- Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objectives: This study aimed to assess the effect of neoadjuvant chemotherapy (NACT) on the rate of lymph node metastasis (LNM) in FIGO stage IB1-IIB cervical cancer patients and compare the LNM between NACT plus surgery and surgery only.
Methods: We identified 34 eligible studies in PubMed, Web of Science, Cochrane Library, and EMBASE from inception to July 27, 2019. Data analyses were performed by Stata (version 13) and Revman (version 5.3).
Results: In these 34 included studies, the pooled incidence of LNM was estimated as 23% (95% CI, 0.20-0.26; I2 = 79.6%, P<0.001). In the subgroup analysis, we identified five factors, including study type, year of publication, continents from which patients came, histological type and the FIGO stage. When taking FIGO stage into consideration, the LNM rate was 13% in stage IB (95% CI: 0.10-0.15; I2 = 5.5%, P=0.385), 23% in stage IIA (95% CI: 0.18-0.28; I2 = 0%, P=0.622), and 27% in stage IIB (95% CI: 0.20-0.33; I2 = 0%, P=0.898), respectively. Through the comparison between NACT plus surgery and surgery only based on the six randomized controlled trials, the incidence of positive lymph nodes was lower in patients receiving NACT plus surgery than surgery only (RR=0.57, 95% CI: 0.39-0.83; I2 = 60.5%, P=0.027). The 5-year OS was higher in the NACT + surgery group than surgery-only group (RR=1.13, 95% CI: 1.03-1.23; I2 = 0.0%, P=0.842).
Conclusions: Among cervical cancer in stage IB1-IIB, the preoperative NACT plus radical surgery resulted in a 23% probability of LNM, which was lower than those receiving radical surgery only. In stage IIA and IIB, the effect of NACT to reduce LNM was more obvious.
Introduction
Despite the development of comprehensive treatment technology, the cases of cervical cancer have increased from 528,000 in 2012 to 570,000 in 2018, and the deaths have increased from 266,000 in 2012 to 311,000 in 2018 (1, 2). The higher regional morbidity incidence was found in developing countries, which was 3 to 10 times higher than developed areas (1, 3, 4). Therefore, cervical cancer has become one of the most important public health challenges. According to the National Comprehensive Cancer Network (NCCN) guidelines, it is recommended that patients with stage IB1 and IIB cervical cancer undergo radical hysterectomy (RH) and/or chemoradiation (5). However, the traditional treatment methods would seriously affect patients’ endocrine and reproductive function, and some patients lost the chance to get effective treatment when diagnosed due to the large tumor size (3, 4). Gradually, preoperative neoadjuvant chemotherapy (NACT) caught clinicians’ attention, which might provide more survival benefits (3–9).
Since neoadjuvant chemotherapy was proposed and applied to the treatment of cervical cancer in the 1980s, more and more studies have focused on NACT (7–14). NACT can treat patients with distant metastases and shows great efficacy in both reducing recurrence and improving survival (7–9, 12) In 2019, the NCCN pointed out that select patients with FIGO stage IB2-IIB disease may accept RH or NACT followed by RH (5).
Actually, there existed many risk factors affecting the prognosis of cervical cancer, among which lymph node metastasis (LNM) was one of the most important high risk factors (3, 4, 6, 8, 9). The presence of LNM and the increase in positive lymph node (LN) number were followed with higher recurrence rates and lower survival rates (15). Therefore, to explain the efficacy of NACT, we need to pay attention to the impact of NACT on LNs, which the previous studies ignored.
Therefore, the aim of this study was to conduct a meta-analysis of the literature on NACT followed by radical surgery and to evaluate the effect of NACT on LNM in FIGO stage IB1-IIB cervical cancer.
Methods
Search Strategy
This study was conducted on the basis of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement and registered in Prospero (PROSPERO CRD42018117658) (16, 17). We searched PubMed, Web of Science, Cochrane Library, and EMBASE for relative studies. In order to search the PubMed database, we used the following combination of terms: (Uterine Cervical Neoplasms[MeSH Terms] OR ((cervix*[Title/Abstract] OR cervical*[Title/Abstract] OR uterine cervix*[Title/Abstract] OR cervix uteri*[Title/Abstract]) AND (cancer*[Title/Abstract] OR tumor*[Title/Abstract] OR tumour*[Title/Abstract] OR neoplas*[Title/Abstract] OR carcinoma*[Title/Abstract] OR malignanc*[Title/Abstract] OR carcinogenesis*[Title/Abstract] OR intraepithelial neoplas*[Title/Abstract]))) AND (Lymphatic Metastasis[MeSH Terms] OR ((lymph node* [Title/Abstract] OR nodal[Title/Abstract] OR node*[Title/Abstract] OR lymphatic[Title/Abstract]) AND (metastasis [Title/Abstract] OR recurrence [Title/Abstract] OR invasion [Title/Abstract] OR Metastatic Ratio[Title/Abstract]))) AND ((neoadjuvant[Title/Abstract] OR preoperat*[Title/Abstract] OR upfront[Title/Abstract] OR primary[Title/Abstract] OR induction[Title/Abstract] OR adjuvant[Title/Abstract]) AND (chemotherapy[Title/Abstract] OR treatment [Title/Abstract] OR therapy[Title/Abstract])). We used an appropriately modified PubMed search strategy to search the other three databases, including Web of Science, Cochrane Library, and EMBASE. The detailed search strategy is shown in Supplementary Appendix 1. The year of publication is limited to the period from inception to July 27, 2019. We also searched the publications that cited those included articles and other related articles.
Inclusion and Exclusion Criteria
The eligible studies must meet the inclusion criteria as follows: (i) the patient was pathologically diagnosed as stage IB1-IIB cervical cancer; (ii) NACT was platinum based; (iii) the surgery was extensive RH; (iv) the study provided complete data, especially LN status.
Studies were excluded if they met any of these criteria: (i) The studies reported patients with other malignant diseases; (ii) the studies included patients receiving other treatments in addition to NACT and radical surgery; (iii) non-English literature; and (iv) reviews, meta-analysis, case reports, conference articles, and articles without clear data.
Two independent researchers (BC and LM) filtered all publications. Disagreements were determined by group discussion with a third researcher (HW).
Data Extraction and Outcomes
Data were extracted by two independent researchers (BC and LM) and filled in standardized data-collection forms (Supplementary Table 1). Opposing opinions were solved by discussion with a third researcher (HW). If the original article did not report detailed data, the researchers would contact the first author by e-mail. Extracted data included authors, country, continent of patients, study type, year of publication, number of patients, age of patients, FIGO stage, histological type, NACT regimen, NACT cycle, and LNM. The endpoint was LNM. The rate of LNM was defined as the ratio of observed number of patients with positive LNs divided by the number of total patients undergoing treatment.
