- 1Department of Nuclear Medicine, Jiangxi Province Key Laboratory of Laboratory Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China
- 3School of Public Health, Nanchang University, Nanchang, China
- 4Jiangxi Provincial Key Laboratory of Preventive Medicine, Nanchang University, Nanchang, China
- 5Department of Clinical Laboratory, Jiangxi Province Key Laboratory of Laboratory Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 6Biological Resource Center, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: Heterogeneous clinical and molecular characteristics are reported in colorectal cancer (CRC) with different tumor laterality. However, the outcome of left- and right-sided patients with stage I–III CRC and the role of chronic inflammation in survival differences between them remain unclear.
Method: A prospective study including 1,181 surgical patients with stage I–III CRC was carried out to investigate the involvement of circulating fibrinogen-to-pre-albumin (Alb) ratio (FPR) and primary tumor sidedness in the clinical outcome of those patients. We further investigated the effect of FPR on adjuvant chemotherapy response and recurrence in stage III patients.
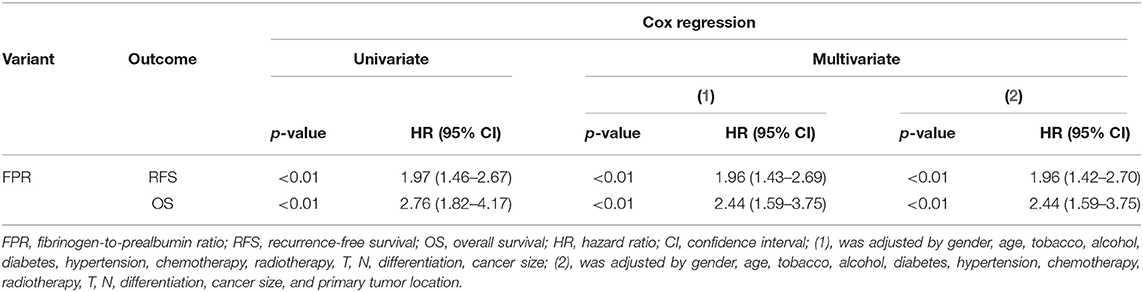
Results: Our study showed that the right tumor location was significantly associated with poor recurrence-free survival (RFS) (p = 0.04, adjusted HR = 1.41, 95% CI = 1.02–1.94) and overall survival (OS) (p = 0.04, adjusted HR = 1.55, 95% CI = 1.01–2.38) only in the stage III disease. In these patients, T4 stage distribution (83.39 vs. 70.94%, p < 0.01) within right-sided cases was significantly higher than left-sided patients. Moreover, preoperative FPR within right-sidedness (p < 0.01), T4 stage (p < 0.05), and large cancer bulk (≥5 cm) (p < 0.05) subgroups was significantly elevated compared to their counterparts, and it was gradually rising following the increased cancer bulk (p trend < 0.01). High-FPR distribution (52.30 vs. 27.00%, p < 0.01) within right-sided patients with the stage III disease was significantly higher than that in the left-sided cases. RFS (plog−rank < 0.01) and OS (plog−rank < 0.01) of the high-FPR patients were extremely inferior to the low-FPR cases, and the significant associations were observed when they were adjusted by other confounders including primary tumor location (p < 0.01, adjusted HR = 1.96, 95% CI = 1.42–2.70 for RFS; p < 0.01, adjusted HR = 2.44, 95% CI = 1.59–3.75 for OS). Additionally, RFS of adjuvant chemotherapy-treated high-FPR patients was superior to the patients without chemotherapy (plog−rank = 0.01) but was inferior to the low-FPR patients undergoing the treatment, especially in the 5-FU- and XELOX-treated subgroup.
Conclusion: These findings indicate that chronic high-grade inflammation weakens chemotherapy efficacy and contributes to the poor prognosis of stage III surgical CRC patients.
Introduction
Colorectal cancer (CRC) is a kind of heterogeneous malignancy with different clinical options and diverse therapeutic outcome (1, 2). Recently accumulated evidence shows that proximal colon cancer and the distal disease including rectal cancer harbor strikingly distinct clinical characteristics and immune and molecular profiles (3–6). Thus, the left-sided (distal colon and rectal cancer) and right-sided (proximal colon cancer) diseases are considered as two distinct entities (7, 8).
A large amount of data consistently shows that metastatic CRC (mCRC) cases with left-sidedness derive meaningful benefit from anti-epidermal growth factor receptor antibody (9–11). Our previous studies also imply that the prognosis of bevacizumab-treated left-sided mCRC patients is better than the counterpart (12, 13). These findings reveal that tumor laterality can affect and predict the efficacy of target therapy within the advanced disease. Meanwhile, several large-scale cohort studies investigated the effect of tumor sidedness on early-stage patients with the treatment of standard chemotherapy (14–18). Unfortunately, no consistent conclusion is achieved and the debate continues. Karim et al. reported no overall survival (OS) and cancer-specific survival (CSS) difference between the right- and left-sided patients with stage I–III or III colon cancer (15). On the contrary, a poor prognosis in terms of recurrence-free survival (RFS) and OS was reported in stage III right-sided colon cancer patients undergoing adjuvant chemotherapy (14, 17). More interestingly, a recent study performed by Ishihara et al. observed a low recurrence rate in stage II–III patients with right-sidedness compared to the left-sided cases; however, 5-year CSS within the left-sided patients was significantly longer than those with the right-sided disease (18).
