- 1Division of Hematology Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung, Taiwan
- 2Division of Hematology Oncology, Chang Gung Memorial Hospital at Linkou and College of Medicine, Chang Gung University, Tao-Yuan, Taiwan
- 3Department of Radiation Oncology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung, Taiwan
- 4Department of Urology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung, Taiwan
- 5Department of Pathology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung, Taiwan
- 6Clinical Trial Center, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Background: Immune checkpoint inhibitors (ICIs) are used widely for treating metastatic urothelial carcinoma (mUC). In practical settings, evidence is lacking on the efficacy of ICIs in some difficult-to-treat patients, such as those with end-stage renal disease (ESRD). Herein, we evaluate the safety and efficacy of ICIs for patients with mUC and ESRD.
Methods: For this retrospective study, patients with mUC who were given ICIs at Kaohsiung Chang Gang Memorial Hospital and Linkou Chang Gung Memorial Hospital between April 2016 and November 2019 were consecutively enrolled. All clinicopathologic data, treatment responses, and adverse events were recorded. The immune-related adverse events (AEs), objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) were compared between ESRD and non-ESRD groups.
Results: In total, 129 patients with mUC were enrolled, with 11 patients categorized as the ESRD group. Among these patients with ESRD receiving ICIs, 7 of 11 (63.6%) had high-grade (grade ≥3) AEs, chiefly hematologic toxicity. Some rarely encountered AEs were noted, including toxic epidermal necrolysis, tuberculosis reactivation, ascites, and cytokine release syndrome. Patients in the ESRD group had numerically higher ORR (54.5% vs. 28.8%, p = 0.09), PFS (7.1 vs. 3.5 months, p = 0.42), and OS (not reached vs. 15.4 months) than the non-ESRD group. A multivariate Cox regression model demonstrated that leukocytosis (hazard ratio [HR]: 2.63; 95% confidence interval [CI]: 1.23–5.63; p = 0.01) and neutrophil-to-lymphocyte ratio (HR 2.91; 95% CI: 1.30–6.53; p = 0.01) were independent prognostic factors.
Conclusion: Administration of ICIs in patients with mUC and ESRD demonstrated a modest antitumor activity, and should be used with caution for increasing risk of hematologic toxicity.
Introduction
Urothelial carcinoma (UC) is a common cancer worldwide, with approximately 500,000 new cases diagnosed annually and an estimated 150,000 cancer-related deaths (1). Early-stage UC can be cured through radical surgery, including cystectomy for bladder cancer and nephroureterectomy for upper tract urothelial carcinoma (UTUC). Nevertheless, approximately 10–30% of these patients experience local recurrence or distant metastasis, leading to mortality from such diseases (2). Cisplatin-based chemotherapy has been the gold standard therapy since 1990, with an objective response rate (ORR) of 40–50% and an overall survival (OS) of 14–15 months (3). As the recent breakthrough of immune checkpoint inhibitors (ICIs) has been widely studied for various cancer types, the paradigm of treatment has shifted to ICIs for patients failing to respond to platinum-based chemotherapy and those who are ineligible for cisplatin (4–8). In the pivotal phase 3 KEYNOTE-045 study, compared with conventional chemotherapy, pembrolizumab conferred a significant survival benefit on patients with metastatic UC (mUC) whose conditions were refractory to first-line platinum-based chemotherapy, regardless of the patients’ PD-L1 expression (4). At this time, five ICIs have been approved by the U.S. Food and Drug Administration (FDA) for mUC treatment.
The efficacy of cisplatin-based chemotherapy in patients with mUC is generally limited by poor Eastern Cooperative Oncology Group (ECOG) performance status or chronic kidney disease. In general, the proportion of patients for whom cisplatin is unsuitable may be 30–50% of the population with stage IV mUC (9). Given their more favorable toxicity profile, ICIs have been investigated as first-line treatments for cisplatin-ineligible patients with mUC. The promising OS results from the IMVigor 210 trial demonstrated that atezolizumab monotherapy provided an excellent OS of 15.8 months, prompting the FDA to grant accelerated approval for ICIs as first-line treatment for cisplatin-ineligible patients with mUC (10). However, many patients have been excluded from prospective trials owing to poor ECOG performance status or having coexisting autoimmune disease or end-stage renal disease (ESRD) requiring hemodialysis. Treatment options for patients with such rare conditions remain uncertain, and related evidence is lacking.
ESRD is a common comorbidity in patients with mUC. UTUC and urothelial carcinoma of the bladder (UCB) independently increase the risk of ESRD, with hazard ratios (HRs) for ESRD up to 7.75 and 3.12 in patients with UTUC and UCB, respectively (11). Patients with ESRD, especially women aged 50 to 60 years, also have a high risk of developing UC (12). As ICIs are eliminated through the reticuloendothelial system and are not excreted through renal filtration, their use in patients receiving dialysis provides an alternative therapeutic choice to avoid cumulative toxicity from conventional chemotherapy (13). Only small case series have provided evidence of the safety and efficacy of ICIs in patients with ESRD, and most of such studies have been on melanoma, lung cancer, and renal cell carcinoma (14, 15). To assist such difficult-to-treat patients, data on the safety and efficacy of ICIs are urgently required. The aim of this retrospective study was to evaluate the safety and efficacy of immune ICIs in patients with mUC and ESRD.
Methods
Patients
We retrospectively reviewed patients with mUC who received ICIs between April 2016 and November 2019 at Kaohsiung Chang Gung Memorial Hospital and Linkou Chang Gung Memorial Hospital in Taiwan. All clinicopathologic data were collected from electrical medical recording systems by physicians and trained assistants. Database variables included age, sex, ECOG performance status, primary tumor site, visceral or lymph node metastasis, PD-L1 expression by tumor proportion score, ICI type, regimen of combination treatment or previous systemic treatment, laboratory data, treatment response, and adverse events (AEs). The study was approved by the Institutional Review Board of Chang Gung Medical Foundation.
Treatment
All patients received an anti-PD-1 (nivolumab, pembrolizumab) or anti-PD-L1 (atezolizumab, durvalumab, or avelumab) medication. The regimen, treatment sequence, and combined treatment regimen were at the discretion of the physician. The regimen of combined treatment included chemotherapy, a cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitor, and a poly ADP-ribose polymerase (PARP) inhibitor.
Response Evaluation and Endpoints
All patients had attended scheduled appointments during treatment until disease progression, treatment intolerance, or death. The follow-up visit procedures included physical examinations, laboratory tests, and imaging studies. Patients were subjected to computed tomography scans of the chest or abdomen for tumor response assessments using the Response Evaluation Criteria in Solid Tumors (version 1.1).
The primary endpoint was treatment-related AEs in patients with ESRD. The observed AEs during any round of ICIs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (Supplementary Table 1). All patients who received at least one cycle of immunotherapy were included in the analysis. The secondary endpoints of the study were treatment response, OS, and progression-free survival (PFS). OS was defined as the time interval from the date of ICIs commencement (any cycle) to the date of death or final patient contact.
Statistical Analysis
All statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA), and survival curves were plotted using GraphPad Prism version 6.04 (GraphPad Software, La Jolla California, USA). The differences between the ESRD subgroup and patients without ESRD were examined using chi-squared (χ2) and t tests for categorical and continuous variables, respectively. We constructed OS and PFS curves using the Kaplan–Meier method. Univariate and multivariate analyses were performed using the Cox proportional hazards regression analysis. A p value <0.05 was considered statistically significant.
