- 1Department of Breast Disease, The First Hospital of Jiaxing & The Affiliated Hospital of Jiaxing University, Jiaxing, China
- 2Cancer Research Center, The First Hospital of Jiaxing & The Affiliated Hospital of Jiaxing University, Jiaxing, China
- 3Department of Breast Surgery, Breast Cancer Center of the Third Affiliated Hospital of Kunming Medical University, Cancer Hospital of Yunnan Province, Kunming, China
- 4Department of Central Laboratory, The First Hospital of Jiaxing & The Affiliated Hospital of Jiaxing University, Jiaxing, China
- 5Department of Pathology, The First Hospital of Jiaxing & The Affiliated Hospital of Jiaxing University, Jiaxing, China
Background: Ductal carcinoma in situ with microinvasion (DCISM) was defined as one or more foci of invasion beyond the basement membrane within 1 mm. The size of primary lesion is associated with axillary status and prognosis in patients with invasive breast cancer; thus, it is of interest to determine whether multiple foci of microinvasion are associated with a higher risk of positive axillary status or worse long-term outcomes in patients with DCISM.
Methods: This study identified 359 patients with DCISM who had undergone axillary evaluation at our institute from January 2006 to December 2015. Patients were categorized as one focus or multiple foci (≥2 foci) according to the pathological results. Clinicopathological features, axillary status, and disease-free survival rate were obtained and analyzed.
Results: Of 359 patients, 233 (64.90%) had one focus of microinvasion and 126 (35.10%) had multiple foci. Overall, 242 (67.41%) and 117 (32.59%) patients underwent sentinel lymph nodes biopsy (SLNB) and axillary lymph nodes dissection (ALND), respectively. Isolated tumor cells were found in four (1.11%) patients and axillary metastasis rate was 2.51%. Neither axillary evaluation methods (P = 0.244) nor axillary metastasis rate (P = 0.559) was significantly different between patients with one focus and multiple foci. In univariate analysis, patients with multiple foci tended to have larger tumor size (P < 0.001), higher nuclear grade (P = 0.001), and higher rate of lymphatic vascular invasion (P = 0.034). Also, the proportion of positive HER2 (P = 0.027) and Ki67 level (P = 0.004) increased in patients with multiple foci, while in multivariate analysis, only tumor size showed significant difference (P = 0.009). Patients with multiple foci were more likely to receive chemotherapy (56.35 vs 40.77%; P = 0.028). At median 5.11 years follow-up, overall survival rate was 99.36%. Patients with multiple microinvasive foci had worse disease-free survival rate compared with one-focus patients (98.29 vs 93.01%, P = 0.032).
Conclusion: Even though the numbers of microinvasion were different and patients with multiple foci of microinvasion tended to have larger tumor size, there was no higher risk of axillary involvement compared with patients with one focus of microinvasion, while patients with multiple microinvasive foci had worse DFS rate. Thus, DCISM patients with multiple foci of microinvasion may be the criterion for more aggressive local–regional treatment. Optimization of adjuvant therapy in DCISM patients is required.
Introduction
Owing to the widespread adoption of screening for breast cancer and the improvements in the sensitivity of mammography, the diagnosis of ductal carcinoma in situ (DCIS) has increased dramatically over the past few decades according to the statistics (1). Due to the increase proportion of DCIS, a rare diagnosis called DCIS with microinvasion (DCISM) is also increasing. According to the staging system of the American Joint Committee on Cancer (AJCC), DCISM was defined as DCIS with one or more microscopic foci of invasion beyond the basement membrane within 1 mm in the longest diameter, which is identified in 10–20% of DCIS cases and approximately 1% of all breast cancers (2, 3). Patients with DCISM are generally considered to have a better prognosis than IDC, while worse than pure DCIS (4, 5).
The rarity of DCISM has made studies difficult, and the few studies of axillary management and outcomes in DCISM showed controversial results (6–14). Previous studies showed that the size of primary lesion is associated with axillary positivity and prognosis in patients with invasive breast cancer (15). Moreover, small, single institute studies have showed that the extent of microinvasion may be a predictive risk for axillary metastasis and worse prognosis (16). Thus, it is of interest to determine whether multiple foci of microinvasion is associated with a higher risk of positive axillary status or worse long-term outcomes in patients with DCISM.
We hypothesized that in the cohort of patients with DCISM, a significant association of multiple foci of microinvasion with axillary metastasis and worse prognosis might exist. In the current study, we report the incidence of axillary involvement and disease-free survival (DFS) in DCISM patients with a single versus multiple foci of microinvasion.
Materials and Methods
We retrospectively reviewed the medical records of patients diagnosed with DCISM who underwent axillary staging at our institute from January 2006 to December 2015. The inclusion criteria were as follows: (1) female patients diagnosed with DCISM on the final pathology; (2) underwent axillary staging. Patients were excluded if they had: (1) neo-adjuvant chemotherapy prior to surgery or (2) history of breast cancer. Data on age, BMI, clinical features, type of surgery and type of axillary procedure, and any adjuvant radiation therapy, hormonal therapy or chemotherapy were collected. Pathologic variables collected included histologic tumor type, histologic and nuclear grade, presence of lymphovascular invasion (LVI), and receptor status [estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2)] in microinvasive sites. Lesion size on pathology was defined as total extent of breast lesion with both DCIS lesion and microinvasive foci. The study was approved by the Ethics Committee of Jiaxing University.
