- 1Tissue Culture and Cytogenetics Unit, Pathology Department, National Cancer Institute, Cairo University, Cairo, Egypt
- 2Medical Biochemistry and Molecular Biology, Cancer Biology Department, National Cancer Institute, Cairo University, Cairo, Egypt
- 3Molecular Virology and Immunology Unit, Cancer Biology Department, National Cancer Institute, Cairo University, Cairo, Egypt
Patients of African ancestry have the poorest outcome and the shortest survival rates from cancer globally. This could be attributed to many variables including racial, biological, socioeconomic and sociocultural factors (either single, multiple or combined), which may be responsible for this major health problem. We sought to assess the most common types of cancer that endanger the health of the African people, and tried to investigate the real differences between African and other Non-African patients regarding incidence, prevalence and mortality rates of different cancers. Therefore, identifying the underlying aetiological causes responsible for the increased incidence and mortality rates of African patients will allow for changing the current plans, to make optimized modalities for proper screening, diagnosis and treatment for those African patients, in order to improve their survival and outcomes.
Introduction
Cancer is a major public health problem worldwide. It is one of the most leading causes of death in several regions depending upon disparities among different people (1). These disparities include socioeconomic, ethnic, racial and cultural factors that differ between low and high-income countries. According to the records obtained from the GLOBOCAN 2018 database of the International Agency for Research on Cancer (IARC) (2), the estimated results of 36 cancer types available from 47 countries of the African region of WHO (AFRO) revealed that there are 811,200 new cancer cases (4.5% of the total world) and 534,000 cancer deaths (7.3% of the total world) reported in the AFRO countries in 2018 (Figure 1).
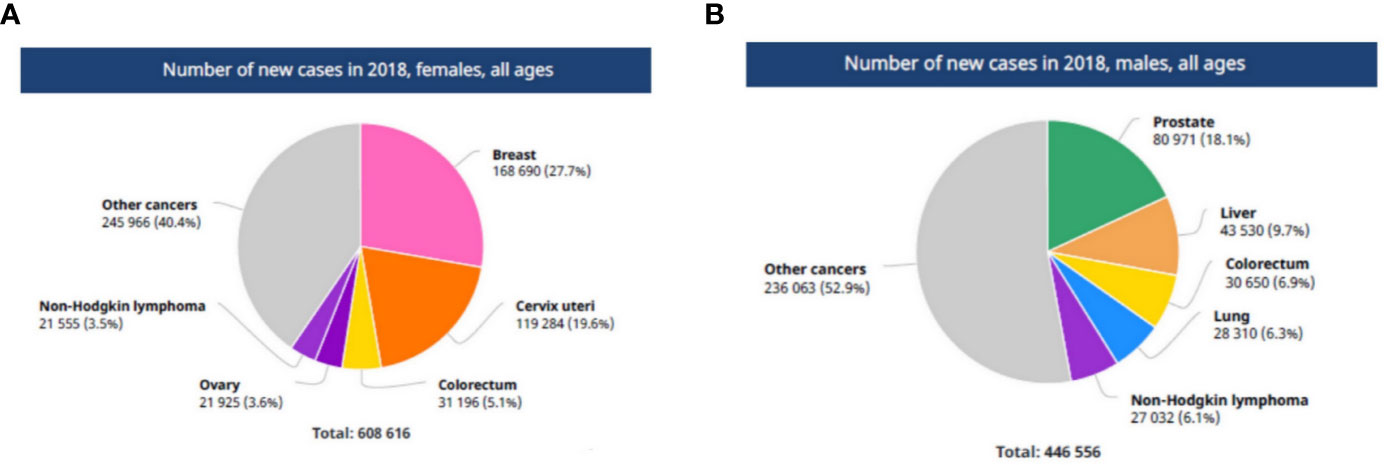
Figure 1 The number of new cancer cases in AFRO region. (A) In African females, (B) in African males. Reproduced from “The Global Cancer Observatory, Africa Globocan 2018” (3).
The estimated cancer burden in the AFRO countries is mainly attributed to breast cancer which represents 27.7% of the total cancer cases, followed by cervical cancer which represented 19.6% of the total cases. Taken together, this represents the most common in African females. Meanwhile, prostate cancer (18.1% of total cases), followed by liver cancer (9.7% of total cases) and colorectal cancers (6.9% of total cases) were the most common in African males (Figure 2). Concerning survival rate of childhood malignancies, the survival rate is as low as 20% in African children and 80% in high income countries (4).
