- Department of Pediatrics, Peking University People’s Hospital, Peking University, Beijing, China
Purpose: While the role of minimal residual disease (MRD) assessment and the significance of achieving an MRD-negative status during treatment have been evaluated in previous studies, there is limited evidence on the significance of MRD re-emergence without morphological relapse in acute lymphoblastic leukemia (ALL). We sought to determine the clinical significance of MRD re-emergence in pediatric ALL patients.
Methods: Between 2005 and 2017, this study recruited 1126 consecutive patients newly diagnosed with ALL. Flow cytometry was performed to monitor MRD occurrence during treatment.
Results: Of 1030 patients with MRD-negative results, 150 (14.6%) showed MRD re-emergence while still on morphological complete remission (CR). Patients with white blood cell counts of ≥50 × 109/L (p = 0.033) and MRD levels of ≥0.1% on day 33 (p = 0.012) tended to experience MRD re-emergence. The median re-emergent MRD level was 0.12% (range, 0.01–10.00%), and the median time to MRD re-emergence was 11 months (range, <1–52 months). Eighty-five (56.6%) patients subsequently developed relapse after a median of 4.1 months from detection of MRD re-emergence. The median re-emergent MRD level was significantly higher in the relapsed cohort than in the cohort with persistent CR (1.05% vs. 0.48%, p = 0.005). Of the 150 patients, 113 continued to receive chemotherapy and 37 underwent transplantation. The transplantation group demonstrated a significantly higher 2-year overall survival (88.7 ± 5.3% vs. 46.3 ± 4.8%, p < 0.001) and cumulative incidence of relapse (23.3 ± 7.4% vs. 64.0 ± 4.6%, p < 0.001) than the chemotherapy group.
Conclusions: MRD re-emergence during treatment was associated with an adverse outcome in pediatric ALL patients. Transplantation could result in a significant survival advantage for these patients.
Introduction
Acute lymphoblastic leukemia (ALL) is the most prevalent hematological malignancy in children (1). Advances in our understanding of the clinical features, immunobiological characteristics, and cytogenetic alterations associated with ALL have led to better risk stratification and risk-directed treatment of ALL patients (2, 3). In pediatric ALL, minimal residual disease (MRD) levels reflect the efficacy of chemotherapy and have shown to be the most powerful prognostic factor. While the role of MRD assessment and the significance of achieving an MRD-negative status at the end of induction and consolidation therapy have been evaluated in previous studies, there is limited evidence on the significance of MRD re-emergence without morphological relapse in ALL, in the context of sequential MRD monitoring. Our previous study showed that MRD re-emergence was an adverse prognostic factor in children at high risk of ALL (4). Pui et al. (5) and Pemmaraju et al. (6) have also reported that MRD re-emergence is associated with a poor outcome in ALL.
Flow cytometry (FCM) was explored as a less labor-intensive, less expensive, and faster MRD technique than polymerase chain reaction (PCR)-based methods and has been used extensively in pediatric ALL patients (7). Since 2005, we have monitored MRD sequentially using FCM at our institution. This study therefore aimed to determine the significance of MRD re-emergence in pediatric ALL patients after achieving an MRD-negative status.
Materials and Methods
Patients
Between January 2005 and December 2017, this trial recruited consecutive patients aged 0 to 18 years who were newly diagnosed with ALL. Patients with mature B-cell leukemia were excluded. The study was approved by the Ethics Committee of Peking University People’s Hospital and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the parents or guardians of the patients. The BOSHI Network Database (https://www.boshicloud.com), an online platform for clinical patient information management and data analysis, was used to retrieve and monitor patient data.
Diagnosis, Minimal Residual Disease Measurement, and Risk Classification
ALL was diagnosed based on morphological, immunophenotypic, and cytogenetic evaluation using standard techniques (8, 9). Fusion transcripts of t(12;21)/ETV6‐RUNX1, t(1;19)/TCF3‐PBX1, t(9;22)/BCR‐ABL1, and 11q23/KMT2A rearrangement (KMT2A-r) were measured using PCR and/or fluorescence in situ hybridization, as previously described (10, 11).
MRD was measured using FCM, with a sensitivity of 0.01% (12, 13). The MRD monitoring schedule was planned in advance, and the scheduled time points for induction therapy were on day 15 and day 33. Patients were classified as either M1 (blast cells, <5%), M2 (5–25%), or M3 (≥25%) based on morphological evaluation. After induction therapy, MRD measurements were performed every 2–3 months during consolidation chemotherapy and every 6 months during maintenance chemotherapy (4). More frequent MRD monitoring was performed for some patients, depending on their conditions.
For initial risk stratification, we referred to the National Cancer Institute (NCI) risk group criteria (14) and cytogenetic subtypes, while the final assessment was based on treatment response and MRD levels during and after induction therapy (15) (Supplementary Figure 1). Standard-risk (SR) patients with an M3 marrow status on day 15 or MRD measurements of 0.01–0.99% on day 33 were upstaged to intermediate risk (IR), whereas IR patients with an M3 marrow status on day 15 were assigned high risk (HR). Patients who did not achieve complete remission (CR) upon completion of induction therapy or had MRD levels of ≥1% on day 33 or ≥0.1% on week 12 were also upstaged to HR.
Definition
CR was defined as a percentage of leukemic blasts of <5% in the bone marrow (BM) sample reviewed at the time of peripheral blood count recovery, the absence of circulating peripheral blasts, and the absence of extramedullary disease. Relapse was defined as the presence of leukemic blasts in any extramedullary location, or in the BM at a level of ≥5%. Moreover, MRD re-emergence was defined as at least two consecutive detectable recurrences of MRD (sensitivity for positive value, ≥0.01%), despite the persistence of morphological CR. The level for MRD positivity was based on first MRD re-emergence level. After the first MRD re-emergence, a second MRD test was scheduled within the next two weeks.
