- 1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Fudan University, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Institute of Thoracic Oncology, Fudan University, Shanghai, China
Background: Pulmonary large cell neuroendocrine cancer (LCNEC) is commonly classified as non-small cell lung cancer (NSCLC). Even for stage I disease, after surgery the survival is always poor, but clinical research on LCNEC is scant and always with unsatisfying sample sizes. Thus, we conduct the first study using the Surveillance, Epidemiology, and End Results (SEER) database to compare survival after surgery between stage I LCNEC and other types of NSCLC.
Methods: From 2004 to 2016, 473 patients with stage IA LCNEC, 17,669 patients with lung adenocarcinoma (LADC) and 8,475 patients with lung squamous cell cancer (LSCC), all treated with surgery were identified. In addition, 1:1 PSM was used, and overall (OS) and cancer-specific survival (CSS) between groups were compared.
Results: The 5-year OS rates and CSS rates for LCNEC were 52.5% and 81.5%, respectively. Overall, both OS and CSS were significantly superior for stage IA LADC than LCNEC (for OS: HR 0.636, 95% CI 0.568-0.712; for CSS: HR 0.688, 95% CI 0.561–0.842, LCNEC as reference), while comparable for LSCC with LCNEC (for OS: HR 0.974, 95% CI 0.869–1.091; for CSS: HR 0.907, 95% CI 0.738–1.115). PSM generated 471 pairs when LCNEC was compared with LADC and both OS and CSS were significantly better in LADC than LCNEC (for OS: HR 0.580, 95% CI 0.491–0.686; for CSS: HR 0.602, 95% CI 0.446–0.814). Of note, for the subgroup of patients ≤ 65 years old, HRs for both OS and CSS were lower (for OS: HR 0.470; for CSS: HR 0.482). As for comparison between LCNEC and LSCC, PSM generated 470 pairs. Differently, only CSS was significantly superior in LSCC than LCNEC (HR 0.563, 95% CI 0.392–0.807), while OS was not. Further grouping by age showed only CSS between two groups for patients with age ≤ 65 years old was significantly different (P = 0.006).
Conclusions: We report the first survival comparison after surgery between stage IA LCNEC and other types of NSCLC by SEER database and PSM. Our results demonstrated after surgery, stage IA LCNEC was worse in survival, especially compared to LADC. Extra clinical care should be paid, especially for younger patients. More studies investigating adjuvant therapy are warranted.
Introduction
Large cell neuroendocrine cancer (LCNEC) of bronchus and lungs is a rare disease and constitutes approximately 3% in lung cancers (1). Although it is one of the four types of pulmonary neuroendocrine tumor (NET), different from other three NET [the typical, atypical carcinoid and small cell lung carcinoma (SCLC)], according to NCCN Guidelines [Version 2.2020, non-small cell lung cancer (NSCLC)], it is most commonly classified as a form of NSCLC (2, 3). Still, we cannot overlook that for LCNEC, there is indeed difference in its biological, clinical, and prognostic characteristics compared with other types of NSCLC [Lung squamous cell cancer (LSCC) and lung adenocarcinoma (LADC)]. As for the treatment of early-stage LCNEC, consistent with early-stage LSCC and LADC, surgery is recommended by guidelines. Even for stage I patients, however, after radical surgery the survival is always poor with a 5-year survival rate of 43%–67% (4–7). The confirmation of this is instrumental clinically. Yet, due to its low incidence, clinical researches on LCNEC are scant and always restricted to retrospective studies with unsatisfying sample sizes (7–10).
Supported by the National Cancer Institute (NCI), the Surveillance, Epidemiology, and End Results (SEER) Program covers clinical data from 18 different population-based cancer registries and covers 30% of the United States population (11). Thus, it provides valuable resources for rare cancer studies such as LCNEC. However, studies using the SEER database comparing survival in stage IA pulmonary LCNEC with other types of NSCLC have not been found. Although one retrospective study (12) using the National Cancer Database and a single-center retrospective study (13) of 125 LCNEC patients have been performed, inconsistent results lied on whether survival in early stage pulmonary LCNEC after surgery was comparable with other types of NSCLC. Larger-scale clinical data and methods to control bias brought by retrospective studies, such as propensity score matching (PSM) which can create matched set consisting of participants in the treatment group and control group with similar propensity scores, thus to approximate a random experiment by balancing covariates, are needed in further studies.
Based on above-mentioned clinical needs, we used the SEER database and PSM to conduct such survival comparison after surgery in stage IA (AJCC TNM-6:T1,N0,M0; AJCC TNM-7: T1a-T1b,N0,M0) NSCLC, and sought to investigate whether the survival difference after surgery exists for very early stage LCNEC.
Patients and Methods
Data Source and Ethics Statement
We used the specialized database “Incidence–SEER 9 Regs Custom Data (with additional treatment fields) of the SEER database Nov 2018 Sub (1975-2016) varying)” to extract data using the SEER*Stat software, version 8.3.6 (Released August 8, 2019). In this study, informed consent was not required, since identifying information of individual patients has been excluded. These data are available publicly and access to the SEER data was obtained by signing the SEER Research Data Agreement. Personal identifying information is not stored in the SEER database.
Patient Selection
Patients from 2004 to 2016 were enrolled in the study. The inclusion criteria were as follows: (1) primary tumor in “Lung and Bronchus” [based on site recode ICD–O–3 (International Classification of Diseases for Oncology, Third Edition)/WHO 2008]; (2) histological confirmation of LCNEC (ICD–O–3 8012/3), LADC(ICD–O–3 8040/3) and LSCC (ICD–O–3 8070/3); (3) stage IA (AJCC TNM-6:T1,N0,M0; AJCC TNM-7: T1a-T1b,N0,M0); (4) received cancer-directed surgery; (5) complete demographic data and follow-up data. It is worth noticing that according to AJCC TNM-6, AJCC TNM-7, and even for AJCC TNM-8, stage IA NSCLC was all defined as tumor with a maximum diameter of 3cm, and with no involvement in visceral pleura, the main branches of the bronchus, lymph nodes and distant sites. Therefore, although recorded according to different versions of the staging, the patients we enrolled were with the same disease level and still represent the same groups of patients in current staging system (AJCC TNM-8).
Statistical Analysis
Categorical variables were compared using Pearson’s chi-square tests. When accessing overall survival (OS), patients recorded as “Alive” were censored. When accessing cancer-specific survival (CSS), patients recorded as “Dead (missing/unknown cause of death)” and “Alive or dead of other cause” were censored. Due to potential differences among patients with stage IA LCNEC, LADC, and LSCC, PSM was used between LCNEC and LADC groups, and between LCNEC and LSCC groups. In short, a propensity score represents the probability that a unit with certain characteristics will be assigned to the treatment group and helps to balance multiple characteristics in retrospective studies to approximate a random experiment. In our study, variables included in the model were age at diagnosis, gender, race, location of primary sites, chemotherapy, and radiotherapy status, which were likely to have an impact on survival for patients with lung cancer according to previous researches (14–18). For PSM between LCNEC and LADC, and between LCNEC and LSCC, 1:1 PSM without replacement was implemented, and nearest neighbor matching method with caliber of 0.001 was used. Overall survival (OS) and CSS between groups were compared by the Kaplan–Meier method and log-rank test. Cox proportional hazard models were also used to estimate hazard ratio (HR) and 95% confidential interval (CI) between groups. Subgroup analyses were performed, and forest plots were created to better present each prognostic factor’s effect on OS and CSS. The patients were stratified to subgroups of different age (≤65 or >65 years), gender (male or female), race (black or white), pathological grade (well-moderate or poor-undifferentiated), location (upper lobe or lower lobe), radiotherapy (yes or no), chemotherapy (yes or no/unknown), and marital status (married or unmarried). Univariate Cox proportional hazard model was used to estimate the HRs. Multivariable Cox proportional hazards models were also performed. Statistical analyses and PSM were performed with SPSS (IBM SPSS Statistics 25.0, Chicago, IL, US). Statistical significance was considered if two-sided p-value < 0.05. Figures were generated by GraphPad Prism (GraphPad Software 6.01, Inc).