Chronic inflammation is one hallmark of malignancies including CRC (19, 20). The inflammatory processes can regulate low expressions of circulating albumin (Alb) and pre-Alb and up-regulated plasma fibrinogen (Fib). Our previous study showed that circulating Fib-to-pre-Alb ratio (FPR) could sensitively imply the body response to chronic inflammation in solid malignancies (21), including hepatocellular carcinoma (22), and gastric cancer (23) as well as CRC (24), and it was superior to other inflammatory ratios or scores to predict the survival of CRC patients (25). The differential FPR and significant survival differences were examined in the right- and left-sided CRC with IV stage (26). So, we speculated that chronic inflammation might be involved in survival differences of the right- and left-sided diseases. Here, we focused on evaluating the possible role of tumor primary location and preoperative FPR in the prognosis of 1,181 radically resected patients with stage I–III CRC. We further investigated the effect of FPR on adjuvant chemotherapy response and RFS to understand the prognostic difference in these patients.
Materials and Methods
Population
The study was undertaken at the Second Affiliated Hospital of Nanchang University (Nanchang, China) from January 2013 to August 2016 and was approved by the Medical Ethics Committee of the hospital. The eligible participants should fit for the following inclusion criteria: (1) newly diagnosed stage I–III CRC patients by clinical characteristics, imaging, clinical laboratory, and pathological examination; (2) agreement to participate and sign an informed consent form; (3) radical resection with histologically negative resection margins; (4) agreement to provide data of clinical characteristics, contact information, and peripheral blood sample. On the contrary, the exclusion criteria for each participant were as follows: (1) patients received any treatment such as neoadjuvant therapy, anticoagulant therapy, or long-term use of non-steroidal anti-inflammatory drugs in recent 3 months before diagnosis; (2) patients were combined with other malignancies, recent bacterium, or virus infection as well as trauma; (3) patients suffered from liver, blood, kidney and autoimmune disease, enterobiasis or cardio-cerebrovascular disease such as stroke, atherosclerosis, coronary heart disease, cardiac infarction, as well as vein thrombosis.
The eligible patients were stratified into the left- and right-sided subgroups according to tumor sidedness. The disease derived from the cecum, ascending colon, hepatic flexure, and transverse colon was recognized as right-sided CRC, whereas the disease originated from splenic flexure, descending colon, sigmoid colon, and rectum was considered as left-sided disease. The baseline characteristics, pathological detection, and surgical operation, as well as adjuvant chemotherapy after surgical operation, were gathered from the medical record. The chemotherapic regimens were classified as follows: single fluorouracil derivative oral anticancer agents such as Capecitabine or Tegafur, Gimeracil, and Oteracil potassium capsules [5-Fluorouracil (5-FU)]; Capecitabine combined with Oxaliplatin regimen (XELOX); combined 5-FU, Leucovorin Calcium, and oxaliplatin regimen (FOLFOX); and combined 5-FU, Leucovorin Calcium, and Irinotecan regimen (FOLFIRI). We obtained the survival data by 3 years' follow-up (3 months a time in the first 2 years, 6 months in the third year) and its deadline was August 30, 2019. The survival endpoints were RFS and OS. RFS was evaluated by standard radiologic criteria; it was measured from the date of surgical resection to documented first radiologic recurrence or distal metastasis and was censored at last follow-up. OS was defined as the time from the first diagnosis to death or the deadline.
Laboratory Detection
To detect FPR, we collected peripheral blood specimens from each included patient from 7:00 to 10:00 am ahead 1 week of surgical operation. Two-milliliter serum and plasma specimens were obtained by 3,000 r/min centrifugation for 10 min. Plasma Fib was measured by Clauss method using SYSMEX CA-7000 machine (Sysmex, Tokyo, Japan). Immuno-turbidimetric assay was used to detect serum pre-Alb with machines of OLYMPUS 5400 (Beckman Coulter, Tokyo, Japan). The inter- and intra-batch coefficients of variation of these biomarkers were <5%. The ratio was calculated according to the following formula: FPR = (plasma Fib/serum pAlb) × 1000. We selected 18.3 as the optimal cutoff value of circulating FPR according to our previous study (24). Then, the patients were classified into high- and low-FPR subgroups according to the cutoff value. Both high- and low-FPR implied chronic high- and low-grade inflammation, respectively.
Statistics
The baseline characteristics of the included patients were presented by number and proportion. Detected data with skewness distribution were shown with median and 25/75 percentile. RFS and OS emerged as median survival months. The Pearson χ2 test was used to compare the distribution differences of counting data in different groups. Mann–Whitney U-test was selected to detect the difference between two groups with skew distribution data. Kaplan–Meier curve with log-rank test and Cox regression analysis were selected to examine survival differences of the patients stratified by tumor location or FPR. Multivariate analysis was conducted by backward stepwise Cox regression modeling with covariates of the baseline and pathological characteristics, treatment, and other confounder factors. The strength between them was measured by hazard ratio (HR) and 95% confidence interval (CI). SPSS software v.17.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 8.2.1 (GraphPad Software Inc., San Diego, USA) were used in all statistical analyses and p < 0.05 was defined as statistical significance in the present study.
Results
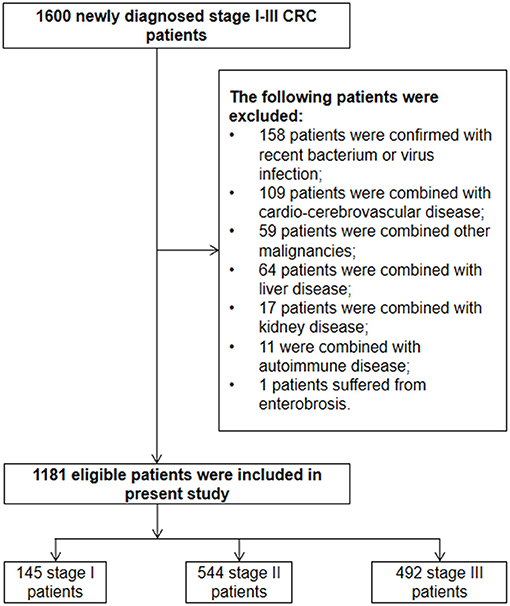
The detailed enrolled procedure is presented in Figure 1. A total of 1,600 newly diagnosed stage I–III CRC patients in the range of January 2013 to August 2016 were enrolled to identify the eligible cases according to the inclusion and exclusion criteria. As a result, 1,181 stage I–III CRC patients were finally enrolled in the present study. Despite the fact that primary rectal and distal colon cancer patients were subjected to different staging procedures and treatment approaches, no survival difference was observed between them (Supplementary Figures 1A–D). So, we considered two of them as CRC with left-tumor location. The demographic and pathological characteristics, as well as treatment data, are displayed in Table 1.
