- 1Department of Pediatric Hematology/Oncology, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy
- 2Department of Surgery - Bambino Gesù Children's Hospital, IRCCS, Rome, Italy
- 3Department of Radiology, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy
Background: Pediatric patients with relapsed or refractory sarcomas have poor outcome and need novel therapies that provide disease control while maintaining an acceptable quality of life. The safety of vincristine, irinotecan, and pazopanib (VIPaz) association has not yet been published in this population.
Methods: A chart review was conducted in children and adolescents with relapsed or refractory bone and soft tissue sarcomas who received VIPaz in our institution.
Results: One hundred sixty-six patients with a diagnosis of soft or bone sarcoma were admitted to our hospital in the period between March 2015 and August 2018, 30 were relapsed or resistant. Seventeen out of 30 resistant or relapsed patients (median age, 14 years) received 114 VIPaz cycles (median six cycles per patient, range 1–17). Sixteen courses (15%) resulted in gastrointestinal toxicity with Grade two diarrhea; 35 courses (30%) resulted in Grade ≥3 neutropenia. One patient presented Grade two hypothyroidism after nine courses, and another one had Grade two hyperbilirubinemia after 12 courses. Two and five patients required a 25% dose reduction of irinotecan (because of diarrhea) and pazopanib (because of neutropenia four and hyperbilirubinemia 1), respectively. No patient experienced heart failure, hypertension, nor posterior reversible encephalopathy syndrome. Pneumothorax was not reported in any case even in lung metastatic patients. After two and four VIPaz cycles, we observed one complete response (CR), five partial responses (PRs), seven stable diseases (SDs), and four progressive diseases (PDs). With a median follow-up of 15 months (range 3–32), five out of 17 (29%) patients were alive, and four patients were in continuous CR after 12 VIPaz cycles.
Conclusions: The VIPaz regimen might be a safe option in children and adolescents with relapsed or refractory sarcomas otherwise unable to be enrolled in other clinical trials; on the other hand, the efficacy of pazopanib observed cannot be sustained from the current study.
Introduction
Sarcomas represent 10% of cancers in children and 8% in adolescents and young adults (1). Except for a few aggressive histotypes, the outcome has greatly improved due to the use of intensive chemotherapy combination, staging refinement, and more effective local control by surgery and irradiation. On the other hand, patients with relapsed or refractory tumors have an extremely poor outcome, with only 10% of patients alive at 5 years from diagnosis (2, 3). Thus, a continuous effort is underway to identify new effective treatments in this patients' setting. In the last two decades, irinotecan, a camptothecin analog, has been largely used as salvage therapy for relapsed or refractory sarcomas. Taking into consideration the two most frequent histotypes, rhabdomyosarcoma (RMS) and Ewing sarcoma (EWS), two irinotecan-containing regimens—associated with vincristine (VI) and temozolomide (IT), respectively—have been reported to improve survival in relapsed or refractory patients (4, 5). Many other international experiences have been published after these early reports, with several schedules associating different antineoplastic drugs with irinotecan (6–11), but without superior results to those with VI or IT. Many trials worldwide considered VI association as the backbone of choice for new drug combinations, being a regimen well-known and well-tolerated.
We decided to combine VI with a new anti-vascular endothelial growth factor (VEGF) molecule, pazopanib (VIPaz), given its promising results achieved in adult sarcomas (12, 13). Pazopanib is an oral pan-tyrosine kinase inhibitor (TKi) targeting VEGF receptor (VEGFR-1,−2,−3), c-Kit, and platelet-derived growth factor receptor (PDGFR) (14). In the largest phase III trial conducted for adult sarcomas (13), pazopanib reduced the risk of progression or death compared to placebo; main reported toxicities were fatigue, hypertension, anorexia, and diarrhea. Sporadically, thromboembolic events and left ventricular ejection fraction drop were reported in the pazopanib arm. This drug is nowadays approved in Italy for the treatment of relapsed sarcoma and advanced renal cell carcinoma in adulthood (15). Despite the promising results of pazopanib in adult soft tissue sarcomas, few experiences of its use in the pediatric setting were published. A phase I study involving 51 children affected by refractory solid tumors identified the maximum tolerated dose (MTD) for both formulations, tablets (450 mg/m2/day) and suspension (160 mg/m2/day) (16). Among evaluable patients, results reported were two partial responses (PRs) [one desmoplastic small round cell tumor (DSRCT) and one hepatoblastoma) and eight stable diseases (SDs) (seven out of with sarcoma) (16). To date, no phase II studies have been disclosed yet. Here we describe our experience on 17 patients with relapsed or refractory sarcomas who received VIPaz regimen as off-label compassionate use. To our knowledge, this is the first report of pazopanib combination in a pediatric and adolescent population.
