- 1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2Department of Gastric Surgery, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Melanoma and Sarcoma Medical Oncology Unit, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: Unplanned excision (UPE) of soft tissue sarcoma (STS) is often chosen in the early phase by general physicians without any radiological evaluation.
Purpose: The present study aimed to evaluate the impact of UPE on the clinical outcomes of patients with STS of the trunk and extremity.
Materials and Methods: Patients with STS of the trunk and extremity who underwent R0 resection between 1998 and 2016 were included and divided into the UPE and planned excision (PE) groups. Propensity score matching (PSM) was used to control the selection bias. The endpoints were disease-specific survival (DSS), local recurrence-free survival (LRFS), and metastasis-free survival (MFS).
Results: In total, 458 patients (277 males, 181 females; median age: 43 years) were included: 329 (71.8%) in the PE group and 129 (28.2%) in the UPE group. The follow-up time ranged from 7.1 to 313.78 months, with a median of 112.18 months. UPE patients were more likely to have a smaller or superficial lesion and were more frequently administered adjuvant therapy. After PSM, compared with the PE group, the UPE group had a longer LRFS (P=0.015), but there was no difference between the two groups regarding DSS and MFS. Residual disease was observed in 77.5% of the re-resected specimens in the UPE group and was a risk factor for DSS (P = 0.046) and MFS (P = 0.029) but was not associated with local recurrence (LR) (P=0.475) or LRFS (P=0.334). Moreover, we found no difference in DSS, LRFS or MFS according to the interval from UPE to definitive resection.
Conclusion: STS treated with UPE had distinct characteristics. Patients who undergo UPE followed by an additional wide R0 resection have similar oncological survival compared to patients who undergo an initial PE, although the high incidence of residual tumor in the UPE group leads to an unfavorable clinical course.
Introduction
Soft tissue sarcoma (STS) represents a rare and heterogeneous group of primary malignancies, accounting for approximately 1% of all adult malignancies (1). Since its incidence is low and it can occur in any part of the body, STS is apt to being ignored by general physicians and is inadvertently excised as a mass assumed to be benign or an inflammatory lesions without wide margins (2). Approximately 19% to 53% of patients who undergo inappropriately unplanned excision (UPE) are referred to sarcoma centers (2–4). Due to the high risk of residual tumors left in the tumor bed after UPE, the current standard therapy suggests additional resection to achieve wide or at least negative margins and ensure local control (5, 6).
Several studies have demonstrated that patients who underwent UPE had worse oncologic outcomes (7, 8). whereas other authors showed similar or even better outcomes than patients who underwent planned excision (PE) initially (9–11). These differing results are probably because the majority of the literature did not consider similar clinicopathological variables. It is difficult to draw conclusions from two comparison groups with disparate tumor features even in multivariable models in which differences can partly be explained (12, 13). Thus, it is necessary to construct an algorithm to balance the differences in baseline characteristics between patients who undergo UPE and PE.
The present study is aimed to compare the oncologic outcomes of patients with STS of the trunk and extremity who underwent UPE with those of patients who underwent PE. We created a group from the study population with balanced baseline characteristics according to some primary characteristics through a propensity score matching (PSM) and then elucidate the potential influence of UPE. Additionally, we evaluated factors were associated with the prognosis and whether residual tumor or delayed re-excision would affect prognosis.
Materials and Methods
Study Population
We reviewed the STS database from the Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China) to identify patients who underwent R0 resection as the final resection status for primary, non-metastatic STS of the trunk and extremity between January 1998 and January 2016. Patients with inadequate medical records (50, 7%) and those who were lost to follow-up (27 unreachable patients at the point of follow-up, 3%) were excluded. Patients with stage IV disease and those who underwent preoperative treatment were also excluded. Finally, 458 patients were included in this study (Supplementary Figure 1).
According to previous reports, UPE was defined as the non-oncologic excision of a suspected benign lesion without consideration the need to remove the normal tissue around the tumor with subsequently pathologically confirmed STS (14, 15). PE was defined as the planned oncologic excision for a preoperatively suspected STS. R0 was defined as the microscopic absence of malignant cells at the resection margin. All tumors were reviewed by experienced pathologists at our institution. Tumor size was determined as the largest diameter described in the pathology reports or measured on imaging, and sarcoma depth was characterized as superficial or deep according to the involvement of the investing muscle fascia. Tumors were staged and graded according to the American Joint Committee on Cancer (AJCC) 8th Edition (16) and the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system respectively (17).
After surgery, all patients were regularly followed-up every 3–6 months during the first 2–3 years and yearly thereafter. Recording of medical history, physical examination, computed tomography (CT) and/or magnetic resonance imaging (MRI) were performed during the follow-up. Additional studies, including positron emission tomography (PET) and biopsy were performed when necessary. The follow-up time was calculated from the date of diagnosis to the date of death or was censored at the end of follow-up (March 1st, 2020). The primary endpoints were disease-specific survival (DSS), local recurrence-free survival (LRFS), and metastasis-free survival (MFS). The time to the occurrence of the event was calculated from the date of R0 surgery to the date when the event was first recorded.
The authenticity of this article was validated by uploading the key raw data to the Research Data Deposit public platform (www.researchdata.org.cn) with the RDD approval number of RDDA2020001446. Our institutional review board (IRB) approved this study (B2020-068-01).
PSM Analysis
The propensity score, defined as the conditional probability of undergoing a therapy given certain covariate factors of covariates, is generally calculated to adjust selection bias in observational studies (18, 19). In our study, one-to-one nearest-neighbour matching without replacement was adopted to control confounding factors in both groups using a 0.1 calliper. PSM was performed by using Empower Stats software (http://www.empowerstats.com/).
Statistical Analysis
Chi-square tests (e.g., Fisher’s exact test and Pearson’s chi-square test) were used for comparisons of categorical data, where appropriate. Survival curves were generated by using the Kaplan-Meier method and compared by using the log-rank test. Prognostic variables associated with DSS, LRFS, and MFS that were significant in the univariate analyses were selected for multivariate Cox proportional hazard model analyses with the stepwise forward selection algorithm, and the results are presented as hazard ratios (HR) and 95% confidence intervals (CI). Two-sided P values < 0.05 were considered statistically significant. All data were analyzed using the IBM SPSS software, version 20.0 (SPSS, Inc., IBM Company, Armonk, New York).
