- 1Norman Cousins Center for PNI, Semel Institute for Neuroscience & Human Behavior, University of California, Los Angeles, CA, United States
- 2Department of Psychiatry & Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
- 3Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, United States
- 4Department of Evolution, Ecology, and Organismal Biology, University of California, Riverside, CA, United States
Several observational studies have found that the risk for breast cancer is significantly reduced in persons who engage in greater amounts of physical activity. Additional observational studies of breast cancer survivors indicate that greater physical activity before or after diagnosis associates with reduced disease-specific mortality. However, no large randomized controlled trials have examined the effect of structured exercise training on disease outcomes in breast cancer. Among the many hurdles in designing such trials lies the challenge of determining how a given regimen of exercise from efficacious preclinical studies can be extrapolated to an equivalent “dose” in humans to guide decisions around treatment regimen in early-phase studies. We argue that preclinical researchers in exercise oncology could better facilitate this endeavor by routinely measuring changes in exercise capacity in the subjects of their breast cancer models. VO2max, the maximal rate of whole-organism oxygen consumption during a progressive exercise test, is emphasized here because it has become a standard measure of cardiorespiratory fitness, is well-integrated in clinical settings, and scales allometrically among nonhuman animals in preclinical research and breast cancer patients/survivors in the clinic. We also conduct secondary analyses of existing whole-transcriptome datasets to highlight how greater uptake and delivery of oxygen during exercise may reverse the typically hypoxic microenvironment of breast tumors, which often associates with more aggressive disease and worse prognosis.
Introduction
Over the past 30 years, exercise therapists and other clinical investigators have conducted randomized controlled trials (RCTs) with cancer patients to examine the effect of structured exercise training on a variety of dependent variables. Although breast cancer has received the most attention, other cancer types studied include prostate, lung, colorectal, gastrointestinal, gynecologic, testicular, bladder, lymphoma, leukemia, and brain (1). The first such RCT was conducted in the late 1980s and found that a structured exercise intervention involving thrice-weekly supervised aerobic activity with breast cancer patients significantly reduced treatment- related nausea, reduced body fat, and increased physical fitness (2–4). However, spurred by emerging observational evidence that physically active women had a lower risk of developing breast cancer, clinical investigators soon began conducting RCTs to examine the effect of exercise on biomarkers that associate with breast cancer prognosis (e.g., serum insulin, C-reactive protein, immune cell activity), doing so during and/or after initial adjuvant treatment (i.e., radiotherapy and/or chemotherapy) (5, 6). Although informative, such studies have not led to any firm conclusions about the ability of structured exercise training to modulate disease outcomes in breast cancer patients (i.e., disease-free survival, cancer-specific mortality, overall mortality). More recently, a “window of opportunity” RCT examined the effect of pre-surgery exercise training on specific tumor biology parameters in excised tumors (e.g., cell proliferation, apoptosis) (7). Although informative and quite novel in its collection of pre- and post-exercise whole-genome gene expression (8), again, this study was unable to provide any firm conclusions about the ability of structured exercise training to alter the course of breast cancer disease.
Definitive conclusions about any treatment effect come from the successful completion of large phase III RCTs (9). However, as outlined by those working in exercise oncology, the path that leads to successful design of such trials contains several unique obstacles for those who wish to determine the effects of a non-pharmacological treatment, such as exercise, on cancer outcomes (10, 11). Among those obstacles lies the challenge of determining how a given regimen of exercise from preclinical studies, which may have been found to be efficacious at inhibiting a given type of tumor, can be extrapolated to an equivalent “dose” in humans to guide decisions around treatment regimen in early phase I/II studies. In conventional drug development, investigators may rely on several established approaches (derived from pharmacokinetics) to estimate an efficacious dose for humans from doses found to work in animals (12, 13). These approaches aim to achieve a pharmacodynamic response in humans that is similar to what was measured in prior supportive animal studies by accounting for known interspecies differences in the pharmacokinetics of the drug class (e.g., absorption, distribution, metabolism, excretion) and applying appropriate scaling factors related to differences in body size (12, 13). In contrast, for a behavioral treatment like exercise, such pharmacological approaches do not seem to readily apply.