Patients and Methods
A chart review of patients affected by sarcoma admitted between March 2015 and August 2018 at the Bambino Gesù Children Hospital, one of the main pediatric oncology centers in Italy, was done. All patients had histologically proven soft or bone sarcoma. For all patients, the histological diagnosis was revised by an experienced pathologist. Patients treated with VIPaz were identified using pharmacy administration records. An institutional review board approved the study, finalized to export data from hospital records; data thus selected are demographics, histology, radiology information, treatment administration, inpatient admissions, and status at data analysis. Data sources include hospital records, histopathologic registries (bone and soft tissue malignancies keywords), radiologic and laboratory records, pharmacy records (pazopanib keywords).
The presented data were censored at December 1, 2019.
Therapy
The treatment schedule was as follows: irinotecan 50 mg/m2/day intravenously for 5 days; vincristine 1.5 mg/m2 intravenously, administrated on days 1 and 8 of each cycle; and pazopanib 450 mg/m2 once daily (maximum 600 mg/day) per os per 21 days of each cycle. Pazopanib was given with clear liquids at least 1 h before or 2 h after a meal. Cefixime, 8 mg/kg once daily (maximum 400 mg/day), was administered orally, during the first week of each cycle to prevent irinotecan-associated diarrhea. If diarrhea occurred, loperamide was given and microbiological investigations were performed. The whole therapy was given on an outpatient basis. Informed consent was obtained from parents or legal guardians.
Assessment of Toxicity and Response
Toxicities were determined and graded using the Common Terminology Criteria for Adverse Events version 4.0 (2009).
Measurable and evaluable disease, as well as disease response (both primary tumor and metastases, if present), was defined and assessed according to the National Cancer Institute (NCI) Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). The objective response rate (ORR) [complete response (CR) + PR] and the tumor control rate (TCR) (CR + PR + SD) were reported after two VIPaz courses with a binomial exact confidence interval as the percentage of participants who have a CR, PR, or SD as determined by investigator assessment of response in accordance with RECIST version 1.1 (17). Overall survival (OS) and time to progression (TTP) were calculated according to the Kaplan–Meier method.
Results
One hundred sixty-six patients with a diagnosis of soft or bone sarcoma were admitted to our hospital in the period between March 2015 and August 2018, of which 25 relapsed and five were resistant to the first-line treatment. Seventeen patients out of 30 were treated at our department with the VIPaz regimen as second-line or third-line therapy.
Patients and tumors characteristics and final outcome are listed in Table 1.
No patient had a previous treatment with other inhibitors of angiogenesis or VEGF or similar drugs. None had previous irinotecan treatments.
The median age was 14 years (range 5–19 years). At the time of treatment start, one patient had a refractory tumor, five patients had progressive disease (PD) (two local; three had combined local and metastatic tumor), and 10 patients had relapsing tumors (three local, seven metastatic). Diagnoses included RMS (n = 8; five alveolar and three embryonal), EWS (n = 5), clear cell sarcoma (n = 1), CIC Fusion with Double-Homeobox (DUX) Transcription Factors (CIC-DUX) fusion-transcript-positive sarcoma (n = 1), undifferentiated sarcoma (n = 1), and DSRCT (n = 1). Previous treatment received by the patients included anthracycline and alkylating agent-based chemotherapy, radiotherapy (seven patients), and in one case, high-dose chemotherapy (busulfan-melphalan).
Toxicity
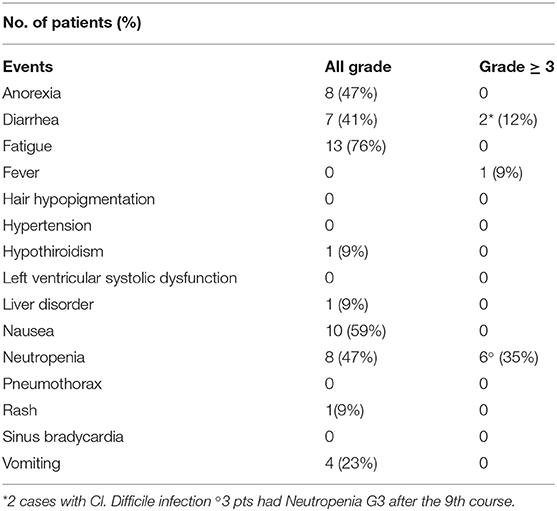
One hundred fourteen cycles of VIPaz (median six cycles per patient; range 1–17) were extrapolated from pharmacy data source. Toxicities were reported in Table 2.
