- Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Purpose: To investigate the effect of chemotherapy and radiotherapy timing after breast conserving surgery (BCS) on recurrence and survival of women with early-stage breast cancer.
Patients and Methods: We retrospectively analyzed 900 patients who underwent BCS followed by both adjuvant chemotherapy and radiotherapy. Of these, 488 women received chemotherapy first (CT-first group) while the other 412 received radiotherapy first (RT-first group). Locoregional recurrence (LRR), distant metastasis (DM), disease-free survival (DFS), and overall survival (OS) rates were calculated using the Kaplan-Meier method and further confirmed with propensity-score matching (PSM) and the Cox proportional hazards model. The optimal cut-off value of interval time from surgery to the start of chemotherapy was calculated by Maxstat.
Results: The median follow-up was 7.1 years. In pre-match analysis, the CT-first group had a significantly higher 8-year DFS than the RT-first group (90.4% vs. 83.1%, P = 0.005). PSM analysis of 528 patients indicated that the 8-year DFS (91.0% vs. 83.3%, P = 0.005) and DM (8.6% vs. 14.6%, P = 0.017) were significantly better in the CT-first group, but that the OS (P = 0.096) and LRR (P = 0.434) were similar. We found the optimal cut-off value of interval from surgery to chemotherapy was 12 weeks. Patients starting chemotherapy later than 12 weeks after surgery had significantly inferior survival outcomes.
Conclusion: For women with breast cancer who require both chemotherapy and radiotherapy after BCS, adjuvant chemotherapy should be started within 12 weeks. Delaying the initiation of radiotherapy, for administration of long-course chemotherapy, does not compromise outcomes.
Introduction
In patients with early-stage invasive breast cancer, breast-conserving therapy offers a similar overall survival to mastectomy (1). Postoperative radiotherapy remains an integral part of breast-conserving therapy, providing remarkably consistent local control and overall survival (2–4). Besides radiotherapy, patients at high risk are always recommended chemotherapy due to the substantial reduction of the risk of relapse and death (5, 6). For those requiring both radiotherapy and chemotherapy after breast conserving surgery (BCS), the optimal sequence of adjuvant therapy needs to be investigated.
Early randomized trials comparing concurrent with sequential chemotherapy and radiotherapy after BCS found no significant difference in survival, but detrimental effects on long-term late toxicities in patients receiving concurrent treatment (7–10). Thus, adjuvant concurrent chemoradiotherapy is generally not recommended in women with breast cancer after BCS. As for sequential treatment, only one small randomized trial has yet evaluated the effect of sequencing radiotherapy and chemotherapy with an anthracycline-based regimen in breast cancer after BCS, and found no significant differences in the rates of freedom from any adverse event or death (11). In addition, retrospective studies demonstrated inconsistent findings owing to a heterogeneous population in terms of patient characteristics, chemotherapy regimens, and radiation techniques (12–17). Improvements in both chemotherapy and radiotherapy, such as taxane-based chemotherapy and hypofractionated radiotherapy, have changed early breast cancer treatment practice (16, 18). However, the optimal sequence of adjuvant treatment needs to be further investigated. Therefore, we conducted this study to determine the optimal timing of initiation of chemotherapy and radiotherapy after BCS.
Patients and Methods
Patient Selection
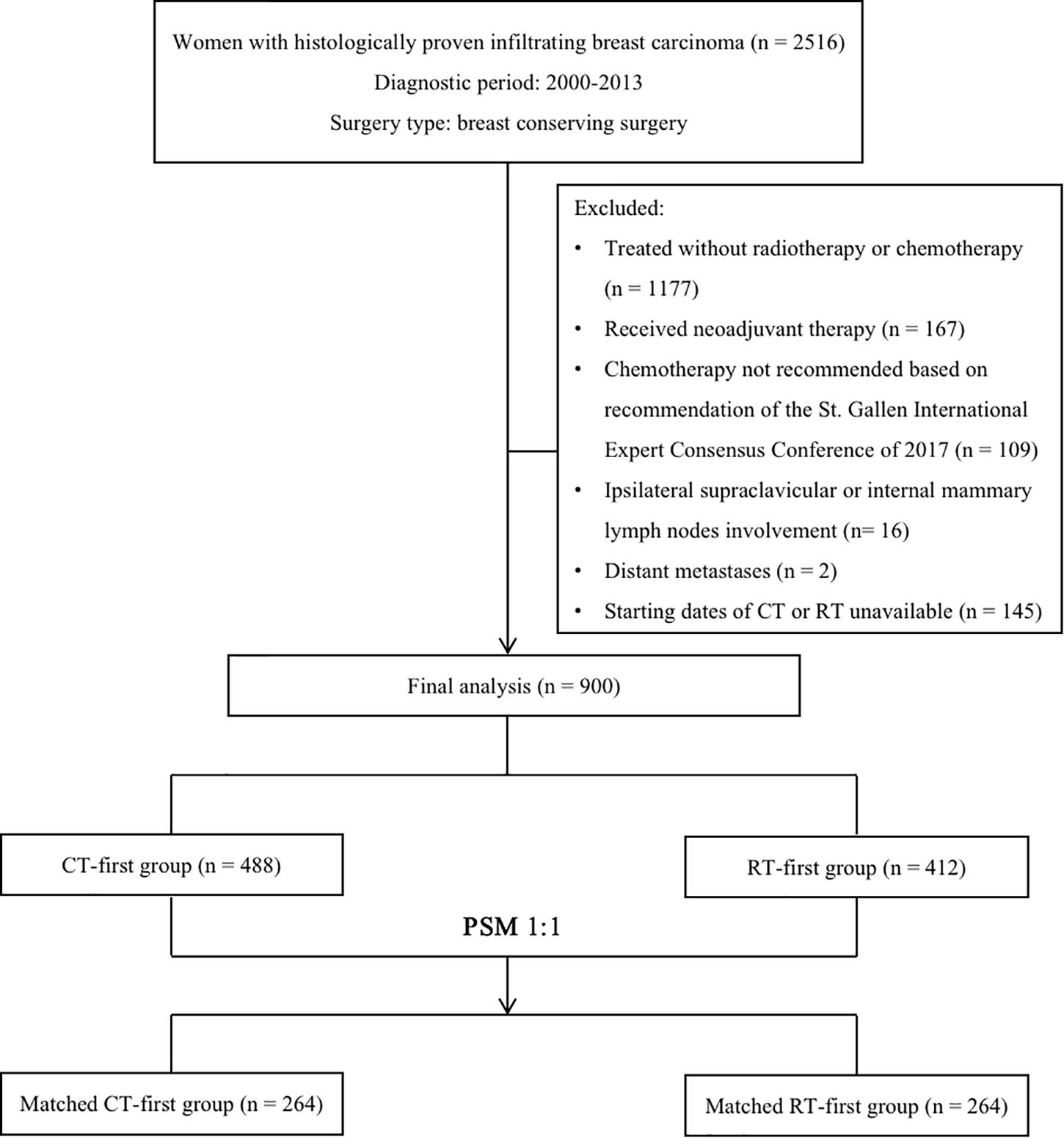
We retrospectively reviewed women with histologically proven infiltrating breast carcinoma treated in the Cancer Hospital of Chinese Academy of Medical Sciences between January 2000 and December 2013. Altogether, 1339 women over 18 years old who received BCS followed by adjuvant chemotherapy and radiotherapy were identified by medical profiles. We excluded patients who received neoadjuvant therapy (n = 167), those whose chemotherapy should be withdrawn according to the latest recommendation of the St. Gallen International Expert Consensus Conference (n = 109) (19), those with ipsilateral supraclavicular or internal mammary lymph node involvement (n = 16), and those with distant metastases at initial diagnosis (n = 2). Patients were also excluded when the initiation date of chemotherapy or radiotherapy was unknown (n = 145). A final total of 900 eligible patients were included in this study. Among them, 488 women received adjuvant chemotherapy first (CT-first group) while the other 412 received radiotherapy first (RT-first group, Figure 1).
