- 1Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Medical Department, Beijing Genecast Biotechnology Co., Beijing, China
- 3Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
- 4Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China
- 5Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Introduction: Although traditional treatments confer survival benefits to patients with gastric cancer (GC), many patients experience relapse soon after postoperative adjuvant therapy. Immune-related mechanisms play an important role in GC, and immunotherapeutic strategies are considered to be a promising direction for the treatment of GC. Thus, our study aimed to investigate the expression and prognostic significance of immune-related genes in GC.
Methods: Formalin-fixed, paraffin-embedded samples were collected from 48 resectable GC patients. The transcriptome data of the tumor immune microenvironment were assessed using an immuno-oncology 395-gene panel RNA sequencing platform. The prognostic value of the 395 genes was analyzed and validated in the KM plotter and GEPIA databases. The data from The Cancer Genome Atlas (TCGA, downloaded from UCSC Xena repository) and Tumor IMmune Estimation Resource (TIMER) were used to evaluate the correlations between prognostic factors and immune signatures.
Results: Among the 395 genes, NOTCH3 was identified as a good prognostic factor for GC patients. Its prognostic value was also suggested in both our GC cohort from Zhongshan Hospital and the public databases (KM plotter and GEPIA database). Mechanistically, high NOTCH3 expression correlated with a lower infiltration of activated CD8+ T cells and a higher infiltration of immunosuppressive cells including Tregs and M2 macrophages in the tumor microenvironment. Moreover, high NOTCH3 expression was accompanied by the increased expression of a series of immune checkpoint inhibitors, resulting in a dampened immune response. Interestingly, NOTCH3 expression had a negative association with well-documented predictive biomarkers of immune checkpoint blockade (ICB) immunotherapy, including tumor mutation burden (TMB), gene expression profiling (GEP) score and innate anti-PD-1 resistance (IPRES) signature.
Conclusion: These findings uncovered a new mechanism by which NOTCH3 participates in the immune tolerance of GC, implying the potential of NOTCH3 as a therapeutic target or predictive marker for GC patients.
Introduction
Gastric cancer (GC) is the fifth most common neoplasm in the world (1). Despite improvements in surgical techniques, radiotherapy, chemotherapy, and neoadjuvant therapy, GC remains the second leading cause of cancer death worldwide (2). Thus, identifying predictive and prognostic markers is an important step for improving current treatment approaches and extending survival.
In previous studies, researchers have established a large number of gene expression signatures for patient stratification. According to these signatures, GC patients are divided into different prognostic risk groups (3–6). Most of the signatures are mainly composed of tumor cell intrinsic genes involved in tumor proliferation and invasion. However, the progression of tumors is controlled not only by malignant cells but also by cells from the host, such as endothelial cells, stromal fibroblasts, and a variety of immune cells. Immune-related mechanisms have been increasingly recognized to play an important role in cancer progression. Cytotoxic T cells, regulatory T cells (Tregs), dendritic cells (DCs), tumor-associated macrophages, and mesenchymal stem cells have been reported to be closely associated with the clinical outcomes of patients with solid tumors (7–9). Three subtypes of tumor microenvironment (TME) cell infiltration showed different survival times in GC. TME cluster-A, which was characterized by increases in the infiltration of immunosuppressive cells, had the poorest outcome. In contrast, TME cluster-C, which showed significant increases in the infiltration of CD8+ T cells, M1 macrophages, and activated memory CD4+ T cells, had a favorable outcome (10).
In addition, immunotherapeutic strategies, especially immune checkpoint blockade (ICB), have become the standard care in various types of cancer. However, only a small proportion of GC patients respond to current ICB therapy. Given the observation that the immune-related TME is essential for the prognosis and efficacy of chemotherapy and immunotherapy (11, 12) in other cancer types, it is worth investigating tumor-immune interactions and identifying novel potential prognostic and therapeutic targets in GC patients.
Herein, transcriptome data of the tumor immune microenvironment from 48 stage III GC patients were assessed using an immuno-oncology (IO) panel RNA sequencing platform. Analysis of the prognostic value of these genes revealed that high mRNA levels of NOTCH3 were associated with poor prognosis in GC, which was validated in two public databases. Importantly, further investigation revealed the correlation of NOTCH3 with tumor-infiltrating immune cells, immune checkpoint gene expression, and well-established biomarkers of ICB therapy. These findings uncovered a new mechanism by which NOTCH3 participates in the progression and immune tolerance of GC, implying the potential of NOTCH3 as a therapeutic target or predictive marker for GC patients.
Materials and Methods
Patient Information and Sample Collection
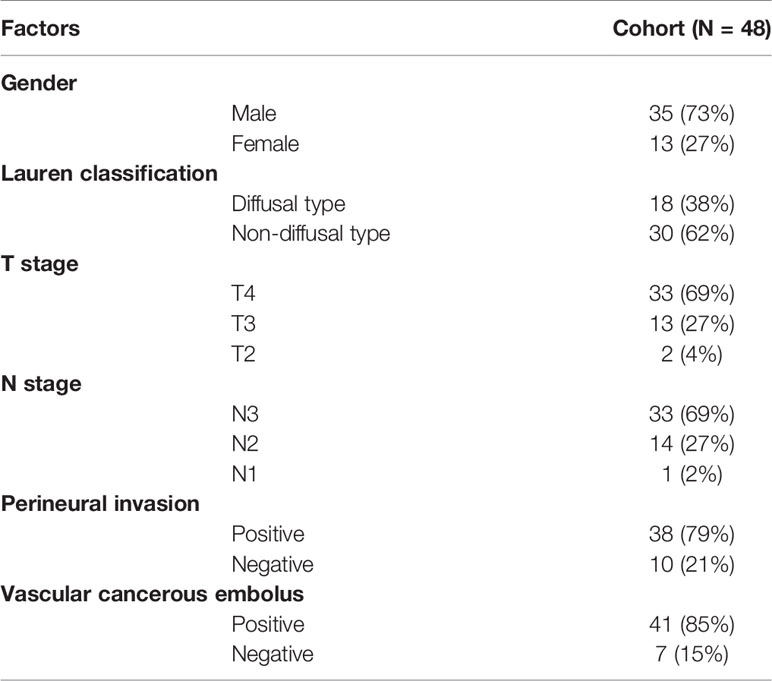
The formalin-fixed, paraffin-embedded (FFPE) samples of 48 GC patients after radical resection were retrieved from the tissue achieve of Zhongshan Hospital (Shanghai, China) in 2015, and the last follow-up date was April 2019. All of the patients were stage III. Clinically recorded information, such as age, sex, tumor histology, and pathologic stage, were collected and the detailed information was shown in Table 1. The institutional review board of Zhongshan Hospital gave explicit approval for the study, and all samples were obtained upon informed consent under an institutional protocol for tissue collection. There were 35 male and 13 female patients. The median age was 62.5 ± 9.82 years, and the median disease-free survival (DFS) was 14.85 ± 0.11 months. Sections from the FFPE blocks were cut and stained with hematoxylin and eosin. Tumor tissues were confirmed by a qualified pathologist. Additional serial sections were cut for RNA extraction.
RNA IO Profiling
The RNA IO profiling platform (Genecast Biotechnology, Beijing, China) is a unique 395-plex gene expression panel that quantifies 395 IO-related genes in human solid cancers that mainly fall into the following categories: tumor markers and essential signaling pathways, tumor-specific antigens, immunological response, infiltrating immune cells, and housekeeping (HK) genes. In brief, RNA was extracted from FFPE tissues by means of the truXTRAC™ FFPE RNA Kit (Covaris, Inc., Woburn, MA). After purification, the RNA yield was quantified using a Qubit™ RNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA). Then, 10 ng of RNA was reverse transcribed into cDNA, and targets were amplified with the primer pool targeting 395 genes. Barcode adapters were ligated to partially digested amplicons. Purified libraries were quantified via an Agilent™ 2100 Bioanalyzer (Agilent, Santa Clara, CA) and then pooled in equal molar amounts prior to enrichment and template preparation using the Ion Chef™ system (Thermo Fisher Scientific, Waltham, MA). For each sample, 200 bp sequencing was performed on the Ion S5 530 chip (Thermo Fisher Scientific, Waltham, MA) to obtain 1–2 M reads. The absolute digital gene expression counts of all samples in the same run were automatically generated in the in-house bioinformatics pipeline. Only sequencing data meeting the quality control (QC) criteria for mapped reads, on-target reads and mean reads were included in the study.