Results
Baseline Clinicopathological Characteristics Prior to PSM
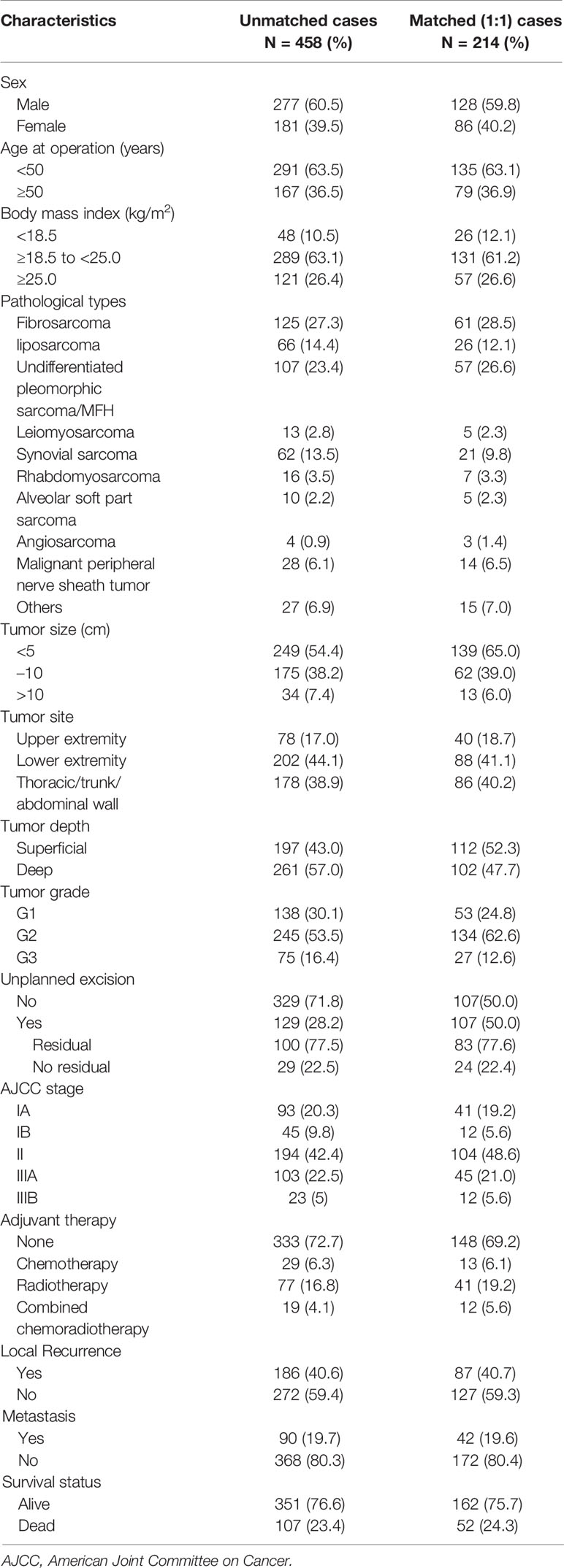
As shown in Table 1, there were 277 male patients and 181 female patients with a male: female ratio of 1.53:1. The mean age was 43 (25th–75th percentile: 31–55) years. Among the 458 patients, 129 patients (28.2%) underwent UPE while 329 patients (71.8%) underwent PE. The most common histological subtypes were fibrosarcoma (n=125, 27.3%) and undifferentiated pleomorphic sarcoma (n=107, 23.4%). A total of 45.6% of lesions were greater than or equal to 5 cm, and 57.0% were deep tumors. A total of 320 (69.9%) patients were histologically classified as G2/G3, 138 (30.1%) patients were classified as stage I, 194 (42.4%) as stage II, and 126 (27.5%) as stage III. Altogether, 27.3% of patients (n=125) underwent adjuvant treatment after surgery with chemotherapy (n=29, 6.3%), radiotherapy (n=77, 16.8%), or combined chemoradiotherapy (n=19, 4.1%).
In addition, with a median follow-up of 112.18 months (range, 7.1–313.78 months), 107 patients (23.4%) died of STS, and the 5-year DSS rate was 86.9%. A total of 186 patients (40.6%) developed local recurrence (LR), of whom 115 were alive at the last follow-up. The median time to LR was 11 months. Distant metastasis (DM) occurred in 90 patients (19.7%), of whom 81 died during the follow-up. The median time to DM was 21 months.
PE Compared With UPE
The PE and UPE groups did not differ in sex, age, body mass index (BMI), tumor location, DM, or death. However, compared with the PE group, the UPE group had a higher proportion of small-diameter lesions (<5 cm: 70.5% vs. 48.0%, P<0.001), lesions in superficial locations (55.8% vs. 38.0%, P=0.001), and adjuvant therapy administered (42.6% vs. 21.3%, P<0.001) and had different distributing trends for tumor grade (P=0.001) and AJCC stage (P=0.003). Based on Kaplan-Meier survival analysis, patients in the UPE group had better LRFS (P=0.008) but a lower 3-year LRFS rate (UPE 78% vs PE 89.1%, P=0.036) than those in the PE group. There was no difference in DSS (P=0.444) or MFS (P=0.658) between the two groups (Figures 1A, C, E; Table 2).
