- 1Department of Hematology, First Affiliated Hospital of Soochow University, Jiangsu Institute of Hematology, National Clinical Research Center for Hematologic Diseases, Suzhou, China
- 2Department of Thrombosis and Hemostasis, Key Laboratory of Thrombosis and Hemostasis of Ministry of Health, Suzhou, China
- 3Institute of Blood and Marrow Transplantation, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, China
- 4Department of Internal Medicine, Yale New Haven Health/Bridgeport Hospital, Bridgeport, CT, United States
Philadelphia chromosome positive (Ph+) in T-lineage acute lymphoproliferative tumors is a rare event in both children and adults. In particular, it has not been reported in T-cell lymphoblastic lymphoma(T-LBL) yet. Here, we describe a patient with Ph+ T-LBL for both cytogenetic abnormality and BCR-ABL1 fusion transcript. Moreover, we review the published cases of Ph+ T-cell acute lymphoblastic leukemia (T-ALL) in the literature and summarize their clinical characteristics, management, and prognosis.
Introduction
Ph+ is the most common cytogenetic abnormality in chronic myeloid leukemia (CML) as well as in a subset of B-lineage acute lymphoblastic leukemias (B-ALL), occurring in about 95 and 30–40% of adult cases, respectively (1, 2). In addition, it can be detected in 2–5% of children with B-ALL and in rare cases of B-lineage lymphoma and acute myelogenous leukemia (AML) (3–8). In several cases, Ph+ may appear in leukemia cells during the course of the disease (9–11). Its presence is an important poor prognostic indicator in children as well as in adults (6, 12, 13), which is associated with short-term of complete remission (CR) and high rate of relapse. Actually, it has been reported that leukemogenesis in Ph+ malignancies is a multi-step process which is characterized by an aggressive presentation and a poor outcome, particularly in T-lineage disorders (2).
T-LBL is a rare and aggressive neoplasm of precursor lymphoblast that occurs predominantly in adolescents and young adults. It is characterized by multiple enlarged lymph nodes and proliferation of immature T lymphoblasts (14, 15). Currently, the most common cytogenetic abnormalities in T-LBL appear in the 14q11–13 region, the site of the T cell receptor (TCR)-alpha (TRA) and TCR-delta (TRD) genes (16).
Here we presented an extremely rare case of T-LBL. To our knowledge, this is the first report of de novo T-LBL with Ph+. In addition, T-ALL accompanied by Ph+ is also a rare event, in which the clinical relevance and the role in leukemogenesis of this translocation are currently unclear. Accordingly, we review the reported cases with Ph+ T-ALL in this article.