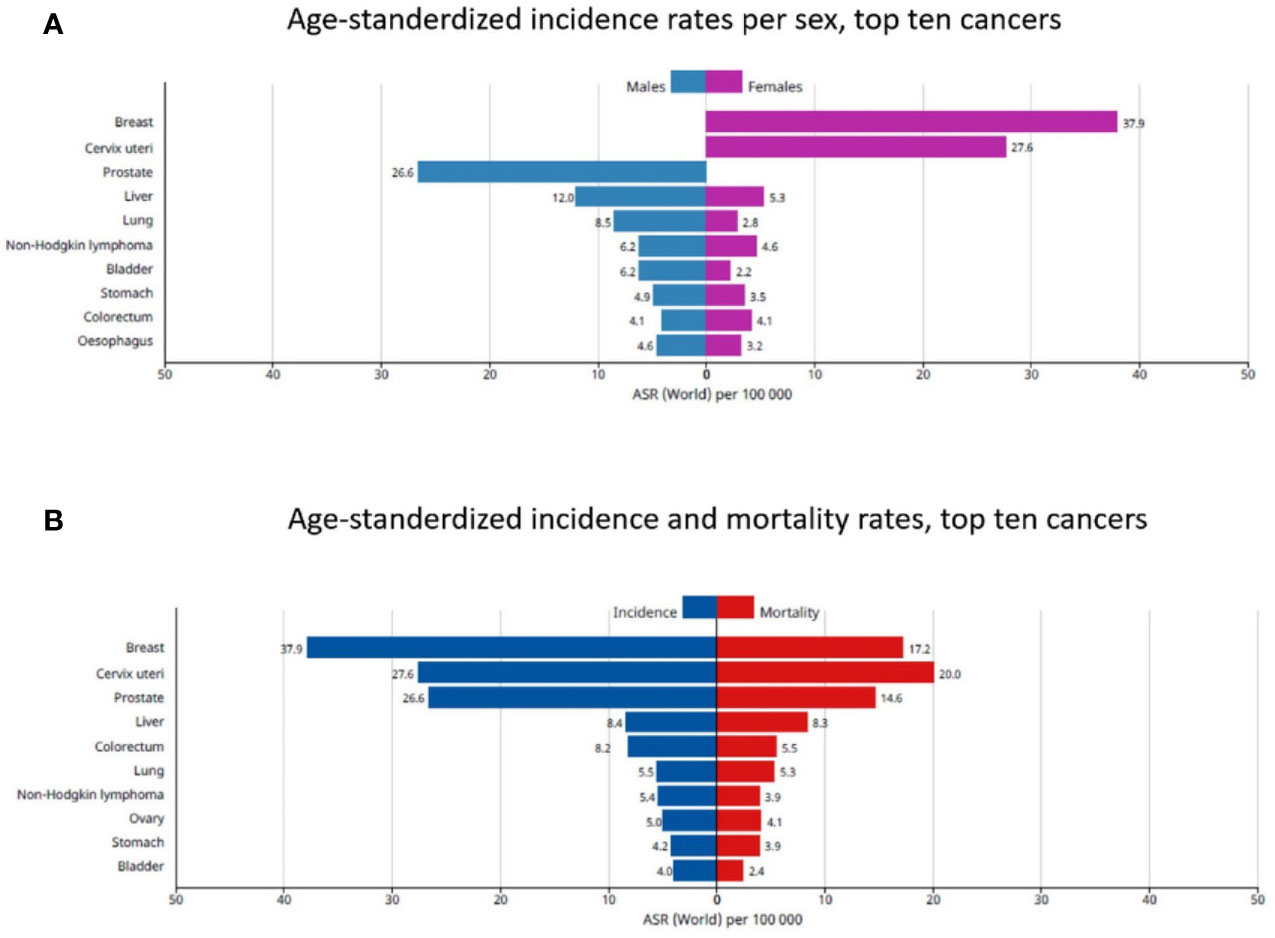
Figure 2 The top 10 cancers in African patients. (A) Age-standardized incidence rates per sex, (B) Age-standardized incidence and mortality rates. Reproduced from “The Global Cancer Observatory, Africa Globocan 2018” (3).
In an interesting study, Pinheiro and his colleagues (5), analysed the cancer mortality data obtained from South Florida for white, Hispanic, and black populations with disaggregation for Cuban, Puerto Rican, South American, African American, and Afro-Caribbean groups, during the period 2012–2016. Pinheiro et al., provided an evidence that, the African American males and females had the highest all sites-combined cancer mortality rates among all groups. As well as the highest mortality rates for many cancers including breast, prostate, lung, stomach, colorectal carcinoma, liver and multiple myeloma. According to their data, the Afro-Caribbean patients had significantly higher mortality rates compared to the white populations especially for stomach, prostate, multiple myeloma, premenopausal breast and endometrial carcinomas. In contrast, lower rates were reported for the other cancer types, particularly the lung cancer. These data are similar to other previous studies reported higher race-specific rates among both Afro-Caribbean and African American populations for endometrial, premenopausal breast, prostate, and multiple myeloma cancers in South Florida’s black population (6, 7). They also reported that lung cancer was the first leading cause of cancer-related death in African American men, followed by Prostate and colorectal cancers. While, for the Afro-Caribbean’s and other Hispanics, prostate cancer was the leading cause of cancer- related death followed by lung and colorectal carcinoma. On the other hand, breast and lung cancers were the first and the second leading causes of cancer- related death in African American females, followed by colorectal cancer, while lung cancer preceded breast and colorectal cancers in the Afro-Caribbeans (5).
It is a well-known fact that, cancer outcome is not equal in all people, and there are many factors that can affect its behaviour and its impact on the patients’ survival or response to treatment. Here, we review the most common types of cancer that endanger the health of the African people or those with African ancestry, and investigate the differences between African and non-African patients regarding incidence, prevalence, and mortality rates of different types of cancer. This will pave the way to produce an appropriate screening method or targeted therapy for such patients.
Prostate Cancer
Prostate cancer is the first leading cause of cancer deaths in African males, and the second leading cause of cancer deaths in the united states (1, 2). It was obviously noted that, racial disparity plays a crucial role in its incidence and mortality rates among American patients (8). In 2007, It has been reported that prostate cancer incidence among black men in the US was 60% higher and its mortality was more than double the estimated rates in white men respectively (9). Later on, Siegel et al. (8), reported in 2014 that African-American men were 2.4 and 5.0 times more likely to die from prostate cancer compared to Americans of European or Asian ancestries, respectively. Various studies tried to investigate this racial disparity in prostate cancer regarding its incidence, prevalence, aggressive behaviour, and mortality rates. Some of these studies proposed that, the poor outcome in black men may be attributed partially to the inaccessible medical care and/or inadequate screening and treatment facilities (10, 11). Meanwhile, other studies mentioned the differences in germline and genetic background between black and white men as a reason (9, 12–14). Moreover, the socioeconomic status and lifestyle variation had also been suggested, however after adjusting for these factors, African ancestry remains a significant risk factor for prostate cancer (15). Supporting these data, Moul et al. and Faisal et al. (16, 17), concluded that the black race has to be considered an independent prognostic factor for disease recurrence, allowing for a more biologically aggressive phenotype. Though, the explanation of this disparity is still unknown and require more in depth studies (18). Tsodikov and his colleagues have also investigated this issue through establishing three predictive models of prostate-specific antigen (PSA) screening patterns in the USA, to compare the prostate cancer natural history in black men compared to the general population using an updated reconstruction of PSA screening. The obtained data were collected through the National Health Interview Survey in 2005, and the prostate cancer incidence from the Surveillance, Epidemiology, and End Results program (SEER) in 1975–2000 (18). They found that 30–43% of black men developed preclinical prostate cancer by the age of 85 years, which was relatively 28–56% higher than in the general population. Also, black men showed a similar risk of diagnosis (35–49%) compared to the general population (32–44%), but their risk of progression to a metastatic disease by the time of diagnosis was 44–75% higher than in the general population. Taken together, these results are consistent with those published by Powell et al. (19), which based on autopsy and surgical pathology data. They observed that black men have an increased risk of transformation to clinically significant cancer compared to white men.