Patients in this study received surgical excision in our institution, including total mastectomy and breast conserving surgery (BCS). Microinvasion was defined as invasive portion no more than 1 mm. Pathological results were reviewed for statements regarding number of invasive foci and categorized as having one focus or multiple foci (≥2 foci). The cutoff was selected according to the language used in the pathology reports. Immunostaining for ER was performed, and cases with more than 1% were considered as positive staining. HER2 positivity was defined as cases where immunohistochemistry staining was 3+ or 2+ with fluorescence in situ hybridization positivity. Sentinel lymph node biopsy (SLNB) positivity was defined as the presence of micrometastasis (>200 cells or >0.2 mm, but <2.0 mm) or macrometastasis (>2.0 mm) identified on hematoxylin and eosin (H&E) staining. SLNs were identified via methylthionine chloride injection and were assessed via serial sections with 100 μm of spacing between sections.
Date and status at last follow-up were collected. Recurrence events were recorded as local-regional (ipsilateral breast and chest wall, ipsilateral axillary or supraclavicular lymph nodes) and distant. Time to recurrence and time to death were measured from date of surgery and censored at the date of last follow-up for event free patients. Time to recurrence was censored at time of death. Patients with less than 6 months follow-up after surgery were not included in the survival analysis. Loss to follow-up was defined as patients with less than 3-year follow-up after surgery.
The clinicopathological characteristics were compared between patients with one focus and multiple foci of microinvasion and between patients who received and did not receive chemotherapy using chi-square test for categorical variables. Time-to-event outcomes were estimated using Kaplan–Meier methods and were compared across groups using the log-rank test. Cox regression model was used for multivariate analysis in survival. All statistical analysis was performed using SPSS statistical software version 18.0 (IBM, Chicago, IL, USA), and P values less than 0.05 were considered significant.
Results
A total of 359 patients with DCISM were identified, of which 233 (64.90%) had one focus of microinvasion and 126 (35.10%) had multiple foci. The median age of this cohort was 49 (range 26–83). The baseline characteristics of the cohort were shown in Table 1. Patients with multiple foci tended to have larger tumor size (P < 0.001), higher nuclear grade (P = 0.001), and higher rate of lymphatic vascular invasion (P = 0.034). Also, the proportion of positive HER2 (P = 0.027) and Ki67 level (P = 0.004) increased in patients with multiple foci. While in multivariate analysis, only tumor size showed significant difference (P = 0.009).
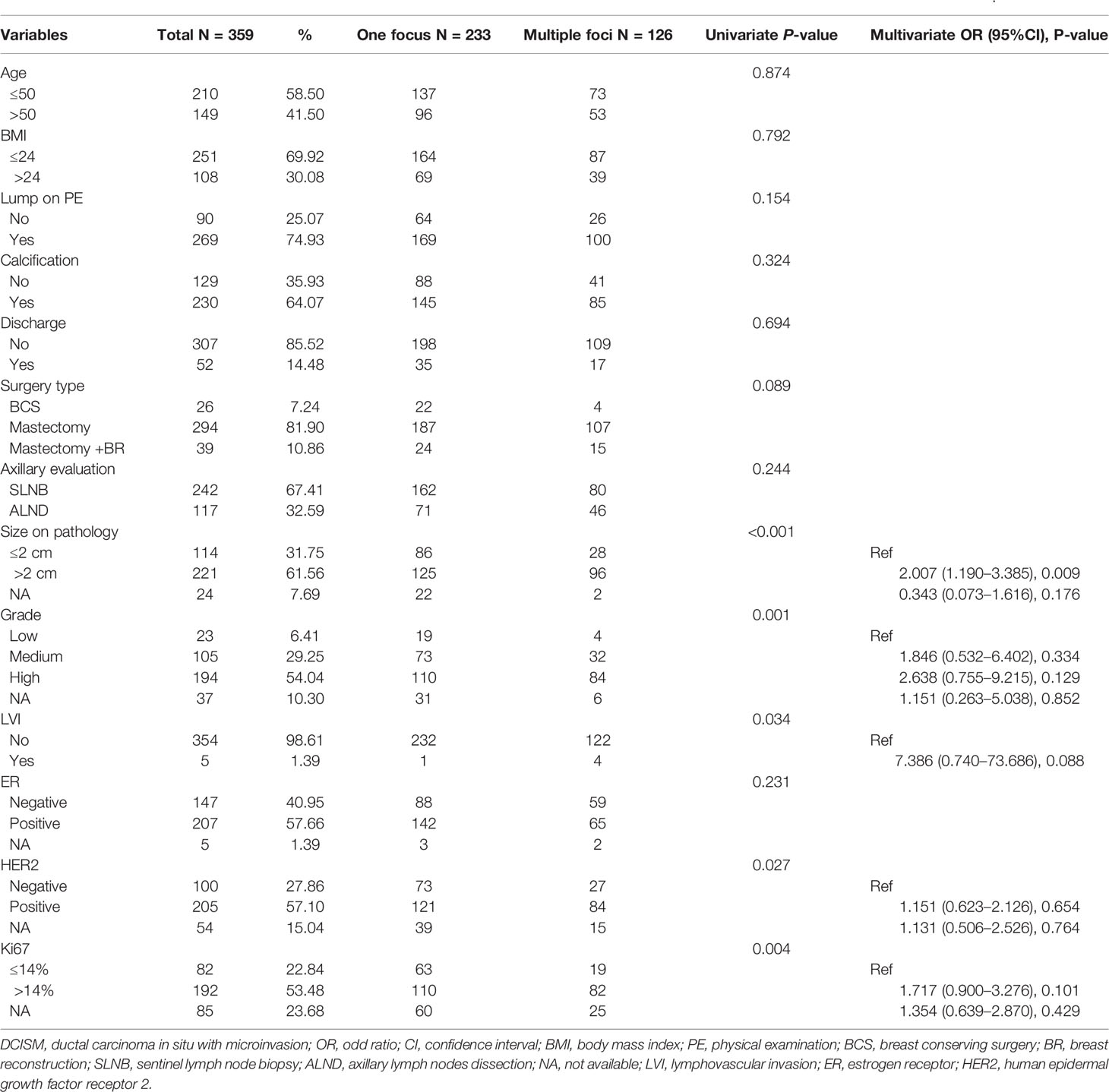
Table 1 Baseline characteristics, univariate and multivariate analysis of clinicopathological factors in DCISM patients with one focus and multiple foci.