Results
Patient Characteristics
In summary, from 2004 to 2016, 343,816 patients were diagnosed with NSCLC, and 41699 of these patients were staged as IA (see Figure 1 for flow diagram of enrollment). Of those patients, 26,617 documented to have received cancer-directed surgery also with complete demographic data and follow-up data were enrolled. Among them, 473 (1.8%) patients were diagnosed as LCNEC, 8,475 (31.8%) were diagnosed as LSCC, and 17,669 (66.4%) were diagnosed as LADC. Patient baseline demographics and pathological characteristics of all enrolled patients were listed and were compared among the three histologic types in Table S1.
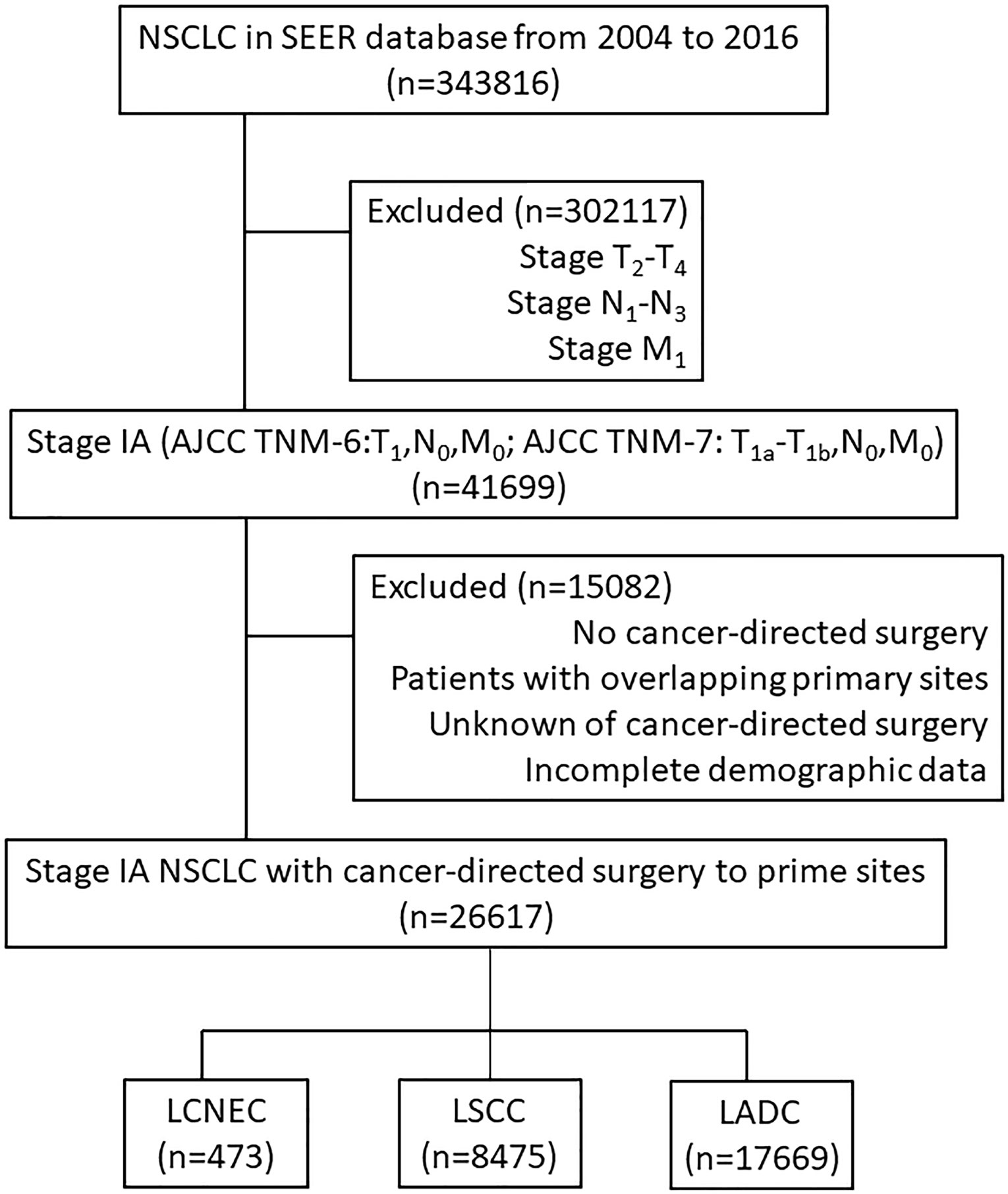
Figure 1 Flow diagram of enrollment. NSCLC, non-small cell lung cancer; the SEER database, Surveillance Epidemiology, and End Results database; AJCC, American Joint Committee on Cancer staging system; LCNEC, large cell neuroendocrine carcinoma; LSCC, lung squamous cell cancer; LADC, lung adenocarcinoma.
Survival Outcome Analysis Before PSM
We first compared survival among the three types of NSCLC before PSM. Both OS and CSS after surgery were significantly superior for patients with stage IA LADC (for OS: HR 0.636, 95% CI 0.568–0.712; for CSS: HR 0.688, 95% CI 0.561–0.842, LCNEC as reference, similarly hereinafter) than LCNEC, while comparable for LSCC (for OS: HR 0.974, 95% CI 0.869–1.091; for CSS: HR 0.907, 95% CI 0.738–1.115) with LCNEC (Figure S1). The 5-year OS rates for stage IA LCNEC, LADC, and LSCC were 52.5%, 66.6%, and 54.6%, respectively. The 5-year CSS rates for stage IA LCNEC, LADC, and LSCC were 81.5%, 86.9%, and 83.6%, respectively.
Survival Outcome Analysis Between LCNEC and LADC After PSM
Patient baseline demographics and pathological characteristics of stage IA LCNEC and stage IA LADC before and after PSM were listed in Table 1. Stage IA LCNEC patients were more likely to be diagnosed at younger age (P < 0.001), with a higher proportion of male patients (P < 0.001), and more patients would undergo chemotherapy (P < 0.001) and radiotherapy (P < 0.001), compared to stage IA LADC. LCNEC was well matched with PSM generating 471 pairs and after PSM, these characteristics were well balanced.
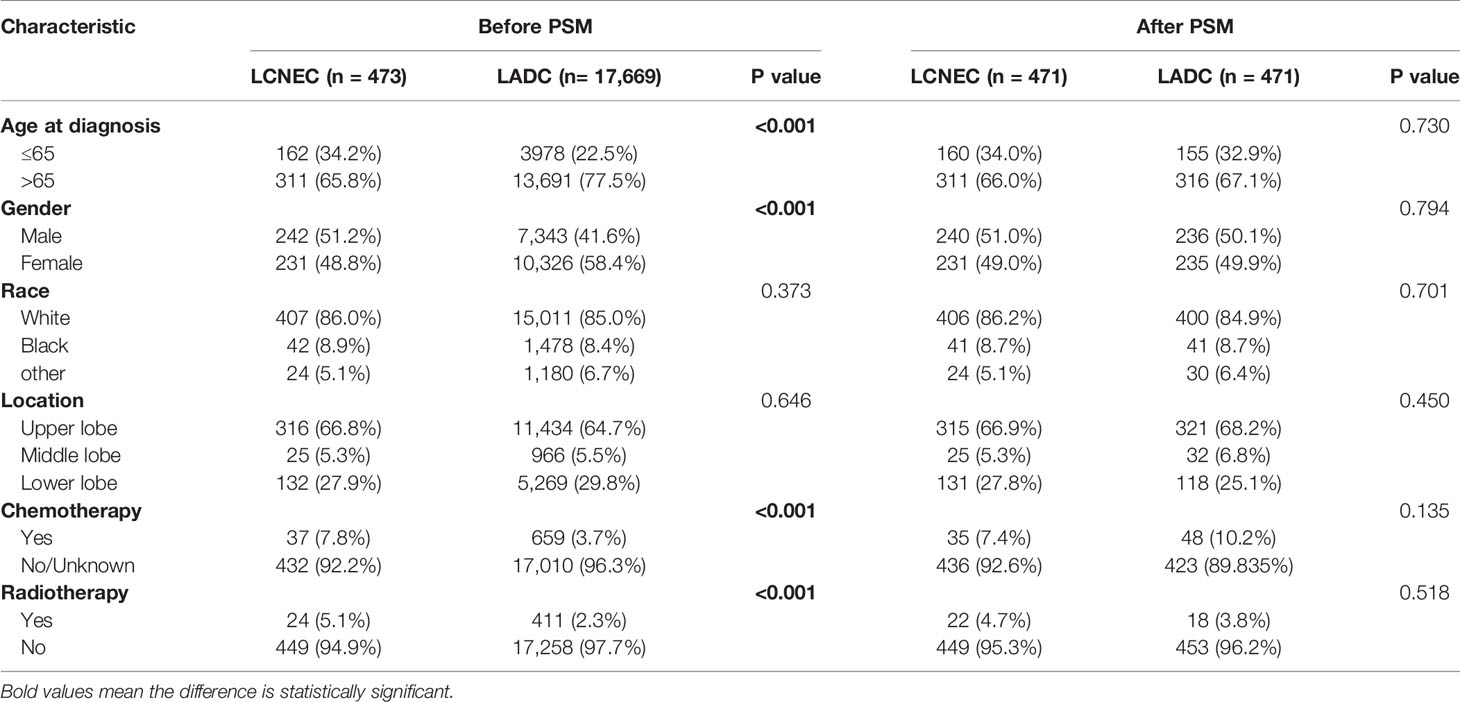
Table 1 Patient baseline demographics and pathological characteristics of LCNEC and LADC before and after PSM.
