- 1Department of Gynecology and Obstetrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Department of Biotherapy, Cancer Center, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, and Collaborative Innovation Center, Chengdu, China
Purpose: To analyze the potential prognostic factors of epithelial ovarian cancer (EOC) in women aged under 35 compared to those aged 60–79.
Methods: Cases were retrospectively obtained from SEER database. Clinical characteristics, such as race, histological type, AJCC stage, laterality of tumors, CA125 results, and surgical strategies, were analyzed in < 35 years group and 60–79 years group. Kaplan-Meier survival curves were used to evaluate overall survival (OS) and cause-specific survival (CSS). Cox proportional hazard model was used to identify the predictors for CSS.
Results: Sixteen thousand eight hundred forty-seven EOC patients diagnosed in 2004–2015 were identified from SEER database, with 1,015 aged under 35 and 15,833 aged 60–79. In < 35 years group, mucinous (32.2%) was the most common histological type, followed by high-grade serous (26.6%) and endometrioid (18.3%), while in 60–79 years group, high-grade serous (68.3%) represented the leading histological type. Most young women were diagnosed at stage I (57.7%), while most old women were diagnosed at stage (48.1%). Both 5-year OS and 5-year CSS were higher in < 35 years group (5-year OS: 76.00% vs 40.18%, p < 0.001; 5-year CSS: 83.56% vs 55.18%, p < 0.001). The multivariate analysis identified histological type and stage as prognostic factors for CSS in both groups. Endometrioid represented a positive predictor for CSS, while carcinosarcoma and malignant Brenner were related to a worse CSS. (< 35 years group: carcinosarcoma vs endometrioid: HR 5.630, p=0.024; malignant Brenner vs endometrioid: HR 4.005, p < 0.001; 60–79 years group: carcinosarcoma vs endometrioid: HR 3.606, p < 0.001; malignant Brenner vs endometrioid: HR 2.291, p < 0.001). Tumors laterality, CA125 levels, surgery and lymphadenectomy failed to be associated with the CSS in < 35 years group, while found to be independent risk factors in 60–79 years group.
Conclusion: EOC women aged under 35 had a better survival outcome over EOC women aged 60–79, owing to high proportion of endometrioid and mucinous types in histology, as well as early-stage diagnosis. Identification of histological types and gene profiles should be underscored in young EOC patients.
Introduction
Ovarian cancer (OC), one of the most lethal gynecological cancers, caused 295,414 new cases and 184,799 deaths across the world in 2018 (1). Although the mortality has decreased due to the improvement of treatment strategies in the past 50 years, the 5-year survival rate is still under 50% because of late diagnosis (2). Epithelial ovarian cancer (EOC), which includes several subtypes with distinctive pathological and clinical features, takes up the majority of OC patients (3). EOC has the highest incidence rate in women in their 6th to 7th decades (4), while is rarely seen in young women, with 1.1% in aged under 25, and 4.1% in aged under 30 (5). Several studies (5–11) have focused on this small group of patients and found that they were associated with distinct patterns of clinicopathological characteristics and a higher overall survival rate. However, few studies included a large population, and some did not analyze the surgical strategies and the associated outcomes in the patient group. Women’s fertility rate sharply decreases after age of 35 (12). Thus, women aged under 35 represent a special group to be considered in many GYN/OB diseases. By retrospectively analyzing the data obtained from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, the current study aims to conduct a thorough analysis including clinicopathologic characteristics, surgical strategies and their relationship with the survival outcome in women aged under 35 compared to women aged 60–79, hoping to provide some helpful evidence for young EOC patients’ counseling and treatment strategies selection.
Methods
Study Population
Women with EOC under the age of 35 were included in < 35 years group, while women with EOC aged between 60 and 79 were identified as 60–79 years group for comparison.
Data Extraction
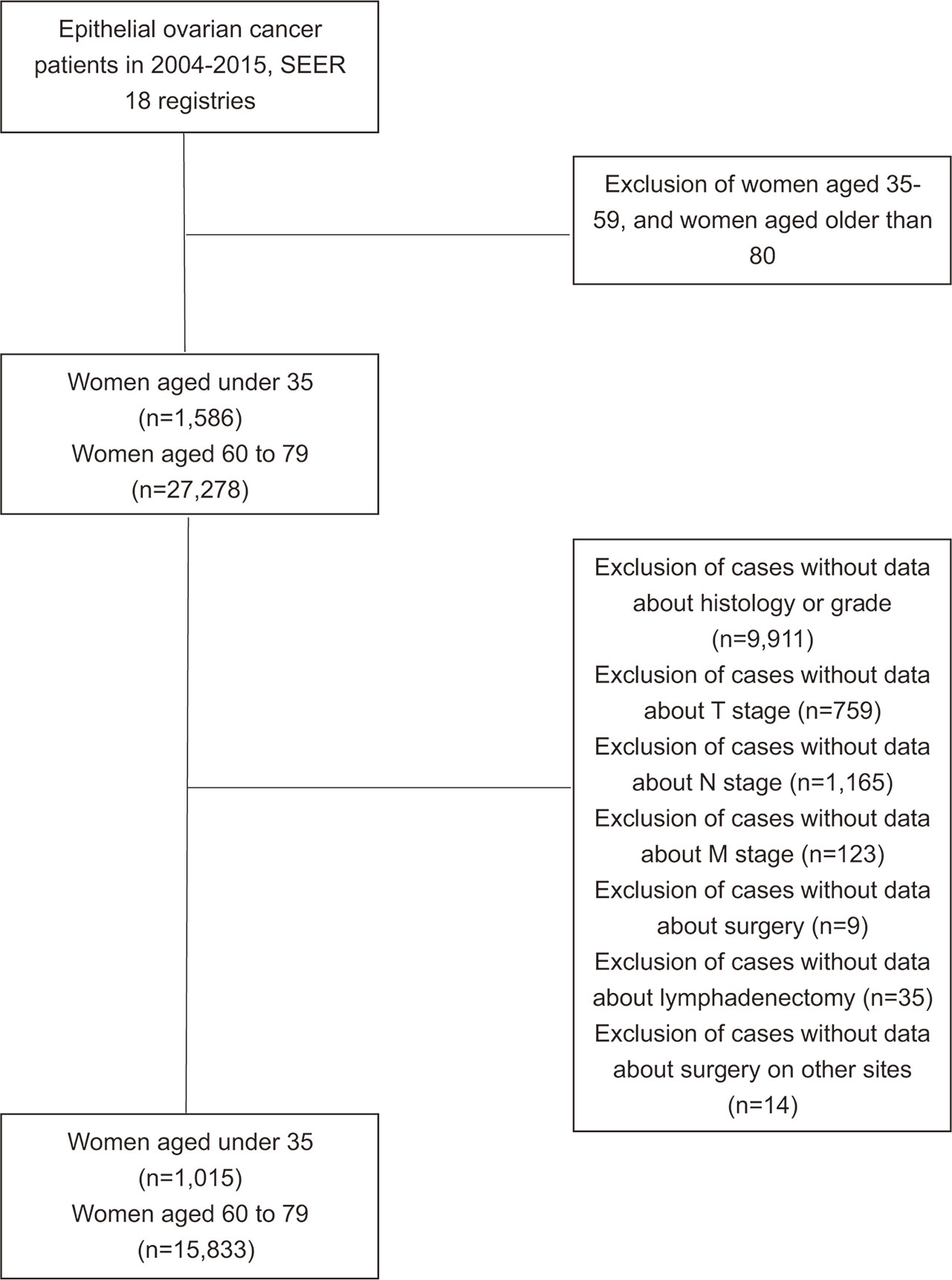
Data in this study was extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program released in November 2016. Women diagnosed with EOC (primary site: C56.9 Ovary) from 2004 to 2015 with the age range of 0–34 or 60–79 were identified. Parameters extracted included demographic, clinicopathological, treatment and survival information. The histological classification was identified according to 2014 WHO EOC histological types and a prior population-based study (13). The histological results were collected from pathology laboratories according to the standards issued by the North American Association of Central Cancer Registries (NAACR). Exclusion criteria were cases with missing information on histological types, AJCC stage, surgery intervention, lymph nodes removement during operation or surgery on remote sites (Figure 1). Surgical methods were classified into no surgery performed, unilateral salpingo-oophorectomy (USO) or bilateral salpingo-oophorectomy (BSO) (without hysterectomy), USO or BSO (with hysterectomy), USO or BSO (NOS), debulking or cytoreductive surgery (NOS), pelvic exenteration, and others.