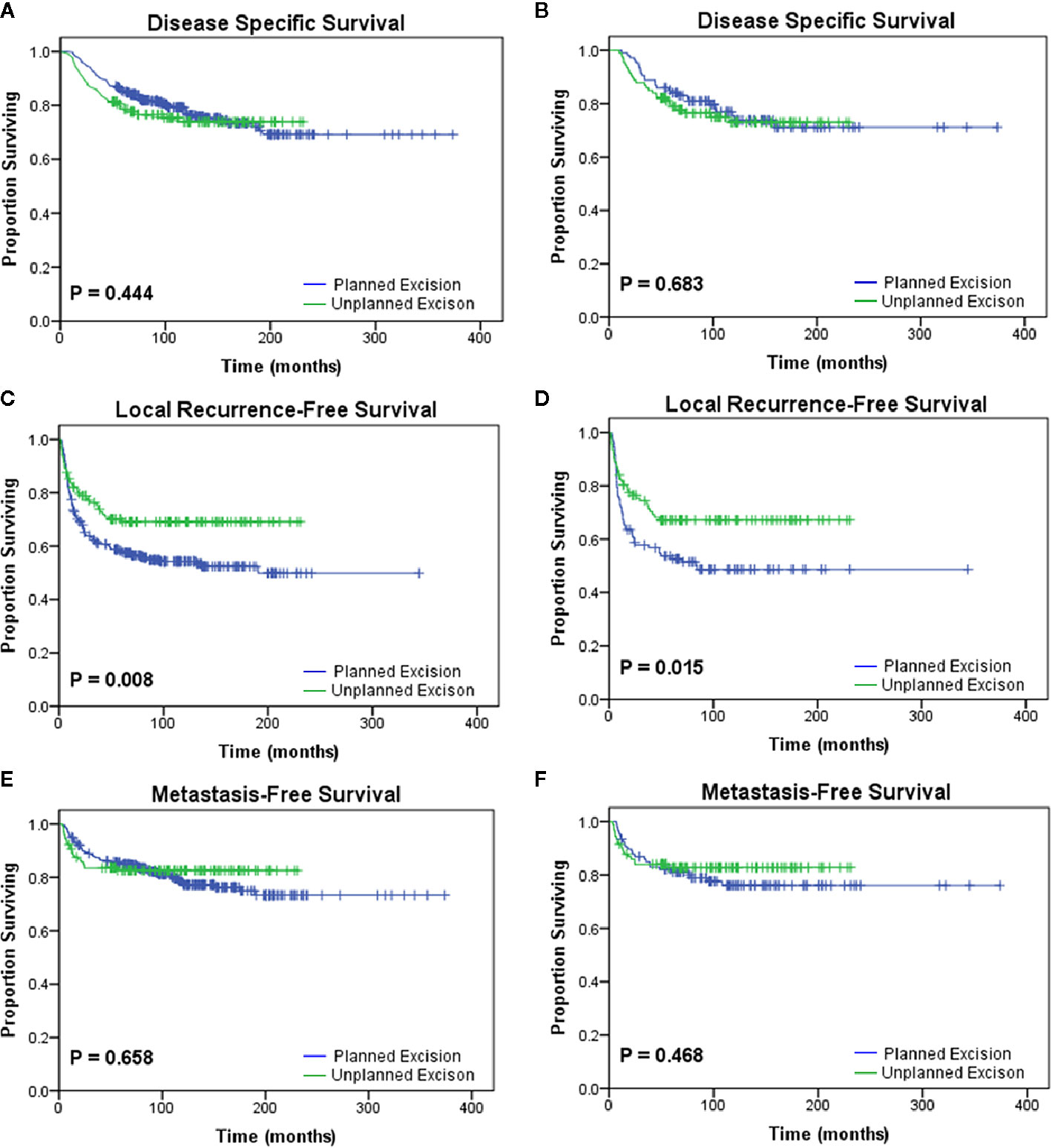
Figure 1 Kaplan-Meier analyses of the oncologic outcomes of the planned excision group and unplanned excision group in the unmatched study cohort (A, C, E) and the matched study cohort (B, D, F). Patients who underwent unplanned excision followed by R0 resection had similar (A, B) disease-specific survival (p=0.444, p=0.683) and (E, F) metastasis-free survival (p=0.658, p=0.468) but improved (C, D) local recurrence-free survival (p=0.008, p=0.015) compared with those who underwent planned excision.
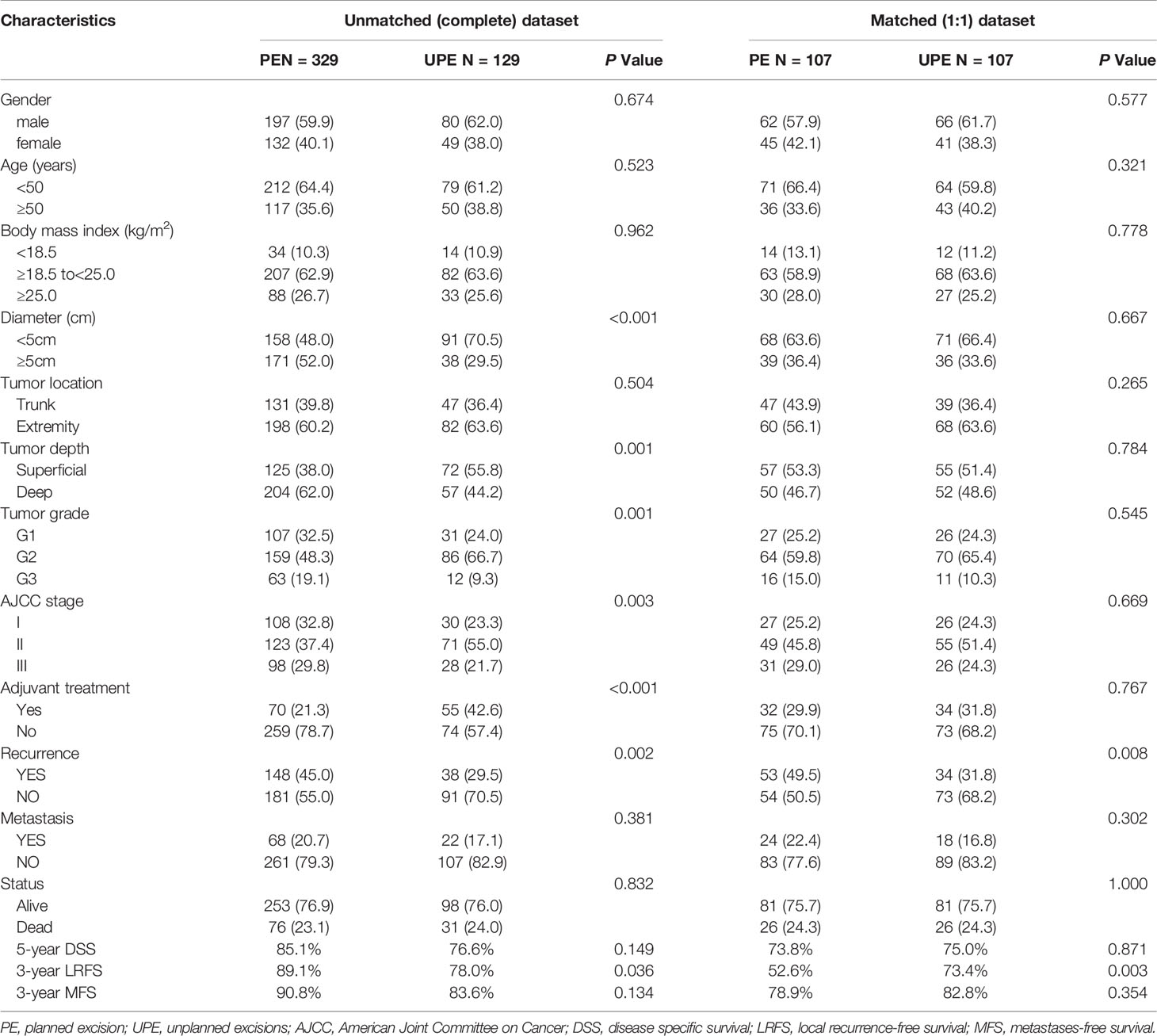
Table 2 Comparison of clinicopathological characteristics between planned excision (PE) group and unplanned excision group (UPE) before and after propensity score matching.