Gene expression normalization was performed as described previously (13). A baseline expression profile for 10 HK genes was established based on the average reads per million (RPM) counts. Each HK gene background-subtracted read was compared against the RPM profile from that internal control sample, which then gave rise to a fold-change ratio for each HK gene: ratio of HK = absolute read count of HK/RPM profile of HK. Then, the normalization ratio for the particular sample was calculated using the median value of all HK ratios. The normalization ratio equals the median (all HK ratios). Next, the nRPM of all genes (G) of the specific sample (S) (nRPM(S,G)) was calculated as:
Data Acquisition
The TCGA gene expression data of stomach adenocarcinoma (STAD), colon adenocarcinoma (COAD), esophageal carcinoma (ESCA), liver hepatocellular carcinoma (LIHC), and pancreatic adenocarcinoma (PAAD) were acquired via the UCSC Xena repository. The scores of 28 immune cell types and functions in these samples were inferred from the gene expression data by single-sample gene set enrichment analysis (ssGSEA) (14). The abundances of M1 macrophages and M2 macrophages were estimated by CIBERSORT (15).
Statistical Analysis
For comparisons between two groups, statistical significance for normally distributed variables was estimated by unpaired Student’s t test, and nonnormally distributed variables were analyzed by the Mann–Whitney U test. Correlation coefficients were computed by Spearman analyses. The Kaplan–Meier method was used to generate survival curves for the subgroups in each data set, and the log-rank (Mantel–Cox) test was used to determine the statistical significance of differences. The hazard ratios for univariate analyses were calculated using a univariate Cox proportional hazards regression model. A multivariate Cox regression model was used to determine independent prognostic factors by SPSS19.
Results
Prognostic Potential of NOTCH3 in Gastric Cancer
To maintain sufficient group sizes for the analysis, 48 resectable patients from Zhongshan Hospital were divided based on the median expression level of 395 immune-related genes. Using univariate Cox regression analysis, we identified 73 genes significantly associated with the DFS of patients (the 73 genes are listed in Supplemental Table 1). Then, we examined the influence of the above 73 genes’ expression on the prognosis of GC in the GEPIA and KM Plotter databases. We observed that among the 73 genes, only NOTCH3 was significantly related to patient survival in both the GEPIA (STAD) and KM Plotter (GC) cohorts when we set the median expression of NOTCH3 as a cutoff to stratify patients (Figure 1A, log-rank test P<0.05). In accordance with the Zhongshan cohort (Figure 1B), patients with high NOTCH3 expression had a shorter DFS or first progression (FP) than patients with low NOTCH3 expression (Figures 1C, D, log-rank test P<0.05). Furthermore, multivariate Cox regression analysis incorporating the clinical features of the 48 GC patients in the Zhongshan cohort revealed that NOTCH3 expression was an independent prognostic factor for GC patients (Table 2).
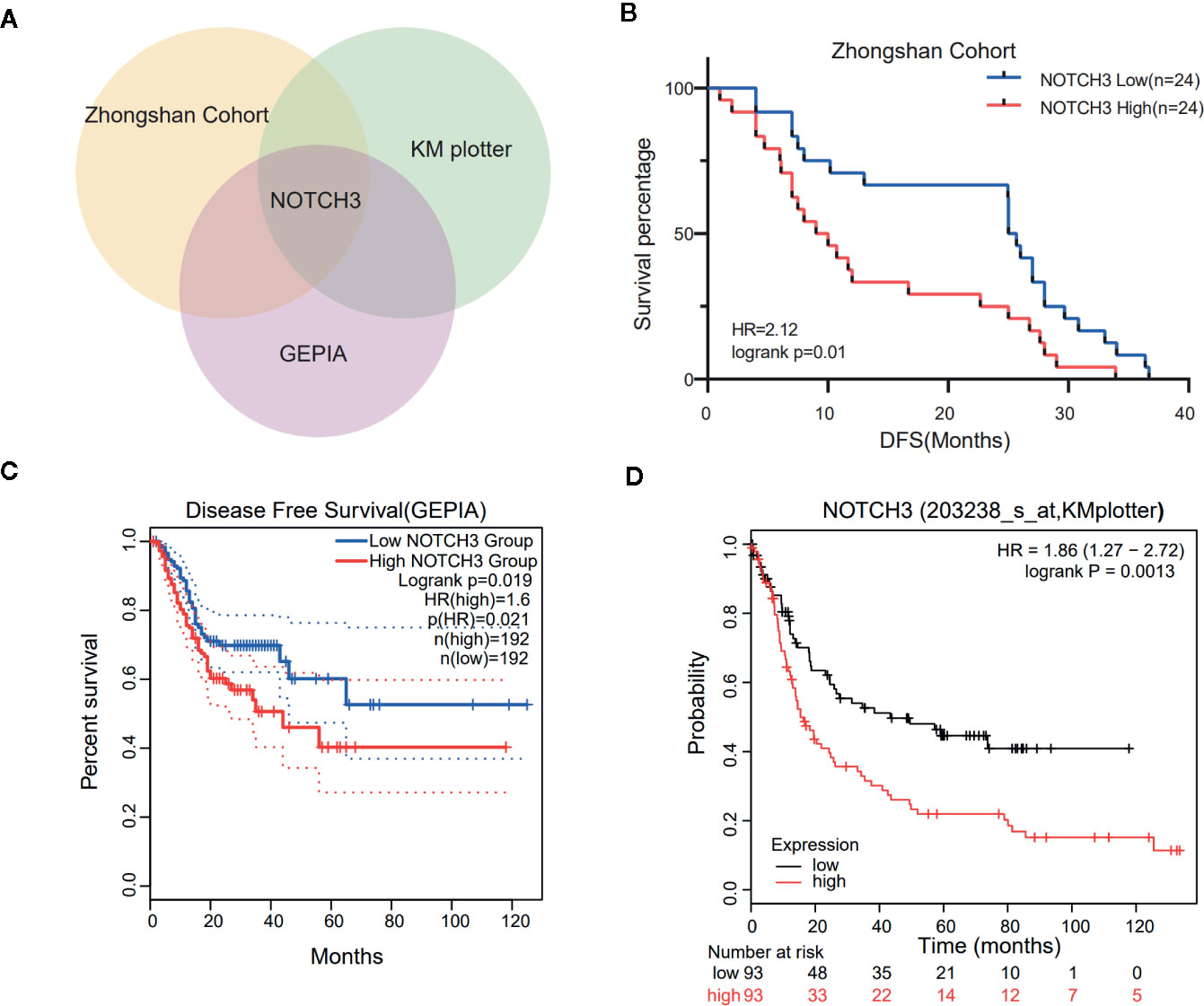
Figure 1 Kaplan-Meier survival curves illustrate the prognostic value of NOTCH3 expression in gastric cancer (GC) patients. (A) Venn diagram showed the genes whose prognostic value could be validated in three cohorts. (B) Survival curves of disease-free survival (DFS) in GC cohort from Zhongshan hospital. (C) Survival curves of DFS in stomach adenocarcinoma (STAD) cohort from GEPIA database. (D) Survival curves of first progression (FP) in GC cohort from KM-plotter database.