The median follow-up time for matched cases was 55 months in LCNEC group and 91 months in LADC group. The results showed that both OS (LADC vs. LCNEC: median OS, 112.0 months vs. 66.0 months; HR 0.580; 95% CI 0.491–0.686; P < 0.001) and CSS (HR 0.602; 95% CI 0.446–0.814; P = 0.001) were significantly better in stage IA LADC group than in LCNEC group (Figures 2A, B). For matched LCNEC and LADC group, the 3- and 5-year OS rates were 67.3% vs. 80% and 52.5% vs. 66.8%, respectively; and 3- and 5-year CSS rates were 86.4% vs. 92.7% and 81.5% vs. 87.6%, respectively.
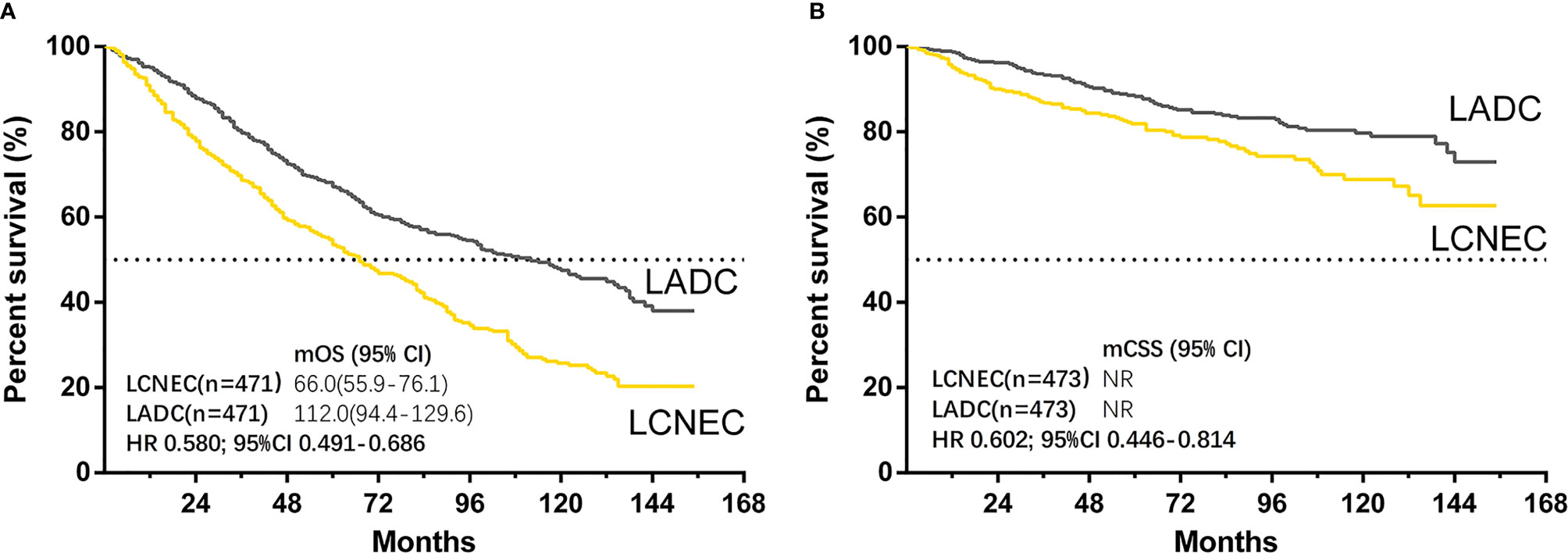
Figure 2 Kaplan–Meier curves for survival outcomes after PSM in LCNEC and LADC group: (A) overall survival (OS) and (B) cancer-specific survival (CSS) in matched patients between large cell neuroendocrine carcinoma (LCNEC) and lung adenocarcinoma (LADC) groups. CI, confidential interval; HR, hazard ratio; NR, not reached. LCNEC as reference.
Forest plots (Figures 3A, B) of HRs for OS and CSS were generated to illustrate subgroup analyses when stratifying patients by age, gender, race, location, chemotherapy, radiotherapy, and marital status. Consistent results showing stage IA LADC group was superior in OS than LCNEC were found in all subgroups (Figure 3A), and in CSS in most subgroups (Figure 3B). Of note, for patients in ≤65 subgroup, HRs for both OS and CSS were lower (for OS: HR 0.470, 95% CI 0.335–0.659, P < 0.001; for CSS: HR 0.482, 95% CI 0.281–0.812, P = 0.006).
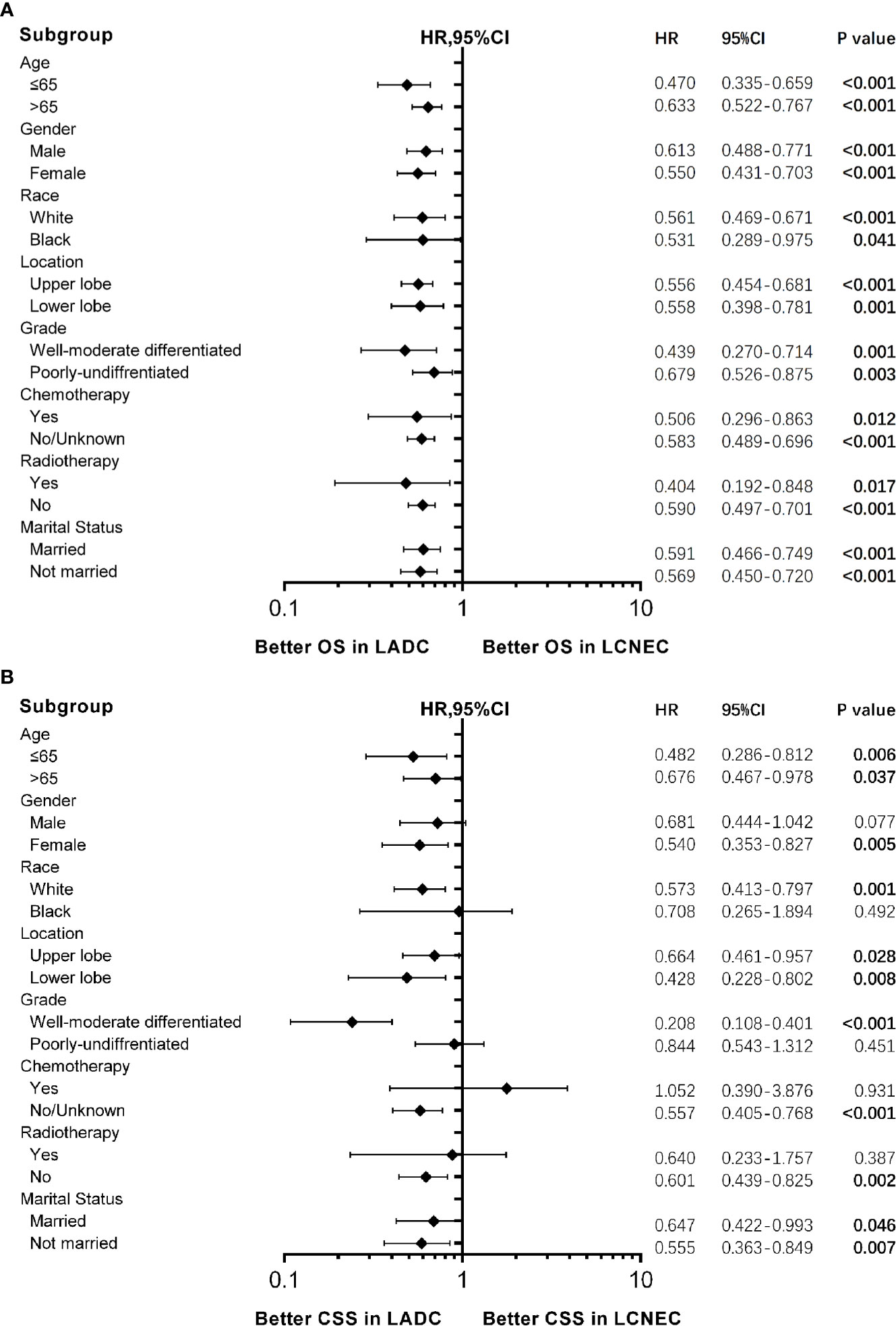
Figure 3 Forest plot of hazard ratios (HRs) for (A) overall survival (OS) and (B) cancer-specific survival (CSS) between stage IA large cell neuroendocrine carcinoma (LCNEC) and lung adenocarcinoma (LADC) in the subgroup analysis. The diamond on the X-axis indicates the HR and the 95% confident interval (CI) of each subgroup. LCNEC as reference.