Gastrointestinal toxicity with Grade 2 diarrhea occurred in 16 cycles (15%), and in two cases, Clostridium difficile was isolated. Grade ≥3 neutropenia occurred in 35 cycles (30%). One patient needed hospitalization and granulocyte colony-stimulating factor (G-CSF) support because of neutropenic fever with a negative blood culture. One patient with alveolar RMS presented Grade 2 hypothyroidism after the 9th VIPaz cycle and needed thyroid hormone supplements; he did not have prior head and neck radiotherapy nor high-dose chemotherapy. Another patient with DSRCT presented Grade two hyperbilirubinemia after the 12th VIPaz cycle not related to the disease. Two and five patients required a 25% dose reduction of irinotecan (because of diarrhea) and pazopanib (because of neutropenia four and hyperbilirubinemia 1), respectively. No patient experienced heart failure nor posterior reversible encephalopathy syndrome or pneumothorax.
Response and Outcome
After two cycles of VIPaz regimen, we observed one CR, five PRs, seven SDs, and four PDs. The ORR was 47% (95% CI, 16–68%), and the TCR was 82% (95% CI, 38–88%). All these responses were confirmed after the fourth course. Local treatment was done after the fourth course of therapy. Only two patients had local treatment: in patient 5, radiotherapy was administered for residual lung metastasis, and in patient 12 after 10 courses, radiotherapy was done before surgery to the vertebra (see Supplementary Table 1).
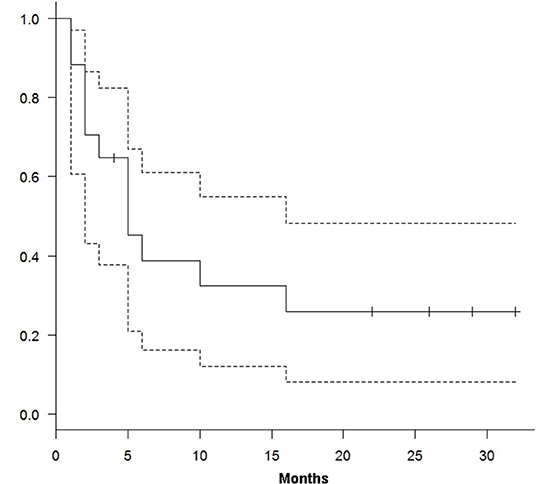
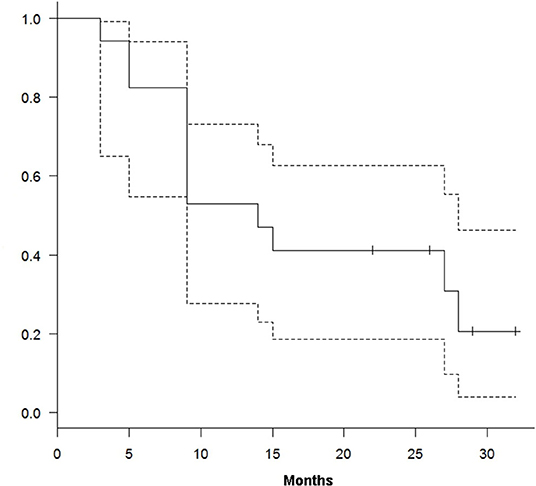
At a median follow-up of 15 months (range 2–32), 12 out of 17 patients have died of disease and five patients are alive (four in CR and one in PD). We obtained a median TTP and OS of 10 (range 1–32) and 15 (range 3–32) months, respectively. From the treatment start, TTP and OS were 39% (95% CI, 16–67%) and 82% (95% CI, 55–94%) at 6 months and 26% (95% CI, 11–81%) and 47% (95% CI, 23–68%) at 12 months (Figures 1, 2).
Discussion
Although the survival of pediatric sarcomas has increased dramatically in the last decades, the prognosis for the refractory/relapsed cases is still dismal.
During the last two decades, several regimens have been adopted as second- and third-line therapies in this patient setting, where it is a strong need for new effective therapies that combine the classic mechanisms of action of chemo with new molecular mechanisms. The VI combination was the precursor of many salvage regimens projected to this scope. From this assumption, we designed VIPaz with the aim to offer a compassionate and safe therapeutic option for patients unable to be enrolled in other ongoing clinical trials.