Assessment of Risk of Bias
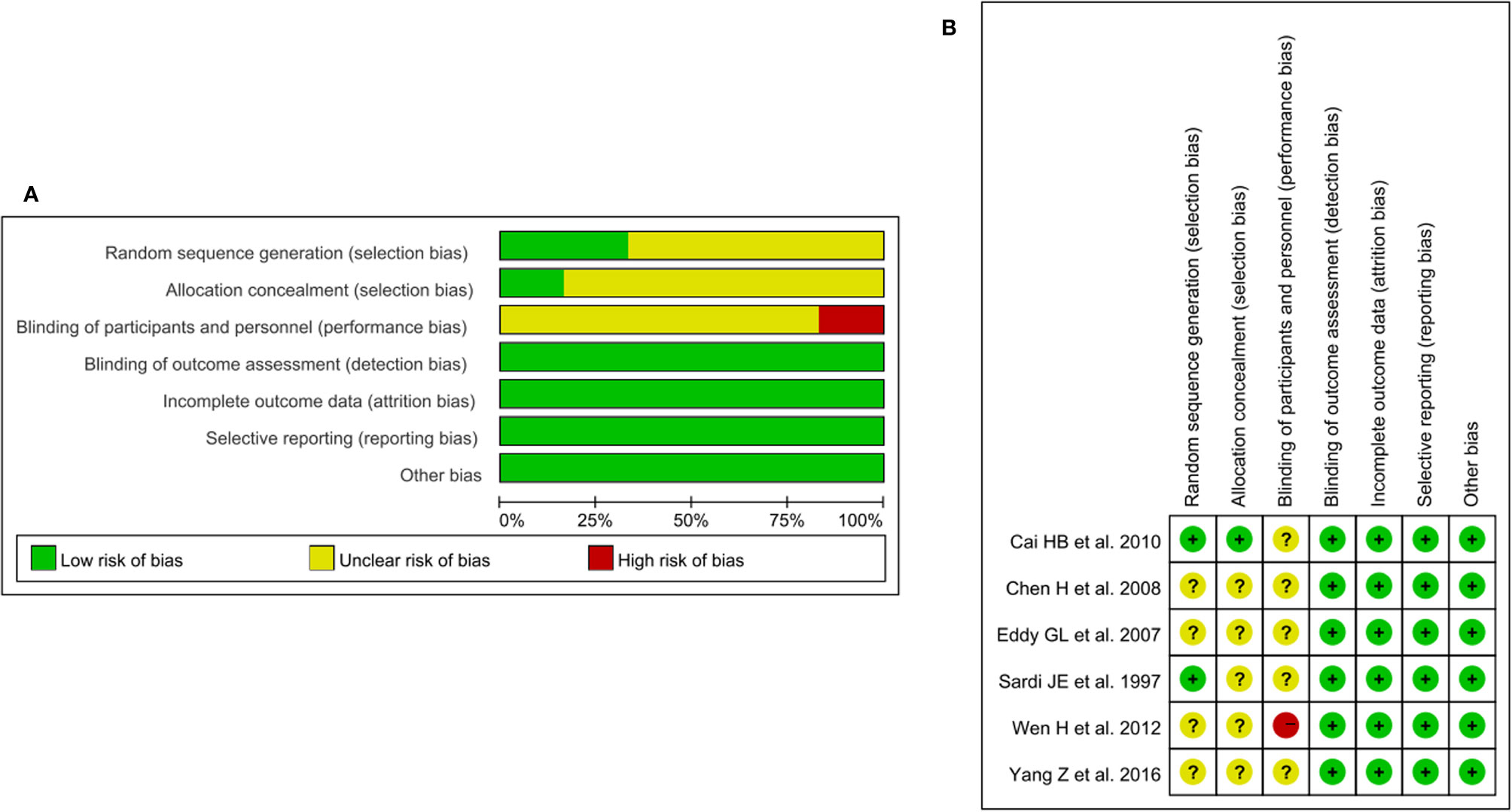
The quality of each identified study was assessed according to a modified version based on the Cochrane Collaboration’s risk of bias tool (Supplementary Tables 2, 3). We took the following five items as criteria: assessment of record, assessment of diagnosis, assessment of LN status, assessment of loss to follow-up, selective inclusion, and exclusion. In each criterion, the study was evaluated as low, unclear, and high risk of bias. The studies were classified as low risk of bias only if the five criteria were all at low risk of bias. The risk of bias was examined by two reviewers (BC and LM) independently, and discrepancies were resolved by consensus. If required, a third reviewer (HW) would join them.
Statistical Analysis
Meta analyses were conducted through Stata (version 13) and Revman (version 5.3). We estimated the LNM rate with Freeman-Turkey double arcsine transformation because there were a large proportion of data that were close to the margins of the possible interval (0% or 100%) (18). Relative risk (RR) was used to compare the LNM rates between two treatment groups. The heterogeneity between the individual studies was quantitatively estimated with the Chi-square and I2 statistics (19, 20). When P was greater than 0.1, it was considered to be no heterogeneity; otherwise, there existed significant heterogeneity. I2 was used to further measure heterogeneity because of the limits of the Chi-square statistic. A threshold of I2 below 50% indicated no significant heterogeneity, and I2 over 50% suggested high heterogeneity. Fixed effects models were considered when there was no between-study heterogeneity. In contrast, we used the random effects model. Sensitivity and subgroup analyses were conducted to evaluate the effect of various variables on outcomes. In order to detect potential publication bias, we performed the visual inspection of funnel plot and Egger’s test (21, 22). All p values are two-sided, and a p value less than 0.05 was considered as statistically significant.