Results
Patient Characteristics
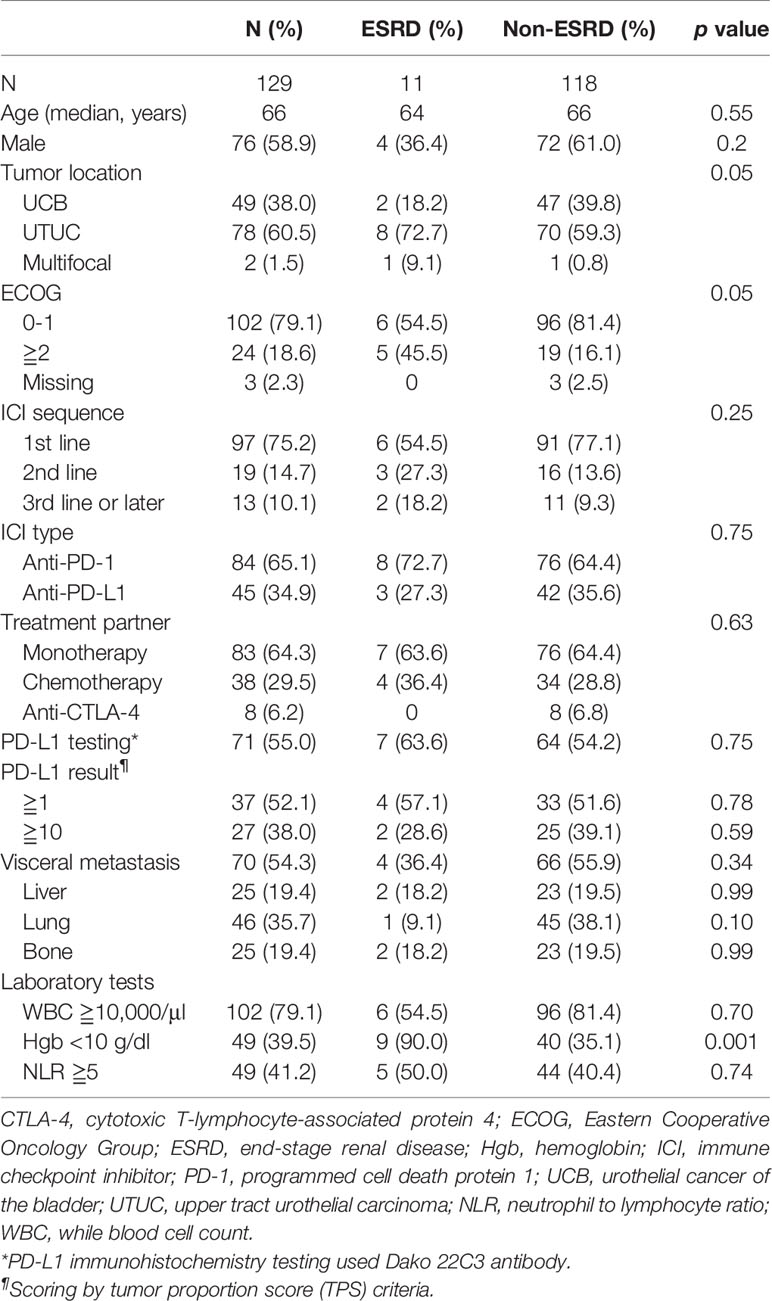
In total, 129 patients were included in this study, including 11 patients (8.5%) with ESRD who were on maintenance hemodialysis; they were categorized into the ESRD group. Basic patient characteristics are shown in Table 1. According to group comparison, the ESRD group had a significantly higher proportion of patients with an ECOG scale score of ≥2 (45.5 vs. 16.1%, p = 0.05), UTUC (72.7% vs. 59.3%, p = 0.05), and anemia (90.0 vs. 35.1%, p = 0.001). No significant difference was noted in age, gender, site of visceral metastasis, tumor proportion score, regimen and sequence of ICIs, white blood cell count, and neutrophil to lymphocyte ratio (NLR) between the two groups. Two-thirds of patients (65.1%) were given anti-PD-1 therapy, and the majority of ICIs were used as monotherapy (64.3%) and as a first-line treatment (75.2%). The individual details of the ESRD group are listed in Table 2.
Treatment-Related AEs
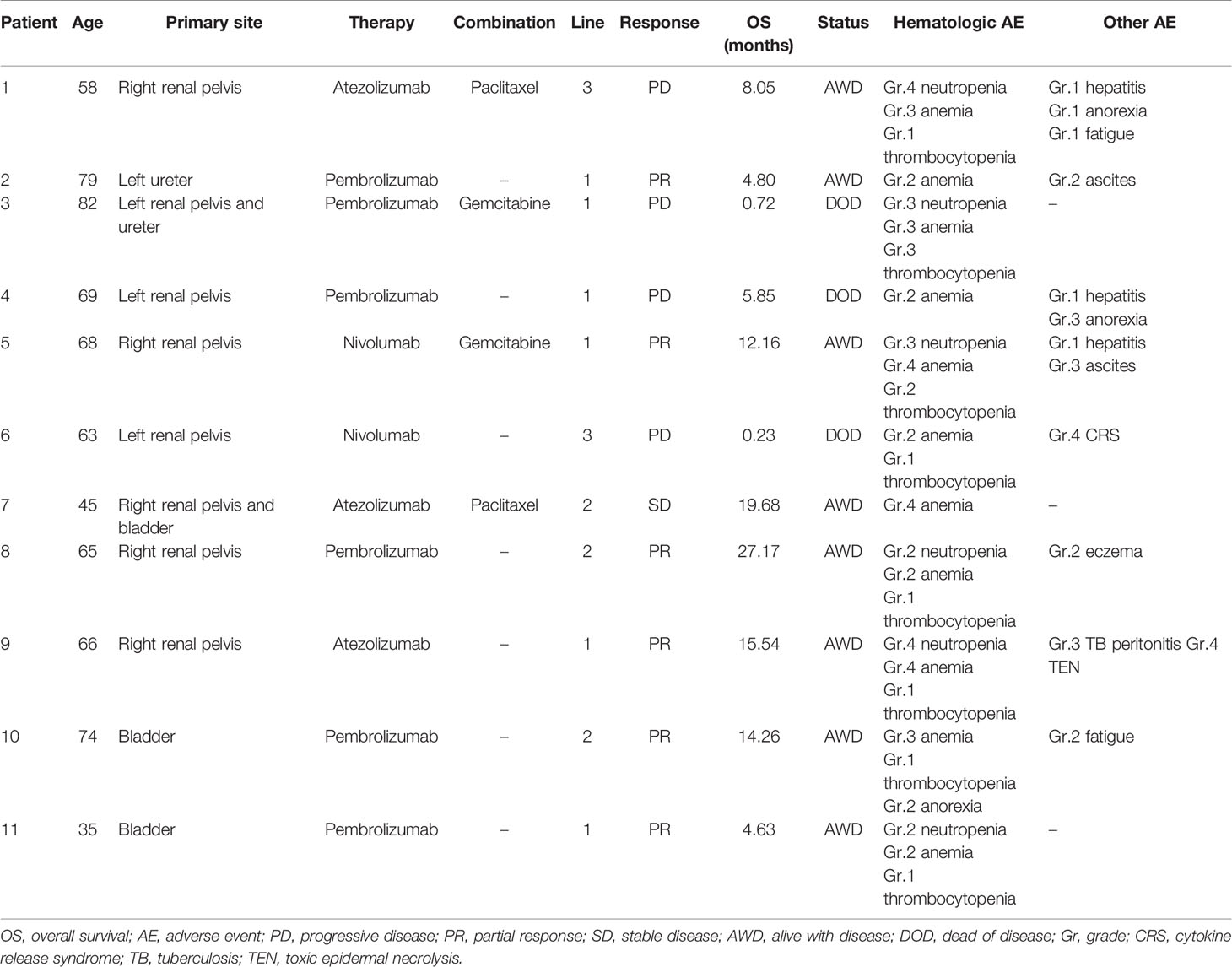
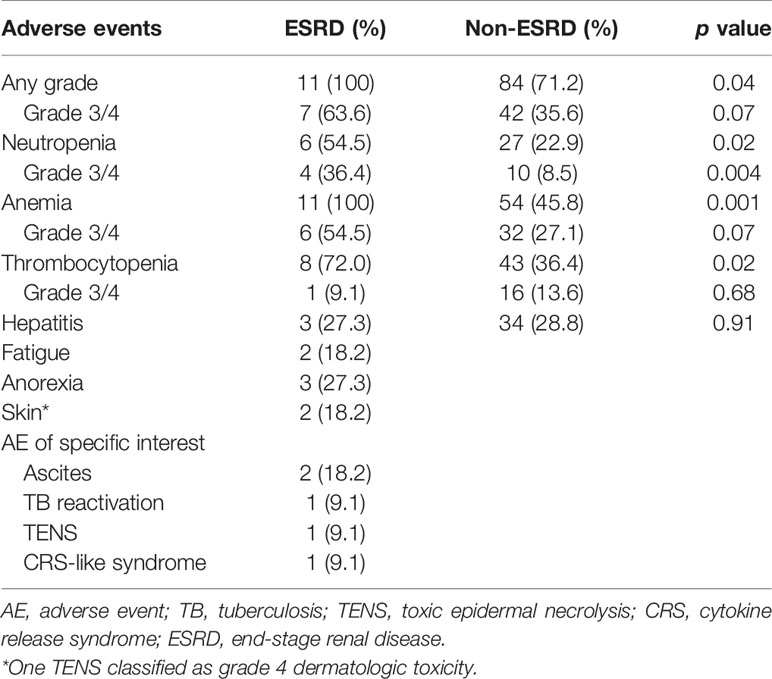
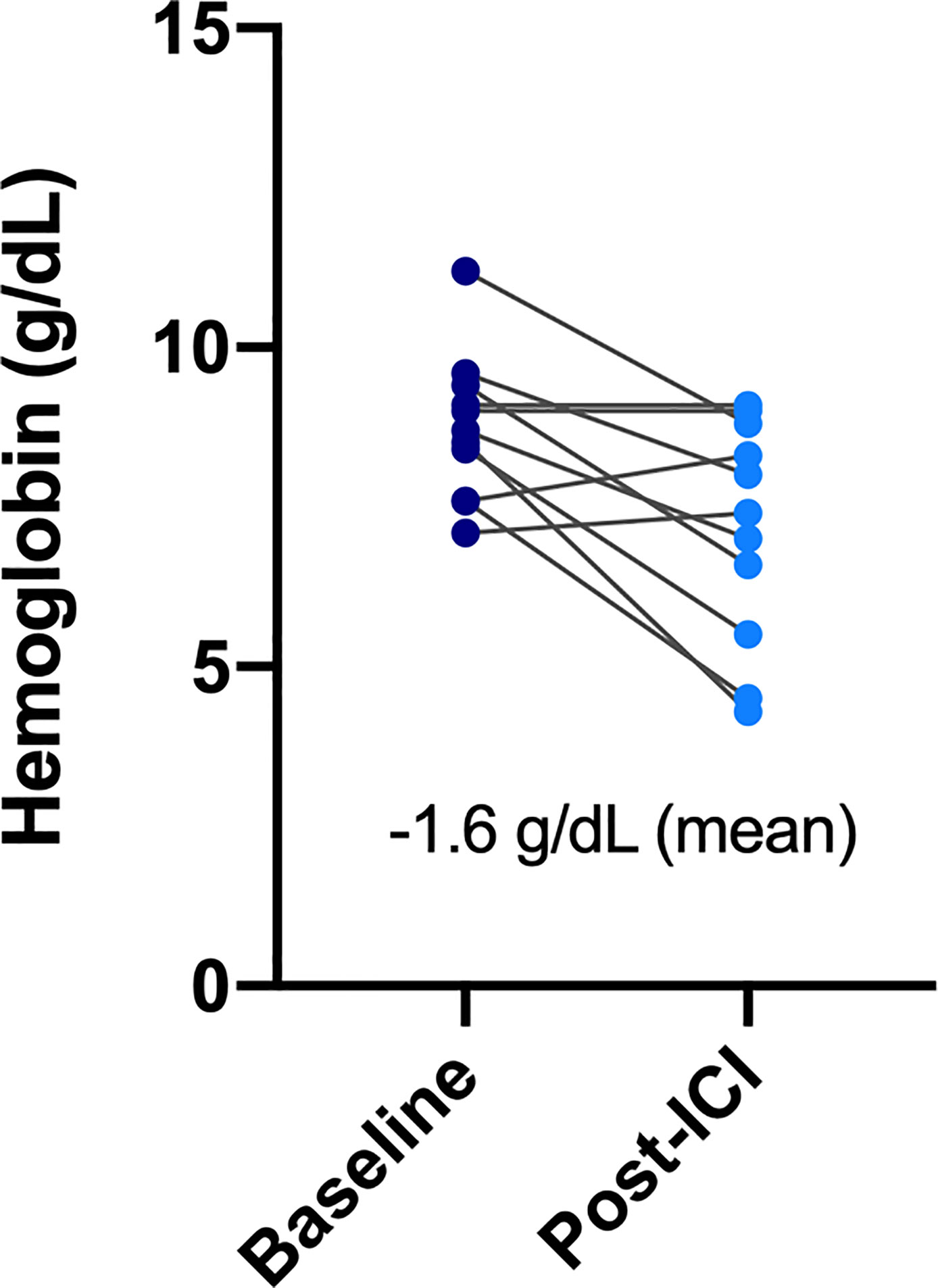
All patients in the ESRD group experienced at least one treatment-related AE during the treatment period, and seven of them (63.6%) had high-grade (grade ≥3) AEs (Table 3). AEs of all grades included hematologic toxicity (neutropenia 54.5%; anemia 100%; and