This is the first report that describes that VIPaz is safe and well-tolerated in children and adolescents with refractory sarcoma.
Because of the small population reviewed, we cannot compare toxicities with an internal cohort of a controlled set of patients in the same period of time.
No severe adverse events or discontinued treatment was reported during treatment; only five patients needed 25% pazopanib dose reduction, and in two patients, a 25% irinotecan dose reduction.
None of our patients had hypertension or pneumothorax. We had a case of hyperbilirubinemia after 12 courses, probably related to the pazopanib, rapidly normalized after dose reduction. Hematological toxicity, mainly neutropenia, was observed with VIPaz. While, with respect to the data emerging from the literature on the association of IT or VI/VIT (4–19), we had similar gastrointestinal toxicities or less, we observed a slightly higher percentage of neutropenia (30%), especially if it compared with the 16% reported by (4) with VI alone.
On the other hand, early data of VIT-0910 trial (NCT01355445), where relapsed or resistant RMS patients were randomized to VIT/VI, showed hematological toxicity grade ≥3 significantly increased in the VIT arm (81 vs. 59%, P = 0.02) (20). Publication is not yet available to argue a possible comparison between the safety of VIT or VIPaz.
In this report, we observed one confirmed CR after four VIPaz cycles in a patient with relapsed alveolar RMS (patient 2, Supplementary Table 1); one persistent PR in a patient with embryonal head and neck RMS who relapsed in the lung and at the time of analysis still in CR after 12 VIPaz courses plus radiotherapy (patient 5, Supplementary Table 1); one patient with vertebral EWS (patient 12, Supplementary Table 1) who progressed during first-line therapy and treated with 10 VIPaz cycles followed by radiotherapy plus radical surgery. Another patient with undifferentiated chemoresistant sarcoma (patient 13, Supplementary Table 1) obtained a durable PR and long-term survival. Interestingly, we observed a long-lasting SD in a patient with DSRCT (patient 15, Supplementary Table 1).
Several clinical trials are ongoing worldwide in order to improve the poor prognosis of resistant sarcomas mainly using VIT regimen. Combining VI to temozolomide (VIT), Raciborska et al. (18) and Setty et al. (19) obtained a TCR (CR + PR + SD) of 68% in EWS and 26% in RMS patients, respectively. In the study by Raciborska et al. (18), the reported 1-year OS was 40%. Certainly, in our study population, we obtained a TCR of 82% and 1-year OS of 47% in a pool of different diseases, and the data shown, albeit anecdotal on RMS, might be better evaluated in a larger sample.
Although it is largely recognized that sarcomas express several pro-angiogenic therapeutic targets, only a few trials with anti-VEGF inhibitors have been published in pediatric sarcomas mainly using bevacizumab (21–24). In preclinical and clinical studies, it has been demonstrated that pazopanib has activity in sarcomas, also as a single-agent therapy. Moreover, in preclinical sarcoma models, pazopanib has shown additive or synergistic effects in combination with chemotherapies (25). Nowadays, pazopanib has been rarely included in pediatric studies. Indeed, there are few clinical trials investigating pazopanib in children: one phase II single-agent trial in relapsed/refractory solid tumors, conducted by COG (NCT01956669), results not yet available; one phase I, open-label, multicenter trial testing pazopanib in combination with irinotecan and temozolomide in children and young adults with relapsed or refractory sarcoma (NCT03139331) and still recruiting.
Lastly, pazopanib has also been included in the COG protocol ARST1321 as a neoadjuvant therapy in non-rhabdo soft tissue sarcoma that can be removed by surgery. Preliminary results of these trials are not yet available.
Despite our limits, due to the monocentric chart review of a small cohort of patients with sarcomas of heterogeneous subtypes, we might state that the VIPaz regimen might be a safe therapy in pediatric patients with refractory or relapsed sarcomas not otherwise treated with other regimens or included in phase I–II clinical trials. Of course, the efficacy of pazopanib cannot be ascertained from the current study.