Results
Characteristics of Studies
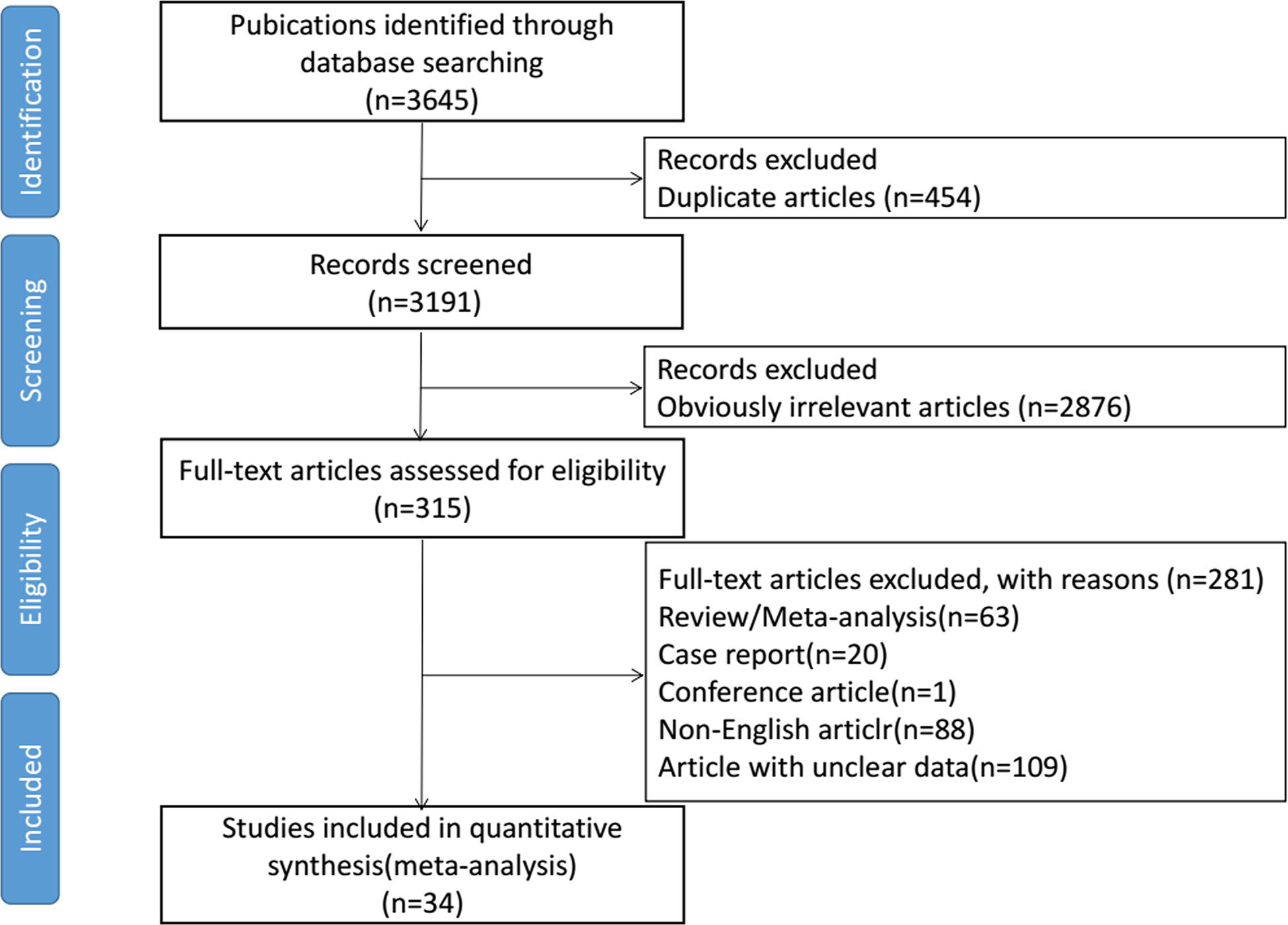
Initially, we identified 3645 eligible studies. After screening, 34 studies were finally included in this meta-analysis, consisting of 3813 patients (6–8, 12–14, 23–50). The selection process is shown in Figure 1. The detailed characteristics for each included article are systematically summarized in Supplementary Table 1. Eight studies were randomized controlled trials (RCTs) with matched data in the experimental arm (6, 13, 14, 24, 32, 41, 45, 47), three were prospective cohorts (23, 40, 49), and the other 23 studies were retrospective cohorts (7, 8, 12, 25–31, 33–39, 42–44, 46, 48, 50). The largest study included 705 women (8), and the smallest included 20 women (42). 73.5% of studies (n=25) contained more than 50 patients. In 61.7% of the studies (n=21), the preoperative chemotherapy cycle was 2–3 weeks. The postoperative adjuvant radiotherapy or chemotherapy was determined by the patient’s surgical outcome and discretion of their clinicians. Through the assessment of risk of bias, 10 of the 34 studies did not meet the requirements for low bias risk (13, 14, 23, 25, 27, 32, 40, 42, 45, 49). In these 10 studies, five were due to loss of follow-up and four were not at low risk in assessment of LN status. One study reported the record of 51 patients without a clear source (Supplementary Table 3).
Summary LNM Rate
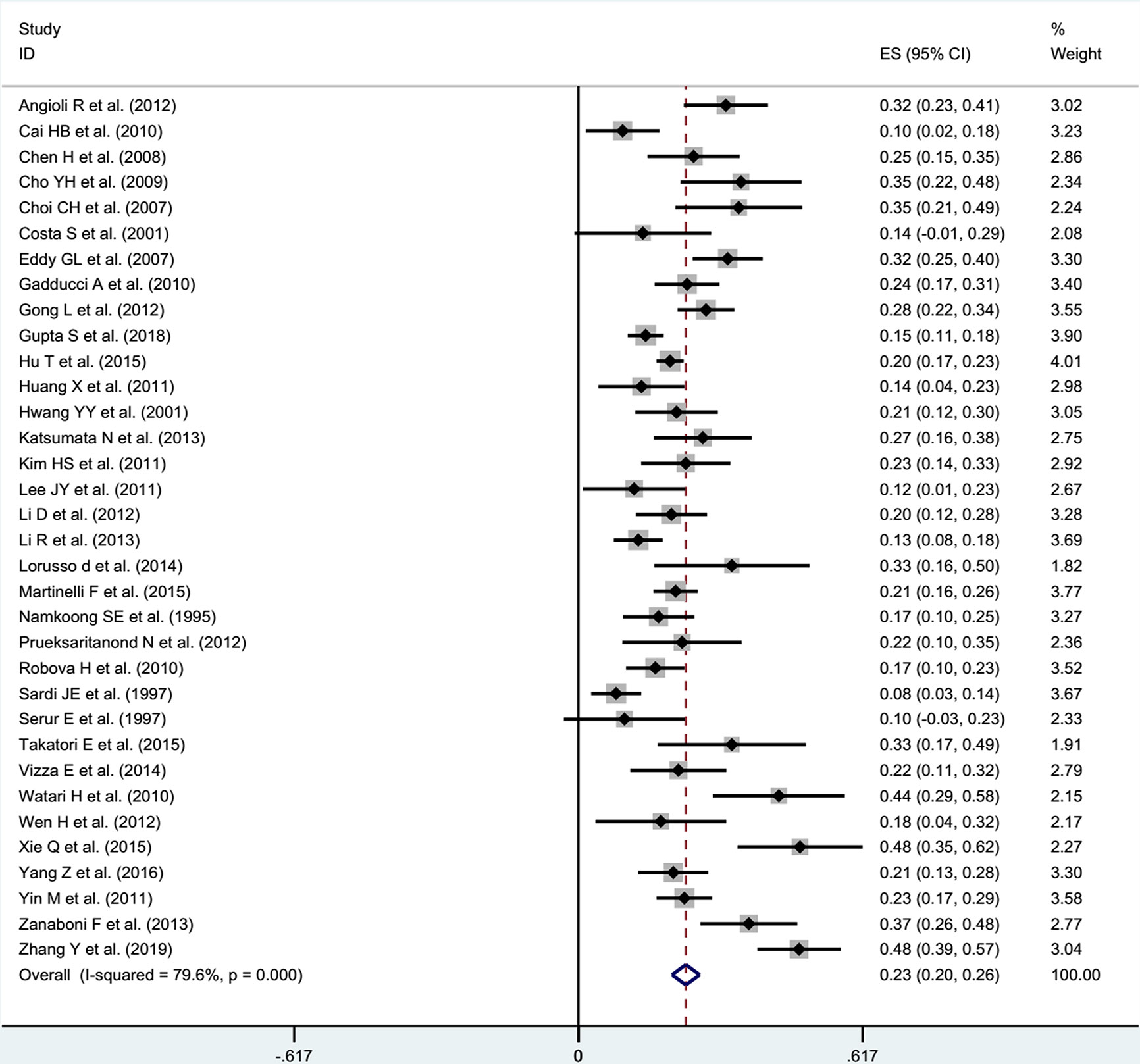
After receiving NACT in patients with stage IB1-IIB cervical cancer, the pooled incidence was 23% (95% CI: 0.20-0.26) with significant heterogeneity (I2 = 79.6%, P<0.001) in the random effects model (Figure 2). Through Freeman-Turkey double arcsine transformation, the transformed estimate of LNM rate was 23.8% (95% CI: 0.209-0.269), which mostly matched the overall random effect estimates (Supplementary Table 4). Further, we removed the literature with high risk of bias and estimated the LNM rate to be 23% (95% CI: 0.20-0.27; I2 = 80.4%, P<0.001) (Figure 3).