Case Report
A 46-year-old male with past medical history of hypertension presented with two-month history of cervical adenopathy. His family members had no history of genetic diseases and similar diseases. Physical examination revealed bilateral multiple enlarged lymph nodes in his neck and axillae. The largest lymph node was located in the left side of the neck (3.0 cm * 3.0 cm), which was firm, fixed, and non-tender.
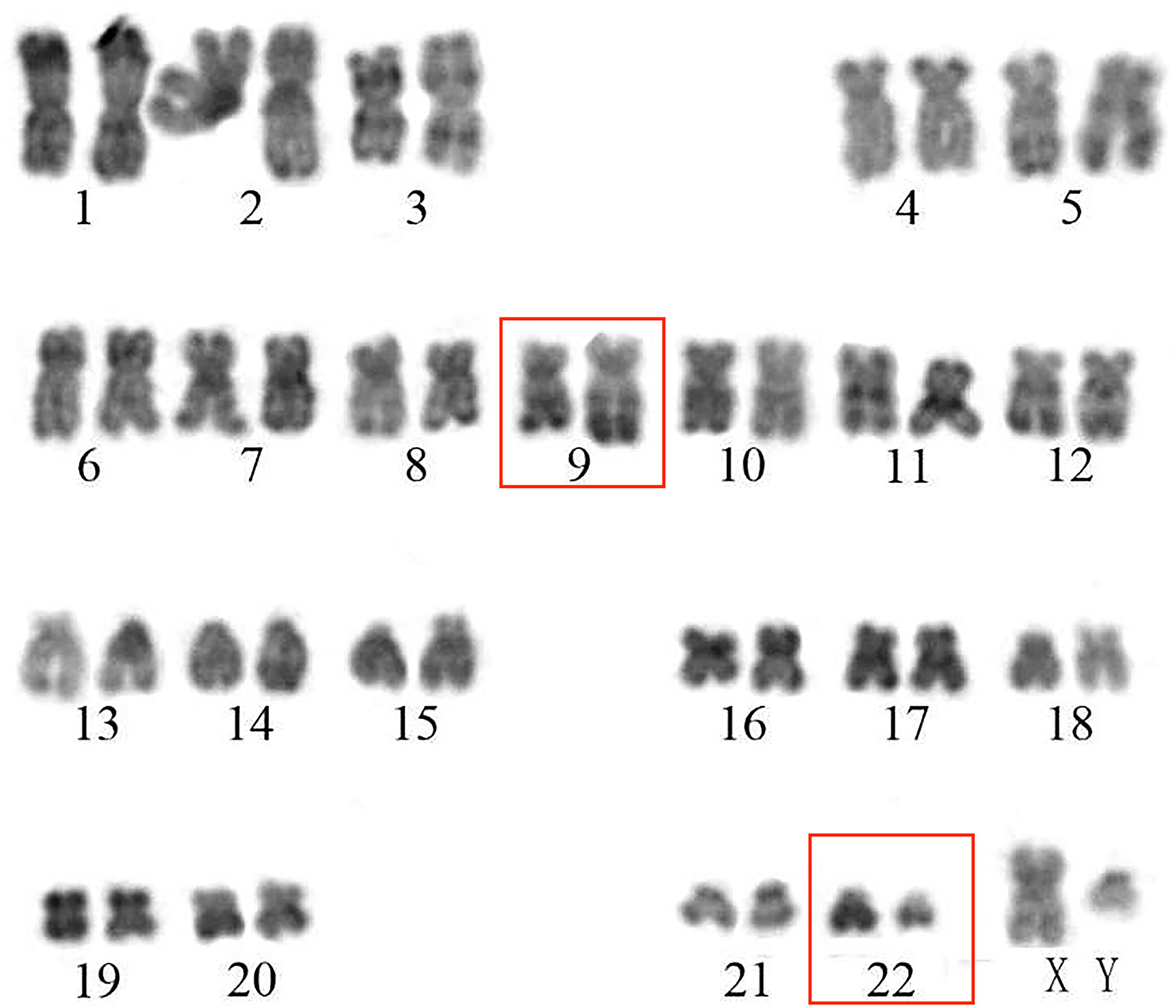
On admission, his complete blood count and metabolic panel were normal. Positron emission tomography (PET) showed the presence of fluorodeoxyglucose avid uptake in multiple parts, including the posterior peritoneum, pelvis, groin, bilateral submaxillary, cervical, and axillary lymph nodes, along with a similar uptake in the 6th anterior rib on the right and the left iliac bone, which was considered as likely lymphoma infiltration. In addition, there was no mediastinal mass or involvement of the central nervous system (CNS) in this patient at diagnosis. The biopsy of the left cervical lymph node (biopsy was completed in other hospital before admission) showed diffuse infiltration with TdT-positive lymphoblasts expressing CD3, BCL2, MYC, and Ki-67. Myeloperoxidase (MPO) and CD20 were negative. Bone marrow (BM) aspiration and flow cytometry analysis revealed a 15.5 and 22% infiltration of immature T-lineage lymphoblastic cells, respectively. Immunophenotype showed a T-lineage phenotype (CD7, CD34, cCD3, CD5), which was similar to that of the lymph node tissue (Figure 1). TCR beta (TRB), TRD, TCR gamma (TRG), immunoglobulin heavy (IgH), light chains kappa (IgK), and lambda (IgL) rearrangement were negative. Cytogenetic analysis was implemented with R-banding showed a noncomplex karyotype: 46XY, (9:22) (q34;q11) [1]/46, XY, [19] (Figure 2), and fluorescence in situ hybridization (FISH) confirmed the presence of a translocation of 9q34 (ABL1) to 22q11 (BCR) in 18% of the nuclei (300 nuclei were analyzed) (Figure 3). Multiplex polymerase chain reaction (PCR) showed the e1a2 BCR-ABL1 fusion transcript. Next-generation sequencing (NGS) revealed DNA methyltransferase 3 alpha (DNMT3A c.2645G>C p.Arg882Pro, mutation rate is 2.3%) and mediator complex subunit 12 (MED12 c.4278G>A p.Trp1426, mutation rate is 4.3%) gene mutation. Diagnosis of T-cell lymphoblastic lymphoma was made based on his clinical presentation, histological and immunological evaluation of lymph node specimens and bone marrow. He was in stage IV according to the Ann Arbor system.