Outcome Definition and Statistical Analysis
Interval time from surgery to chemotherapy (SCIT) was defined as the time from BCS to the start of chemotherapy. Interval time from surgery to radiotherapy (SRIT) was defined as the time from BCS to the start of radiotherapy.
The endpoints included disease-free survival (DFS), overall survival (OS), locoregional recurrence (LRR), and distant metastasis (DM). DFS was defined as the time from surgery to the first evidence of recurrence (locoregional or distant) or death from any cause. OS was defined as the time from surgery to death from any cause. LRR was defined as any tumor recurrence within the ipsilateral breast, or within the axillary, supraclavicular, or internal mammary nodes during follow-up. DM was defined as any failure outside the locoregional area defined above.
Baseline clinical characteristics were compared between different groups using the Chi-square test. Survival curves were estimated by the Kaplan-Meier method and compared with a log-rank test. Cox proportional hazards regression was performed for multivariate analysis. To address the imbalance of potential confounders of pretreatment variables, one-to-one patient matching without replacement was performed to pair cohorts of each group with a caliper size of 0.001 by propensity-score matching (PSM). We also used the Maxstat method to identify the optimal cut-off value of SCIT for outcomes (17). Statistical analyses were performed using SPSS Statistics v25.0 (IBM Corp., Armonk, NY, United States) and the “Maxstat” and “Matching” packages in R v3.4.41. A p-value < 0.05 was considered stastistically significant.
Results
Baseline Characteristics and Outcomes
The baseline characteristics of the whole group is shown in Table 1. The median age was 44 years (range, 20-74). The majority of patients were treated between 2008 and 2013 (75.6%), had stage I/II disease (89.0%), and had hormone receptor–positive disease (74.2%). We determined molecular subtypes by tumor grade, estrogen and progesterone receptors (ER/PR), and human epidermal growth factor receptor 2 (HER2) status. In the 884 (98.2%) patients who had these data available, 210 (23.3%) were classified as Luminal A, 287 (31.8%) Luminal B1, 149 (16.6%) Luminal B2, 62 (6.9%) HER2 overexpression, and 176 (19.6%) were triple negative.
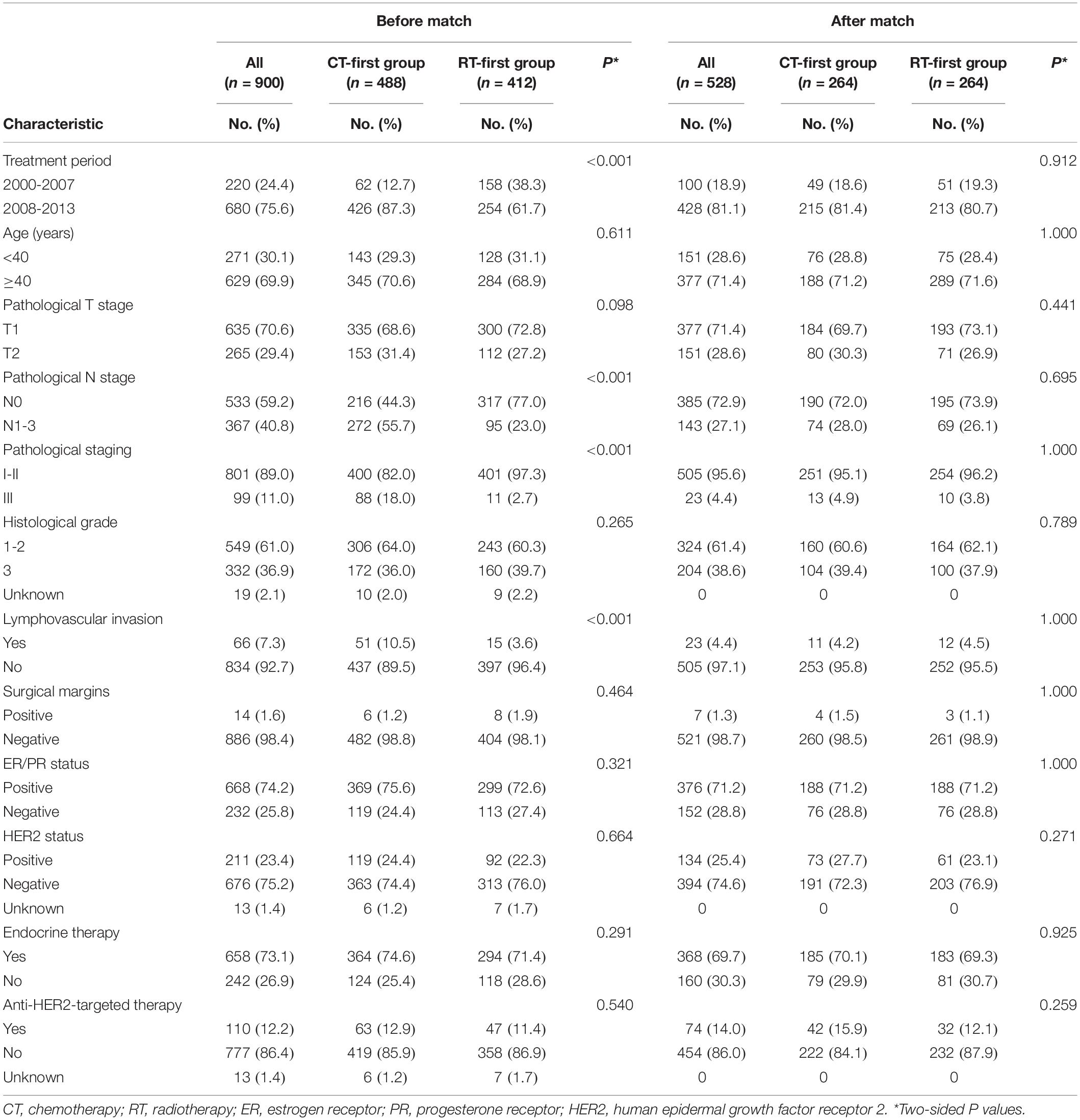
Table 1. Baseline characteristics of all patients stratified by receiving chemotherapy or radiotherapy first before and after matching.
Patients received lumpectomy combined with either axillary dissection (n = 667, 74.1%) or sentinel lymph node biopsy (n = 233, 25.9%). All patients received adjuvant chemotherapy with a median of 6 cycles (range, 1-8). The most commonly used regimen was anthracycline combined with taxanes in 425 (47.2%) patients, followed by an anthracycline-based regimen in 253 (28.1%) patients, a taxane-based regimen in 171 (19.0%) patients, and other regimens in 51 (5.7%) patients. Nearly all patients with ER/PR positive tumor (n = 658/668, 98.5%) received endocrine therapy. While only half of patients with HER2-positive disease (n = 110/211, 52.1%) received anti-HER2-targeted therapy combined with chemotherapy, with the regimens of doxorubicin plus cyclophosphamide followed by paclitaxel plus trastuzumab (n = 93); or docetaxel, cyclophosphamide, and trastuzumab (n = 17).