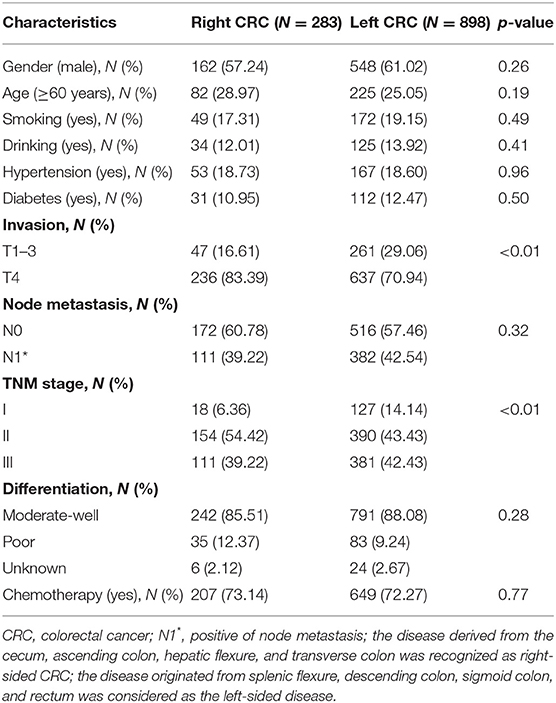
Table 1. The baseline characteristics of 1,181 stage I–III surgical colorectal cancer patients in the study.
Eight hundred and ninety-eight and 283 patients were diagnosed as left- and right-sided CRC; 12.28, 46.06, and 41.66% of them were stage I (127 for left, 18 for right), stage II (390 for left, 154 for right), and stage III (381 for left, 111 for right) patients, respectively. Significant distribution differences of TNM (p < 0.01) and T (p < 0.01) stage were observed between the right- and left-sided cases. However, no distribution difference of other clinical characteristics was observed between the two subgroups.
The median follow-up period was 24 months (range, 3–36 months) after surgical resection, and 7.87% of them were lost to follow-up. Two hundred and eighty-nine cases were recurrent, 166 cases were dead in the follow-up period. Moreover, 8.28, 16.54, and 38.15% of stage I, II, and III patients experienced radiologic recurrence; the death rates within each stage were 3.45, 11.03, and 20.33%, respectively. No survival difference was observed in the subgroups stratified by clinical characteristics and therapeutic regimens (Table 2). We also did not observe survival differences between the right- and left-sided patients in overall (p = 0.10 for RFS and p = 0.184 for OS), stage I (p = 0.88 for RFS and p = 0.97 for OS), and stage II (p = 0.90 for RFS and p = 0.76 for OS) subgroup (Table 2), whereas recurrence rate (45.95 vs. 38.01%, p = 0.05) and mortality (27.02 vs. 18.37%, p < 0.01) of the right-sided individuals with stage III disease were significantly higher than the left-sided patients (Figures 2A,B). Moreover, survival outcome (plog−rank = 0.03, adjusted HR = 1.41, and 95% CI = 1.02–1.94 for RFS; plog−rank = 0.04, adjusted HR = 1.55, and 95% CI = 1.01–2.38 for OS) within the stage III right-sided CRC patients were significantly worse than the left-sided cases (Figures 2C,D and Table 2).
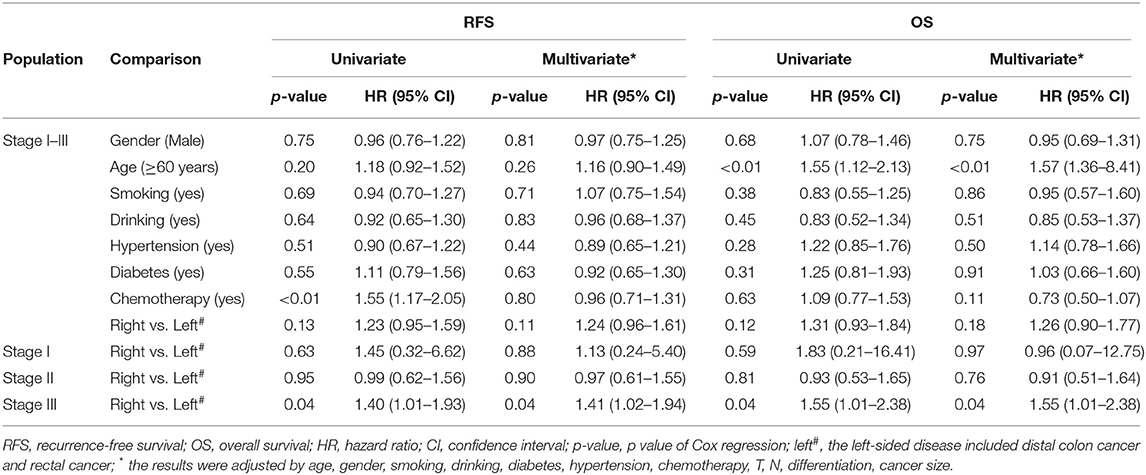
Table 2. Cox analysis of common clinical characteristics and primary tumor location within 1,181 surgical patients with stage I–III CRC and subgroups stratified by TNM stage in the study.