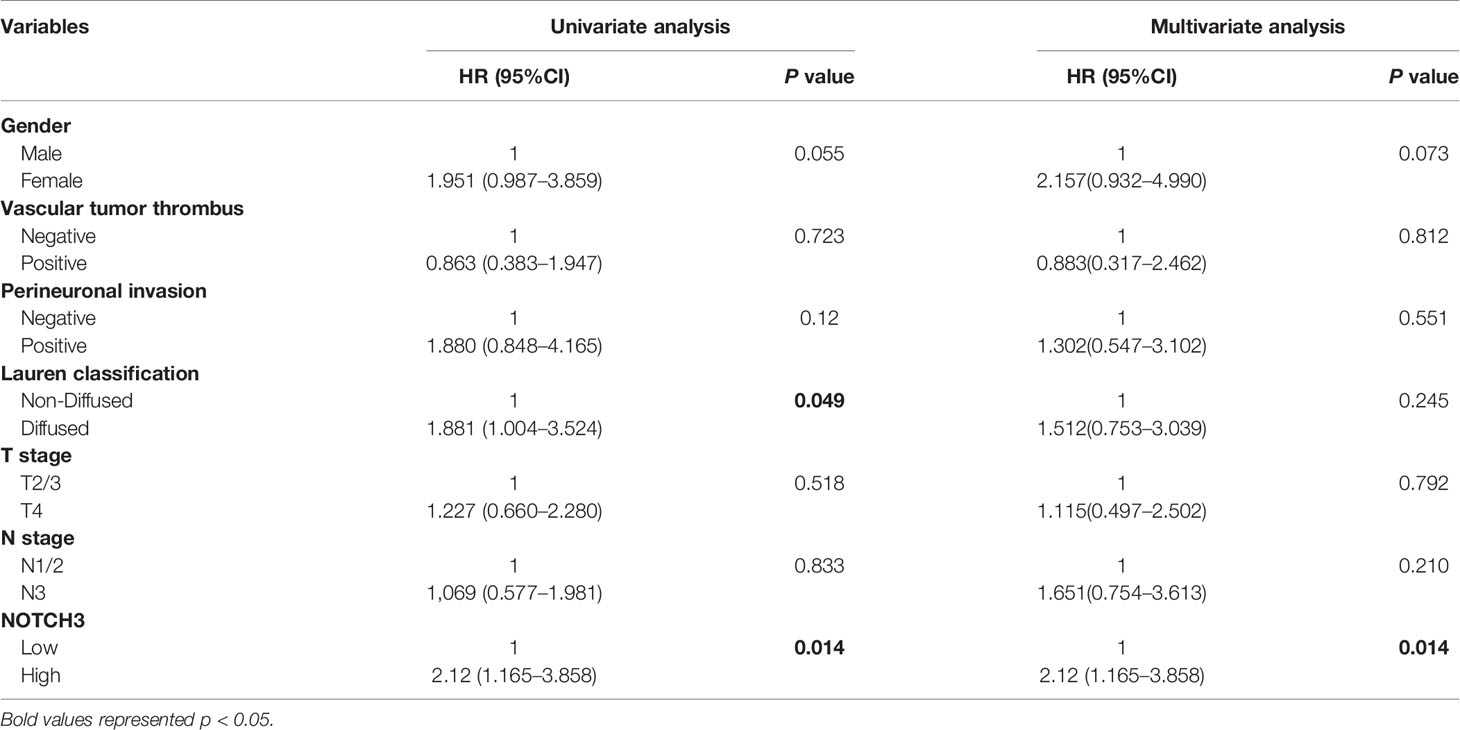
Table 2 Univariate and multivariable Cox regression analyses for disease-free survival (DFS) time of 48 patients with gastric cancer from Zhongshan Cohort.
Given the observation that NOTCH3 has prognostic value in GC, the RNA expression data in The Cancer Genome Atlas (TCGA) database were also used to analyze the prognostic potential of NOTCH3 in other gastrointestinal (GI) cancers, including LIHC, ESCA, COAD, and PAAD. The optimal cutoff values of NOTCH3 expression to determine the prognosis of patients in the TCGA cohort were calculated on the basis of the prognostic significance using X-Tile software (16). High NOTCH3 expression levels were associated with a poor prognosis of DFS and progression-free survival (PFS) in COAD, PAAD and ESCA. Moreover, high NOTCH3 expression was also correlated with shorter overall survival (OS) in COAD patients. However, NOTCH3 expression has no prognostic value for patients with liver cancer (Figure S1). Taken together, these results suggest the prognostic value of NOTCH3 expression in GC and other GI cancers.
NOTCH3 Expression Is Correlated With Immune Infiltration in Gastric Cancer
Lymphocyte infiltration in the TME is associated with the clinical prognosis of many kinds of cancers, including GC, melanoma, urothelial cancer, lung cancer, and breast cancer (17–20). The NOTCH signaling pathway has also been reported to be involved in the proliferation, differentiation and activation of lymphocytes in the thymus or peripheral lymphoid organs (21, 22). However, less is known about whether NOTCH signaling has an impact on the infiltration of lymphocytes in the TME. Therefore, we aimed to explore the correlation of NOTCH3 expression with immune cell infiltration in GI cancers. First, the association of tumor-infiltrating immune cell (TIIC) abundance with NOTCH3 expression was estimated in GC from the Tumor IMmune Estimation Resource (TIMER) database. Interestingly, the expression of NOTCH3 was positively correlated with the infiltration of CD4+ T cells and macrophages in STAD patients (Figures 2A, B). In contrast, NOTCH3 expression had a poor correlation with the enrichment of B cells, CD8+ T cells, neutrophils and DCs (Figures 2C–F). As expected, the positive association of NOTCH3 expression with CD4+ T cells and macrophages was also observed in COAD, LIHC and PAAD (Figure S2).
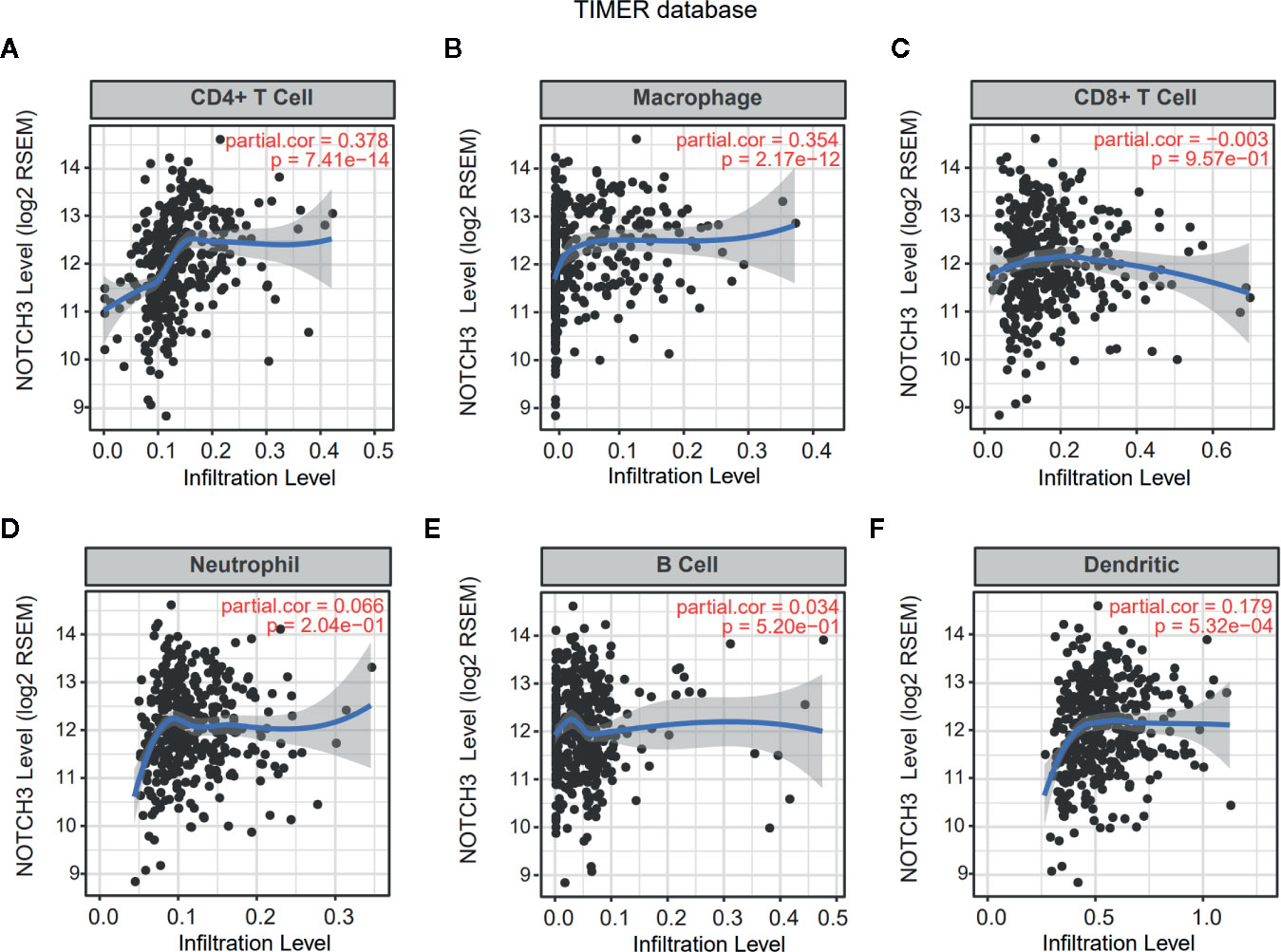
Figure 2 Correlation of NOTCH3 expression with immune infiltration in gastric cancer (GC) patients from Tumor IMmune Estimation Resource (TIMER) database. NOTCH3 expression has a positive correlation with infiltration of CD4+ T cells (A) and macrophages (B), but a very weak correlation with CD8+ T cells (C), neutrophils (D), B cells (E), dendritic cells (F) in stomach adenocarcinoma (STAD) (n=414) patients.