Blackburn and his colleagues (20) tried to investigate the association between the underlying genetic differences for prostate cancer with the racial variations among peoples. They reported a lower frequency for TMPRSS2‐ERG fusion which is inversely associated with aggressive prostate cancer in black South Africans males compared to those from European ancestry. Similarly, Zhou et al. (21), performed a meta-analysis study and reported that the highest incidence of TMPRSS2‐ERG fusion was recorded in 49% in men of European ancestry. While, lower incidence rates were found in Asian (27%) and African (25%) male ancestries. Moreover, Magi-Galluzzi et al. (22) reported a racial discordance in the mechanism(s) of TMPRSS2‐ERG fusion occurrence, since the African Americans more commonly had TMPRSS2‐ERG fusion through deletion, whereas the European and Asian Americans had TMPRSS2‐ERG fusion through translocation.
For more confirmation of these data, an interesting study was done by Jaratlerdsiri et al. (23), who performed deep whole-genome sequencing for paired tumor-normal tissues obtained from African patients compared to non-African patients. The results of the study revealed a 1.8-fold increase in the small somatic variants, and also elevated oncogenic driver mutations in the African- derived tumors in comparison to the European counterpart. The ERG fusions and PIK3CA mutations were absent, PTEN loss was less frequent, whereas CCND1 and MYC were frequently gained. In addition, out of the commonly affected prostate cancer gene pathways, genes regulating the calcium ion-ATPase signal transduction were disrupted in the African tumors. Therefore, it is quite clear that, a special screening program for the black men of African ancestry is highly required, and this should be done depending upon their own genetic makeup.
Breast Cancer
Breast cancer (BC) is the most commonly diagnosed cancer in the African females, and it also represents the second leading cause of cancer- related deaths following cancer cervix in sub-Saharan Africa (SSA) (2). Its incidence had been increased in the last six years by more than 23% (from 1.7 million new patients in 2012 to 2.1 million in 2018) (24, 25). In addition, its five-year survival rate is less than 40% in SSA, compared to 86% in the United States (26). In their observational study on BC patients from USA, Iqbal et al. (27), reported that black women with small-sized tumors had 9.0% increase in the risk of death compared to the non-Hispanic white women who had only 4.6% increased risk of death. These data are in accordance with many previously published studies which showed that black women usually have higher risk of BC recurrence regardless of the age, tumor size or tumor grade. Based on these data, the African ancestry, by itself, should be considered an independent predictor factor for poor survival rates (28, 29).
Although BC mortality rate is now decreasing in the developed countries due to the implementation of screening programs including mammography, which is the gold standard for early detection and successful management of BC. The screening of BC in Africa is still a great challenge (30). This is attributed mainly to the lack of financial and technical support, in addition to decreased numbers of well-trained radiologists and technicians (31, 32). It was reported in some previous studies that, the age of peak incidence of BC is lower in SSA, with most of the women had advanced-stage disease at the time of diagnosis (33). At the same time, mammography is less effective for detecting tumors at advanced stages, as well as in younger women due to changes in breast tissue density according to the hormonal profile of the patients (34). Moreover, mammography is not available in most countries of SSA, and it is only available in urban centers, that made it rater costy for women living in semi-urban or rural areas to compensate for the travel and accommodation (35, 36). Another major obstacle which could also be responsible for the poor outcomes of the BC patients in Africa, is the failure to deliver the proper treatment to the patients. This is because that the treatment options for advanced stages of breast cancer are limited and restricting mainly to mastectomy, in addition to lacking other modalities including chemotherapy and/or radiotherapy facilities (37, 38). Taken together, these factors prevent many women from getting their proper medical treatment(s) for their disease. They seek for other non- medical and non- effective options such as prayer camps and herbs, and accordingly, they usually present with advanced high grade and advanced stage tumors (37). Based on the previous data we can conclude that, breast cancer is the major health problem threatening African women, owing to poverty, social and cultural barriers, as well as limited diagnostic and treatment facilities. Black et al. (30), suggested in their study that increasing public awareness for breast self-examination and clinical breast examination (CBE) could help, at least partially, in down staging of BC in the African females. This has also been supported by the relatively recent study of Dos Santos et al. (39), who reported in their study, which was done in Sudan and Tanzania, that training health workers for CBE together with awareness campaigns can effectively improve the patients’ outcomes.
Uterine Cervical Cancer
carcinoma of the cervix uteri is among the most preventable malignancies worldwide (40), however it remains the first leading cause of cancer deaths in African women [(2), Figure 3]. Human papillomavirus (HPV) types 16 and 18 are the most common etiological factors for the pathogenesis of cervical cancer in Africa (42). The reported prevalence rate of HPV was 97.0% in Malawi (43), 92.1% in South Africa (44), 90.7% in Ibadan Nigeria (45), and 69.8% in Maiduguri and Nigeria (42). In fact, the HPV infection is usually cleared in the immunocompetent women (46). However, in women with underlying human immuno-deficiency virus (HIV) infection; as a common situation in Africa, there is an increasing risk of developing cancer cervix rather than in women without HIV infection, with the annual detection rates are 1.4 versus 0.4 per 100 persons per year; respectively (47–49). It was reported by de Martel et al. (50), that SSA had the highest age standardized incidence rate (ASIR) of HPV attributable cancer all over the world (ASIR 19.3 cases per 100,000 person/years). A recent meta-analysis study performed by Drolet et al. (51), including midline studies published between February 1, 2014, and October 11, 2018, reported that nearly two thirds of all cervical cancers cases caused by HPV16 and HPV18 could be prevented with the currently available HPV vaccines. At the same time, Cervical screening programs either with cytology, HPV testing, or both could prevent most of the remainder of cases especially in developed countries. However, in Africa it is rather challenged by many factors including limited resources, lack of knowledge about the cervical cancer and unavailability of screening centers (52, 53). It was estimated that the overall cervical cancer screening in Ethiopia was 0.8% according to the ICO Information Centre on HPV and Cancer 2017 (54). Similarly, it was reported to be 1% in another study done by Getachew et al. (52). All these factors contributed to inefficient early detection and consequently later diagnosis and poorer survival rates.