Interspecies scaling factors utilized in conventional drug development have their origin in a long history of studies that have empirically determined allometric scaling equations for translating several normal physiological parameters between nonhuman animals and humans. Moreover, changes in such physiological parameters likely associate with exercise's mechanism(s) of action on cancer inhibition in both animal models and humans alike (14). Among the several physiological parameters known to associate with exercise is resting heart rate (HR), which varies allometrically with body mass (BM) among land-dwelling mammals as HR = 212 ∙ BM−0.22 beats per min (BM in kg) (15). During restful periods, the operation of HR in conjunction with other parameters—including cardiac output and respiratory minute volume—results in approximately the same percentage of oxygen being extracted from ventilated air and delivered to the body regardless of the mammal's size (16). However, the dynamics surrounding oxygen consumption change during exercise, and the overall result can be measured by another physiological parameter—the maximal rate of whole-organism oxygen consumption during a progressive exercise test (VO2max).
Translating Exercise Dose: Focus on the Response
VO2max, the maximal rate of whole-organism oxygen consumption (typically over 1 min) during a progressive exercise test, has become the standard measure of cardiorespiratory fitness and a robust indicator of exposure to routine aerobic/endurance activity (17). Investigators have long utilized VO2max to investigate the effect of exercise training on cardiorespiratory fitness and other risk factors for cardiovascular disease (CVD) [e.g., (18)]. Not surprisingly, exercise oncologists have also commonly used this measure (or the related VO2peak) to determine the effects of structured exercise interventions in numerous RCTs. A recent meta-analysis of 48 such RCTs (the most prevalent being for breast cancer) found that VO2peak in cancer patients was significantly increased by exercise therapy in comparison to cancer patients in the control group (1), consistent with many other studies of unaffected individuals (19).
Given that it serves as a robust indicator of exposure to routine aerobic/endurance activity (19), pre–post changes in measures of cardiorespiratory fitness like VO2max have been thought of as the “pharmacokinetic equivalent” for an exercise trial or as a manipulation check wherein the exercise regimen is shown to have done its job (8). This is prudent because it is becoming increasingly clear that a given regimen of exercise does not induce the same training effect across all subjects who equally complete the same regimen (be they human or animal model) (20). Individual variations in both baseline and acquired VO2max following exercise exposure have a substantial genetic component (21). However, this issue is not unique to VO2max, as several other measures of training effect show heterogeneity [e.g., running capacity, submaximal heart rate, submaximal systolic blood pressure, fasting high-density lipoprotein (HDL) cholesterol levels] (20, 22). Thus, given the supposition that global physiological alteration from greater physical activity contains the likely mediator(s) between exercise and cancer inhibition, perhaps it is less important to attempt extrapolation of the exercise regimen per se from preclinical studies and more important to extrapolate the measure of global physiological alteration.
Preclinical research in exercise oncology could better inform clinical investigation if the former began to routinely measure pre- and post-intervention exercise capacity. VO2max is emphasized here because, as noted above, it is a gold standard and has become well-integrated into clinical settings, including oncology. Exercise physiologists and clinicians alike note the power of VO2max to noninvasively and objectively determine the efficacy of aerobic, endurance-exercise training programs in clinical settings (23, 24). If more preclinical research in the exercise oncology realm did the same, then such exercise data could be analyzed in relation to a plethora of mechanistic tumor biology data that can be captured in a well-controlled preclinical experiment. More pertinent to the point at hand, the connections that are discovered with VO2max could be more readily translated for consideration at the clinical level, as VO2max cuts across all orders of the class Mammalia (25).
As with the exercise-related physiological parameters noted above, VO2max scales allometrically across mammals. The most recent large-scale empirical determination utilized phylogenetically informed statistics when analyzing a total of 77 species, including humans, and determined the relationship to be VO2max = 0.303 ∙ BM0.837 ml/min (excepting bat, horse, and pronghorn, which have unusually high values) (BM in g) (25). The VO2max of a 35-g adult mouse is predicted to be, on average, about 5.94 ml/min, which falls within the measured range for sedentary laboratory mice of this size [e.g., (26)]. But because of the fractional exponent for BM in this equation (i.e., as the species gets larger, VO2max gets larger, though less than proportionately), the VO2max of an average adult human (62 kg) is predicted to be around 3,109 ml/min, which also falls within the measured range for adult men and women in national reference standards (17).