All patients underwent whole breast irradiation with a boost to the tumor bed. Of them, 719 (79.9%) received tangential field-based 3D conformal or intensity-modulated radiation therapy, while the other 181 (21.1%) received 2D tangential field therapy only. Altogether, 756 (84.0%) patients received conventional fractionated radiation: 50 Gy in 25 fractions over 5 weeks for the whole breast plus a tumor bed boost of 10 to 20 Gy in 5 to 10 fractions. The remaining 144 (16.0%) received hypofractionated radiotherapy of 43.5 Gy in 15 fractions over 3 weeks for the whole breast, with a boost dose of 8.7 Gy in 3 fractions (18). Meanwhile, 123 (13.7%) patients, mostly with N2/3 disease, also received additional supraclavicular regional irradiation by conventional fractionation.
Within the median follow-up time of 7.1 years (range, 1.2-18.6), a total of 113 (12.6%) women relapsed. Of these, 18 (15.9%) were due to isolated LRR, 67 (59.3%) due to isolated DM, and 28 (24.8%) due to both LRR and DM. During follow-up, 70 (7.8%) patients died. Of these, sixty-seven were due to breast cancer, two were due to leukemia, and one was due to severe pneumonia. The OS and DFS rates were 97.0% and 91.7% respectively at 5 years, dropping to 92.1% and 86.5% respectively at 8 years. The LRR and DM rates were 3.0% and 7.1% at 5 years, increasing to 5.2% and 11.0% at 8 years.
Treatment Intervals
The median interval between surgery and the start of adjuvant treatment was 4 weeks (range, 1-13; Figure 2A). The median interval time from surgery to chemotherapy (SCIT) was 6 weeks (range, 1-21) for all patients; for the CT-first group this was 4 weeks (range, 1-15); and for the RT-first group this was 13 weeks (range, 4-21; Figure 2B). The median interval time from surgery to radiotherapy (SRIT) was 14 weeks (range, 2-32) for all patients; for the CT-first group this was 22 weeks (range, 5-32); and for the RT-first group this was 5 weeks (range, 2-16; Figure 2C). All patients started radiotherapy within 32 weeks after BCS.
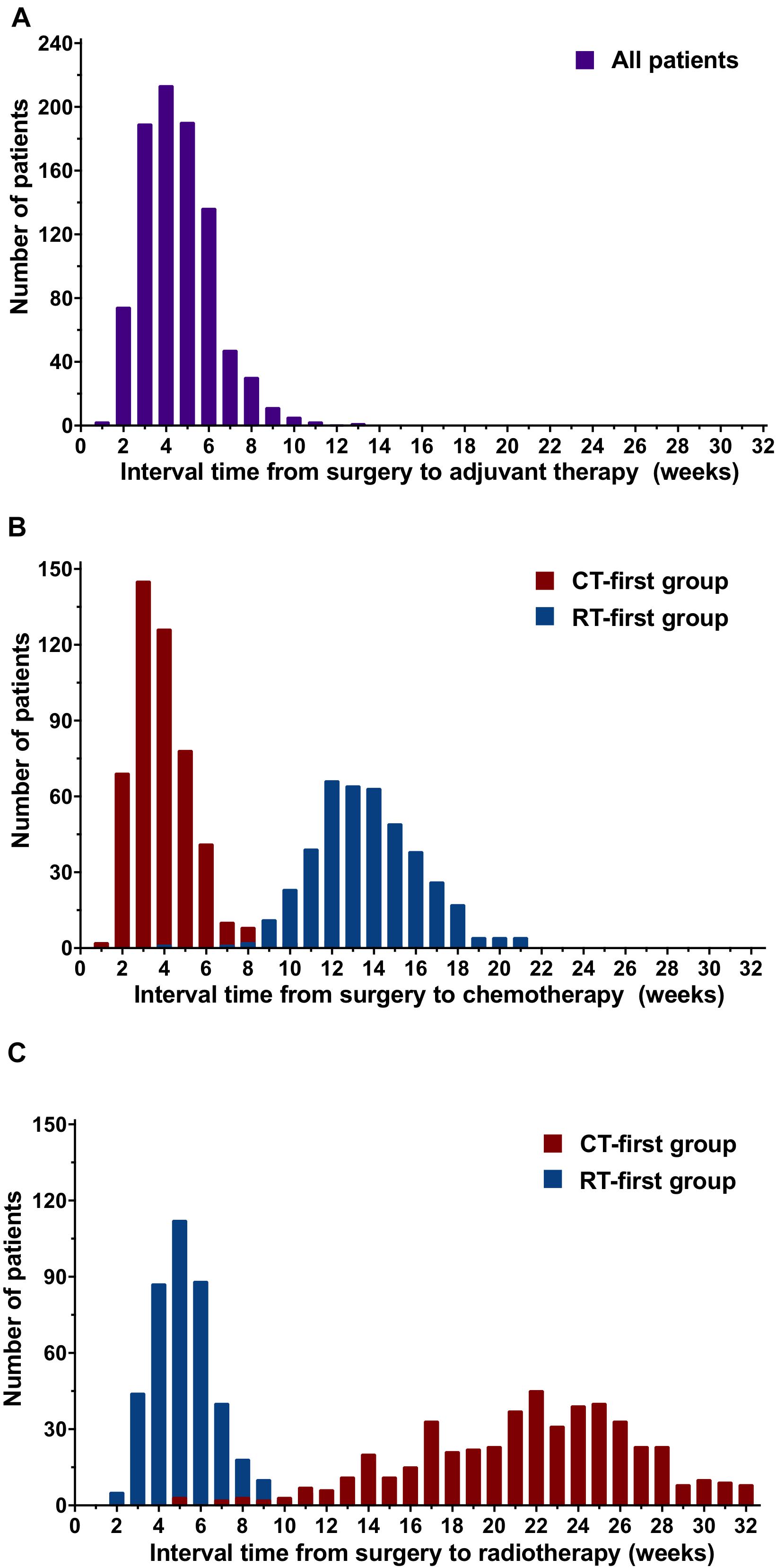
Figure 2. Distribution of the interval time from surgery to adjuvant treatment, chemotherapy, or radiotherapy for patients in the chemotherapy-first (CT-first) group and radiotherapy-first (RT-first) group. (A) Interval time from surgery to adjuvant treatment, (B) Interval time from surgery to chemotherapy (SCIT), (C) Interval time from surgery to radiotherap (SRIT).
Comparison of CT-First and RT-First Groups
Compared with the RT-first group, more patients in the CT-first group had high-risk factors, such as node-positive disease, stage III, and presence of lymphovascular invasion (Table 1). Since more patients in the CT-first group were treated in the time-period between 2008 and 2013 (Table 1), the median follow-up was 6.3 years (range, 1.2-15.8) for the CT-first group, whereas 8.5 years (range, 1.3-16.6) for the RT-first group. Patients in the CT-first group achieved a better DFS than those in the RT-first group (HR 0.58; 95% CI 0.39-0.85). The 5-year and 8-year DFS rates were 92.7% and 90.4% for the CT-first group, higher than those for the RT-first group (90.5% and 83.1% respectively, P = 0.005, Figure 3A). However, there was no significant difference between the two groups in terms of OS (P = 0.459, Figure 3B), DM (P = 0.070, Figure 3C), or LRR (P = 0.184, Figure 3D).