Overall, 242 (67.41%) and 117 (32.59%) patients underwent sentinel lymph nodes biopsy (SLNB) and axillary lymph nodes dissection (ALND), respectively. The medium number of SLNs was three [interquartile range (IQR) 2–4]. Isolated tumor cells (ITCs) were found in four (1.11%) patients and axillary metastasis rate was 2.51%. Neither axillary evaluation methods (P = 0.244) nor axillary metastasis rate (2.07 vs 3.42%, P = 0.559) was significantly different between patients with one focus and multiple foci (Table 2).
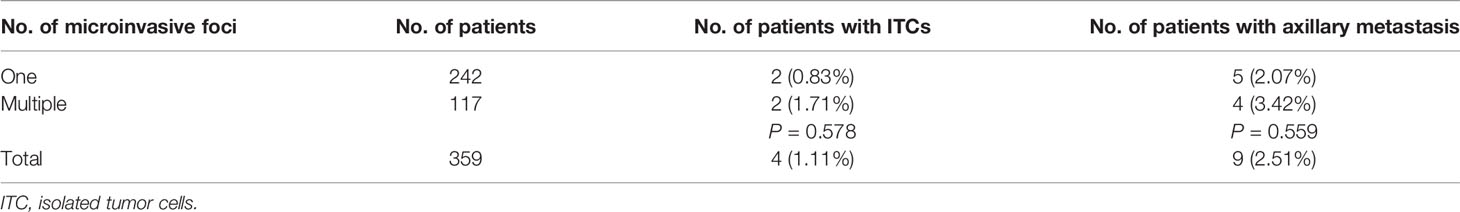
Table 2 Number of microinvasive foci and rate of axillary metastasis with P values for comparison of rates between groups.
For patients who received BCS, 53.85% (14/26) received adjuvant radiation therapy. There were 17 patients who received adjuvant radiation therapy; all of them had one focus of microinvasion. There were differences in receipt of adjuvant systemic therapy in the two groups. Hormonal therapy was used more frequently in the one -focus group (49.36 vs 35.71%; P = 0.051), while chemotherapy was used more frequently in the multiple-foci group (56.35 vs 40.77%; P = 0.028), which can partly be explained by the different clinicopathological factors in these two groups. Also, we found that both the use of hormonal therapy and chemotherapy was more common among patients with positive axillary. Hormonal therapy was given to 55.56% of axillary-positive patients compared to 44.29% of axillary-negative patients (P = 0.111), which did not reach significant differences. Moreover, 66.67 and 45.71% of axillary-positive and axillary-negative patients received chemotherapy, respectively (P = 0.003).
In the subset of patients with negative axillary, multiple foci of microinvasion were also associated with adjuvant systemic therapy, suggesting that this pathologic factor may be used in decision making for adjuvant systemic therapy. Compared with patients with one focus of microinvasion, patients with multiple foci were more likely to receive chemotherapy: 55.74% (68/122) vs 40.35% (92/228), respectively (P = 0.029). However, they were less likely to receive hormonal therapy: 33.61% (41/122) vs 50.00% (114/228), respectively (P = 0.019). Furthermore, we divided patients with negative axillary into two groups by the usage of chemotherapy to further investigate whether the number of microinvasive foci was independently associated with chemotherapy (Table 3). In the univariate analysis, we found that most of the pathological factors were associated with chemotherapy. However, only tumor size, ER, and HER2 showed significant differences in multivariate analysis. Patients with larger tumor size (P = 0.008), negative ER (P = 0.023) and positive HER2 (P < 0.001) were more likely to receive chemotherapy. Thus, the number of microinvasive foci was not significantly associated with chemotherapy in patients with negative axillary.