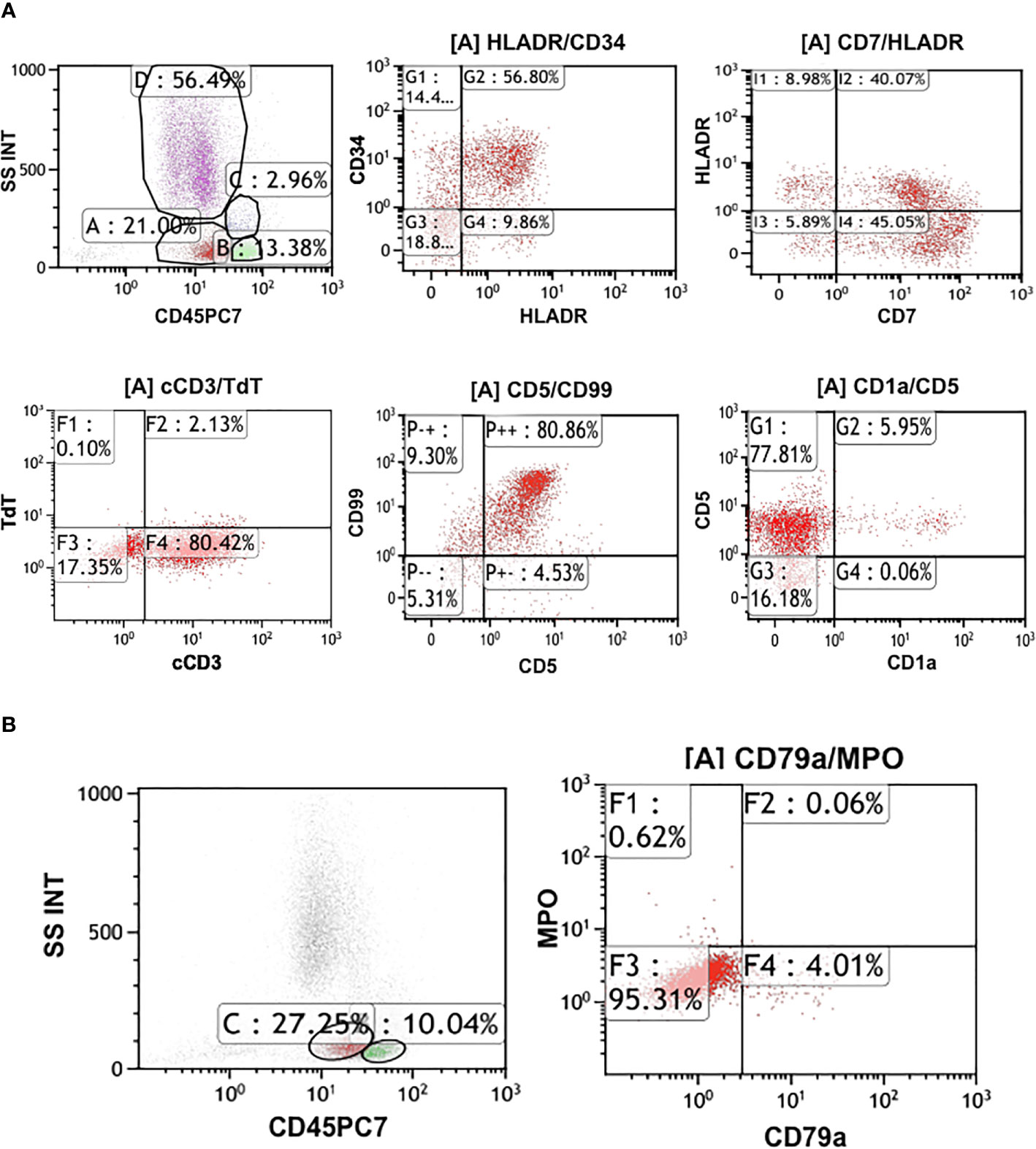
Figure 1 (A) Flow cytometry analysis of the patient. It shows approximately 22% infiltration of immature T-lineage lymphoblastic cells. Immunophenotype presented CD7, CD34, cCD3, CD5 are positive. (B) The immunophenotype of the flow cytometry showed CD79a, MPO are negative.
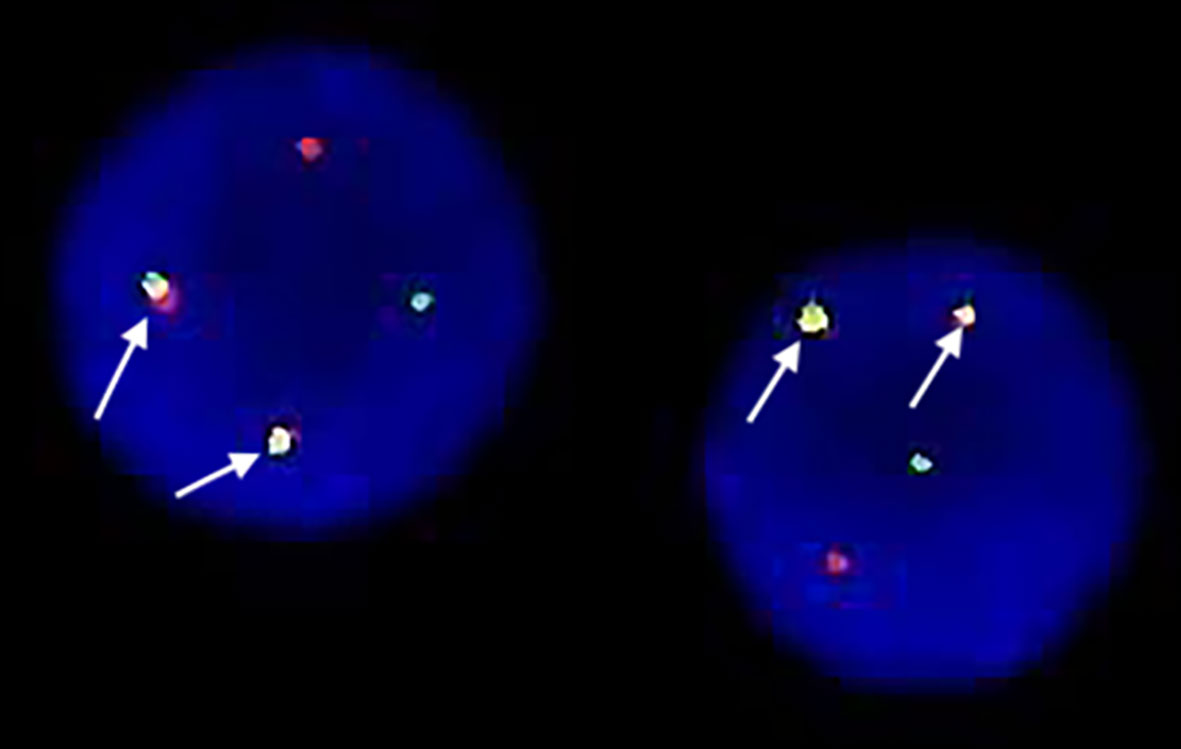
Figure 3 Fluorescence in-situ hybridization(FISH) showed location of the probes. One green BCR signal, one red ABL signal and two yellow fusion signals: BCR-ABL and ABL-BCR indicating the presence of BCR-ABL translocation in the lymphoblasts.
