- 1The Department and Key Laboratory of Endocrinology and Metabolism, The First Medical Center of PLA General Hospital, Beijing, China
- 2Department of Endocrinology, The 940th Hospital of Joint Logistics Support force of Chinese PLA, Lanzhou, China
- 3Department of Endocrinology, Hainan Hospital of PLA General Hospital, Sanya, China
- 4The 8th Medical Center of Chinese PLA General Hospital, Beijing, China
- 5Department of Clinical Pharmacy, Shenyang Pharmaceutical University, Shenyang, China
Purpose: Genetic mutations may play an important role in the progression and invasion of thyroid carcinoma (TC), and their coexistence may result in mutational synergy. The presence of the BRAFV600E mutation, as well as mutations affecting the TERT promoter, RAS, CHEK2 and RET/PTC, may all have an impact on prognosis. The aim of this study was to explore whether synergy between the coexistent mutations predicts histopathological prognostic factors that influence disease outcome.
Methods: A comprehensive literature search of PubMed, Embase and the Cochrane Library, from their inception until January 2020. Primary outcomes included: disease stage, lymph node metastasis, extrathyroidal extension and distant metastasis; while, secondary outcomes included: tumor recurrence, mortality, invasion of thyroid capsule, multiplicity, presented as an odds ratio (OR) with 95% credible intervals (CrI).
Results: 27 publications (comprising 9 active intervention arms), involving 8,388 TC patients, were selected. Network meta-analytic estimates of active interventions contrasted with other active interventions, with random effects, were calculated. In terms of outcomes focus on overall TC, BRAFV600E + TERT co-mutation ranked highest for diseases stage (OR = 5.74, 95% CrI: 3.09–10.66), as well as lymph node metastasis, extrathyroidal extension (5.74, 4.06–8.10), tumor recurrence (7.21, 3.59–14.47), and invasion of the thyroid capsule (3.11, 1.95–4.95). BRAFV600E + TERT co-mutation ranked secondary in distant metastasis, mortality, and multiplicity that ranked highest was TERT+RAS or RAS. When we were limited to the study of patients with papillary TC (PTC), BRAFV600E + TERT always ranked highest for primary outcomes: disease stage (6.39, 3.13–13.04), lymph node metastasis, extrathyroidal extension (5.80,3.89–8.64) and distant metastasis (7.33, 3.00–17.89), while BRAFV600E + TERT again ranked highest in secondary outcomes: tumor recurrence (7.23,3.37–15.51), mortality (9.26, 3.02–28.42), invasion of thyroid capsule (3.20,2.01–5.11), and multiplicity.
Conclusions: In this molecular marker mutation-based systematic review and network meta-analysis, we found that coexistent BRAFV600E + TERT genetic co-mutations predicted poor histopathological prognosis, including progression, invasion, and metastasis, especially in PTC. For the overall TC, the BRAFV600E + TERT + RAS triple mutations may have a greater impact on the prognosis, and further research should related to potentially important features. This study is registered with PROSPERO, number CRD42019143242.
Introduction
Thyroid carcinoma (TC) is the most common type of endocrine malignancy, the incidence of which, has undergone a steady increase over the last two decades worldwide, becoming the sixth leading cause of malignant neoplasms in women (1). According to the various molecular origins of TC, its pathological type and sub-type can be defined as either papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), poorly differentiated thyroid carcinoma (PDTC), or anaplastic thyroid carcinoma (ATC). In addition, medullary thyroid carcinoma (MTC), which originates from parafollicular cells, also accounts for a small proportion of thyroid malignancies. Differentiated thyroid cancer mainly includes PTC and FTC, of which, PTC represents the most common clinical pathological type, accounting for more than 80% of all TC cases (2, 3). Although at present, the mortality rate for TC has not risen rapidly, as the degree of malignancy is generally low, meaning that the majority of TC patients achieve a good therapeutic outcome. For patients with no metastasis, surgery represents in usually the first-line treatment. Although the differentiation of TC is good, the degree of malignancy is low, and I131 treatment is the main treatment after traditional thyroidectomy or near-total thyroidectomy. However, some TC (especially PTC) tumors are highly invasive, postoperative recurrence, metastasis or even death occur frequently. Therefore, novel therapeutic strategies are urgently needed (4, 5).
In the era of precision medicine, the ultimate goal pursued by clinicians is to accurately assess the patient’s condition and prepare the most appropriate individualized treatment plan (6). Therefore, research on the mutational profile in thyroid carcinomas is a priority. Recent medical research has resulted in great progress in the study of thyroid tumorigenesis at the molecular level. Numerous studies have found that certain genetic mutations are significantly correlated with the development, progression, prognosis, and diagnosis of TC (7–10). Moreover, the coexistence of several key mutations may lead to mutational synergy. Therefore, there is an increasing requirement for more accurate prognostic molecular markers, to be used as tools in the prediction of histopathological prognostic factors, which may impact disease outcome.
Genetic mutations play an important role in the etiology, progression, and invasion of TC. To this end, recent studies have focused on the identification of genetic mutations as molecular markers, which will of utmost clinical importance in predicting the progression and prognosis of TC (7–10). Mutations targeting components of the well-characterized mitogen-activated protein kinase (MAPK) signaling pathway have been identified as driver mutations (11, 12). Molecular alterations affecting MAPK signaling include: i) point mutations in the B-Raf proto-oncogene (BRAF) and RAS genes, ii) chromosomal rearrangements of RET/papillary thyroid cancer (PTC) and PAX8/peroxisome proliferator-activated receptor γ (PPARγ) (13, 14), and iii) the recently identified Telomerase Reverse Transcriptase (TERT) promoter mutations (15, 16). The most frequently-occurring mutation in the BRAF gene is V600E (BRAFV600E), which promotes the constitutive activation of BRAF kinase (17, 18) and is widely accepted as a highly specific molecular marker for PTC.
Although several studies have shown that these individual genetic mutations may be associated with certain histopathological features and outcomes (19, 20), their coexistence may have a synergistic effect, thus having a higher impact on disease prognosis. Moon et al. demonstrated that coexistence of the BRAFV600E and TERT promoter mutations has a synergistic effect on the clinical outcomes in PTC, whereas each mutation alone exerts only a modest effect (21). Therefore, the aim of our systematic review and network meta-analysis was to provide a more accurate measure of TC prognosis by identifying the impact of coexisting mutations.
Methods
This network meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and PRISMA extension guidelines (22, 23). A prospective protocol was created and uploaded to the PROSPERO online platform using the registration number CRD42019143242 (24).
Search Strategy
To perform the systematic review and network meta-analysis, we searched PubMed, Embase and the Cochrane Library for relevant records published in English and Chinese (from database inception date to January 2020) using the search terms “genetic mutations” OR “gene mutations” AND “thyroid carcinoma” OR “thyroid cancer,” and their Medical Subject Headings (MeSH) terms combined with a list of all included studies (see details in Supplementary Table 1). We included clinical data comparing coexistent genetic mutations with single genetic mutations as molecular markers for predicting the histopathological features associated with prognosis.
Eligibility and Exclusion Criteria
Studies had to include at least two of the following genetic mutations molecular marker types: the BRAFV600E gene mutation, TERT promoter mutations, RAS gene mutations, CHEK2 mutations and RET/PTC gene rearrangements. Participants had to be adults (≥18 years old and of both genders) with a primary diagnosis of TC, with no specific TC type restrictions. We excluded conference abstracts, reviews, meta-analyses, letters, and records, which did not meet our criteria, such as not reporting coexisting genetic mutations etc. After removing duplicate records and performing a preliminary screening of titles and abstracts, two researchers (WL and ZL) independently assessed full-text and supplementary materials of the selected records for final inclusion. Potentially relevant full-text published articles were also retrieved and assessed. Disagreements were resolved by consensus or by requesting an additional round of reviewing by ZYS or LZH.