Statistical Analysis
Median age of each group was calculated by independent samples t-test, and the rest variables were evaluated by chi-squared test, Fisher exact test, or post hoc test. Kaplan-Meier survival curves and log-rank test with 95% confidence intervals (CIs) were applied to evaluate the outcomes between the two groups. Risk factors were analyzed using Cox proportional hazards regression model with 95%CI. We considered p < 0.05 as statistically significant. For the post hoc test, the adjusted standardized value > 3 was regarded statistically significant. Data extraction was completed in SEER*Stat, and all analyses were performed using the SPSS Statics software, version 25.0 (IBM Corporation, NY, USA).
Results
Included Patient Characteristics
As shown in Table 1, 16,847 EOC patients were extracted from the SEER database, with 1,015 patients younger than 35 years, and 15,833 aged 60–79. Mucinous (32.2%) was the most common histological type in the < 35 years group, followed by high-grade serous (26.6%) and endometrioid (18.8%) tumors, while high-grade serous (68.3%) was most commonly observed type in the 60–79 years group. As for AJCC stage, over half of the young women (57.7%) were diagnosed at Stage I, while nearly half of the old women (48.1%) were diagnosed at stage III. The association between histological type and AJCC stage were analyzed in both groups (Table S1). Patients who were diagnosed at stage III were more likely to have high-grade serous tumors (56.3% in < 35 years group, 56.4% in 60–79 years group) and low-grade serous tumors (60.3% in < 35 years group, 47.4% in 60–79 years group), while those diagnosed at stage I were more likely to have endometrioid tumors (80.1% in < 35 years group, 70.5% in 60–79 years group) and mucinous tumors (85.3% in < 35 years group, 65.4% in 60–79 years group).The CA125 level elevated in most patients in both group (55.7% and 71.6%, respectively). The statistical significances of clinicopathological characteristics were evaluated by post hoc test (Table S2).
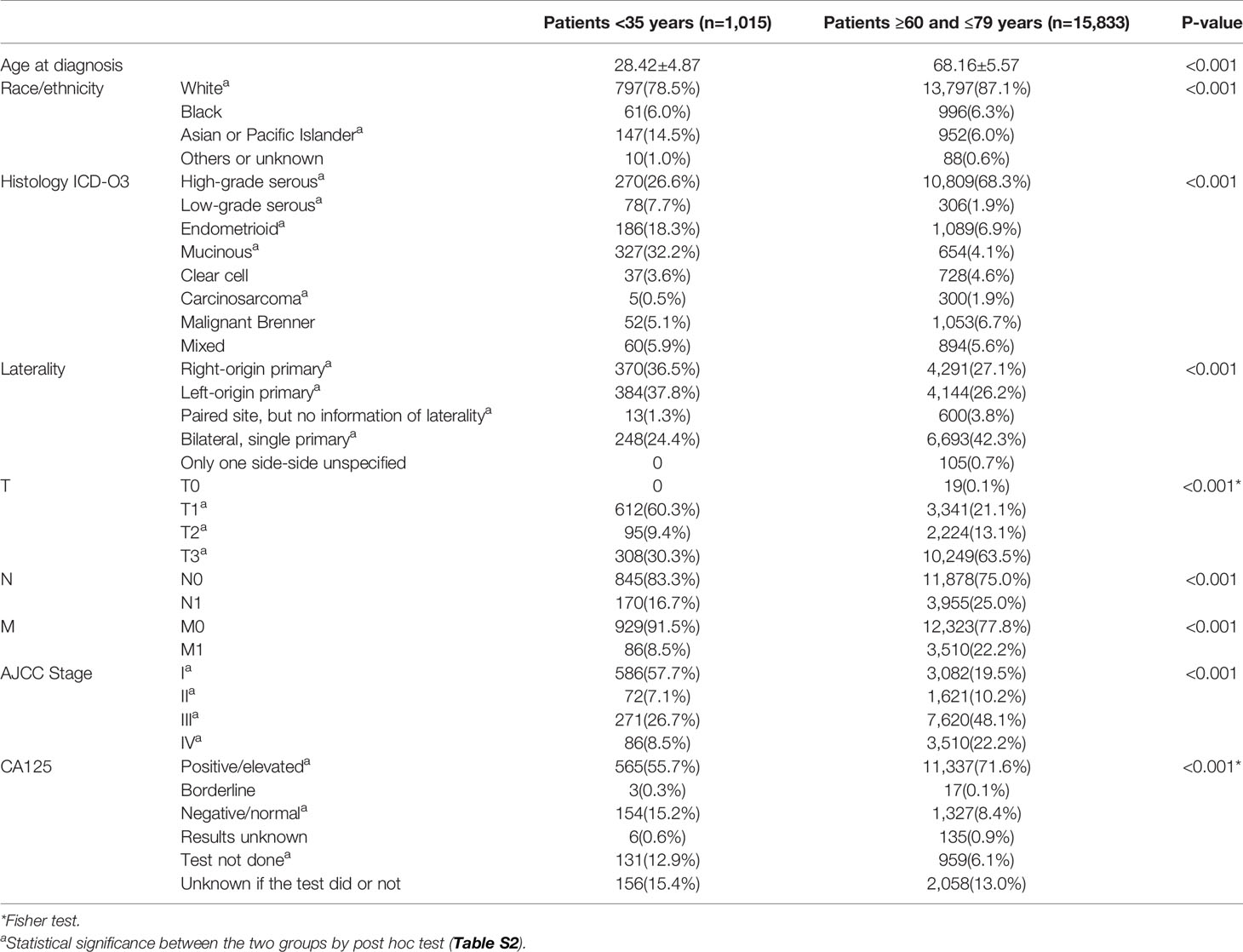
Table 1 Clinicopathological characteristics of young and old patients with epithelial ovarian cancer in 2004–2015, SEER 18 registries.