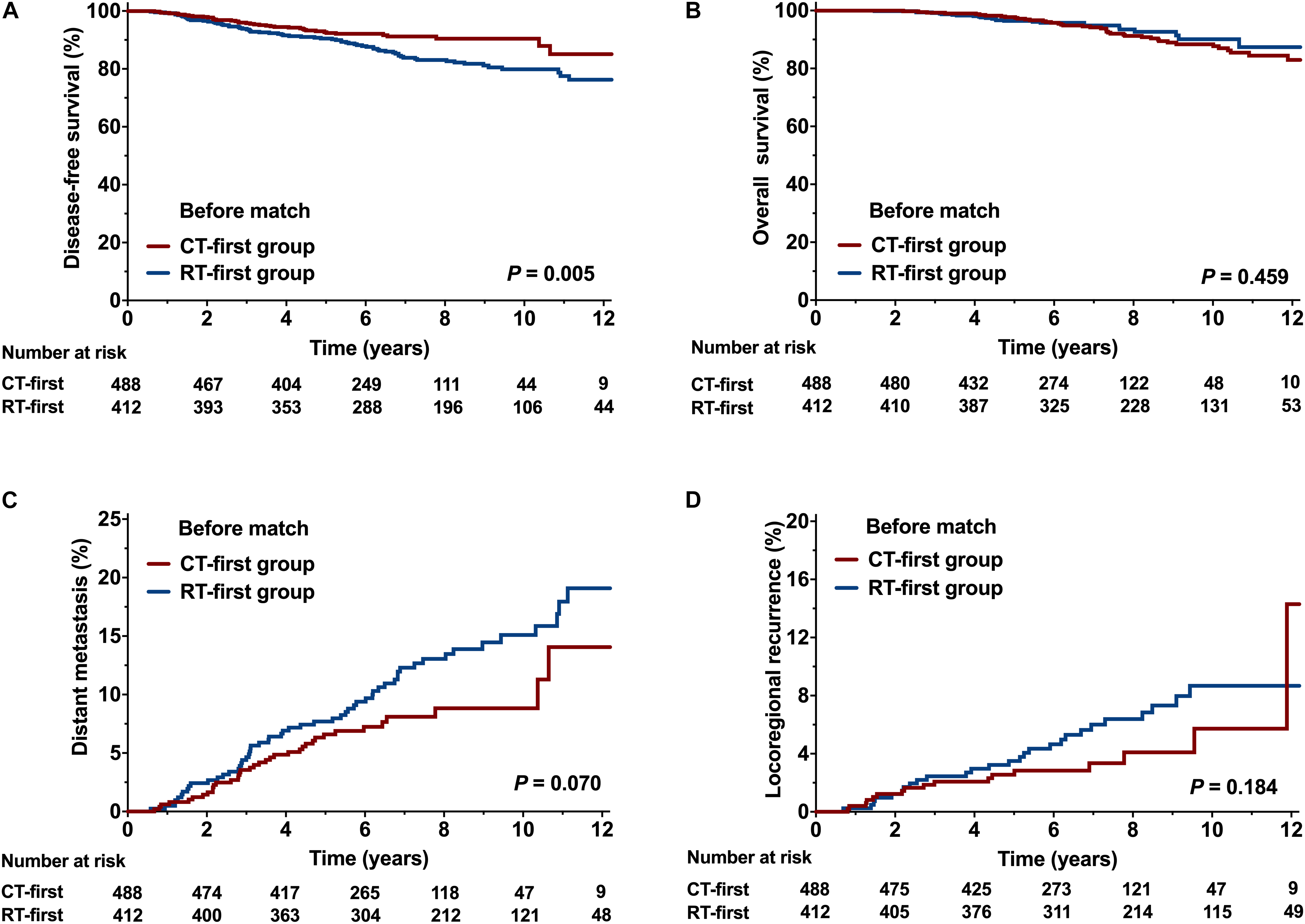
Figure 3. Comparison of the CT-first and RT-first groups before propensity score matching (PSM). (A) Disease-free survival (DFS), (B) Overall survival (OS), (C) Distant metastasis (DM), and (D) Locoregional recurrence (LRR).
Overall, 528 (58.7%) patients were selected by PSM, with 264 in each group. After adjusting for confounding variables, all clinical features were well balanced (Table 1). The CT-first group showed better DFS and DM compared with the RT-first group. The 8-year DFS rate of the CT-first group was 91.0%, significantly higher than the RT-first group (83.3%, P = 0.005, Figure 4A); the CT-first group had a 8-year DM rate of 8.6%, significantly lower than the RT-first group (14.6%, P = 0.017, Figure 4C). There was no significant difference in 8-year OS (94.2% and 90.9%, P = 0.096, Figure 4B) and 8-year LRR (4.2% vs. 5.3%, P = 0.434, Figure 4D) between the two groups.
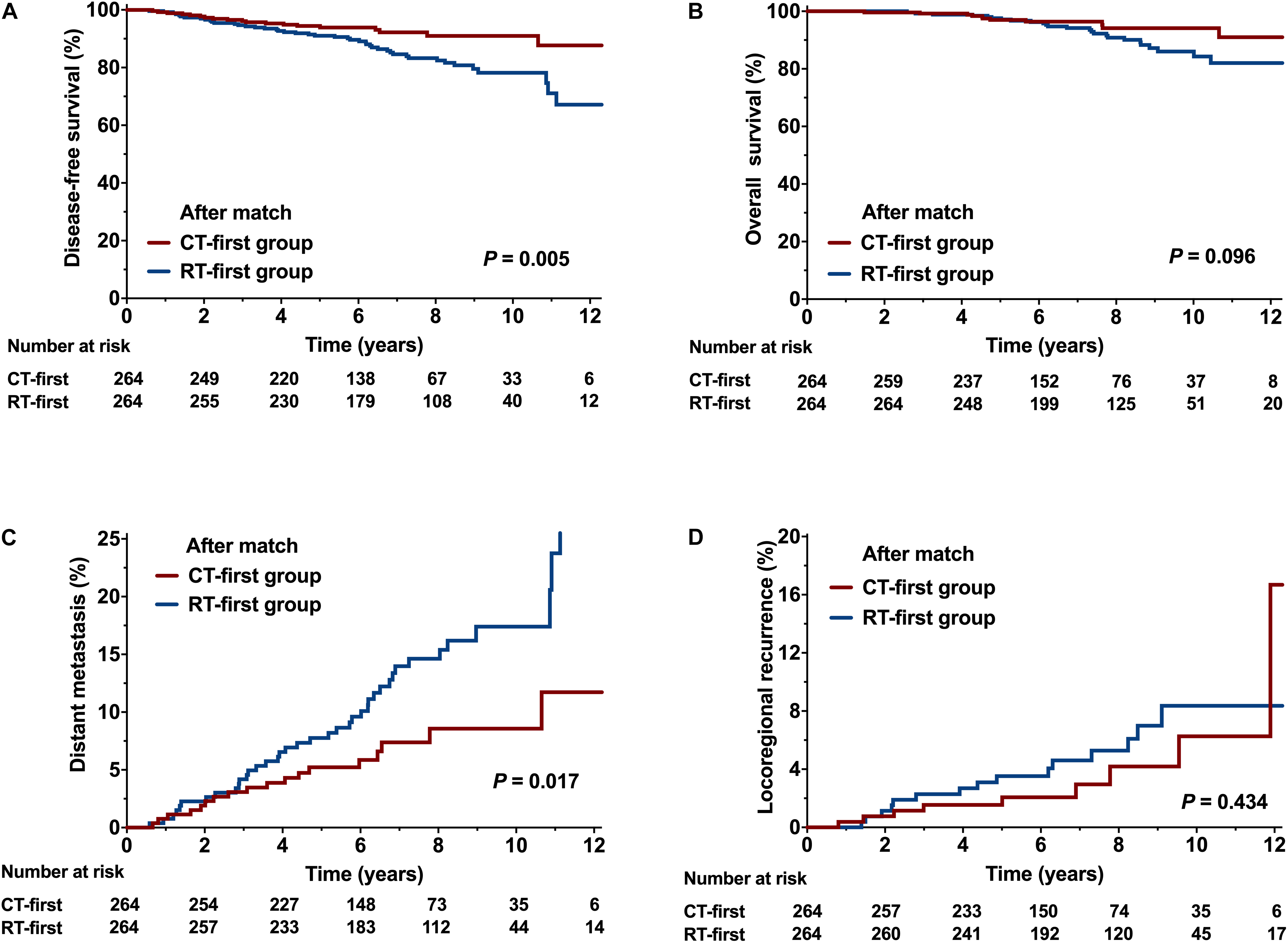
Figure 4. Comparison of the CT-first and RT-first groups after propensity score matching (PSM). (A) Disease-free survival (DFS), (B) Overall survival (OS), (C) Distant metastasis (DM), and (D) Locoregional recurrence (LRR).