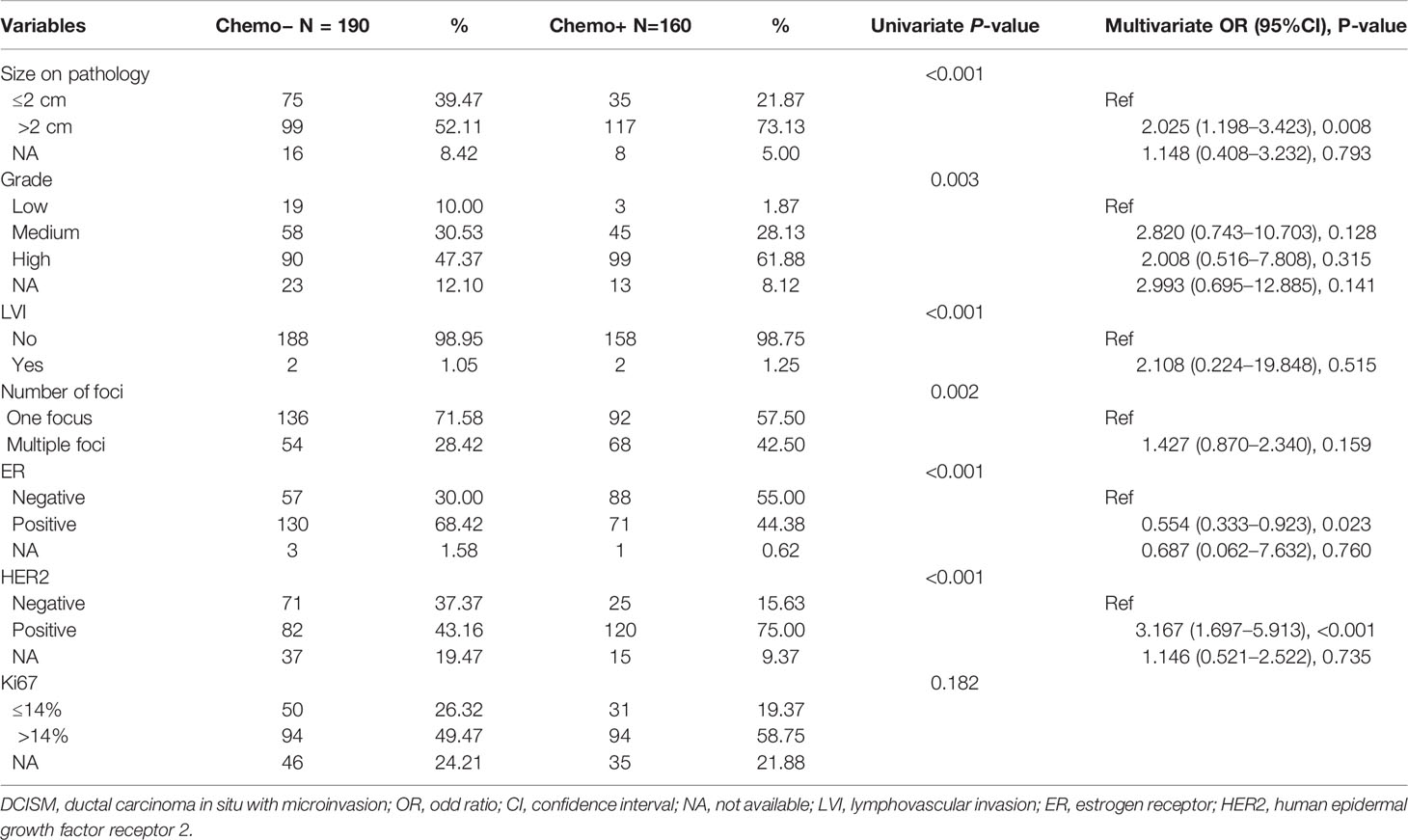
Table 3 Univariate and multivariate analysis of impact factors on chemotherapy in DCISM patients with negative axillary.
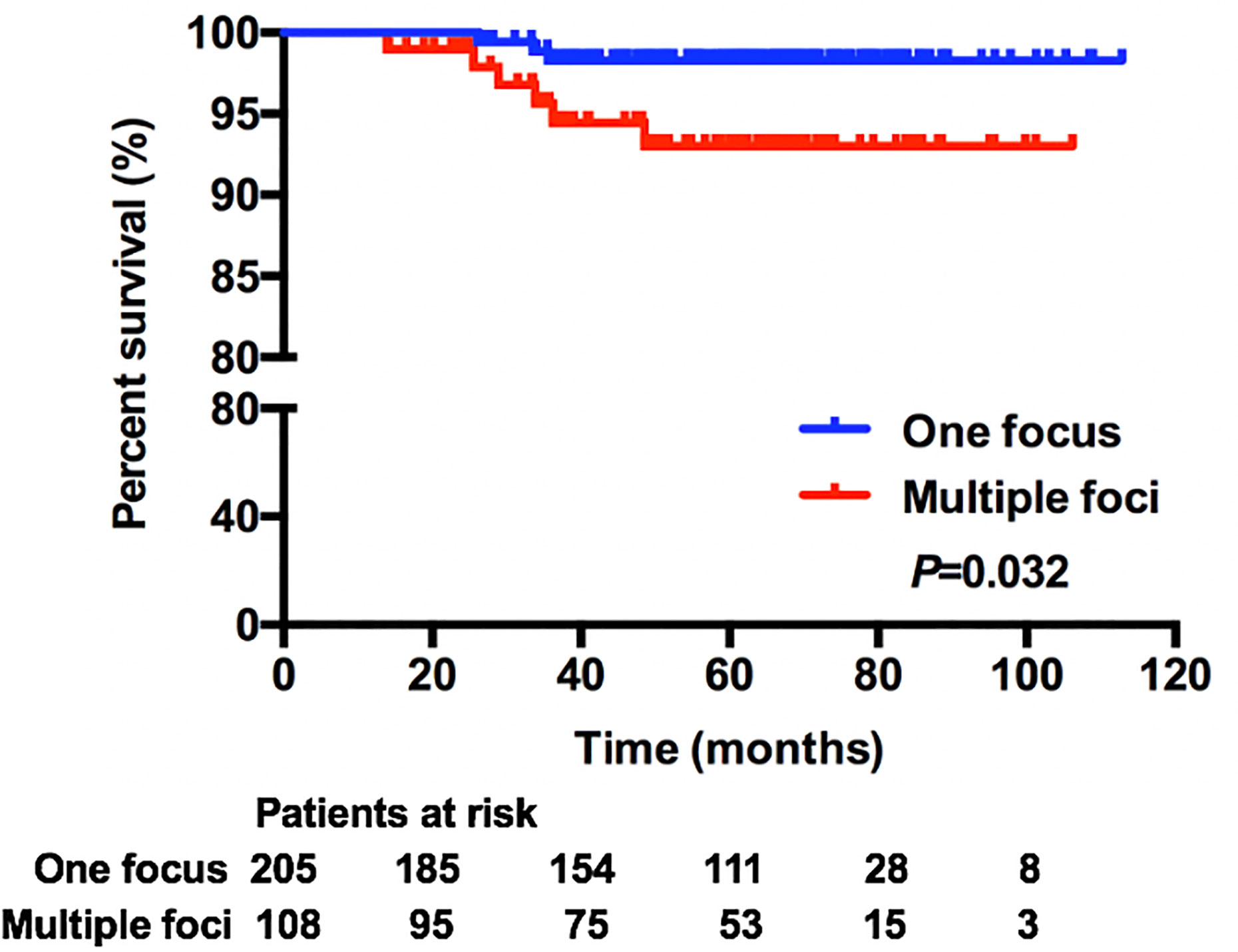
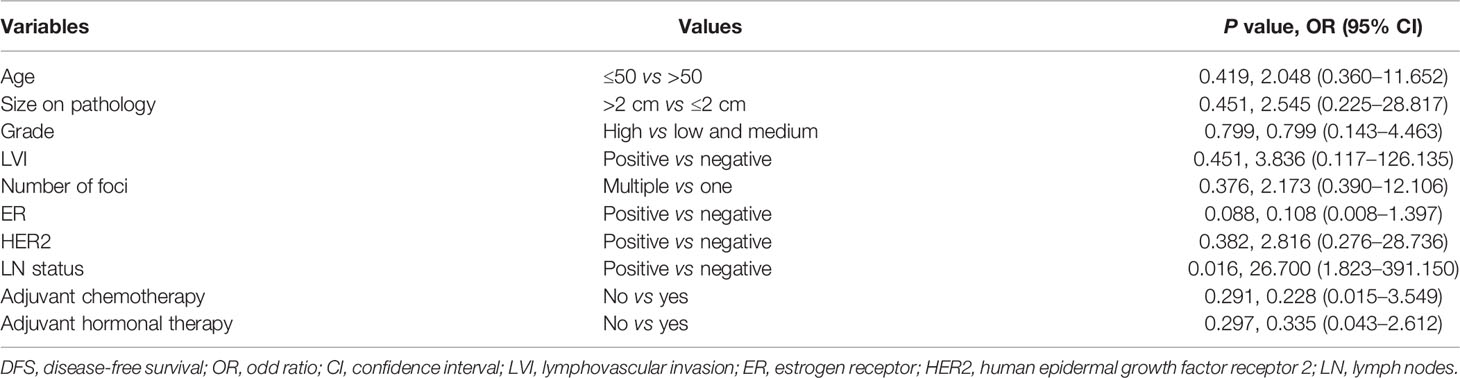
In this cohort, 313 (87.19%) patients were included in the survival analysis. The median follow-up was 61.27 months, which was 5.11 years for the total population and was similar for one-focus and multiple-foci groups. Overall survival rate was 99.36% for the total population. There were nine (2.88%) recurrences: four local and regional; four distant; and one concurrent local and distant (Supplementary Table 1). Kaplan–Meier analysis showed that patients with multiple microinvasive foci had worse DFS rate compared with one-focus patients (98.29 vs 93.01%, P = 0.032) (Figure 1). While, multivariate analysis showed that the only independent predictor for worse DFS was axillary metastasis status (Table 4). Compared with patients with no axillary metastasis, patients with positive axillary were 26.70-fold more likely to have worse DFS (P = 0.016).
Discussion
DCISM is rare, and there are controversial results reported on the outcomes of this subtype compared with pure DCIS and invasive breast cancer. Generally, the biological behavior and survival outcomes of DCISM were intermediate between those diagnosed with DCIS and invasive breast cancer. However, previous studies have shown conflicting results on both axillary metastasis and survival outcome of DCISM patients. In this current study, we focused on the association between extent of microinvasion foci in DCIS with axillary status and survival outcome.
Previous studies showed that the biological behavior of DCISM was worse than pure DCIS, such as larger tumors, higher tumor grade, human epidermal growth factor receptor 2 positivity, and a high Ki-67 index (14, 17, 18). In our study, we also found that DCIS patients with multiple foci of microinvasion had more aggressive biology than those with one focus of microinvasion. Patients with multiple foci tended to have larger tumor size, higher nuclear grade, and higher rate of lymphatic vascular invasion, which were all potential impact factors of worse prognosis. Also, there were more HER2 positivity and higher Ki67 level in patients with multiple foci. In multivariate analysis, we found that only tumor size showed a significant difference, indicating that DCIS patients with larger tumor (>2 cm) were more likely to have multiple invasive foci.