Treatment
All patients underwent a modified version of the ALL-Berlin-Frankfurt-Munster (BFM) protocol described previously (4). Briefly, the patients underwent induction therapy, including vincristine, idarubicin, cyclophosphamide, prednisone/dexamethasone, and L-asparaginase (COIPL), followed by consolidation therapy with one to two cycles of re-induction and maintenance therapy (Supplementary Figure 1 and Supplementary Table 1). The consolidation chemotherapy regimen included high-dose methotrexate (HDMTX) (targeted steady-state concentration of 16 μM/L for SR patients and 24 μM/L for IR/HR patients), high-dose cytarabine (HDAra-C) (cytarabine for SR patients and cytarabine + idarubicin for IR/HR patients), and ifosfamide (IFO) (only for HR patients), which were given alternately. Re-induction comprised 1 course of COIPL for SR patients and 2 courses of COIPL for IR/HR patients. Maintenance therapy included daily mercaptopurine and weekly methotrexate. Re-induction was administered every 6 months during the consolidation chemotherapy. The scheduled consolidation chemotherapy comprised 9 rounds of HDMTX and 2 rounds of HDAra-C for SR patients, 11 rounds of HDMTX and 2 rounds of HDAra-C for IR patients, and 13 rounds of HDMTX and 3 rounds of HDAra-C for HR patients. Since 2010, for patients diagnosed with BCR-ABL1 ALL (days 8–15 of induction), imatinib mesylate was initiated at a dose of 260 to 340 mg/m2/day. The total doses of idarubicin and L-asparaginase were 80 mg/m2 and 200 000 units/m2 for SR patients, and 100 mg/m2 and 300,000 units/m2 for IR/HR patients, respectively.
All patients regularly received triple intrathecal therapy to prevent central nervous system (CNS) leukemia. The total number of intrathecal therapies administered ranged from 16 in SR patients to 23 in IR/HR patients. Patients presenting with CNS leukemia received twice-weekly intrathecal chemotherapy until normalization of cerebrospinal fluid levels, after which they received weekly CNS therapy for four more doses. The total duration of treatment was 3 years for SR patients and 3.5 years for IR/HR patients. Patients in the HR group who achieved CR were offered the option of undergoing allogeneic hematopoietic cell transplantation (allo-HSCT). The transplant conditioning regimens were administered as previously described (4).
Statistical Analysis