The patient received initial induction with hyper CVAD regimen: hyper-fractionated cyclophosphamide (CTX) given 300 mg/m2 intravenously over 2 h every 12 h for 6 doses on days 1 to 3 with 600 mg/m2 Mesna per day intravenously via continuous infusion on days 1 to 3 beginning 1 h prior to CTX and completed by 12 h after the last dose of CTX; 2.5 mg/m2 vincristine intravenously on days 4 and 11; 50 mg/m2 doxorubicin intravenously over 24 h via central venous catheter on day 4; and 40 mg dexamethasone daily intravenously on days 1 to 4 and days 11 to 14 (17–23). In addition, dasatinib 100 mg daily was started as soon as cytogenetic analysis and FISH examination verified the existence of Ph chromosome. Complete hematological and cytogenetic remission was achieved after the induction chemotherapy, but nested real time-polymerase chain reaction (RT-PCR) was still positive for the BCR-ABL1 (ela2) transcript. The enlarged lymph nodes located at groins and posterior peritoneal resolved, but adenopathy still existed in the cervical and axillary regions after the induction chemotherapy. Later, the patient achieved complete molecular remission (CMR) after the first consolidation therapy. Meanwhile, the patient underwent CNS prophylaxis with the triple intrathecal therapy (methotrexate, cytarabine, dexamethasone). He then underwent an allogeneic hematopoietic stem cell transplantation (allo-HSCT) from a HLA-haploidentical daughter with the improved TBI/CY conditioning regimen (total body irradiation 8 Gy on days −8 to −6, 2g/m2 cytarabine intravenously over 3 h on day −5, CTX was given 1.8 g/m2 intravenously over 3 h on days −4 to −3 with 600 mg/m2 Mesna per day intravenously via continuous infusion on days −4 to −3 beginning 1 h prior to CTX and completed by 12 h after the last dose of CTX). There were no major complications, and he is currently alive in continuous CMR 2 years after allo-HSCT.
Discussion
Ph+ has always been considered as a poor prognostic factor of patients with ALL and is treated with intensive therapy to achieve remission. This patient is, to our knowledge, the first case of de novo T-LBL with Ph+. Although there are differences between the T-LBL and T-ALL in gene expression and immunophenotypes, their clinical characteristics and response to chemotherapy or HSCT are very similar. Subsequently, we reviewed and summarized the clinical characteristics, additional cytogenetic abnormalities, therapeutic regimens, and outcome of reported cases of Ph+ T-ALL. Currently, a total of 30 cases were reported (Table 1). Specifically, it appears to be male-predominant with 25 males and 5 females. 13 cases were children with a median age of 8 years old (range from 5 to 17), while 17 cases were adults with a median age of 47 years old (range from 18 to 72). There were seven cases presented with an anterior mediastinal mass on X-ray or computed tomography (CT). Almost all cases were found to have the Ph+ at the initial diagnosis except for three cases, in which Ph+ was detected when the disease relapsed. Moreover, according to our literature review, BCR-ABL1 fusion transcripts were analyzed in 21 cases. The most common BCR-ABL1 fusion gene type was minor breakpoint transcript (m-bcr) which presented in 18 cases. One patient had two types of BCR-ABL1 transcripts. The majority of patients achieved CR following induction chemotherapy. Five patients received treatment of combination of chemotherapy and TKIs. 10 cases with or without CR1 underwent HSCT. The overall prognosis was dismal. Only nine patients were alive until the last follow-up, and 15 patients were reported dead with a median survival of 7 months (range from 0.1 to 60). However, induction chemotherapy with a combination of hyper-CVAD and TKIs may prolong the CR duration and survival in some patients.