Treatment Strategies
Only 1.1% young women and 3.6% old women did not have surgery. In < 35 years group, more patients underwent uterine-preserving surgery than those in 60–79 years group (43.7% vs 10.1%), while more old women underwent debulking or cytoreductive surgery than their young counterparts (47.0% vs 21.3%). Besides, more young women underwent lymphadenectomy than old women (68.4% vs 55.2%, p < 0.001). There was no significance in the tendency of surgery on distant sites in the two groups (Table 2)
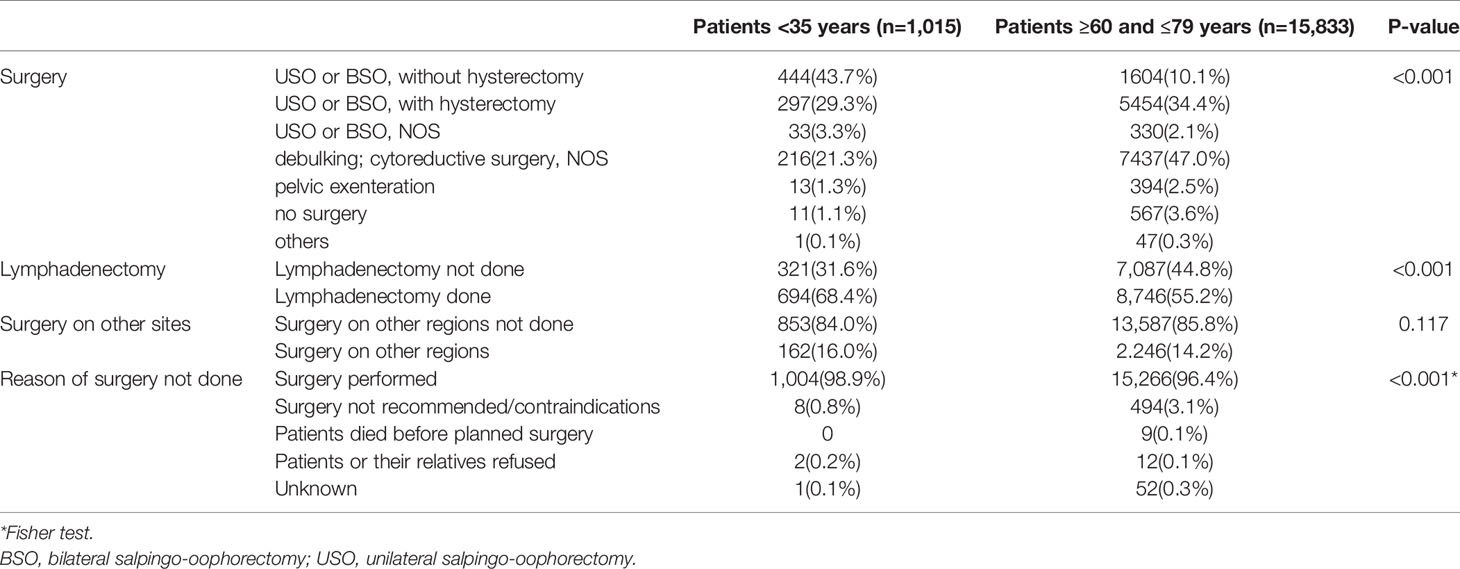
Table 2 Surgery details of young and old patients with epithelial ovarian cancer in 2004–2015, SEER 18 registries.
Survival Outcomes
The survival outcomes of the two groups were illustrated in Figure 2. Compared to women aged between 60 and 79 years, young women had a better 5-year overall survival (OS) (76.00% vs 40.18%, p < 0.001) and cause-specific survival (CSS) (83.56% vs 55.18%, p < 0.001).
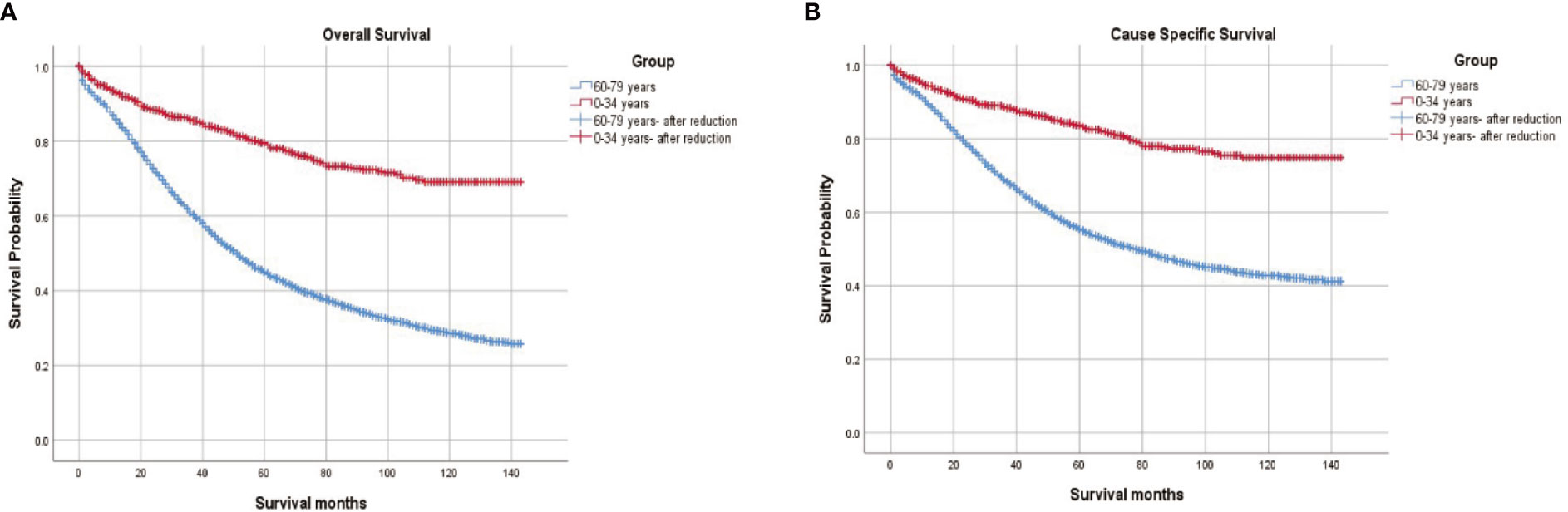
Figure 2 (A) Overall survival and (B) Cause-specific survival in women aged <35 and women aged 60 to 79 diagnosed with epithelial ovarian cancer.