The outcome data used in the analysis were last updated on April 15, 2020. Overall survival (OS) was defined as the time between the date of diagnosis and the date of death due to any reason or the date of last contact. Event-free survival (EFS) was defined as the time between the date of diagnosis and the date of an event (e.g., relapse, second malignancy, death due to any reason) or the date of the last follow-up. The Kaplan-Meier method was used to estimate the survival rates, and log-rank tests were used to compare their differences. Multivariate analyses were performed using a Cox proportional hazards model. The cumulative incidence of relapse (CIR) for competing events was constructed using the Kalbfleisch-Prentice method. The OSMRD-r and CIRMRD-r were evaluated from the time of MRD re-emergence. Fisher’s exact test was used to compare differences between categorical variables among the groups. Logistic regression was used to evaluate factors affecting the re-emergence of MRD. R software version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 26.0 (SPSS Inc., Chicago, IL) were used for statistical analyses.
Results
Patient Characteristics and Treatment Outcomes
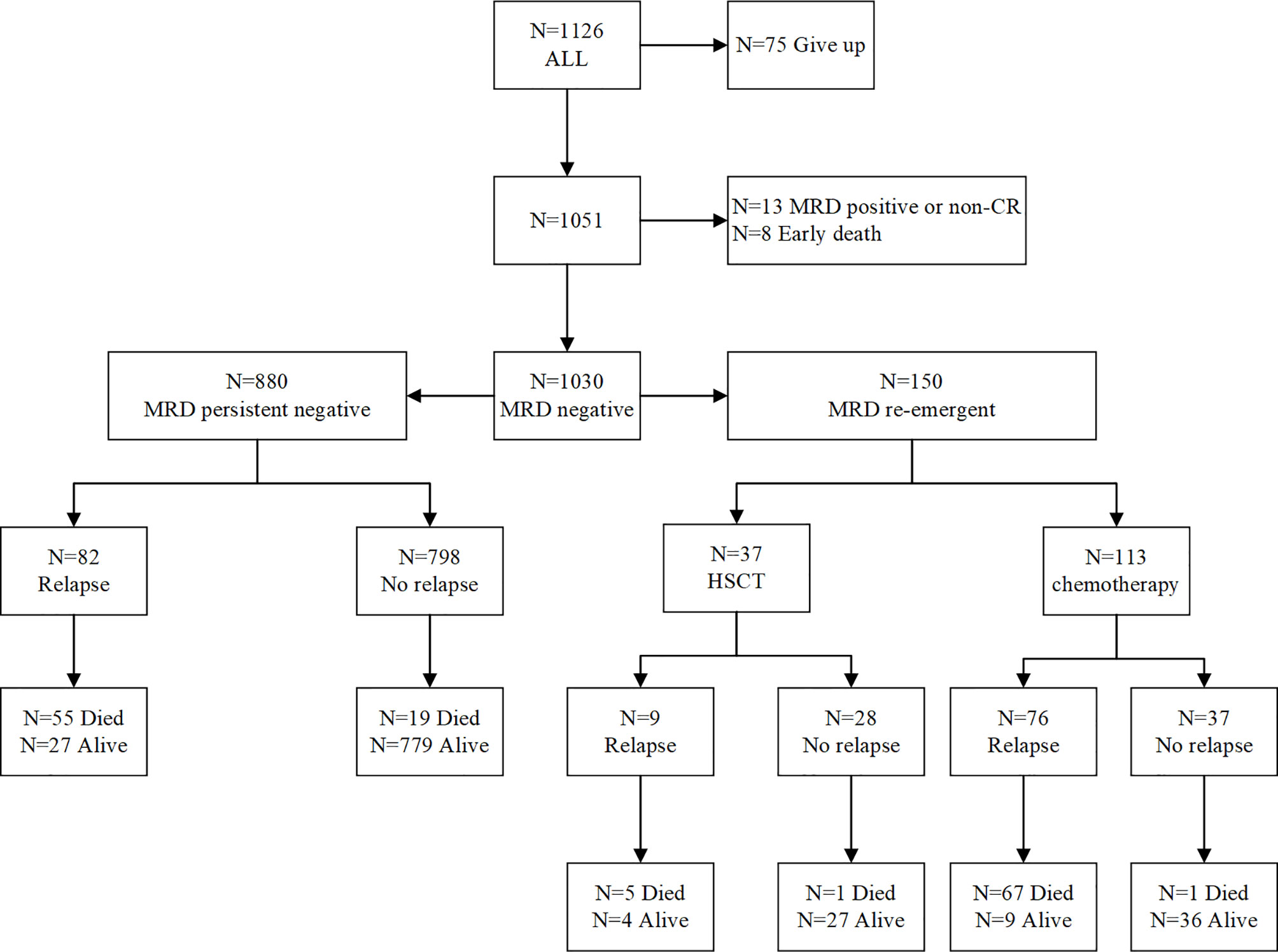
There were 1126 patients with newly diagnosed ALL during the study period in our center. Among them, 25 (2.2%) did not complete the induction treatment and lost contact, while 50 (4.4%) who were on CR without serious toxicities gave up treatment because of financial difficulties, of whom 40 were in the early intensification phase and 10 were in the consolidation phase. Ultimately, 1051 patients were enrolled in the study. At a median follow-up of 60.6 months (range, 0.8–184.5 months), the estimated 5-year OS, EFS, and CIR in the 1051 patients were 84.0 ± 1.0%, 79.0 ± 1.0%, and 17.8 ± 1.2%, respectively.
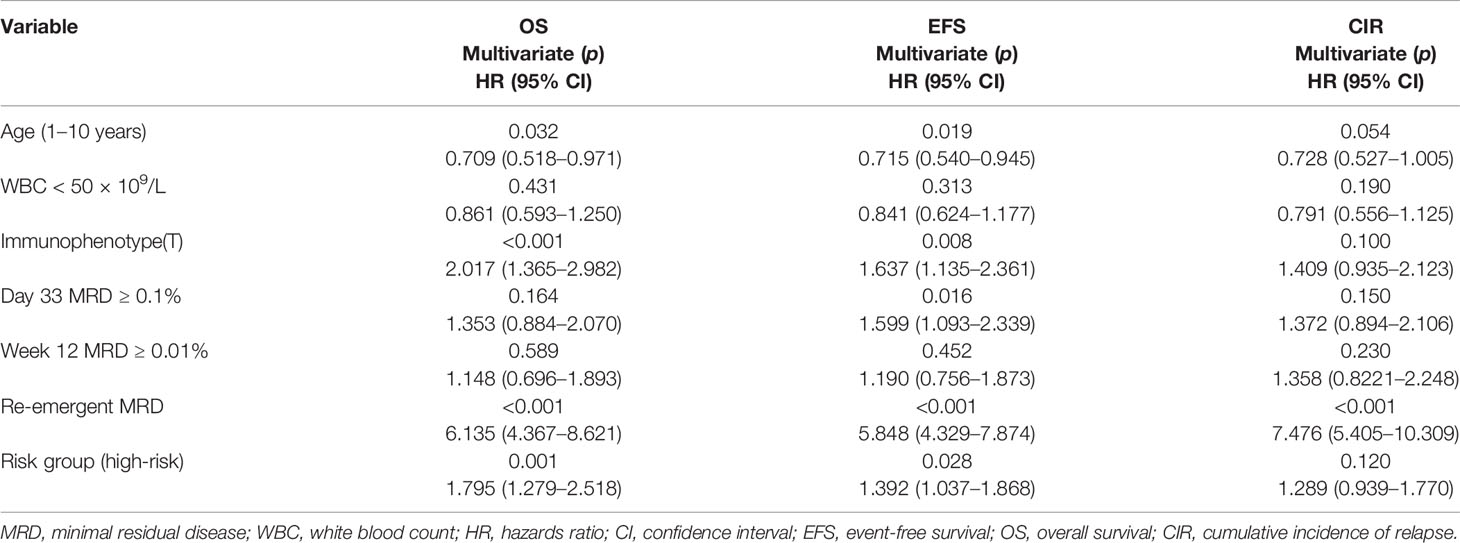
Multivariate predictors of outcome in pediatric ALL are presented in Table 1. In the multivariate analysis, the re-emergence of MRD during treatment was the most powerful prognostic factor for OS (p < 0.001, hazard ratio = 6.135), EFS (p < 0.001, hazard ratio = 5.848), and CIR (p < 0.001, hazard ratio = 7.476). The 5-year OS, EFS, and CIR for patients with re-emergent MRD were 49.8 ± 4.3%, 38.4 ± 4.2%, and 60.2 ± 4.3%, respectively. In patients with persistently MRD-negative results, the corresponding values were significantly better, at 91.7 ± 1.0%, 88.5 ± 1.1%, and 9.2 ± 1.0% (p < 0.001) (Figures 1A–C).