Data Extraction
Although the ‘histopathological features associated with a worsened prognosis’ was our outcome of interest, this term was too broad for describing mutation-specific TC phenotypes. Instead, we broke the term ‘histopathological features associated with worsened prognosis’ into primary outcomes, such as disease stage, lymph node metastasis, extrathyroidal extension, and distant metastasis, and secondary outcomes including tumor recurrence, mortality, invasion of the thyroid capsule, and multiplicity. These outcomes were deemed to represent a suitable alternative for assessing histopathological prognostic features in the majority of the selected studies.
Two researchers (LW and LZ) independently used a standardized electronic form to extract and summarize the following data: study first author, publication year, region, TC type, sample size, specimen type, detection method, molecular markers (coexisting mutations, single mutations, and no mutation), and available outcomes.
Quality Assessment and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Rating Scale
Two reviewers (LW and LZ) evaluated the risk of bias in our analyses, based on the original records and their supplementary materials, using the Critical Appraisal Skills Programme (CASP) scales (25), which were designed for the assessment of observational studies. Twelve aspects were assigned an assessment index associated with the risk of bias as ‘yes,’ ‘no,’ or ‘cannot tell.’ Moreover, we used the GRADE framework to develop and present summaries of evidence (26).
Data Synthesis and Statistical Analysis
To estimate the effect sizes for the categorical outcomes using our outcome data, we computed the odds ratio (OR) with 95% confidence intervals (CI, for standardized meta-analysis) and 95% credible intervals (CrI, for network meta-analysis). In order to address heterogeneity relating to the outcomes documented in each of the selected study records, we used the random effects model, which is best suited to resolving heterogeneity in standardized meta-analyses (27, 28), to record the two-sided P value and I2 statistic (the ratio of true heterogeneity to total observed variation) measures.
To visualize network geometry and node connectivity, we generated network plots for the primary outcomes. Moreover, we undertook consistency testing via both direct and indirect evidence using the random effects model, and were satisfied with the level of consistency in our network meta-analysis. We use the inconsistency factor (IF) to determine the factors that affect the authenticity of network meta-analysis. If the IF value is close to 0, then it means that direct evidence and indirect evidence are very consistent. Mean rank and surface under the cumulative ranking curve (SUCRA) values were produced for primary and secondary outcomes (29, 30). Publication bias was determined by adjusting funnel plot asymmetry. Meta-analysis was carried out using the “mvmeta” and “network” packages of Stata MP software, version 14.0.
Results
Systematic Review and Characteristics
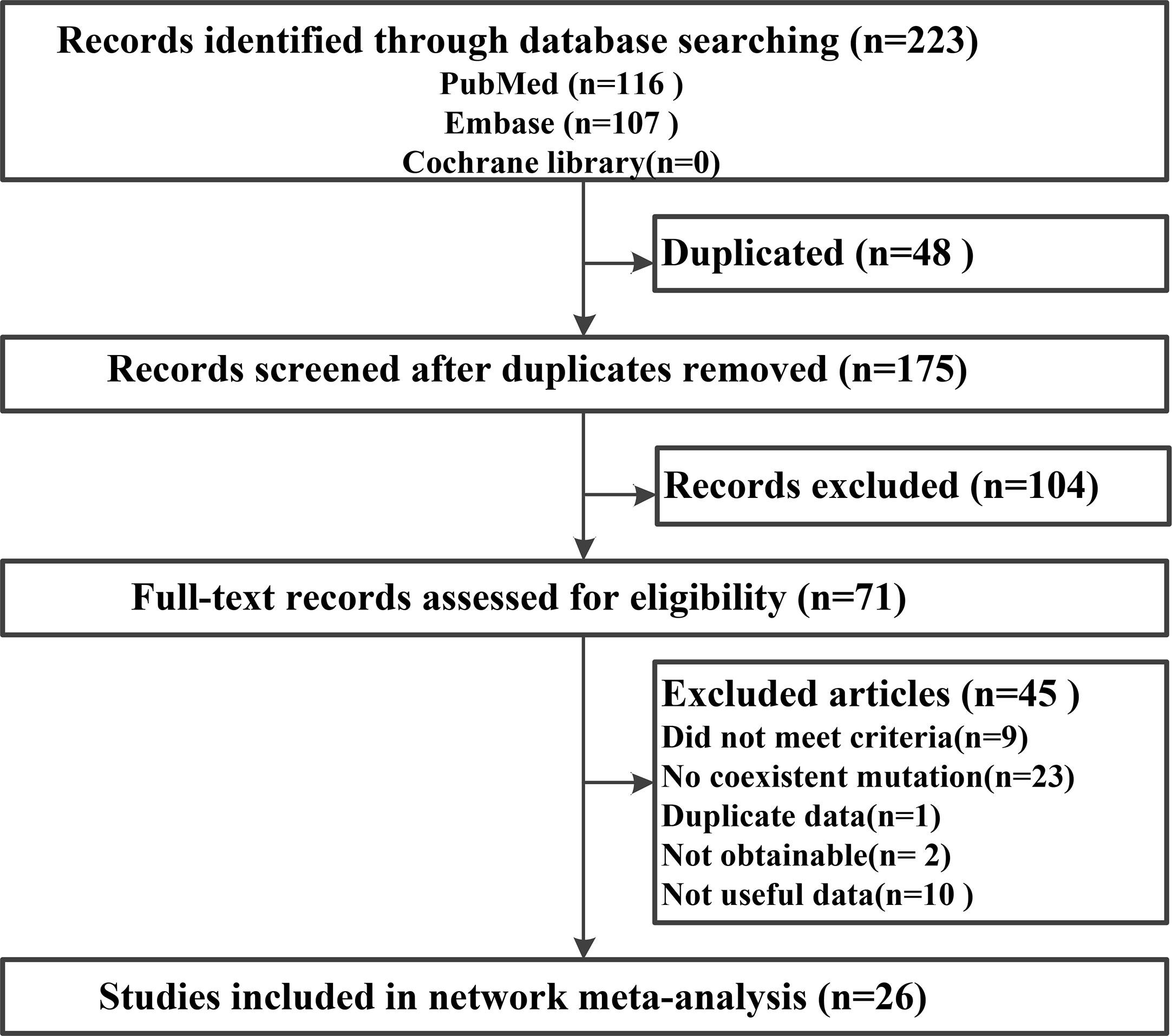
Electronic searches identified a total of 223 potentially eligible records. Following the elimination of duplicate records and a preliminary review, 71 full-text records were assessed. Further exclusion of unsuitable articles yielded a final 26 studies (31–56) for use in network meta-analysis (Figure 1). Overall, data relating to the histopathological features collected from 8,388 patients and documented in 26 studies met our inclusion criteria (Table 1).
We next evaluated four pairs of coexistent genetic mutations: BRAFV600E + TERT, BRAFV600E + CHEK2, TERT + RAS, and BRAFV600E + RET/PTC, in addition to four isolated genetic mutations involving the same signaling proteins: BRAFV600E, TERT, RAS, CHEK2, and RET/PTC. Figure 2 shows the network of eligible comparisons for lymph node metastasis. According to the meta-analysis plots, circles represent a coexistent or single genetic mutation. Circle size is proportional to the total number of patients with thyroid carcinoma, while the line width is proportional to the number of studies used in the head-to-head comparisons. The most common coexisting and single genetic mutation comparisons, which made a large contribution to each network estimations, were high frequency BRAFV600E + TERT versus BRAFV600E; and BRAFV600E + TERT versus TERT. CASP scales indicated that the 26 selected studies were of adequate quality (Supplementary Table 2).