Strikingly, it is worthwhile noting some special cases among these published documents. For instance, Ragg et al. (24) reported a case with Ph+ T-ALL and proposed hypothesis that BCR-ABL1 fusion may occur in early lymphoid progenitor. Monma et al. (25) reported the first case of bilineage T-ALL and AML with Ph+. They proposed the mechanism that both T and myeloid cells had BCR-ABL1 fusion gene and the same clonal rearrangement of the TCR gene may result from the original leukemic clone with the BCR-ABL1 fusion gene derived from the precursor T cells and transformed into myelomonoblasts. Abla et al. (26) represented a case of an adolescent, who presented with sudden onset pancytopenia and septic shock with multiorgan dysfunction and finally was diagnosed as T-ALL with BCR-ABL1 fusion transcript. However, the parvovirus B19 was detected in his BM by PCR analysis. They speculated that the fulminant presentation of ALL may be associated with parvovirus B19 infection. Miller et al. (10) documented a case of childhood T-ALL with late developing Ph+ at relapse whereas it was negative at the initial diagnosis in 1984. Despite intensive chemotherapy, the patient achieved partial remission (PR) and relapsed again 1 month later. He died 11 months after presentation because of leukemia progression. Consistently, Tchirkov et al. (9) also found a child of T-ALL who was discovered to have the BCR-ABL1 (b2a2) transcript at disease relapse. Despite achieving a second remission, the patient relapsed again 3 months later and died of leukemia progression 14 months after presentation. Rapidly elevated number of BCR-ABL1 transcripts at the second relapse and dismal outcome might reflect the close association between the BCR-ABL1-positive leukemic clone and progression of the disease. Both pediatric patients were found to have the Ph+ at the time of relapse, but the results were negative at the initial diagnosis. The disease progressed rapidly with poor response to the treatment. The late development of the Ph+ might reflect that leukemogenesis in Ph+ T-ALL is a multi-step process.
Accordingly, whether the BCR-ABL1 fusion in acute leukemia accompanied with T-cell characteristics derives from either CML with T-cell blastic phase (CML-BP) or de novo T-ALL remains controversial. In fact, the work of Preetesh et al. showed that it was about 0.01% patients had T-cell lymphoid CML-BP and approximately 1.3% patients with de novo Ph+ T-ALL (27). In addition, it’s really difficult to distinguish between T-cell lymphoid CML-BP vs de novo T-ALL. However, patients tend to be diagnosed with CML-BP when the following clinical traits present: history of prior CML, presence of non-ela2 BCR-ABL1 transcripts, adult age group, extramedullary disease, massive splenomegaly, presence of increased number of residual circulating granulocytic precursors, eosinophils and basophils, presence of major bcr-abl breakpoint transcript, absence of lymphoblastic leukemia in BM, and excellent response to chemotherapy with TKIs (27, 28).
Interestingly, Wei et al. reported a case presenting with lymphadenopathy two months after the diagnosis of Ph+ CML in the chronic phase. The biopsy of the lymph node indicated an extramedullary blast crisis resembling T-LBL. However, the bone marrow cytology and biopsy still revealed CML and did not show T-cell lymphoblasts. Finally, this patient was diagnosed as CML resembling T-LBL and achieved complete remission after treatment with dasatinib and systemic chemotherapy (hyper-CVAD) (29).
In our case, the patient had no history of prior CML. He presented with multiple enlarged lymph nodes without any other symptoms, and the complete blood count was normal. BM aspiration and flow cytometry analysis revealed the infiltration of immature T-lineage lymphoblastic cells. FISH analysis showed the BCR-ABL1 fusion gene within the blastic tumor cell nuclei. Moreover, Raanani et al. described that none of the CML-BP cases tested by RT-PCR showed the m-bcr and a p190 fusion protein. In line with this point, Shailendra et al. proposed that none of the CML cases in T-cell lymphoblastic crisis showed bcr-abl involving the m-bcr by RT-PCR (28). Nevertheless, most patients with Ph+ ALL have breakpoints in the m-bcr (30). The result of RT-PCR in our case revealed m-bcr, which is inconsistent with the diagnosis of CML-BP. Furthermore, all cases of T-ALL showed medullary involvement with lymphoblastic leukemia while only in about half of the cases of CML-BP (30). Meanwhile, the lymph node biopsy, bone marrow cytology, and flow cytometry analysis also supported the diagnosis of T-LBL. Therefore, the diagnosis of de novo T-LBL is clear after excluding the extramedullary blast phase of CML. The good response to the treatment of our case supports the indication of combining chemotherapy with dasatinib for Ph+ T-LBL, but the long-term therapeutic effect is still under observation.
Also, it should be noted that Ph+ may be underestimated because the results of conventional karyotype of most patients were normal (31, 32). Therefore, FISH and molecular examination should be included in routine diagnostic process to detect the existence of BCR-ABL1 fusion so that we can figure out lineage-specific population and have a better understanding of the characteristic of the BCR-ABL1-positive leukemia subtypes. Moreover, the presence of additional cytogenetic abnormalities is common and can provide prognostic value (33).