Optimal Interval Between Surgery and Chemotherapy
Maxstat indicated that the optimal cut-off value of SCIT influencing DFS was 12 weeks. Accordingly, we divided patients into two groups: SCIT < 12 weeks (n = 581) and SCIT ≥ 12 weeks (n = 319). Of the patients with a SCIT < 12 weeks, there were 484 (99.2%) in the CT-first group and 97 (23.5%) in the RT-first group. Compared with the group of SCIT ≥ 12 weeks, more patients in the group of SCIT < 12 weeks had high-risk factors (Supplemental Table).
Table 2 shows the results of univariate analysis of the association between clinical variables and survival outcomes. Compared to a SCIT < 12 weeks, a SCIT ≥ 12 weeks was associated with a significantly lower 8-year DFS rate (80.7% vs. 90.3%, P < 0.001; Figure 5A); and 8-year OS rate (88.6% vs. 94.7%, P = 0.035; Figure 5B); a higher 8-year DM rate (14.8% vs. 8.8%, P = 0.013; Figure 5C) and 8-year LRR rate (7.6% vs. 3.8%, P = 0.015; Figure 5D). Since 98.5% of hormone receptor-positive patients received endocrine therapy, the variable of hormone receptor was excluded in the multivariable analysis in order to avoid the interaction of these two variables. Multivariable analysis demonstrated that a SCIT ≥ 12 weeks was independently associated with increased risk of LRR (HR 2.08, 95% CI 1.11-3.91, P = 0.023) and DM (HR 1.89, 95% CI 1.23-2.87, P = 0.003), as well as adverse DFS (HR 2.35, 95% CI 1.57-3.55; P < 0.001) and OS (HR 1.88, 95% CI 1.13-3.11; P = 0.015; Figure 6).
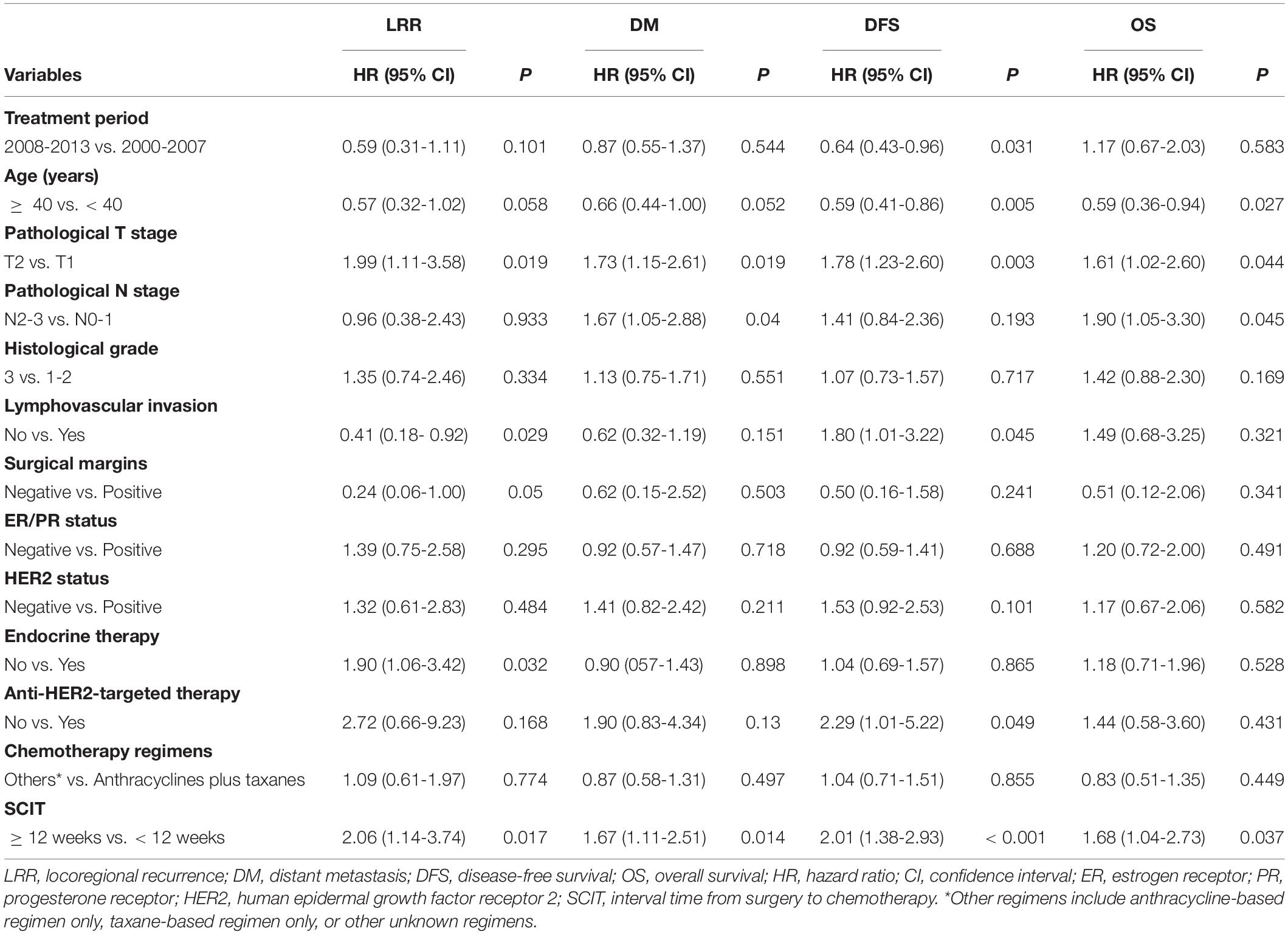
Table 2. Univariate analysis of the association between clinical variables and survival outcomes for all patients.