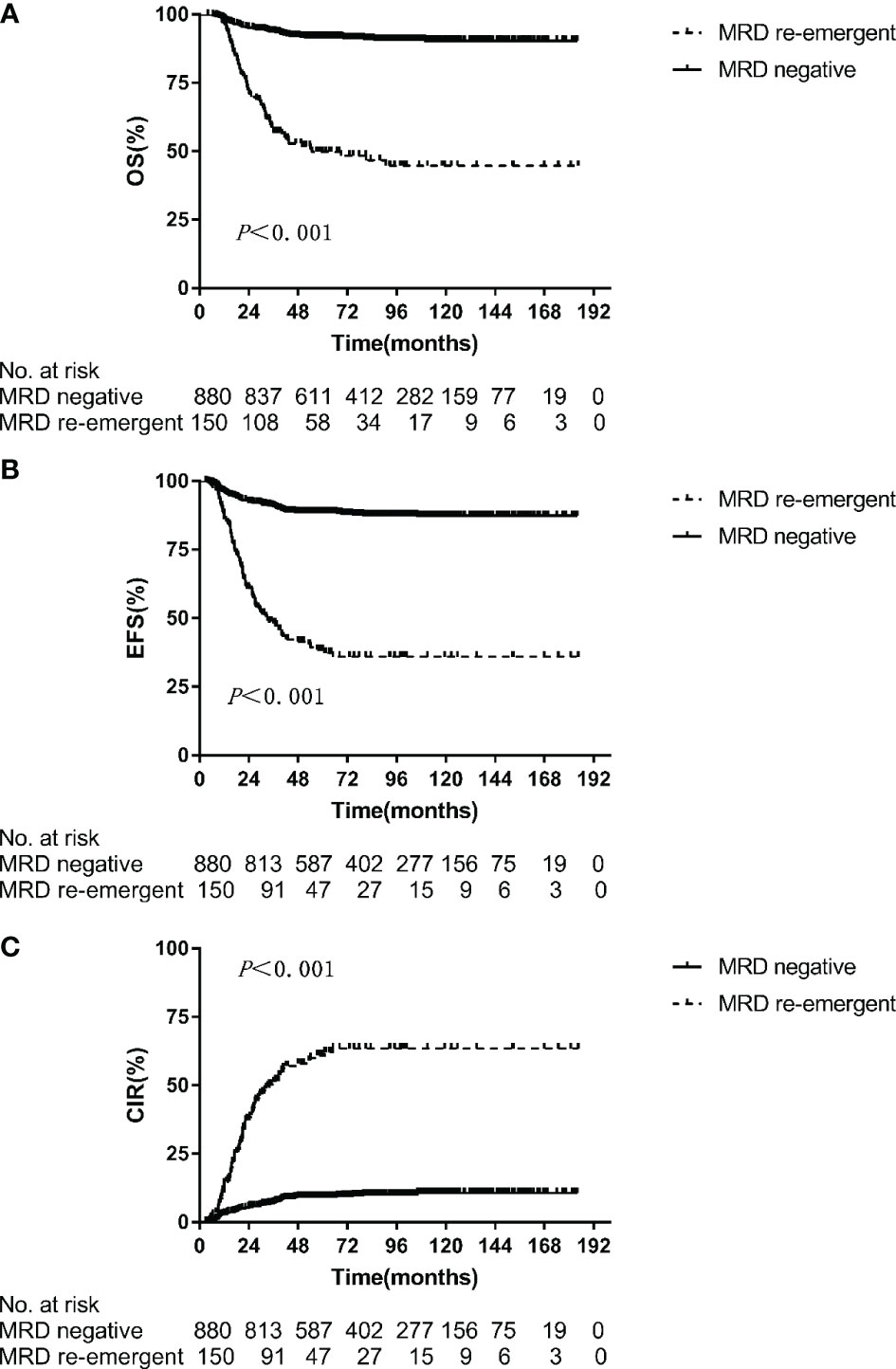
Figure 1 Overall survival (A), event-free survival (B), cumulative incidence of relapse (C) for MRD negative and MRD re-emergent group.
Re-Emergence of Minimal Residual Disease
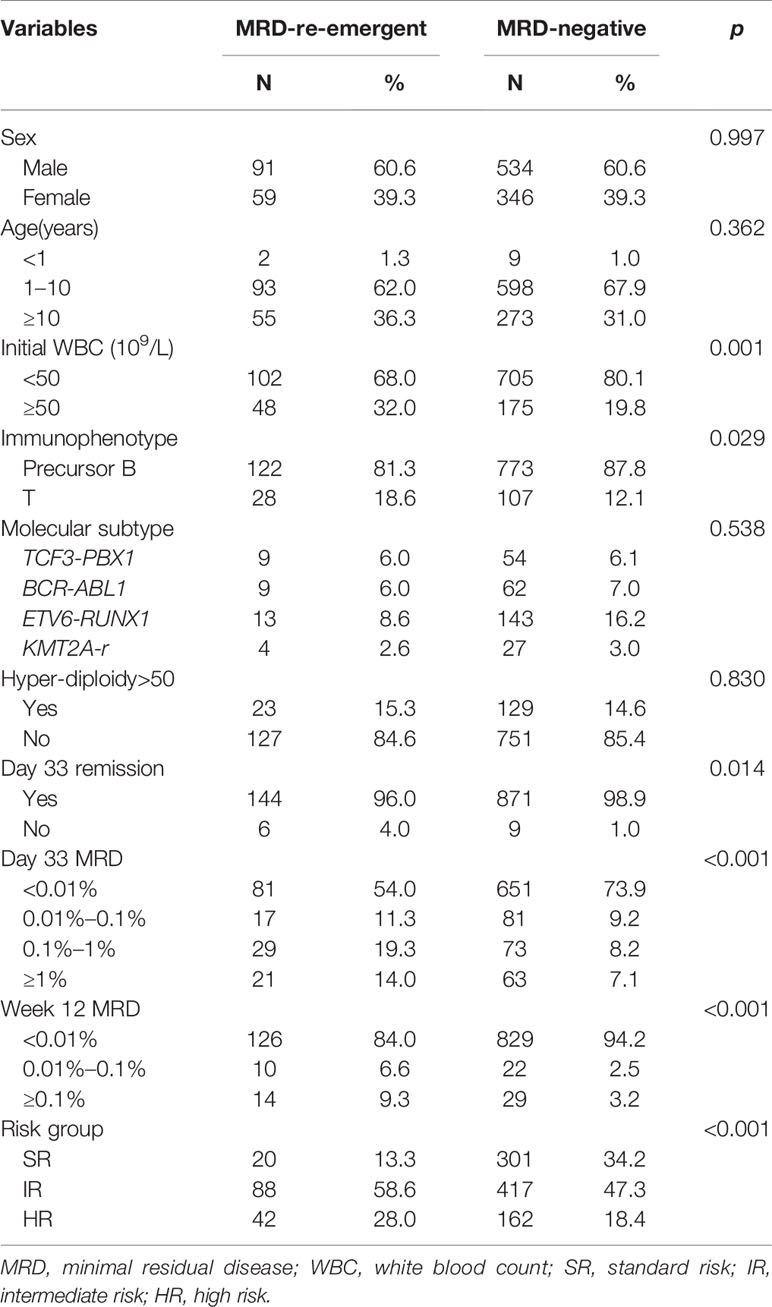
Among the 1051 patients, 8 died during induction therapy and 13 maintained a persistently MRD-positive status until relapse. Finally, 1030 patients achieved an MRD-negative status on BM examination. Of these patients, 150 (14.6%) ultimately developed re-emergent MRD while still on morphological CR and were the focus of this analysis. Figure 2 depicts the study flowchart for patient disposition. Further, we analyzed the characteristics of patients with persistently MRD-negative results and re-emergent MRD (Table 2), and found that those with white blood cell (WBC) counts of ≥50 × 109/L (hazard ratio, 1.609; 95% confidence interval [CI], 1.034–2.488; p = 0.033) and MRD levels of ≥0.1% on day 33 (hazard ratio, 1.908; 95% CI, 1.145–3.145; p = 0.012) tended to have re-emergent MRD.
The overall median level for MRD positivity in the 150 patients was 0.12% (range, 0.01–10.00%). The median duration from MRD negativity to MRD re-emergence was 11 months (range, <1–52 months). Eighty-five (56.6%) patients subsequently developed relapse (78 patients with BM leukemia, 4 with BM + CNS leukemia, 