After PSM, two paired cohorts of 107 patients each were generated for both the UPE and PE groups where the baseline covariates (including size, depth, grade, AJCC stage, adjuvant therapy, etc.) were properly balanced. In the matched cohorts, the UPE group had a longer median LRFS (UPE 23.60 months vs. PE13.80 months, P=0.015) than the PE group, but no difference in DSS (P=0.683) or MFS (P=0.468) was found (Figures 1B, D, F). Additionally, UPE with subsequent R0 resection improved the 3-year local control rate of the tumor (3-year LRFS: UPE 73.4% vs. PE 52.6%, P = 0.003) compared with PE, which was exactly the opposite of the unmatched results. Oncological outcomes, including the 5-year DSS rate (PE 73.8% vs. UPE 75.0%, P = 0.871) and 3-year MFS rate (PE 78.9% vs. UPE 82.8%, P = 0.354), were not significantly different between the two groups (Table 2).
Predictive Factors for Oncologic Outcomes
In the univariate analysis, tumor size, tumor depth, tumor grade, and AJCC stage were prognostic factors for DSS, LR, and DM (Table 3). Multivariate analysis demonstrated that tumor size and tumor grade remained independent predictors for both DSS (P=0.007, P<0.001) and DM (P=0.031, P< 0.001), but the resection status (UPE or PE) was not related to the DSS or DM. It is also worth noting that receiving adjuvant treatment was independently associated with worse DSS (with vs. without: HR 0.64, 95% CI: 0.43–0.94, P = 0.022) and an increased risk of DM (with vs. without: HR 0.54, 95% CI: 0.36–0.82, P = 0.004). Subsequent analysis revealed that location in the trunk (P = 0.020), lower tumor grade (P = 0.001), and receiving UPE (P = 0.027) were independent protective factors for LR (Table 4).
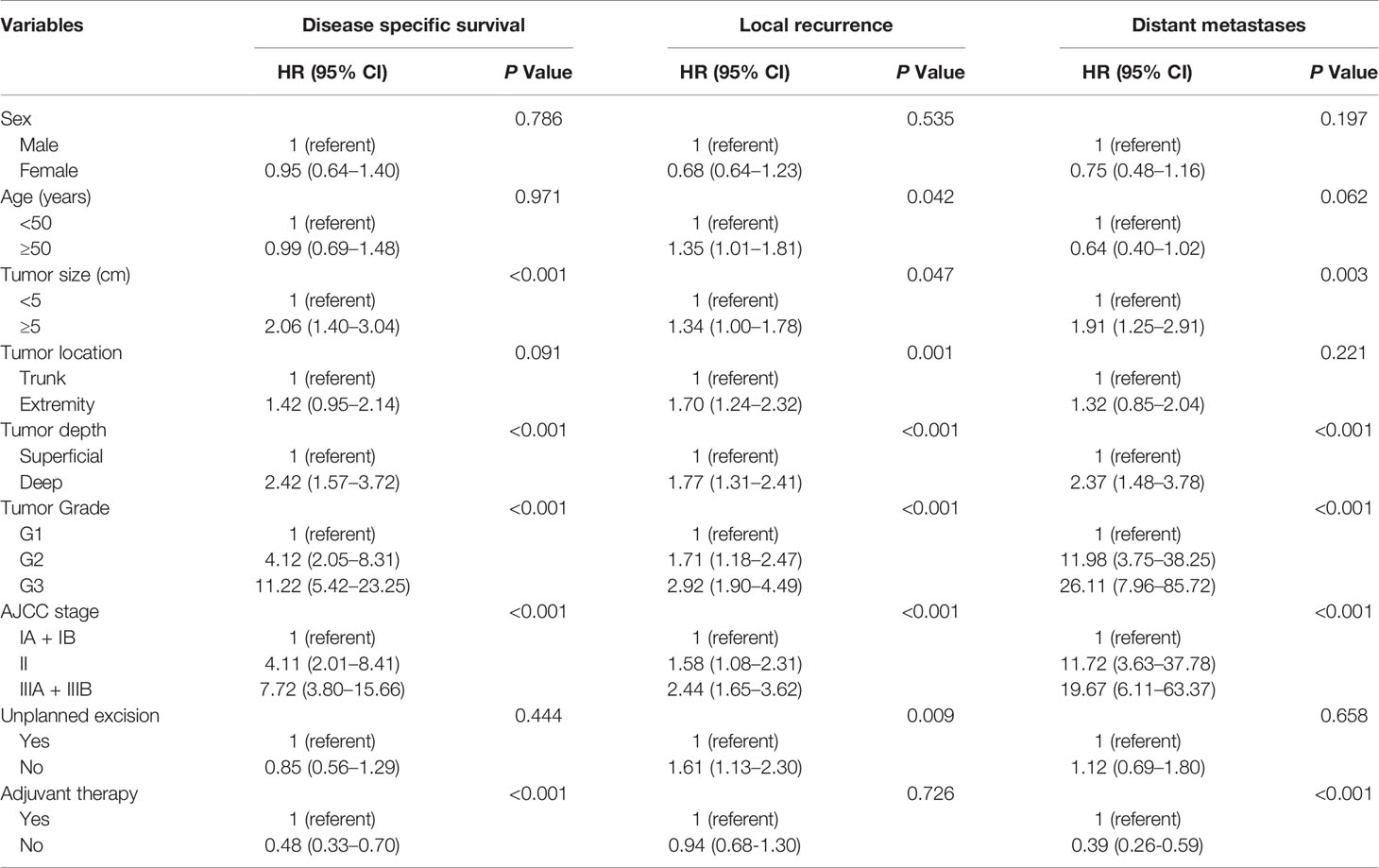
Table 3 Univariate analyses of variables for disease specific survival, local recurrence and distant metastases (unmatched complete datasets).