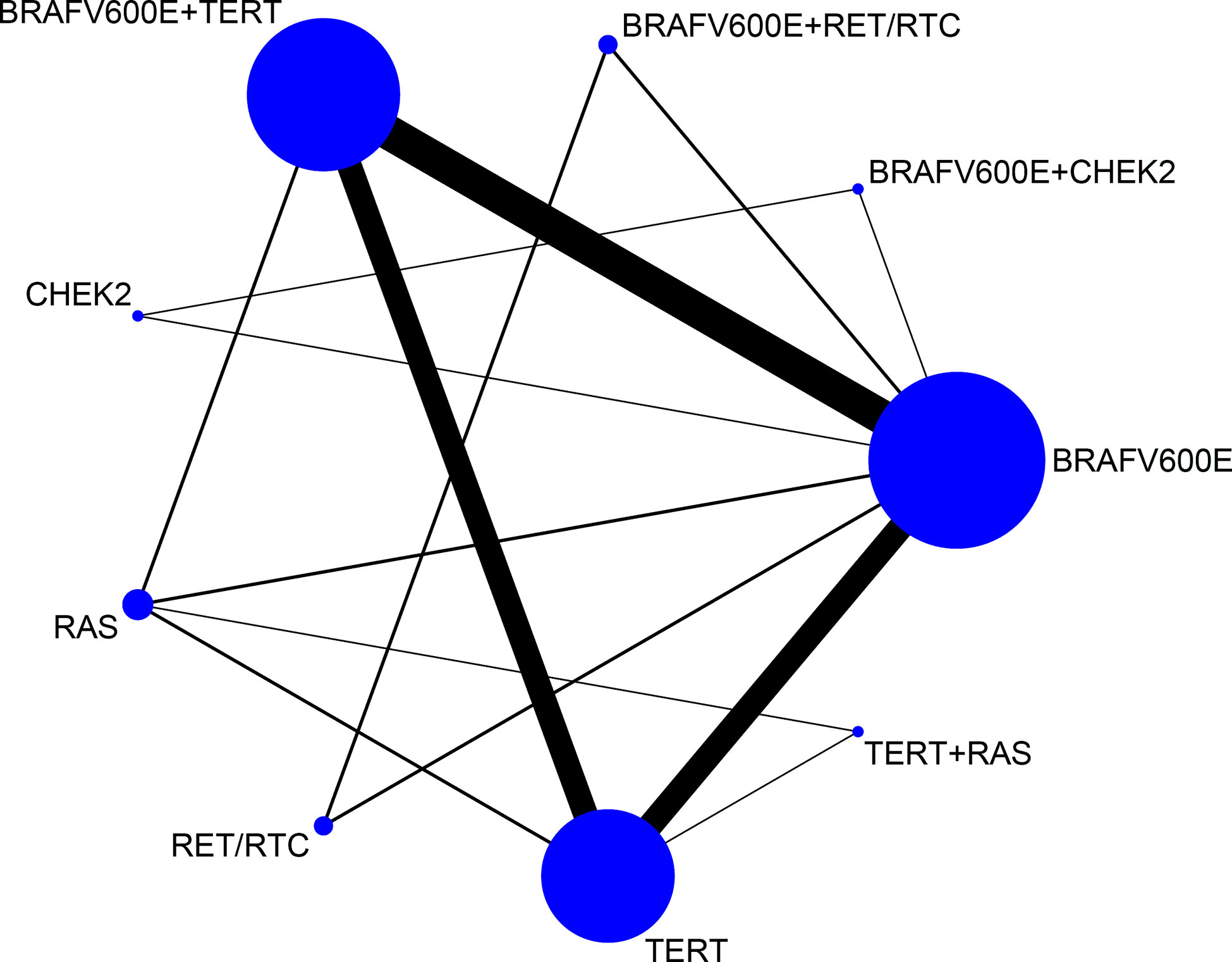
Figure 2 Network meta-analysis plots relating to the eligible comparisons of lymph node metastasis outcomes. Each circular node represents a coexistent or single genetic mutation. The circle size is proportional to the total number of patients with thyroid carcinoma, while the line width is proportional to the number of studies used in the head-to-head comparisons.
TC-Based Network Meta-Analysis: Primary Outcomes
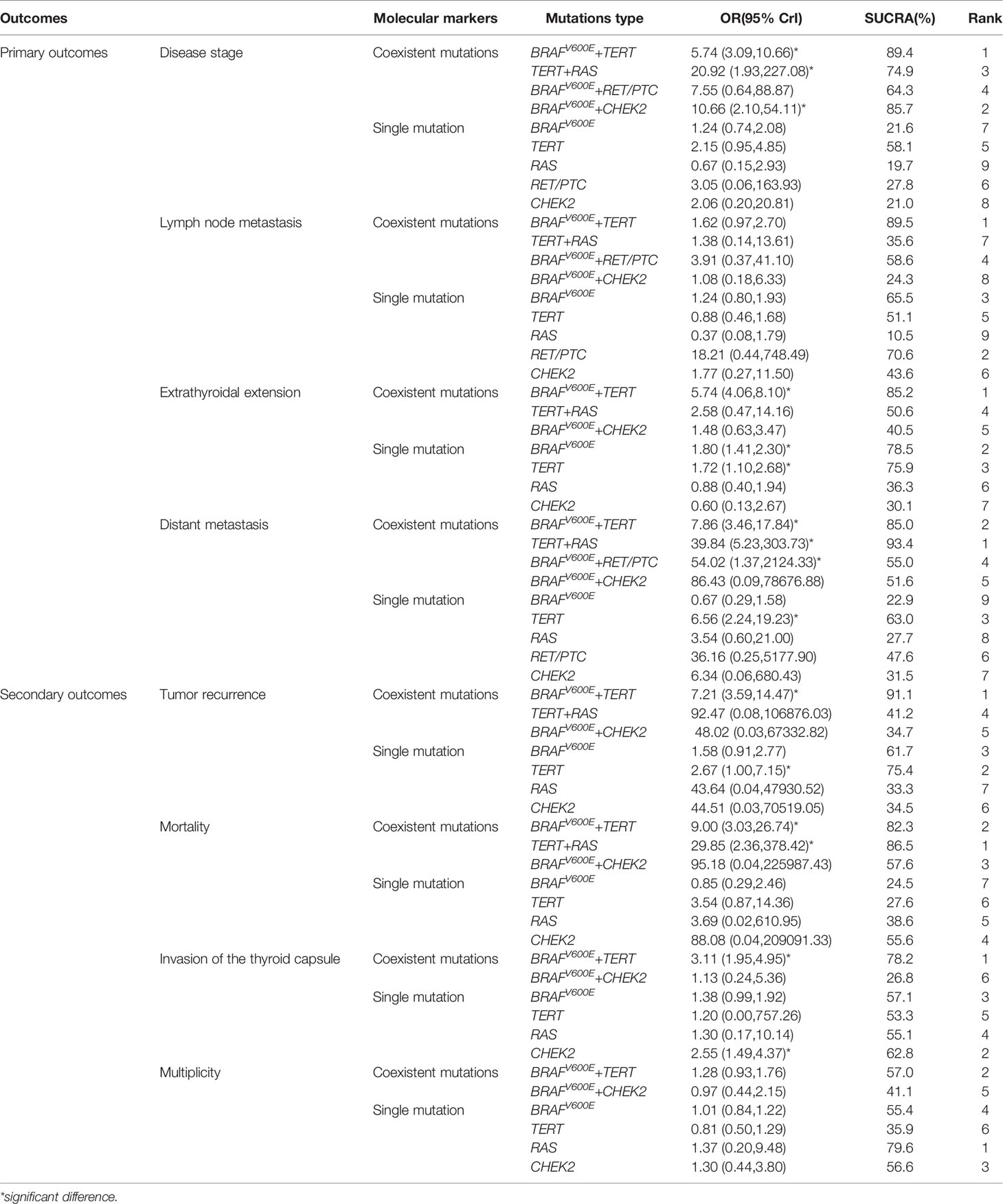
Table 2 show the network meta-analysis results for the primary outcomes, including disease stage, lymph node metastasis, extrathyroidal extension, and distant metastasis. In the evaluation of disease stage (32, 33, 36–39, 44, 45, 47–56), incorporated nine active mutant arms from 18 of the selected studies. Compared with wild-type, BRAFV6000E + TERT mutations ranked highest with significant differences (OR = 5.74, 95% CrI: 3.09–10.66), followed by BRAFV6000E + CHEK2 (10.66, 2.10–54.11), TERT + RAS, BRAFV6000E + RET/PTC, TERT, RET/PTC, BRAFV6000E, CHEK2, and RAS. For the lymph node metastasis outcome form 23 studies (31–42, 44, 45, 48–56), BRAFV6000E + TERT also ranked highest, followed by RET/PTC, BRAFV6000E, BRAFV6000E + RET/PTC, TERT, CHEK2, TERT+RAS, BRAFV6000E + CHEK2, and RAS. Comparisons between the no molecular markers yielded significant result, although both were accompanied by a very low GRADE score.