thrombocytopenia 72%), hepatitis (27.3%), fatigue (18.2%), anorexia (27.3%), and dermatologic toxicity (18.2%). Regarding hematologic toxicity, four patients (36.4%) had grade 3 neutropenia or higher, six (54.5%) had grade 3 anemia or higher, and one (9.1%) had grade 3 thrombocytopenia or higher. However, given the nature of defective function on hematopoiesis for patients with ESRD, the median baseline hemoglobin (Hb) of ESRD group was 8.75 g/dl. The low level of baseline Hb in ESRD group can actually be categorized in CTCAE grade 2 anemia, indicating that any decline of Hb will classified into grade 3 anemia. Although a considerable number of grade 3–4 anemia were observed in the ESRD group, the decrease in mean Hb between baseline and post-ICI administration was 1.6 g/dl, which was not substantially significant (Figure 1). For one who developed toxic epidermal necrolysis (TEN), a grade 4 dermatologic AE was recorded. Two patients presented with refractory ascites after receiving a PD-1 inhibitor. The ascites subsided after ICI usage was discontinued and recurred again after the re-administration of ICIs for disease relapse. One patient had disseminated tuberculosis reactivation. A cytokine release syndrome (CRS)-like syndrome was observed in one patient who presented with intermittent spiking fever and respiratory failure after receiving a PD-1 inhibitor.