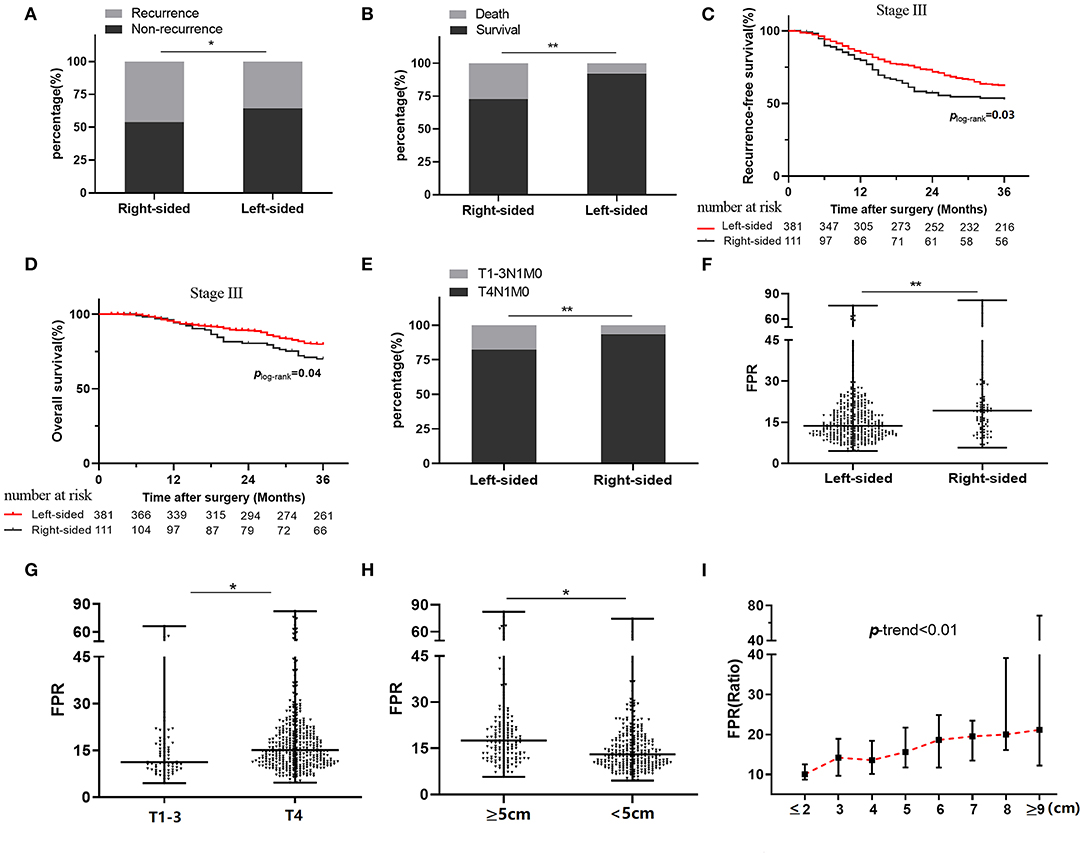
Figure 2. Relationship between clinical outcome, tumor sidedness, and preoperative FPR in stage III colorectal cancer patients. (A) Recurrence status in stage III patients stratified by tumor sidedness. (B) Death status in stage III patients stratified by tumor sidedness. (C) Kaplan–Meier curves for RFS in stage III patients with right-sided and left-sided cancer. (D) Kaplan–Meier curves for OS in stage III patients with right-sided and left-sided cancer. (E) Frequency distribution of T4 stage in left-sided and right-sided cancer. (F) Preoperative FPR in left-sided and right-sided cancer. (G) Preoperative FPR in T1–3 and T4 subgroup. (H) Preoperative FPR in subgroups with large (≥5 cm) or small (<5 cm) cancer size. (I) The change of median preoperative FPR in different cancer sizes. FPR, Fib to pre-Alb ratio; *≤0.05. **≤0.01.
In stage III patients, the frequency distribution of the T4 stage within the left-sided cases was significantly lower than that it in the right-sided patients (p < 0.01) (Figure 2E). Preoperative FPR was higher in subgroups with the right-sided disease (p < 0.05), T4 stage (p < 0.05), and cancer bulk ≥5 cm (p < 0.05) compared to the counterparts (Figures 2F–H). Moreover, circulating FPR was gradually rising in the patient with increased cancer bulk (p trend < 0.01) (Figure 2I).
In this study, a high-frequency distribution of evaluated FPR was also observed in the right-sided patients (p < 0.01 for 52.30 vs. 27.00%) (Figure 3A). Prognosis of the high-FPR patients was extremely inferior to the cases with low-FPR (plog−rank < 0.01 for RFS, plog−rank < 0.01 for OS) (Figures 3B,C), and the significant associations were observed when they were adjusted by confounders including clinical baseline and pathological characteristics, treatment, and primary tumor location (p < 0.01, adjusted HR = 1.96 and 95% CI = 1.42–2.70 for RFS; p < 0.01, adjusted HR = 2.44 and 95% CI = 1.459–3.75 for OS) (Table 3). However, there was no survival difference within the high- or low-FPR patients with different tumor locations (Supplementary Figures 1E,F).