For the evaluation of extrathyroidal extension, 16 studies (7 active arms; Table 2; (31–34, 36–38, 40, 44, 45, 48–50, 53–55) were included. Of all molecular markers compared with wild-type, BRAFV6000E + TERT ranked highest (5.74, 4.06-8.10), followed by BRAFV6000E (1.80, 1.41-2.30), TERT (1.72, 1.10–2.68), TERT + RAS, BRAFV6000E + CHEK2, RAS, and CHEK2. In the analysis of distant metastasis (only eight studies, nine active arms; (32, 36, 44, 45, 50, 52, 54, 56), the following mutations were observed in ascending order: TERT + RAS ranked highest, followed by BRAFV6000E + TERT, BRAFV6000E, TERT, and RAS. These above results imply that the coexistence of BRAFV6000E + TERT mutations predicted a worse prognosis for disease stage, extrathyroidal extension in TC patients with significant differences.
TC-Based Network Meta-Analysis: Secondary Outcomes
We next evaluated the secondary outcomes: tumor recurrence, mortality, invasion of the thyroid capsule and multiplicity (Table 2). With regards to tumor recurrence, the coexistence of BRAFV6000E + TERT mutations still ranked the highest in tumor recurrence (eight studies, seven active arms; (32, 36, 44–46, 49, 50, 54), with significant results (7.21, 3.59–14.47) followed by TERT (2.67, 1.00–7.15), BRAFV6000E, TERT + RAS, BRAFV6000E + CHEK2, and CHEK2. With respect to mortality rate [five studies, seven active arms; (36, 43–45, 50)], TERT + RAS ranked the highest (29.85, 2.36–378.42), followed by BRAFV6000E + TERT (9.00, 3.03–26.74), BRAFV6000E + CHEK2, CHEK2, RAS, TERT, and BRAFV6000E. For invasion of the thyroid capsule, nine studies covering six active arms were analyzed [Table 2; (32–34, 38, 41, 44, 48, 52, 54)]. The coexisting BRAFV6000E + TERT mutations ranked highest (3.11, 1.95–4.95), followed by CHEK2 (2.55, 1.49–4.37), BRAFV6000E, RAS, TERT, and BRAFV6000E + CHEK2. In terms of multiplicity, the RAS mutations ranked highest (13 studies, 6 active arms; (31–33, 35, 36, 40, 41, 44, 45, 48, 49, 51, 54), followed by BRAFV6000E + TERT, CHEK2, BRAFV6000E, BRAFV6000E + CHEK2, and TERT. With the two above indicators receiving low GRADE scores.
In summary, the combined mutation of BRAFV6000E and TERT ranked highest in disease stage, lymph node metastasis, extrathyroidal extension, tumor recurrence and invasion of the thyroid capsule; while ranked secondly in distant metastasis, mortality and multiplicity followed by TERT + RAS or RAS alone. This inconsistency may be due to that the research object is all types of TC. We limited the research object to PTC and observed the efficacy of co-mutation of genes in prognosis.
PTC-Based Network Meta-Analysis: Primary Outcomes
We found that the BRAFV600E + TERT coexistent mutations ranked highest among the majority of studied outcomes. In order to assess study accuracy, we subsequently performed a network meta-analysis of PTC published research (n = 21), by evaluating the role of BRAFV600E + TERT co-mutations in tumor invasion and recurrence.
Figure 3 summarizes four typical outcomes with respect to PTC metastasis, invasion and recurrence, including disease stage and lymph node metastasis (A), extrathyroidal extension, and distant metastasis (B). Data relating to PTC disease stage were available from 14 of the selected publications (eight active arms; (32, 33, 36–39, 45, 48–52, 54, 56). For all genetic mutant arms, the BRAFV600E + TERT coexistent mutation ranked highest with significant different compared with wild-type (6.39, 3.13–13.04), followed by BRAFV6000E + CHEK2 (11.00, 1.91–63.26), BRAFV6000E + RET/PTC, BRAFV6000E, TERT, RAS, CHEK2, and RET/PTC. Comparisons between the following molecular markers yielded significant results: BRAFV6000E+ TERT versus TERT, BRAFV6000E + TERT and BRAFV6000E, as well as BRAFV6000E + CHEK2 versus BRAFV6000E. Data observing to PTC lymph node metastasis were available from 20 articles (eight active arms; (31–42, 45, 48–52, 54, 56). The BRAFV600E + TERT coexistent mutation ranked highest, again, with no significant different, followed by BRAFV600E + RET/PTC, BRAFV6000E + CHEK2, BRAFV600E, RET/PTC, RAS, CHEK2, and TERT, with no significant results among all comparisons (Figure 3A), and had a very low GRADE score from such above outcomes.