To investigate whether NOTCH3 expression has an impact on lymphocyte infiltration with further evidence at the level of sub-immune cell types, we downloaded the RNA-expression data of 414 GC (STAD) patients from the TCGA database. ssGSEA was applied to calculate the enrichment of 28 immune cell types. We defined the patients with NOTCH3 expression higher than the 75th percentile as the “NOTCH3 High group” (n=104) and patients with NOTCH3 expression lower than the 25th percentile as the “NOTCH3 Low group” (n=104). Notably, most of the immunosuppressive cells, such as Tregs, macrophages, mast cells and myeloid-derived suppressor cells (MDSCs), exhibited significantly higher enrichment levels in the NOTCH3 High group (Figure S3, Figures 3A, B). Surprisingly, activated CD8+ T cells showed a reduction in the NOTCH3 High group (Figure 3C). Thus, the ratio of activated CD8+ T cells to Tregs was elevated in the NOTCH3 Low group (Figure 3D), suggesting a graver imbalance of acquired immunity in the TME of patients with higher expression levels of NOTCH3.
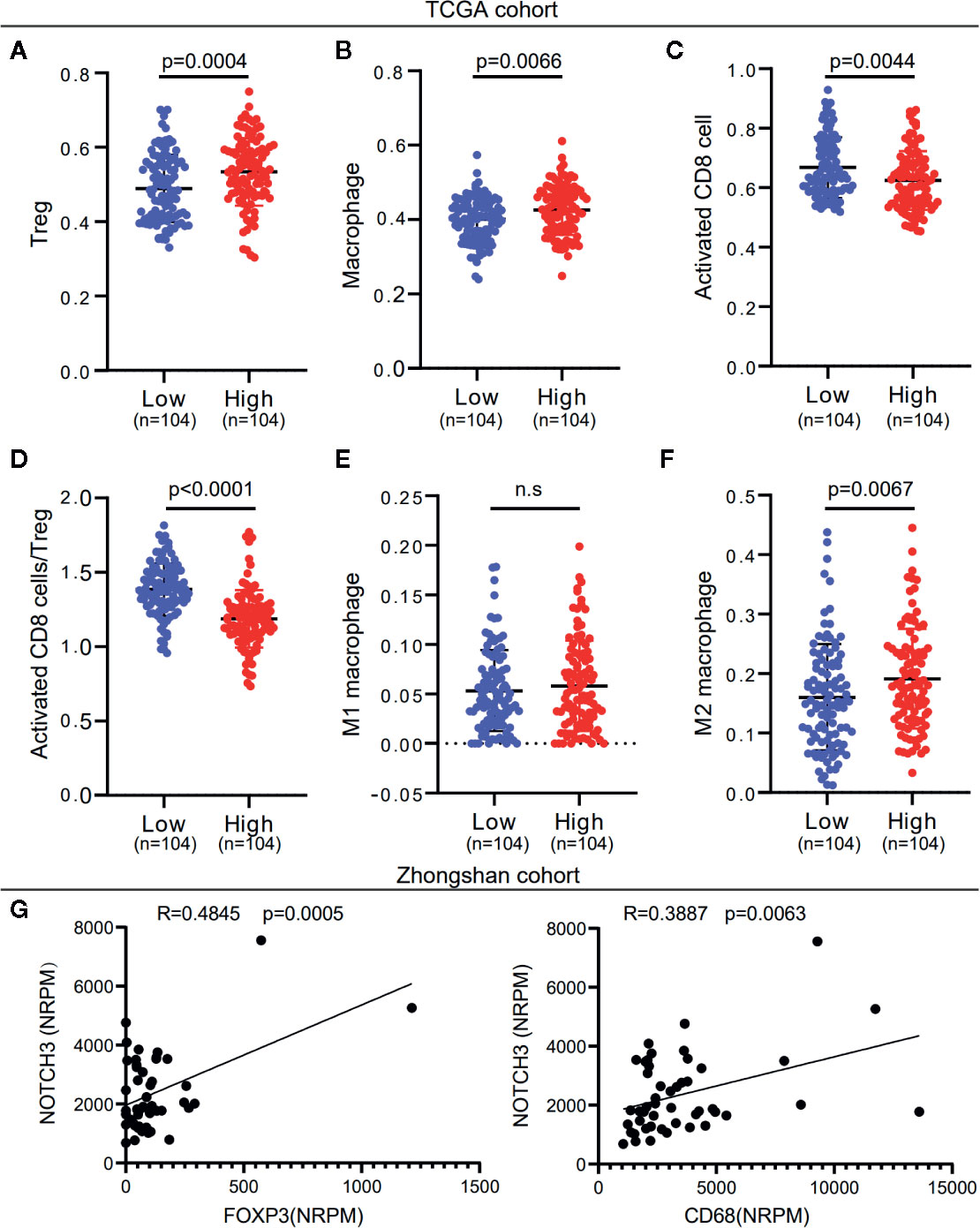
Figure 3 NOTCH3 upregulation is associated with increased immune suppressive cell enrichment in tumor microenvironment (TME) of gastric cancer (GC) patients. (A–C) Tregs (A), macrophages (B), and activated CD8+ cells (C) abundance was quantified by ssGSEA. (D) The boxplot showed the ratio of activated CD8+ T cell versus Tregs in indicated groups. (E, F) Comparison of M1 (E) and M2 (F) macrophage fractions between NOTCH3 High and Low group. The analyzed data of (A–F) was obtained from The Cancer Genome Atlas (TCGA) stomach adenocarcinoma (STAD) cohort. The percentages of M1 and M2 macrophages in each tumor sample were quantified by CIBERSORT. (G) The NOTCH3 expression had positive correlations with FOXP3 (left) and CD68 (right) in patients from Zhongshan cohort.
To further determine whether NOTCH3 expression is correlated with M1 or M2 macrophages, we used the CIBERSORT deconvolution method to calculate the enrichment of M1 and M2 macrophages. As shown in Figures 3E, F, macrophages in the GC TME were mainly composed of M2 macrophages. M2 but not M1 macrophages were more abundant in the NOTCH3 High group. Concordantly, in the 48 GC patients in this study, NOTCH3 expression had a significant positive correlation with the expression of FOXP3 and CD68, which are the biomarkers of Tregs and macrophages, respectively (Figure 3G). Therefore, our analysis indicated that high NOTCH3 expression is positively correlated with immunosuppressive cells and inversely correlated with cytotoxic T cells, which contributes to the shorter survival of GC patients.
