- 1College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
- 2Department of Medical Oncology, Flinders Medical Centre, Adelaide, SA, Australia
- 3School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA, Australia
Introduction: Beta-blockers (BB) are commonly used to manage cardiovascular disease and may have benefits in controlling complications of anti-HER2 therapies. This study aimed to evaluate the association of pre-existing BB use with survival outcomes in patients initiating anti-HER2 therapy for advanced breast cancer (ABC).
Materials and Methods: Data from clinical trials EMILIA, TH3RESA, MARIANNE, and CLEOPATRA was pooled. Cox proportional analysis was used to assess the association between pre-existing BB use with survival outcomes in patients initiating anti-HER2 therapies.
Results: Of the 2,777 patients with HER2 positive ABC, 266 were using a BB at the time of anti-HER2 therapy initiation. BB use was associated with worse overall survival (OS) (adjusted HR = 1.27, 95% CI: 1.04–1.55). Sensitivity analysis in patients with pre-existing cardiovascular disease (CVD) also indicated that BB use was associated with worse OS (1.29, 1.02–1.63).
Conclusion: In large high-quality data, BB use at the time of anti-HER2 therapy initiation for ABC was independently associated with worse OS, regardless of CVD status. The finding is contrary to pre-study hypotheses and findings in other BC subtypes. Future research should aim to gain a deeper understanding of the effects of BBs on specific BC subtypes, cancer types, and cancer treatments.
Introduction
Human epidermal growth factor receptor 2 (HER2) amplification is associated with an increased incidence of metastasis in breast cancer (BC) (1). Monoclonal antibodies targeting HER2 and associated pathways, such as trastuzumab, have revolutionized the standard of care for patients with both early and advanced HER2 positive BC (2, 3). Whilst, anti-HER2 therapies are generally well-tolerated, they are associated with cardiotoxic effects, which may be further augmented when used in combination with anthracyclines (4, 5).
Pre-clinical evidence indicates that beta-blockers (BB) may have anticancer and immune-boosting effects, and clinical trials investigating the effects of BB on cancer are being initiated (6, 7). Guidelines also support the prophylactic use of BB to reduce anti-HER2 therapy-induced cardiotoxicities (8, 9). Specifically, BB may suppress the effects of catecholamine released through the stress associated activation of the sympathetic nervous system, which has been linked to cell survival, proliferation, and motility (10). Additionally, stimulation of β2 receptors may promote resistance to anti-HER2 therapies; thus it has also been hypothesized that BB may re-sensitize patients to anti-HER2 therapy (11). Despite this, the effects of BB have not been investigated in patients with HER2 positive advanced breast cancer (ABC) within large high-quality data. This is important as research has indicated that BB effects may not be the same between cancer subtypes and treatments (12–14).
This study aimed to evaluate the association of pre-existing oral BB use with overall survival (OS) and progression-free survival (PFS) in patients with HER2 positive ABC initiating anti-HER2 therapies.
Materials and Methods
Patient Population
Individual participant data (IPD) from the Roche sponsored phase III clinical trials CLEOPATRA (NCT00567190) (15–17), MARIANNE (NCT01120184) (18, 19), EMILIA (NCT00829166) (20, 21) and TH3RESA (NCT01419197) (22, 23) was utilized in this retrospective pooled analysis study.
All 4 trials were randomized controlled trials (RCT) and included patients with locally recurrent, unresectable or metastatic HER2 positive BC (15–23). CLEOPATRA and MARIANNE included treatment naïve patients (not accounting hormonal patients) (15–19). CLEOPATRA patients were randomly assigned in a 1:1 ratio to receive either placebo or pertuzumab, with trastuzumab plus docetaxel (15–17). Whilst, MARIANNE participants were randomly assigned 1:1:1 to trastuzumab plus a taxane, trastuzumab emtansine (T-DM1) plus placebo, or T-DM1 plus pertuzumab (18, 19). EMILIA and TH3RESA included patients with documented progression of disease despite prior therapies (20–23). Participants were randomly assigned 1:1 to either lapatinib plus capecitabine, or T-DM1 in EMILIA (20, 21). While in TH3RESA patients were randomly assigned 1:2 to the treatment of physician's choice, or T-DM1(22, 23).