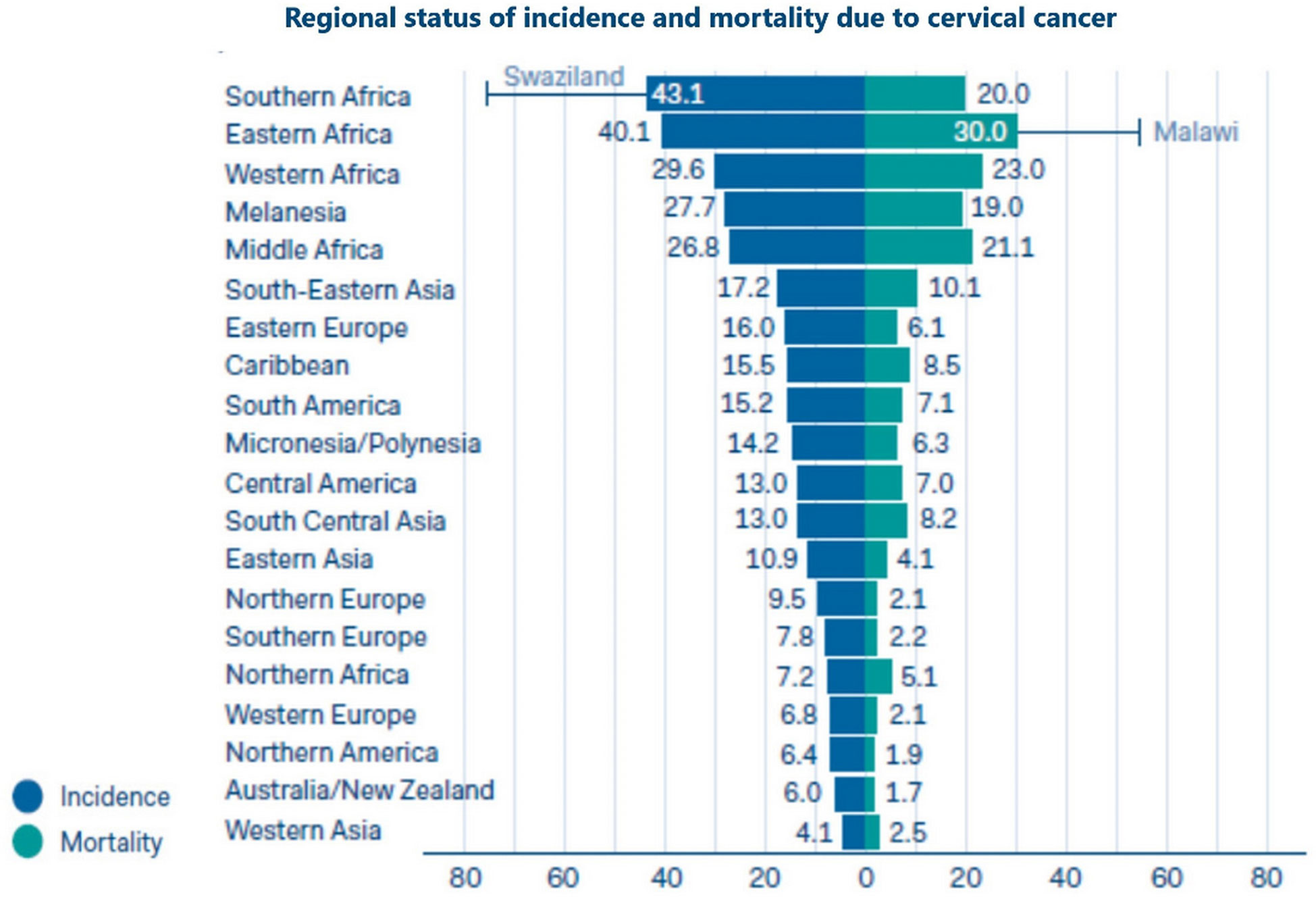
Figure 3 Regional status of incidence and mortality due to uterine cervical cancer (41).
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related death in Africa, and a major health problem all over the world (1). It was recorded that 80% of HCC cases occurred in the SSA and eastern Asia according to Cancer Today, which is an international agency for research and cancer (24). The prevalence of HCC is heterogeneous because it has variable risk factors, since hepatitis B (HBV) and aflatoxin exposure are the major risk factors for HCC in SSA, whereas hepatitis C (HCV) is the major risk factor for HCC in USA, Europe, and Japan (55). In a large, retrospective observational study done by Yang et al. (56), which included 2,566 patients who were treated in 21 tertiary referral centers from different countries in Africa, they observed that the African patients presented with HCC were at a younger age (median of 45 years), with advanced disease stage, severe liver dysfunction and poor performance status. Additionally, Mak et al. (57), reported that the mortality rate of HCC black African patients is higher than that in white patients. Many studies had addressed this disparity between the black and the white population, among those are Ladep et al. (58), who concluded that this disparity might be due to different biological and etiological risk factors that should be urgently identified, as those patients represent high-risk group patients who need a prompt effective treatment. Other studies attributed this poor outcome to the absence of comprehensive surveillance programs for HCC, inaccessible expert medical care, socioeconomic and sociocultural factors that affect treatment decision making (59, 60). In addition to the previously mentioned etiological factors, it is clear that the HIV epidemic has had a major demographic and health impact on the black African population, which also should be assessed (57).
Lung Cancer
Lung cancer remains the first leading cause of cancer- related deaths in the United States (1), with the highest lung cancer mortality rate being detected in the African-American population (61, 62). Indeed, there were a conflicting data regarding the racial disparity of the prevalence and outcome of patients with lung cancer. Many studies reported a significantly lower frequencies of EGFR mutations in black compared white patients (63, 64). However, other groups failed to find any significant association between EGFR mutations and patients’ races (65, 66). An important study done by Campbell et al. (67), who performed genomic sequencing for a panel of 504 cancer genes in lung cancer tissue specimens obtained from 245 black patients compared to 264 white patients. Based on the data of their study, they concluded that there was no significant difference regarding mutational frequencies and copy number changes between the black and white patients. Also, there was no significant difference in the genetic alterations of the receptor tyrosine kinase/Ras/Raf pathway including EGFR and KRAS. Additionally, Mitchell et al. (68), reported no significant association between lung cancer survival and ethnic variations especially in West African ancestry. These data were confirmed by previous studies suggested that genetic ancestry did not adversely contribute to lung cancer risk or survival (69, 70). Therefore, some investigators suggested that other factors including socioeconomic, environmental or cultural variables could explain these disparities (68, 69).