Association of NOTCH3 Expression With Immune Checkpoint Inhibitors
Tumor cells escape immune surveillance and progress through different mechanisms, such as the overexpression of inhibitory immune checkpoint molecules that impair the antitumor immune response (23, 24). In this study, we first analyzed the association of NOTCH3 expression with a series of immune checkpoint inhibitors in the TCGA database. As shown in Figure S4A, multiple inhibitory checkpoint molecules, including CD274, CTLA4, CD276, HAVCR2 and CD200, had positive correlations with NOTCH3 mRNA levels in GC and other GI cancers.
Additionally, the expression of immune checkpoint genes, as mentioned above, was significantly higher in the NOTCH3 High group (n=104) than in the NOTCH3 Low group (n=104) in GC patients (Figure S4B). It is worth mentioning that the expression of three genes, ADORA2A, CD276 (B7-H3) and TNFRSF4, correlated with NOTCH3 in the TCGA STAD database (Figures 4A, B). This correlation was validated in the Zhongshan cohort of 48 patients (Figure 4C). The expression of these three genes was also significantly reduced in the NOTCH3 Low group when the 48 patients were stratified according to the median expression of NOTCH3 as the cutoff (Figure 4D). These data suggest that multiple immune checkpoint pathways, not only the PD-1/PD-L1 axis, cause immune tolerance and escape in patients with high NOTCH3 expression and result in poor outcomes in GC patients.
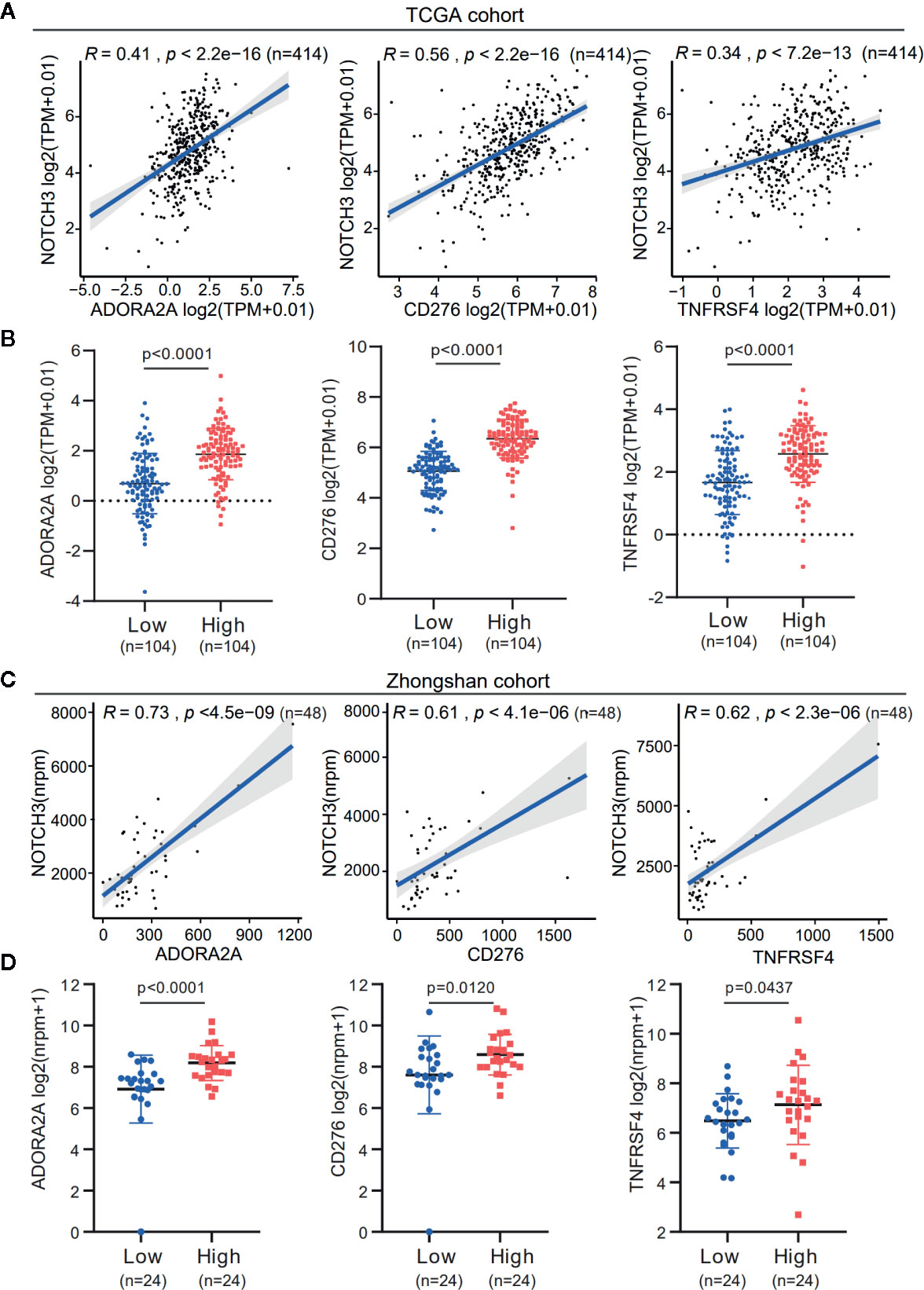
Figure 4 The expression association of NOTCH3 with immune checkpoint inhibitors in gastric cancer (GC) patients. (A) Association analysis between expression of NOTCH3 and ADORA2A, CD276, TNFRSF4 in The Cancer Genome Atlas (TCGA) stomach adenocarcinoma (STAD) database. (B) Boxplot distributions between groups with different NOTCH3 expression for ADORA2A, CD276, TNFRSF4 according to TCGA STAD database. (C) In the cohort from Zhongshan Hospital, linear regression curves displayed the correlation between expression of NOTCH3 and ADORA2A, CD276, TNFRSF4. (D) The boxplot illustrated the distribution of ADORA2A, CD276, TNFRSF4 expression in NOTCH3 differentially expressed patients from Zhongshan cohort.
Relationship Between NOTCH3 Expression and the Currently Adopted Predictive Biomarkers for Immune Checkpoint Blockade Therapy
PD-L1 (CD274) and CTLA4 are two well-studied immune checkpoints targeted for the treatment of patients with cancer (25–28). Numerous studies revealed that in addition to PD-L1 expression stained by IHC, biomarkers such as the density of tumor-infiltrating lymphocytes (TIL), tumor mutation burden (TMB), mismatch repair (MMR) deficiency, IFN-γ signature, and the 18-gene T-cell–inflamed gene expression profiling (GEP) score have been associated with the treatment effect of anti-PD-1/PD-L1 therapy (29–32). Given the observation that NOTCH3 expression had an impact on the infiltration of lymphocytes (Figure 3) and checkpoint inhibitor expression (CD274, CTLA4) (Figure S4B), we wondered whether NOTCH3 expression has an effect on other features predictive of ICB therapy. As shown in Figures 5A, B, in the TCGA STAD cohort, both TMB and GEP score, which have been demonstrated to be positively correlated with the prognosis of patients receiving pembrolizumab treatment (29, 32), were lower in the NOTCH3 High-expressed group.