Currently, the extent of microinvasion in DCIS is not a factor included in the staging of breast cancer. Although the size of the tumor has clearly been associated with axillary status, and overall prognosis in patients diagnosed with invasive breast cancer, multiple foci of microinvasion in DCIS have not been consistently linked to outcomes above (13, 19). It seems possible that an increased disease burden with multiple foci of microinvasion can lead to worse outcomes, yet due to the rarity of this specific pathological pattern, the impact of the extent of microinvasion in DCIS on axillary metastasis and patient survival outcome has not been clearly described. Cindy B. Matsen et al. reported that extent of microinvasion in DCIS was not associated with axillary metastasis, showing that there was no higher risk of nodal involvement with multiple foci of microinvasion as compared to one focus (20). While, in other research, rates of axillary involvement differed in DCIS patients with different numbers of microinvasive foci, showing that 2.1% axillary metastasis in patients with one focus and 15.6% for multiple foci (P = 0.037) (21). There were few data on the long-term outcome of this specific pathological subtype, and results were inconsistent. Some indicated that different extent of microinvasive foci in DCIS had similar survival outcome, while others did not (21–23). A study from Canada showed that multiple foci of microinvasion were associated with higher risk of invasive local recurrence in DCIS patients received BCS (23). Another study also showed that multifocality in DCIS was associated with a worse survival in chemotherapy and trastuzumab-naive patients, while in multivariate regression analysis, the difference was statistically insignificant (21). In our study, the incidence of pathologically positive axillary metastasis in DCISM patients was 2.51%, which was comparable with previous studies (21, 24). We found that the axillary metastasis rate was similar in patients with one focus and multiple foci (2.07 vs 3.42%, P = 0.559). However, the extent of microinvasion was associated with DFS in DCIS patients. Compared with one-focus patients, patients with multiple foci had worse DFS rate (93.01 vs 98.29%, P = 0.032).