CSS by lymphadenectomy were further identified (Figure 3). In < 35 years group, lymphadenectomy did not indicate a significantly better outcome (5-year CSS 84.21% vs 82.12%, p=0.318). In 60–79 years group, however, those who had their lymph nodes removed had a much higher CSS rate (63.22% vs 44.78%, p < 0.001). Furthermore, we analyzed the survival curves on lymphadenectomy by stages in both groups. In < 35 years group, lymphadenectomy made no significant differences for patients diagnosed at stage I/II (93.19% vs 94.72%, p=0.686) or at stage III/IV (64.37% vs 63.19%, p=0.828), while in 60–79 years group, both patients diagnosed at stage I/II (88.49% vs 80.39%, p < 0.001) and those diagnosed at stage III/IV (47.36% vs 35.86%, p < 0.001) benefited from lymphadenectomy (Figure S1).
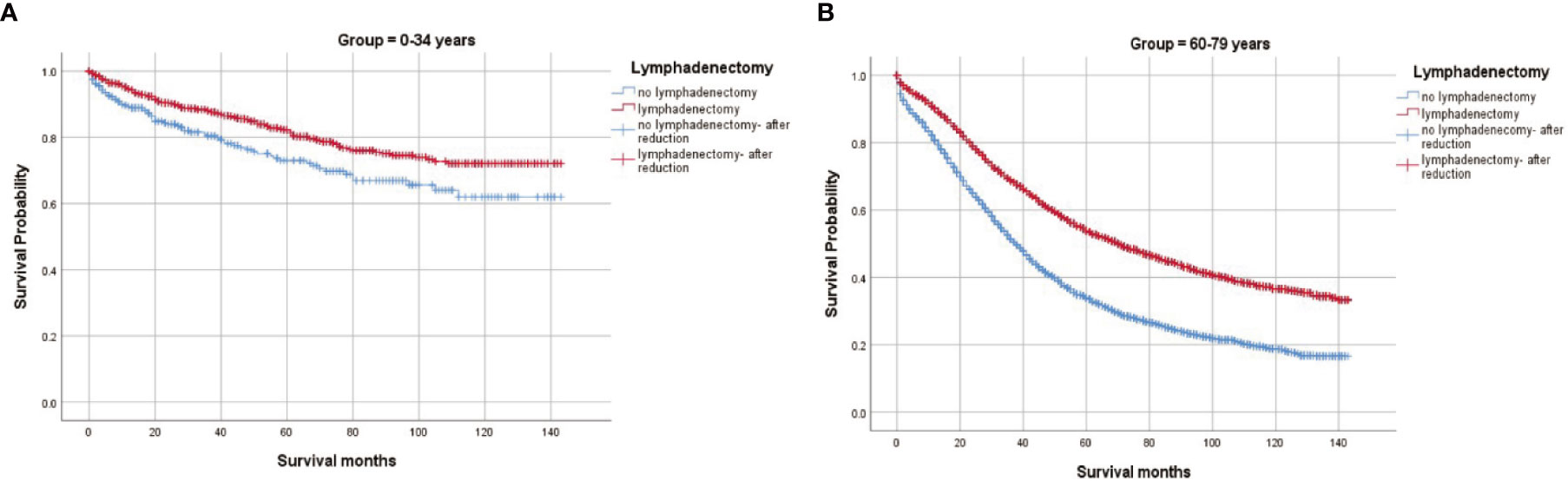
Figure 3 Cause-specific survival by lymphadenectomy in women aged <35 and women aged 60 to 79 diagnosed with epithelial ovarian cancer. (A) Lymphadenectomy in women aged <35; (B) Lymphadenectomy in women aged 60.
Risk Factors of Cause-Specific Survival
In the < 35 years group, histological type, laterality of tumors, AJCC stage, level of CA125 before therapies, surgery, and surgery on other sites (all p < 0.001) were risk factors for CSS in univariate survival analysis (Table 3). In multivariate analysis, however, only histological type and AJCC stage remained as independent prognostic factors. Compared to endometrioid histological type, carcinosarcomas (Hazard ratio (HR) 5.630 95%CI 1.256, 25.226, p=0.024) and malignant Brenner tumors (HR 4.005 95%CI 1.880, 8.531, p < 0.001) were related to a worse CSS. As for the AJCC stage, the risk increased as the stage advanced (T2 versus T1, HR 2.896, 95%CI 1.381, 5.962, p=0.005; T3 versus T1 HR 8.724, 95%CI 5.355, 14.213, p < 0.001; T4 versus T1 HR 26.856, 95%CI 16.009, 45.054, p < 0.001).