The inclusion criteria for each of the trials included left ventricular ejection fraction (LVEF) ≥ 50%.
Participants of EMILIA and TH3RESA who respectively received either lapatinib plus capecitabine or treatment of physician's choice were excluded from the current pooled analyses as the study aimed to evaluate the effect of concomitant oral BB use on survival outcomes in patients initiating trastuzumab backboned therapies (i.e., trastuzumab, pertuzumab, and T-DM1 are associated with cardiotoxic effects).
Predictors and Outcomes
The primary assessed outcome was OS, with PFS assessed as a secondary outcome. OS was defined as the time from randomization to the last follow-up visit or death from any cause in all studies. PFS was defined as the time from randomization to disease progression or death from any cause, with progression assessed by the investigators using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 within CLEOPATRA and EMILIA while RECIST version 1.1 within MARIANNE and TH3RESA.
Across the four pooled clinical trials, available pre-treatment characteristic data included BB use status, age, body mass index (BMI; Normal, Overweight, Obese or Underweight), race (Asian or Non-Asian), presence of brain metastasis and visceral disease, albumin below the lower limit of normal (< LLN), Eastern Cooperative Oncology Group performance status (ECOG PS), estrogen/progesterone receptor status (ER/PR), any prior taxane, anthracycline or trastuzumab use, presence of arrhythmia, heart failure, cerebrovascular disease, hypertension, coronary artery disease, other cardiovascular diseases (CVD) or diabetes mellitus—this allowed examination of BB use in the univariable and adjusted analysis.
Secondary analysis of anonymized shared clinical trial data was confirmed negligible risk research by the Southern Adelaide Local Health Network, Office for Research and Ethics, and was exempted from review.
Statistical Analysis
Cox proportional hazard analysis was used to assess the association between pre-existing concomitant BB use with OS and PFS. Results were reported as hazard ratios (HR) with 95% confidence intervals (95%CI). Statistical significance was set at a threshold of P < 0.05 and was determined via the likelihood ratio test. All analyses were stratified by study and treatment. Regression adjusted analyses by age, BMI, race, presence of brain metastasis and visceral disease, albumin, ECOG PS, ER/PR status, any prior taxane, anthracycline or trastuzumab use, presence of arrhythmia, heart failure, cerebrovascular disease, hypertension, coronary artery disease, other CVD or diabetes mellitus were conducted. Analyses using doubly robust estimation—[regression model adjustment plus propensity score weighting adjustment (propensity score estimated using logistic regression)] were undertaken to confirm identified associations (24).
Sensitivity analysis of the association between pre-existing BB use with OS and PFS in patients with pre-existing CVD at baseline was conducted.
Kaplan-Meier analysis was used for plotting and estimating OS/PFS probabilities. All analyses were conducted using R version 3.4.3.
Results
Patient Population
Data was available from 2,777 patients (Supplementary Table 1), of which 266 (10%) were using an oral BB at the time of anti-HER2 therapy initiation. Supplementary Table 2 presents patient characteristic data by BB use status. Median follow-up was 50 [95% CI: 49–51] months in CLEOPATRA, 35 [34–36] months in MARIANNE, 47 [46–49] months in EMILIA and 35 [34–36] months in TH3RESA. Within the total 2,777 patients, 762 had pre-existing CVD at the time of anti-HER2 therapy initiation, of which 217 (29%) were using a BB.
Of the total 266 patients using a BB, 212 were using a selective BB, 51 a non-selective BB, and three were using a BB with intrinsic sympathomimetic activity (ISA). Bisoprolol (n = 68), atenolol (n = 67), metoprolol (n = 57), carvedilol (n = 23), and propranolol (n = 21) were the most used BB. Of the total 266 (10%) patients using a BB, 60 (7%) were in CLEOPATRA, 103 (10%) in MARIANNE, 51 (10%) in EMILIA and 52 (13%) in TH3RESA (P[χ2] = 0.021).