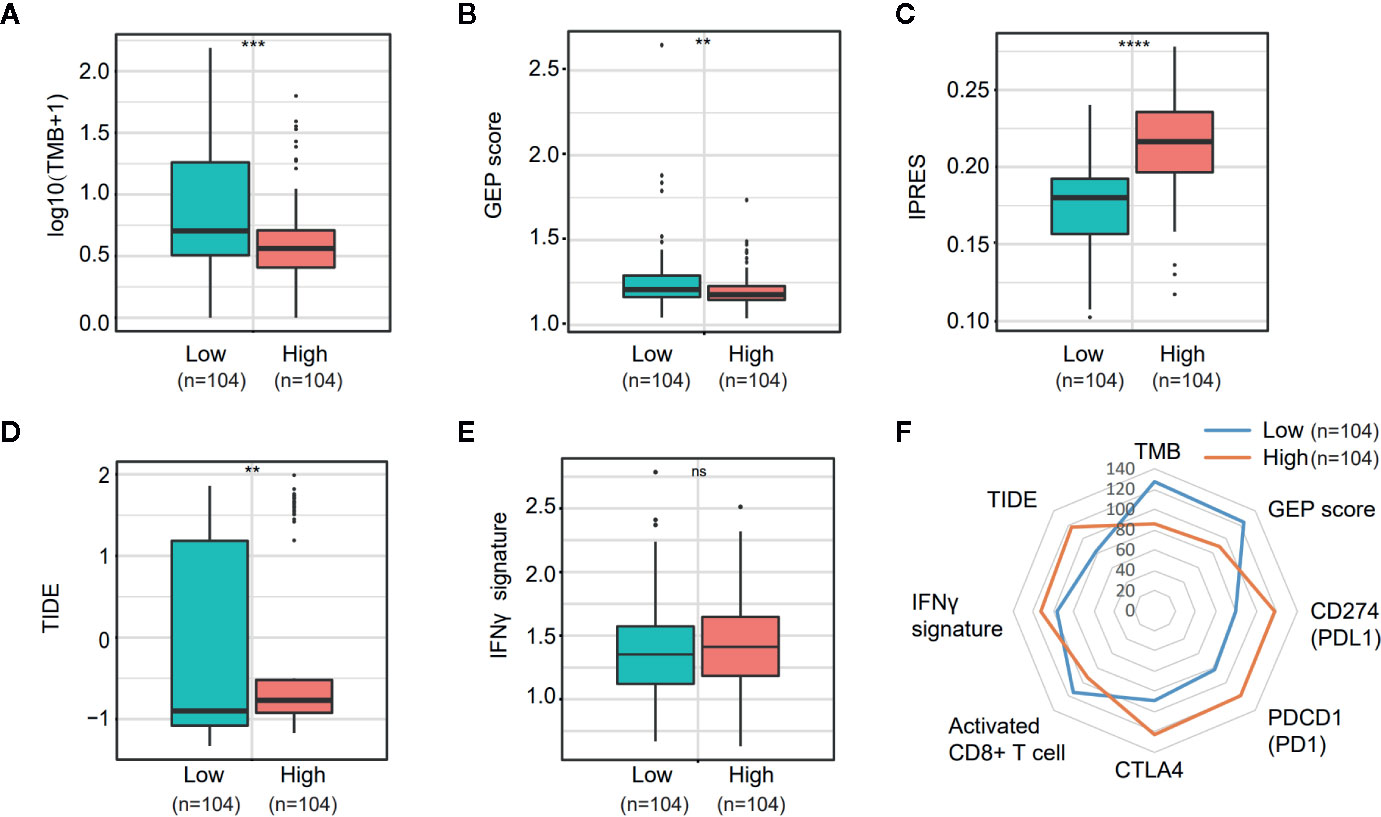
Figure 5 Comparison of the biomarkers for immune checkpoint blockade therapy between NOTCH3 High and Low group in The Cancer Genome Atlas (TCGA) database. (A, B) The boxplot indicated tumor mutation burden (TMB) (A) and gene expression profiling (GEP) score (B) were reduced in NOTCH3 High group comparing with NOTCH3 Low group. (C, D) The boxplot illustrated the higher innate anti-PD-1 resistance (IPRES) (C) and Tumor Immune Dysfunction and Exclusion (TIDE) score (D) in NOTCH3 High group. (E) The IFNγ signature showed no difference in NOTCH3 High and Low groups. (F) The radar chart summarized the association between NOTOCH3 expression and the currently-adopted predictive biomarkers for immune checkpoint blockade therapy. Each point indicates the median rank of predictive biomarkers in NOTCH3 High and Low groups of gastric cancer (GC) patients.
Conversely, the innate PD-1 resistance (IPRES) gene signature (33) for the prediction of resistance to PD-L1 or CTLA-4 blockade was much higher in the NOTCH3 High group (Figure 5C). Similarly, patients with high NOTCH3 expression had a higher Tumor Immune Dysfunction and Exclusion (TIDE) signature (34), implying the failure of ICB therapy in the NOTCH3 High group (Figure 5D). Regarding the IFN-γ signature (35), there was no significant difference between the two groups (Figure 5E). Overall, despite the differential expression of PD-1, PD-L1 and CTLA4 at the mRNA level (Figure S4B, Figure 5F), the impact of NOTCH3 expression on the other biomarkers of ICB therapy can be inferred as patients with high NOTCH3 expression may be more intrinsically resistant to ICB therapy.
Discussion
Immune cells and cytokines in the TME have a crucial influence on the occurrence and development of malignant cancers. In this study, we conducted immune-related RNA IO panel sequencing on 48 GC patients. Combining the data from two public databases, we identified NOTCH3 as a prognostic factor in GC and other GI cancers. We also delineated the molecular properties of immunogenicity associated with NOTCH3 expression, providing a potential target or predictive factor for GC immunotherapy.
The NOTCH signaling pathway is essential for cell fate and organogenesis. Accumulated evidence indicates that members of the NOTCH family play a central role in tumor malignancies, and they might serve as biomarkers for the diagnosis and prognosis of cancer (36–38). There are four NOTCH receptors (NOTCH1-4) in mammals. It has been reported that high NOTCH1 and NOTCH3 expression levels are related to poor OS rates in cancer (39, 40). However, low NOTCH2 expression levels are related to poor outcomes in cancer (41). To date, the contribution of NOTCH3 to GC development has not been fully elucidated. In our study, NOTCH3 was verified to be associated with the survival of GC patients using our cohort and public databases, and the prognostic value of NOTCH3 in GC patients was confirmed.
Increasing evidence has shown that the NOTCH pathway facilitates tumorigenesis and progression by initiating the transcription of target genes involved in tumor cell proliferation, differentiation, invasion and angiogenesis (42). It is also recognized that the progression of tumors is not only controlled by the intrinsic changes in cancerous cells but also dependent on the activity of nonmalignant cells in the TME, especially lymphocyte infiltration and activation (43). By the CIBERSORT analysis of TCGA RNA data, we found that activated CD8+ T cells were enriched in the NOTCH3 Low group (Figure 3C). At the same time, NOTCH3 expression was positively correlated with genes of immune checkpoint inhibitors, including ADORA2A (A2a receptor) and CD276 (B7-H3) (Figure 4). Accordingly, Yu and his colleagues reported that NOTCH signaling plays a negative role in regulating the antitumor activity of CD8+ T cells by upregulating the expression of PD-1 (44). Therefore, complementary to the lower infiltration of CD8+ T cells, these checkpoint inhibitors form a counter-regulatory mechanism in cancer cells putatively driven by the high expression of NOTCH3, indicating a new mechanism of NOTCH signaling for facilitating immune escape. Cytotoxic T cells play a crucial role in determining the clinical outcomes of patients in various types of cancer. Jung Soo Lee and his colleagues found that the high expression of TILs, mainly CD8+ T cells, may be a potential prognostic biomarker in patients with GC (45). It was reported that GC patients with high-density groups of CD3, CD8, and CD45RO cells had significantly longer survival times than those with low-density groups of these cells (46). Thus, the high expression of NOTCH3 with low activity of cytotoxic adaptive immunity probably contributes to the poor prognosis of GC patients.