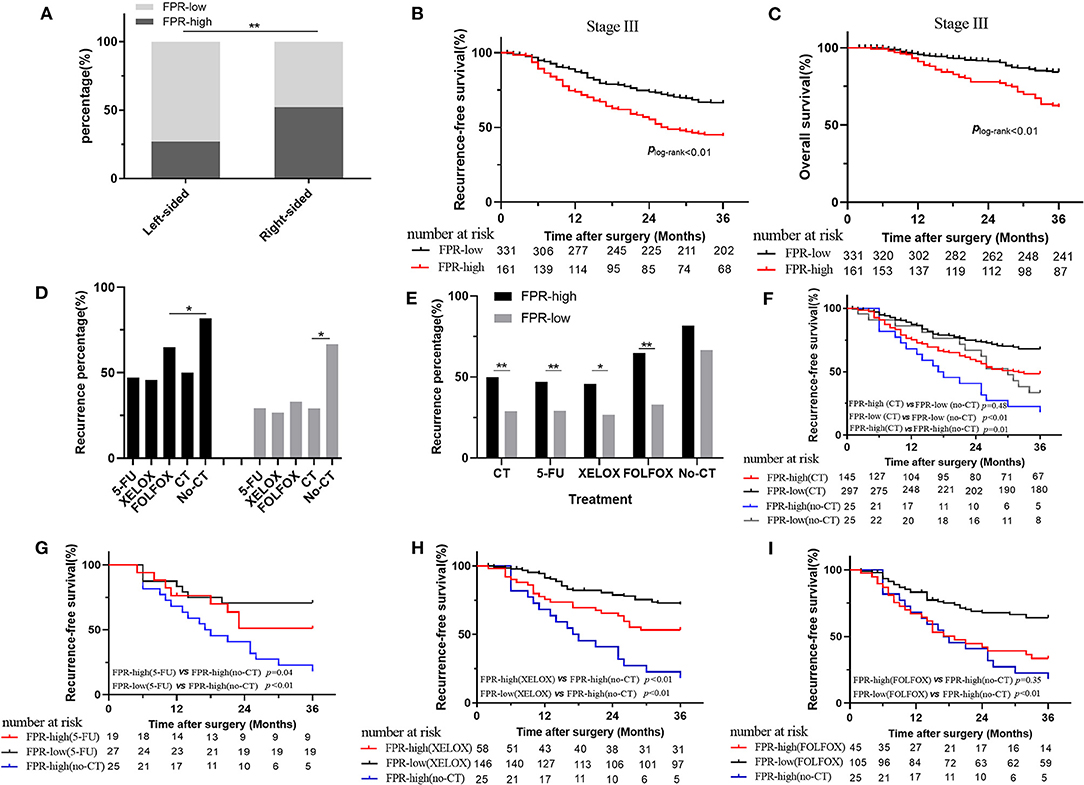
Figure 3. Association of preoperative FPR with clinical outcome in stage III colorectal cancer patients. (A) The distribution of high- and low-FPR in left-sided and right-sided cancer. (B) Kaplan–Meier curves for RFS in the stage III patients with high and low FPR. (C) Kaplan–Meier curves for OS in the stage III patients with high and low FPR. (D,E) The recurrence rate comparison in stage III patients with or without chemotherapy treatment. (F) Kaplan–Meier curves for RFS in high- and low-FPR chemotherapy-treated subgroups and non-chemotherapy-treated subgroup. (G) Kaplan–Meier curves for RFS in non-chemotherapy-treated patients, low- and high-FPR subgroups with the treatment of 5-FU. (H) Kaplan–Meier curves for RFS in non-chemotherapy-treated patients, low- and high-FPR subgroups with the treatment of XELOX. (I) Kaplan–Meier curves for RFS in non-chemotherapy-treated patients, low- and high-FPR subgroups with the treatment of FOLFOX; HR, hazard ratio; FPR, Fib to pre-Alb ratio; *≤0.05; **≤0.01.
Due to high medical costs and poor physical conditions, 50 of the stage III CRC patients did not receive any adjuvant therapy after surgical resection. Four hundred and forty-two patients received adjuvant chemotherapy, among which 51 patients received adjuvant chemoradiotherapy. In the chemotherapy-treated patients, 46, 204, 150, and 5 of the stage III cases received the treatments of single 5-FU, XELOX, FOLFOX, and FOLFIRI, respectively. However, 37 patients received the treatment without a defined regimen. In chemotherapy-treated and the non-treated groups, high- and low-FPR patients harbored 48.74% and 29.48, 81.82, and 66.67% recurrence rates, respectively. The recurrence rate of adjuvant chemotherapy-treated patients was significantly lower than the patient without the treatment, regardless of FPR status (Figure 3D). The high-FPR patients harbored significantly high recurrence rate compared to the low FPR cases in chemotherapy-treated patients (p < 0.01 for 48.74 vs. 29.48%), especially in 5-FU (p < 0.01 for 47.10 vs. 29.20%), XELOX (p = 0.02 for 45.15 vs. 26.63%), and FOLFOX (p < 0.01 for 61.59 vs. 33.78%) subgroups (Figure 3E). However, no recurrence difference was observed in non-chemotherapy-treated high- and low-FPR patients (Figure 3E). Low-FPR patients harbored the best RFS with the treatment of chemotherapy, and the outcomes of chemotherapy-treated low- and high-FPR patients were superior to those without the treatment (Figure 3F and Supplementary Figure 1G). Low-FPR patients with treatment of 5-FU, XELOX, FOLFOX, and the high FPR patients without chemotherapy harbored the best and worst RFS. The survival of high-FPR patients without chemotherapy was inferior to the cases undergoing the treatment, especially in 5-FU- and XELOX-treated subgroup (Figures 3G–I). However, no RFS difference was observed in high- and low-FPR patients with adjuvant radiotherapy (Supplementary Figure 1H).