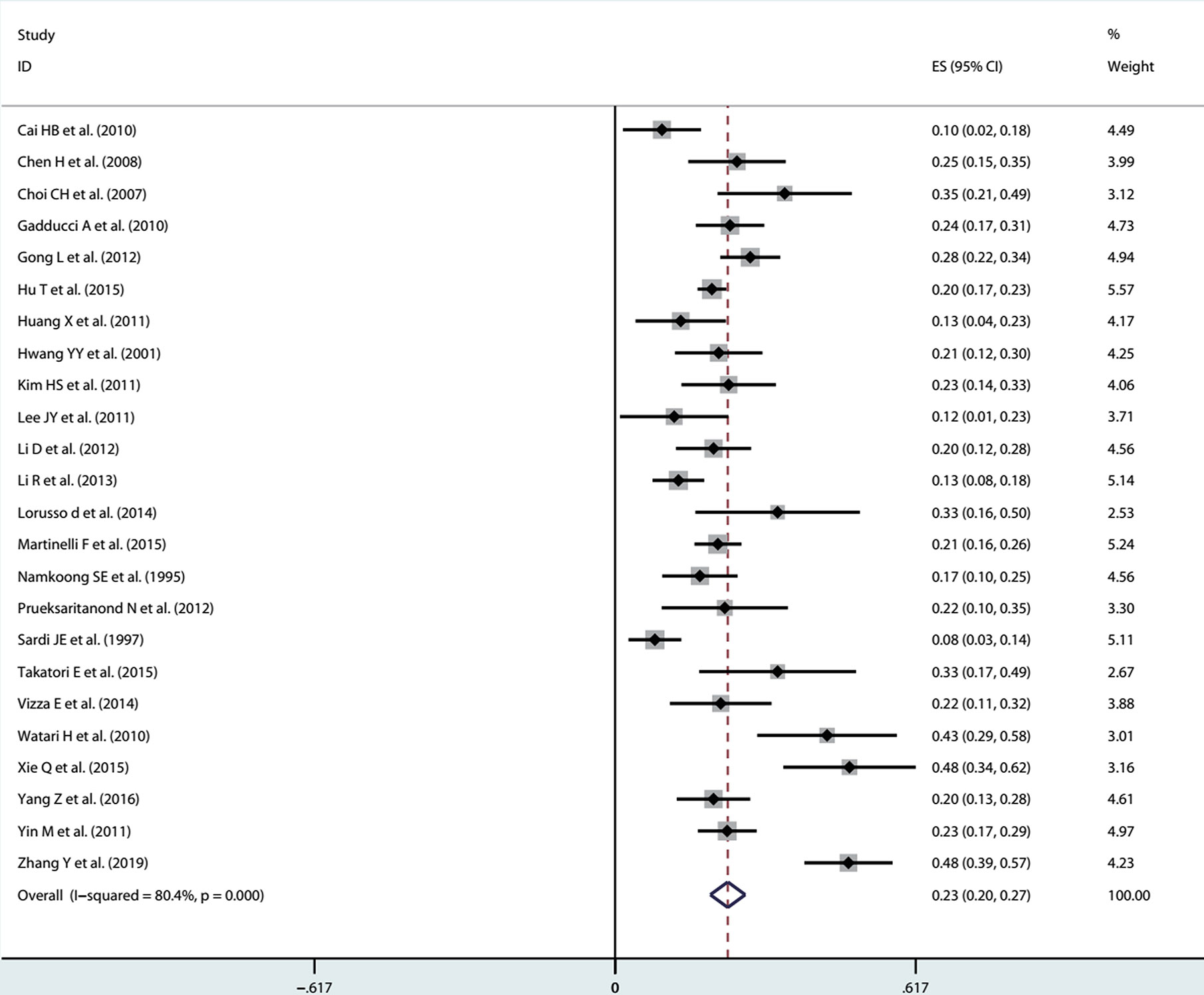
Figure 3 After removing the literature with high risk of bias, the forest plot of estimated LNM rate.
Subgroup Analysis
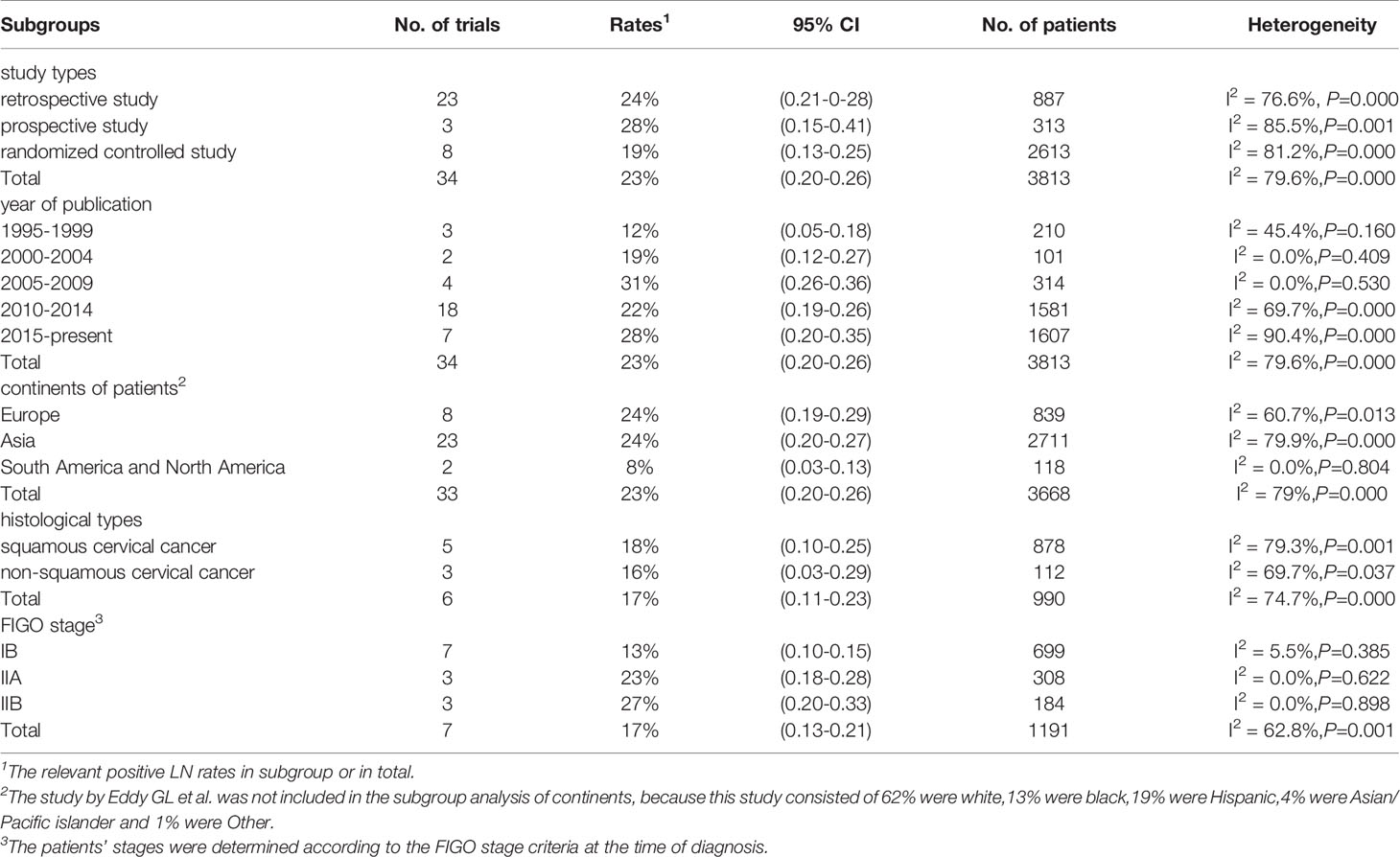
We took the following factors into consideration for the subgroup analysis: study types, the year of publication, patients’ continents, histological types, and FIGO stage (Table 1). Supplementary Figures 4A, B shows the rate of positive LNs in subgroups defined by the year of publication and FIGO stage. From 1995 to 2009, LNM rates showed an upward trend (1995-1999: 12%, 95% CI: 0.05-0.18; 2000-2004: 19%, 95% CI: 0.12-0.27; 2005-2009: 31%, 95% CI: 0.26-0.36). From 2010 to present, LNM rates fluctuated (2010-2014: 22%, 95% CI: 0.19-0.26; 2015-present: 28%, 95% CI: 0.20-0.35). Supplementary Figure 4B shows that the LNM rate was 13% (95% CI: 0.10-0.15) in stage IB, 23% (95% CI: 0.18-0.28) in stage IIA, and 27% (95% CI: 0.20-0.33) in stage IIB.