Association Between Concomitant BB Use and Survival
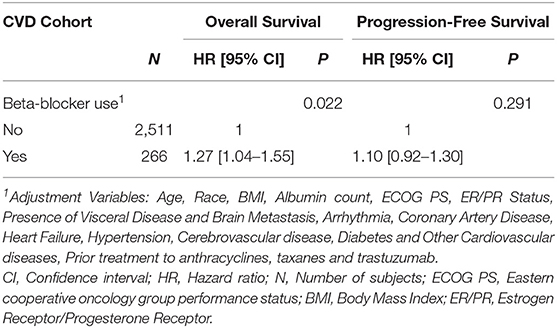
BB use was associated with worse OS (adjusted HR= 1.27, 95% CI:1.04–1.55). No statistically significant association between BB use with PFS was identified (adjusted HR = 1.10, 95% CI: 0.92–1.30) (Table 1). On univariable analysis, similar associations between BB use with OS and PFS were observed (Supplementary Table 3). Further, doubly robust estimation produced similar associations between BB use with OS (HR= 1.35, 95% CI: 1.03–1.77) and PFS (HR= 1.18, 95% CI: 0.94–1.49).
Figure 1 presents Kaplan-Meier estimates of OS and PFS by the status of BB use. No substantial heterogeneity in effect size of BB use was apparent between studies or treatment (Supplementary Table 4).
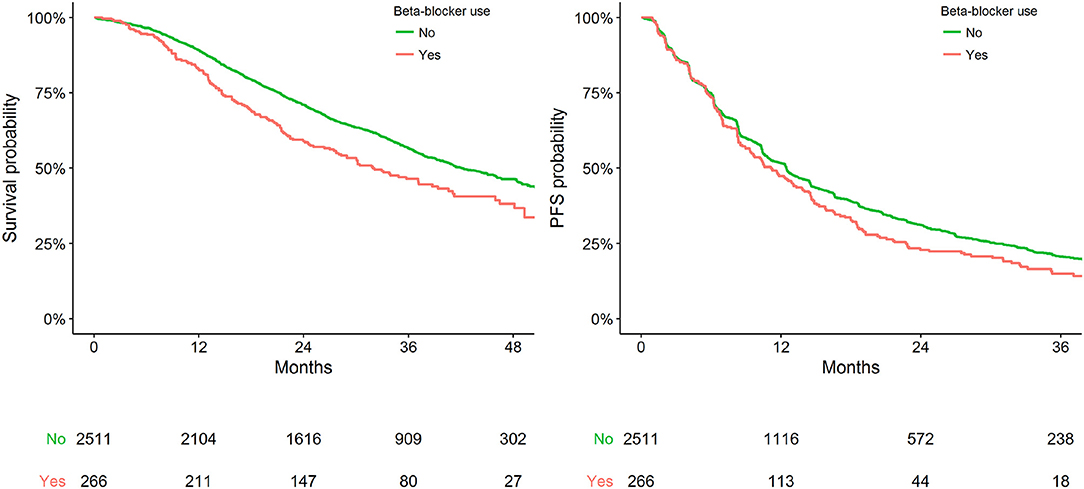
Figure 1. Kaplan-Meier plot representing survival outcomes in the pooled cohort by status of BB use.
Sensitivity Analysis
Within the available population, 762 patients had pre-existing CVD at the time of anti-HER2 therapy initiation, of which 217 (29%) were using a BB. BB use was significantly associated with worse OS in the sensitivity cohort (adjusted HR = 1.29, 95% CI: 1.02–1.63). No statistically significant association between BB use with PFS was identified (adjusted HR = 1.04, 95% CI: 0.85–1.26) (Table 2). Figure 2 presents Kaplan Meier estimates of OS and PFS by the status of BB use in patients with pre-existing CVD.