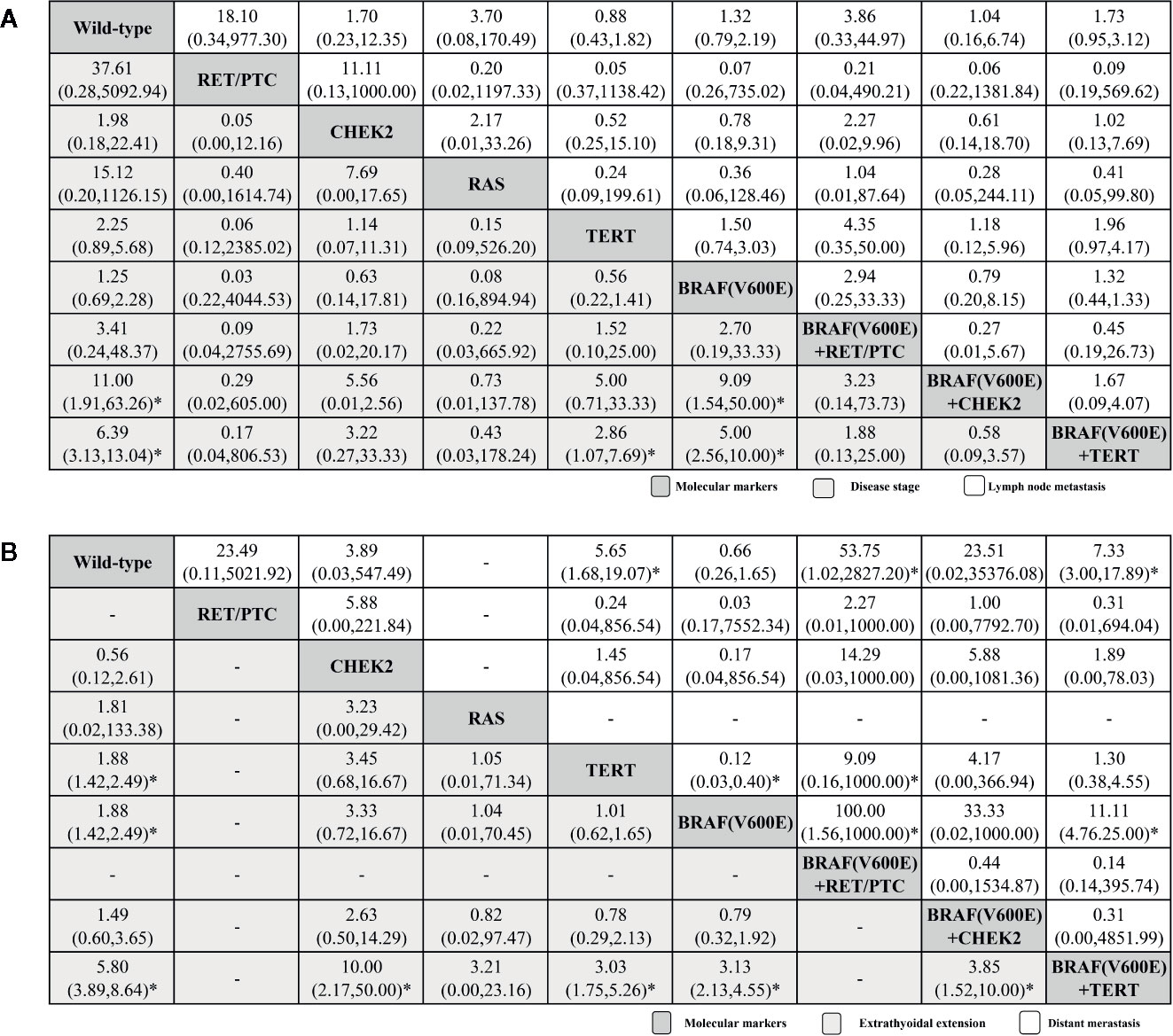
Figure 3 Histopathological feature profiles for the disease stage and lymph node metastasis (A), extrathyroidal extension, and distant metastasis (B) based PTC outcomes. From left to right, molecular markers for disease stage and lymph node metastasis (A), extrathyroidal extension and distant metastasis (B) are ranked by mean rank and SUCRA score. Information relating to the ORs and 95% CrI is listed in the column, with the rows displaying molecular marker identity. OR values higher than 1 favor the column-defining treatment (i.e., the left-most in order), indicating histopathological features associated with a worse prognosis. To obtain OR values for comparisons in the opposite direction, reciprocals should be taken. *Statistical significance.
Extrathyroidal extension outcome analysis included data form 13 of the research papers (six active arms; Figure 3B; (31–34, 36–38, 40, 45, 48–50, 54). The BRAFV600E + TERT coexistent mutation once again ranked highest (5.80, 3.89–8.64), while followed by BRAFV6000E (1.88, 1.42–2.49), TERT (1.88, 1.42–2.49), RAS, BRAFV6000E + CHEK2, and CHEK2. Comparisons between the following molecular markers yielded significant differences: BRAFV600E + TERT versus BRAFV600E, BRAFV600E + TERT versus TERT, BRAFV600E + TERT versus BRAFV6000E +CHEK2 and BRAFV600E + TERT versus CHEK2. For distant metastasis for seven original researches (seven active arms; Figure 3B; (32, 36, 45, 50, 52, 54, 56). BRAFV600E + TERT coexistent mutation ranked highest again (7.33, 3.00–17.89), which is inconsistent with the overall TC result, followed by BRAFV600E + RET/PTC (53.75, 1.02–2927.20), TERT (5.65, 1.68–19.07), BRAFV6000E + CHEK2, RET/PTC, CHEK2, and BRAFV600E, significant results could be found in BRAFV600E + TERT versus BRAFV600E. Generally, BRAFV600E + TERT always ranked first in our primary outcomes.
PTC-Based Network Meta-Analysis: Secondary Outcomes
The tumor recurrence results were obtained from six of the selected publications (five arms; Figure 4A; (32, 36, 45, 49, 50, 54) and showed that the BRAFV600E + TERT coexistent mutation also ranked highest (7.23, 3.37–15.51), followed by BRAFV600E (4.35, 2.17–9.09), TERT, CHEK2, and BRAFV6000E + CHEK2. For mortality outcome from four studies (three arms; Figure 4A; (36, 43, 45, 50), BRAFV600E + TERT ranked first (9.26, 3.02–28.42), followed by BRAFV6000E and TERT. Significance tested in BRAFV600E + TERT versus TERT and TERT versus BRAFV600E groups.
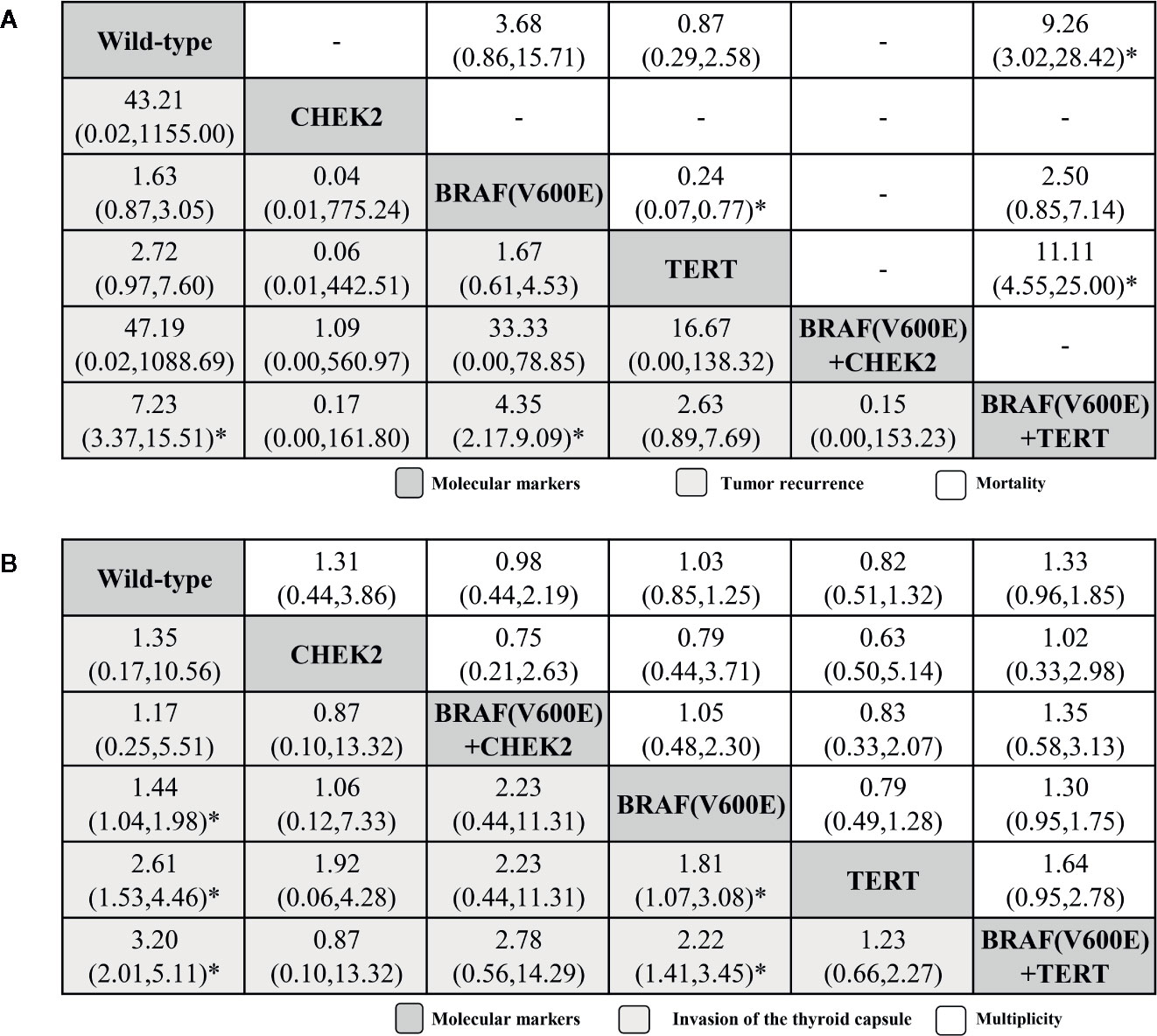
Figure 4 Histopathological feature profiles for the tumor recurrence and mortality (A), invasion of the thyroid capsule and multiplicity (B) based PTC outcomes. From left to right, molecular markers for tumor recurrence and mortality (A), invasion of the thyroid capsule and multiplicity (B) are ranked by mean rank and SUCRA score. Information relating to the ORs and 95% CrI is listed in the column, with the rows displaying molecular marker identity. OR values higher than 1 favor the column-defining treatment (i.e., the left-most in order), indicating histopathological features associated with a worse prognosis. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. *Significant results.