Sensitivity Analysis and Publication Bias
The sensitivity analyses were performed by excluding one study at a time and did not noticeably affect the results (Supplementary Figure 1). The visual inspection of funnel plots and the Egger’s test showed no evidence of the presence of small study effects (Supplementary Figure 2, 3).
NACT Plus Surgery Versus Surgery
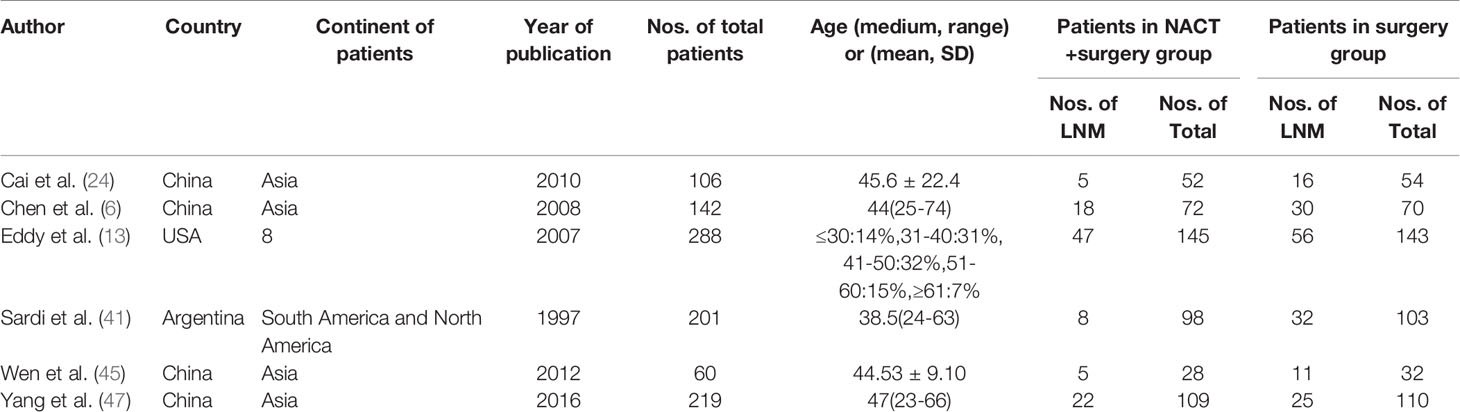
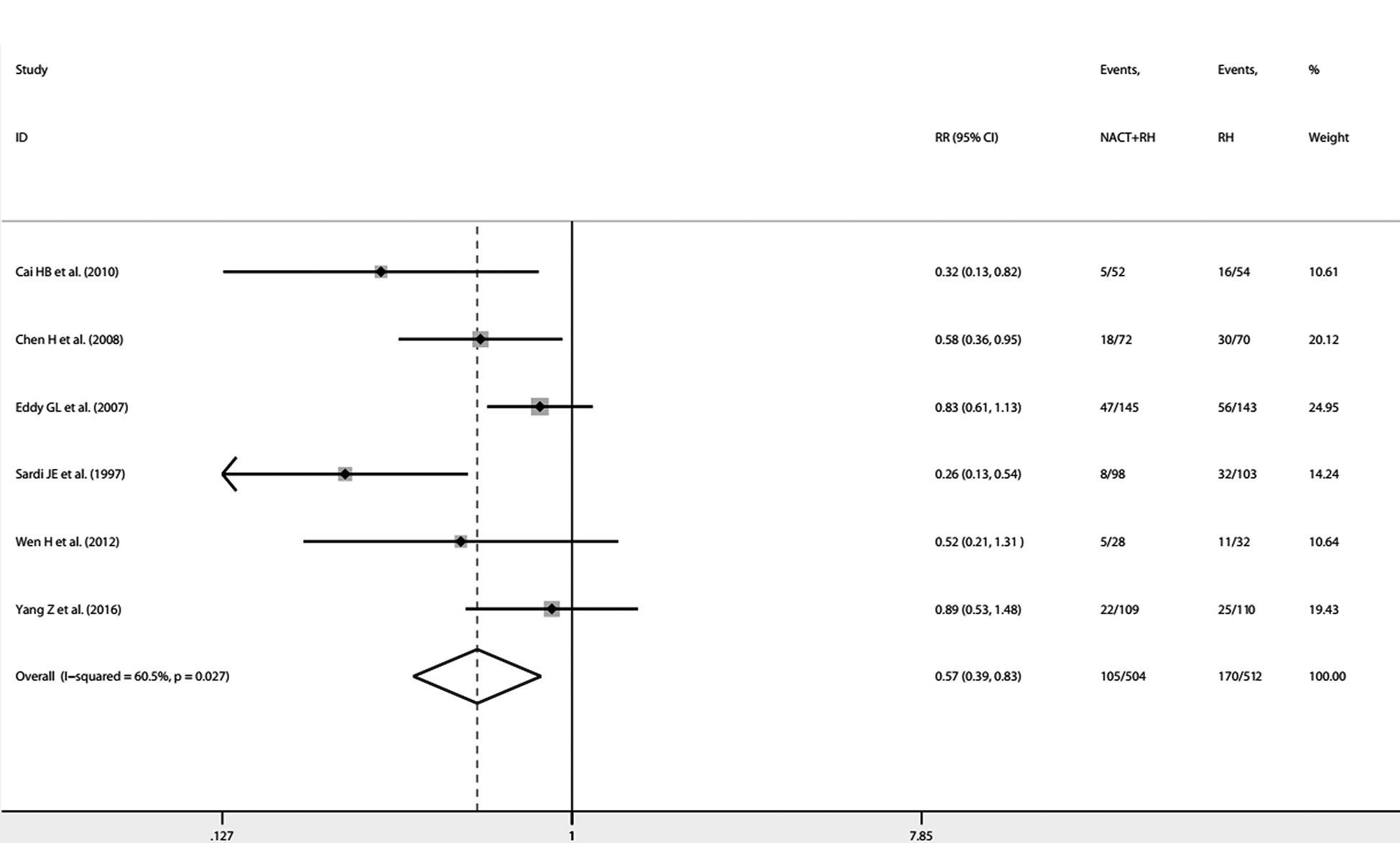
From the 34 included studies, we identified six two-arm RCTs including 1016 patients (6, 13, 24, 41, 45, 47). The characteristics for the six RCTs are summarized in Table 2. Revman (version 5.3) was used to conduct the assessment of the risk of bias for each RCT on the basis of the Cochrane Collaboration tool (51). The results are shown in Figures 4A, B. Because I2 = 60.5%, P=0.027, we used the random effects model. The pooled RR of 0.57 (95% CI=0.39-0.83) suggests a significant lower risk of LNM in the NACT plus RH group than RH group (Figure 5). Furthermore, after extracting the 5-year OS in 5 RCTs, the RR of OS was estimated as 1.13 (95% CI: 1.03-1.23; I2 = 0.0%, P=0.842), which suggests that the 5-year OS of the NACT+RH group was higher than that of the RH group (Supplementary Table 5, Supplementary Figure 5).