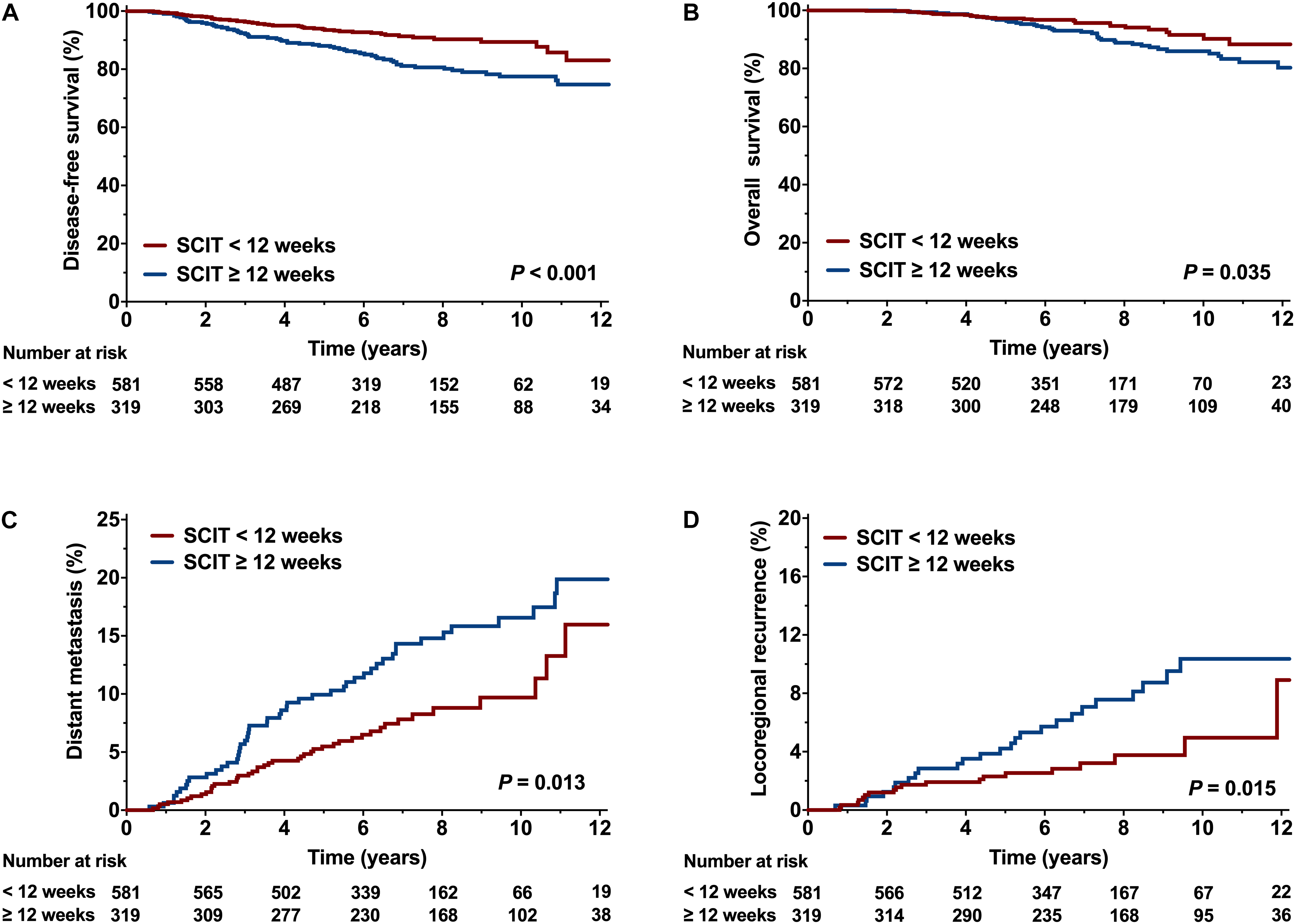
Figure 5. Comparison of survival curve between the interval time from surgery to chemotherapy (SCIT) < 12 weeks and SCIT ≥ 12 weeks. (A) Disease-free survival (DFS), (B) Overall survival (OS), (C) Distant metastasis (DM), and (D) Locoregional recurrence (LRR).
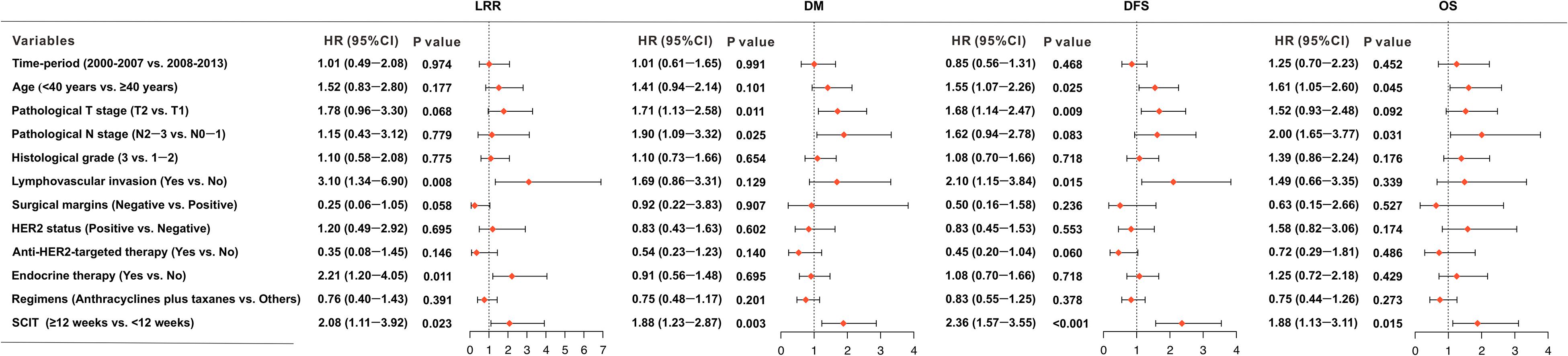
Figure 6. Forest plots indicating the independent prognostic effects of the interval time from surgery to chemotherapy (SCIT) < 12 weeks and SCIT ≥ 12 weeks, or clinical variables on locoregional recurrence (LRR), distant metastasis (DM), disease-free survival (DFS), and overall survival (OS).
Discussion
The optimal timing and sequence of chemotherapy and radiotherapy after BCS for early stage breast cancer has not been well defined, especially in the modern treatment era. This retrospective study demonstrated that patients receiving chemotherapy first had better DFS than those receiving radiotherapy first. Chemotherapy delayed beyond 12 weeks after BCS had a significant adverse effect on clinical outcomes.