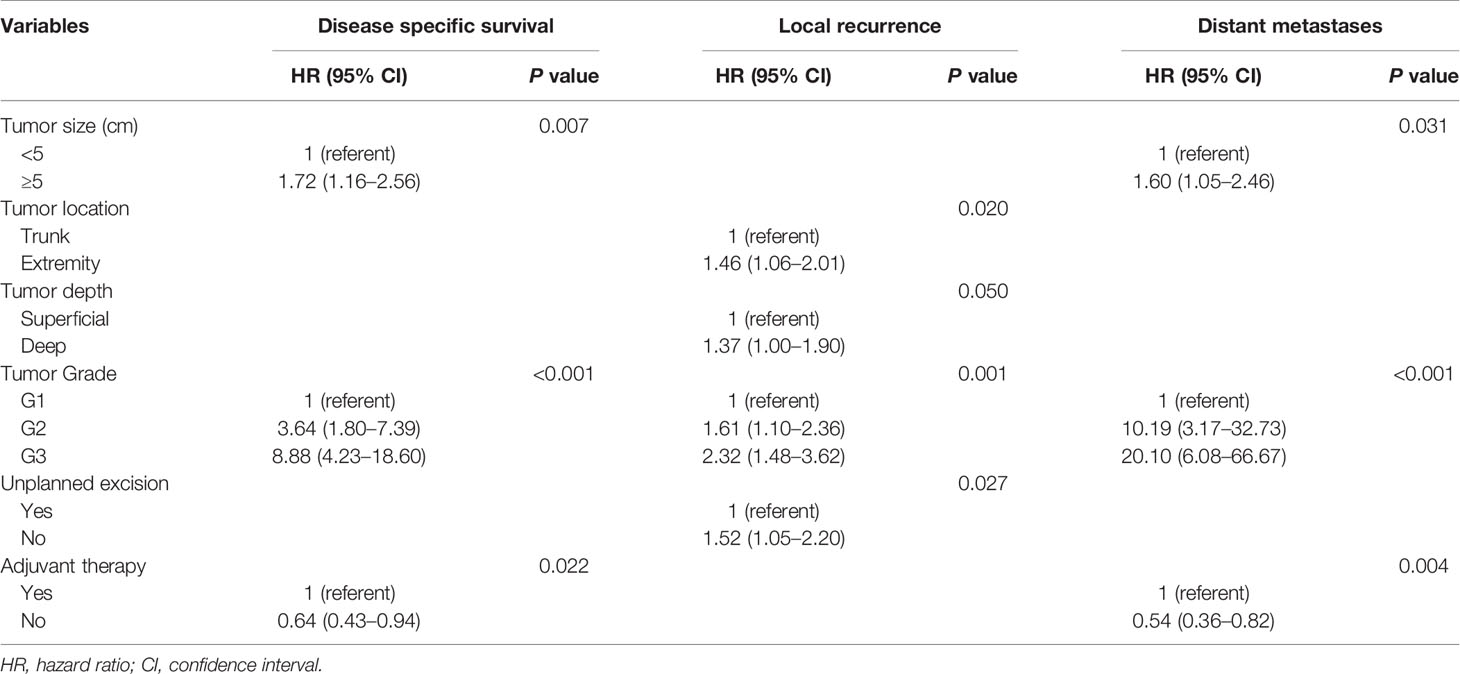
Table 4 Multivariate analyses of variables for disease specific survival, local recurrence and distant metastases (unmatched complete datasets).
Subgroup Analysis of the UPE Group
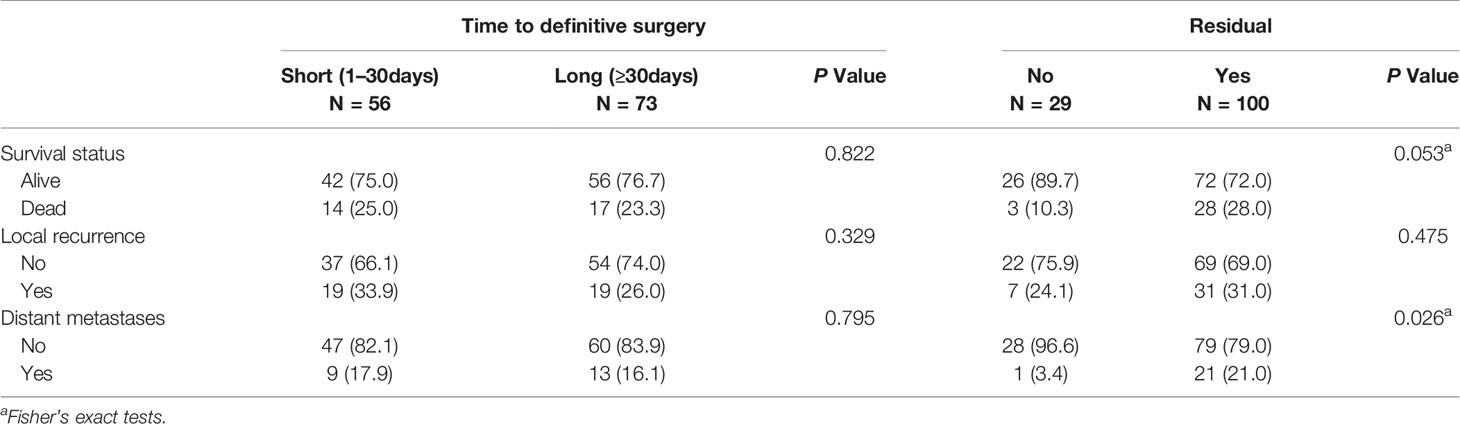
Patients who underwent UPE were divided into the residual group (RG, n=100, 77.5%) and the no residual group (NRG, n=29, 22.5%) according to the presence or absence of macroscopic or microscopic tumors in the re-excised specimens. The RG showed significantly worse outcomes than the NRG in terms of DSS (P=0.046) and MFS (P=0.029) (Figure 2). Moreover, the residual tumor was associated with increased rates of DM (NRG 3.4% vs. RG 21%, P=0.026), but the trend towards higher mortality and LR rate were not statistically significant (Table 5).
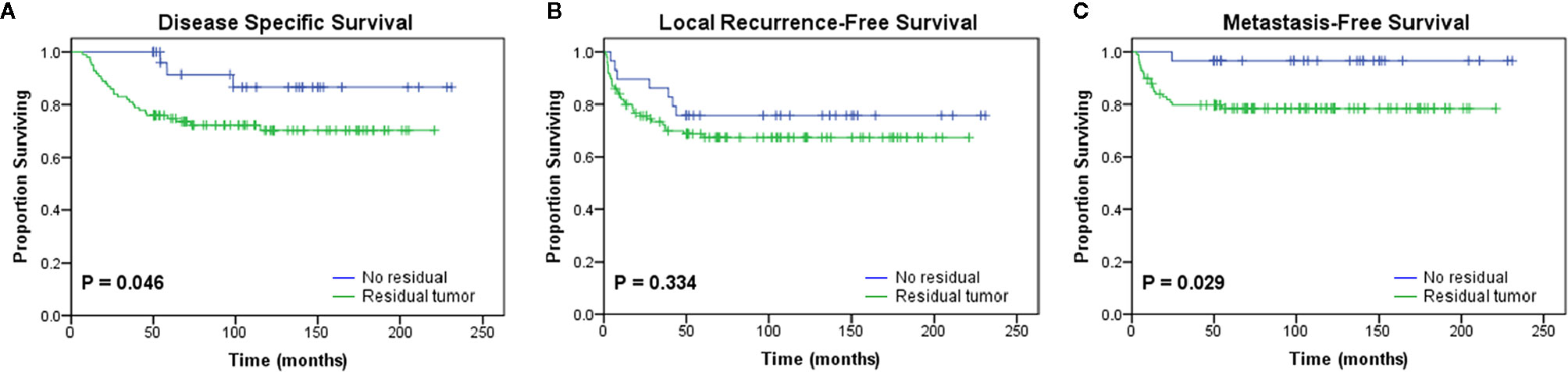
Figure 2 Kaplan-Meier curves showing the (A) disease-specific survival (p = 0.046), (B) local recurrence-free survival (p = 0.334), and (C) metastasis-free survival (p = 0.029) of patients after initial unplanned excision based on the presence or absence of residual tumor in the re-excision specimen.