Discussion
LNM is one of the most important risk factors affecting the prognosis of cervical cancer, so we explored the impact of NACT on LNM rates. In FIGO stage IB1-IIB cervical cancer, the positive LN rate of NACT followed by RH was 23% (95% CI: 0.20-0.26). The NACT plus RH brings more benefits in reducing LNM among the stage IIA and IIB patients. Through the comparison between NACT plus RH and RH, we further confirmed that the incidence of nodal metastasis was lower in the NACT plus RH group (RR=0.57, 95% CI: 0.39-0.83, I2 = 60.5%, P=0.027).
Based on this research, LNM occurs in 23% of patients with stage IB1-IIB cervical cancer after NACT plus RH (95% CI: 0.20-0.26, I2 = 79.6%, P<0.001), which was close to those earlier analyses (52–55). Our systematic review and meta-analysis included 34 studies, consisting of 3813 patients. Such a large scale can increase the applicability of our findings. Furthermore, the subsequent data processing and analysis, such as Freeman-Turkey double arcsine transformation and subgroup analysis, could ensure the accuracy of the findings.
The incidence of positive LNs from 1995 to 2019 show an upward trend through the subgroup analysis by the year of publication. The reason for the difference over time was speculated to be stage migration and development of diagnostic techniques. With the improvement of imaging technology and surgical methods, the accuracy and sensitivity of finding positive LNs were greatly improved. Similarly, a study based on squamous cell carcinoma of the anus noted an increase in observed positive LN rate over time, which was summarized as the Will Rogers phenomenon (56, 57).
If we divide patients into three subgroups as IB, IIA, and IIB, the between-study heterogeneity is not significant. The estimated incidence was 13% (95% CI: 0.10-0.15) in stage IB, 23% (95% CI: 0.18-0.28) in stage IIA, and 27% (95% CI: 0.20- 0.33) in stage IIB. According to the previous literature including patients receiving surgery only, the nodal metastasis rates of the FIGO stage IB, IIA, and IIB were 11.5-22%, 26.7-33%, and 39.2%-63%, respectively (52–55). Therefore, NACT plus RH was more effective in reducing LNM for patients with stage IIA and IIB.
When we consider the LNM rate in patients undergoing preoperative chemotherapy plus RH and RH only, the pooled RR of 0.57 (95% CI: 0.39-0.83, I2 = 60.5%, P=0.027) revealed the lower risk of LNM in the NACT + RH group than the RH group. When considering 5-year OS as the tumor outcome, the pooled RR of 1.13 (95% CI: 1.03-1.23; I2 = 0.0%, P=0.842) suggests a higher 5-year OS of the NACT + RH group compared with the RH group. Actually, the beneficial effects of NACT have been mentioned in many other studies. Napolitano U et al. compared the OS and disease-free survival (DFS) between the NACT group and conventional surgery or radiotherapy alone groups and found that NACT plus RH can enable more patients to undergo surgical treatment and improve OS and DFS (58). Also, Rydzewska L et al. conducted a series of related studies on NACT plus surgery for cervical cancer. In 2010, he analyzed pathological response and found a significant decrease in LNM in the NACT plus surgery group compared with the surgery group (OR: 0.54, 95% CI: 0.39-0.73; heterogeneity: P ≤ 0.001) (59). In 2012, Rydzewska L et al. conducted further research and got a similar significant decrease in LNM (OR: 0.54, 95% CI: 0.40-0.73; heterogeneity: P ≤ 0.001) (60).
However, some studies propose different perspectives. Gong L et al. provided a phase III trial based on locally advanced cervical cancer (LACC) patients to detect the incidence of nodal metastasis. Their analysis showed that the proportion of positive-node patients in the NACT plus RH group (27.8%) was similar to that in the RH group (28.8%) (61). From these data, Gong L et al. concluded that NACT plus RH did not show a significant advantage compared with RH only. However, according to the clinical features, patients in the NACT plus RH group had a higher incidence of anemia before treatment (36% vs. 16%, p < 0.001), larger diameter of tumor (5.5 vs. 4.9 cm, p < 0.001), and a higher proportion in advanced stage, especially stage IIB (57% vs. 11%, p < 0.001), compared with the RH group. Therefore, the conclusion lacked strong evidence.
Recently, an RCT by Gupta S et al. caught great attention (14). Through comparing the efficacy of NACT plus RH versus concomitant chemotherapy and radiotherapy (CTRT) in LACC patients, they found no advantage in pelvic positive LN rate (14.6% vs 14.2%) (14). However, their assessment of LN status was limited to the pelvis and was not based on surgical outcomes, so the results did not accurately reflect the actual status of LNs. What is more, among the included patients with different disease stages, their analysis did not fully answer the questions for patients with operable cervical cancer (stages IB2 and IIA).
There also existed several limitations. First, to limit heterogeneity, subgroup analysis was performed based on six terms. Some subgroup analysis might be limited to only 3 studies with small sample sizes. Second, several important factors, such as depth of invasion and lymph-vascular space invasion (LVSI), can significantly affect the prognosis of patients with cervical cancer, but these included articles did not provide the incidence of LNM in patients grouped by depth of invasion or LVSI. Therefore, the subgroup analysis to explore the impact of infiltration depth and LVSI was not performed. Third, some studies used different evaluation methods. Four only provided the status of LNs by preoperative MRI. Another 30 studies determined the status of both para-aortic LNs and pelvis LNs based on surgical findings. When only including the studies reporting LNM based on surgical findings, the analysis demonstrated similar results (Supplementary Figure 6).