Although intensive chemotherapy can improve remission, it can also cause severe complications, which shorten the remission duration (34). The outcome of patients with Ph+ ALL improved substantially with the introduction of the TKIs (35). Currently, plenty of evidence validated that imatinib-based regimens provided significantly enhanced CR (36–38). For older patients, the dose of induction therapy may be reduced due to side effects. The balance between benefits and adverse effects of the chemotherapy should be evaluated in the elderly patients (33). In addition, CNS prophylaxis should be administered during the treatment. Dombret et al. recapitulated that allo-HSCT in the first CR was the best treatment option in adults with Ph+ ALL currently (37). Chiaretti et al. reported a scheme based on imatinib plus steroids as induction followed by consolidation with HAM regimen (cytarabine, mitoxantrone and granulocyte colony-stimulating factor) chemotherapy plus imatinib with or without HSCT. The results showed 96% patients achieved CR with decreased deaths and reduced toxicity, which provide an effective and safe induction treatment for adult Ph+ ALL (39). Accordingly, it has been proposed that autologous hematopoietic cell transplantation (auto-HCT) can be a potential option for Ph+ ALL since the induction of TKIs and precise monitoring of the minimal residual disease (MRD) (40, 41). On the other hand, Daver et al. presented the results of the 13-year follow-up on their previous study of hyper-CVAD regimen and imatinib with Ph+ ALL. They confirmed the effectiveness of the above therapeutic avenue and proposed HSCT may not be beneficial to all patients with Ph+ ALL because the median overall survival was not significantly improved for patients who underwent transplantation. Nevertheless, they recommended regular monitoring of MRD and early consideration of allo-HSCT for patients with residual molecular disease at 3 months (42).
In addition, Cazzaniga et al. suggested that a better MRD response was associated with a more favorable outcome in patients in both good and poor risk groups. Accordingly, they proposed MRD monitoring might be beneficial to optimize the use of TKIs and help select patients who need allo-HSCT in CR (43). Molecular detections such as RT-PCR for BCR-ABL1 transcripts or TCR gene rearrangements or amplicon-based NGS of TCR seem to be more sensitive than traditional cytogenetic analysis (44, 45).
In summary, we described a case of T-LBL with Ph+ for the first time. This patient showed a favorable outcome after receiving chemotherapy with dasatinib followed by allo-HSCT, but the long-term efficacy is still under investigation. Moreover, much more work is needed to understand the clinical characteristics, underlying genetic lesions, response to treatment strategy and prognosis in Ph+ diseases, especially in T-ALL and T-LBL.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XL and NP collected the clinical data and wrote the paper. YW, LG, LZ, XX, ZZ, and ZBZ provided patient care. YX gave some advice for this manuscript writing. CR and DW presented amendments and suggestion for the paper. SC and ZJ had full access to all data and carried final responsibility for submitting the paper for publication. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grant from the National Key R&D Program of China (2019YFA0111000), the National Natural Science Foundation of China (81570138, 81570139, 81600116, 81600114, 81700140, 81873449, 81970142, 81900130, 81970136), the Natural Science Foundation of the Jiangsu Higher Education Institution of China (18KJA320005), the Natural Science Foundation of Jiangsu Province (BK20190180), China Postdoctoral Science Foundation (2018M632372), the priority academic program development of Jiangsu Higher Education Institution, the Innovation Capability Development Project of Jiangsu Province (BM2015004), Jiangsu Provincial Key Medical Center (YXZXA2016002) and National Science and Technology Major Project (2017ZX09304021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the patient and the staff of the Hematology Department of the First Affiliated Hospital of Soochow University and Jiangsu Institute of Hematology. We thank the sample from Jiangsu Biobank of Clinical Resources.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.584149/full#supplementary-material
Supplementary Figure 1 | The picture of bone marrow (BM) aspiration analysis.
Supplementary Figure 2 | Additional flow cytometry analysis of the BM.
Supplementary Figure 3 | A table showcasing a timeline with relevant data from the episode of care.
References
1. Liu-Dumlao T, Kantarjian H, Thomas DA, O’Brien S, Ravandi F. Philadelphia-positive acute lymphoblastic leukemia: current treatment options. Curr Oncol Rep (2012) 14(5):387–94. doi: 10.1007/s11912-012-0247-7
2. Lo Nigro L, Sainati L, Mirabile E, Lanciotti M, Poli A, Leszl A, et al. Association of cytogenetic abnormalities with detection of BCR-ABL fusion transcripts in children with T-lineage lymphoproliferative diseases (T-ALL and T-NHL). Pediatr Blood Cancer (2004) 42(3):278–80. doi: 10.1002/pbc.10453
3. Preti HA, O’Brien S, Giralt S, Beran M, Pierce S, Kantarjian HM. Philadelphia-chromosome-positive adult acute lymphocytic leukemia: characteristics, treatment results, and prognosis in 41 patients. Am J Med (1994) 97(1):60–5. doi: 10.1016/0002-9343(94)90049-3
4. Westbrook CA, Hooberman AL, Spino C, Dodge RK, Larson RA, Davey F, et al. Clinical significance of the BCR-ABL fusion gene in adult acute lymphoblastic leukemia: a Cancer and Leukemia Group B Study (8762). Blood (1992) 80(12):2983–90. doi: 10.1182/blood.V80.12.2983.bloodjournal80122983
5. Schrappe M, Arico M, Harbott J, Biondi A, Zimmermann M, Conter V, et al. Philadelphia chromosome-positive (Ph+) childhood acute lymphoblastic leukemia: good initial steroid response allows early prediction of a favorable treatment outcome. Blood (1998) 92(8):2730–41. doi: 10.1182/blood.V92.8.2730.