Previous reports found conflicting results: some studies found that delaying the initiation of radiotherapy after BCS led to an increased risk of local failure (12, 20, 21), while others showed that radiotherapy started after the completion of chemotherapy but within 7 months after surgery did not affect tumor control or survival (14, 15, 22). Most patients in these studies were treated with outdated chemotherapy regimens (cyclophosphamide, methotrexate, fluorouracil; CMF); and endocrine therapy was not widely used. Recent studies using anthracycline-based regimens did not find an increased risk of local recurrence when radiotherapy was postponed after chemotherapy (11, 16, 23). Bellon et al. reported the findings of a prospective randomized trial on sequencing adjuvant treatment in node-positive patients after BCS, all of whom received 12 weeks of adjuvant anthracycline-based chemotherapy either before or after radiotherapy. There were no significant differences between the CT-first and RT-first arms in terms of time to any adverse event, distant metastasis, or death (11). The National Comprehensive Cancer Network (NCCN) breast cancer guidelines recommend that women receive adjuvant chemotherapy before radiotherapy after BCS based on the study by Bellon et al., but it does not have enough statistical power to rule out clinically important survival benefit for either sequence (11, 24). The addition of paclitaxel to anthracycline-based regimens was associated with better local control for node-positive disease (16). One study showed no effect on clinical outcomes of delaying radiotherapy for more than 32 weeks after BCS and completing four adjuvant cycles of doxorubicin/cyclophosphamide and four cycles of taxane (17). A study from the MD Anderson Cancer Center also showed no significant difference in recurrence-free survival between patients with node-negative disease who started radiotherapy < 25 weeks and ≥ 25 weeks after BCS when chemotherapy was delivered (25). In our study, the patients were young, 40.8% of them had node-positive disease, 11% had stage III disease, 36.9% had grade 3 tumors, and almost half received anthracycline plus taxane regimens. We also found that delaying the start of radiotherapy up to 32 weeks did not affect local tumor control.
Additionally, clarifying the pattern of recurrence is helpful for determining an adjuvant therapy schedule. Our study found that recurrence at a distant site was the main failure pattern for patients with early stage breast cancer, which is consistent with the literatures (26, 27), indicating the value of systemic therapy. Specifically, we found that delaying the initiation of chemotherapy beyond 12 weeks after BCS has an independently significant adverse effect on clinical endpoints after balancing these potential confounders by multivariable analysis. Therefore, early initiation of anthracycline and/or taxane-based chemotherapy regimens appears to be very important.
Consistent with our study, other research also showed that delaying the initiation of adjuvant chemotherapy had detrimental effects on survival outcomes. A systematic review indicated that overall survival (OS) decreased by 15% for every 4-week delay in initiation of chemotherapy with CMF or anthracycline-based regimens (28). Two large-cohort retrospective studies showed that initiation of adjuvant chemotherapy beyond 12 or 13 weeks from surgery was associated with inferior survival (29, 30). A pooled analysis of three clinical trials also demonstrated that SCIT longer than six weeks had a negative effect on OS and DFS in hormone receptor-negative patients, while delaying radiotherapy by more than six months after surgery did not affect outcomes in patients receiving long-course chemotherapy (31). All these studies included patients treated with both BCS and mastectomy, and the analysis did not differentiate between the two.
Almost all patients treated with BCS are recommended to receive radiotherapy, whereas in patients treated with mastectomy, radiotherapy is reserved for those at high risk. There is little dispute on the sequence of chemotherapy and radiotherapy in patients treated with mastectomy: chemotherapy followed by radiotherapy is commonly used in practice. Our study focused on patients treated with BCS, and emphasized the importance of early delivery of chemotherapy even in early-stage breast cancer. The important strengths of our study are: 1) the exclusion of patients who did not have an indication for chemotherapy based on the St. Gallen International Expert Consensus Conference of 2017 (19); and 2) most patients received anthracycline and/or taxane-based chemotherapy regimens; 3) the use of Maxstat to calculate the optimal cut-off value of SCIT. With hypofractionated radiotherapy becoming standard in breast cancer (18, 32, 33), the sequence of chemotherapy and radiotherapy will not be influenced to a great degree by the findings of our study, because patients could easily complete a 3-week radiotherapy course without delaying initiation of chemotherapy beyond 12 weeks after BCS.
Limitations of this study should be acknowledged. First, given the retrospective nature of the present study, selection biases cannot be avoided. CT-first group had more patients with high-risk features, and most patients in the CT-first group were treated during the recent time-period. However, after adjusting for the potential risk factors, including treatment period, we found SCIT of 12 weeks was an independent prognostic factor. Second, anti-HER2 targeted therapy has significantly improved DFS of patients with HER2-positive breast cancer in addition to chemotherapy (34). In our study, only 52.1% of patients with HER2-positive disease received anti-HER2 targeted therapy, which may exaggerate the effect of delay of chemotherapy due to the inadequate use of systemic treatment. Third, the 13-year span of patient inclusion was very long; therefore, changes in the diagnosis and treatment of breast cancer might have affected patients’ prognoses. Fortunately, we found that the treatment period did not significantly influence the prognoses of patients in this cohort. Last, as the majority of patients had stage I/II or ER/PR-positive diseases, who had a continuously risk of relapse beyond 5 years, thus longer-term follow-up is needed.
In summary, delaying the initiation of chemotherapy beyond 12 weeks after surgery is associated with inferior survival and tumor control in patients after BCS. However, delaying the initiation of radiotherapy up to 32 weeks, for administration of long-course chemotherapy, does not compromise patient outcomes. Longer-term follow up is warranted to validate our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
S-LW contributed and designed the research. S-LW and S-YC collected and analyzed data. S-YC, S-LW, YuT, and Y-XL wrote the article. All authors provided study materials or patients and approved the article.
Funding
This work was supported by the National Key Projects of Research and Development of China (2016YFC0904600), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2016-12M-1-001), the National Natural Science Foundation of China (81670185), and Beijing Hope Run Special Fund of Cancer Foundation of China (LC2016B08).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.571390/full#supplementary-material
Footnotes
References
1. van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. (2016) 17:1158–70. doi: 10.1016/S1470-2045(16)30067-5
2. Early Breast Cancer Trialists’ Cllaborative Group Darby, S McGale, P Correa, C Taylor, C Arriagada, R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
3. Speers C, Pierce LJ. Postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer: a review. JAMA Oncol. (2016) 2:1075–82. doi: 10.1001/jamaoncol.2015.5805
4. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
5. Early Breast Cancer Trialists’ Collaborative Group Peto, R Davies, C Godwin, J Gray, R Pan, HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. (2012) 379:432–44. doi: 10.1016/S0140-6736(11)61625-5
6. Henry NL, Somerfield MR, Abramson VG, Allison KH, Anders CK, Chingos DT, et al. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: American society of clinical oncology endorsement of cancer care ontario guideline recommendations. J Clin Oncol. (2016) 34:2303–11. doi: 10.1200/JCO.2015.65.8609
7. Pinnaro P, Rambone R, Giordano C, Giannarelli D, Strigari L, Arcangeli G. Long-term results of a randomized trial on the sequencing of radiotherapy and chemotherapy in breast cancer. Am J Clin Oncol. (2011) 34:238–44. doi: 10.1097/COC.0b013e3181dea9b8
8. Rouesse J, de la Lande B, Bertheault-Cvitkovic F, Serin D, Graic Y, Combe M, et al. A phase III randomized trial comparing adjuvant concomitant chemoradiotherapy versus standard adjuvant chemotherapy followed by radiotherapy in operable node-positive breast cancer: final results. Int J Radiat Oncol Biol Phys. (2006) 64:1072–80. doi: 10.1016/j.ijrobp.2005.10.011
9. Toledano A, Azria D, Garaud P, Fourquet A, Serin D, Bosset JF, et al. Phase III trial of concurrent or sequential adjuvant chemoradiotherapy after conservative surgery for early-stage breast cancer: final results of the ARCOSEIN trial. J Clin Oncol. (2007) 25:405–10. doi: 10.1200/JCO.2006.07.8576
10. Pinnaro P, Giordano C, Farneti A, Strigari L, Landoni V, Marucci L, et al. Impact of sequencing radiation therapy and chemotherapy on long-term local toxicity for early breast cancer: results of a randomized study at 15-year follow-up. Int J Radiat Oncol Biol Phys. (2016) 95:1201–9. doi: 10.1016/j.ijrobp.2016.03.016
11. Bellon JR, Come SE, Gelman RS, Henderson IC, Shulman LN, Silver BJ, et al. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol. (2005) 23:1934–40. doi: 10.1200/JCO.2005.04.032
12. Recht A, Come SE, Gelman RS, Goldstein M, Tishler S, Gore SM, et al. Integration of conservative surgery radiotherapy, and chemotherapy for the treatment of early-stage, node-positive breast cancer: sequencing timing, and outcome. J Clin Oncol. (1991) 9:1662–7. doi: 10.1200/JCO.1991.9.9.1662
13. Livi L, Borghesi S, Saieva C, Meattini I, Rampini A, Petrucci A, et al. Radiotherapy timing in 4,820 patients with breast cancer: university of florence experience. Int J Radiat Oncol Biol Phys. (2009) 73:365–9. doi: 10.1016/j.ijrobp.2008.04.066
14. Karlsson P, Cole BF, Price KN, Gelber RD, Coates AS, Goldhirsch A, et al. Timing of radiation therapy and chemotherapy after breast-conserving surgery for node-positive breast cancer: long-term results from international breast cancer study group trials VI and VII. Int J Radiat Oncol Biol Phys. (2016) 96:273–9. doi: 10.1016/j.ijrobp.2016.06.2448
15. Yock TI, Taghian AG, Kachnic LA, Coen JJ, Assaad SI, Powell SN. The effect of delaying radiation therapy for systemic chemotherapy on local-regional control in breast cancer. Breast Cancer Res Treat. (2004) 84:161–71. doi: 10.1023/B:BREA.0000018414.36274.72
16. Sartor CI, Peterson BL, Woolf S, Fitzgerald TJ, Laurie F, Turrisi AJ, et al. Effect of addition of adjuvant paclitaxel on radiotherapy delivery and locoregional control of node-positive breast cancer: cancer and leukemia group B 9344. J Clin Oncol. (2005) 23:30–40. doi: 10.1200/JCO.2005.12.044
17. Koh HK, Shin KH, Kim K, Lee ES, Park IH, Lee KS, et al. Effect of time interval between breast-conserving surgery and radiation therapy on outcomes of node-positive breast cancer patients treated with adjuvant doxorubicin/cyclophosphamide followed by taxane. Cancer Res Treat. (2016) 48:483–90. doi: 10.4143/crt.2015.111
18. Wang SL, Fang H, Song YW, Wang WH, Hu C, Liu YP, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. (2019) 20:352–60. doi: 10.1016/S1470-2045(18)30813-1
19. Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. (2017) 28:1700–12. doi: 10.1093/annonc/mdx308
20. Buchholz TA, Austin-Seymour MM, Moe RE, Ellis GK, Livingston RB, Pelton JG, et al. Effect of delay in radiation in the combined modality treatment of breast cancer. Int J Radiat Oncol Biol Phys. (1993) 26:23–35. doi: 10.1016/0360-3016(93)90169-v
21. Hartsell WF, Recine DC, Griem KL, Murthy AK. Delaying the initiation of intact breast irradiation for patients with lymph node positive breast cancer increases the risk of local recurrence. Cancer. (1995) 76:2497–503. doi: 10.1002/1097-0142(19951215)76:123.0.co;2-6
22. Wallgren A, Bernier J, Gelber RD, Goldhirsch A, Roncadin M, Joseph D, et al. Timing of radiotherapy and chemotherapy following breast-conserving surgery for patients with node-positive breast cancer. International breast cancer study group. Int J Radiat Oncol Biol Phys. (1996) 35:649–59. doi: 10.1016/0360-3016(96)00186-1
23. Cefaro GA, Genovesi D, Marchese R, Di Tommaso M, Di Febo F, Ballone E, et al. The effect of delaying adjuvant radiation treatment after conservative surgery for early breast cancer. Breast J. (2007) 13:575–80. doi: 10.1111/j.1524-4741.2007.00511.x
24. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:310–20. doi: 10.6004/jnccn.2018.0012
25. Buchholz TA, Hunt KK, Amosson CM, Tucker SL, Strom EA, McNeese MD, et al. Sequencing of chemotherapy and radiation in lymph node-negative breast cancer. Cancer J Sci Am. (1999) 5:159–64.
26. Lyngholm CD, Laurberg T, Alsner J, Damsgaard TE, Overgaard J, Christiansen PM. Long-term results of the Danish Breast Cancer Group (DBCG) 89 TM cohort. Acta Oncol. (2016) 55:983–92. doi: 10.3109/0284186X.2016.1156741
27. Geurts YM, Witteveen A, Bretveld R, Poortmans PM, Sonke GS, Strobbe LJA, et al. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat. (2017) 165:709–20. doi: 10.1007/s10549-017-4340-3
28. Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. (2013) 13:240. doi: 10.1186/1471-2407-13-240
29. Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. (2006) 24:4888–94. doi: 10.1200/JCO.2005.01.6089
30. Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. (2016) 2:322–9. doi: 10.1001/jamaoncol.2015.3856
31. Abdel-Rahman, O. Impact of timeliness of adjuvant chemotherapy and radiotherapy on the outcomes of breast cancer; a pooled analysis of three clinical trials. Breast. (2018) 38:175–80. doi: 10.1016/j.breast.2018.01.010
32. Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. (2018) 8:145–52. doi: 10.1016/j.prro.2018.01.012
33. Wang EH, Mougalian SS, Soulos PR, Rutter CE, Evans SB, Haffty BG, et al. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: a national cancer data base analysis. Int J Radiat Oncol Biol Phys. (2014) 90:993–1000. doi: 10.1016/j.ijrobp.2014.06.038
Keywords: breast neoplasm, breast-conserving surgery, chemotherapy, radiotherapy, timing
Citation: Chen S-Y, Tang Y, Wang S-L, Song Y-W, Fang H, Wang J-Y, Jing H, Zhang J-H, Sun G-Y, Zhao X-R, Jin J, Liu Y-P, Chen B, Qi S-N, Li N, Tang Y, Lu N-N, Ren H, Yu Z-H and Li Y-X (2020) Timing of Chemotherapy and Radiotherapy Following Breast-Conserving Surgery for Early-Stage Breast Cancer: A Retrospective Analysis. Front. Oncol. 10:571390. doi: 10.3389/fonc.2020.571390
Received: 10 June 2020; Accepted: 03 September 2020;
Published: 23 September 2020.
Edited by:
Michael Gnant, Medical University of Vienna, AustriaReviewed by:
Shereen Elazzazy, Hamad Medical Corporation, QatarMiranda Clements, National Institutes of Health (NIH), United States
Copyright © 2020 Chen, Tang, Wang, Song, Fang, Wang, Jing, Zhang, Sun, Zhao, Jin, Liu, Chen, Qi, Li, Tang, Lu, Ren, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Lian Wang, d3NsMjAwNDAxMThAeWFob28uY29t; Ye-Xiong Li, eWV4aW9uZzEyQDE2My5jb20=
†These authors have contributed equally to this work
 Si-Ye Chen†
Si-Ye Chen† Yu Tang
Yu Tang Shu-Lian Wang
Shu-Lian Wang Guang-Yi Sun
Guang-Yi Sun Ye-Xiong Li
Ye-Xiong Li