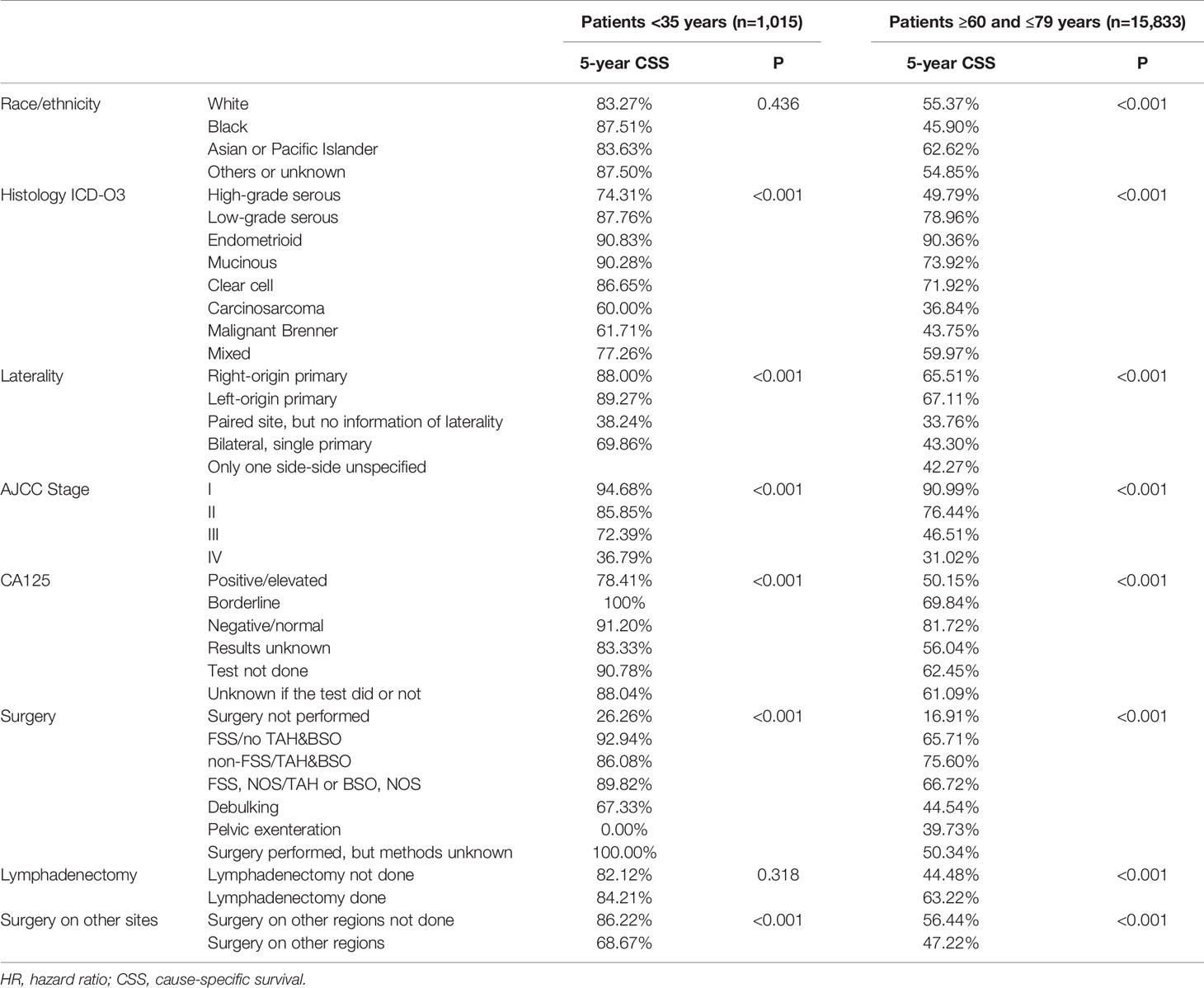
Table 3 Univariate analysis by Cox regression for CSS in both young and old women group with epithelial ovarian cancer in 2004–2015, SEER 18 registries.
In the 60–79 years group, race, histological type, laterality of tumors, AJCC stage, level of CA125 before therapies, surgery, lymphadenectomy, and surgery on other sites (all p < 0.001) were all associated with CSS. Only surgery on other sites (p=0.715) was excluded in the multivariate survival analysis. Bilateral tumors represented risk factors for CSS (right-origin primary tumor as a reference, paired sites, NOS HR 1.210, 95%CI 1.051, 1.393, p=0.008; bilateral, single primary, HR 1.228, 1.147, 1.316, p<0.001) (Table 4).
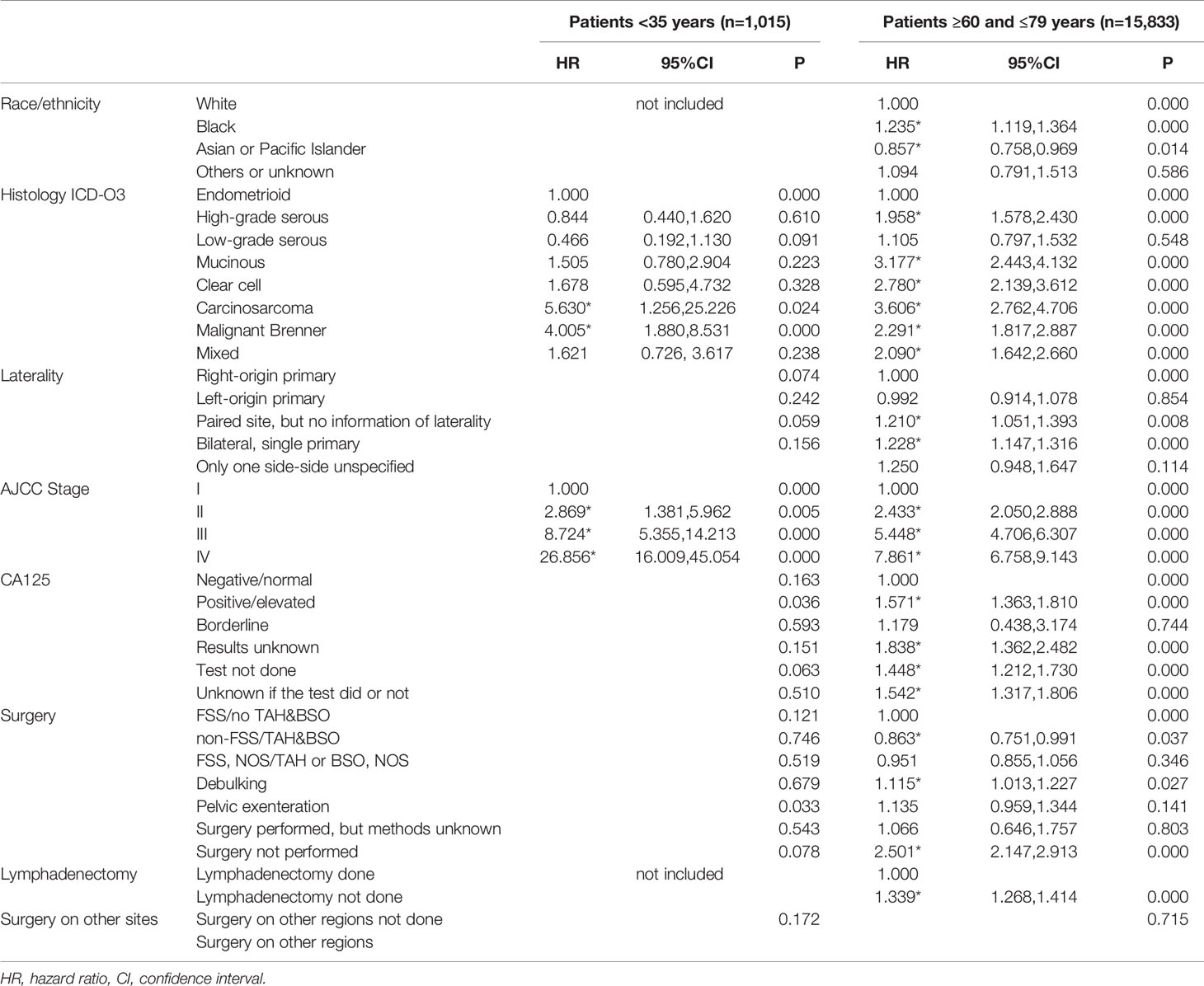
Table 4 Multivariate analysis by Cox regression for CSS in both young and old women group with epithelial ovarian cancer in 2004–2015, SEER 18 registries.