Discussion
In this study, we investigated the role of tumor laterality and chronic inflammation in the survival outcome of stage I–III CRC individuals. We found that the prognosis of the right-sided stage III patients who harbored high T4 stage was worse than the right-sided cases. Preoperative high FPR was observed in stage III right-sided, T4 stage, and large cancer bulk subgroups, and it was significantly associated with poor prognosis of the patients regardless of tumor location. Moreover, the clinical outcome of high-FPR patients with treatment of adjuvant chemotherapy was inferior to the low-FPR cases with the same treatment, but was superior to the patients without chemotherapy after surgical resection.
Colorectal tissue within different locations is derived from different embryonic tissues (27). The distinct pathological and genetic features of the left- and right-sided CRC contribute to heterogeneous outcomes (28). In our study, the clinical outcome of the right-sided stage III patient was inferior to the left-sided cases, not the patients with the early-stage disease, indicating that tumor laterality was associated with prognosis in the stage III patients, and the right-sided cases were more likely to experience recurrence and metastasis after the radical operation. The result is consistent with the finding reported by Petrelli et al. (29). We also observed a significant difference in preoperative FPR and T4 patients' distribution in the left- and right-sided cases, the T4 patients harbored extremely high FPR, and preoperative FPR was found to be positively associated with cancer size. These findings demonstrated that the disease triggered level of chronic inflammation mainly relying on cancer bulk, and the right-sided patients with T4 stage and large cancer size harbored chronic high-grade inflammation compared to the left-sided cases. Moreover, no survival difference between the right- and left-sided patients was observed in subgroups stratified by FPR, suggesting that FPR was related to the poor survival of patients regardless of primary tumor location. High FPR was still significantly associated with poor prognosis of the cases when it was adjusted by other confounders including tumor laterality, revealing that chronic high-grade inflammation was an independent prognostic factor for the disease. Our previous study also indicated that elevated neutrophil-to-lymphocyte ratio (NLR) was significantly associated with diminished RFS, OS, and cancer-specific survival in surgical patient with stage I–III CRC. These findings indicated that common inflammatory ratios, FPR and NLR, could be independent prognostic factors for the disease.
Our previous study showed radio-chemotherapy resistance in right-sided metastatic CRC patients (26). In our study, we found that low FPR patients harbored the lowest recurrence rate and best RFS with the treatment of adjuvant chemotherapy, and the non-recurrence rate and survival of high FPR patients with the same treatment were superior to the patients without any adjuvant chemotherapy. These findings illustrated that survival benefit magnitude from adjuvant chemotherapy differed by the grade of chronic inflammation, low-grade inflammation could benefit more from adjuvant chemotherapy, while chronic high-grade inflammation might weaken sensitivity to common adjuvant chemotherapy, leading to heterogeneous clinical chemotherapy response and outcome in stage III surgical cases.
It is well-known that the interaction of genetic and environmental factors contributes to the onset and metastasis of CRC (30). Distinct genetic features might be one cause leading to survival differences in stage III patients with various tumor locations. Deficient DNA mismatch repair (MMR) proteins could impair genomic stability, leading to tumorigenesis, chemoresistance, and progression of the disease (31–34). Consensus molecular subtype (CMS) 1 and 3 with hypermutation of KRAS, BRAF, PIK3CA, and MMR pathway gene are commonly observed in the right-sided disease, while the left-sided disease usually harbors mediate and high copy number variation (35). Moreover, microsatellite-stable patients have been reported to benefit more from fluorouracil-based adjuvant chemotherapy than the cases with microsatellite instability (36, 37). On the other hand, rich expression of IFN-γ, CXCL9, and CXCL10 and significantly high CTLA-4 or PD-1+ CD8/CD4 T cells were observed in the tumor microenvironment of CMS 1 patients (35). An increasing gut microbial richness was also reported from the right-sided colon to the left-sided disease (38). Escherichia-Shigella and Prevotella, which were highly enriched in the right-sided CRC, appeared to be linked to elevated IL-17-producing cells and up-regulation of STAT3 and IL-6 in the mucosa of CRC patients (38, 39). Elevated IL-6 produced from the cancer microenvironment could effectively inhibit the synthesis of Alb and pre-Alb (40), whereas IL-17 facilitated cisplatin resistance in CRC by inhibiting cancer cell apoptosis through targeting p-Akt, Bax, Bcl-2, and mTOR (41), promoting its pro-proliferative and antiapoptotic properties (42). Moreover, hyperfibrinogenemia is a common clinical phenomenon in CRC patients, especially in the advanced disease. Fib has been reported to function as a scaffold to promote cancer progression (43). Thus, FPR implied chronic high-grade inflammation triggered by CRC cell, infiltrated gut commensal microorganisms, and immune cells as well as stromal cells conferred to impaired chemosensitivity, resulting in poor response to adjuvant chemotherapy and unsatisfactory survival in the right-sided cases.
In this study, we confirmed that high-grade inflammation within the left- and right-sided stage III surgical patients impaired survival benefit from adjuvant chemotherapy, leading to poor survival of the stage III cases. We also found that primary tumor location might be just a confounder factor for the disease while high-grade chronic inflammation was an independent prognostic factor for the patients. Despite the interesting findings, several limitations should be addressed as follows. We categorized transverse colon cancer as a right-sided disease, which might influence our findings. We could not obtain the pathological sample from each enrolled patient. RAS and BRAF, as well as microsatellite instability status, were unavailable from each enrolled patient in our study. Hence, we did not investigate the role of RAS and BRAF status in the survival of patients with right- and left-sided diseases.