Prospective trials are necessary to further explore such treatment options in a selected population.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Bambino Gesù Children's hospital ethics committee. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
GM drew the study and scheme, wrote the paper, and performed statistical analysis. EM, VD, and AD edited and revised the manuscript. IR, AS, AM, and AC managed the patients and revised manuscript. PD reviewed all radiological studies.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Il sorriso di Costanza and Il cuore grande di Flavio Onlus for their support. VD is a recipient of a 2020-FUV (Fondazione Umberto Veronesi) postdoctoral fellowship.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01228/full#supplementary-material
Abbreviations
VIPaz, Vincristine/irinotecan/pazopanib; RMS, Rhabdomyosarcoma; EWS, Ewing sarcoma; VI, Irinotecan/vincristine; IT, Irinotecan/temozolomide; TKi, Tyrosine kinase inhibitor; VEGFR, Vascular endothelial growth factor receptor; PDGFR, Platelet-derived growth factor receptor; MTD, Maximum tolerated dose; DSRCT, Desmoplastic small round cell tumor; TC, Computed tomography; MRI, Magnetic resonance imaging; NCI, National Cancer Institute; RECIST, Response Evaluation Criteria in Solid Tumors; CR, Complete response; PR, Partial response; PD, Progressive disease; SD, Stable disease; ORR, Objective response rate; TCR, Tumor control rate; OS, Overall survival; TTP, Time to progression; VIT, Vincristine/irinotecan/temozolomide.
References
1. Amankwah EK, Conley AP, Reed DR. Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol. (2013) 5:147–162 doi: 10.2147/CLEP.S28390
2. Raney RB Jr, Crist WM, Maurer HM, Foulkes MA. Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response: a report from the Intergroup Rhabdomyosarcoma Study I. Cancer. (1983) 52:44–50. doi: 10.1002/1097-0142(19830701)52:1<44::AID-CNCR2820520110>3.0.CO
3. Pappo AS, Anderson JR, Crist WM, Wharam MD, Breitfeld PP, Hawkins D, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. (1999) 17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487
4. Pappo AS, Lyden E, Breitfeld P, Donaldson SS, Wiener E, Parham D, et al. Children's Oncology Group. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children's Oncology Group. J Clin Oncol. (2007). 25:362–9. doi: 10.1200/JCO.2006.07.1720
5. Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, et al. Temozolomide and intravenous irinotecan for treatment of advanced ewing sarcoma. Pediatr Blood Cancer. (2007) 48:132–9. doi: 10.1002/pbc.20697
6. Dharmarajan KV, Wexler LH, Wolden SL. Concurrent radiation with irinotecan and carboplatin in intermediate- and high-risk rhabdomyosarcoma: a report on toxicity and efficacy from a prospective pilot phase II study. Pediatr Blood Cancer. (2013) 60:242–7. doi: 10.1002/pbc.24205
7. McGregor LM, Spunt SL, Furman WL, Stewart CF, Schaiquevich P, Krailo MD, et al. Phase 1 study of oxaliplatin and irinotecan in pediatric patients with refractory solid tumors: a children's oncology group study. Cancer. (2009) 115:1765–75. doi: 10.1002/cncr.24175
8. Hartmann C1, Weinel P, Schmid H, Grigull L, Sander A, Linderkamp C, et al. Oxaliplatin, irinotecan, and gemcitabine: a novel combination in the therapy of progressed, relapsed, or refractory tumors in children. J Pediatr Hematol Oncol. (2011) 33:344–9. doi: 10.1097/MPH.0b013e31820994ec
9. Zak D, Styler MJ, Rosenbluth JZ, Brodsky I. Combination of gemcitabine and irinotecan for recurrent metastatic osteogenic sarcoma. Clin Adv Hematol Oncol. (2005) 3:297–9.