Notably, in our study, the mastectomy rate and ALND rate were both high. Over 80% of DCISM patients received mastectomy, and over 30% received ALND. This may be related to the characteristic of DCIS on preoperative imaging and potential underestimate of invasive breast cancer in patients diagnosed with DCIS by core needle biopsy. As we have known, the extent of DCIS lesions on preoperative imaging can be large and multicentric. Also, microinvasive foci were more likely to be found in larger background of DCIS than smaller lesion. Thus, these DCISM patients were more likely to receive mastectomy than BCS. Furthermore, the potential upstaging of DCISM to invasive breast cancer was an important issue for surgical options, especially axillary evaluation. Previous studies showed that about 14–32% of patients diagnosed with DCIS on core needle biopsy could be upstaged to invasive diseases after surgical excision (25–27). Also, the volume of breasts in eastern patients were smaller than western patients, which led to less BCS. And the increasing application of breast reconstruction was another important issue for surgical choices preferring mastectomy.
In this current study, we found that the usage of adjuvant therapy was different in patients with one focus or multiple foci of microinvasion, mostly due to the different clinicopathological factors in these two groups. All patients received adjuvant radiation therapy had one focus of microinvasion. Patients with one focus of microinvasion were more likely to had smaller tumor, which led to more possibility of BCS. Thus, adjuvant radiation therapy was more common among patients with one focus of microinvasion. Also, we found that both hormonal therapy and chemotherapy were more common among patients with positive axillary, which was reasonable because nodal metastasis was one of the powerful predictors of recurrence and survival in breast cancer. In patients with negative axillary, the extent of microinvasion in DCIS was not associated with adjuvant chemotherapy, while tumor size, ER, and HER2 did appear to influence this decision. Thus, multiple microinvasions alone was not a significant impact factor for decision on adjuvant chemotherapy.
For small breast tumors, especially DCIS or DCISM, whether they receive adjuvant therapy is always a dilemma. Some previous studies showed that patients with small tumors receiving systemic therapy were significantly younger and had lymph node metastasis, higher tumor grade, negative ER, and positive HER2 status (28–30). While other studies showed that no benefit was observed for adjuvant chemotherapy in very small tumors, such as T1mi, T1a, and T1b HER2-positive or triple-negative breast cancers with no axillary metastasis (31, 32). Also, studies showed that either aromatase inhibitors or tamoxifen could be an effective adjuvant treatment options in order to lower the risk of recurrent DCIS (33, 34). For DCIS or DCISM patients who received BCS, radiation therapy remains to be the standard of care (35, 36). Up to now, whether it is necessary to receive chemotherapy and target therapy in DCISM patients has not been addressed clearly. According to our findings, patients with larger tumor (>2cm), negative ER, and positive HER2 were more likely to receive chemotherapy. Also, previous studies have proved that tumor size, ER negativity, and HER2 overexpression promoted factors on invasion and metastasis in breast cancer (37, 38). Thus, we suggest that, in high-risk DCISM patients, adjuvant systemic therapy can be properly applied.
Interestingly, we found that almost all the recurrence patients in the cohort had overexpressed HER2, which was well-known by accelerating cell proliferation and enhancing malignant behavior (39, 40). For HER2 positive patients with adjuvant chemotherapy (n = 122), only 14 patients also administered Trastuzumab. With limited cases, we still found that patients who received Trastuzumab had no recurrence in a median follow-up of 24.80 months, while patients without Trastuzumab had 5.66% recurrence rate in a median follow-up of 24.20 months. In addition, compared with patients without Trastuzumab, patients received Trastuzumab had larger tumor size on average (3.9 vs 3.6 cm) and higher rate of multiple foci of microinvasion (64.3 vs 43.5%). Thus, HER2-positive patients with multiple foci of microinvasion could be considered for optimization of target therapy. Previous studies reported that HER2 overexpression was an independent predictor of invasive disease and poor prognosis, indicating that HER2 expression might be associated with an important pathway through which DCIS lesions may progress toward invasive lesions (18, 21, 41). Thus, HER2 overexpression in DCIS patients might be a predictor of the presence of invasive foci and long-term recurrence after surgery. For HER2 positive DCISM patients with other high risks of poor outcome, both adjuvant chemotherapy and target therapy could be considered.
There were several limitations to the present study. First, it was a retrospective study, which led to lower level of evidence. However, this study had a relatively large dataset with uniform inclusion and exclusion criteria. Second, some information was missing, which could lead to bias in the analysis. Furthermore, the pathological features were mixed. Grading criteria were for in situ lesions, while ER and HER2 status was in microinvasive sites because the invasive component was too small to adequately grade. Further assessment is needed to accurately select low-risk DCISM patients who can safely avoid axillary staging and adjuvant systemic therapy.