Differences between animals in pre-intervention exercise capacity (i.e., baseline VO2max) or in the relative change at post-intervention (i.e., within-subject % increase in VO2max) could be used to test for association with tumor inhibition and/or mechanistic variables in the tumor microenvironment. For example, for one given cancer model with specific tumor biology features, it may be found that a certain minimum amount of exercise capacity, measured by VO2max (i.e., a threshold), needs to be reached by the subject in order to achieve a monolithic inhibitory effect. Conversely, a different cancer model with a different tumor biology may show that the relative increase in VO2max from baseline has tumor-inhibitory effects in a dose-response manner, or, after a certain threshold is achieved, the relative increase exerts greater amounts of tumor inhibition in a dose-response manner. In either case, the amount of VO2max found to serve as a threshold in the efficacious preclinical study may be more translatable (and relevant) than the animal exercise regimen that was used to derive the VO2max. Suppose a given tumor model is found to exhibit a threshold VO2max value that is 10% above the predicted average value for the animals in the study, given their BM. Such a finding may then suggest that future clinical investigations consider a phase I/II trial where cancer survivors (with similar residual tumor biology to that of the model) are exposed to a structured exercise regimen in the intervention arm that aims to increase (and maintain) a VO2max that is 10% above the predicted average value for each person based on each person's specific BM.
VO2max: Direct Effects on Tumor Biology?
Exercise-induced changes in cardiorespiratory fitness may not always correlate with other exercise-induced changes that might have more direct bearing for the tumor biology of a given model. Exercise oncologists, focusing on cancer treatment-induced cardiovascular toxicity, have pointed to research that finds slightly different exercise regimens can have essentially the same augmenting effect on peak oxygen consumption but differ in their salutary effects on plasma lipoproteins (27, 28). Similar divergence across different regimens for VO2max and breast cancer risk covariates (e.g., insulin, adipocytokines, inflammatory proteins, etc.) (29) may also exist.
Notwithstanding possible dissociations with VO2max, it seems appropriate to note that the primary constituent of VO2max, i.e., greater whole-body oxygen consumption, may in itself have direct effects on breast tumor biology that become substantial in the course of increasing and maintaining a relatively higher VO2max. Tumors tend to be hypoxic microenvironments, and greater tumor hypoxia is associated with poorer response to radiation treatment, poorer response to chemotherapy, greater likelihood of metastasis, and worse survival (30–32). However, in preclinical models of breast cancer, voluntary wheel running has been found to reduce tumor hypoxia (33, 34). Thus, it would appear that, in the course of blood flow alteration and greater tissue oxygenation for working muscles during physical exercise (35), there is a collateral increase in oxygenation of mammary tissue and/or the tumors that inhabit this area.
Preclinical evidence suggests that tumor vessel normalization is a key mechanism by which exercise is able to reduce hypoxia and greatly improve the antitumor effectiveness of chemotherapy in breast cancer (33). Such evidence is consistent with other research that finds tumor vessel normalization with the drug bevacizumab can improve the direct antitumor effect of chemotherapy in both early- and late-stage breast cancer (36, 37). However, the effect of bevacizumab is short-lived as drug resistance develops (38), whereas the effect of exercise may endure (33), at least if the exercise regimen is maintained. We emphasize, though, that there has been no direct comparison of exercise vs. bevacizumab on tumor vessel normalization and the effects of antitumor chemotherapy.
Other research suggests that normalizing the tumor vasculature and increasing tissue oxygenation may improve immunosurveillance and immunotherapy (39, 40). Given the less than ideal response of breast cancer to checkpoint blockade immunotherapy, which antagonizes negative feedback signaling to antitumor immune cells, investigators have speculated that greater benefit may be achieved by combining such checkpoint blockade drugs with other treatments (41). To this end, it is argued that the next generation of preclinical exercise studies in cancer should evaluate the interaction between exercise and novel immunotherapies (42). Breast cancer has long been thought of as a non-immunogenic or “immunologically cold” malignancy (43), which may be due, in part, to the high propensity of myeloid cells in breast tumors to exhibit an immunosuppressive role (44). However, emerging evidence shows that such cells may become less immunosuppressive in tissue environments that are less hypoxic as a result of greater physical activity [reviewed in (14, 45)].