Discussion
This population-based study retrospectively analyzed different patterns of clinicopathological characteristics, treatment and outcomes between women with EOC aged under 35, those aged 60–79. Mucinous tumors and stage I represented the most common histological type and AJCC stage observed in young women, respectively, and both of them indicated better survival outcomes. For old women, however, high-grade serous tumors and stage III were most commonly seen, and they indicated worse survival outcomes. Histological type and AJCC stage were prognostic factors for CSS in both groups. For EOC patients aged 60–79, laterality, CA125 levels, surgery techniques, and lymphadenectomy were risk factors for CSS only. To our knowledge, the current study is the first study analyzing clinicopathological characteristics, treatment and survival outcomes in a large group of young EOC patients.
Mucinous (32.2%) represented the most common histological type in women aged under 35, followed by high-grade serous (26.6%), and endometrioid (18.3%) tumors. Our results are similar to a Japanese population-based study that demonstrated mucinous (36.7%) as the most prevalent histological type, followed by clear-cell (28.7%) and endometrioid (19.6%) in EOC women aged 40 and younger (14). However, other retrospective studies (6, 8–10, 13) found that serous histological type took up the largest portion in EOC women aged under 35 or 40. The difference could be explained by the following reasons. Firstly, most studies did not apply 2014 WHO EOC histology classification, which divides serous histological type into high-grade and low-grade. Secondly, the different age ranges and different ethnicity of included patients could also cause the difference. It should be noted that some included patients with mucinous histological type might have mucinous carcinomas that originated from the gastrointestinal tract, because mucinous carcinomas originated from different sites share very similar pathological characteristics (15). Currently, there are no immunohistochemical (IHC) algorithms for mucinous EOC, and an accurate diagnosis of primary mucinous EOC warrants a combination of the IHC, biomarkers and imaging results (16). Shimada et al. reviewed the pathological results of mucinous EOC patients and found only 33.9% were diagnosed with mucinous invasive carcinomas (17). It is agreed that with improved histopathology techniques and a greater understanding of mucinous carcinomas, the incidence of mucinous EOC drops to 3% (18). Therefore, the percentage of mucinous EOC in both < 35 years and 60–79 years group would be lower than the current results.
In both < 35 years group and 60–79 years group, endometrioid, mucinous, low-grade serous, and clear cell tumors indicated a higher 5-year CSS, while carcinomas, malignant Brenner tumors, and high-grade serous indicated lower 5-year CSS in the univariate survival analysis. The results are similar to the study of Aihua Lan (19), which is based on a SEER database without age stratification. In the multivariate analysis, however, mucinous was related to a poorer survival rate in 60–79 years group. In < 35 years group, although there was no statistical significance, the hazard rate of mucinous histological type was 1.505. The difference of mucinous-associated survival outcomes could result from several factors. Firstly, as we mentioned above, patients with mucinous carcinomas metastasized from the gastrointestinal tract were also included in our study and this group of patients would have much worse survival outcomes. Secondly, mucinous EOC diagnosed at an early stage had favorable prognosis, while those diagnosed at a late stage and recurrent tumors had poor survival outcomes because of the insensitivity to chemotherapy (13, 20–23). Increasing studies focused on unfolding the genetic secret of mucinous EOC in recent years. The mutation of KRAS protein, which is associated with the RAS/RAF/MARK pathway, might play an important role in the beginning event of mucinous EOC (24, 25). The amplification of HER2 and p53 genes mutation were also reported in patients with mucinous EOC, and both of them were associated with the malignant transformation in the stepwise progression of mucinous EOC (15, 22). Considering the high incidence of mucinous EOC in young women, gene diagnosis for prognosis and therapy consultation could be arranged in this patient group in the future.
The early-stage diagnosis of young women with EOC has been demonstrated in several studies (10, 26, 27). Mucinous and endometrioid histological types, which were commonly observed in young women, often present as localized masses, while high-grade serous tumors, commonly diagnosed in old women, often spread beyond pelvis at diagnosis (27, 28). Moreover, endometriosis-associated ovarian cancer (EAOC) including endometrioid and clear-cell histological types, are more often reported in young women. EAOC usually presents chronic pelvic pain, dyspareunia, dysmenorrhea and infertility, as well as significantly elevated CA125 levels, making them easier to be detected at an early stage (29–31). With the development of advanced molecular techniques, mutations in several genes such as ARID1A, PIK3CA, and CTCF have been found involved in the progression from benign endometriosis to EAOC (32). As a result, it is important for physicians to screen for EAOC in patients with endometriosis, and to initiate management as well as regular monitoring for those with a high risk of EAOC. It is known that positive family history and BRCA1/2 mutation could be detected especially in patients with early-onset EOC, indicating genetics as a key risk factor for young EOC patients (33). Therefore, combing the results of gene profiles with prediction models such as Risk of ovarian malignancy algorithm (ROMA), Copenhagen index (CPH-I), Risk of ovarian cancer algorithm (ROCA), LR2, and the Assessment of Different NEoplasias in the AdneXa (ADNEX) model, would be helpful in early recognition of young women with high risk of EOC and in the further monitoring process (34, 35).
The laterality of tumors was found to be a risk factor for the prognosis of old women, with bilateral tumors indicating a poorer outcome. This could be explained by the findings of Ditto et al. that bilaterality of EOC tumors is associated with lymph node metastases (36). Laterality failed to be a risk factor for young women could be due to the relatively small enrolled number. However, since we lacked the information on chemotherapy, which could act as a confounding factor as a number of old women did not receive chemotherapy due to their poor performance status, resulting in a false–positive result. More literature about the laterality of EOC tumors is thus warranted for further study.