Additionally, the median time interval between UPE and re-excision was 30 days (range, 4 to 136 days; interquartile range, 22–43 days). To investigate the effect of a delayed re-resection on the study end points, the patients were divided into two cohorts: the short-interval group (<30 days) and the long-interval group (≥30 days) according to the median value. We observed no significant difference in prognosis between the two groups (Figure 3; Table 5).
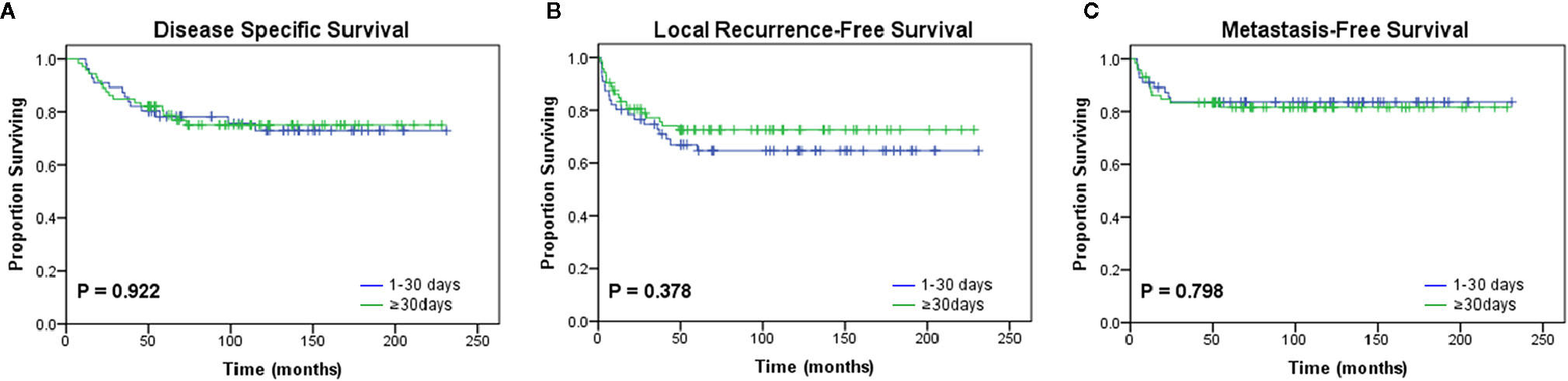
Figure 3 Kaplan-Meier curves for oncologic outcomes according to the interval from unplanned excision to definitive surgery. No differences in (A) disease-specific survival (p = 0.922), (B) local recurrence-free survival (p = 0.378), or (C) metastasis-free survival (p = 0.798) were observed between the two groups divided by the median interval value (30 days).
Discussion
This study was designed to investigate the impact of unplanned, non-oncologic excision on the outcomes of patients with STS of the trunk and extremity, with particular attention to similar baseline characteristics. To date, there is no consensus with regard to the potential survival effects of UPE. A recent retrospective study by Munoz et al. (20) reported that the risk of LR and DM was higher in patients who underwent re-resection than in those who underwent planned primary surgery. Worse oncologic outcomes were also reported by Qureshi et al. (7) and Saeed et al. (8) where the UPE group had worse LRFS and progression-free survival. These results might ascribe the poor prognosis in the UPE group to the residual tumor cells contained in muscular or fascial boundaries or fragmented excision (21). However, the findings of subsequent studies were inconsistent with the above conclusions and demonstrated similar or even better LRFS, MFS, and DSS in patients who underwent re-excision than in those with a planned definitive cancer resection as the primary surgical procedure (9, 11, 13, 22). This contradiction in results can be caused by the difficulty in comparing patients who undergo UPE with patients who undergo PE due to the more favorable tumor features and better biological characteristics (such as smaller size, more superficial location, and more benign) of patients with UPE. Additionally, some studies included the patients with various treatments after UPE, including re-excision, observation, and radiation, which affects the conclusions that can be reached (7). We believe that the conclusions are more convincing if the outcomes are compared between the two groups based on similar baseline characteristics.
Therefore, in our study, we used the PSM approach for possible confounding factors, which makes the analyses more precise. Compared to other relevant studies (9, 20, 21, 23), the patients included in our study were more likely to have smaller tumor size, and fibrosarcoma and undifferentiated sarcoma were the most common types of histology in our study. Based on our analysis, we found that UPE followed by R0 resection was associated with a better LRFS and 3-year LRFS rates than PE. According to our univariable and multivariable analyses, UPE decreased the risk of local recurrence and was confirmed as an independent predictive factor of LR. The risk of LR for PE patients was 1.52 times higher than that for UPE patients. Moreover, the UPE was not associated with worse DSS or a high risk of DM. More optimistic than our findings, Bianchi et al. (24) reported that UPE had a better sarcoma-specific survival and higher LR- and DM-free rates, which was likely driven by the complete re-excision after UPE. The above findings imply that for patients who undergo UPE, subsequent definitive oncologic re-excision is able to result in an acceptable outcome. This is of great value in areas with inadequate medical knowledge and poor technology. Unexpectedly, we also showed that adjuvant therapy was considered a significant contributing factor for death and metastasis. One reason may be that only patients with more aggressive tumors would choose postoperative treatment, and these patients had an inherently poor prognosis and were prone to metastasis. The highly malignant and invasive biology may significantly dilute the effectiveness of adjuvant therapy.