To further investigate the factors influencing OS and CSS in these patients, a multivariate analysis was performed (Table 2). Consistent with prior results, histologic type was an independent factor for both OS and CSS (For OS, HR: 0.587, 95% CI: 0.452–0.762, P < 0.001; and for CSS, HR = 0.493, 95% CI: 0.314–0.776, P = 0.002). Intriguingly, we also noticed age was an independent factor for OS (P < 0.001) while not for CSS (P = 0.397). Thus, we also performed Kaplan-Meier analysis to show the influence of age on OS and CSS between matched groups (Figure S2), and the difference in OS and CSS was still significantly different between LCNEC and LADC in both ≤65 years old and >65-year-old subgroups.
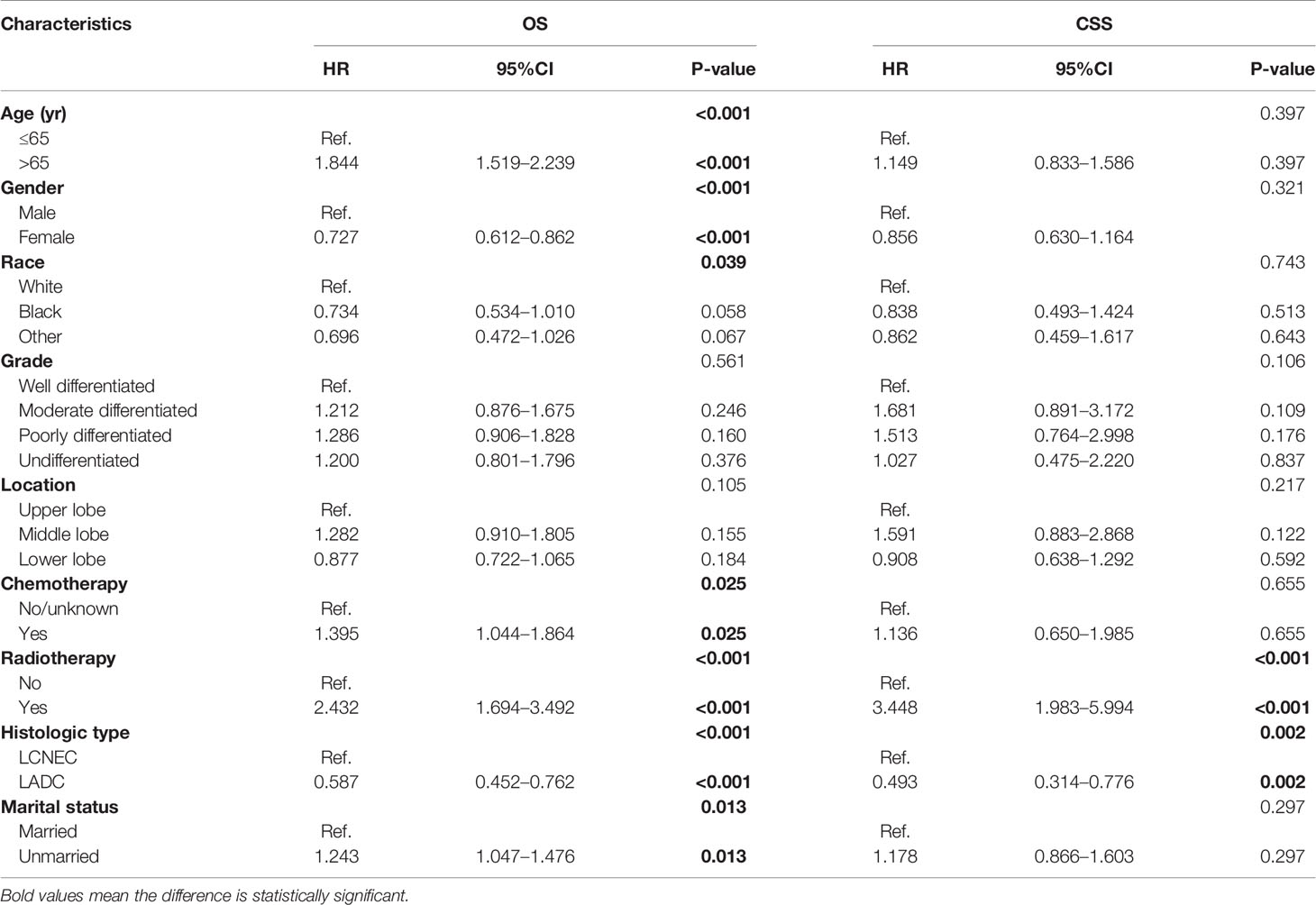
Table 2 Multivariate analysis of overall survival (OS) and cancer-specific survival (CSS) predictors using cox proportional hazard model.
Survival Outcome Analysis Between LCNEC and LSCC After PSM
Patient baseline demographics and pathological characteristics of stage IA LCNEC and stage IA LSCC before and after PSM were listed in Table 3. As was listed, stage IA LCNEC were more likely to be diagnosed at younger age (P < 0.001), with different races distribution (P = 0.027), and more patients would undergo chemotherapy (P < 0.001), compared to stage IA LSCC. LCNEC was well matched in PSM and PSM generated 470 pairs. After PSM, these different characteristics were well balanced.
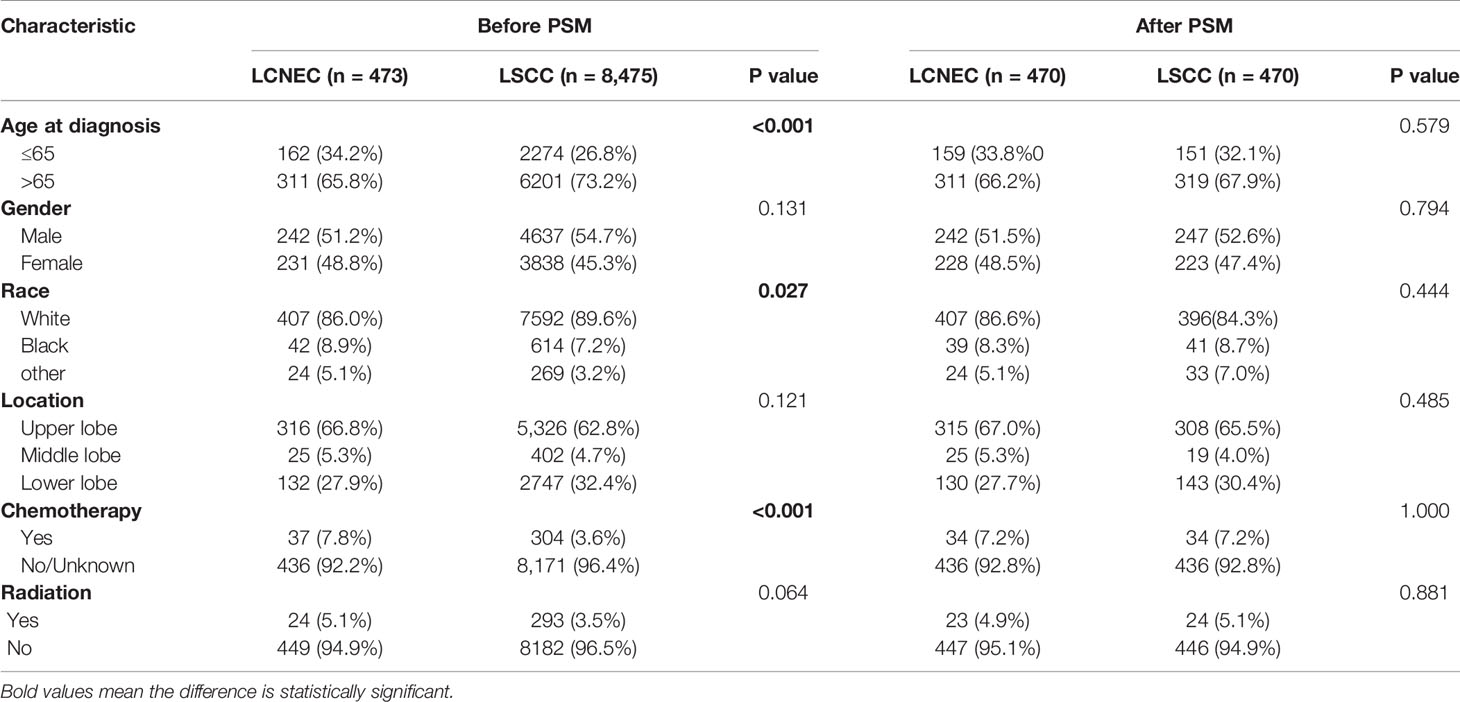
Table 3 Patient baseline demographics and pathological characteristics of LCNEC and LSCC before and after PSM.