2 with BM + testicular leukemia, and 1 with leukemia in other extramedullary sites) after a median of 4.1 months (range, <1–47.4 months) from the detection of MRD re-emergence. Among the 150 patients with re-emergent MRD, the median level for the first MRD-positive result was significantly higher in the relapsed cohort than in the cohort with persistent CR (1.05% vs. 0.48%, p = 0.005) (Figure 3A). To further investigate the predictive role of re-emergent MRD in relapse, we performed a receiver operating characteristic (ROC) curve analysis of the first re-emergent MRD level and the actual development of relapse. It turned out that the area under the ROC curve (AUC) was 0.631 (95% CI, 0.540–0.708; p = 0.004). Further, we investigated the diagnostic accuracy using different MRD levels as cutoff points. The optimal cutoff point to predict relapse was 0.15%, with a sensitivity and specificity of 61.65% and 71.69%, respectively. The median duration from MRD negativity to MRD re-emergence tended to be shorter for patients who experienced a subsequent relapse, although the differences did not reach statistical significance (11.9 vs. 15.0 months, p = 0.068) (Figure 3B).
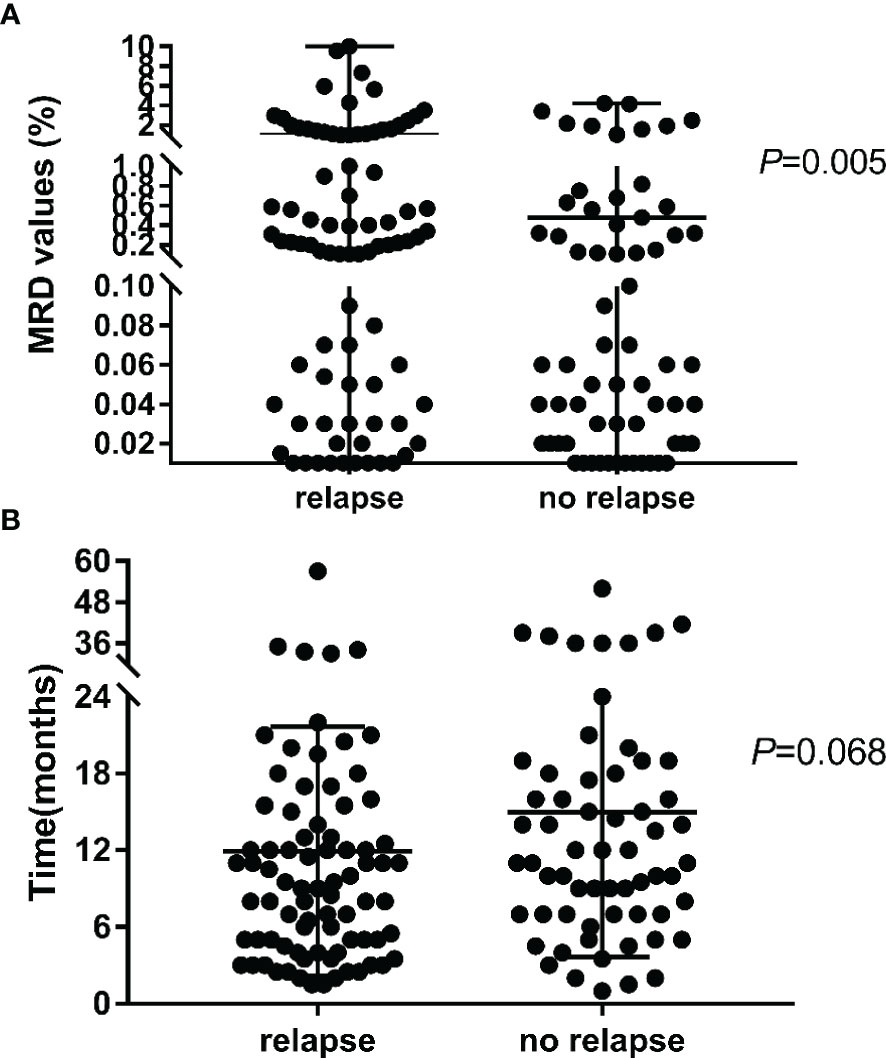
Figure 3 The MRD value (A) and duration time (B) for the first MRD re-emergent in the subsequent relapse and no relapse cohort.