In contrast to CD8+ T cells, Tregs are immunosuppressive cells in the TME. Through IL-10 and IL-35 cytokine production, tumor-infiltrating Tregs help tumor cells escape immune surveillance (47, 48). In addition to Tregs, M2 macrophages are another important immunosuppressive innate immune cell that is abundant in the TME. M2 macrophages promote tumor growth, progression, invasion and metastasis by producing anti-inflammatory cytokines, such as IL-10 and TGF-β (49, 50). Both Tregs and M2 macrophages protect tumor cells from discovery and elimination by the normal immune system, resulting in poor outcomes in GC (51, 52). The important role of NOTCH signaling in regulating these cell types has been confirmed by different researchers (53–56). Notwithstanding, most of the studies were performed in the context of mouse models or isolated cultured cells. Our results from the analysis of the TCGA database showed that high NOTCH3 expression was correlated with a high content of immunosuppressive Tregs and M2 macrophages (Figures 3A, F). This finding was also supported by the positive correlation between NOTCH3 and FOXP3 or CD68 expression in Zhongshan cohort (Figure 3G). These conclusions were drawn based on the results from the clinical specimens of cancer patients, and the correlation between NOTCH signaling and the infiltration of these immunosuppressive lymphocytes in the TME was verified.
The suppression of immune checkpoints with monoclonal antibodies blocking PD-L1 and PD1 has been proven to be an unprecedented anticancer therapy in different kinds of cancers (57–59). However, the responses occur in only a small fraction of patients. In particular, in GC, the overall response rate of anti-PD-1/PD-L1 treatment was only approximately 12%, and some patients were prone to developing hyperprogressive disease (HPD) (57, 60). Several biomarkers, including TMB, PD-1/PD-L1 expression, microsatellite instability (MSI) status and GEP score, have been well established for predicting the benefits of ICB. To date, in GC patients, no clinically validated biomarkers could completely distinguish responders from nonresponders despite the T-cell–inflamed GEP score (57). Intriguingly, patients with high NOTCH3 expression had significantly lower TMB and GEP scores than those with low NOTCH3 expression. In contrast, the IPRES signature, which represents the resistance to ICB therapy, and the TIDE score, which describes the dysfunction and exhaustion of CD8+ T cells, were both higher in patients with high NOTCH3 expression (Figure 5). Therefore, NOTCH expression has the potential to predict the efficiency of ICB therapy. There are few studies directly linking NOTCH signaling with ICB therapy in addition to Donghai Xiong’s finding that in one patient with esophageal squamous cell carcinoma (ESCC) who developed HPD after anti-PD-1 immunotherapy, somatic mutations of NOTCH4 were not present in the pretherapy tumor (61). Although our findings have important implications for the treatment of GC patients with different NOTCH3 expression levels, whether GC patients with low NOTCH3 expression could benefit from ICB therapy needs to be evaluated directly in clinical practice.
In conclusion, our research provided convincing data on the prognostic value of NOTCH3 expression in GC patients. Mechanistically, we found that the effect of NOTCH signaling on the infiltration and activity of immune cells, especially CD8+ T cells, Tregs and M2 macrophages, in the TME is involved in its contribution to the prognosis of GC. In particular, NOTCH signaling was correlated with the well-documented predictive biomarkers of immunotherapy, which would shed new light on the discovery of potential predictive biomarkers for GC patients.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: https://db.cngb.org/cnsa/project/CNP0001464/reviewlink/, accession number CNP0001464.
Ethics Statements
The studies involving human participants were reviewed and approved by The institutional review board of Zhongshan Hospital gave explicit approval for the study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YC wrote the first draft of manuscript. TL and HZ contributed to the conception and design of the research. QL, XS, and FL contributed to the experiment and analysis of the data. BM contributed to the analysis and interpretation of the data. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the ① Study on the prevention and control of major chronic non-infectious diseases, National Key Research and Development Plan of China, 2017YFC1308900, and ② The Science and Technology Commission of Shanghai Municipality, 19ZR1409500.
Conflict of Interest
The authors FL, XS, and BM were employed by Beijing Genecast Biotechnology Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We acknowledge the support from Medical Oncology Department of Zhongshan Hospital. We are grateful to Professor Sun Yihong and his team because they provided the data of cases from the General Surgery Department. We must give our sincere thanks to the GC patients who took part in this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.574937/full#supplementary-material
Abbreviations
COAD, colon adenocarcinoma; DCs, dendritic cells; DFS, disease-free survival; ESCA, esophageal carcinoma; GC, gastric cancer; GEP, gene expression profiling; ICB, immune checkpoint blockade; IO, immuno-oncology; IPRES, innate anti-PD-1 resistance; LIHC, liver hepatocellular carcinoma; OS, overall survival; PAAD, pancreatic adenocarcinoma; PFS, progression-free survival; ssGSEA, single-sample gene set enrichment analysis; STAD, stomach adenocarcinoma; TCGA, The Cancer Genome Atlas; TIIC, tumor-infiltrating immune cell; TIMER, Tumor Immune Estimation Resource; TMB, tumor mutation burden; TME, tumor microenvironment; Tregs, regulatory T cells.
References
1. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad Gastroenterologiczny (2019) 1:26–38. doi: 10.5114/pg.2018.80001
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clinicians (2019) 1:7–34. doi: 10.3322/caac.21551
3. Wang P, Wang Y, Hang B, Zou X, Mao JH. A novel gene expression-based prognostic scoring system to predict survival in gastric cancer. Oncotarget (2016) 34:55343–51. doi: 10.18632/oncotarget.10533
4. Xu ZY, Chen JS, Shu YQ. Gene expression profile towards the prediction of patient survival of gastric cancer. Biomedicine Pharmacotherapy = Biomed Pharmacotherapie (2010) 2:133–9. doi: 10.1016/j.biopha.2009.06.021
5. Takeno A, Takemasa I, Seno S, Yamasaki M, Motoori M, Miyata H, et al. Gene expression profile prospectively predicts peritoneal relapse after curative surgery of gastric cancer. Ann Surg Oncol (2010) 4:1033–42. doi: 10.1245/s10434-009-0854-1
6. Deng X, Xiao Q, Liu F, Zheng C. A gene expression-based risk model reveals prognosis of gastric cancer. PeerJ (2018) 6:e4204. doi: 10.7717/peerj.4204
7. Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet (2018) 10135:2128–39. doi: 10.1016/S0140-6736(18)30789-X
8. Hadler-Olsen E, Wirsing AM. Tissue-infiltrating immune cells as prognostic markers in oral squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer (2019) 7:714–27. doi: 10.1038/s41416-019-0409-6
9. Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell (2019) 4:588–602.e10. doi: 10.1016/j.ccell.2019.02.009
10. Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, et al. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol Res (2019) 5:737–50. doi: 10.1158/2326-6066.CIR-18-0436
11. Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, et al. Tumor-infiltrating Neutrophils is Prognostic and Predictive for Postoperative Adjuvant Chemotherapy Benefit in Patients With Gastric Cancer. Ann Surg (2018) 2:311–8. doi: 10.1097/SLA.0000000000002058
12. Waniczek D, Lorenc Z, Snietura M, Wesecki M, Kopec A, Muc-Wierzgon M. Tumor-Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Archivum Immunol therapiae experimentalis (2017) 5:445–54. doi: 10.1007/s00005-017-0463-9
13. Paluch BE, Glenn ST, Conroy JM, Papanicolau-Sengos A, Bshara W, Omilian AR, et al. Robust detection of immune transcripts in FFPE samples using targeted RNA sequencing. Oncotarget (2017) 2:3197–205. doi: 10.18632/oncotarget.13691
14. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell (2015) 1-2:48–61. doi: 10.1016/j.cell.2014.12.033
15. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods (2015) 5:453–7. doi: 10.1038/nmeth.3337
16. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 21:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
17. Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol (2015) 11:669–82. doi: 10.1038/nri3902
18. Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol (2017) 11:655–68. doi: 10.1038/nrclinonc.2017.88
19. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature (2018) 7693:544–8. doi: 10.1038/nature25501
20. Lee K, Hwang H, Nam KT. Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer. Gut liver (2014) 2:131–9. doi: 10.5009/gnl.2014.8.2.131
21. Garcia-Leon MJ, Fuentes P, de la Pompa JL, Toribio ML. Dynamic regulation of NOTCH1 activation and Notch ligand expression in human thymus development. Development (2018) 16:dev165597. doi: 10.1242/dev.165597
22. Miller AM, Schoenberger SP. Notch signaling maintains T cell memories. Nat Med (2015) 1:16–8. doi: 10.1038/nm.3784
23. Orabona C, Mondanelli G, Puccetti P, Grohmann U. Immune Checkpoint Molecules, Personalized Immunotherapy, and Autoimmune Diabetes. Trends Mol Med (2018) 11:931–41. doi: 10.1016/j.molmed.2018.08.005
24. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med (2018) 12:165. doi: 10.1038/s12276-018-0191-1
25. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 4:252–64. doi: 10.1038/nrc3239
26. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med (2012) 26:2443–54. doi: 10.1056/NEJMoa1200690
27. McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol (2016) 8:833–42. doi: 10.1200/JCO.2015.63.7421
28. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. New Engl J Med (2018) 2:158–68. doi: 10.1056/NEJMra1703481
29. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. New Engl J Med (2018) 22:2093–104. doi: 10.1056/NEJMoa1801946
30. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer (2016) 5:275–87. doi: 10.1038/nrc.2016.36
31. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol (2016) 12:e542–e51. doi: 10.1016/S1470-2045(16)30406-5
32. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science (2018) 6411:eaar3593. doi: 10.1126/science.aar3593
33. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell (2017) 3:542. doi: 10.1016/j.cell.2017.01.010
34. Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med (2018) 10:1550–8. doi: 10.1038/s41591-018-0136-1
35. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest (2017) 8:2930–40. doi: 10.1172/JCI91190
36. Zhang Y, Li D, Feng F, An L, Hui F, Dang D, et al. Progressive and Prognosis Value of Notch Receptors and Ligands in Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Sci Rep (2017) 1:14809. doi: 10.1038/s41598-017-14897-6
37. Polychronidou G, Kotoula V, Manousou K, Kostopoulos I, Karayannopoulou G, Vrettou E, et al. Mismatch repair deficiency and aberrations in the Notch and Hedgehog pathways are of prognostic value in patients with endometrial cancer. PloS One (2018) 12:e0208221. doi: 10.1371/journal.pone.0208221
38. Liu ZY, Wu T, Li Q, Wang MC, Jing L, Ruan ZP, et al. Notch Signaling Components: Diverging Prognostic Indicators in Lung Adenocarcinoma. Medicine (2016) 20:e3715. doi: 10.1097/MD.0000000000003715
39. Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res (2005) 18:8530–7. doi: 10.1158/0008-5472.CAN-05-1069
40. Chu D, Li Y, Wang W, Zhao Q, Li J, Lu Y, et al. High level of Notch1 protein is associated with poor overall survival in colorectal cancer. Ann Surg Oncol (2010) 5:1337–42. doi: 10.1245/s10434-009-0893-7
41. Chu D, Zhang Z, Zhou Y, Wang W, Li Y, Zhang H, et al. Notch1 and Notch2 have opposite prognostic effects on patients with colorectal cancer. Ann Oncol (2011) 11:2440–7. doi: 10.1093/annonc/mdq776
42. Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer (2011) 5:338–51. doi: 10.1038/nrc3035
43. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer (2017) 5:761–73. doi: 10.7150/jca.17648
44. Mathieu M, Cotta-Grand N, Daudelin JF, Thebault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8(+) T-cell activation. Immunol Cell Biol (2013) 1:82–8. doi: 10.1038/icb.2012.53
45. Zheng X, Song X, Shao Y, Xu B, Chen L, Zhou Q, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget (2017) 34:57386–98. doi: 10.18632/oncotarget.18065
46. Chiaravalli AM, Feltri M, Bertolini V, Bagnoli E, Furlan D, Cerutti R, et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Archiv an Int J Pathol (2006) 3:344–53. doi: 10.1007/s00428-005-0066-4
47. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol (2010) 3:170–81. doi: 10.1038/nri2711
48. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol (2008) 7:523–32. doi: 10.1038/nri2343
49. Qi L, Yu H, Zhang Y, Zhao D, Lv P, Zhong Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget (2016) 44:71673–85. doi: 10.18632/oncotarget.12317
50. Liu Z, Kuang W, Zhou Q, Zhang Y. TGF-beta1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int J Mol Med (2018) 6:3395–403. doi: 10.3892/ijmm.2018.3923
51. Perrone G, Ruffini PA, Catalano V, Spino C, Santini D, Muretto P, et al. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer (2008) 13:1875–82. doi: 10.1016/j.ejca.2008.05.017
52. Huang X, Pan Y, Ma J, Kang Z, Xu X, Zhu Y, et al. Prognostic significance of the infiltration of CD163(+) macrophages combined with CD66b(+) neutrophils in gastric cancer. Cancer Med (2018) 5:1731–41. doi: 10.1002/cam4.1420
53. Anastasi E, Campese AF, Bellavia D, Bulotta A, Balestri A, Pascucci M, et al. Expression of activated Notch3 in transgenic mice enhances generation of T regulatory cells and protects against experimental autoimmune diabetes. J Immunol (2003) 9:4504–11. doi: 10.4049/jimmunol.171.9.4504
54. Barbarulo A, Grazioli P, Campese AF, Bellavia D, Di Mario G, Pelullo M, et al. Notch3 and canonical NF-kappaB signaling pathways cooperatively regulate Foxp3 transcription. J Immunol (2011) 11:6199–206. doi: 10.4049/jimmunol.1002136
55. Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science (2014) 6186:921–5. doi: 10.1126/science.1252510
56. Wang SH, Lu QY, Guo YH, Song YY, Liu PJ, Wang YC. The blockage of Notch signalling promoted the generation of polymorphonuclear myeloid-derived suppressor cells with lower immunosuppression. Eur J Cancer (2016) 68:90–105. doi: 10.1016/j.ejca.2016.08.019
57. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol (2018) 5:e180013. doi: 10.1001/jamaoncol.2018.0013
58. Frederickson AM, Arndorfer S, Zhang I, Lorenzi M, Insinga R, Arunachalam A, et al. Pembrolizumab plus chemotherapy for first-line treatment of metastatic nonsquamous non-small-cell lung cancer: a network meta-analysis. Immunotherapy (2019) 5:407–28. doi: 10.2217/imt-2018-0193
59. Neoadjuvant Nivolumab Safe, Led to Major Response Rates in Resectable Lung Cancer. Cancer (2018) 16:3283. doi: 10.1002/cncr.31672
60. Kato K, Satoh T, Muro K, Yoshikawa T, Tamura T, Hamamoto Y, et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2019) 2:344–54. doi: 10.1007/s10120-018-0899-6
Keywords: gastric cancer, NOTCH signaling, prognosis, immune tolerance, biomarker
Citation: Cui Y, Li Q, Li W, Wang Y, Lv F, Shi X, Tang Z, Shen Z, Hou Y, Zhang H, Mao B and Liu T (2021) NOTCH3 is a Prognostic Factor and Is Correlated With Immune Tolerance in Gastric Cancer. Front. Oncol. 10:574937. doi: 10.3389/fonc.2020.574937
Received: 22 June 2020; Accepted: 13 November 2020;
Published: 05 January 2021.
Edited by:
Yun Dai, Peking University First Hospital, ChinaReviewed by:
Xiuying Xiao, Shanghai JiaoTong University, ChinaHe Ren, Affiliated Hospital of Qingdao University, China
Copyright © 2021 Cui, Li, Li, Wang, Lv, Shi, Tang, Shen, Hou, Zhang, Mao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianshu Liu, bGl1dGlhbnNodTE5NjlAMTI2LmNvbQ==; Beibei Mao, bWFvYmVpYmVpemh5QDE2My5jb20=
†These authors have contributed equally to this work
 Yuehong Cui
Yuehong Cui Qian Li1†
Qian Li1† Fang Lv
Fang Lv Xinying Shi
Xinying Shi Zhaoqing Tang
Zhaoqing Tang Yingyong Hou
Yingyong Hou Henghui Zhang
Henghui Zhang Beibei Mao
Beibei Mao Tianshu Liu
Tianshu Liu