We examined the effect of a structured exercise regimen for breast cancer patients on tumor hypoxia by analyzing whole transcriptome data from the innovative RCT by Ligibel et al. (7) (NCBI GEO accession number GSE129508). Patients randomized to the exercise condition completed two 60–90-min trainer-supervised exercise sessions per week over a median period of about 4 weeks and completed additional aerobic exercise on their own between supervised sessions with a pedometer to track activity amounts. Using a hypoxia gene expression signature that was developed in part from analysis of several breast cancer studies (46) (see Supplementary Data File 1), transcriptome representation analysis (TRA) (47) indicated that pre- to post-intervention change in hypoxia was more reduced in tumors of exercising patients than in the tumors of control patients, though the effect was marginal (P = 0.06) (Figure 1A). Given the meta-analytic finding that high tumor grade significantly correlates with tumor hypoxia in breast cancer patients (32), we analyzed patients with low-grade (1/2) and high-grade (3) tumors separately and found that the effect of exercise on hypoxia was significantly stronger for patients with high-grade tumor (P = 0.004) (Figure 1B).
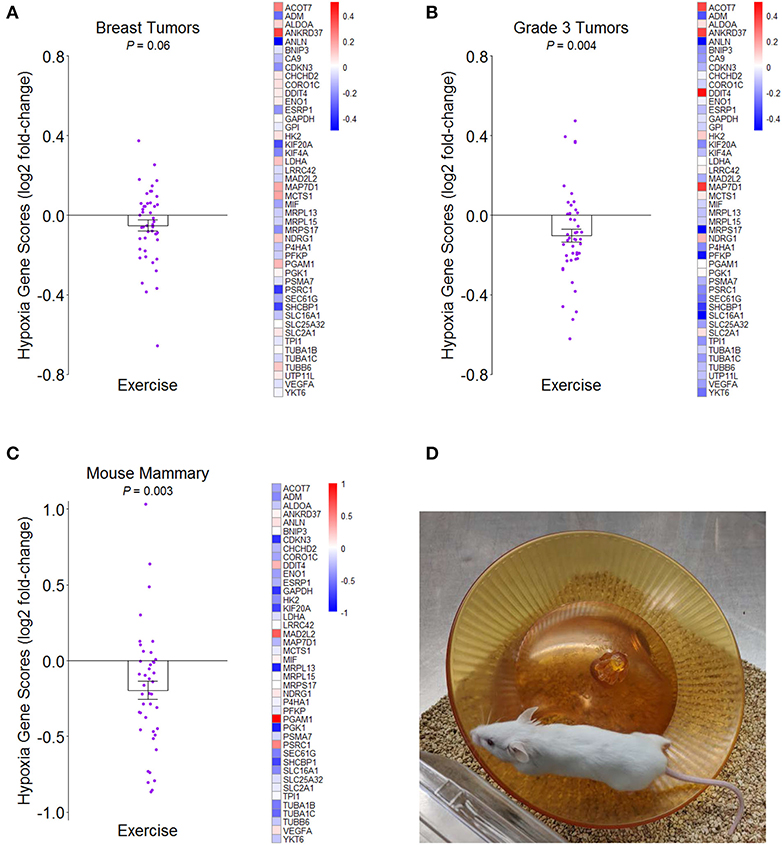
Figure 1. Effects of greater routine physical activity on hypoxia in mammary tissue. (A) Mean ± SEM fold-change for hypoxia genes from tumors of breast cancer patients randomized to exercise vs. control conditions in Ligibel et al. (7) on log2 scale. Reference hypoxia gene set from Buffa et al. (46). Distinct data points given for each gene in plot. Heatmap of mean fold-changes for distinct hypoxia genes in exercise group vs. control group on log2 scale. P-value indicates significance of difference between groups in mean change scores from transcriptome representation analysis (TRA). (B) Mean ± SEM and heatmap as in (A) but for breast cancer patients with grade 3 tumors. (C) Mean ± SEM fold-change for same reference hypoxia gene set in mammary tissues of mice randomized to exercise vs. control in NCBI GEO accession number GSE150620 on log2 scale. Heatmap of mean fold-changes for distinct hypoxia genes in exercise vs. control group on log2 scale. TRA P-value indicates significance of difference between groups in mean expression scores of genes. (D) Representative image of activity paradigm for mice in (C).
We then determined whether this same hypoxia gene expression signature would indicate less hypoxia in the mammary tissues of mice after being randomized to voluntary wheel running for 2 weeks prior to mammary cancer cell engraftment (NCBI GEO accession number GSE150620). Compared to sedentary control mice that also received mammary cancer, wheel-running mice had less hypoxia gene expression (P = 0.003) (Figures 1C,D). Together, the results suggest that greater routine physical activity can reduce hypoxia in breast tumors and surrounding mammary tissue in both humans and mice. Although VO2max was not measured in either of these studies, we speculate that it would likely correlate with tissue hypoxia in an inverse manner.