When the OSMRD-r was evaluated from the time of MRD re-emergence to the last follow-up, its median value was 20.6 months in the 150 patients. To determine if the time of MRD re-emergence had an effect on the outcome of ALL, patients were divided into two groups: <12 months and ≥12 months from MRD negativity to MRD re-emergence. No statistically significant differences in 2-year OSMRD-r (60.6 ± 6.2% vs. 54.0 ± 5.5%, p = 0.745) and 2-year CIRMRD-r (49.7 ± 6.4% vs. 56.8 ± 5.5%, p = 0.582) were found between the two groups. Regarding treatments prior to morphological relapse among these 150 patients, 113 patients continued to receive maintenance chemotherapy according to the specified treatment protocol and 37 underwent allo-HSCT (30 from haploidentical donors, 5 from HLA-identical sibling donors, and 2 from matched unrelated donors). Moreover, the HSCT group showed significantly better 2-year OSMRD-r (88.7 ± 5.3% vs. 46.3 ± 4.8%, p < 0.001) and 2-year CIRMRD-r (23.3 ± 7.4% vs. 64.0 ± 4.6%, p < 0.001) than the chemotherapy group (Figures 4A, B).
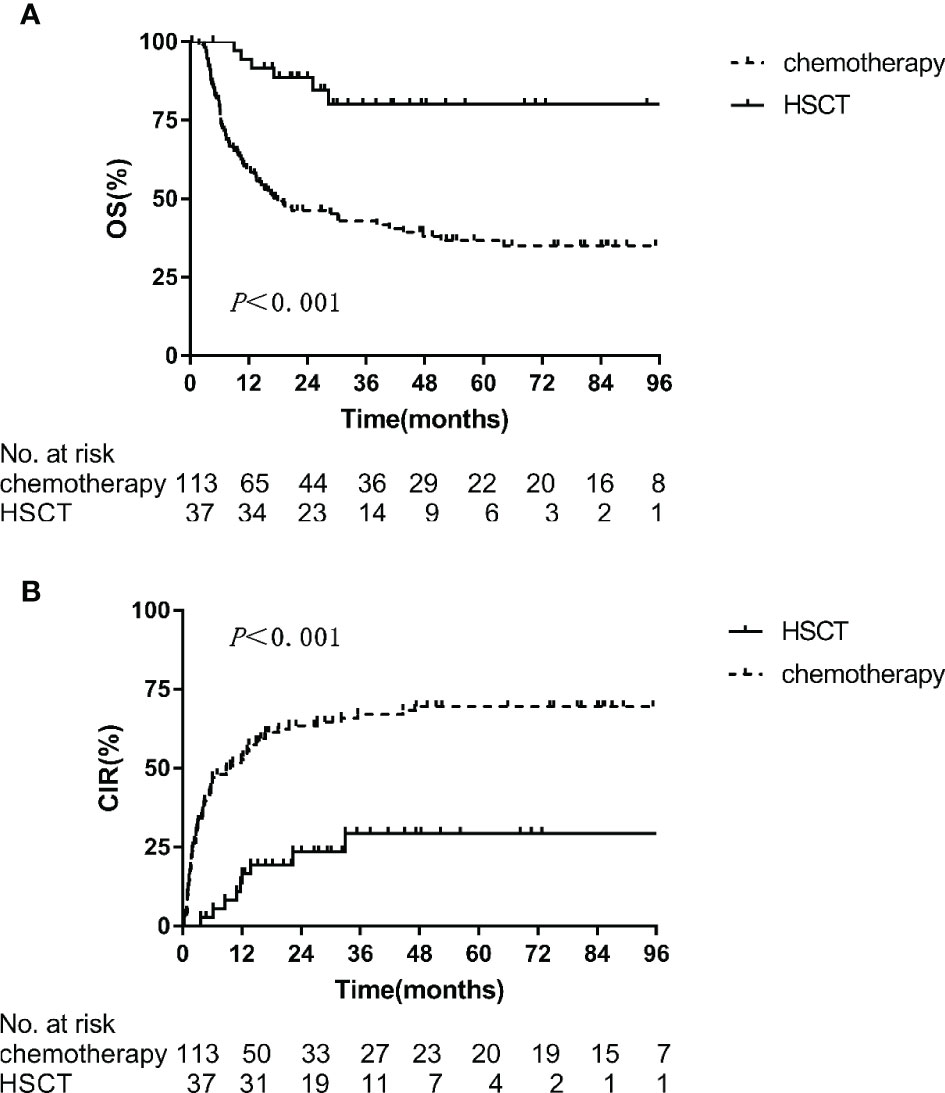
Figure 4 Kaplan-Meier estimates of 2-year outcomes in the HSCT and chemotherapy arms in MRD re-emergent group. (A) OS, (B) CIR.