Conclusion
DCIS with microinvasion is a rare subgroup in breast cancer patients and presents a therapeutic conundrum. Even though the numbers of microinvasion were different and patients with multiple foci of microinvasion tended to have larger tumor size, there was no higher risk of axillary involvement compared with patients with one focus of microinvasion. While, patients with multiple microinvasive foci had worse DFS rate. Thus, DCISM patients with multiple foci of microinvasion may be the criterion for more aggressive local–regional treatment, especially in HER2 positive patients. Optimization of adjuvant therapy in DCISM patients is required.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by First Hospital of Jiaxing. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JS wrote this article. RG did the statistics. HP, XL, ZG, C, LX, and DX collected data. WW and CC gave administration support. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from National Natural Science Foundation of China (Grant No. 81902674) and Innovation Subject of Jiaxing (Grant No. 2019-cx-04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.607502/full#supplementary-material
References
1. Lynge E, Ponti A, James T, Majek O, von Euler-Chelpin M, Anttila A, et al. Variation in detection of ductal carcinoma in situ during screening mammography: A survey within the International Cancer Screening Network. Eur J Cancer (2014) 50(1):185–92. doi: 10.1016/j.ejca.2013.08.013
2. Bianchi S, Vezzosi V. Microinvasive carcinoma of the breast. Pathol Oncol Res (2008) 14(2):105–11. doi: 10.1007/s12253-008-9054-8
3. Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol (2002) 20(17):3628–36. doi: 10.1200/JCO.2002.02.026
4. Wong JH, Kopald KH, Morton DL. The Impact of Microinvasion on Axillary Node Metastases and Survival in Patients with Intraductal Breast-Cancer. Arch Surg-Chicago (1990) 125(10):1298–302. doi: 10.1001/archsurg.1990.01410220082011
5. Vieira CC, Mercado CL, Cangiarella JF, Moy L, Toth HK, Guth AA. Microinvasive ductal carcinoma in situ: Clinical presentation, imaging features, pathologic findings, and outcome. Eur J Radiol (2010) 73(1):102–7. doi: 10.1016/j.ejrad.2008.09.037
6. Shatat L, Gloyeske N, Madan R, O’Neil M, Tawfik O, Fan F. Microinvasive breast carcinoma carries an excellent prognosis regardless of the tumor characteristics. Hum Pathol (2013) 44(12):2684–9. doi: 10.1016/j.humpath.2013.07.010
7. Sue G, Lannin DR, Killelea BK, Horowitz NR, Chagpar AB. Predictors of microinvasion and its prognostic role in ductal carcinoma in situ. Am J Surg (2013) 206(4):478–81. doi: 10.1016/j.amjsurg.2013.01.039
8. Lyons JM, Stempel M, Van Zee KJ, Cody HS. Axillary node staging for microinvasive breast cancer: Is it justified? Ann Surg Oncol (2012) 19(11):3416–21. doi: 10.1245/s10434-012-2376-5
9. Gray RJ, Mulheron B, Pockaj BA, Degnim A, Smith SL. The optimal management of the axillae of patients with microinvasive breast cancer in the sentinel lymph node era. Am J Surg (2007) 194(6):845–9. doi: 10.1016/j.amjsurg.2007.08.034
10. Parikh RR, Haffty BG, Lannin D, Moran MS. Ductal Carcinoma in Situ with Microinvasion: Prognostic Implications, Long-Term Outcomes, and Role of Axillary Evaluation. Int J Radiat Oncol (2012) 82(1):7–13. doi: 10.1016/j.ijrobp.2010.08.027
11. Zavagno G, Belardinelli V, Marconato R, Carcoforo P, Franchini Z, Scalco G, et al. Sentinel lymph node metastasis from mammary ductal carcinoma in situ with microinvasion. Breast (2007) 16(2):146–51. doi: 10.1016/j.breast.2006.08.002
12. Li Y, Zhang S, Wei X, Zhang J. The clinical features and management of women with ductal carcinoma in situ with microinvasion: A retrospective Cohort study. Int J Surg (2015) 19:91–4. doi: 10.1016/j.ijsu.2015.05.013
13. Sopik V, Sun P, Narod SA. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Tr (2018) 167(3):787–95. doi: 10.1007/s10549-017-4572-2
14. Wang W, Zhu W, Du F, Luo Y, Xu B. The Demographic Features, Clinicopathological Characteristics and Cancer-specific Outcomes for Patients with Microinvasive Breast Cancer: A SEER Database Analysis. Sci Rep (2017) 7:42045. doi: 10.1038/srep42045
15. Bevilacqua JL, Kattan MW, Fey JV, Cody HS,3, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol (2007) 25(24):3670–9. doi: 10.1200/JCO.2006.08.8013
16. Kapoor NS, Shamonki J, Sim MS, Chung CT, Giuliano AE. Impact of multifocality and lymph node metastasis on the prognosis and management of microinvasive breast cancer. Ann Surg Oncol (2013) 20(8):2576–81. doi: 10.1245/s10434-013-2924-7
17. Yao JJ, Zhan WW, Chen M, Zhang XX, Zhu Y, Fei XC, et al. Sonographic Features of Ductal Carcinoma In Situ of the Breast With Microinvasion: Correlation With Clinicopathologic Findings and Biomarkers. J Ultrasound Med (2015) 34(10):1761–8. doi: 10.7863/ultra.15.14.07059
18. Champion CD, Ren Y, Thomas SM, Fayanju OM, Rosenberger LH, Greenup RA, et al. DCIS with Microinvasion: Is It In Situ or Invasive Disease? Ann Surg Oncol (2019) 26(10):3124–32. doi: 10.1245/s10434-019-07556-9
19. Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol (2005) 23(30):7497–502. doi: 10.1200/JCO.2005.02.1147
20. Matsen CB, Hirsch A, Eaton A, Stempel M, Heerdt A, Van Zee KJ, et al. Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol (2014) 21(10):3330–5. doi: 10.1245/s10434-014-3920-2