Conclusions
The evidence of a beneficial link between physical activity and breast cancer has been around for a long time. Thus, it may be somewhat surprising that a large phase III RCT for the effect of structured exercise training on disease outcomes has not been launched. By now, multiple observational studies have found that the risk for breast cancer is significantly reduced in persons who engage in greater amounts of physical activity (48), and several more observational studies of breast cancer survivors indicate that greater physical activity either before or after diagnosis associates with reduced disease-specific mortality (49, 50). Likewise, meta-analysis of preclinical exercise studies in rodent models of breast cancer (i.e., mice and rats) finds an overall inhibitory effect in this cancer type (51), even though not all preclinical exercise studies in rodents have shown efficacy across all tumor types examined thus far [see review by (42)]. Thus, it is important to note that exercise may not be as beneficial for other cancer types. Nevertheless, given the enormity of the epidemiological findings for breast cancer, the World Health Organization's IARC (International Agency for Research on Cancer) has assigned its strongest evidence designation to physical activity as a preventative factor in breast cancer (52).
However, as noted at the beginning of this Perspective article, we face several challenges to determining the effects of a non-pharmacological treatment like exercise on cancer outcomes in the clinical setting. We have focused specifically on one—the challenge of extrapolating the overall exercise dose from efficacious preclinical studies to help inform clinical treatment regimens. Currently, the basic exercise dose that is recommended for cancer survivors largely follows general physical activity guidelines for adults with chronic conditions, which aims for at least 150 min per week of aerobic activity, with two or more days per week of resistance training (53). Although VO2max may be an imperfect indicator of exercise exposure (given the heterogeneity of all physiological training effects noted above, with likely genetic contributions), we contend that its reliability, validity, and translatability make its use a highly worthy endeavor. We also think that greater use of an objective measure of exercise exposure at the preclinical level will facilitate identification of the essential biology that is at work in the effect between physical activity and cancer inhibition (for those cancer types that are responsive to exercise). Exercise oncologists presciently understand that this is another challenge facing successful clinical trial development for the effect of structured exercise training on cancer outcomes. Most large definitive trials of “nonregulated” therapies for cancer (i.e., non-pharmaceutical) have failed to show any benefit, which may likely be due to a lack of prior studies (preclinical or otherwise) successfully identifying doses and scheduling that effectively altered the relevant underlying biology (11). Thus, investigators in exercise oncology do not want to go down that same fruitless path.
To conduct a rational, optimal trial design for a given tumor type, clinical investigators will benefit from knowing what downstream biological endpoint(s) to aim for with a structured exercise regimen. They will also benefit from knowing how much downstream biological endpoint is needed to induce inhibition of the given tumor type and, then, how much exercise is needed to bring about an efficacious amount of the downstream biological endpoint. Preclinical researchers can make great contributions to this venture by measuring the effect of exercise on tumor biology in their cancer models. They can make greater contributions by also measuring the dose of exercise in their cancer models in a way that is reliable, valid, and translatable.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/ (GSE150620, GSE129508).
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board, Dana-Farber/Harvard Cancer Center. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Institutional Animal Use and Care Committee, UCLA.
Author Contributions
DL wrote the initial draft of the article, in consultation with TG. DL conducted secondary analyses on existing datasets and constructed accompanying figures for the article. TG made critical revisions to the manuscript. Both authors contributed to manuscript revision and read and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the US National Institutes of Health (K07CA188237) and the UCLA Norman Cousins Center for PNI.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.575657/full#supplementary-material
References
1. Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. (2018) 36:2297–305. doi: 10.1200/JCO.2017.77.5809
2. Winningham ML, MacVicar MG. The effect of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. (1988) 15:447–50.
3. Winningham ML, MacVicar MG, Bondoc M, Anderson JI, Minton JP. Effect of Aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. (1989) 16:683–9.
4. MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients' functional capacity. Nurs Res. (1989) 38:348–51. doi: 10.1097/00006199-198911000-00007
5. Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. (2013) 52:195–215. doi: 10.3109/0284186X.2012.742564
6. Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. (2013) 30 (Suppl.):S75–87. doi: 10.1016/j.bbi.2012.05.001
7. Ligibel JA, Dillon D, Giobbie-Hurder A, McTiernan A, Frank E, Cornwell M, et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: Results from the pre-operative health and body (PreHAB) Study. Clin Cancer Res. (2019) 25:5398–406. doi: 10.1158/1078-0432.CCR-18-3143
8. Koelwyn GJ, Jones LW. Exercise as a candidate antitumor strategy: a window into the future. Clin Cancer Res. (2019) 25:5179–81. doi: 10.1158/1078-0432.CCR-19-1318
9. Friedman LM, Furberg CD, DeMets DL, Reboussin DM, Granger CB. Fundamentals of Clinical Trials. 5th ed. New York, NY: Springer (2015).
10. Jones LW. Precision oncology framework for investigation of exercise as treatment for cancer. J Clin Oncol. (2015) 33:4134–7. doi: 10.1200/JCO.2015.62.7687
11. Iyengar NM, Jones LW. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. (2019) 5:1620–7. doi: 10.1001/jamaoncol.2019.2585
12. Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. (2012) 11:751–61. doi: 10.1038/nrd3801
13. Mahmood I. Misconceptions and issues regarding allometric scaling during the drug development process. Expert Opin Drug Metab Toxicol. (2018) 14:843–54. doi: 10.1080/17425255.2018.1499725
14. Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. (2017) 17:620–32. doi: 10.1038/nrc.2017.78
15. Mortola JP. The heart rate - breathing rate relationship in aquatic mammals: A comparative analysis with terrestrial species. Curr Zool. (2015) 61:569–77. doi: 10.1093/czoolo/61.4.569
16. Lindstedt SL, Schaeffer PJ. Use of allometry in predicting anatomical and physiological parameters of mammals. Lab Anim. (2002) 36:1–19. doi: 10.1258/0023677021911731
17. Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R. A reference equation for normal standards for VO2 max: analysis from the fitness registry and the importance of exercise national database (FRIEND Registry). Prog Cardiovasc Dis. (2017) 60:21–9. doi: 10.1016/j.pcad.2017.03.002
18. Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, et al. Dose response to exercise in women aged 45-75 yr (DREW): design and rationale. Med Sci Sports Exerc. (2004) 36:336–44. doi: 10.1249/01.MSS.0000113738.06267.E5
19. Batacan RB Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. (2017) 51:494–503. doi: 10.1136/bjsports-2015-095841
20. Ross R, Goodpaster BH, Koch LG, Sarzynski MA, Kohrt WM, Johannsen NM, et al. Precision exercise medicine: understanding exercise response variability. Br J Sports Med. (2019) 53:1141–53. doi: 10.1136/bjsports-2018-100328
21. Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985). (1999) 87:1003–8. doi: 10.1152/jappl.1999.87.3.1003
22. Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. (2001) 33:S446–51. doi: 10.1097/00005768-200106001-00013
23. Poole DC, Jones AM. Measurement of the maximum oxygen uptake Vo 2max: Vo 2peak is mo longer acceptable. J Appl Physiol (1985). (2017) 122:997–1002. doi: 10.1152/japplphysiol.01063.2016
24. Williams CA, Saynor ZL, Barker AR, Oades PJ, Tomlinson OW. Measurement of Vo2max in clinical groups is feasible and necessary. J Appl Physiol (1985). (2017) 123:1017. doi: 10.1152/japplphysiol.00538.2017
25. Dlugosz EM, Chappell MA, Meek TH, Szafranska PA, Zub K, Konarzewski M, et al. Phylogenetic analysis of mammalian maximal oxygen consumption during exercise. J Exp Biol. (2013) 216:4712–21. doi: 10.1242/jeb.088914
26. Swallow JG, Garland T Jr, Carter PA, Zhan WZ, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). J Appl Physiol (1985). (1998) 84:69–76. doi: 10.1152/jappl.1998.84.1.69
27. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. (2018) 137:1176–91. doi: 10.1161/CIRCULATIONAHA.117.024671
28. Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. (2002) 347:1483–92. doi: 10.1056/NEJMoa020194
29. Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2017) 26:355–65. doi: 10.1158/1055-9965.EPI-16-0602
30. Harris AL. Hypoxia–a aey regulatory factor in tumour growth. Nat Rev Cancer. (2002) 2:38–47. doi: 10.1038/nrc704
31. Daimiel I. Insights into hypoxia: non-invasive assessment through imaging modalities and its application in breast cancer. J Breast Cancer. (2019) 22:155–71. doi: 10.4048/jbc.2019.22.e26
32. Zhao Z, Mu H, Li Y, Liu Y, Zou J, Zhu Y. Clinicopathological and prognostic value of hypoxia-inducible factor-1α in breast cancer: A meta-analysis including 5177 patients. Clin Transl Oncol. (2020) 22:1892–906. doi: 10.1007/s12094-020-02332-8
33. Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. (2015) 107:djv040. doi: 10.1093/jnci/djv040
34. Buss LA, Dachs GU. Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE(-/-) mice. J Appl Physiol (1985). (2018) 24:938–49. doi: 10.1152/japplphysiol.00738.2017
35. Korthuis RJ. Chapter 4: Exercise hyperemia and regulation of tissue oxygenation during muscular activity. In: Skeletal Muscle Circulation. San Rafael, CA: Morgan & Claypool Life Sciences (2011).