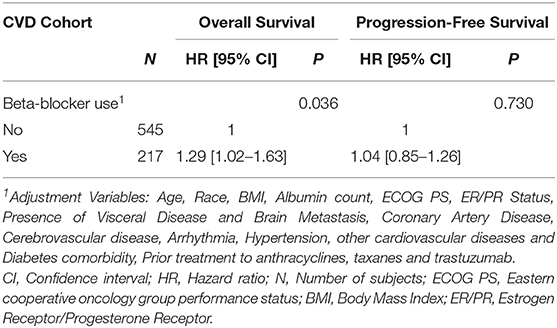
Table 2. Adjusted analysis of pre-existing BB use with OS and PFS in the cardiovascular disease cohort.
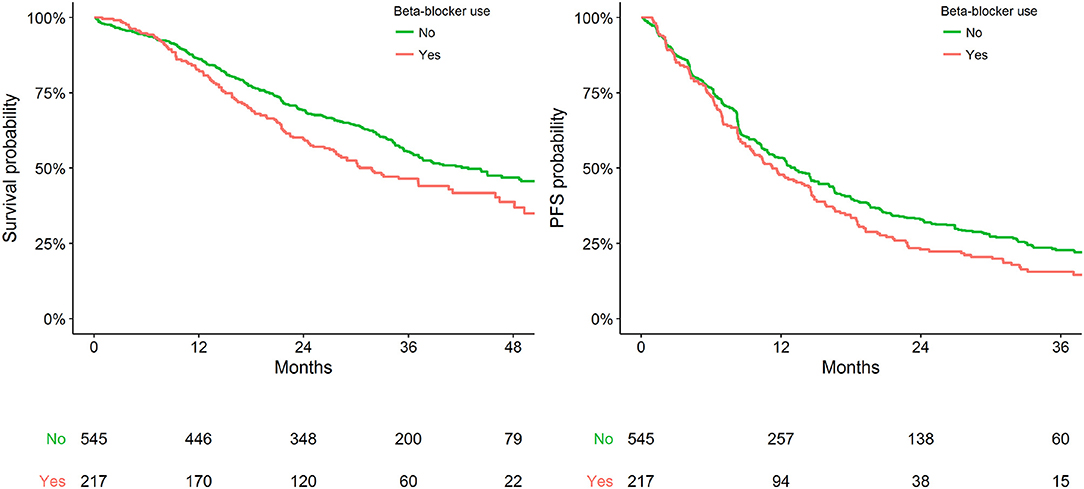
Figure 2. Kaplan-Meier plot representing survival outcomes in patients with pre-existing CVD by status of BB use.
Discussion
In post-hoc analysis of large high-quality data from prospective clinical trials, pre-existing oral BB use was independently associated with inferior OS in ABC patients initiating anti-HER2 treatments. Inferior OS was maintained in analyses adjusted for CVD and sensitivity subgroup analysis of those with pre-existing CVD.
Prior studies have investigated the effect of pre-existing BB use on survival outcomes in patients initiating treatments for early BC and advanced triple negative-breast cancer (TNBC). However, little is known about BB effects in patients with HER2 positive ABC. In advanced TNBC, BB use has demonstrated associations with improved survival outcomes in retrospective analyses of clinical trial and patient medical data (10, 25). Further, contemporary evidence supports the use of BB for protection from cardiotoxic regimens (8, 26). Specifically, an RCT indicated that post-diagnostic BB use was associated with improved cardiotoxic free survival compared to placebo in patients initiating trastuzumab and anthracycline therapy for HER 2 positive non-metastatic BC (26).
Despite prior findings in other BC subtypes, in our study of patients initiating anti-HER2 therapy for HER2 positive ABC, the pre-existing use of a BB was independently associated with worse OS. The biological basis of these findings remains unknown, despite adjusting analyses for age, BMI, race, presence of brain metastasis and visceral disease, albumin, ECOG PS, ER/PR status, any prior taxanes, anthracycline or trastuzumab use, and the presence of hypertension, heart failure, coronary artery disease, cerebrovascular disease, arrhythmia, other CVDs or diabetes mellitus. Furthermore, the association was observed in a sensitivity analysis of patients with pre-existing CVD. Whilst the analyses have been adjusted there may be still confounders, for example, the frailty of non-BB users.