In summary, our study reveals that chronic high-grade inflammation confers impaired sensitivity to common adjuvant chemotherapy, leading to the poor outcome of the patients with stage III disease. Further study is warranted to validate our findings and to investigate the clinical utility of anti-inflammation therapeutic target antibody combined with adjuvant chemotherapy for the high-FPR patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Second Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-QY provided the idea, established the study design, performed the statistics, and written the manuscript. X-HY screened and selected eligible patients, laboratory detection, and follow-up. Y-CL contributed to data preparation, performed, and verified the statistic results. FS contributed to sample collection, and manuscript preparation. X-XC provided the idea and research funding for this study and revised and approved this study.
Funding
This report was supported by the National Natural Science Foundation of China (grant number: 81702090), the Natural Science Youth Foundation of Jiangxi Province (grant number: 20171BAB215054), and the Key Technology Research and Development Program of Jiangxi Province (grant number: 20171BBG70049).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.580455/full#supplementary-material
Supplementary Figure 1. Survival comparison in overall or stage III population. (A) Kaplan–Meier curves for Recurrence-free survival (RFS) in stage I-III patients with rectal and left-sided (excluded rectum) cancer; (B) Kaplan–Meier curves for overall survival (OS) in stage I-III patients with rectal and left-sided (excluded rectum) cancer; (C) Kaplan–Meier curves for RFS in stage III patients with rectal and left-sided (excluded rectum) cancer; (D) Kaplan–Meier curves for OS in stage III patients with rectal and left-sided (excluded rectum) cancer; (E) Kaplan–Meier curves for RFS in FPR-high or FPR-low patients with right-sided or left-sided stage III cancer; (F) Kaplan–Meier curves for OS in FPR-high or FPR-low patients with right-sided or left-sided stage III cancer; (G) Kaplan–Meier curves for RFS in non-chemotherapy treated patients, low FPR chemotherapy-treated cases and first-line 5-FU, XELOX, FOLFOX treated high FPR subgroups; (H) Kaplan–Meier curves for RFS in FPR-high or FPR-low patients receiving radiotherapy. FPR, Fib to pre-Alb ratio; CT, chemotherapy; 5-FU, 5-fluorouracil; XELOX, capecitabine, leucovorin, oxaliplatin; FOLFOX, fluorouracil, leucovorin, oxaliplatin; *≤0.05; **≤0.01.
References
1. Stintzing S. Recent advances in understanding colorectal cancer. FRes. (2018) 7:14604. doi: 10.12688/f1000research.14604.1
2. Merlano MC, Granetto C, Fea E, Ricci V, Garrone O. Heterogeneity of colon cancer: from bench to bedside. ESMO Open. (2017) 2:e000218. doi: 10.1136/esmoopen-2017-000218
3. Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. (2017) 15:411–9. doi: 10.6004/jnccn.2017.0038
4. Gao XH, Yu GY, Gong HF, Liu LJ, Xu Y, Hao LQ, et al. Differences of protein expression profiles, KRAS and BRAF mutation, and prognosis in right-sided colon, left-sided colon and rectal cancer. Sci Rep. (2017) 7:7882. doi: 10.1038/s41598-017-08413-z
5. Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. (2015) 148:88–99. doi: 10.1053/j.gastro.2014.09.041
6. Gallois C, Pernot S, Zaanan A, Taieb J. Colorectal cancer: why does side matter? Drugs. (2018) 78:789–98. doi: 10.1007/s40265-018-0921-7
7. Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. (2012) 61:794–7. doi: 10.1136/gutjnl-2012-302014
8. Papagiorgis P. Colorectal cancer: dichotomous or continuum model? Perhaps, a combination of both. Gut. (2013) 62:1519–20. doi: 10.1136/gutjnl-2013-305209
9. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. (2017) 3:194–201. doi: 10.1001/jamaoncol.2016.3797
10. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. (2017) 70:87–98. doi: 10.1016/j.ejca.2016.10.007
11. Benavides M, Díaz-Rubio E, Carrato A, Abad A, Guillén C, Garcia-Alfonso P, et al. Tumour location and efficacy of first-line EGFR inhibitors in KRAS/RAS wild-type metastatic colorectal cancer: retrospective analyses of two phase II randomised Spanish TTD trials. ESMO Open. (2019) 4:e000599. doi: 10.1136/esmoopen-2019-000599
12. You XH, Wen C, Xia ZJ, Sun F, Li Y, Wang W, et al. Primary tumor sidedness predicts bevacizumab benefit in metastatic colorectal cancer patients. Front Oncol. (2019) 9:723. doi: 10.3389/fonc.2019.00723
13. You XH, Jiang YH, Fang Z, Sun F, Li Y, Wang W, et al. Chemotherapy plus bevacizumab as an optimal first-line therapeutic treatment for patients with right-sided metastatic colon cancer: a meta-analysis of first-line clinical trials. ESMO Open. (2020) 4:605. doi: 10.1136/esmoopen-2019-000605
14. Taieb J, Kourie HR, Emile JF, Le Malicot K, Balogoun R, Tabernero J, et al. Association of prognostic value of primary tumor location in stage III colon cancer with RAS and BRAF mutational status. JAMA Oncol. (2018) 4:e173695. doi: 10.1001/jamaoncol.2018.1718
15. Karim S, Brennan K, Nanji S, Berry SR, Booth C. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol. (2017) 3:1386–92. doi: 10.1001/jamaoncol.2017.1016
16. Kerr DJ, Domingo E, Kerr R. Is sidedness prognostically important across all stages of colorectal cancer? Lancet Oncol. (2016) 17:1480–2. doi: 10.1016/S1470-2045(16)30431-4