In this study, we have demonstrated that young women did not benefit from lymphadenectomy, while old women with lymphadenectomy had a higher CSS compared to those without lymph nodes removal. The difference might be explained by our finding that young women with EOC were mostly at stage I, while the old were mostly at stage III, which suggests that lymph nodes were more likely to metastasize in old patients; thus those undetected lymph nodes containing metastatic cancer cells in old women would be removed. It could also be explained by the possible selective bias that women underwent lymphadenectomy in the control group might be relatively younger, and with a better health status compared to those without lymphadenectomy. Lymphadenectomy still represents a controversial issue in the surgery for ovarian cancer. The recent LION trial (37) focused on advanced ovarian patients with R0 cytoreductive surgery and negative lymph nodes detected both preoperatively and during the operation. In this patient group, the systemic pelvic and paraaortic lymphadenectomy were not associated with a better OS or progression-free survival (PFS). Similar results were observed in patients with ovarian cancer at early stages (38) and those who underwent lymphadenectomy after primary surgery (39). Due to the limited data on lymph nodes removal such as number, location, postoperative complication, and recurrence rate, we could not get further results. According to the evidence we have, we suggest that for young women there is no need for lymphadenectomy unless lymph nodes detected by radiologic examination or tested positive during the surgery, or fertility-sparing is required.
Some limitations could not be overlooked in the current study. Missing data on histology, stage and surgery might cause selective bias. Secondly, information on residual disease, cytoreduction surgery and neoadjuvant chemotherapy was included since 2010, which restricted us to analyze their roles in survival outcomes in the young patient group. Experience of surgeons, the specific type of lymphadenectomy, the recurrence incidence of EOC, and fertility outcomes of those who underwent uterine preserving surgeries were not included in the SEER database, thus we could not analyze their relationship with the outcomes of EOC patients. Currently, most researchers hold the view that combined biomarkers, instead of single one, are encouraging in the screening process (35, 40).For example, human epididymis protein 4 (HE4) and CA125 are included in ROMA with a sensitivity of 71% and specificity of 88% (41). However, only CA125 results were available in the SEER database.
In summary, women with EOC aged under 35 have higher OS and CSS compared to women aged 60–79, which could be due to a large percentage of mucinous and endometrioid histological types and early-diagnosis in the young EOC women group. Physicians can provide more positive prognosis information for young EOC patients, and the identification of histological types should be underscored in the diagnosis of this patient group. Moreover, gene diagnosis might play an important role in further prognosis assessments and clinical decisions.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because the data in the study was extracted from the SEER database, and the authors have obtained the approval to use the database.
Author Contributions
YH, XM, and ZyL designed the study. YH, BjL, and XM extracted and analyzed the data. YH and BjL wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We would like to thank Lin L from Stony Brook University for language editing and suggestions in statistics section. We appreciate Zhang Jj and Bi Sw from Sichuan University for language edition.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.595789/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin (2018) 68(4):284–96. doi: 10.3322/caac.21456
3. Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, et al. Patterns and Progress in Ovarian Cancer Over 14 Years. Obstet Gynecol (2006) 108(3, Part 1):521–8. doi: 10.1097/01.AOG.0000231680.58221.a7
4. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin (2011) 61(3):183–203. doi: 10.3322/caac.20113
5. Rodriguez M, Nguyen HN, Averette HE, Steren AJ, Penalver MA, Harrison T, et al. National survey of ovarian carcinoma XII. Epithelial ovarian malignancies in women less than or equal to 25 years of age. Cancer (Philadelphia) (1994) 73(4):1245–50. doi: 10.1002/1097-0142(19940215)73:4<1245::AID-CNCR2820730419>3
6. Pectasides D, Fountzilas G, Aravantinos G, Bamias A, Kalofonos HP, Skarlos D, et al. Epithelial ovarian carcinoma in younger vs older women: is age an independent prognostic factor? The Hellenic Oncology Cooperative Group experience. Int J Gynecol Cancer (2007) 17(5):1003–10. doi: 10.1111/j.1525-1438.2007.00912.x
7. Lalrinpuii E, Bhageerathy PS, Sebastian A, Jeyaseelan L, Vinotha T, Thomas A, et al. Ovarian Cancer in Young Women. Indian J Surg Oncol (2017) 8(4):540–7. doi: 10.1007/s13193-017-0680-z
8. Tang L, Zheng M, Xiong Y, Ding H, Liu FY. Clinical characteristics and prognosis of epithelial ovarian cancer in young women. Chin J Cancer (2008) 27(9):951–5. doi: 10.3321/j.issn:1000-467X.2008.09.011
9. Elzakkers JCJ, van der Aa MA, van Altena AM, de Hullu JA, Harmsen MG. Further insights into the role of tumour characteristics in survival of young women with epithelial ovarian cancer. Gynecol Oncol (2019) 155(2):213–9. doi: 10.1016/j.ygyno.2019.08.018
10. Massi D, Susini T, Savino L, Boddi V, Amunni G, Colafranceschi M. Epithelial ovarian tumors in the reproductive age group: Age is not an independent prognostic factor. Cancer (1996) 77(6):1131–6. doi: 10.1002/(SICI)1097-0142(19960315)77:63.0.CO;2-2
11. Duska LR, Chang YC, Flynn CE, Chen AH, Fuller AF, Goodman A, et al. Epithelial ovarian carcinoma in the reproductive age group. Cancer (1999) 85(12):2623–9. doi: 10.1002/(SICI)1097-0142(19990615)85:123.0.CO;2-O
12. Meczekalski B, Czyzyk A, Kunicki M, Podfigurna-Stopa A, Plociennik L, Jakiel G, et al. Fertility in women of late reproductive age: the role of serum anti-Mullerian hormone (AMH) levels in its assessment. J Endocrinol Invest (2016) 39(11):1259–65. doi: 10.1007/s40618-016-0497-6