We also compared the incidence of all grade AE and hematologic AE between ESRD and non-ESRD groups. As shown in Table 3, patients with ESRD on ICIs treatment had a higher incidence of all grade of neutropenia (54.5 vs. 22.9%, p = 0.02), anemia (100 vs. 45.8%, p = 0.001) and thrombocytopenia (72.0 vs. 36.4%, p = 0.02) than non-ESRD patients. Except for hematologic toxicity, there was no new additional safety concerns emerged from this comparative study between ESRD and non-ESRD group.
Treatment Responses
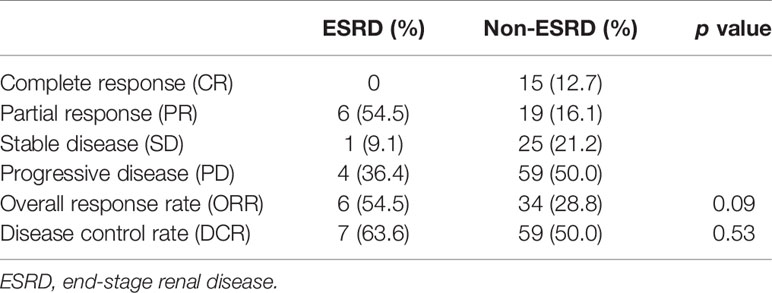
The objective response rate (ORR) was significantly higher in the ESRD group than in the non-ESRD group (54.5 vs. 28.8%, p = 0.09). In terms of the disease control rate (DCR), the ESRD group benefited more (63.6%) than the non-ESRD group did (50.0%); in the ESRD group, six patients achieved partial response (54.5%), and one patient achieved stable disease status (9.1%). All details are provided in Table 4.
Survival Outcomes
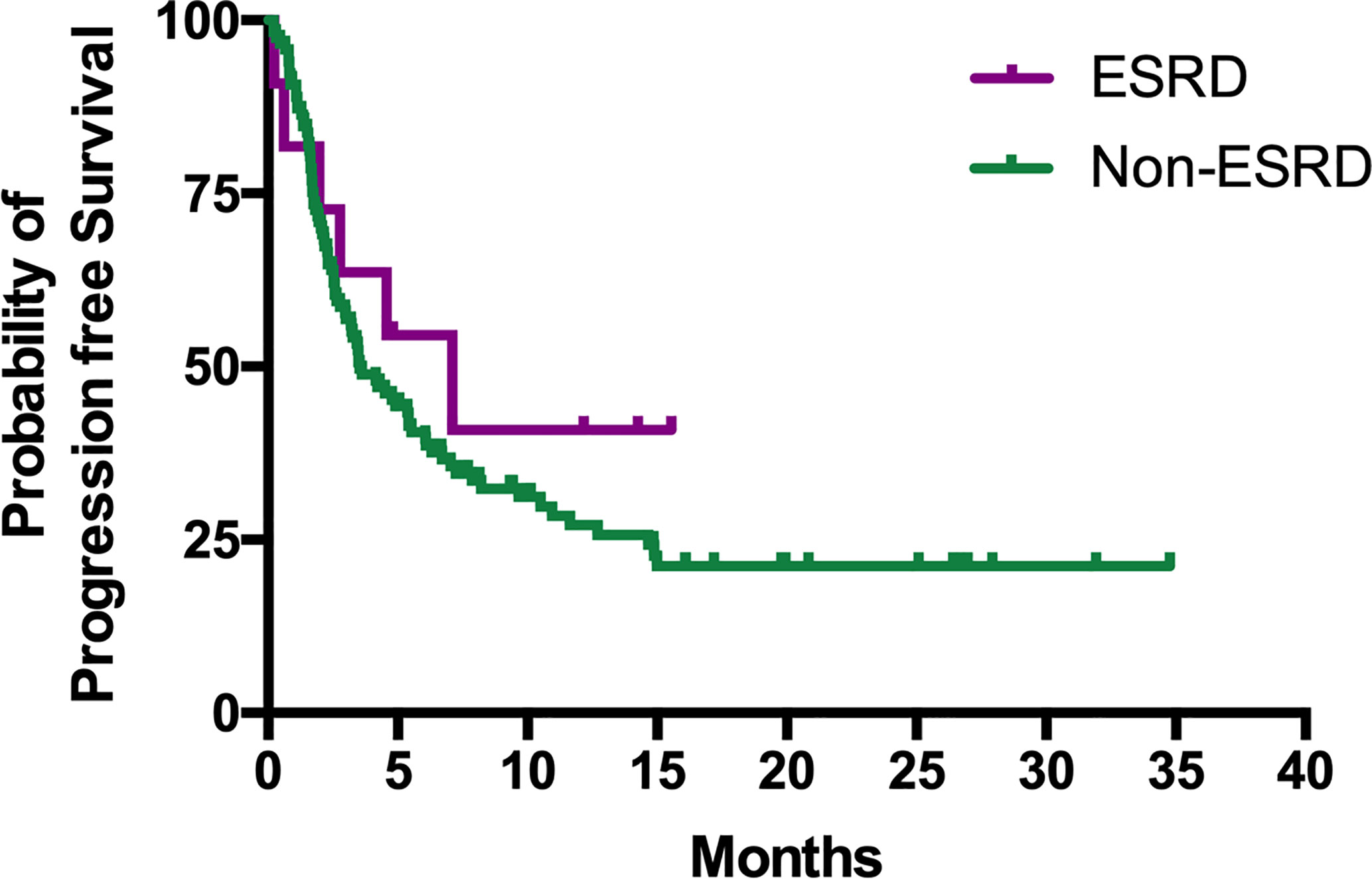
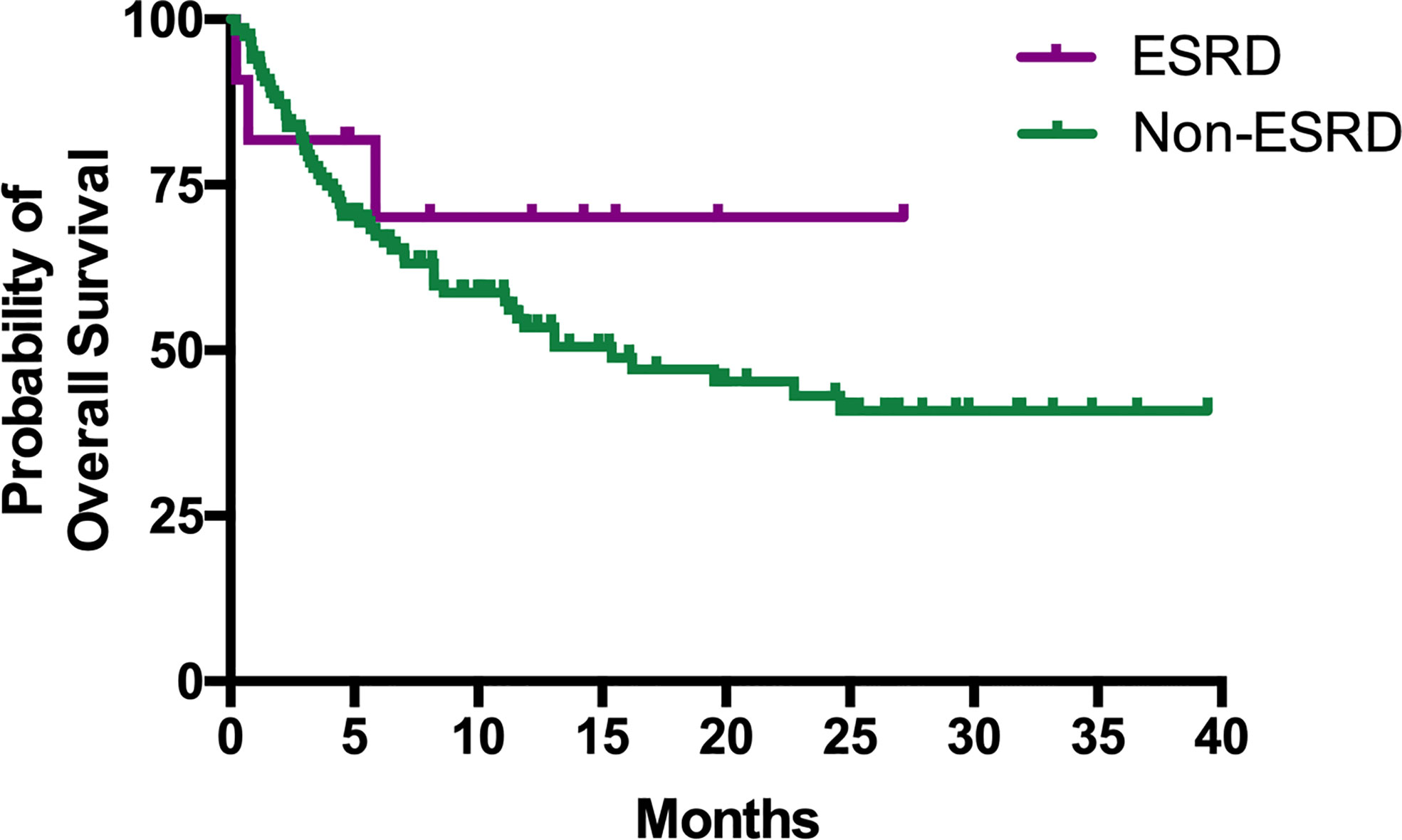
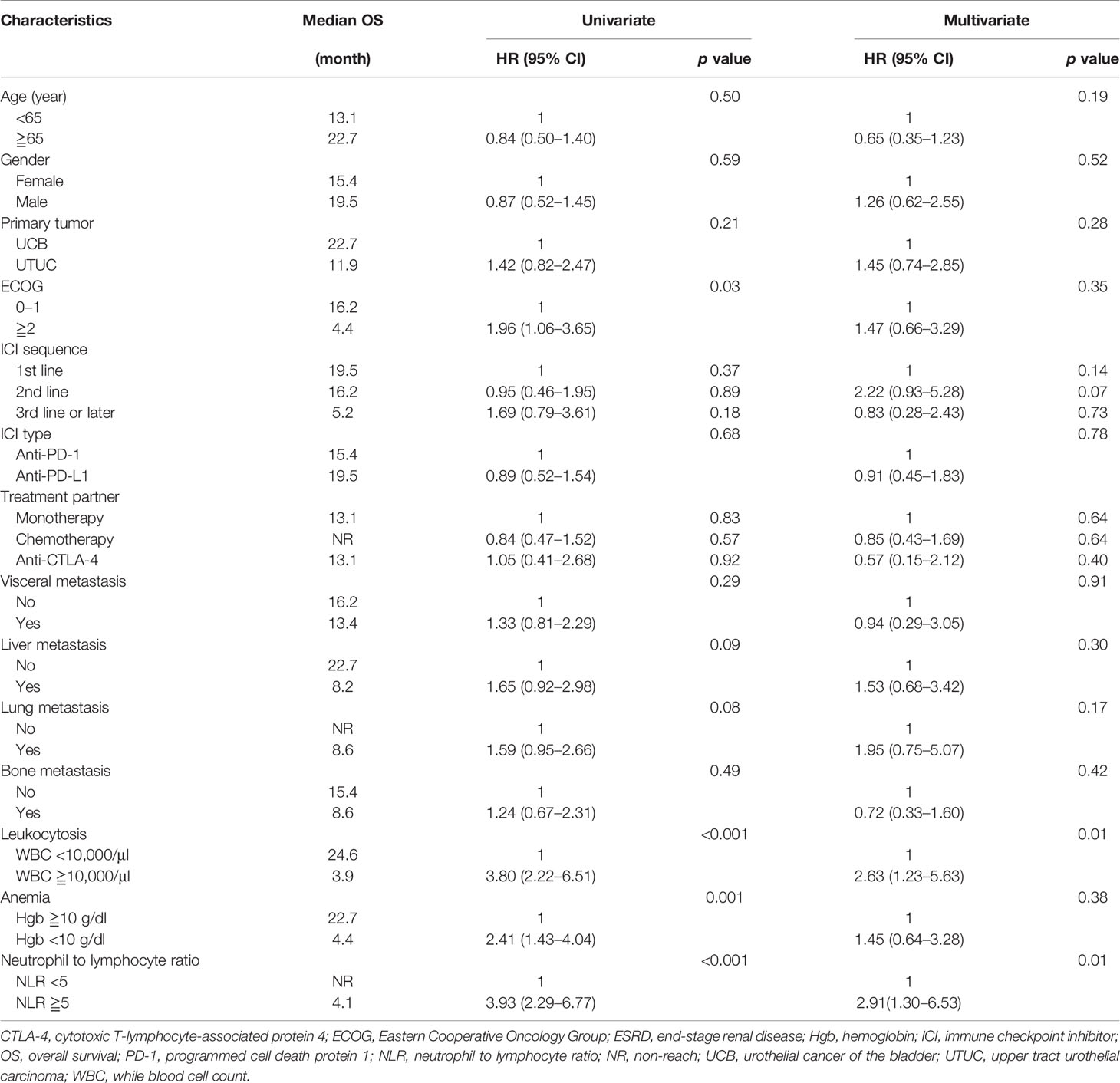