Another future research interest is possible differences in the effects of non-selective vs. selective BB on cancer care. Biological studies suggest that antagonism of β2 receptors is responsible for the anti-cancer effects of BB rather than β1 antagonism. Hence non-selective BB could be more effective in improving survival outcomes (11). Prolonged BB exposure can also lead to β receptor upregulation, hampering the effectiveness of BB (10). This phenomenon has been demonstrated in observational studies where post-diagnostic BB use was associated with significant improvements in survival outcomes compared to pre-diagnostic BB use (27). In the present study, the sample of patients on non-selective BB was insufficient for an appropriately powered subgroup analysis (n = 51), and data on the length of prior BB use was not available.
A key limitation of this study is the unplanned post-hoc nature of the analysis (28). Nonetheless, this study pooled large (n = 2,777) and high-quality data from four contemporary RCTs (CLEOPATRA, MARIANNE, TH3RESA, and EMILIA), increasing study power and generalizability. Despite this, the sample was insufficient to allow subgroup analysis of BB effects by specific CVD types (e.g., arrhythmia, coronary artery disease). Furthermore, a lack of BB dose data inhibited exploratory analysis of the dose-response relationship was not possible (29, 30). While BB use was independently and significantly associated with worsened OS in the total and sensitivity cohort, the associations with PFS was not statistically significant. The reasons for this discrepancy are unclear, however, PFS is an imperfect surrogate that may be affected by variability in the timing of assessments, investigators, and measurement biases (31, 32). Additionally, OS associations may be confounded by subsequent therapies or cross over (31, 32). This indicates that the effect of BB may be compounded in patients with HER2 positive ABC rather than isolated to therapies including trastuzumab.
In large high-quality data, pre-existing use of BB in HER2 positive ABC patients initiating anti-HER2 therapies was independently associated with worse OS. The finding is contrary to pre-study hypotheses, findings in other BC subtypes, and emerging preclinical data for effects of BB on cancer. While this study does not justify the cessation of BB, and CVD should continue to be appropriately treated within HER2 positive ABC, the study presents important evidence that the effect of BB may not be the same between cancers or BC subtypes—with the impact potentially being negative within HER2 positive ABC. Future research is urgently required to validate findings in large real-world databases and prospective studies. Subsequent studies should aim to gain a deeper understanding of the effects of BB while considering dose responses, duration of prior exposure, and BB selectivity on specific BC subtypes, cancer types, and cancer treatments.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: clinicalstudydatarequest.com.
Ethics Statement
The study was an independent secondary analysis of anonymized shared clinical trial data. Secondary analysis of anonymized shared clinical trial data was confirmed negligible risk research by the Southern Adelaide Local Health Network, Office for Research and Ethics and was exempt from review. Data were accessed according to Roche's policy and process for clinical study data sharing. Additionally, these trials which were conducted by Roche were performed in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. Protocol approval was obtained from an independent ethics committee at each study site. All patients provided written informed consent.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
Research was undertaken with the financial support of Cancer Council South Australia's Beat Cancer Project on behalf of its donors and the State Government through the Department of Health (Grant IDs: 1159924 and 1127220). MS, AR, and RM receive financial fellowship support from the Cancer Council's Beat Cancer Project with support from its donors and the South Australian Department of Health. BK was supported by the National Breast Cancer Foundation Practitioner grant. AH is a researcher funded by a Postdoctoral Fellowship from the National Breast Cancer Foundation, Australia (PF-17-007).