Conclusion
The rate of positive LN in stage IB1-IIB cervical cancer patients was 23% after receiving NACT plus RH. The LNM rate in the NACT plus RH group was lower than the RH group. NACT plus RH showed the more obvious effect of eliminating positive LNs in patients with stage IIA and IIB compared with previously reported surgical patients. Therefore, NACT can be considered as a valuable and reasonable treatment option in patients with stage IB1-IIB cervical cancer.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
BC, LW, CR, HS, WD, DZ, LM, and HW contributed to the conception and design of the study. BC, LM, and HW organized the databases and provided methodological supports. BC and LM performed the statistical analysis. BC wrote the draft of the manuscript. LM and HW contributed to the supervision of the study. All authors critically reviewed and revised the manuscript for important intellectual content and approved the final version of the manuscript before submission. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a key program from the National Natural Science Foundation of China (No.81830074).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.570258/full#supplementary-material
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Haie-Meder C, Morice P, Castiglione M, ESMO Guidelines Working Group. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2010) 21 Suppl 5:v37–40. doi: 10.1093/annonc/mdq162
4. Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2012) 23 Suppl 7:vii27–32. doi: 10.1093/annonc/mds268
5. Koh W-J, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Comp Canc Netw (2019) 17(1):64–84. doi: 10.6004/jnccn.2019.0001
6. Chen H, Liang C, Zhang L, Huang S, Wu X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study. Gynecol Oncol (2008) 110(3):308–15. doi: 10.1016/j.ygyno.2008.05.026
7. Kim HS, Kim JY, Park NH, Kim K, Chung HH, Kim YB, et al. Matched-case comparison for the efficacy of neoadjuvant chemotherapy before surgery in FIGO stage IB1-IIA cervical cancer. Gynecol Oncol (2010) 119(2):217–24. doi: 10.1016/j.ygyno.2010.06.017
8. Hu T, Li S, Chen Y, Shen J, Li X, Huang K, et al. Matched-case comparison of neoadjuvant chemotherapy in patients with FIGO stage IB1-IIB cervical cancer to establish selection criteria. Eur J Cancer (2012) 48(15):2353–60. doi: 10.1016/j.ejca.2012.03.015
9. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(suppl_4):iv72–83. doi: 10.1093/annonc/mdx220
10. Frei ER. Clinical cancer research: an embattled species. Cancer (1982) 50(10):1979–92. doi: 10.1002/1097-0142(19821115)50:103.0.CO;2-D
11. Friedlander M, Kaye SB, Sullivan A, Atkinson K, Elliott P, Coppleson M, et al. Cervical carcinoma: a drug-responsive tumor–experience with combined cisplatin, vinblastine, and bleomycin therapy. Gynecol Oncol (1983) 16(2):275–81. doi: 10.1016/0090-8258(83)90102-6
12. Vizza E, Corrado G, Zanagnolo V, Tomaselli T, Cutillo G, Mancini E, et al. Neoadjuvant chemotherapy followed by robotic radical hysterectomy in locally advanced cervical cancer: a multi-institution study. Gynecol Oncol (2014) 133(2):180–5. doi: 10.1016/j.ygyno.2014.02.035
13. Eddy GL, Bundy BN, Creasman WT, Spirtos NM, Mannel RS, Hannigan E, et al. Treatment of (“bulky”) stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecol Oncol (2007) 106(2):362–9. doi: 10.1016/j.ygyno.2007.04.007
14. Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients with Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J Clin Oncol (2018) 36(16):1548–55. doi: 10.1200/JCO.2017.75.9985
15. Kim SM, Choi HS, Byun JS. Overall 5-year survival rate and prognostic factors in patients with stage IB and IIA cervical cancer treated by radical hysterectomy and pelvic lymph node dissection. Int J Gynecol Cancer (2000) 10(4):305–12. doi: 10.1046/j.1525-1438.2000.010004305.x
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C. The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.2427/5768
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. PLoS Med (2009) 7(6):e1000097. doi: 10.1371/journal.pmed.1000097
18. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health (2014) 72(1):39. doi: 10.1186/2049-3258-72-39
19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
21. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analysis of controlled trials with binary endpoints. Stat Med (2006) 25(20):3443–57. doi: 10.1002/sim.2380
22. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
23. Angioli R, Plotti F, Montera R, Aloisi A, Luvero D, Capriglione S, et al. Neoadjuvant chemotherapy plus radical surgery followed by chemotherapy in locally advanced cervical cancer. Gynecol Oncol (2012) 127(2):290–6. doi: 10.1016/j.ygyno.2012.07.104
24. Cai HB, Chen HZ, Yin HH. Randomized study of preoperative chemotherapy versus primary surgery for stage IB cervical cancer. J Obstet Gynaecol Res (2006) 32(3):315–23. doi: 10.1111/j.1447-0756.2006.00404.x
25. Cho YH, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Comparative study of neoadjuvant chemotherapy before radical hysterectomy and radical surgery alone in stage IB2-IIA bulky cervical cancer. J Gynecol Oncol (2009) 20(1):22–7. doi: 10.3802/jgo.2009.20.1.22
26. Choi CH, Kim TJ, Lee JW, Kim BG, Lee JH, Bae DS. Phase II study of neoadjuvant chemotherapy with mitomycin-c, vincristine and cisplatin (MVC) in patients with stages IB2-IIB cervical carcinoma. Gynecol Oncol (2007) 104(1):64–9. doi: 10.1016/j.ygyno.2006.07.006
27. Costa S, Terzano P, Santini D, Ceccarelli C, Martoni A, Angelelli B, et al. Neoadjuvant chemotherapy in cervical carcinoma: regulators of cell cycle, apoptosis, and proliferation as determinants of response to therapy and disease outcome. Am J Clin Pathol (2001) 116(5):729–37. doi: 10.1309/8B4E-57PR-T50F-VRQT
28. Gadducci A, Cosio S, Zola P, Tisi G, Ferrero A, Piovano E, et al. Pretreatment platelet and hemoglobin levels are neither predictive nor prognostic variables for patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy: a retrospective Italian study. Int J Gynecol Cancer (2010) 20(8):1399–404. doi: 10.1111/IGC.0b013e3181f1574e
29. Gong L, Lou JY, Wang P, Zhang J, Liu H, Peng Z. Clinical evaluation of neoadjuvant chemotherapy followed by radical surgery in the management of stage IB2-IIB cervical cancer. Int J Gynaecol Obstet (2012) 117(1):23–6. doi: 10.1016/j.ijgo.2011.11.017
30. Huang X, Lan C, Huang H, Zhang Y, Huang H, Cao X, et al. Neoadjuvant docetaxel combined with cisplatin and followed by radical surgery for the treatment of locally advanced (stage IB2 - IIB) cervical cancer: preliminary results of a single-institution experience. Expert Opin Pharmacother (2011) 12(2):165–73. doi: 10.1517/14656566.2011.530657
31. Hwang YY, Moon H, Cho SH, Kim KT, Moon YJ, Kim SR, et al. Ten-year survival of patients with locally advanced, stage ib-iib cervical cancer after neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol (2001) 82(1):88–93. doi: 10.1006/gyno.2001.6204
32. Katsumata N, Yoshikawa H, Kobayashi H, Saito T, Kuzuya K, Nakanishi T, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer (2013) 108(10):1957–63. doi: 10.1038/bjc.2013.179
33. Lee JY, Kim YH, Kim MJ, Kim K, Chung HH, Park NH, et al. Treatment of stage IB2, IIA bulky cervical cancer: a single-institution experience of neoadjuvant chemotherapy followed by radical hysterectomy and primary radical hysterectomy. Arch Gynecol Obstet (2011) 284(2):477–82. doi: 10.1007/s00404-010-1685-9
34. Li D, Cai J, Kuang Y, Cao J, Wang Z. Surgical-pathologic risk factors of pelvic lymph node metastasis in stage Ib1-IIb cervical cancer. Acta Obstet Gynecol Scand (2012) 91(7):802–9. doi: 10.1111/j.1600-0412.2012.01415.x
35. Li R, Lu ST, Si JG, Liu B, Wang H, Mei Y, et al. Prognostic value of responsiveness of neoadjuvant chemotherapy before surgery for patients with stage IB(2)/IIA(2) cervical cancer. Gynecol Oncol (2013) 128(3):524–9. doi: 10.1016/j.ygyno.2012.11.006
36. Lorusso D, Ramondino S, Mancini M, Zanaboni F, Ditto A, Raspagliesi F. Phase II trial on cisplatin-adriamycin-paclitaxel combination as neoadjuvant chemotherapy for locally advanced cervical adenocarcinoma. Int J Gynecol Cancer (2014) 24(4):729–34. doi: 10.1097/IGC.0000000000000115
37. Martinelli F, Bogani G, Ditto A, Carcangiu M, Papadia A, Lecce F. How often parametrial involvement leads to post-operative adjuvant treatment in locally advanced cervical cancer after neoadjuvant chemotherapy and type C radical hysterectomy? Eur J Surg Oncol (2015) 41(8):1089–96. doi: 10.1016/j.ejso.2015.03.228
38. Namkoong SE, Park JS, Kim JW, Bae SN, Han GT, Lee JM, et al. Comparative study of the patients with locally advanced stages I and II cervical cancer treated by radical surgery with and without preoperative adjuvant chemotherapy. Gynecol Oncol (1995) 59(1):136–42. doi: 10.1006/gyno.1995.1280
39. Prueksaritanond N, Chaisarn P, Yanaranop M. The efficacy of neoadjuvant paclitaxel-carboplatin chemotherapy followed by radical hysterectomy compared to radical hysterectomy alone in bulky stage IB2-IIA cervical cancer. J Med Assoc Thai (2012) 95 Suppl 3:S55–61.