The median PFS of patients in the ESRD and non-ESRD groups was 7.1 and 3.5 months, respectively (p = 0.42; the PFS curve is plotted in Figure 2). The median OS of patients in the ESRD group was not reached and was 15.4 months in the non-ESRD group (the OS curve is plotted in Figure 3). In the univariate analysis of OS, the prognostic factors included ECOG (≥2 vs. <1; HR: 1.96; 95% CI: 1.06–3.65; p <0.03), leukocytosis (≥10,000/μl vs. <10,000/μL; HR: 3.80; 95% CI: 2.22–6.51; p <0.001), anemia (<10 g/dl vs. ≥10 g/dl; HR: 2.41; 95% CI: 1.43–4.04; p = 0.001) and NLR (≥5 vs. <5; HR: 3.93; 95% CI: 2.29–6.77; p <0.001). In the univariate analysis, a trend of survival benefits was observed for patients without liver metastasis (HR: 1.65; 95% CI: 0.92–2.98; p = 0.09) and without lung metastasis (HR: 1.59; 95% CI: 0.95–2.66; p = 0.08). After adjustments were made for all potential prognostic factors in the multivariate analysis, the only independent factor was leukocytosis (HR: 2.63; 95% CI: 1.23–5.63; p = 0.01) and NLR (HR: 2.91; 95% CI: 1.30–6.53; p = 0.01). All details are presented in Table 5.