6. Bloomfield CD, Goldman AI, Alimena G, Berger R, Borgstrom GH, Brandt L, et al. Chromosomal abnormalities identify high-risk and low-risk patients with acute lymphoblastic leukemia. Blood (1986) 67(2):415–20. doi: 10.1182/blood.V67.2.415.415
7. Mitani K, Sato Y, Tojo A, Ishikawa F, Kobayashi Y, Miura Y, et al. Philadelphia chromosome positive B-cell type malignant lymphoma expressing an aberrant 190 kDa bcr-abl protein. Br J Haematol (1990) 76(2):221–5. doi: 10.1111/j.1365-2141.1990.tb07875.x
8. Fujii H, Yashige H, Misawa S, Tanaka S, Urata Y, Matuyama F. Ph chromosome in a patient with non-leukemic non-Hodgkin B-cell lymphoma. Am J Hematol (1990) 35(3):213–5. doi: 10.1002/ajh.2830350315
9. Tchirkov A, Bons JM, Chassagne J, Schoepfer C, Kanold J, Briancon G, et al. Molecular detection of a late-appearing BCR-ABL gene in a child with T-cell acute lymphoblastic leukemia. Ann Hematol (1998) 77(1-2):55–9. doi: 10.1007/s002770050412
10. Miller BA, Reid MM, Nell M, Lipton JM, Sallan SE, Nathan DG, et al. T-cell acute lymphoblastic leukaemia with late developing Philadelphia chromosome. Br J Haematol (1984) 56(1):139–46. doi: 10.1111/j.1365-2141.1984.tb01279.x
11. Coad JE, Arthur DC, Gajl-Peczalska KJ, Litz CE. Late-developing Philadelphia chromosomes in a case of T-cell acute lymphoblastic leukemia. Leukemia (1994) 8(5):889–94.
12. Burke MJ, Cao Q, Trotz B, Weigel B, Kumar A, Smith A, et al. Allogeneic hematopoietic cell transplantation (allogeneic HCT) for treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer (2009) 53(7):1289–94. doi: 10.1002/pbc.22263
13. Fletcher JA, Lynch EA, Kimball VM, Donnelly M, Tantravahi R, Sallan SE. Translocation (9,22) is associated with extremely poor prognosis in intensively treated children with acute lymphoblastic leukemia. Blood (1991) 77(3):435–9. doi: 10.1182/blood.V77.3.435.bloodjournal773435
14. Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol (2016) 96(5):447–60. doi: 10.1111/ejh.12722
15. Portell CA, Sweetenham JW. Adult lymphoblastic lymphoma. Cancer J (2012) 18(5):432–8. doi: 10.1097/PPO.0b013e31826b1232
16. Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol (2011) 79(3):330–43. doi: 10.1016/j.critrevonc.2010.12.003
17. Towatari M, Yanada M, Usui N, Takeuchi J, Sugiura I, Takeuchi M, et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Blood (2004) 104(12):3507–12. doi: 10.1182/blood-2004-04-1389
18. Thomas DA, Faderl S, Cortes J, O’Brien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood (2004) 103(12):4396–407. doi: 10.1182/blood-2003-08-2958
19. Thomas DA, O’Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood (2004) 104(6):1624–30. doi: 10.1182/blood-2003-12-4428
20. Hunault M, Truchan-Graczyk M, Caillot D, Harousseau JL, Bologna S, Himberlin C, et al. Outcome of adult T-lymphoblastic lymphoma after acute lymphoblastic leukemia-type treatment: a GOELAMS trial. Haematologica (2007) 92(12):1623–30. doi: 10.3324/haematol.10882
21. Ravandi F, O’Brien S, Thomas D, Faderl S, Jones D, Garris R, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood (2010) 116(12):2070–7. doi: 10.1182/blood-2009-12-261586
22. Ravandi F, O’Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer (2015) 121(23):4158–64. doi: 10.1002/cncr.29646
23. Hoelzer D, Gokbuget N, Digel W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood (2002) 99(12):4379–85. doi: 10.1182/blood-2002-01-0110
24. Ragg S, Zehentner BK, Loken MR, Croop JM. Evidence for BCR/ABL1-positive T-cell acute lymphoblastic leukemia arising in an early lymphoid progenitor cell. Pediatr Blood Cancer (2019) 66(9):e27829. doi: 10.1002/pbc.27829
25. Monma F, Nishii K, Ezuki S, Miyazaki T, Yamamori S, Usui E, et al. Molecular and phenotypic analysis of Philadelphia chromosome-positive bilineage leukemia: possibility of a lineage switch from T-lymphoid leukemic progenitor to myeloid cells. Cancer Genet Cytogenet (2006) 164(2):118–21. doi: 10.1016/j.cancergencyto.2005.06.021
26. Abla O, Gassas A, Stevens R, Grant R, Abdelhaleem M. bcr-abl-positive T-cell acute lymphoblastic leukemia associated with parvovirus B19 infection. J Pediatr Hematol Oncol (2006) 28(2):98–9. doi: 10.1097/01.mph.0000199589.21797.dd
27. Jain P, Kantarjian H, Jabbour E, Kanagal-Shamanna R, Patel K, Pierce S, et al. Clinical characteristics of Philadelphia positive T-cell lymphoid leukemias-(De novo and blast phase CML). Am J Hematol (2017) 92(1):E3–4. doi: 10.1002/ajh.24579