17. Cascinu S, Poli D, Zaniboni A, Lonardi S, Labianca R, Sobrero A, et al. The prognostic impact of primary tumour location in patients with stage II and stage III colon cancer receiving adjuvant therapy. A GISCAD analysis from three large randomised trials. Eur J Cancer. (2019) 111:1–7. doi: 10.1016/j.ejca.2019.01.020
18. Ishihara S, Murono K, Sasaki K, Yasuda K, Otani K, Nishikawa T, et al. Impact of primary tumor location on postoperative recurrence and subsequent prognosis in nonmetastatic colon cancers: a multicenter retrospective study using a propensity score analysis. Ann Surg. (2018) 267:917–21. doi: 10.1097/SLA.0000000000002206
19. Nathan C, Ding A. Nonresolving inflammation. Cell. (2010) 140:871–82. doi: 10.1016/j.cell.2010.02.029
20. Murray PJ. Nonresolving macrophage-mediated inflammation in malignancy. FEBS J. (2018) 285:641–53. doi: 10.1111/febs.14210
21. Li SQ, You XH, Sun F, Xia ZJ, Fang Z, Wang W, et al. Albumin to fibrinogen ratio and fibrinogen to pre-albumin ratio are economical, simple and promising prognostic factors for solid malignancy. J Thorac Dis. (2019) 11:S2036–8. doi: 10.21037/jtd.2019.08.96
22. Zhang L, Chen QG, Li SQ, Zhang J, Min QH, Gao QF, et al. Preoperative fibrinogen to prealbumin ratio as a novel predictor for clinical outcome of hepatocellular carcinoma. Future Oncol. (2019) 15:13–22. doi: 10.2217/fon-2018-0376
23. Zhang J, Li SQ, Liao ZH, Jiang YH, Chen QG, Huang B, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. (2017) 8:75195–205. doi: 10.18632/oncotarget.20661
24. Sun F, Peng HX, Gao QF, Li SQ, Zhang J, Chen QG, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II-III colorectal cancer patients. Cancer Manag Res. (2018) 10:2151–61. doi: 10.2147/CMAR.S167398
25. Ying HQ, Liao YC, Sun F, Peng HX, Cheng XX. The role of cancer-elicited inflammatory biomarkers in predicting early recurrence within stage II-III colorectal cancer patients after curable resection. J Inflamm Res. (2021) 14:115–29. doi: 10.2147/JIR.S285129
26. Chen QG, Zhang L, Sun F, Li SQ, You XH, Jiang YH, et al. Elevated FPR confers to radiochemoresistance and predicts clinical efficacy and outcome of metastatic colorectal cancer patients. Aging. (2019) 11:1716–32. doi: 10.18632/aging.101864
27. Boeckx N, Janssens K, Van Camp G, Rasschaert M, Papadimitriou K, Peeters M, et al. The predictive value of primary tumor location in patients with metastatic colorectal cancer: a systematic review. Crit Rev Oncol Hematol. (2018) 121:1–10. doi: 10.1016/j.critrevonc.2017.11.003
28. Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. (2014) 25:1995–2001. doi: 10.1093/annonc/mdu275
29. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs. right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. (2017) 3:211–9. doi: 10.1001/jamaoncol.2016.4227
30. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
31. Jover R, Nguyen TP, Pérez-Carbonell L, Zapater P, Payá A, Alenda C, et al. 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology. (2011) 140:1174–81. doi: 10.1053/j.gastro.2010.12.035
32. Li SKH, Martin A. Mismatch repair and colon cancer: mechanisms and therapies explored. Trends Mol Med. (2016) 22:274–89. doi: 10.1016/j.molmed.2016.02.003
33. Alex AK, Siqueira S, Coudry R, Santos J, Alves M, Hoff PM, et al. Response to chemotherapy and prognosis in metastatic colorectal cancer with DNA deficient mismatch repair. Clin Colorectal Cancer. (2017) 16:228–39. doi: 10.1016/j.clcc.2016.11.001
34. De' Angelis GL, Bottarelli L, Azzoni C, De' Angelis N, Leandro G, Di Mario F, et al. Microsatellite instability in colorectal cancer. Acta Biomed. (2018) 89:97–101. doi: 10.17179/excli2017-948
35. Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. (2017) 17:268. doi: 10.1038/nrc.2017.24
36. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. (2003) 349:247–57. doi: 10.1056/NEJMoa022289
37. Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. (2007) 25:767–72. doi: 10.1200/JCO.2006.05.8172
38. Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. (2015) 6:20. doi: 10.3389/fmicb.2015.00020
39. Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE. (2011) 6:e16393. doi: 10.1371/journal.pone.0016393
40. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. (2012) 33:209–90. doi: 10.1016/j.mam.2011.12.002
41. Sui G, Qiu Y, Yu H, Kong Q, Zhen B. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol Lett. (2019) 17:944–50. doi: 10.3892/ol.2018.9645
42. Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. (2013) 19:1114–23. doi: 10.1038/nm.3291
Keywords: primary tumor location, chronic inflammation, FPR, colorectal cancer, chemosensitivity
Citation: Ying HQ, You XH, Liao YC, Sun F and Cheng XX (2021) High-Grade Inflammation Attenuates Chemosensitivity and Confers to Poor Survival of Surgical Stage III CRC Patients. Front. Oncol. 11:580455. doi: 10.3389/fonc.2021.580455
Received: 06 July 2020; Accepted: 22 February 2021;
Published: 23 April 2021.
Edited by:
Jing He, Guangzhou Medical University, ChinaReviewed by:
Zeming Liu, Zhongnan Hospital of Wuhan University, ChinaZhijie Xu, Central South University, China
Copyright © 2021 Ying, You, Liao, Sun and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Xin Cheng, Y3h4bmN1QDE2My5jb20=
†These authors have contributed equally to this work
 Hou-Qun Ying
Hou-Qun Ying Xia-Hong You2†
Xia-Hong You2† Fan Sun
Fan Sun