Consistent with these results, Murphy et al. (71), concluded that African Americans consumed greater amounts of nicotine per cigarette compared to other American ancestry groups. This was measured by the urinary total nicotine equivalents (TNE), which is a more objective measure of smoking intensity than the number of cigarettes per day (CPD). Accordingly, TNE is correlated with the uptake of the well-known tobacco carcinogens such as nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and polycyclic aromatic hydrocarbons (72). Therefore, it seems that, exomic mutations does not contribute to the observed racial disparities between black and white populations regarding lung cancer development and outcome. However, further investigations are suggested into other genomic variations such as mutations in noncoding regions and epigenetic changes, or assessment of other socioeconomic factors including smoking behavior and access to health care facilities (67).
Based on our previous discussion, we can conclude that cancer is a major public health problem in Africa, with increased incidence, financial toxicities and mortality (Figures 4, 5). Racial disparities seem to played an important role for the increasing incidence and prevalence of many cancers including prostate and breast cancers which are genetically more common in black patients rather than in white population. However, the increased incidence of other cancer types including lung, hepatocellular and uterine cervical cancers could be attributed to many factors other than racial disparities. Actually, Africa is challenged by many problems including mainly the prevalence of oncogenic viruses such as HIV for non-Hodgkin lymphoma, HHV-8 for Kaposi sarcoma, HPV for cervical cancer, HBV and HCV for HCC. Other factors including limited screening programs as PAS, TURS for prostate cancer, and mammography for breast cancer. Also included is poor implementation of HPV vaccines as for uterine cervical cancer and HBV for HCC. Moreover, African patients were challenged by poor economic circumstances, low life standard, inaccessible medical care and poor medical services. All these factors together with the racial disparities contributed to increased cancer incidence and mortality among African patients. Therefore, identifying the underlying aetiological causes for increased cancer death in Africans will contribute to better modalities for screening, diagnosis, treatment and prevention.
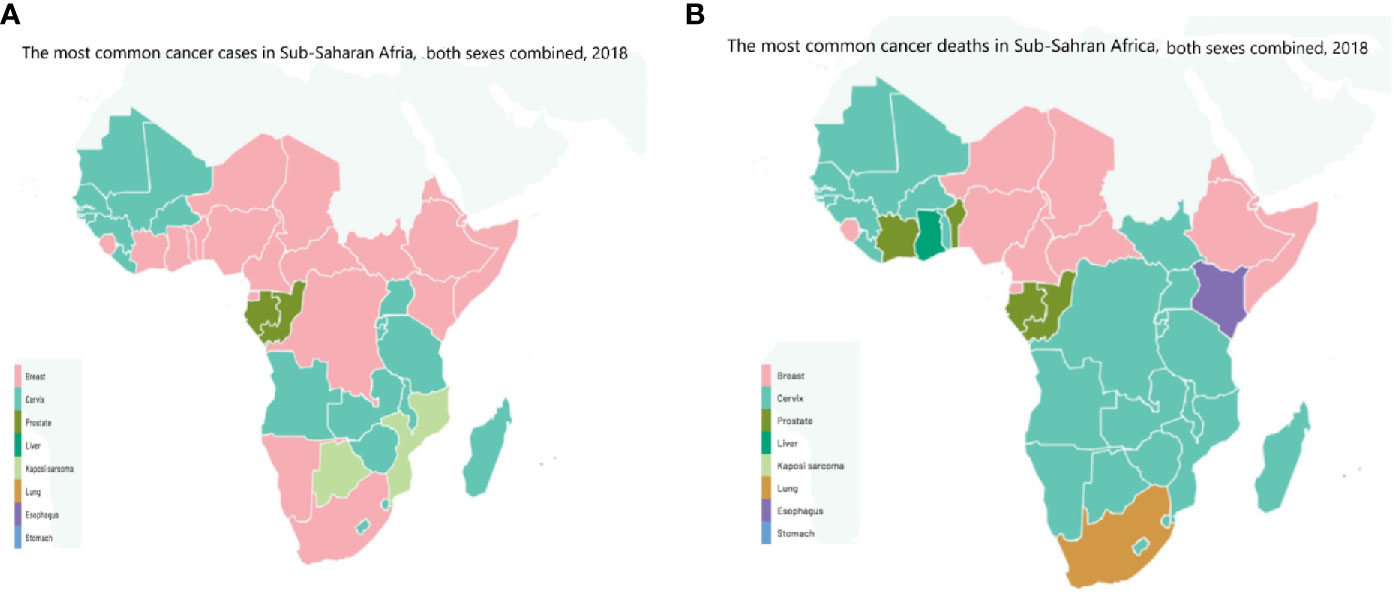
Figure 4 The most common (A) cancer cases, (B) cancer deaths in Sub-Saharan Africa, both sexes combined, 2018 (3). Reproduced from “The Cancer Atlas,” canceratlas.cancer.org (73).