28. Verrma SP, Dutta TK, Vinod KV, Dubashi B, Ariga KK. Philadelphia chromosome positive pre-T cell acute lymphoblastic leukemia: a rare case report and short review. Indian J Hematol Blood Transfus (2014) 30(Suppl 1):177–9. doi: 10.1007/s12288-013-0314-8
29. Wei J, Huang M, Wang Y, Zhou J. Sudden extramedullary blast crisis of chronic myeloid leukemia manifesting as T-cell lymphoblastic lymphoma. Onkologie (2013) 36(3):119–22. doi: 10.1159/000348681
30. Raanani P, Trakhtenbrot L, Rechavi G, Rosenthal E, Avigdor A, Brok-Simoni F, et al. Philadelphia-chromosome-positive T-lymphoblastic leukemia: acute leukemia or chronic myelogenous leukemia blastic crisis. Acta Haematol (2005) 113(3):181–9. doi: 10.1159/000084448
31. Alshomar A, El Fakih R. Philadelphia chromosome-positive T-cell acute lymphoblastic leukemia: A case report and review of the literature. Hematol Oncol Stem Cell Ther (2020). doi: 10.1016/j.hemonc.2020.02.004
32. Prebet T, Mozziconacci MJ, Sainty D, Arnoulet C, Lafage M, Dastugue N, et al. Presence of a minor Philadelphia-positive clone in young adults with de novo T-cell ALL. Leuk Lymphoma (2009) 50(3):485–7. doi: 10.1080/10428190802601148
33. Fielding AK. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood (2010) 116(18):3409–17. doi: 10.1182/blood-2010-01-242750
34. Chao NJ, Blume KG, Forman SJ, Snyder DS. Long-term follow-up of allogeneic bone marrow recipients for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood (1995) 85(11):3353–4. doi: 10.1182/blood.V85.11.3353.bloodjournal85113353
35. Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer (2011) 117(8):1583–94. doi: 10.1002/cncr.25690
36. Lee KH, Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia (2005) 19(9):1509–16. doi: 10.1038/sj.leu.2403886
37. Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia–results of the prospective multicenter LALA-94 trial. Blood (2002) 100(7):2357–66. doi: 10.1182/blood-2002-03-0704
38. Wassmann B, Pfeifer H, Goekbuget N, Beelen DW, Beck J, Stelljes M, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood (2006) 108(5):1469–77. doi: 10.1182/blood-2005-11-4386
39. Chiaretti S, Vitale A, Vignetti M, Piciocchi A, Fazi P, Elia L, et al. A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica (2016) 101(12):1544–52. doi: 10.3324/haematol.2016.144535
40. Giebel S, Labopin M, Gorin NC, Caillot D, Leguay T, Schaap N, et al. Improving results of autologous stem cell transplantation for Philadelphia-positive acute lymphoblastic leukaemia in the era of tyrosine kinase inhibitors: a report from the Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Eur J Cancer (2014) 50(2):411–7. doi: 10.1016/j.ejca.2013.08.027
41. Wetzler M, Watson D, Stock W, Koval G, Mulkey FA, Hoke EE, et al. Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica (2014) 99(1):111–5. doi: 10.3324/haematol.2013.085811
42. Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica (2015) 100(5):653–61. doi: 10.3324/haematol.2014.118588
43. Cazzaniga G, De Lorenzo P, Alten J, Rottgers S, Hancock J, Saha V, et al. Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies. Haematologica (2018) 103(1):107–15. doi: 10.3324/haematol.2017.176917
44. Bruggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv (2017) 1(25):2456–66. doi: 10.1182/bloodadvances.2017009845
Keywords: T-cell lymphoblastic lymphoma, Philadelphia chromosome, clinical characteristics, managements, prognosis
Citation: Li X, Ping N, Wang Y, Xu X, Gao L, Zeng Z, Zhang L, Zhang Z, Xie Y, Ruan C, Wu D, Jin Z and Chen S (2021) Case Report: A Case With Philadelphia Chromosome Positive T-Cell Lymphoblastic Lymphoma and a Review of Literature. Front. Oncol. 10:584149. doi: 10.3389/fonc.2020.584149
Received: 16 July 2020; Accepted: 19 November 2020;
Published: 20 January 2021.
Edited by:
Jose-Maria Ribera, Germans Trias i Pujol Health Science Research Institute (IGTP), SpainReviewed by:
Belinda Pinto Simoes, University of São Paulo, BrazilHabibe Kurt, Brown University, United States
Copyright © 2021 Li, Ping, Wang, Xu, Gao, Zeng, Zhang, Zhang, Xie, Ruan, Wu, Jin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengming Jin, amluemhlbmdtaW5nMjAxOEAxNjMuY29t; Suning Chen, Y2hlbnN1bmluZ0BzdWRhLmVkdS5jbg==; Changgeng Ruan, Y3NoY21hQG1lZG1haWwuY29t
†These authors have contributed equally to this work
 Xuewei Li
Xuewei Li Nana Ping1,3†
Nana Ping1,3† Lijuan Gao
Lijuan Gao Suning Chen
Suning Chen