Discussion
The present study reports the treatment experience of 11 consecutive patients with ESRD who received ICIs for mUC. Although some unexpected AEs occurred, generally, in patients with ESRD, the ICIs were well tolerated without additional toxicity. Furthermore, the major efficacy endpoints of ORR, PFS, and OS suggested benefits of ICI use in patients with ESRD. To our knowledge, this is the largest case series on the safety and efficacy of ICIs for patients with cancer who require maintenance hemodialysis. Our real-world data indicate that the administration of ICIs may be beneficial in such difficult treatment scenarios.
A few case reports and case series had examined the efficacy and safety of administrating ICIs in patients with ESRD on dialysis. In reviewing literature, only 41 patients had been reported; most of them were metastatic melanoma, NSCLC and renal cell carcinoma (RCC), only five cases were mUC (13–31) (Table 6) Vitale et al. reported eight ESRD patients with metastatic RCC who received dialysis (seven on hemodialysis, one on peritoneal dialysis) and nivolumab as cancer treatment. Only two patients (25%) experienced grade 3 AEs (diarrhea, asthenia, and anorexia), and five patients (62.5%) had grade 1–2 AEs, including cutaneous toxicities, anorexia, diarrhea, nausea, vomiting, arthralgia, and hematologic toxicities. These irAEs were appropriately managed with systemic corticosteroid and symptomatic treatment (15). Strohbehn et al. presented a brief report of treatment response and side effects in 19 ESRD patients received ICI therapy. However, the study population were quite heterogeneous in cancer types (six genitourinary cancer, three melanoma, three merkel cell carcinoma, three head and neck cancer), ICI regimen (90% anti-PD-1/PD-L1, 5% anti-CTLA-4 and 5% combined anti-PD-1/CTLA-4), and dialysis modality (79% hemodialysis, 21% peritoneal dialysis), which limited to achieve a definite conclusion (32). Compared with previous reports, our study revealed more hematologic AEs, 36.4% of which were grade 3–4 neutropenia. However, a standard chemotherapy regimen, either of gemcitabine plus cisplatin or MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), caused more than 70% of patients to experience grade 3–4 neutropenia (3). Given concerns related to neutropenia and risk of infection, ICI is a safe treatment for patients with mUC and ESRD.
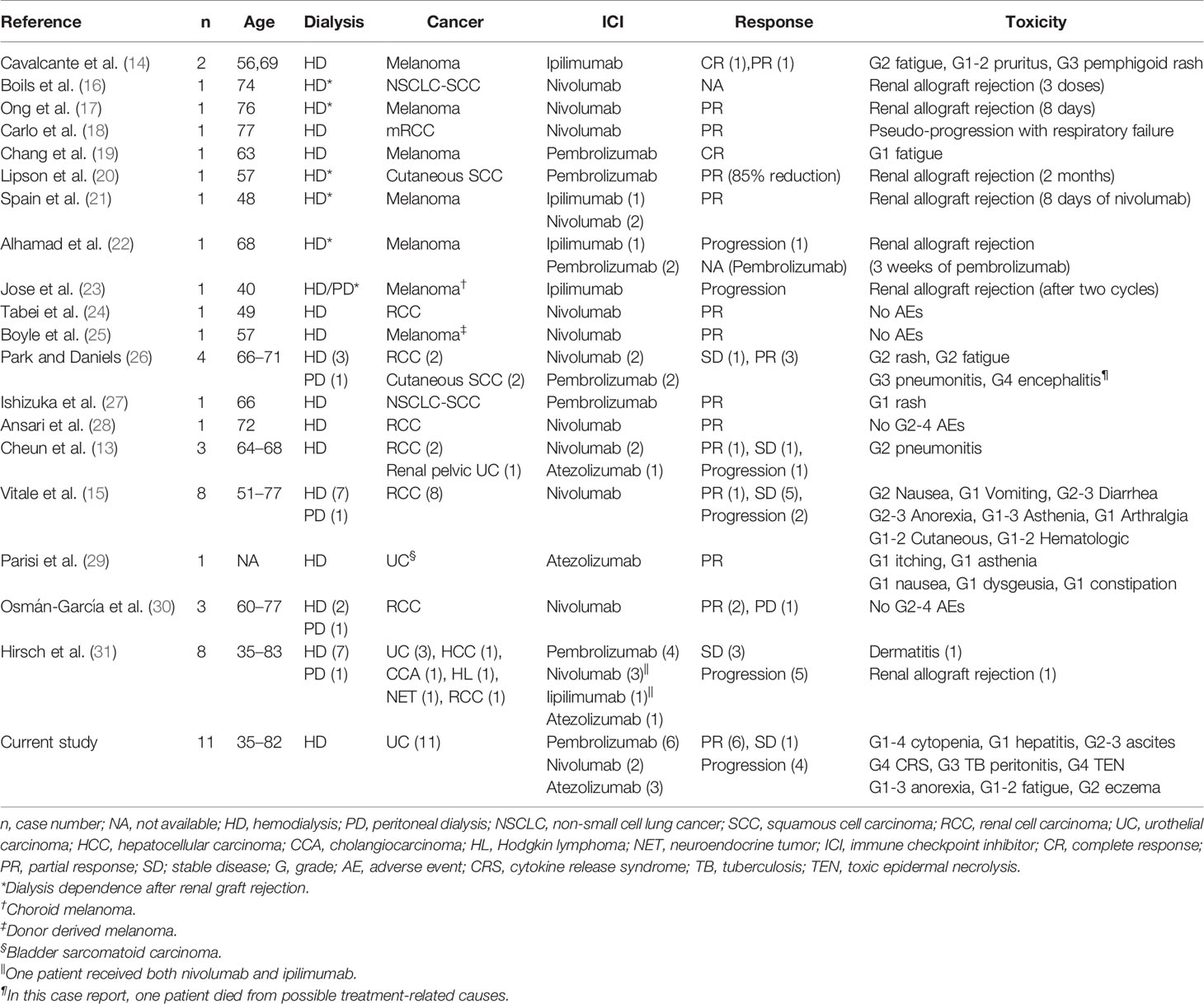
Table 6 Summary of 41 published cases of the use of immune checkpoint inhibitors in dialysis patients.