13. Peres LC, Cushing-Haugen KL, Kobel M, Harris HR, Berchuck A, Rossing MA, et al. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst (2019) 111(1):60–8. doi: 10.1093/jnci/djy071
14. Yoshikawa N, Kajiyama H, Mizuno M, Shibata K, Kawai M, Nagasaka T, et al. Clinicopathologic features of epithelial ovarian carcinoma in younger vs. older patients: analysis in Japanese women. J Gynecol Oncol (2014) 25(2):118–23. doi: 10.3802/jgo.2014.25.2.118
15. Babaier A, Ghatage P. Mucinous Cancer of the Ovary: Overview and Current Status. Diagnostics (2020) 10:52. doi: 10.3390/diagnostics10010052
16. Hart WR. Mucinous tumors of the ovary: A review. Int J Gynecol Pathol (2005) 24:4–25. doi: 10.1097/01.pgp.0000148335.39146.a7
17. Thigpen JT. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Yearbook Oncol (2009) 2009:42–3. doi: 10.1016/s1040-1741(09)79380-7
18. Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol (2016) 27(Suppl 1):i53–7. doi: 10.1093/annonc/mdw087
19. Lan A, Yang G. Clinicopathological parameters and survival of invasive epithelial ovarian cancer by histotype and disease stage. Future Oncol (2019) 15(17):2029–39. doi: 10.2217/fon-2018-0886
20. Mackay HJ, Brady MF, Oza AM, Reuss A, Pujade-Lauraine E, Swart AM, et al. Prognostic Relevance of Uncommon Ovarian Histology in Women With Stage III/IV Epithelial Ovarian Cancer. Int J Gynecol Cancer (2010) 20(6):945–52. doi: 10.1111/IGC.0b013e3181dd0110
21. Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol (2011) 205(5):480 e1–8. doi: 10.1016/j.ajog.2011.06.049
22. Moioli M, Barra F, Maramai M, Valenzano Menada M, Vellone VG, Costantini S, et al. Mucinous ovarian cancer: current therapeutic targets, preclinical progress, and experimental drugs. Expert Opin Invest Drugs (2019) 28(12):1025–9. doi: 10.1080/13543784.2019.1693999
23. Ramalingam P. Morphologic, Immunophenotypic, and Molecular Features of Epithelial Ovarian Cancer. Oncology (2016) 30(2):166–76. doi: 10.1111/his.13984
24. Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Boyd J. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol (2003) 90(2):378–81. doi: 10.1016/s0090-8258(03)00264-6
25. Lee YJ, Lee MY, Ruan A, Chen CK, Liu HP, Wang CJ, et al. Multipoint Kras oncogene mutations potentially indicate mucinous carcinoma on the entire spectrum of mucinous ovarian neoplasms. Oncotarget (2016) 7(50):82097–103. doi: 10.18632/oncotarget.13449
26. Chan JK, Urban R, Cheung MK, Osann K, Shin JY, Husain A, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer (2006) 95(10):1314–20. doi: 10.1038/sj.bjc.6603457
27. Lockley M, Stoneham SJ, Olson TA. Ovarian cancer in adolescents and young adults. Pediatr Blood Cancer (2019) 66(3):e27512. doi: 10.1002/pbc.27512
28. Harrison ML, Jameson C, Gore ME. Mucinous ovarian cancer. Int J Gynecol Cancer (2008) 18(2):209–14. doi: 10.1111/j.1525-1438.2007.01022.x
29. Lyttle B, Bernardi L, Pavone ME. Ovarian cancer in endometriosis: clinical and molecular aspects. Minerva Ginecol (2014) 66(2):155–64.
30. Barreta A, Sarian L, Ferracini AC, Eloy L, Brito ABC, de Angelo Andrade L, et al. Endometriosis-Associated Ovarian Cancer: Population Characteristics and Prognosis. Int J Gynecol Cancer (2018) 28(7):1251–7. doi: 10.1097/IGC.0000000000001317
31. Check JH. CA-125 as a biomarker for malignant transformation of endometriosis. Fertil Steril (2009) 91(5):e35–5. doi: 10.1016/j.fertnstert.2009.02.036
32. Herreros-Villanueva M, Chen CC, Tsai EM, Er TK. Endometriosis-associated ovarian cancer: What have we learned so far? Clin Chim Acta (2019) 493:63–72. doi: 10.1016/j.cca.2019.02.016
33. Arts-de Jong M, Manders CM, Hoogerbrugge N, Ligtenberg MJ, Massuger LF, de Hullu JA, et al. Added value of family history in counseling about risk of BRCA1/2 mutation in early-onset epithelial ovarian cancer. Int J Gynecol Cancer (2013) 23(8):1406–10. doi: 10.1097/IGC.0b013e3182a1cf71
34. Terzic M, Aimagambetova G, Norton M, Della Corte L, Marin-Buck A, Lison JF, et al. Scoring systems for the evaluation of adnexal masses nature: current knowledge and clinical applications. J Obstet Gynaecol (2020) 29:1–8. doi: 10.1080/01443615.2020.1732892
35. Giampaolino P, Della Corte L, Foreste V, Vitale SG, Chiofalo B, Cianci S, et al. Unraveling a difficult diagnosis: the tricks for early recognition of ovarian cancer. Minerva Med (2019) 110(4):279–91. doi: 10.23736/S0026-4806.19.06086-5
36. Ditto A, Martinelli F, Reato C, Kusamura S, Solima E, Fontanelli R, et al. Systematic para-aortic and pelvic lymphadenectomy in early stage epithelial ovarian cancer: a prospective study. Ann Surg Oncol (2012) 19(12):3849–55. doi: 10.1245/s10434-012-2439-7
37. Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N Engl J Med (2019) 380(9):822–32. doi: 10.1056/NEJMoa1808424
38. Dell Anna T, Signorelli M, Benedetti-Panici P, Maggioni A, Fossati R, Fruscio R, et al. Systematic lymphadenectomy in ovarian cancer at second-look surgery: A randomised clinical trial. Br J Cancer (2012) 107(5):785–92. doi: 10.1038/bjc.2012.336
39. Dell Anna T, Signorelli M, Benedetti-Panici P, Maggioni A, Fossati R, Fruscio R, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer (2006) 95(6):699–704. doi: 10.1038/sj.bjc.6603323
40. Maggioni A, Benedetti Panici P, Dell Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Role of biomarkers for early detection of ovarian cancer recurrence. Gland Surg (2020) 9(4):1102–11. doi: 10.21037/gs-20-544
Keywords: Surveillance Epidemiology and End Results database, AJCC stage, survival, histological characteristics, epithelial ovarian cancer
Citation: Huang Y, Ming X, Li B and Li Z (2020) Histological Characteristics and Early-Stage Diagnosis Are Associated With Better Survival in Young Patients With Epithelial Ovarian Cancer: A Retrospective Analysis Based on Surveillance Epidemiology and End Results Database. Front. Oncol. 10:595789. doi: 10.3389/fonc.2020.595789
Received: 17 August 2020; Accepted: 19 November 2020;
Published: 23 December 2020.
Edited by:
Tonya J. Webb, University of Maryland, Baltimore, United StatesReviewed by:
Renzo Luciano Boldorini, Università degli Studi del Piemonte Orientale, ItalyAntoni Llueca Abella, University of Jaume I, Spain
Copyright © 2020 Huang, Ming, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyu Li, emhlbmd5dWxpMDFAMTI2LmNvbQ==
 Yue Huang
Yue Huang Xiu Ming1,2
Xiu Ming1,2 Bingjie Li
Bingjie Li Zhengyu Li
Zhengyu Li