Conflict of Interest
AR, MS, and RM report grants from Pfizer, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01130/full#supplementary-material
References
1. Peake BF, Nahta R. Resistance to HER2-targeted therapies: a potential role for FOXM1. Breast Cancer Manag. (2014) 3:423–31. doi: 10.2217/bmt.14.33
2. Mitri Z, Constantine T, O'regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract. (2012) 2012:7. doi: 10.1155/2012/743193
3. Florido R, Smith KL, Cuomo KK, Russell SD. Cardiotoxicity from human epidermal growth factor receptor-2 (HER2) targeted therapies. J Am Heart Assoc. (2017) 6:e006915. doi: 10.1161/JAHA.117.006915
4. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochr Syst Rev Interven. (2012) 2012:CD006243. doi: 10.1002/14651858.CD006243.pub2
5. Yu A, Singh J, Wang R, Liu J, Eaton A, Oeffinger K, et al. Cardiac safety of dual anti-HER2 therapy in the neoadjuvant setting for treatment of HER2-positive breast cancer. Oncologist. (2017) 22:1–6. doi: 10.1634/theoncologist.2016-0406
6. Knight JM, Kerswill SA, Hari P, Cole SW, Logan BR, D'souza A, et al. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer. (2018) 18:593. doi: 10.1186/s12885-018-4509-0
7. Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang J-MB, et al. Pre-operative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a Phase II randomized trial. Clin Cancer Res. (2019) 26:1803–11. doi: 10.1158/1078-0432.CCR-19-2641
8. Curigliano G, Cardinale D, Suter T, Plataniotis G, De Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines†. Ann Oncol. (2012) 23:vii155–66. doi: 10.1093/annonc/mds293
9. Ma Y, Bai F, Qin F, Li J, Liu J, Li D, et al. Beta-blockers for the primary prevention of anthracycline-induced cardiotoxicity: a meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol. (2019) 20:11. doi: 10.1186/s40360-019-0298-6
10. Spera G, Fresco R, Fung H, Dyck JRB, Pituskin E, Paterson I, et al. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol. (2017) 28:1836–1841. doi: 10.1093/annonc/mdx264
11. Liu D, Yang Z, Wang T, Yang Z, Chen H, Hu Y, et al. β2-AR signaling controls trastuzumab resistance-dependent pathway. Nat Oncogene. (2016) 35:47–8. doi: 10.1038/onc.2015.58
12. Yap A, Lopez-Olivo MA, Dubowitz J, Pratt G, Hiller J, Gottumukkala V, et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth. (2018) 121:45–57. doi: 10.1016/j.bja.2018.03.024
13. Coelho M, Squizzato A, Cassina N, Marino F, Ribeiro LV, Cosentino M. Effect of beta-blockers on survival of lung cancer patients: a systematic review and meta-analysis. Eur J Cancer Prev. (2019) 29:306–14. doi: 10.1097/CEJ.0000000000000544
14. Udumyan R, Montgomery S, Fang F, Valdimarsdottir U, Hardardottir H, Ekbom A, et al. Beta-blocker use and lung cancer mortality in a nationwide cohort study of patients with primary non–small cell lung cancer. Cancer Epidemiol Biomark Prev. (2020) 29:119–26. doi: 10.1158/1055-9965.EPI-19-0710
15. Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. (2012) 366:109–19. doi: 10.1056/NEJMoa1113216
16. Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. (2013) 14:461–71. doi: 10.1016/S1470-2045(13)70130-X
17. Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. (2015) 372:724–34. doi: 10.1056/NEJMoa1413513
18. Perez EA, Barrios C, Eiermann W, Toi M, Im Y-H, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2–positive, advanced breast cancer: primary results from the phase III MARIANNE Study. J Clin Oncol. (2016) 35:141–8. doi: 10.1200/JCO.2016.67.4887