The invasion of the thyroid capsule results were from only eight research papers (five active arms; (32–34, 38, 41, 48, 52, 54). The BRAFV600E + TERT coexistent mutation again ranked highest (3.20, 2.01–5.11), followed by TERT (2.61, 1.53–4.46), BRAFV600E (1.44, 1.04–1.98), BRAFV6000E + CHEK2, and CHEK2. Comparisons between the following molecular markers yielded significant results: BRAFV6000E + TERT versus BRAFV6000E and TERT versus BRAFV6000E. For multiplicity outcome (five active arms; (31–36, 40, 41, 45, 48, 49, 51, 54), ranking order were BRAFV6000E + TERT, BRAFV6000E, CHEK2, BRAFV6000E + CHEK2, and TERT.
In summary, the co-mutation of BRAFV600E + TERT was more significant in PTC, which always ranking first. And the significant results were found in the outcomes disease stage, extrathyroid extension, distant metastasis, tumor recurrence, mortality, and invasion of thyroid capsule. Which means the BRAFV600E + TERT co-mutation plays an important role in the invasion and recurrence of TC, and above all, PTC.
Discussion
Our systematic review and network meta-analysis, evaluating the coexistence of genetic mutations as a valuable means of predicting the histopathological features associated with TC prognosis, included 8388 patients from 26 quality original research articles. Firstly, among the primary outcomes, coexistence of the BRAFV600E + TERT mutations ranked: i) highest in the disease stage and extrathyroidal extension outcomes; ii) second in distant metastasis and mortality outcome. Furthermore, the BRAFV600E + TERT co-mutation ranked highest in all of the following secondary outcomes: tumor recurrence, mortality, invasion of thyroid capsule; and ranked second in multiplicity outcome. Moreover, on performing another network meta-analysis of the outcomes related to patients with PTC, we noticed that the coexistent BRAFV600E + TERT mutation also ranked highest in all of the outcomes. Our research complies with the PRISMA guidelines and was registered with the PROSPERO cooperative, in order to assure that the study is both systematic and gradual in nature.
Of the eight outcomes (primary outcomes: lymph node metastasis, disease stage, distant metastatis, and extrathyroidal extension; and secondary outcomes: tumor recurrence, mortality, invasion of the thyroid capsule, and multiplicity) analyzed, the coexistent BRAFV600E + TERT mutation ranked highest five times (Table 2), demonstrating that it has a profound impact on the histopathological features associated with a worse prognosis. Giorgenon et al. documented a significant association between the dual TERTp/BRAFV600E mutation and advanced stage, compared with the control group that was negative for two mutations (33), consistent with our results. Kim and colleagues concluded that, compared with the presence of a single mutation, concomitant TERT and BRAF mutations worsened the survival rate of papillary cancer patients (57). Similarly, in our network meta-analysis of PTC patients, coexistent mutations always ranked highest as molecular markers of invasion, progression and recurrence (Figures 3 and 4). Our findings are in keeping with work by Jin et al., who demonstrated a significant role of BRAFV600E and TERT promoter mutations in PTC, which is particularly aggressive in cases when the two mutations coexist (48).
We also found an indication of the TERT promoter mutation, either alone or in combination with the BRAFV600E mutation (which ranked second followed by TERT promoter single mutation), having a certain effect on the invasion of thyroid capsule outcome (Figure 4). This observation confirms that the TERT promoter mutation with or without BRAFV600E mutation represents an independent prognostic factor for poor prognosis. Similar results were found in the study by Kim et al., in which they concluded that concomitant TERT and BRAF mutations worsened the survival rate of patients with papillary cancer (57). Moreover, a study by Melo and colleagues, reported that distant metastases were enriched for TERTp mutations but depleted in BRAF mutations. TERTp mutations may play a role in distant metastases, which is consistent with our results (58).
Our research proves that when the research type is TC, two outcome indicators (distant metastasis and mortality) showed that TERT+RAS ranked first from network meta-analysis, and another outcome indicator (multiplicity) showed that RAS ranked first. The above results suggest that RAS mutations may also be one of the main reasons affecting long-term prognosis. These analyses were performed for TC in general, and similar study by Bellevicine C’s research found that RAS was strictly related to the risk of malignancy of TC (59), and previous studies have demonstrated that the presence of RAS mutations in a thyroid nodule provides evidence for neoplasia (60). Thus, we made a conclusion that BRAFV600E, TERT, and RAS triple mutations may herald a worse prognosis. For research type, which is limited to PTC, we confirm that coexistent BRAFV600E + TERT genetic mutations are the best predictors of poor TC prognosis and have the highest impact on the tumor progression, invasion, and recurrence in patients with PTC.
Despite the systematic nature of our work, there are several limitations to this study. Firstly, we only performed network meta-analysis. Although the results included head-to-head results, there was no direct comparison between all combination of mutations (e.g., BRAFV600E + TERT versus BRAFV600E + RET/PTC), which could have only been obtained through inaccurate indirect comparisons. In addition, our result GRADE scores ranged from low and very low, due to the exclusion of randomized control trials, and the inclusion of indirect comparisons. Moreover, the ‘no mutation’ controls were different for each group. For instance, a given study may have only comprised data relating to the BRAFV600E + TERT genetic co-mutations and BRAFV600E and TERT single mutations while not considering RAS mutation, CHEK2 mutation, or RET/PTC rearrangements, which may have an impact on the overall outcomes, which is also the reason for our limitations. In such cases, it is possible that the no mutation group actually included other kinds of mutations, which may have a certain impact on the overall results. What’s more, AJCC staging system 8th edition begins to be used internationally from Jan 2019, and only two studies using the new edition AJCC staging system (32, 33), which maybe also a limitation of our research. Last but not least, we were only able to select 21 studies relating to PTC, and were therefore limited by patient numbers. We decided not to include FTC studies within the PTC analysis group for the sake of increasing our samples size, as this would limit the accuracy of results. Notwithstanding these limitations, our network meta-analysis is the first comprehensive study to document the effect of genetic co-mutations on the prognosis of TC patients.
The synergistic impact of the BRAFV600E + TERTp co-mutations on the invasiveness and progression of PTC may be explained in part by increased TERT expression, which may result from the BRAF-induced up-regulation of several E26 transcription factors (36). Coincidentally, another study has claimed that the BRAFV600E-activated MAPK pathway may selectively up-regulate mutant TERT proteins, thus promoting cooperative oncogenesis (61). The BRAF gene belongs to the RAF gene family. It is a downstream signaling molecule of RET and RAS. It can encode a silk/threonine-specific kinase and is the most effective activator in the MAPK/extracellular regulated protein kinases (ERK) pathway. RAF is also an activator with the strongest kinase activity in the family, continuous activation of the MAPK signaling pathway, and the slenderness leads to abnormal cell proliferation, differentiation, uncontrolled cell cycle, and in circulation, thereby forming tumors. So from the perspective of mechanism, the results we have obtained are valid.