The median follow-up time for matched cases was 55 months in LCNEC group and 45 months in LSCC group. The results showed that only CSS was significantly better in LSCC group than LCNEC group (HR, 0.563; 95% CI, 0.392–0.807; P = 0.002), while OS was not (HR, 0.893; 95% CI, 0.750–1.064; P = 0.204) (Figures 4A, B). For matched LCNEC and LSCC group, the 3- and 5-year OS rates were 67.1% vs. 74.2% and 52.4% vs. 58.8%; and 3- and 5-year CSS rates were 86.3% vs. 93.2% and 81.7% vs. 88.4%, respectively. We further grouped by age (Figure 5) and for patients with age ≤ 65, only CSS between two groups was significantly different (P = 0.006). In patients with age ≤ 65, there was a trend (P = 0.171) showing OS of LSCC was superior, and Kaplan-Meier analysis for OS also showed disjoint curves, however, the difference was not significant. In elder patients, there was also a trend (P = 0.054) that in patients with age > 65, CSS of LSCC was superior around before 120 months and the curve intersected. Still, these results reflected for stage IA LCNEC and LSCC patients after surgery to primary site, LSCC was superior in survival than LCNEC, while the difference was moderate.
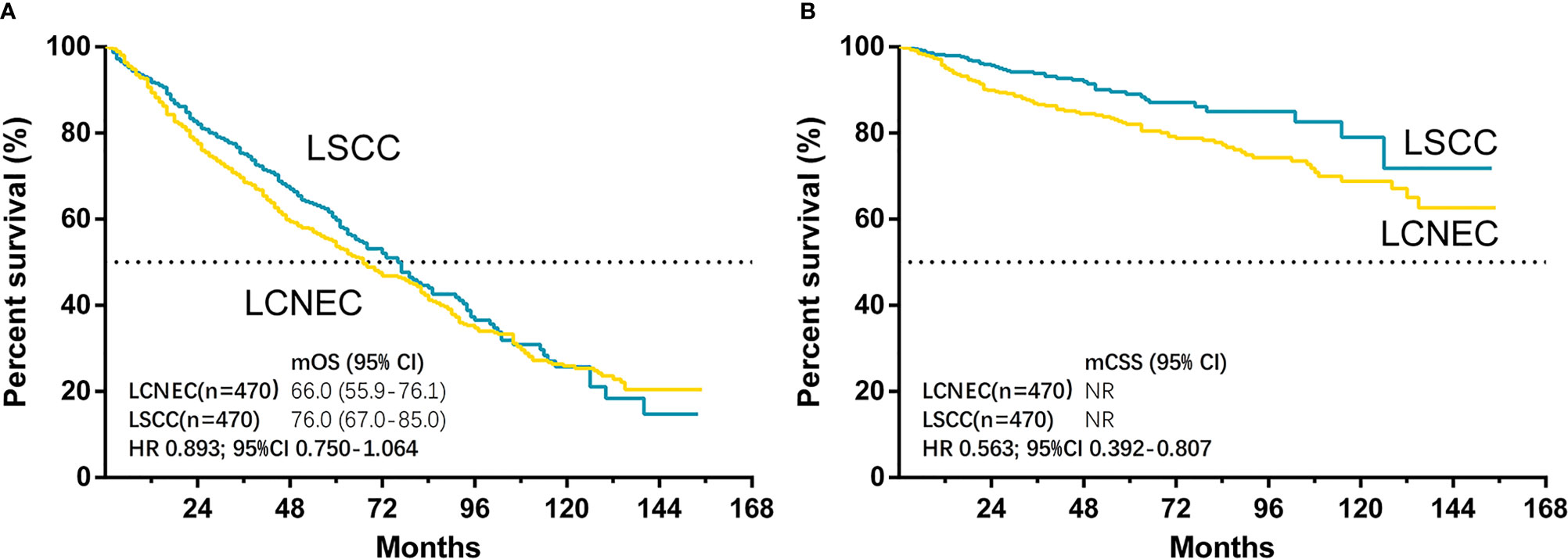
Figure 4 Kaplan–Meier curves for survival outcomes after PSM in LCNEC and LSCC group: (A) overall survival (OS) and (B) cancer-specific survival (CSS) in matched patients between large cell neuroendocrine carcinoma (LCNEC) and lung squamous cell cancer (LSCC) groups. CI, confidential interval; HR, hazard ratio; NR, not reached. LCNEC as reference.
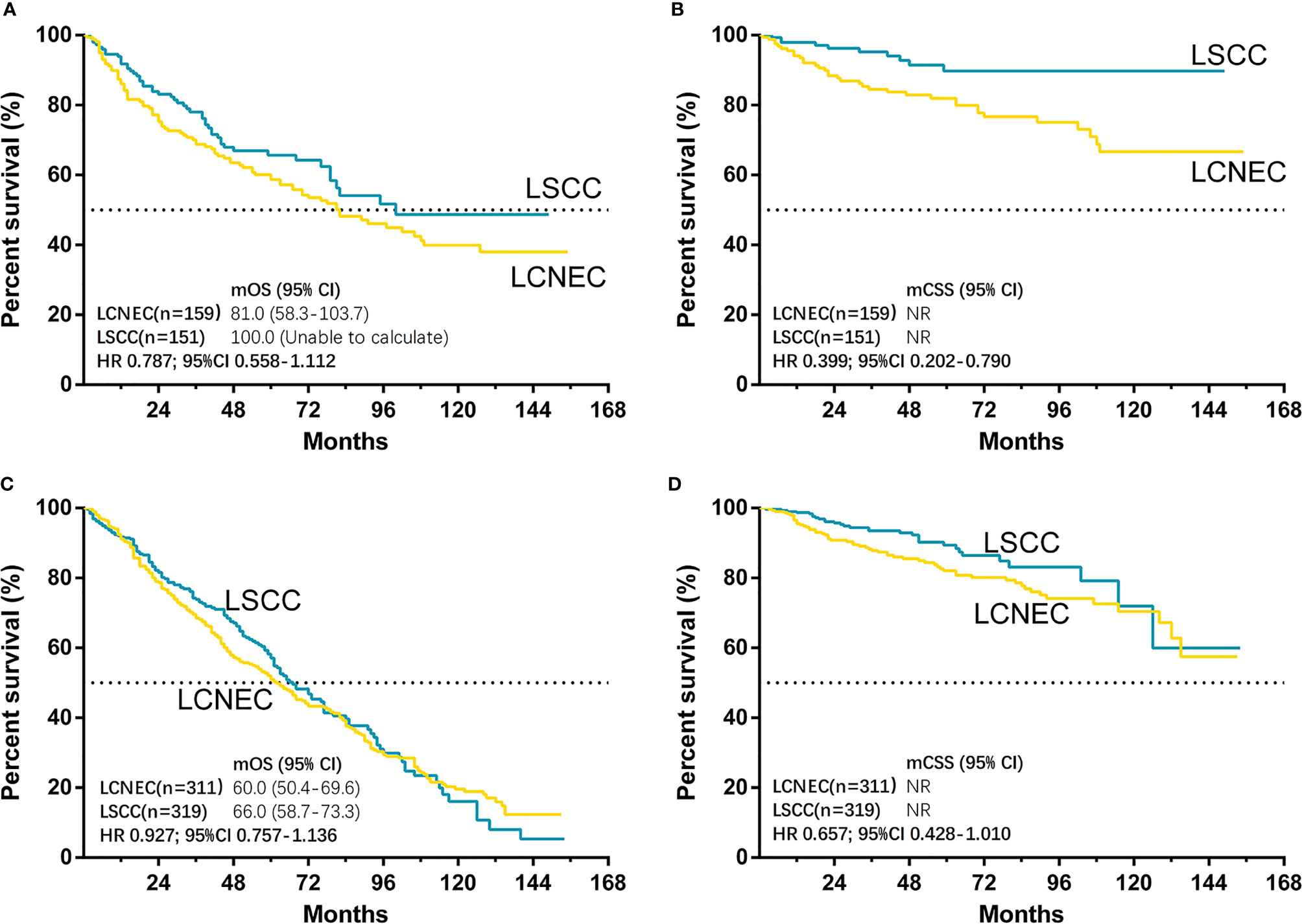
Figure 5 Kaplan–Meier curves for survival outcomes after PSM in LCNEC and LSCC ≤65 years old and >65 years old subgroups: (A) overall survival (OS) and (B) cancer-specific survival (CSS) in matched patients between large cell neuroendocrine carcinoma (LCNEC) and lung squamous cell cancer (LSCC) ≤65 years old subgroup; and (C) OS and (D) CSS in >65 years old subgroups. CI, confidential interval; HR, hazard ratio; NR, not reached. LCNEC as reference.