19. Perez EA, Barrios CH, Eiermann W, Toi M, Im Y-H, Conte PF, et al. Phase III, randomized study of first-line trastuzumab emtansine (T-DM1) ± pertuzumab (P) vs. trastuzumab + taxane (HT) treatment of HER2-positive MBC: Final overall survival (OS) and safety from MARIANNE. J Clin Oncol. (2017) 35:1003. doi: 10.1200/JCO.2017.35.15_suppl.1003
20. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. New Engl J f Med. (2012) 367:1783–91. doi: 10.1056/NEJMoa1209124
21. Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:732–42. doi: 10.1016/S1470-2045(17)30312-1
22. Krop IE, Kim S-B, González-Martín A, Lorusso PM, Ferrero J-M, Smitt M, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. (2014) 15:689–99. doi: 10.1016/S1470-2045(14)70178-0
23. Krop IE, Kim S-B, Martin AG, Lorusso PM, Ferrero J-M, Badovinac-Crnjevic T, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. (2017) 18:743–54. doi: 10.1016/S1470-2045(17)30313-3
24. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. (2011) 173:761–7. doi: 10.1093/aje/kwq439
25. Munzone E, Botteri E, Rotmensz N, Cipolla CM, Zanelotti A, Viale LA, et al. Prognostic effect of beta blockers (BB) in triple-negative breast cancer (TNBC) patients. J Clin Oncol. (2017) 31:1061. doi: 10.1200/jco.2013.31.15_suppl.1061
26. Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, Mccaskill-Stevens W, et al. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. (2019) 73:2859–68. doi: 10.1016/j.jacc.2019.03.495
27. Zhong S, Yu D, Zhang X, Chen X, Yang S, Tang J, et al. β-Blocker use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Eur J Cancer Prev. (2016) 25:440–8. doi: 10.1097/CEJ.0000000000000192
28. Douglas CE, Henry M. Post-hoc data analysis: benefits and limitations. Curr Opin Aller Clin Immunol. (2013) 223–4. doi: 10.1097/ACI.0b013e3283609831
29. Calip GS, Yu O, Hoskins KF, Boudreau DM. Associations between diabetes medication use and risk of second breast cancer events and mortality. Cancer Causes Control. (2015) 26:1065–77. doi: 10.1007/s10552-015-0599-z
30. Chen L, Chubak J, Boudreau DM, Barlow WE, Weiss NS, Li C. Use of antihypertensive medications and risk of adverse breast cancer outcomes in a SEER–medicare population. Cancer Epidemiol Biomark Prev. (2017) 26:1603–10. doi: 10.1158/1055-9965.EPI-17-0346
31. Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. (2008) 13 (Suppl. 2):19–21. doi: 10.1634/theoncologist.13-S2-19
Keywords: beta-blockers, survival, breast cancer, HER2 positive, targeted therapy
Citation: Modi ND, Tan JQE, Rowland A, Koczwara B, Kichenadasse G, McKinnon RA, Wiese MD, Sorich MJ and Hopkins AM (2020) The Influence of Pre-Existing Beta-Blockers Use on Survival Outcomes in HER2 Positive Advanced Breast Cancer: Pooled Analysis of Clinical Trial Data. Front. Oncol. 10:1130. doi: 10.3389/fonc.2020.01130
Received: 03 February 2020; Accepted: 05 June 2020;
Published: 14 July 2020.
Edited by:
Aga Syed Sameer, King Saud bin Abdulaziz University for Health Sciences, Saudi ArabiaReviewed by:
Marianna De Camargo Cancela, National Cancer Institute (INCA), BrazilMujeeb Zafar Banday, Government Medical College (GMC), India
Copyright © 2020 Modi, Tan, Rowland, Koczwara, Kichenadasse, McKinnon, Wiese, Sorich and Hopkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natansh D. Modi, bW9kaTAwMzdAZmxpbmRlcnMuZWR1LmF1
†These authors have contributed equally to this work
 Natansh D. Modi
Natansh D. Modi Jin Quan Eugene Tan
Jin Quan Eugene Tan Andrew Rowland
Andrew Rowland Bogda Koczwara1,2
Bogda Koczwara1,2 Ganessan Kichenadasse
Ganessan Kichenadasse Ross A. McKinnon
Ross A. McKinnon Michael J. Sorich
Michael J. Sorich Ashley M. Hopkins
Ashley M. Hopkins