The present study revealed that 77.5% of patients had residual tumors in re-excised specimens (RTRS), which is within the range of 43% to 83% found in the literature (4, 24–26), supporting the significance of additional wide resection for patients undergoing UPE. Our results also showed that the RG presented worse oncological outcomes, including DSS and MFS, and the presence of RTRS was a risk factor for DM. However, we did not confirm a connection between the RTRS and LR, which was not in line with conventional understanding and several previous studies demonstrating that patients with RTRS were inclined to have shorter LRFS (14, 27–29). This might be attributable to the small sample size, since there were only 29 patients in the NRG in the present study. Our cohort was too small to reach statistical significance and further studies are needed to confirm this trend. Moreover, Han et al. (30) found that there were no difference in the prognosis and oncologic outcomes according to the time until definitive resection, which was in accordance with our results. Based on these findings, we support the view that any effect of delayed definitive surgery is likely to be of minor clinical significance (31).
Our study also shows that patients who underwent UPE were more likely to have a smaller or superficial lesion and were more often administered adjuvant therapy than those who underwent PE. These results showed that clinicians often adopted UPE strategies for lesions with good biological features. And in the traditional concept, the patients with UPE may have a worse prognosis than the patients underwent PE. Considering that these sarcoma patients underwent UPE, postoperative treatments were more inclined to be performed to reduce the potential “adverse” effects of UPE, even though the conditions were not that serious. In addition, over the past several decades, the postoperative adjuvant therapy of PE patients in China was non-standard, and the doctors did not determine adjuvant treatments recommendations based on standard pathologic factors of patients. Thus, the proportion of the UPE group receiving postoperative treatments is relatively higher than the PE group in our study. Therefore, since completely avoiding the occurrence of UPE in patients with STS is impractical, we recommend three principles of diagnosis and treatment to prevent deteriorated conditions from occurring: (a) identify and diagnose tumors based on the clinical history and imaging manifestations carefully before the initial surgery, having more awareness of the possibility of malignancy in lesions with a small size and superficial location; (b) emphasize the importance of eliminating the whole lesion completely and achieving negative margins initially; and (c) perform reoperation with a multidisciplinary approach as a salvage measure after UPE has occurred, regardless of the interval between UPE and re-excision. Certainly, considering the remaining high incidence of UPE, more widespread recognition of such an inadequate procedure is needed.
There are some limitations to our study. First, in this research, a relatively high LR rate was found (n=186, 40.6%), which was slightly higher than that in other relevant studies (32). Possible explanations are as follows: we included a cohort of patients with a long follow-up time (some for over 18 years), based on which the risk for LR was observed to increase accordingly. Nearly 70% of the enrolled patients in this study had an advanced tumor stage (II-III) or high grade (G2-G3) at the time of initial diagnosis. In fact, over a decade ago, many patients did not seek treatment until their limbs were heavily swollen, and doctors were often their last choice. Furthermore, due to a lack of awareness of the guidelines by general surgeons and the lack of standardized management, the resection margin rarely meets the requirements of the extended resection, and the proportion of patients receiving adjuvant treatment remained relatively low (27.3%), especially chemoradiotherapy (4.1%). Second, as a retrospective study, there was intrinsic selection bias (i.e., loss to follow-up and clinical decisions made based on the economic condition of the patients). However, for natural reasons, UPE cannot be studied prospectively. Third, this was a single-center study; therefore, the characteristics of the enrolled patients and the results of this study may not be generalizable to other populations, but our results still offer a good description of patients referred after UPE of sarcoma excision and serve as valuable references. Furthermore, we used PSM analysis to reduce bias. However, the small numbers in the matched dataset might impact the statistical probabilities of our results. Therefore, our conclusions should be verified in a larger population of STS patients from multiple centers.
In conclusion, STS treated with UPE had distinct characteristics, including smaller lesion sizes, superficial location, more benign features, and high risk of residual tumor. Our propensity score data provided evidence that, compared with PE, UPE followed by R0 resection has a major impact on local control and could result in comparable long-term oncologic outcomes in patients with STS of the trunk and extremity. Considering the large number of cases with residual disease found in re-excised specimens with residual disease, which is an unfavorable factor associated with the prognosis, a definitive salvage reoperation with multidisciplinary treatments should be performed for STS patients with UPE, regardless of the time interval.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Sun Yat-sen University Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YL, Z-WZ, and XZ conceived and designed the study. YL, T-HG, and B-SX collected the data. YL, T-HG, B-SX, D-CH, and H-BQ performed data analysis and interpretation. YL, T-HG, and B-SX contributed to writing and review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (no. 81902736).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.617590/full#supplementary-material
Supplementary Figure 1 | Flow chart of the exclusion and inclusion criteria.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA. A Cancer J Clinicians (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br (2008) 90(2):203–8. doi: 10.1302/0301-620X.90B2.19760
3. Noria S, Davis A, Kandel R, Levesque J, O’Sullivan B, Wunder J, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am (1996) 78(5):650–5. doi: 10.2106/00004623-199605000-00003
4. Charoenlap C, Imanishi J, Tanaka T, Slavin J, Ngan SY, Chander S, et al. Outcomes of unplanned sarcoma excision: impact of residual disease. Cancer Med (2016) 5(6):980–8. doi: 10.1002/cam4.615