Discussion
With a median follow-up of 60.6 months, this single-institution trial showed that the 5-year OS and EFS of the 1051 pediatric patients with ALL were 84.0 ± 1.0% and 79.0 ± 1.0%, which are comparable to the results of other studies (15–18). Multiple studies have established MRD detection as an independent prognostic factor for ALL and have demonstrated that achievement of an MRD-negative status could lead to better clinical outcomes (3, 19, 20). In our study, we applied FCM for sequential post-remission MRD measurement and found that patients with higher end-induction MRD levels (≥0.1%) and positive levels of MRD on week 12 (≥0.01%) exhibited a worse EFS and OS. However, in the multivariate analysis of the whole cohort, MRD at any particular time point did not show a strong prognostic significance. We calculated that there may be two reasons for this result. First, the risk stratification of the patients in this study was adjusted based on the MRD level at end-induction and week 12. Therefore, risk stratification-oriented treatment may affect the results. Second, MRD may not show a strong prognostic significance on the context of MRD-guided therapy and MRD alone was not sufficient to fully predict outcomes. The significance of MRD on treatment outcomes varied depending on leukemia subtypes and measurement time, such as different genotypes. Meanwhile, re-emergent MRD during treatment was the most powerful adverse prognostic indicator, even after adjusting for other risk factors. The 5-year OS (91.7 ± 1.0% vs. 49.8 ± 4.3%, p < 0.001) and EFS (88.5 ± 1.1% vs. 38.4 ± 4.2%, p < 0.001) were significantly better in the persistently MRD-negative group. This obvious survival gap strongly confirmed the poor prognostic significance of MRD re-emergence in ALL.
In this study, 14.6% (150/1030) of pediatric ALL patients experienced MRD re-emergence while still on morphological CR. Patients with a high leukemia burden (WBC ≥ 50 × 109/L) and a poor response to early treatment (MRD levels ≥ 0.1% on day 33) were prone to MRD re-emergence, which indicates the need for further strengthening MRD monitoring in these patients. Several previous investigations demonstrated the clinical potential and prognostic value of FCM- or PCR-based MRD quantification in the post-remission setting, producing lead times from clinical relapse of 3.6 to 4.1 months (6, 21). In our analysis, 85 (56.6%) patients subsequently developed relapse after a median of 4.1 months from the detection of re-emergent MRD, and this finding was consistent with those of previous studies. Additionally, a strong correlation was observed between re-emergent MRD levels and clinical relapse, suggesting that a higher re-emergent MRD level (cutoff, 0.15%) may signify an impending relapse. It was worth mentioning that a total of five patients had a re-emergence of MRD > 2%, but never developed a morphologic relapse. One of the patients had a large deletion of IKZF gene. He started taking tyrosine kinase inhibitors after MRD recurrence, and continued to survive disease-free. As of the last follow-up date, he had been followed up for 61.6 months. The other four patients all chose further transplantation rescue treatment after MRD recurrence, and all of them survived disease-free.
As re-emergent MRD can reliably predict clinical relapse, we should monitor MRD sequentially to expand the time window for a more effective preemptive treatment against a potential relapse (22, 23). In this retrospective study, patients with re-emergent MRD were given the choice between HSCT or chemotherapy according to their preference. The results showed that the HSCT group had a significantly higher survival advantage than the chemotherapy group. Re-emergent MRD may be a group of residual leukemia cells that are out of the detection range of FCM and resistant to chemotherapy (3, 20). Although the intensification of chemotherapy may not fully eliminate re-emergent MRD, the strong graft-versus-leukemia effect of HSCT may help (24). However, the outcome of the HSCT group in this study was unsatisfactory, highlighting the urgent need for novel, less toxic strategies and enrollment of subjects in clinical trials specifically designed for ALL patients with MRD persistence or re-emergence.
This study has a few limitations. It is a retrospective single-center study without predetermined enrollment criteria. Limited by the availability of donors and patients’ preference of whether to undergo HSCT, we were unable to define the indications of transplantation in advance. Furthermore, technical constraints such as low tumor burden, immunophenotypic shifts, and clonal selection may have contributed to a decreased sensitivity of measurements, leading to more false-negative results, as suggested by the occurrence of relapse in patients with negative FCM-MRD findings (25). Recent studies have described a highly sensitive next-generation sequencing platform to monitor MRD and have observed the conversion of an MRD-negative status to a positive one as early as 25.6 weeks prior to clinical relapse (21). Besides, patients in this study did not undergo a unified treatment escalation after the recurrence of MRD, because although multiple studies have confirmed the poor prognosis of MRD re-emergence, the current international standards have not yet reached a consensus on the treatment of risk escalation after the recurrence of MRD. However, we believe that the findings of this study will provide more powerful evidence to support the future treatment options for patients with re-emergent MRD.
In conclusion, this study revealed that MRD re-emergence at any time after induction and consolidation therapy was associated with relapse in pediatric ALL patients. We also found that patients with re-emergent MRD could benefit from HSCT, reflecting the necessity of sequential MRD monitoring for better risk stratification and earlier preemptive therapies against impending relapse, thus potentially improving outcome for pediatric B-ALL. Prospective studies on sequential MRD monitoring coupled with less toxic strategies such as chimeric antigen receptor T cell therapy designed to eradicate MRD are warranted to address the unmet medical needs of pediatric ALL patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Material preparation and data collection were performed by Y-PJ, Y-XZ, and A-DL. Data analysis was performed and the first draft of the manuscript was written by YW and Y-JX, and they contributed equally to this work. L-PZ designed the research and was the chief person in charge of the manuscript. All authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Foundation of 2018 Beijing Key Clinical Specialty Construction Project-Pediatrics (2199000726).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.596677/full#supplementary-material
References
1. Bonaventure A, Harewood R, Stiller CA, Gatta G, Clavel J, Stefan DC, et al. Worldwide comparison of survival from childhood leukaemia for 1995-2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol (2017) 4:e202–17. doi: 10.1016/S2352-3026(17)30052-2
2. Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood (2017) 129:1913–8. doi: 10.1182/blood-2016-12-725804
3. Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis. JAMA Oncol (2017) 3:e170580. doi: 10.1001/jamaoncol.2017.0580
4. Xue YJ, Suo P, Huang XJ, Lu AD, Wang Y, Zuo YX, et al. Superior survival of unmanipulated haploidentical haematopoietic stem cell transplantation compared with intensive chemotherapy as post-remission treatment for children with very high-risk philadelphia chromosome negative B-cell acute lymphoblastic leukaemia in first complete remission. Br J Haematol (2020) 188:757–67. doi: 10.1111/bjh.16226
5. Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol (2015) 16:465–74. doi: 10.1016/S1470-2045(15)70082-3
6. Pemmaraju N, Kantarjian H, Jorgensen JL, Jabbour E, Jain N, Thomas D, et al. Significance of recurrence of minimal residual disease detected by multi-parameter flow cytometry in patients with acute lymphoblastic leukemia in morphological remission. Am J Hematol (2017) 92:279–85. doi: 10.1002/ajh.24629
7. Short NJ, Jabbour E. Minimal Residual Disease in Acute Lymphoblastic Leukemia: How to Recognize and Treat It. Curr Oncol Rep (2017) 19:6. doi: 10.1007/s11912-017-0565-x
8. Pui CH, Evans WE. Acute lymphoblastic leukemia. New Engl J Med (1998) 339:605–15. doi: 10.1056/NEJM199808273390907
9. Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia (1995) 9:1783–6.