Forest plots of HRs for OS (Figure S3) and CSS (Figure 6) was generated to illustrate the exploratory subgroup analyses when stratifying patients by age, gender, race, location, chemotherapy, radiotherapy, and marital status. Results showing stage IA LSCC group was superior in CSS than LCNEC were found in most subgroups, but no significant difference was found in age > 65 years old, black, female, lower lobe, poorly-undifferentiated, radiotherapy, and chemotherapy subgroups. It is also worth noticing that in subgroup analysis for patients with age≤65, a lower HR for CSS was observed (HR 0.400, 95CI 0.202–0.290, P = 0.008).
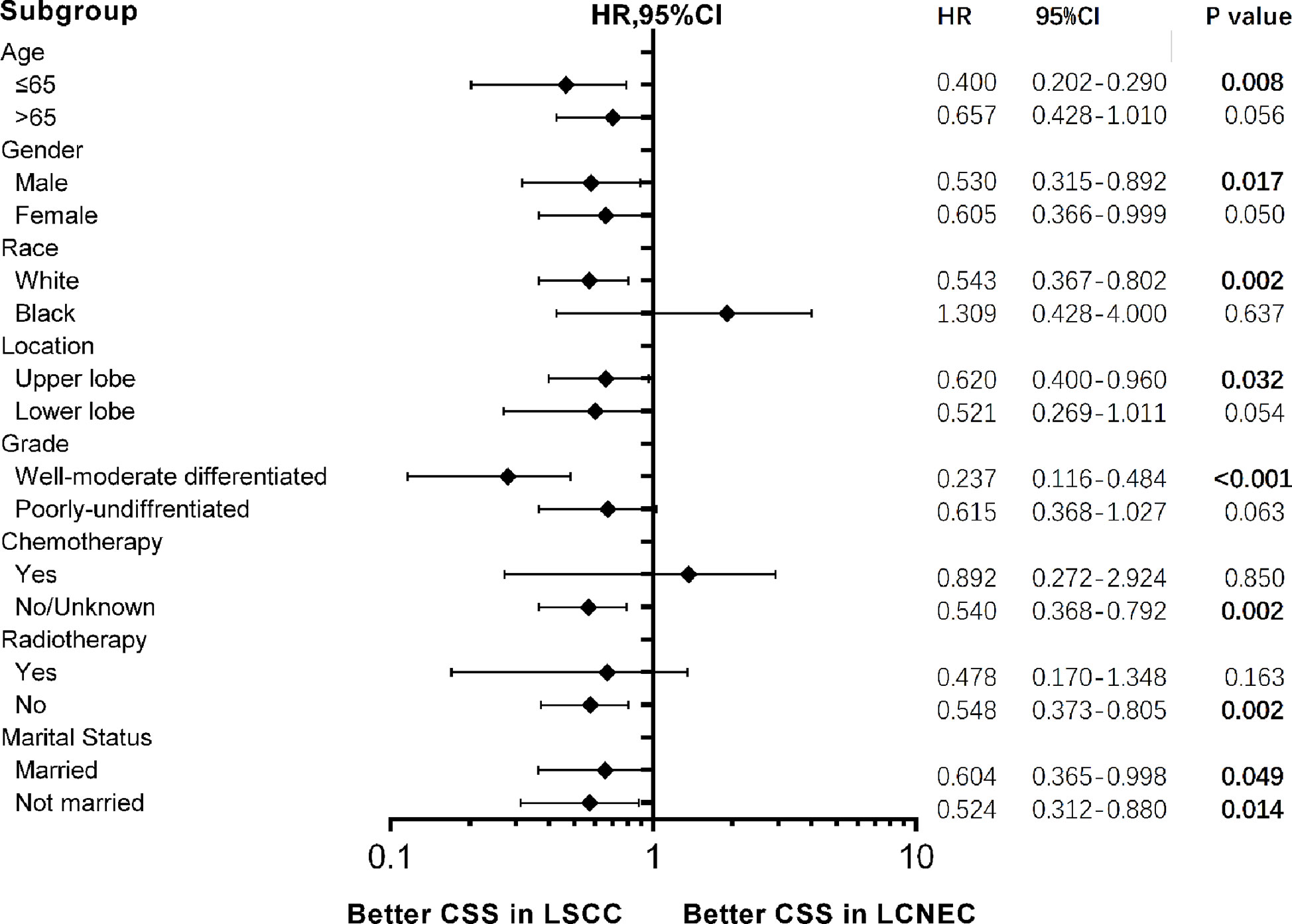
Figure 6 Forest plot of hazard ratios (HRs) for cancer-specific survival (CSS) between stage IA large cell neuroendocrine carcinoma (LCNEC) and lung squamous cell cancer (LSCC) in the subgroup analysis. The diamond on the X-axis indicates the HR and the 95% confident interval (CI) of each subgroup. LCNEC as reference.
Multivariate analysis was performed to further investigate the factors influencing OS and CSS in matched patients (Table 4). On multivariate analysis, consistent with prior results, histologic type was an independent factor for CSS (HR: 0.404, 95% CI: 0.248–0.659, P < 0.001), but not for OS (HR: 0.924, 95% CI: 0.724–1.181, P = 0.530). Also, similar to the comparison with LADC, age was an independent factor for OS (HR: 1.587, 95% CI: 1.301-1.935, P < 0.001) but not for CSS (HR: 1.079, 95% CI: 0.751–1.551, P = 0.679).