In this systematic review and network meta-analysis, we have identified clinically-important differences between the histopathological prognostic features associated with coexistent versus single mutations in TC. We found that the BRAFV600E + TERT co-mutations predicted poor histopathological prognosis, including progression, invasion, and metastasis, especially in PTC. Also, further research should related to potentially important features such as molecular profile and clinical outcome. For the overall TC, the BRAFV600E + TERT + RAS triple mutations may have a greater impact on the prognosis.
Data Availability Statement
All datasets analyzed for this study are included in the article/Supplementary Material.
Author Contributions
Conceptualization: ZL. Methodology (data collection): YZ and LW. Statistical analysis: YZ, LW, LZ, XJ, XH, PP, SZ, YW, and JW. Writing (original draft preparation): YZ and LW. Review and editing: ZL. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.540238/full#supplementary-material
Supplementary Figure 1 | Inconsistency plot for the lymph node metastasis outcome in TC.
Supplementary Figure 2 | Funnel plot for the lymph node metastasis outcome in TC.
Supplementary Figure 3 | Inconsistency plot for the extrathyroidal extension outcome in TC.
Supplementary Figure 4 | Inconsistency plot for the lymph node metastasis outcome in PTC.
Supplementary Table 1 | Search strategies.
Supplementary Table 2 | Quality assessment of included of studies by Critical Appraisal Skills Programme (CASP) scales.
Supplementary Table 3 | Assessment of the quality of evidence using the Grading of Recommendations Assessment.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551
2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
3. Chen B, Zhong L, Dong D, Zheng J, Fang M, Yu C, et al. Computed Tomography Radiomic Nomogram for Preoperative Prediction of Extrathyroidal Extension in Papillary Thyroid Carcinoma. Front Oncol (2019) 9:829. doi: 10.3389/fonc.2019.00829
4. Yu ST, Chen WZ, Xu DB, Xie R, Zhou T, Yu JC. Minimally Invasive Video-Assisted Surgical Management for Parapharyngeal Metastases From Papillary Thyroid Carcinoma: A Case Series Report. Front Oncol (2019) 9:1226. doi: 10.3389/fonc.2019.01226
5. Feng J, Zhou Q, Yi H, Ma S, Li D, Xu Y, et al. A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death Dis (2019) 10:433. doi: 10.1038/s41419-019-1637-7
6. Striano P, Vari MS, Mazzocchetti C, Verrotti A, Zara F. Management of genetic epilepsies: From empirical treatment to precision medicine. Pharmacol Res (2016) 107:426–9. doi: 10.1016/j.phrs.2016.04.006
7. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 23(159):676–90. doi: 10.1016/j.cell.2014.09.050
8. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest (2016) 126:1052–66. doi: 10.1172/JCI85271
9. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res (2018) 24:3059–68. doi: 10.1158/1078-0432.CCR-18-0373
10. Insilla AC, Proietti A, Borrelli N, Macerola E, Niccoli C, Vitti P, et al. TERT promoter mutations and their correlation with BRAF and RAS mutations in a consecutive cohort of 145 thyroid cancer cases. Oncol Lett (2018) 15:2763–70. doi: 10.3892/ol.2017.7675
11. Han S, Ehrhardt J Jr, Shukla S, Elkbuli A, Nikiforov YE, Gulec SA. A Case of Papillary Thyroid Carcinoma and Kostmann Syndrome: A Genomic Theranostic Approach for Comprehensive Treatment. Am J Case Rep (2019) 20:1027–34. doi: 10.12659/AJCR.916143
12. Halim CE, Xinjing SL, Fan L, Bailey Vitarbo J, Arfuso F, Tan CH, et al. Ahn KS 2019 Anti-cancer effects of oxymatrine are mediated through multiple molecular mechanism(s) in tumor models. Pharmacol Res (2019) 147:104327. doi: 10.1016/j.phrs.2019.104327
13. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet (2013) 381:1058–69. doi: 10.1016/S0140-6736(13)60109-9
14. Roskoski R Jr, Sadeghi-Nejad A. Role of RET protein-tyrosine kinase inhibitors in the treatment RET-driven thyroid and lung cancers. Pharmacol Res (2018) 128:1–17. doi: 10.1016/j.phrs.2017.12.021
15. Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, et al. TERT promoter mutations in thyroid Cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab (2013) 98:E1562–6. doi: 10.1210/jc.2013-2383
16. Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res (2019) 144:19–50. doi: 10.1016/j.phrs.2019.03.006
17. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417:949–54. doi: 10.1038/nature00766
18. Li M, Chai HF, Peng F, Meng YT, Zhang LZ, Zhang L, et al. Estrogen receptor β upregulated by lncRNA-H19 to promote cancer stem-like properties in papillary thyroid carcinoma. Cell Death Dis (2018) 9:1120. doi: 10.1038/s41419-018-1077-9
19. Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol (2009) 27:2977–82. doi: 10.1200/JCO.2008.20.1426
20. Vuong HG, Altibi AM, Abdelhamid AH, Ngoc PU, Quan VD, Tantawi MY, et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: a systematic review. Oncotarget (2017) 8:10637–49. doi: 10.18632/oncotarget.12885
21. Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of Coexistent BRAFV600E and TERT Promoter Mutations on Poor Clinical Outcomes in Papillary Thyroid Cancer: A Meta-Analysis. Thyroid (2017) 27:651–60. doi: 10.1089/thy.2016.0350
22. Liberati A1, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
23. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med (2015) 162:777–84. doi: 10.7326/M14-2385
24. PROSPERO. Centre for Reviews and Dissemination. Systematic Reviews: CRD"s Guidance for Undertaking Reviews in Health Care. York, England: University of York (2009). Available at: https://www.crd.york.ac.uk/prospero/
25. Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA (1993) 270:2598–601. doi: 10.1001/jama.270.21.2598
26. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PloS One (2014) 9:e99682. doi: 10.1371/journal.pone.0099682
27. Feng F, Zhang Y, Hou J, Cai J, Jiang Q, Li X, et al. Can music improve sleep quality in adults with primary insomnia? A systematic review and network meta-analysis. Int J Nurs Stud (2018) 77:189–96. doi: 10.1016/j.ijnurstu.2017.10.011
28. Yang C, Gong G, Jin E, Han X, Zhuo Y, Yang S, et al. Topical application of honey in the management of chemo/radiotherapy-induced oral mucositis: A systematic review and network meta-analysis. Int J Nurs Stud (2019) 89:80–7. doi: 10.1016/j.ijnurstu.2018.08.007
29. Ma Y, Wang C, Zhang Q, Peng X, Feng Y, Meng X. The effects of polysaccharides from Auricularia auricula (Huaier) in adjuvant anti-gastrointestinal cancer therapy: A systematic review and network meta-analysis. Pharmacol Res (2018) 132:80–9. doi: 10.1016/j.phrs.2018.04.010
30. Feng F, Jiang Q, Jia H, Sun H, Chai Y, Li X, et al. Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res (2018) 135:89–101. doi: 10.1016/j.phrs.2018.06.021
31. Colombo C, Muzza M, Proverbio MC, Tosi D, Soranna D, Pesenti C, et al. Impact of Mutation Density and Heterogeneity on Papillary Thyroid Cancer Clinical Features and Remission Probability. Thyroid (2019) 29:237–51. doi: 10.1089/thy.2018.0339
32. Gąsior-Perczak D, Kowalik A, Walczyk A, Siołek M, Gruszczyński K, Pałyga I, et al. Coexisting Germline CHEK2 and Somatic BRAFV600E Mutations in Papillary Thyroid Cancer and Their Association with Clinicopathological Features and Disease Course. Cancers (Basel) (2019) 11:1774. doi: 10.3390/cancers11111744
33. Giorgenon TMV, Carrijo FT, Arruda MA, Cerqueira TLO, Barreto HR, Cabral JB, et al. Preoperative detection of TERT promoter and BRAFV600E mutations in papillary thyroid carcinoma in high-risk thyroid nodules. Arch Endocrinol Metab (2019) 63:107–12. doi: 10.20945/2359-3997000000116
34. Hou XF, Tian YX, Liu QJ, Xue JC. The Role of BRAF and TERT promoter mutations in thyroid cancer. Gansu Med J (2019) 38:385–8.