40. Robova H, Halaska M, Pluta M, Skapa P, Strnad P, Lisy J, et al. The role of neoadjuvant chemotherapy and surgery in cervical cancer. Int J Gynecol Cancer (2010) 20(11 Suppl 2):S42–6. doi: 10.1111/IGC.0b013e3181f60d73
41. Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, et al. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecol Oncol (1997) 67(1):61–9. doi: 10.1006/gyno.1997.4812
42. Serur E, Mathews RP, Gates J, Levine P, Maiman M, Remy JC. Neoadjuvant chemotherapy in stage IB2 squamous cell carcinoma of the cervix. Gynecol Oncol (1997) 65(2):348–56. doi: 10.1006/gyno.1997.4645
43. Takatori E, Shoji T, Omi H, Kagabu M, Miura F, Takeuchi S, et al. Analysis of prognostic factors for patients with bulky squamous cell carcinoma of the uterine cervix who underwent neoadjuvant chemotherapy followed by radical hysterectomy. Int J Clin Oncol (2015) 20(2):345–50. doi: 10.1007/s10147-014-0702-6
44. Watari H, Kanuma T, Ohta Y, Hassan MH, Mitamura T, Hosaka M, et al. Clusterin expression inversely correlates with chemosensitivity and predicts poor survival in patients with locally advanced cervical cancer treated with cisplatin-based neoadjuvant chemotherapy and radical hysterectomy. Pathol Oncol Res (2010) 16(3):345–52. doi: 10.1007/s12253-009-9235-0
45. Wen H, Wu X, Li Z, Wang H, Zang R, Sun M, et al. A prospective randomized controlled study on multiple neoadjuvant treatments for patients with stage IB2 to IIA cervical cancer. Int J Gynecol Cancer (2012) 22(2):296–302. doi: 10.1097/igc.0b013e31823610a1
46. Xie Q, Liang J, Rao Q, Xie X, Li R, Liu Y, et al. Aldehyde Dehydrogenase 1 Expression Predicts Chemoresistance and Poor Clinical Outcomes in Patients with Locally Advanced Cervical Cancer Treated with Neoadjuvant Chemotherapy Prior to Radical Hysterectomy. Ann Surg Oncol (2016) 23(1):163–70. doi: 10.1245/s10434-015-4555-7
47. Yang Z, Chen D, Zhang J, Yao D, Gao K, Wang H, et al. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: A randomized multicenter study. Gynecol Oncol (2016) 141(2):231–9. doi: 10.1016/j.ygyno.2015.06.027
48. Yin M, Zhao F, Lou G, Zhang H, Sun M, Li C, et al. The long-term efficacy of neoadjuvant chemotherapy followed by radical hysterectomy compared with radical surgery alone or concurrent chemoradiotherapy on locally advanced-stage cervical cancer. Int J Gynecol Cancer (2011) 21(1):92–9. doi: 10.1111/IGC.0b013e3181fe8b6e
49. Zanaboni F, Grijuela B, Giudici S, Cormio G, Babilonti L, Ghezzi F, et al. Weekly topotecan and cisplatin (TOPOCIS) as neo-adjuvant chemotherapy for locally-advanced squamous cervical carcinoma: Results of a phase II multicentric study. Eur J Cancer (2013) 49(5):1065–72. doi: 10.1016/j.ejca.2012.10.008
50. Zhang Y, Yan H, Li R, Guo Y, Zheng R, et al. High expression of survivin predicts poor prognosis in cervical squamous cell carcinoma treated with paclitaxel and carboplatin. Med (Baltimore) (2019) 98(20):e15607. doi: 10.1097/MD.0000000000015607
51. Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
52. Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer (1999) 85(7):1547–54. doi: 10.1002/(sici)1097-0142(19990401)85:7
53. van Meurs H, Visser O, Buist MR, Kate FJWT, van der Velden J. Frequency of pelvic lymph node metastases and parametrial involvement in stage IA2 cervical cancer: a population-based study and literature review. Int J Gynecol Cancer (2009) 19(1):21–6. doi: 10.1111/IGC.0b013e318197f3ef
54. Rogers LJ, Luesley DM. Stage IA2 cervical carcinoma: how much treatment is enough? Int J Gynecol Cancer (2009) 19(9):1620–4. doi: 10.1111/IGC.0b013e3181a446b3
55. Togami S, Kamio M, Yanazume S, Yoshinaga M, Douchi T. Can pelvic lymphadenectomy be omitted in stage IA2 to IIB uterine cervical cancer? Int J Gynecol Cancer (2014) 24(6):1072–6. doi: 10.1097/IGC.0000000000000163
56. Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med (1985) 312(25):1604–8. doi: 10.1056/NEJM198506203122504
57. Sekhar H, Zwahlen M, Trelle S, Malcomson L, Kochhar R, Saunders MP, et al. Nodal stage migration and prognosis in anal cancer: a systematic review, meta-regression, and simulation study. Lancet Oncol (2017) 18(10):1348–59. doi: 10.1016/S1470-2045(17)30456-4
58. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol (2009) 27(28):4649–55. doi: 10.1200/JCO.2009.21.8909
59. Napolitano U, Imperato F, Mossa B, Framarino ML, Marziani R, Marzetti L, et al. The role of neoadjuvant chemotherapy for squamous cell cervical cancer (Ib-IIIb): a long-term randomized trial. Eur J Gynaecol Oncol (2003) 24(1):51–9. doi: 10.1016/S0010-7824(02)00442-0
60. Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev (2010) (1):CD007406. doi: 10.1002/14651858.CD007406.pub2
Keywords: uterine cervical neoplasms, neoadjuvant chemotherapy, surgery, lymphatic metastasis, lymph node metastasis
Citation: Chen B, Wang L, Ren C, Shen H, Ding W, Zhu D, Mao L and Wang H (2020) The Effect of Neoadjuvant Chemotherapy on Lymph Node Metastasis of FIGO Stage IB1-IIB Cervical Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 10:570258. doi: 10.3389/fonc.2020.570258
Received: 07 June 2020; Accepted: 23 September 2020;
Published: 05 November 2020.
Edited by:
Assia Konsoulova, Complex Oncological Center (Burgas), BulgariaReviewed by:
Pengpeng Qu, Tianjin Central Hospital for Gynecology and Obstetrics, ChinaTakuro Ariga, University of the Ryukyus Hospital, Japan
Copyright © 2020 Chen, Wang, Ren, Shen, Ding, Zhu, Mao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wang, aHVpdDcxQHNvaHUuY29t
 Bingxin Chen
Bingxin Chen Hui Wang
Hui Wang