Chen Y, Zhuang W, Zhang YP, Chen ZD, et al. Phase 3 study of camrelizumab vs chemotherapy for locally advanced/metastatic esophageal cancer: the ESCORT Study. In: Oral Presentation at the 15th OESO World Conference for Esophaeal Diseases (2019).
50. Wang F, Ren C, Zhao Q, Xu N, Shen L, Dai GH, et al. Association of frequent amplification of chromosome 11q13 in esophageal squamous cell cancer with clinical benefit to immune check point blockad. J Clin Oncol. (2019) 37 (Suppl. 15):4036. doi: 10.1200/JCO.2019.37.15_suppl.4036
51. FDA Approves Pembrolizumab for Advanced Esophageal Squamous Cell Cancer. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-esophageal-squamous-cell-cancer
52. He Y, Rivard CJ, Rozeboom L, Yu H, Ellison K, Kowalewski A, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. (2016) 107:1193–7. doi: 10.1111/cas.12986
53. Deng J, Zhao S, Zhang X, Jia K, Wang H, Zhou C, et al. OX40 (CD134) and OX40 ligand, important immune checkpoints in cancer. Onco Targets Ther. (2019) 12:7347–53. doi: 10.2147/OTT.S214211
54. Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. (2017) 76:167–82. doi: 10.1016/j.ejca.2017.01.011
55. Chen J, Luo S, Qin S, Cheng Y, Li Z, Fan Y, et al. Pembrolizumab vs chemotherapy in patients with advanced/metastatic adenocarcinoma (AC) or squamous cell carcinoma (SCC) of the esophagus as second-line therapy: analysis of the Chinese subgroup in KEYNOTE-181. Anal Oncol. (2019) 30 (Suppl. 5):mdz247.086. doi: 10.1093/annonc/mdz247.086
56. Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. (2019) 37:318–27. doi: 10.1200/JCO.2018.78.2276
57. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the Phase 2 KEYNOTE-180 study. JAMA Oncol. (2018) 5:546–50. doi: 10.1001/jamaoncol.2018.5441
58. Xu RH, Wang FH, Shi JH, Feng JF, Shen L, Yang SJ, et al. Recombinant humanized anti-PD-1 monoclonal antibody (JS001) as salvage treatment for advanced esophageal squamous cell carcinoma: preliminary results of an open-label, multi-cohort, phase Ib/II clinical study. J Clin Oncol. (2018) 36 (Suppl. 4):116. doi: 10.1200/JCO.2018.36.4_suppl.116
59. Noble F, Mellows T, Matthews LHM, Bateman AC, Harris S, Underwood TJ, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother. (2016) 65:651–62. doi: 10.1007/s00262-016-1826-5
60. Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. (2016) 4:59. doi: 10.1186/s40425-016-0165-6
61. Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. (2011) 167:207–10. doi: 10.1016/j.jss.2009.08.029
62. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. (2016)17:e542–51. doi: 10.1016/S1470-2045(16)30406-5
63. Tumeh PC, Harview CL, Yearley JH, Shintaka P, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) 515:568–71. doi: 10.1038/nature13954
64. Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. (2016) 6:827–37. doi: 10.1158/2159-8290.CD-15-1545
65. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. (2015) 348:124–8. doi: 10.1126/science.aaa1348
66. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. (2014) 371:2189–99. doi: 10.1056/NEJMoa1406498
67. Rech AJ, Balli D, Mantero A, Ishwaran H, Nathanson KL, Stager BZ, et al. Tumor immunity and survival as a function of alternative neopeptides in human cancer. Cancer Immunol Res. (2018) 6:276–87. doi: 10.1158/2326-6066.CIR-17-0559
68. Zang YS, Dai C, Xu XM, Cai X, Wang G, Wei J, et al. Comprehensive analysis of potential immunotherapy genomic biomarkers in 1000 Chinese patients with cancer. Cancer Med. (2019) 8:4699–708. doi: 10.1002/cam4.2381
69. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
70. Kato R, Yamasaki M, Urakawa S, Nishida K, Makino T, Morimoto-Okazawa A, et al. Increased Tim-3+ T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother. (2018) 67:1673–83. doi: 10.1007/s00262-018-2225-x
Keywords: esophageal cancer, programmed death-1, programmed death-ligand 1, combination therapy, predictive biomarkers
Citation: Yang H, Wang K, Wang T, Li M, Li B, Li S and Yuan L (2020) The Combination Options and Predictive Biomarkers of PD-1/PD-L1 Inhibitors in Esophageal Cancer. Front. Oncol. 10:300. doi: 10.3389/fonc.2020.00300
Received: 12 November 2019; Accepted: 20 February 2020;
Published: 05 March 2020.
Edited by:
Benjamin Frey, University of Erlangen Nuremberg, GermanyReviewed by:
Umberto Malapelle, University of Naples Federico II, ItalyFrederique Vegran, INSERM U1231 Lipides, Nutrition, Cancer (LNC), France
Vincent Bourbonne, Centre Hospitalier Regional Universitaire (CHU) de Brest, France
Copyright © 2020 Yang, Wang, Wang, Li, Li, Li and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Yuan, aG5obnlsQDEyNi5jb20=
 Hui Yang
Hui Yang Kunlun Wang
Kunlun Wang