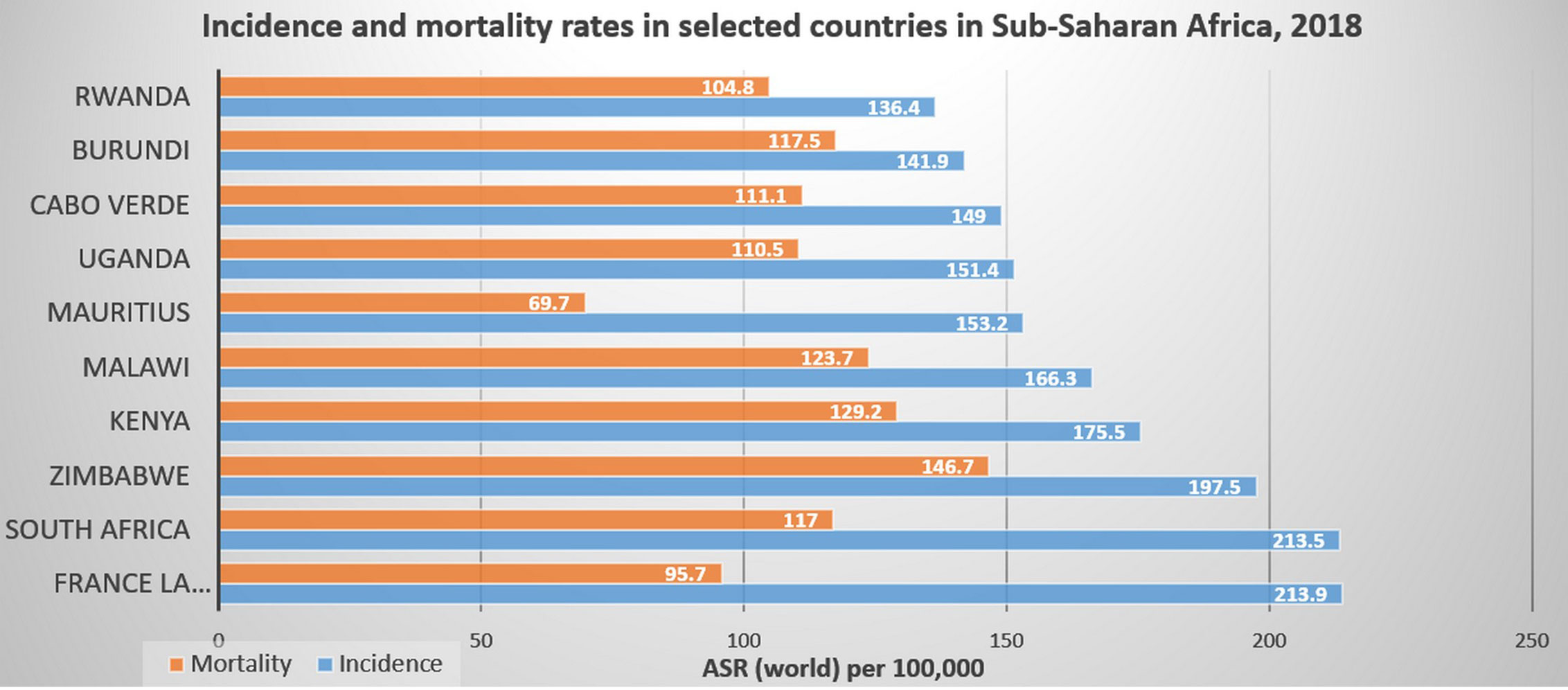
Figure 5 Incidence and mortality rates in selected countries in Sub-Saharan Africa, 2018 (3). Reproduced from “The Cancer Atlas,” canceratlas.cancer.org (73).
Author Contributions
AB: revised the manuscript. MA: collecting data and writing the manuscript. A-RZ: directing the work and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
3. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (2018). Available at: http://gco.iarc.fr/today/data/factsheets/populations/903-africa-fact-sheets.pdf.
4. Stefan C, Bray F, Ferlay J, Liu B, Maxwell Parkin D. Cancer of childhood in sub-Saharan Africa. Ecancermed Sci (2017) 11:755. doi: 10.3332/ecancer.2017.755
5. Pinheiro PS, Callahan KE, Koru-Sengul T, Ransdell J, Bouzoubaa L, Brown CP, et al. Risk of Cancer Death Among White, Black, and Hispanic Populations in South Florida. Prev Chronic Dis (2019) 16:E83. doi: 10.5888/pcd16.180529
6. Pinheiro PS, Callahan KE, Ragin C, Hage RW, Hylton T, Kobetz EN. Black heterogeneity in cancer mortality: USblacks, Haitians, and Jamaicans. Cancer Contr (2016) 23(4):347–58. doi: 10.1177/107327481602300406
7. Pinheiro PS, Callahan KE, Boscoe FP, Balise RR, Cobb TR, Lee DJ, et al. Cancer site-specific disparities in New York, including the 1945-1965 birth cohort’s impact on liver cancer patterns. Cancer Epidemiol Biomarkers Prev (2018) 27(8):917–27. doi: 10.1158/1055-9965.EPI-18-0194
8. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin (2014) 64:9–29. doi: 10.3322/caac.21208
9. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol (2007) 177:444–9. doi: 10.1016/j.juro.2006.09.024
10. Underwood W, De Monner S, Ubel P, Fagerlin A, Sanda MG, Wei JT. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol (2004) 171:1504–7. doi: 10.1097/01.ju.0000118907.64125.e0
11. Schwartz K, Powell IJ, Underwood W3, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology (2009) 74:1296–302. doi: 10.1016/j.urology.2009.02.058
12. Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Brufsky A, Talcott J, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci (1997) 94(7):3320–3. doi: 10.1073/pnas.94.7.3320
13. Bensen JT, Xu Z, Smith GJ, Mohler JL, Fontham ET, Taylor JA. Genetic polymorphism and prostate cancer aggressiveness: a case-only study of 1,536 GWAS and candidate SNPs in African-Americans and European-Americans. Prostate (2013) 73:11–22. doi: 10.1002/pros.22532
14. Faisal FA, Sundi D, Tosoian JJ, Choeurng V, Alshalalfa M, Ross AE, et al. Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. Eur Urol (2016) 70(1):14–7. doi: 10.1016/j.eururo.2015.09.031
15. Park SY, Haiman CA, Cheng I, Park SL, Wilkens LR, Kolonel LN, et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the Multiethnic Cohort Study. Cancer Causes Control (2015) 26:1507–15. doi: 10.1007/s10552-015-0644-y
16. Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol (1996) 155:1667–73. doi: 10.1016/S0022-5347(01)66160-3
17. Faisal FA, Sundi D, Cooper JL, Humphreys EB, Partin AW, Han M, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology (2014) 84(6):1434–41. doi: 10.1016/j.urology.2014.08.039
18. Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer (2017) 123(12):2312–9. doi: 10.1002/cncr.30687
19. Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol (2010) 183:1792–6. doi: 10.1016/j.juro.2010.01.015
20. Blackburn J, Vecchiarelli S, Heyer EE, Patrick SM, Lyons RJ, Jaratlerdsiri W, et al. TMPRSS2-ERG fusions linked to prostate cancer racial health disparities: A focus on Africa. Prostate (2019) 79(10):1191–6. doi: 10.1002/pros.23823
21. Zhou CK, Young D, Yeboah ED, Coburn SB, Tettey Y, Biritwum RB, et al. TMPRSS2:ERG gene fusions in prostate cancer of West African men and a meta-analysis of racial differences. Am J Epidemiol (2017) 186(12):1352–61. doi: 10.1093/aje/kwx235
22. Magi-Galluzzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, Esgueva R, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate (2011) 71(5):489–97. doi: 10.1002/pros.21265
23. Jaratlerdsiri W, Chan EK, Gong T, Petersen DC, Kalsbeek AM, Venter PA, et al. Whole-genome sequencing reveals elevated tumor mutational burden and initiating driver mutations in African men with treatment-naïve, high-risk prostate cancer. Cancer Res (2018) 78(24):6736–46. doi: 10.1158/0008-5472.CAN-18-0254
24. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer, Lyon (2018). Available at: https://gco.iarc.fr/today (Accessed September 18, 2018).
25. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
26. Cumbera S, Nchanji K, Tsoka-Gwegweni J. Breast cancer among women in sub-Saharan Africa: prevalence and a situational analysis. South Afr J Gyn Onc (2017) 9(2):35–7. doi: 10.1080/20742835.2017.1391467
27. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA (2015) 313(2):165–73. doi: 10.1001/jama.2014.17322
28. Maskarinec G, Sen C, Koga K, Conroy SM. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond Engl) (2011) 7(6):677–87. doi: 10.2217/WHE.11.67
29. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol (2006) 24(9):1342–9. doi: 10.1200/JCO.2005.03.3472
30. Black E, Richmond R. Improving early detection of breast cancer in sub-Saharan Africa: why mammography may not be the way forward. Global Health (2019) 15(1):3. doi: 10.1186/s12992-018-0446-6
31. Denny L, de Sanjose S, Mutebi M, Anderson BO, Kim J, Jeronimo J, et al. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. Lancet (2017) 389(10071):861–70. doi: 10.1016/S0140-6736(16)31795-0
32. Corbex M, Burton R, Sancho-Garnier H. Breast cancer early detection methods for low and middle income countries, a review of the evidence. Breast (2012) 21(4):428–34. doi: 10.1016/j.breast.2012.01.002
33. Harford J. Breast cancer early detection in low-income and middle-income countries: do what you can versus one size fits all. Lancet Onc (2011) 12(3):306–12. doi: 10.1016/S1470-2045(10)70273-4
34. Tsu V, Jeronimo J, Anderson B. Why the time is right to tackle breast and cervical cancer in low-resource settings. Bull World Health Organ (2013) 91:683–90. doi: 10.2471/BLT.12.116020
35. Brinton LA, Figueroa JD, Awuah B, Yarney J, Wiafe S, Wood SN, et al. Breast cancer in sub-Saharan Africa: opportunities for prevention. Breast Cancer Res Treat (2014) 144(3):467–78. doi: 10.1007/s10549-014-2868-z
36. Smith R, Caleffi M, Albert U, Chen T, Duffy S, Franceschi D, et al. Breast cancer in limited-resource countries: early detection and access to care. Breast J (2006) 12(Suppl 1):S16–26. doi: 10.1111/j.1075-122X.2006.00200.x
37. Tetteh D, Faulkner S. Sociocultural factors and breast Cancer in sub-Saharan Africa: implications for diagnosis and management. Women’s Health (2016) 12(1):147–56. doi: 10.2217/whe.15.76
38. Clegg-Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in Ghana? A Pilot Study Ghana Med J (2009) 43(3):127–31. doi: 10.4314/gmj.v43i3.55338
39. Dos Santos I, McCormack V, Jedy-Agba E, Adebamowo C. Downstaging breast cancer in sub-Saharan Africa: A realistic target. Cancer Control (2017). http://www.cancercontrol.info/wp-content/uploads/2017/12/46-52-Silva.pdf.
40. Campos NG, Sharma M, Clark A, Lee K, Geng F, Regan C, et al. The health and economic impact of scaling cervical cancer prevention in 50 low- and lower-middle-income countries. Int J Gynaecol Obstet (2017) 138(suppl 1):47–56. doi: 10.1002/ijgo.12184
41. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
42. Kabir A, Bukar M, Nggada HA, Rann HB, Gidado A, Musa AB. Prevalence of human papillomavirus genotypes in cervical cancer in Maiduguri, Nigeria. Pan Afr Med J (2019) 33:284. doi: 10.11604/pamj.2019.33.284.18338
43. Howitt BE, Herfs M, Tomoka T, Kamiza S, Gheit T, Tommasino M, et al. Comprehensive Human Papillomavirus Genotyping in Cervical Squamous Cell Carcinomas and Its Relevance to Cervical Cancer Prevention in Malawian Women. J Glob Oncol (2017) 3(3):227–34. doi: 10.1200/JGO.2015.001909
44. Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer (2014) 134(6):1389–98. doi: 10.1002/ijc.28425
45. Okolo C, Franceschi S, Adewole I, Thomas JO, Follen M, Snijder PJF, et al. Human papillomavirus infection in women with and without cervical cancer in Ibadan, Nigeria. Infect Agents Cancer (2010) 5(1):24. doi: 10.1186/1750-9378-5-24
46. Lowy DR, Solomon D, Hildesheim A, Schiller JT, Schiffman M. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer (2008) 113(7):1980–93. doi: 10.1002/cncr.23704
47. Looker KJ, Rönn MM, Brock PM, Brisson M, Drolet M, Mayaud P, et al. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc (2018) 21(6):e25110. doi: 10.1002/jia2.25110
48. Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS (2018) 32(6):795–808. doi: 10.1097/QAD.0000000000001765
49. Massad LS, Xie X, D’Souza G, Darragh TM, Minkoff H, Wright R, et al. Incidence of cervical precancers among HIV-seropositive women. Am J Obstet Gynecol (2015) 212(5):606.e1–8. doi: 10.1016/j.ajog.2014.12.003
50. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global Health (2020) 8(2):e180–90. doi: 10.1016/S2214-109X(19)30488-7
51. Drolet M, Bénard É, Pérez N, Brisson M, Ali H, Boily MC, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet (2019) 394(10197):497–509. doi: 10.1016/S0140-6736(19)30298-3
52. Getachew S, Getachew E, Gizaw M, Ayele W, Addissie A, Kantelhardt EJ. Cervical cancer screening knowledge and barriers among women in Addis Ababa, Ethiopia. PLoS One (2019) 14(5):e0216522. doi: 10.1371/journal.pone.0216522
53. Lyimo FS, Beran TN. Demographic, knowledge, attitudinal, and accessibility factors associated with uptake of cervical cancer screening among women in a rural district of Tanzania: three public policy implications. BMC Public Health (2012) 12:22. doi: 10.1186/1471-2458-12-22
54. Ameya G, Yerakly F. Characteristics of cervical disease among symptomatic women with histopathological sample at Hawassa University referral hospital, Southern Ethiopia. BMC Womens Health (2017) 17(1):91. doi: 10.1186/s12905-017-0444-5.
55. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology (2012) 142:1264–73. doi: 10.1053/j.gastro.2011.12.061
56. Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol (2017) 2(2):103–11. doi: 10.1016/S2468-1253(16)30161-3
57. Mak D, Sengayi M, Chen WC, Babb de Villiers C, Singh E, Kramvis A. Liver cancer mortality trends in South Africa: 1999-2015. BMC Cancer (2018) 18(1):798. doi: 10.1186/s12885-018-4695-9
58. Ladep NG, Lesi OA, Mark P, Lemoine M, Onyekwere C, Afihene M, et al. Problem of hepatocellular carcinoma in West Africa. World J Hepatol (2014) 6(11):783–92. doi: 10.4254/wjh.v6.i11.783
59. Tognarelli J, Ladep NG, Crossey MM, Okeke E, Duguru M, Banwat E, et al. Reasons why West Africa continues to be a hotbed for hepatocellular carcinoma. Niger Med J (2015) 56:231–5. doi: 10.4103/0300-1652.165032
60. Olivier J, Tsimpo C, Gemignani R, Shojo M, Coulombe H, Dimmock F, et al. Understanding the roles of faith-based health-care providers in Africa: review of the evidence with a focus on magnitude, reach, cost, and satisfaction. Lancet (2015) 386(10005):1765–75. doi: 10.1016/S0140-6736(15)60251-3
61. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975–2013. Bethesda, MD: National Cancer Institute (2016).
62. DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin (2016) 66:290–308. doi: 10.3322/caac.21340
63. Yang SH, Mechanic LE, Yang P, Landi MT, Bowman ED, Wampfler J, et al. Mutations in the tyrosine kinase domain of the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res (2005) 11(6):2106–10. doi: 10.1158/1078-0432.CCR-04-1853
64. Leidner RS, Fu P, Clifford B, Hamdan A, Jin C, Eisenberg R, et al. Genetic abnormalities of the EGFR pathway in African American Patients with non-small-cell lung cancer. J Clin Oncol (2009) 27(33):5620–6. doi: 10.1200/JCO.2009.23.1431
65. Araujo LH, Lammers PE, Matthews-Smith V, Eisenberg R, Gonzalez A, Schwartz AG, et al. Somatic mutation spectrum of non-small-cell lung cancer in african americans: a pooled analysis. J Thorac Oncol (2015) 10(10):1430–6. doi: 10.1097/JTO.0000000000000650
66. Yamaguchi N, Vanderlaan PA, Folch E, Boucher DH, Canepa HM, Kent MS, et al. Smoking status and self-reported race affect the frequency of clinically relevant oncogenic alterations in non-small-cell lung cancers at a United States-based academic medical practice. Lung Cancer (2013) 82(1):31–7. doi: 10.1016/j.lungcan.2013.07.013
67. Campbell JD, Lathan C, Sholl L, Ducar M, Vega M, Sunkavalli A, et al. Comparison of Prevalence and Types of Mutations in Lung Cancers Among Black and White Populations. JAMA Oncol (2017) 3(6):801–9. doi: 10.1001/jamaoncol.2016.6108
68. Mitchell KA, Shah E, Bowman ED, Zingone A, Nichols N, Pine SR, et al. Relationship between West African ancestry with lung cancer risk and survival in African Americans. Cancer Causes Control (2019) 30(11):1259–68. doi: 10.1007/s10552-019-01212-z
69. Lathan CS. Lung cancer care: the impact of facilities and area measures. Transl Lung Cancer Res (2015) 4(4):385–91. doi: 10.3978/j.issn.2218-6751.2015.07.23
70. Jones CC, Mercaldo SF, Blume JD, Wenzlaff AS, Schwartz AG, Chen H, et al. Racial Disparities in Lung Cancer Survival: The Contribution of Stage, Treatment, and Ancestry. J Thorac Oncol (2018) 13(10):1464–73. doi: 10.1016/j.jtho.2018.05.032
71. Murphy SE, Park SL, Balbo S, Haiman CA, Hatsukami DK, Patel Y, et al. Tobacco biomarkers and genetic/epigenetic analysis to investigate ethnic/racial differences in lung cancer risk among smokers. NPJ Precis Oncol (2018) 2:17. doi: 10.1038/s41698-018-0057-y
72. Patel YM, Park SL, Carmella SG, Paiano V, Olvera N, Stram DO, et al. Metabolites of the polycyclic aromatic hydrocarbon phenanthrene in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. PLoS One (2016) 11(6):e0156203. doi: 10.1371/journal.pone.0156203
Keywords: Africa, cancer, incidence, survival, mortality
Citation: Bahnassy AA, Abdellateif MS and Zekri A-RN (2020) Cancer in Africa: Is It a Genetic or Environmental Health Problem? Front. Oncol. 10:604214. doi: 10.3389/fonc.2020.604214
Received: 08 September 2020; Accepted: 19 October 2020;
Published: 14 December 2020.
Edited by:
Clayton Yates, Tuskegee University, United StatesReviewed by:
Faruk Mohammed, Ahmadu Bello University, NigeriaFrancis Makokha, Mount Kenya University, Kenya
Copyright © 2020 Bahnassy, Abdellateif and Zekri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdel-Rahman N. Zekri, bmNpemVrcmlAeWFob28uY29t
 Abeer A. Bahnassy
Abeer A. Bahnassy Mona S. Abdellateif
Mona S. Abdellateif Abdel-Rahman N. Zekri
Abdel-Rahman N. Zekri