35. Huang M, Yan C, Xiao J, Wang T, Ling R. Relevance and clinicopathologic relationship of BRAF V600E, TERT and NRAS mutations for papillary thyroid carcinoma patients in Northwest China. Diagn Pathol (2019) 14:74. doi: 10.1186/s13000-019-0849-6
36. Song YS, Yoo SK, Kim HH, Jung G, Oh AR, Cha JY, et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr Relat Cancer (2019) 26:629–41. pii: ERC–17-0562.R2. doi: 10.1530/ERC-17-0562
37. Argyropoulou M, Veskoukis AS, Karanatsiou PM, Manolakelli A, Kostoglou-Athanassiou I, Vilaras G, et al. Low Prevalence of TERT Promoter, BRAF and RAS Mutations in Papillary Thyroid Cancer in the Greek Population. Pathol Oncol Res (2020) 26:347–54. doi: 10.1007/s12253-018-0497-2
38. Dai LL. A Study on Relation Between BRAFV600E mutation and TERT Promoter mutation with Clinicopathologic Characteristics of papillary thyroid carcinoma. Jinan University (2018).
39. Deng F. Relationship between BRAFv6ooE gene and TERT promoter mutations and cervical lymph node metastasis in papillary thyroid carcinoma. Zhengzhou University (2018).
40. Ren H, Shen Y, Hu D, He W, Zhou J, Cao Y, et al. Co-existence of BRAFV600E and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag Res (2018) 10:1005–13. doi: 10.2147/CMAR.S159583
41. Rusinek D, Pfeifer A, Krajewska J, Oczko-Wojciechowska M, Handkiewicz-Junak D, Pawlaczek A, et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. Int J Mol Sci (2018) 19:2647. doi: 10.3390/ijms19092647
42. Zhou D, Li Z, Bai X. BRAF V600E and RET/PTC Promote the Activity of Nuclear Factor-κB, Inflammatory Mediators, and Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Study of 50 Patients in Inner Mongolia. Med Sci Monit (2018) 24:6795–808. doi: 10.12659/MSM.909205
43. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol (2017) 3:202–8. doi: 10.1001/jamaoncol.2016.3288
44. Marques IJ, Moura MM, Cabrera R, Pinto AE, Simões-Pereira J, Santos C, et al. Identification of somatic TERT promoter mutations in familial nonmedullary thyroid carcinomas. Clin Endocrinol (2017) 87:394–9. doi: 10.1111/cen.13375
45. Shen X, Liu R, Xing M. A six-genotype genetic prognostic model for papillary thyroid cancer. Endocr Relat Cancer (2017) 24:41–52. doi: 10.1530/ERC-16-0402
46. Song YS, Lim JA, Min HS, Kim MJ, Choi HS, Cho SW, et al. Changes in the clinicopathological characteristics and genetic alterations of follicular thyroid cancer. Eur J Endocrinol (2017) 177:465–73. doi: 10.1530/EJE-17-0456
47. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J Nucl Med (2017) 58:258–65. doi: 10.2967/jnumed.116.180240
48. Jin L, Chen E, Dong S, Cai Y, Zhang X, Zhou Y, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget (2016) 7:18346–55. doi: 10.18632/oncotarget.7811
49. Lee SE, Hwang TS, Choi YL, Han HS, Kim WS, Jang MH, et al. Prognostic Significance of TERT Promoter Mutations in Papillary Thyroid Carcinomas in a BRAF(V600E) Mutation-Prevalent Population. Thyroid (2016) 26:901–10. doi: 10.1089/thy.2015.0488
50. Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer (2016) 122:1370–9. doi: 10.1002/cncr.29934
51. Sun J, Zhang J, Lu J, Gao J, Ren X, Teng L, et al. BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Carcinoma in Chinese Patients. PloS One (2016) 11:e0153319. doi: 10.1371/journal.pone.0153319
52. Gandolfi G, Ragazzi M, Frasoldati A, Piana S, Ciarrocchi A, Sancisi V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur J Endocrinol (2015) 172:403–13. doi: 10.1530/EJE-14-0837
53. Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab (2014) 99:E1130–6. doi: 10.1210/jc.2013-4048
54. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol (2014) 32:2718–26. doi: 10.1200/JCO.2014.55.5094
55. Liu XL. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Jilin University (2013).
56. Henderson YC, Shellenberger TD, Williams MD, El-Naggar AK, Fredrick MJ, Cieply KM, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res (2009) 15:485–91. doi: 10.1158/1078-0432.CCR-08-0933
57. Kim TH, Kim YE, Ahn S, Kim JY, Ki CS, Oh YL, et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer (2016) 23:813–23. doi: 10.1530/ERC-16-0219
58. Melo M, Gaspar da Rocha A, Batista R, Vinagre J, Martins MJ, Costa G, et al. TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J Clin Endocrinol Metab (2017) 102:1898–907. doi: 10.1210/jc.2016-2785
59. Bellevicine C, Migliatico I, Sgariglia R, Nacchio M, Vigliar E, Pisapia P, et al. Tiroide Network. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg alterations in different Bethesda diagnostic categories: A multicentric prospective study on the validity of the 7-gene panel test in 1172 thyroid FNAs deriving from different hospitals in South Italy. Cancer Cytopathol (2020) 128:107–18. doi: 10.1002/cncy.22217
60. Xing M. Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med (2016) 14:12. doi: 10.1186/s12916-016-0559-9
Keywords: coexistent genetic mutations, thyroid carcinoma, histopathological features, prognosis, BRAFV600E + TERT
Citation: Zhao L, Wang L, Jia X, Hu X, Pang P, Zhao S, Wang Y, Wang J, Zhang Y and Lyu Z (2020) The Coexistence of Genetic Mutations in Thyroid Carcinoma Predicts Histopathological Factors Associated With a Poor Prognosis: A Systematic Review and Network Meta-Analysis. Front. Oncol. 10:540238. doi: 10.3389/fonc.2020.540238
Received: 04 March 2020; Accepted: 30 September 2020;
Published: 03 November 2020.
Edited by:
Qing Chun Zhao, Shenyang Pharmaceutical University, ChinaReviewed by:
Agnieszka Walczyk, Holy Cross Cancer Center, PolandXiaopei Shen, Fujian Medical University, China
Fan Feng, The 302th Hospital of PLA, China
Copyright © 2020 Zhao, Wang, Jia, Hu, Pang, Zhao, Wang, Wang, Zhang and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Lyu, bWV0YWJvbGlzbTMwMUAxMjYuY29t; Yingshi Zhang, emhhbmd5aW5nc2hpNTI2QDE2My5jb20=
†These authors have contributed equally to this work
 Ling Zhao1,2†
Ling Zhao1,2† Lin Wang
Lin Wang Xiaodong Hu
Xiaodong Hu Ping Pang
Ping Pang Sitong Zhao
Sitong Zhao Yingshi Zhang
Yingshi Zhang Zhaohui Lyu
Zhaohui Lyu