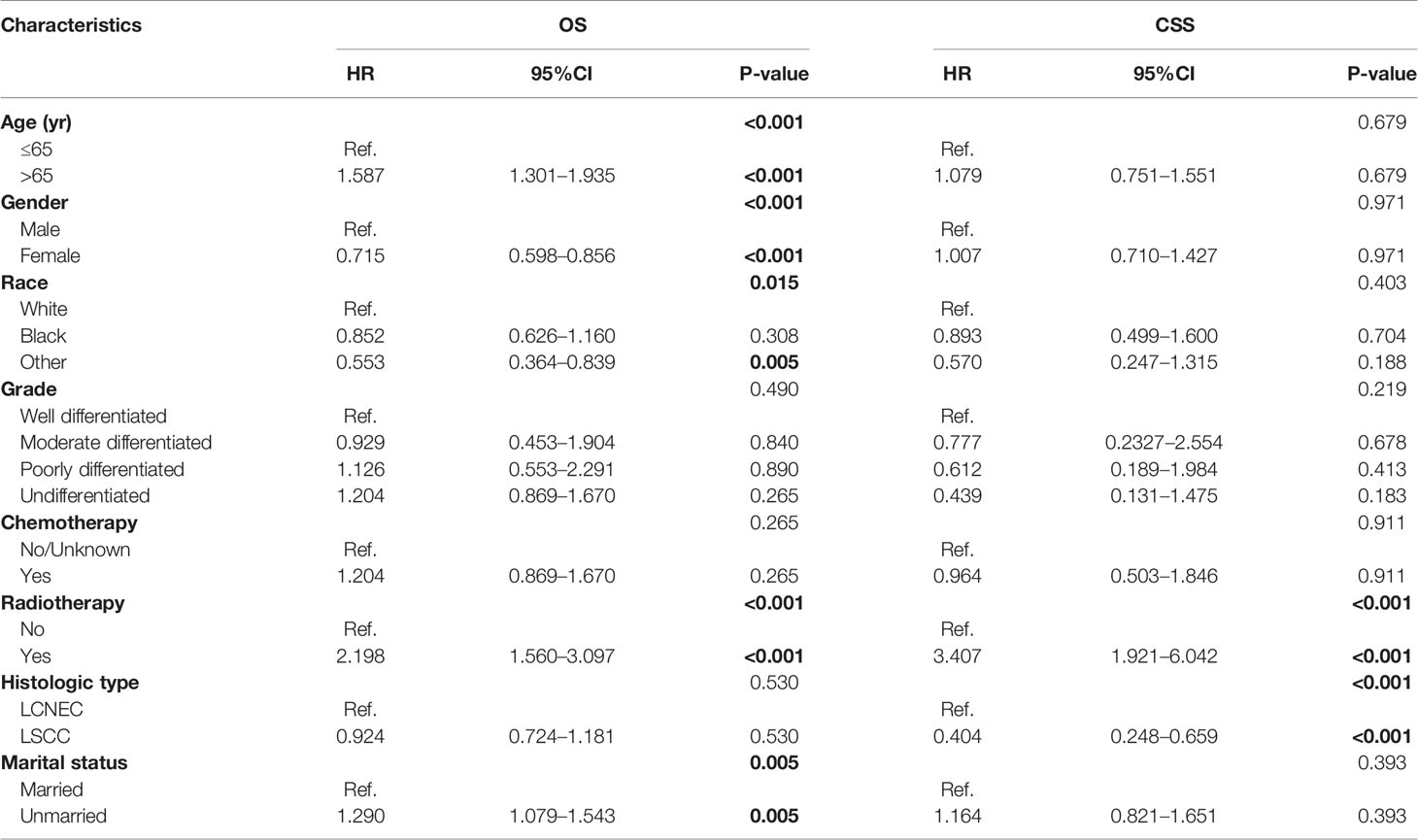
Table 4 Multivariate analysis of overall survival (OS) and cancer-specific survival (CSS) predictors using cox proportional hazard model.
Discussion
LCNEC of bronchus and lungs is a rare disease and is one of the four types of pulmonary NETs, according to NCCN Guidelines (Version 2.2020, NSCLC). Like early stage LSCC and LADC, surgery is recommended by guidelines. However, in our study for stage IA LCNEC, the survival after radical surgery is still poor with a 5-year OS rate of 52.5%, consistent with the 5-year survival rate of 43%–67% in previous studies (4–7). By comparison in our research, both OS and CSS after surgery were significantly superior for patients with stage IA LADC than LCNEC (for OS: HR 0.636; for CSS: HR 0.688), while comparable for LSCC with LCNEC (for OS: HR 0.974; for CSS: HR 0.907). The 5-year OS rate for stage IA LCNEC, LADC, and LSCC were 52.5%, 66.6%, and 54.6%, respectively. The survival data was basically consistent with previous study (12). Slightly different from our study, the results of previous study (12) indicated stage I LCNEC was worse in OS after surgery compared with LADC and LSCC.
To better control the bias brought by retrospective clinical data, PSM was used to create a highly comparable control group. PSM generated 471 pairs when LCNEC group was compared with LADC group, and both OS (HR, 1.724) and CSS (HR, 1.661) were significantly worse in stage IA LCNEC group than in LADC group. Consistent results showing stage IA LADC group was superior for OS than LCNEC were found in all subgroups and for CSS in most subgroups. Of note, for the subgroup of patients ≤ 65 years of age, HRs for both OS and CSS were lower (for OS: HR, 0.470; for CSS: HR, 0.482). On multivariate analysis, consistent with prior results, histologic type was an independent factor for both OS and CSS. As for comparison between LCNEC and LSCC, PSM generated 470 pairs. Only CSS was significantly better in LSCC than LCNEC (HR, 0.563), while OS was not. Further grouping by age showed only CSS between two groups for patients with age≤65 years old was significantly different (P = 0.006), and in subgroup analysis for patients with age ≤ 65, a lower HR for CSS was observed (HR, 0.400). Collectively, these results demonstrated stage IA NSCLC was worse in survival, especially than LADC. Clinically special attention should be paid to younger patients with LCNEC, because they showed significantly worse survival compared to other types of NSCLC.
Thus, even for disease at extremely early stage, besides radical surgery, more clinical studies investigating whether adjuvant therapy should also be considered in treatment modality are warranted. Although available literature regarding the treatment of early-stage LCNEC is scant, current evidence indicates adjuvant or neo-adjuvant chemotherapy may play a major role (19). On the one hand, previous small sample analysis indicated adjuvant chemotherapy may bring survival benefits. In a multicenter retrospective analysis (7), 144 patients with LCNEC who underwent lung resection were reviewed, and in stage I (n = 73) disease, adjuvant or induction chemotherapy had a trend (P = 0.077) showing better outcome compared with no chemotherapy. Another retrospective study (9) reviewed 45 patients with LCNEC who underwent surgery, and it also showed despite the small sample size, the survival benefit of adjuvant chemotherapy can still be observed even in the stage I LCNEC cases [surgery plus adjuvant chemotherapy (n = 11), surgery alone (n = 16)]. On the other hand, in terms of treatment regimen, there are also several other prospective (20) and retrospective studies (8, 21–25) showing platinum-based (usually cisplatin + etoposide or irinotecan) chemotherapy plus surgery achieved better survival than surgery alone. Also, a randomized phase III trial (26) is now ongoing in Japan to compare the adjuvant cisplatin + etoposide regimen with cisplatin + irinotecan regimen in patients with completely resected high-grade LCNEC.
This study had certain limitations. First, although our study also showed better survival in stage IA LSCC than LCNEC, unlike previous analysis (12), the difference in our study was only significant in some subgroups, perhaps attributed to our smaller sample size and larger bias of previous study. Larger scale data with smaller bias are called for. Still, both studies consistently emphasize extra attention should be paid to LCNEC compared to other early-stage NSCLC. Second, although PSM was performed, the analyses are essentially retrospective and it inevitably comes with a selection bias. Finally, details of chemotherapy and radiotherapy are limited in the SEER registry and it is not prudent to address the specific effect of the addition of chemotherapy or radiotherapy based on current data, thus current chemotherapy and radiotherapy data were only used for PSM but cannot be used to address whether the adjuvant therapy can achieve better survival than surgery alone. In the design of the experiment, we also assumed that the number of lymph nodes dissected may have an impact on the prognosis. SEER did record the number of local lymph nodes dissected and the number of lymph nodes biopsied. However, in the number of lymph nodes resected, half of the patients were recorded none or unknown; also, in the number of lymph nodes biopsied, half of the patients were recorded as no lymph nodes was examined, or although the examination was performed but the specific number was not recorded, or status of lymph nodes biopsied was not recorded in the medical history. These made us think that this part of the data is not convincing enough to analyze. Despite these limitations, information that plays a vital role in the PSM was available and analyzed in this study.
In conclusion, we report the first survival comparison between stage IA LCNEC and other types of NSCLC patients who received surgery from data of the SEER database by using PSM analysis. Our results demonstrated that after surgery, stage IA LCNEC was worse in survival than LADC and LSCC, especially compared with LADC. Even for disease at extremely early stage, extra clinical care should be paid compared with other types of NSCLC, and besides radical surgery, especially for younger patients, more clinical studies investigating whether adjuvant therapy should also be considered in treatment modality are warranted.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found at the Surveillance, Epidemiology, and End Results (SEER) database.