21. Fang Y, Wu J, Wang W, Fei X, Zong Y, Chen X, et al. Biologic behavior and long-term outcomes of breast ductal carcinoma in situ with microinvasion. Oncotarget (2016) 7(39):64182–90. doi: 10.18632/oncotarget.11639
22. de Mascarel I, MacGrogan G, Mathoulin-Pelissier S, Soubeyran I, Picot V, Coindre JM. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer (2002) 94(8):2134–42. doi: 10.1002/cncr.10451
23. Rakovitch E, Sutradhar R, Lalani N, Nofech-Mozes S, Gu S, Goldberg M, et al. Multiple foci of microinvasion is associated with an increased risk of invasive local recurrence in women with ductal carcinoma in situ treated with breast-conserving surgery. Breast Cancer Res Treat (2019) 178(1):169–76. doi: 10.1007/s10549-019-05364-z
24. Gojon H, Fawunmi D, Valachis A. Sentinel lymph node biopsy in patients with microinvasive breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol (2014) 40(1):5–11. doi: 10.1016/j.ejso.2013.10.020
25. Si J, Yang B, Guo R, Huang N, Quan C, Ma L, et al. Factors associated with upstaging in patients preoperatively diagnosed with ductal carcinoma in situ by core needle biopsy. Cancer Biol Med (2019) 16(2):312–8. doi: 10.20892/j.issn.2095-3941.2018.0159
26. Watanabe Y, Anan K, Saimura M, Koga K, Fujino M, Mine M, et al. Upstaging to invasive ductal carcinoma after mastectomy for ductal carcinoma in situ: predictive factors and role of sentinel lymph node biopsy. Breast Cancer (2018) 25(6):663–70. doi: 10.1007/s12282-018-0871-7
27. Jakub JW, Murphy BL, Gonzalez AB, Conners AL, Henrichsen TL, Maimone ST, et al. A Validated Nomogram to Predict Upstaging of Ductal Carcinoma in Situ to Invasive Disease. Ann Surg Oncol (2017) 24(10):2915–24. doi: 10.1245/s10434-017-5927-y
28. Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A. Management of small T1a/b breast cancer by tumor subtype. Breast Cancer Res Treat (2017) 163(1):111–8. doi: 10.1007/s10549-017-4168-x
29. Schroeder MC, Lynch CF, Abu-Hejleh T, Chrischilles EA, Thomas A. Chemotherapy use and surgical treatment by receptor subtype in node-negative T1a and T1b female breast cancers, Iowa SEER Registry, 2010 to 2012. Clin Breast Cancer (2015) 15(1):e27–34. doi: 10.1016/j.clbc.2014.07.009
30. Vaz-Luis I, Ottesen RA, Hughes ME, Mamet R, Burstein HJ, Edge SB, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol (2014) 32(20):2142–50. doi: 10.1200/JCO.2013.53.1608
31. Parsons BM, Uprety D, Smith AL, Borgert AJ, Dietrich LL. A US Registry-Based Assessment of Use and Impact of Chemotherapy in Stage I HER2-Positive Breast Cancer. J Natl Compr Canc Netw (2018) 16(11):1311–20. doi: 10.6004/jnccn.2018.7058
32. Migdady Y, Sakr BJ, Sikov WM, Olszewski AJ. Adjuvant chemotherapy in T1a/bN0 HER2-positive or triple-negative breast cancers: application and outcomes. Breast (2013) 22(5):793–8. doi: 10.1016/j.breast.2013.02.014
33. Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet (2016) 387(10021):866–73. doi: 10.1016/S0140-6736(15)01129-0
34. Margolese RG, Cecchini RS, Julian TB, Ganz PA, Costantino JP, Vallow LA, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet (2016) 387(10021):849–56. doi: 10.1016/S0140-6736(15)01168-X
35. Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst (2011) 103(6):478–88. doi: 10.1093/jnci/djr027
36. Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med (2004) 350(14):1430–41. doi: 10.1056/NEJMra031301
37. Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Danish Breast Cancer Cooperative G: Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol (2008) 26(9):1419–26. doi: 10.1200/JCO.2007.14.5565
38. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol (2008) 26(14):2373–8. doi: 10.1200/JCO.2007.14.4287
39. Zhang W, Gao EL, Zhou YL, Zhai Q, Zou ZY, Guo GL, et al. Different distribution of breast ductal carcinoma in situ, ductal carcinoma in situ with microinvasion, and invasion breast cancer. World J Surg Oncol (2012) 10:262. doi: 10.1186/1477-7819-10-262
40. Ozkan-Gurdal S, Cabioglu N, Ozcinar B, Muslumanoglu M, Ozmen V, Kecer M, et al. Factors predicting microinvasion in Ductal Carcinoma in situ. Asian Pac J Cancer Prev (2014) 15(1):55–60. doi: 10.7314/APJCP.2014.15.1.55
41. Tunon-de-Lara C, Chauvet MP, Baranzelli MC, Baron M, Piquenot J, Le-Bouedec G, et al. The Role of Sentinel Lymph Node Biopsy and Factors Associated with Invasion in Extensive DCIS of the Breast Treated by Mastectomy: The Cinnamome Prospective Multicenter Study. Ann Surg Oncol (2015) 22(12):3853–60. doi: 10.1245/s10434-015-4476-5
Keywords: ductal carcinoma in situ, microinvasion, recurrence, foci, survival outcome
Citation: Si J, Guo R, Pan H, Lu X, Guo Z, Han C, Xue L, Xing D, Wu W and Chen C (2020) Multiple Microinvasion Foci in Ductal Carcinoma In Situ Is Associated With an Increased Risk of Recurrence and Worse Survival Outcome. Front. Oncol. 10:607502. doi: 10.3389/fonc.2020.607502
Received: 17 September 2020; Accepted: 05 November 2020;
Published: 03 December 2020.
Edited by:
Alberto Farolfi, Romagnolo Scientific Institute for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Gilles Houvenaeghel, Institut Paoli-Calmettes (IPC), FranceGamze Durhan, Hacettepe University, Turkey
Copyright © 2020 Si, Guo, Pan, Lu, Guo, Han, Xue, Xing, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Si, c2lqaW5nMTAwOEAxNjMuY29t
†These authors have contributed equally to this work
 Jing Si
Jing Si Rong Guo3†
Rong Guo3†