We also reported some notable irAEs in this study. A 65-year-old woman had disseminated tuberculosis reactivation and TEN after anti-PD-L1 administration. The patient fully recovered from TEN after systemic steroid administration and intensive skin care, and her tuberculosis was appropriately controlled by anti-tuberculosis agents. It is worthwhile to highlight the relationship between ICI use and TB reactivation. Barber et al. hypothesized that ICIs may boost TH1 function and increase the level of interferon γ-producing Mycobacterium tuberculosis-specific CD4 T-cells in the blood (33). The pathogenesis of TEN is also related to cell-mediated cytotoxic reactions and the clonal expansion of drug-specific T-cells with cytotoxicity against keratinocytes directly and indirectly through the recruitment of other cells (34). Cavalcante et al. reported that a patient with ESRD developed a grade 3 pemphigoid rash and bullous lesion after ipilimumab administration, achieving a complete response (14). Further studies are required to clarify the incidence of severe dermatologic irAEs in patients with ESRD and to elucidate the relationship between the intensity of cell-mediated cytotoxic reactions and the durable response rate.
One patient in our study presented with daily spiking fever, hypotension, altered mental status, hypoxia, and respiratory failure after administration of the first cycle of anti-PD-1 treatment. The clinical manifestation was thought to be severe sepsis but also resembled an unusual form of CRS, an inflammatory systemic disorder resulting from an overwhelming elevation of cytokine levels and T-cell engagement and proliferation. CRS severity can range from mild symptoms to a fulminant disease with multiple organ failure and death. CRS has been observed to be triggered by several monoclonal antibodies, systemic interleukin-2, and more recently, the CD19-CD3 chimeric antigen receptor T-cell therapy (35). A few case reports have detailed life-threatening CRS in patients after the administration of ICIs, with occurrences ranging from cycles 1 to 17 (36–39). The culprit medications were anti-PD-1 and anti-LAG-3. Alexander et al. reported the case of a patient with stage IV melanoma who received nivolumab on cycle 17 and had a CRS episode; it was controlled by tocilizumab initially, but the patient died 6 weeks later because of another CRS episode (39). Seth et al. also reported a patient with alveolar soft part sarcoma who received nivolumab and had a CRS event that was resolved by tocilizumab and corticosteroids (38). Although CRS is an uncommon complication associated with ICIs, early recognition and prompt management of CRS is crucial owing to its high mortality risk.
Among patients with ESRD in this report, ICIs conferred a significantly higher ORR and better DCR on patients with ERSD than those without. The response rate benefits reflect the trends of better PFS and median OS. Our results showed that the efficacy of ICIs for patients with ESRD was not inferior to that for patients without ESRD. A possible explanation of the superior antitumor efficacy of ICIs may be related to pharmacokinetics. Renal failure or hemodialysis seems to have no effect on the pharmacokinetics of ICIs, possibly because the clearance of ICIs is governed by numerous physiological mechanisms; this clearance predominantly occurs through nonspecific degradation within plasma and tissues. This nonspecific route of degradation reduces the influence of age, hepatic impairment, and renal failure on clearance (40). Considering the large molecular weights of ICIs (nivolumab: 146 kDa; ipilimumab: 148 kDa; pembrolizumab: 149 kDa; atezolizumab: 145 kDa), which cannot penetrate dialysis pores, drug removal and elimination through hemodialysis are unlikely (13). The pharmacokinetic characteristics of ICIs, which are unaffected by renal failure and hemodialysis, were also demonstrated by a similar incidence of AEs among patients in the ESRD and non-ESRD groups.
This study had some inevitable limitations owing to its retrospective nature; furthermore, it was limited by the relatively small sample size of the ESRD group. However, it is difficult to conduct a prospective clinical trial through recruiting patients with advanced UC or mUC to receive ICIs. The difficulty is not simply due to sample size; additionally, ESRD may develop during the treatment period among such patients with UC. Finally, the study had unpreventable bias in terms of the choice of ICIs being governed by physicians’ decisions, patients’ financial considerations, and the instructions of the National Health Insurance system in Taiwan. However, our results demonstrated that the administration of ICIs in patients with ESRD resulted in them having a better survival trend than did patients without ESRD, and no notable safety concerns arose.
In conclusion, our study revealed that administration of ICIs in patients with mUC and ESRD demonstrated a modest antitumor activity, and should be used with caution for increasing risk of hematologic toxicity. Further confirmatory studies are required to validate our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Chang Gung Medical Foundation. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
M-CK analyzed and interpreted data, prepared the tables, and wrote the original manuscript. Y-LS designed the conceptualization and methodology, prepared the figures, and reviewed and edited the manuscript. P-JS, C-CH, H-LL, T-JC, S-HL, C-CW, T-TL, Y-TC, and C-HK contributed the resources. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the multidisciplinary team of the genitourinary cancer at our hospital for their generous assistance and cooperation. The study was supported in part by a grant from Chang Gung Memorial Hospital, Kaohsiung, Taiwan (CMRPG8H1381, CMRPG8G1432). This manuscript was edited by Wallace Academic Editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.584834/full#supplementary-material
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2014) 136:E359–86. doi: 10.1002/ijc.29210
2. Mari A, Campi R, Tellini R, Gandaglia G, Albisinni S, Abufaraj M, et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: a comprehensive review of the literature. World J Urol (2018) 36:157–70. doi: 10.1007/s00345-017-2115-4
3. Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol (2000) 17(17):3068–77. doi: 10.1200/JCO.2000.18.17.3068
4. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
5. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
6. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18(3):312–22. doi: 10.1016/S1470-2045(17)30065-7
7. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma [abstract]. J Clin Oncol (2018) 35. doi: 10.1200/JCO.2017.35.6_suppl.286
8. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol (2017) 35(19):2117–24. doi: 10.1200/JCO.2016.71.6795
9. Gómez De Liaño A, Duran I. The continuing role of chemotherapy in the management of advanced urothelial cancer. Ther Adv Urol (2018) 10(12):455–80. doi: 10.1177/1756287218814100
10. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389:67–76. doi: 10.1016/S0140-6736(16)32455-2