Author Contributions
LZ, TG, and LY contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No. 81872461, 81703024).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.572462/full#supplementary-material
References
1. Fasano M, Della Corte CM, Papaccio F, Ciardiello F, Morgillo F. Pulmonary Large-Cell Neuroendocrine Carcinoma: From Epidemiology to Therapy. J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer (2015) 10(8):1133–41. doi: 10.1097/jto.0000000000000589
2. Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med (2010) 134(11):1628–38. doi: 10.1043/2009-0583-rar.1
3. Varlotto JM, Medford-Davis LN, Recht A, Flickinger JC, Schaefer E, Zander DS, et al. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer (2011) 6(6):1050–8. doi: 10.1097/JTO.0b013e318217b6f8
4. Eichhorn F, Dienemann H, Muley T, Warth A, Hoffmann H. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann Thoracic Surg (2015) 99(3):983–9. doi: 10.1016/j.athoracsur.2014.10.015
5. Iyoda A, Hiroshima K, Moriya Y, Sekine Y, Shibuya K, Iizasa T, et al. Prognostic impact of large cell neuroendocrine histology in patients with pathologic stage Ia pulmonary non-small cell carcinoma. J Thoracic Cardiovasc Surg (2006) 132(2):312–5. doi: 10.1016/j.jtcvs.2006.02.046
6. Iyoda A, Jiang SX, Travis WD, Kurouzu N, Ogawa F, Amano H, et al. Clinicopathological features and the impact of the new TNM classification of malignant tumors in patients with pulmonary large cell neuroendocrine carcinoma. Mol Clin Oncol (2013) 1(3):437–43. doi: 10.3892/mco.2013.80
7. Veronesi G, Morandi U, Alloisio M, Terzi A, Cardillo G, Filosso P, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases. Lung Cancer (Amsterdam Netherlands) (2006) 53(1):111–5. doi: 10.1016/j.lungcan.2006.03.007
8. Abedallaa N, Tremblay L, Baey C, Fabre D, Planchard D, Pignon JP, et al. Effect of chemotherapy in patients with resected small-cell or large-cell neuroendocrine carcinoma. J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer (2012) 7(7):1179–83. doi: 10.1097/JTO.0b013e3182572ead
9. Saji H, Tsuboi M, Matsubayashi J, Miyajima K, Shimada Y, Imai K, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anti Cancer Drugs (2010) 21(1):89–93. doi: 10.1097/CAD.0b013e328330fd79
10. Sarkaria IS, Iyoda A, Roh MS, Sica G, Kuk D, Sima CS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thoracic Surg (2011) 92(4):1180–6; discussion 6-7. doi: 10.1016/j.athoracsur.2011.05.027
11. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol (2016) 40(12):e94–102. doi: 10.1097/pas.0000000000000749
12. Raman V, Jawitz OK, Yang CJ, Voigt SL, Tong BC, D’Amico TA, et al. Outcomes for Surgery in Large Cell Lung Neuroendocrine Cancer. J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer (2019) 14(12):2143–51. doi: 10.1016/j.jtho.2019.09.005
13. Roesel C, Terjung S, Weinreich G, Gauler T, Theegarten D, Stamatis G, et al. A Single-Institution Analysis of the Surgical Management of Pulmonary Large Cell Neuroendocrine Carcinomas. Ann Thoracic Surg (2016) 101(5):1909–14. doi: 10.1016/j.athoracsur.2015.12.009
14. Kehl KL, Lathan CS, Johnson BE, Schrag D. Race, Poverty, and Initial Implementation of Precision Medicine for Lung Cancer. J Natl Cancer Institute (2019) 111(4):431–4. doi: 10.1093/jnci/djy202
15. Meza R, Meernik C, Jeon J, Cote ML. Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One (2015) 10(3):e0121323. doi: 10.1371/journal.pone.0121323
16. Shugarman LR, Mack K, Sorbero ME, Tian H, Jain AK, Ashwood JS, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care (2009) 47(7):774–81. doi: 10.1097/MLR.0b013e3181a393fe
17. Jakobsen E, Rasmussen TR, Green A. Mortality and survival of lung cancer in Denmark: Results from the Danish Lung Cancer Group 2000-2012. Acta Oncol (Stockholm Sweden) (2016) 55 Suppl 2:2–9. doi: 10.3109/0284186x.2016.1150608
18. Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol (2016) 893:1–19. doi: 10.1007/978-3-319-24223-1_1
19. Lo Russo G, Pusceddu S, Proto C, Macerelli M, Signorelli D, Vitali M, et al. Treatment of lung large cell neuroendocrine carcinoma. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2016) 37(6):7047–57. doi: 10.1007/s13277-016-5003-4
20. Iyoda A, Hiroshima K, Moriya Y, Takiguchi Y, Sekine Y, Shibuya K, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thoracic Surg (2006) 82(5):1802–7. doi: 10.1016/j.athoracsur.2006.05.109
21. Iyoda A, Hiroshima K, Moriya Y, Iwadate Y, Takiguchi Y, Uno T, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thoracic Cardiovasc Surg (2009) 138(2):446–53. doi: 10.1016/j.jtcvs.2008.12.037
22. Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, Sartori G, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol Off J Am Soc Clin Oncol (2005) 23(34):8774–85. doi: 10.1200/jco.2005.02.8233
23. Tanaka Y, Ogawa H, Uchino K, Ohbayashi C, Maniwa Y, Nishio W, et al. Immunohistochemical studies of pulmonary large cell neuroendocrine carcinoma: a possible association between staining patterns with neuroendocrine markers and tumor response to chemotherapy. J Thoracic Cardiovasc Surg (2013) 145(3):839–46. doi: 10.1016/j.jtcvs.2012.03.036
24. Kenmotsu H, Niho S, Ito T, Ishikawa Y, Noguchi M, Tada H, et al. A pilot study of adjuvant chemotherapy with irinotecan and cisplatin for completely resected high-grade pulmonary neuroendocrine carcinoma (large cell neuroendocrine carcinoma and small cell lung cancer). Lung Cancer (Amsterdam Netherlands) (2014) 84(3):254–8. doi: 10.1016/j.lungcan.2014.03.007
25. Fournel L, Falcoz PE, Alifano M, Charpentier MC, Boudaya MS, Magdeleinat P, et al. Surgical management of pulmonary large cell neuroendocrine carcinomas: a 10-year experience. Eur J Cardio Thoracic Surg Off J Eur Assoc Cardio Thoracic Surg (2013) 43(1):111–4. doi: 10.1093/ejcts/ezs174
26. Eba J, Kenmotsu H, Tsuboi M, Niho S, Katayama H, Shibata T, et al. A Phase III trial comparing irinotecan and cisplatin with etoposide and cisplatin in adjuvant chemotherapy for completely resected pulmonary high-grade neuroendocrine carcinoma (JCOG1205/1206). Japanese J Clin Oncol (2014) 44(4):379–82. doi: 10.1093/jjco/hyt233
Keywords: large cell lung neuroendocrine, stage IA, propensity score matching, SEER database, surgery
Citation: Zou L, Guo T, Ye L, Zhou Y, Chu L, Chu X, Ni J, Zhu Z and Yang X (2020) Outcomes for Surgery in Stage IA Large Cell Lung Neuroendocrine Compared With Other Types of Non-Small Cell Lung Cancer: A Propensity Score Matching Study Based on the Surveillance, Epidemiology, and End Results (SEER) Database. Front. Oncol. 10:572462. doi: 10.3389/fonc.2020.572462
Received: 14 June 2020; Accepted: 23 October 2020;
Published: 26 November 2020.
Edited by:
Ticiana A. Leal, University of Wisconsin-Madison, United StatesReviewed by:
Arnold Manfred Herskovic, Rush University, United StatesChristopher Gerard Azzoli, Brown University, United States
Copyright © 2020 Zou, Guo, Ye, Zhou, Chu, Chu, Ni, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengfei Zhu, ZnVzY2N6emZAMTYzLmNvbQ==; Xi Yang, bnRnZW9yZ2VAcXEuY29t
†These authors have contributed equally to this work
 Liqing Zou
Liqing Zou Tiantian Guo
Tiantian Guo Luxi Ye
Luxi Ye Yue Zhou
Yue Zhou Li Chu
Li Chu Xiao Chu
Xiao Chu Jianjiao Ni
Jianjiao Ni Zhengfei Zhu
Zhengfei Zhu Xi Yang
Xi Yang