5. Jones DA, Shideman C, Yuan J, Dusenbery K, Carlos Manivel J, Ogilvie C, et al. Management of Unplanned Excision for Soft-Tissue Sarcoma With Preoperative Radiotherapy Followed by Definitive Resection. Am J Clin Oncol (2016) 39(6):586–92. doi: 10.1097/COC.0000000000000095
6. Brinkmann EJ, Ahmed SK, Houdek MT. Extremity Soft Tissue Sarcoma: Role of Local Control. Curr Treat Options Oncol (2020) 21(2):13. doi: 10.1007/s11864-020-0703-9
7. Qureshi YA, Huddy JR, Miller JD, Strauss DC, Thomas JM, Hayes AJ. Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol (2012) 19(3):871–7. doi: 10.1245/s10434-011-1876-z
8. Saeed H, King DM, Johnstone CA, Charlson JA, Hackbarth DA, Neilson JC, et al. Preoperative Radiation Therapy Followed by Reexcision May Improve Local Control and Progression-Free Survival in Unplanned Excisions of Soft Tissue Sarcomas of the Extremity and Chest-Wall. Int J Surg Oncol (2016) 2016:5963167. doi: 10.1155/2016/5963167
9. Zaidi MY, Ethun CG, Liu Y, Poultsides G, Howard JH, Mogal H, et al. The impact of unplanned excisions of truncal/extremity soft tissue sarcomas: A multi-institutional propensity score analysis from the US Sarcoma Collaborative. J Surg Oncol (2019) 120(3):332–9. doi: 10.1002/jso.25521
10. Morii T, Aoyagi T, Tajima T, Yoshiyama A, Ichimura S, Mochizuki K. Unplanned resection of a soft tissue sarcoma: clinical characteristics and impact on oncological and functional outcomes. J Orthop Sci (2015) 20(2):373–9. doi: 10.1007/s00776-014-0689-x
11. Smolle MA, Tunn PU, Goldenitsch E, Posch F, Szkandera J, Bergovec M, et al. The Prognostic Impact of Unplanned Excisions in a Cohort of 728 Soft Tissue Sarcoma Patients: A Multicentre Study. Ann Surg Oncol (2017) 24(6):1596–605. doi: 10.1245/s10434-017-5776-8
12. Vansteelandt S, Daniel RM. On regression adjustment for the propensity score. Stat Medicine (2014) 33(23):4053–72. doi: 10.1002/sim.6207
13. Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg (2000) 231(5):655–63. doi: 10.1097/00000658-200005000-00005
14. Davis AM, Kandel RA, Wunder JS, Unger R, Meer J, O’Sullivan B, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol (1997) 66(2):81–7. doi: 10.1002/(SICI)1096-9098(199710)66:2<81::AID-JSO2>3.0.CO;2-H
15. Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol (1985) 3(10):1344–8. doi: 10.1200/JCO.1985.3.10.1344
16. Cates JMM. AJCC eighth edition for soft tissue sarcoma of the extremities and trunk. Ann Oncol (2018) 29(9):2023. doi: 10.1093/annonc/mdy247
17. Neuville A, Chibon F, Coindre J-M. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology (2014) 46(2):113–20. doi: 10.1097/PAT.0000000000000048
18. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol (1999) 150(4):327–33. doi: 10.1093/oxfordjournals.aje.a010011
19. Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res (2014) 49(5):1701–20. doi: 10.1111/1475-6773.12182
20. Munoz Munoz P, Bajawi Carretero M, Gonzalez Barranquero A, Mena Mateos A, Corral Moreno S, Sanjuanbenito Dehesa A, et al. Impact of unplanned resection and re-excision of a soft tissue sarcoma on prognosis. Cir Esp (2020) 98(5):281–7. doi: 10.1016/j.cireng.2020.04.009
21. Gingrich AA, Elias A, Michael Lee CY, Nakache YN, Li CS, Shah DR, et al. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J Surg Res (2017) 208:26–32. doi: 10.1016/j.jss.2016.08.096
22. Morattel B, Mustaki L, Montemurro M, Letovanec I, Durham AD, Becce F, et al. Oncological outcome, functional results and costs after unplanned excision of musculoskeletal soft tissue sarcoma. Eur J Surg Oncol (2020) 46(5):898–904. doi: 10.1016/j.ejso.2020.01.025
23. Bateni SB, Gingrich AA, Jeon SY, Hoch JS, Thorpe SW, Kirane AR, et al. Clinical Outcomes and Costs Following Unplanned Excisions of Soft Tissue Sarcomas in the Elderly. J Surg Res (2019) 239:125–35. doi: 10.1016/j.jss.2019.01.055
24. Bianchi G, Sambri A, Cammelli S, Galuppi A, Cortesi A, Righi A, et al. Impact of residual disease after “unplanned excision” of primary localized adult soft tissue sarcoma of the extremities: evaluation of 452 cases at a single Institution. Musculoskelet Surg (2017) 101(3):243–8. doi: 10.1007/s12306-017-0475-y
25. Qureshi SS, Prabhu A, Bhagat M, Kembhavi S, Vora T, Chinnaswamy G, et al. Re-excision after unplanned resection of nonmetastatic nonrhabdomyosarcoma soft tissue sarcoma in children: Comparison with planned excision. J Pediatr Surg (2017) 52(8):1340–3. doi: 10.1016/j.jpedsurg.2017.01.006
26. Traub F, Griffin AM, Wunder JS, Ferguson PC. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer (2018) 124(19):3868–75. doi: 10.1002/cncr.31648
27. Nakamura T, Kawai A, Sudo A. Analysis of the patients with soft tissue sarcoma who received additional excision after unplanned excision: report from the Bone and Soft Tissue Tumor Registry in Japan. Jpn J Clin Oncol (2017) 47(11):1055–9. doi: 10.1093/jjco/hyx123
28. Arai E, Sugiura H, Tsukushi S, Nakashima H, Urakawa H, Kozawa E, et al. Residual tumor after unplanned excision reflects clinical aggressiveness for soft tissue sarcomas. Tumour Biol (2014) 35(8):8043–9. doi: 10.1007/s13277-014-2043-5
29. Venkatesan M, Richards CJ, McCulloch TA, Perks AG, Raurell A, Ashford RU, et al. Inadvertent surgical resection of soft tissue sarcomas. Eur J Surg Oncol (2012) 38(4):346–51. doi: 10.1016/j.ejso.2011.12.011
30. Han I, Kang HG, Kang SC, Choi JR, Kim HS. Does Delayed Reexcision Affect Outcome After Unplanned Excision for Soft Tissue Sarcoma? Clin Orthop Relat R (2011) 469(3):877–83. doi: 10.1007/s11999-010-1642-8
31. Decanter G, Stoeckle E, Honore C, Meeus P, Mattei JC, Dubray-Longeras P, et al. Watch and Wait Approach for Re-excision After Unplanned Yet Macroscopically Complete Excision of Extremity and Superficial Truncal Soft Tissue Sarcoma is Safe and Does Not Affect Metastatic Risk or Amputation Rate. Ann Surg Oncol (2019) 26(11):3526–34. doi: 10.1245/s10434-019-07494-6
Keywords: soft tissue sarcoma, trunk and extremity, oncologic outcomes, planned excision, unplanned excision
Citation: Liang Y, Guo T-H, Xu B-S, Hong D-C, Qiu H-B, Zhou Z-W and Zhang X (2021) The Impact of Unplanned Excision on the Outcomes of Patients With Soft Tissue Sarcoma of the Trunk and Extremity: A Propensity Score Matching Analysis. Front. Oncol. 10:617590. doi: 10.3389/fonc.2020.617590
Received: 15 October 2020; Accepted: 18 December 2020;
Published: 22 January 2021.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Valerie Patrice Grignol, The Ohio State University, United StatesRobert J. Canter, University of California, Davis, United States
Copyright © 2021 Liang, Guo, Xu, Hong, Qiu, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Wei Zhou, emhvdXpod0BzeXN1Y2Mub3JnLmNu; Xing Zhang, emhhbmd4aW5nQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
 Yao Liang
Yao Liang Tian-Hui Guo1,3†
Tian-Hui Guo1,3† Dong-Chun Hong
Dong-Chun Hong Hai-Bo Qiu
Hai-Bo Qiu