10. Crews KR, Stewart CF, Liu T, Rodriguez-Galindo C, Santana VM, Daw NC. Effect of fractionated ifosfamide on the pharmacokinetics of irinotecan in pediatric patients with osteosarcoma. J Pediatr Hematol Oncol. (2004) 26:764–7. doi: 10.1097/00043426-200411000-00016
11. Yoon JH, Kwon MM, Park HJ, Park SY, Lim KY, Joo J, Park BK1. A study of docetaxel and irinotecan in children and young adults with recurrent or refractory Ewing sarcoma family of tumors. BMC Cancer. (2014). 14:622. doi: 10.1186/1471-2407-14-622
12. Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organization for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. (2009) 27:3126–32. doi: 10.1200/JCO.2008.21.3223
13. Van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet. (2012) 379:1879–86. doi: 10.1016/S0140-6736(12)60651-5
14. Harris PA1, Boloor A, Cheung M, Kumar R, Crosby RM, Davis-Ward RG, et al. Discovery of 5-[[4-[(2,3-dimethyl-2Hindazol-6-yl) methylamino]-2-pyrimidinyl]amino]-2- methylbenzenesulfonamide(pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. (2008) 51:4632–40. doi: 10.1021/jm800566m
15. Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. (2010) 28:1061–8. doi: 10.1200/JCO.2009.23.9764
16. Glade Bender JL, Lee A, Reid JM, Baruchel S, Roberts T, Voss SD, et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol. (2013) 31:3034–43. doi: 10.1200/JCO.2012.47.0914
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
18. Raciborska A, Bilska K, Drabko K, Chaber R, Pogorzala M, Wyrobek E, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer. (2013) 60:1621–5. doi: 10.1002/pbc.24621
19. Setty BA, Stanek JR, Mascarenhas L, Miller A, Bagatell R, Okcu F, et al. VIncristine, irinotecan, and temozolomide in children and adolescents with relapsed rhabdomyosarcoma. Pediatr Blood Cancer. (2018) 65:e26728. doi: 10.1002/pbc.26728
20. Defachelles AS, Bogart E, Casanova M, Merks H, Bisogno G, Calareso G, et al. Randomized phase 2 trial of the combination of vincristine and irinotecan with or without temozolomide, in children and adults with refractory or relapsed rhabdomyosarcoma (RMS). J Clin Oncol. (2019) 37. doi: 10.1200/JCO.2019.37.15_suppl.10000
21. Wagner L, Turpin B, Nagarajan R, Weiss B, Cripe T, Geller J. Pilot study of vincristine, oral irinotecan, and temozolomide (VOIT regimen) combined with bevacizumab in pediatric patients with recurrent solid tumors or brain tumors. Pediatr Blood Cancer. (2013) 60:1447–51. doi: 10.1002/pbc.24547
22. Okada K, Yamasaki K, Tanaka C, Fujisaki H, Osugi Y, Hara J. Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Jpn J Clin Oncol. (2013) 43:1073–9. doi: 10.1093/jjco/hyt124
23. Chisholm JC, Merks JHM, Casanova M, Bisogno G, Orbach D, Gentet JC, et al. European paediatric Soft tissue sarcoma Study Group (EpSSG) and the European Innovative Therapies for Children with Cancer (ITCC) Consortium. Open-label, multicentre, randomised, phase II study of the EpSSG and the ITCC evaluating the addition of bevacizumab to chemotherapy in childhood and adolescent patients with metastatic soft tissue sarcoma (the BERNIE study). Eur J Cancer. (2017). 83:177–84. doi: 10.1016/j.ejca.2017.06.015
24. Kuo C, Kent PM, Logan AD, Tamulonis KB, Dalton KL, Batus M, et al. Docetaxel, bevacizumab, and gemcitabine for very high risk sarcomas in adolescents and young adults: a single-center experience. Pediatr Blood Cancer. (2017) 64:e26265. doi: 10.1002/pbc.26265
Keywords: vincristine, irinotecan, pazopanib, pediatric sarcomas, new drugs
Citation: Russo I, Di Paolo V, Crocoli A, Mastronuzzi A, Serra A, Di Paolo PL, Di Giannatale A, Miele E and Milano GM (2020) A Chart Review on the Feasibility and Safety of the Vincristine Irinotecan Pazopanib (VIPaz) Association in Children and Adolescents With Resistant or Relapsed Sarcomas. Front. Oncol. 10:1228. doi: 10.3389/fonc.2020.01228
Received: 29 April 2020; Accepted: 16 June 2020;
Published: 06 August 2020.
Edited by:
Rimas J. Orentas, Seattle Children's Research Institute, United StatesReviewed by:
Abha Gupta, Hospital for Sick Children, CanadaPeter Adamson, Sanofi Genzyme, United States
Copyright © 2020 Russo, Di Paolo, Crocoli, Mastronuzzi, Serra, Di Paolo, Di Giannatale, Miele and Milano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Maria Milano, Z2l1c2VwcGVtYXJpYS5taWxhbm9Ab3BiZy5uZXQ=
 Ida Russo1
Ida Russo1 Virginia Di Paolo
Virginia Di Paolo Alessandro Crocoli
Alessandro Crocoli Angela Mastronuzzi
Angela Mastronuzzi Angela Di Giannatale
Angela Di Giannatale Evelina Miele
Evelina Miele Giuseppe Maria Milano
Giuseppe Maria Milano