36. Trédan O, Lacroix-Triki M, Guiu S, Mouret-Reynier MA, Barrière J, Bidard FC, et al. Angiogenesis and tumor microenvironment: bevacizumab in the breast cancer model. Target Oncol. (2015) 10:189–98. doi: 10.1007/s11523-014-0334-9
37. Arjaans M, Schröder CP, Oosting SF, Dafni U, Kleibeuker JE, de Vries EG. VEGF pathway targeting agents, vessel normalization and tumor drug uptake: from bench to bedside. Oncotarget. (2016) 7:21247–58. doi: 10.18632/oncotarget.6918
38. Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. (2011) 10:417–27. doi: 10.1038/nrd3455
39. Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. (2008) 453:410–4. doi: 10.1038/nature06868
40. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. (2017) 544:250–4. doi: 10.1038/nature21724
41. Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res. (2017) 23:2640–6. doi: 10.1158/1078-0432.CCR-16-2569
42. Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. (2016) 76:4032–50. doi: 10.1158/0008-5472.CAN-16-0887
43. Bense RD, Sotiriou C, Piccart-Gebhart MJ, Haanen JBAG, van Vugt MATM, de Vries EGE, et al. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst. (2016) 109:djw192. doi: 10.1093/jnci/djw192
44. Kim IS, Gao Y, Welte T, Wang H, Liu J, Janghorban M, et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol. (2019) 21:1113–26. doi: 10.1038/s41556-019-0373-7
45. Buss LA, Dachs GU. Effects of exercise on the tumour microenvironment. Adv Exp Med Biol. (2020) 1225:31–51. doi: 10.1007/978-3-030-35727-6_3
46. Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer. (2010) 102:428–35. doi: 10.1038/sj.bjc.6605933
47. Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. (2013) 110:16574–9. doi: 10.1073/pnas.1310655110
48. Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. (2016) 176:816–25. doi: 10.1001/jamainternmed.2016.1548
49. Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. (2012) 104:815–40. doi: 10.1093/jnci/djs207
50. Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. (2016) 22:4766–75. doi: 10.1158/1078-0432.CCR-16-0067
51. Figueira ACC, Cortinhas A, Soares JP, Leitão JC, Ferreira RP, Duarte JA. Efficacy of exercise on breast cancer outcomes: a systematic review and meta-analysis of preclinical data. Int J Sports Med. (2018) 39:327–42. doi: 10.1055/s-0044-101149
52. Vainio H, Bianchini F. (eds). IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity. Vol. 6. Lyon: IARC Press (2002).
Keywords: breast cancer, exercise dose, exercise oncology, physical activity, preclinical research, translational science, tumor hypoxia, VO2max
Citation: Lamkin DM and Garland T Jr (2020) Translating Preclinical Research for Exercise Oncology: Take It to the VO2max. Front. Oncol. 10:575657. doi: 10.3389/fonc.2020.575657
Received: 24 June 2020; Accepted: 21 August 2020;
Published: 02 October 2020.
Edited by:
Imtiaz Ahmad Siddiqui, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Neetika Khurana, Northern Illinois University, United StatesAbhishek Roy, Virginia Commonwealth University, United States
Copyright © 2020 Lamkin and Garland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donald M. Lamkin, ZGxhbWtpbkB1Y2xhLmVkdQ==
 Donald M. Lamkin
Donald M. Lamkin Theodore Garland Jr.4
Theodore Garland Jr.4