10. Qin Y, Zhu H, Jiang B, Li J, Lu X, Li L, et al. Expression patterns of WT1 and PRAME in acute myeloid leukemia patients and their usefulness for monitoring minimal residual disease. Leuk Res (2009) 33:384–90. doi: 10.1016/j.leukres.2008.08.026
11. Qin YZ, Zhu HH, Liu YR, Wang YZ, Shi HX, Lai YY, et al. PRAME and WT1 transcripts constitute a good molecular marker combination for monitoring minimal residual disease in myelodysplastic syndromes. Leuk Lymphoma (2013) 54:1442–9. doi: 10.3109/10428194.2012.743656
12. Liu YR, Zhang LP, Chang Y, Cheng YF, Fu JY, Li LD, et al. [Clinical significance for minimal residual disease detection by 4 color flow cytometry in adult and childhood B lineage acute lymphoblastic leukemia]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi (2006) 27:302–5.
13. Zhao XS, Liu YR, Zhu HH, Xu LP, Liu DH, Liu KY, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol (2012) 91:183–92. doi: 10.1007/s00277-011-1285-1
14. Winick N, Devidas M, Chen S, Maloney K, Larsen E, Mattano L, et al. Impact of Initial CSF Findings on Outcome Among Patients With National Cancer Institute Standard- and High-Risk B-Cell Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. J Clin Oncol (2017) 35:2527–34. doi: 10.1200/JCO.2016.71.4774
15. Cui L, Li ZG, Chai YH, Yu J, Gao J, Zhu XF, et al. Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: The first nation-wide prospective multicenter study in China. Am J Hematol (2018) 93:913–20. doi: 10.1002/ajh.25124
16. Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol (2012) 30:2384–92. doi: 10.1200/JCO.2011.40.5936
17. Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia (2018) 32:606–15. doi: 10.1038/leu.2017.265
18. Takahashi H, Kajiwara R, Kato M, Hasegawa D, Tomizawa D, Noguchi Y, et al. Treatment outcome of children with acute lymphoblastic leukemia: the Tokyo Children’s Cancer Study Group (TCCSG) Study L04-16. Int J Hematol (2018) 108:98–108. doi: 10.1007/s12185-018-2440-4
19. Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood (2009) 113:4153–62. doi: 10.1182/blood-2008-11-185132
20. Abou Dalle I, Jabbour E, Short NJ. Evaluation and management of measurable residual disease in acute lymphoblastic leukemia. Ther Adv Hematol (2020) 11:2040620720910023. doi: 10.1177/2040620720910023
21. Cheng S, Inghirami G, Cheng S, Tam W. Simple deep sequencing-based post-remission MRD surveillance predicts clinical relapse in B-ALL. J Hematol Oncol (2018) 11:105. doi: 10.1186/s13045-018-0652-y
22. Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. New Engl J Med (2018) 378:449–59. doi: 10.1056/NEJMoa1709919
23. Liu J, Zhong JF, Zhang X, Zhang C. Allogeneic CD19-CAR-T cell infusion after allogeneic hematopoietic stem cell transplantation in B cell malignancies. J Hematol Oncol (2017) 10:35. doi: 10.1186/s13045-017-0405-3
24. Ravandi F, Jorgensen JL, O’Brien SM, Jabbour E, Thomas DA, Borthakur G, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol (2016) 172:392–400. doi: 10.1111/bjh.13834
25. Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood (2008) 111:5477–85. doi: 10.1182/blood-2008-01-132837
Keywords: acute lymphoblastic leukemia, pediatric, minimal residual disease, re-emergence, hematopoietic cell transplantation (HSCT)
Citation: Wang Y, Xue Y-J, Jia Y-P, Zuo Y-X, Lu A-D and Zhang L-P (2021) Re-Emergence of Minimal Residual Disease Detected by Flow Cytometry Predicts an Adverse Outcome in Pediatric Acute Lymphoblastic Leukemia. Front. Oncol. 10:596677. doi: 10.3389/fonc.2020.596677
Received: 20 August 2020; Accepted: 21 December 2020;
Published: 05 February 2021.
Edited by:
Emmanuel Katsanis, University of Arizona, United StatesReviewed by:
Holly Pariury, University of Arizona Medical Center, United StatesRabi Hanna, Cleveland Clinic Children’s, United States
Aron Flagg, Yale University, United States
Copyright © 2021 Wang, Xue, Jia, Zuo, Lu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai-Dong Lu, bHVhaWRvbmdAcGt1cGguZWR1LmNu; Le-Ping Zhang, cm15eWVrNjAwNEAxNjMuY29t
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share last authorship
 Yu Wang†
Yu Wang† Le-Ping Zhang
Le-Ping Zhang