11. Hung PH, Tsai HB, Hung KY, Muo CH, Chung MC, Chang CH, et al. Patients with urothelial carcinoma have poor renal outcome regardless of whether they receive nephrouretectomy. Oncotarget (2016) 7(38):61679–89. doi: 10.18632/oncotarget.11223
12. Wang SM, Lai MN, Chen PC, Wang JD. Increased risk of urothelial cancer in young and middle aged patients with end-stage renal disease. J Formos Med Assoc (2015) 114:52–7. doi: 10.1016/j.jfma.2013.10.022
13. Cheun H, Kim M, Lee H, Oh KH, Keam B. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs (2019) 37(3):579–83. doi: 10.1007/s10637-018-0673-y
14. Cavalcante L, Amin A, Lutzky J. Ipilimumab was safe and effective in two patients with metastatic melanoma and end-stage renal disease. Cancer Manage Res (2015) 7:47–50. doi: 10.2147/CMAR.S73389
15. Vitale MG, Baldessari C, Milella M, Buti S, Militello AM, Di Girolamo S, et al. Immunotherapy in Dialysis-Dependent Cancer Patients: Our Experience in Patients With Metastatic Renal Cell Carcinoma and a Review of the Literature. Clin Genitourin Cancer (2019) 17(5):E903–8. doi: 10.1016/j.clgc.2019.06.009
16. Postow MA, Callahan MK, Wolchok JD. Use of the PD-1 Pathway Inhibitor Nivolumab in a Renal Transplant Patient With Malignancy. Am J Transplant (2016) 16:2496–7. doi: 10.1111/ajt.13786
17. Ong M, Ibrahim AM, Bourassa-Blanchette S, Canil C, Fairhead T, Knoll G, et al. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer (2016) 4:64. doi: 10.1186/s40425-016-0171-8
18. Carlo MI, Feldman DR. Response to Nivolumab in a Patient With Metastatic Clear Cell Renal Cell Carcinoma and End-stage Renal Disease on Dialysis. Eur Urol (2016) 70(6):1082–3. doi: 10.1016/j.eururo.2016.05.040
19. Chang R, Shirai K. Safety and efficacy of pembrolizumab in a patient with advanced melanoma on haemodialysis. BMJ Case Rep (2016) 2016:bcr2016216426. doi: 10.1136/bcr-2016-216426
20. Lipson EJ, Bagnasco SM, Moore J Jr, Jang S, Patel MJ, Zachary AA, et al. Tumor Regression and Allograft Rejection after Administration of Anti-PD-1. N Engl J Med (2016) 374(9):896–8. doi: 10.1056/NEJMc1509268
21. Spain L, Higgins R, Gopalakrishnan K, Turajlic S, Gore M, Larkin J. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol (2016) 27(6):1135–7. doi: 10.1093/annonc/mdw130
22. Alhamad T, Venkatachalam K, Linette GP, Brennan DC. Checkpoint Inhibitors in Kidney Transplant Recipients and the Potential Risk of Rejection. Am J Transplant (2016) 16:1332–3. doi: 10.1111/ajt.13711
23. Jose A, Yiannoullou P, Bhutani S, Denley H, Morton M, Picton M, et al. Renal Allograft Failure After Ipilimumab Therapy for Metastatic Melanoma: A Case Report and Review of the Literature. Transplant Proc (2016) 48(9):3137–41. doi: 10.1016/j.transproceed.2016.07.019
24. Tabei T, Natsume I, Kobayashi K. Successful treatment of metastatic clear cell carcinoma with nivolumab in a patient receiving dialysis treatment. Int J Urol (2017) 24(9):708–10. doi: 10.1111/iju.13420
25. Boyle SM, Ali N, Olszanski AJ, Park DJ, Xiao G, Guy S, et al. Donor-Derived Metastatic Melanoma and Checkpoint Inhibition. Transplant Proc (2017) 49(7):1551–4. doi: 10.1016/j.transproceed.2017.06.007
26. Park S, Daniels GA. Anti-PD-1 therapy in patients with end-stage renal disease on dialysis: A single-center case series. J Clin Oncol (2017) 35(15_suppl):e14553–3. doi: 10.1200/JCO.2017.35.15_suppl.e14553
27. Ishizuka S, Sakata S, Yoshida C, Takaki A, Saeki S, Nakamura K, et al. Successful treatment by pembrolizumab in a patient with end-stage renal disease with advanced non-small cell lung cancer and high PD-L1 expression. Respir Investig (2018) 56(4):361–4. doi: 10.1016/j.resinv.2018.03.005
28. Ansari J, Ali M, Farrag A, Ali AM, Alhamad A. Efficacy of Nivolumab in a Patient with Metastatic Renal Cell Carcinoma and End-Stage Renal Disease on Dialysis: Case Report and Literature Review. Case Rep Immunol (2018) 2018:1623957. doi: 10.1155/2018/1623957
29. Parisi A, Cortellini A, Cannita K, Bersanelli M, Ficorella C. Safe Administration of anti-PD-L1 Atezolizumab in a Patient with Metastatic Urothelial Cell Carcinoma and End-Stage Renal Disease on Dialysis. Case Rep Oncol Med (2019) 2019:3452762. doi: 10.1155/2019/3452762
30. Osmán-García I, Congregado-Ruiz CB, Lendínez-Cano G, Baena-Villamarin C, Conde-Sanchez JM, Medina-López RA. Outcomes and Safety of Biweekly and Monthly Nivolumab in Patients with Metastatic Renal Cell Carcinoma and Dialysis: Three Case Reports and Literature Review. Urol Int (2020) 104(3-4):323–6. doi: 10.1159/000504515
31. Hirsch JS, Wanchoo R, Ng JH, Khanin Y, Jhaveri KD. Use of Immune Checkpoint Inhibitors in End Stage Kidney Disease Patients, Single Center Experience and Review of the Literature. Kidney360 (2020) 1(5):399–402. doi: 10.34067/KID.0000422020
32. Strohbehn IA, Lee M, Seethapathy H, Chute D, Rahma O, Guidon A, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients on Dialysis: A Retrospective Case Series. Am J Kidney Dis (2020) 76(2):299–302. doi: 10.1053/j.ajkd.2020.02.451
33. Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med (2019) 11(475):eaat2702. doi: 10.1126/scitranslmed.aat2702
34. Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol (2011) 128(6):1266–76.e11. doi: 10.1016/j.jaci.2011.08.013
35. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J ImmunoTher Cancer (2018) 6:56. doi: 10.1186/s40425-018-0343-9
36. Dimitriou F, Matter AV, Mangana J, Urosevic-Maiwald M, Micaletto S, Braun RP, et al. Cytokine Release Syndrome During Sequential Treatment With Immune Checkpoint Inhibitors and Kinase Inhibitors for Metastatic Melanoma. J Immunother (2019) 42:29–32. doi: 10.1097/CJI.0000000000000236
37. Oda H, Ishihara M, Miyahara Y, Nakamura J, Kozuka Y, Iwasa M, et al. First Case of Cytokine Release Syndrome after Nivolumab for Gastric Cancer. Case Rep Oncol (2019) 12:147–56. doi: 10.1159/000496933
38. Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer (2017) e26642. doi: 10.1002/pbc.26642
39. Slota A, Khan R, Rahman A, Warner EA. Cytokine Release Syndrome As a Rare Complication of Nivolumab: A Case Report. Blood (2019) 134(Supplement_1):5630. doi: 10.1182/blood-2019-127586
Keywords: immune checkpoint inhibitor, end-stage renal disease, metastatic urothelial carcinoma, safety, survival
Citation: Kuo M-C, Su P-J, Huang C-C, Luo H-L, Chiu T-J, Li S-H, Wu C-C, Liu T-T, Cheng Y-T, Kang C-H and Su Y-L (2020) Safety and Efficacy of Immune Checkpoint Inhibitors for Patients With Metastatic Urothelial Carcinoma and End-Stage Renal Disease: Experiences From Real-World Practice. Front. Oncol. 10:584834. doi: 10.3389/fonc.2020.584834
Received: 18 July 2020; Accepted: 29 October 2020;
Published: 27 November 2020.
Edited by:
Sumit Kumar Subudhi, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jamie S. Lin, University of Texas MD Anderson Cancer Center, United StatesMehmet Asim Bilen, Emory University, United States
Copyright © 2020 Kuo, Su, Huang, Luo, Chiu, Li, Wu, Liu, Cheng, Kang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Li Su, eW9saXN1QG1hYy5jb20=
 Ming-Chun Kuo
Ming-Chun Kuo Po-Jung Su2
Po-Jung Su2 Chun-Chieh Huang
Chun-Chieh Huang Hao-Lun Luo
Hao-Lun Luo