- 1Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan
- 2Department of Radiation Oncology, Fu Jen Catholic University Hospital, New Taipei City, Taiwan
- 3School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
- 4Department of Computer Science and Information Engineering, National Taiwan University, Taipei, Taiwan
- 5Division of Radiation Oncology, Departments of Oncology, National Taiwan University Hospital, Taipei, Taiwan
- 6Graduate Institutes of Oncology, Taipei, Taiwan
- 7Clinical Medicine, National Taiwan University College of Medicine, Taipei, Taiwan
Purpose: Beam angle optimization is a critical issue for modern radiotherapy (RT) and is a challenging task, especially for large body sizes and noncoplanar designs. Noncoplanar RT techniques may have dosimetric advantages but increase the risk of mechanical collision. We propose a software solution to accurately predict colliding/noncolliding configurations for coplanar and noncoplanar beams.
Materials and Methods: Individualized software models for two different linear accelerators were built to simulate noncolliding gantry orientations for phantom/patient subjects. The sizes and shapes of the accelerators were delineated based on their manuals and on-site measurements. The external surfaces of the subjects were automatically contoured based on computed tomography (CT) simulations. An Alderson Radiation Therapy phantom was used to predict the accuracy of spatial collision prediction by the software. A gantry collision problem encountered by one patient during initial setup was also used to test the validity of the software. Results: In the comparison between the software estimates and on-site measurements, the noncoplanar collision angles were all predicted within a 5-degree difference in gantry position. The confusion matrix was calculated for each of the two empty accelerator models, and the accuracies were 98.7% and 97.3%. The true positive rates were 97.7% and 96.9%, while the true negative rates were 99.8% and 97.9%, respectively. For the phantom study, the collision angles were predicted within a 5-degree difference. The software successfully predicted the collision problem encountered by the breast cancer patient in the initial setup position and generated shifted coordinates that were validated to correspond to a noncolliding geometry.
Conclusion: The developed software effectively and accurately predicted collisions for accelerator-only, phantom, and patient setups. This software may help prevent collisions and expand the range of spatially applicable beam angles.
Introduction
Collision prevention for patient safety is a key issue in radiation therapy (RT) (1). The spatial optimization of beam angles remains essential and critical for RT; however, challenges of increased uncertainty can arise with large-size patients or noncoplanar beams, leading to potential gantry collisions. Noncoplanar RT techniques, which potentially have dosimetric advantages by virtue of the additional degrees of freedom, have been used in several clinical situations, including the treatment of lung cancer and liver cancer (2, 3). However, the increased risk of gantry collision demands special attention and prohibits frequent use. Obstacles remain with regard to the necessary replanning work and the delay in treatment when a problem of unexpected gantry collision is encountered. The conservative use of noncoplanar beams or pretreatment dry runs compromises the broad application of such techniques. Hence, the ability to select noncoplanar beam angles that will not result in gantry collisions mainly depends on individual skill and experience. A few preliminary methods have been proposed to prevent collisions (2, 4–10), such as a chart of couch-gantry combinations (7), prediction software for collision avoidance (8, 10), and the use of supplemental cameras (5, 11) or a 3-dimensional scanner (4). Graphical simulations (12, 13) or experimental reference measurements (14) also offered some possible approaches to try to solve above problem. Unfortunately, no user-friendly, patient- and equipment-specific method has yet been presented that can conveniently predict collisions. Our goal is to establish a software platform that can integrate patient- and accelerator-specific information into the estimation of the noncolliding space for coplanar/noncoplanar beam selection before the planning process in order to improve patient safety and prevent hardware damage.
Materials and Methods
Geometrical Linear Accelerator Models
Geometrical models of two accelerators were built for simulations based on polygonal geometry. A model with a cantilever, with the accelerator head anchored at one end and a vertical support, and a rotatable couch was built for each system. The machine dimensions were set in accordance with the manuals and on-site measurements. Linear accelerators from two different vendors, the Elekta Synergy® (Elekta, Crawley, UK) and the Varian TrueBeam™ STx (Varian Medical Systems, Palo Alto, CA, USA), were used. The models were established in Unity™ 3D (Unity Technologies, San Francisco, CA, USA).
Automatic Computed Tomography (CT) Contouring and Image Exporting
The simulation CT images were subjected to image segmentation, image intensity transformation, and region of interest (ROI) processing, and the contours were exported as Digital Imaging and Communications in Medicine (DICOM) files. For comprehensive collision prediction, the contoured subjects included the body surface, auxiliary equipment such as a shell or a vacuum bag, and other accessories above the CT couch plate. A user interface (UI) was designed to help users choose their own intensity transformation specifications. We applied the grayscale morphological closing technique from the field of computer vision to smooth the images. The functions of dilation, erosion, and contour finding for grayscale images were implemented using the Open Source Computer Vision (OpenCV) library. The isocenters of the CT images were defined manually. Finally, the DICOM images were exported and saved in the secured space.
Collider Models
Each DICOM file was exported with three contour sequences including the isocenter, the CT plate height, and a collider. A collider is any object which can collide with the gantry, either the patient body or any auxiliary equipment. Each contour is retrieved from the CT image in a 2-dimensional space. Each contour on the single CT slice is a set of consecutive points located on an XY plane. In order to construct the 3-dimensional (3D) structure from 2-dimensional (2D) images, we implemented an algorithm to incorporate the Z-axis coordinate into the 2D image stacks with contour interpolation. Linear interpolation was used between the two corresponding contours on the closest adjacent slices to convert a set of 2D contours into a 3D mesh (15). A 3D mesh in Unity™ 3D therefore is created based on the patient’s external contour and consists of a set of vertices and a set of triangles. Triangulation is applied to split the polygon into triangles.
Collision Detection
We used a mesh collider function built in Unity™ 3D to perform collision detection (16). In our study, we used the trigger for a mesh to detect collision events. We did not use the collider function for a rigid body because Unity™ 3D does not allow two rigid bodies to overlap. Our work was designed to simulate all the possible gantry and couch combinations including configurations involving collisions. Three types of triggers within Unity were used: [OnTriggerEnter], [OnTriggerStay], and [OnTriggerExit]. When an object collides with another object, Unity calls [OnTriggerEnter]. [OnTriggerExit] is called when the colliding event ends. In between [OnTriggerEnter] and [OnTriggerExit] is called [OnTriggerStay].
Model Validations for an Empty Couch, A Phantom, and A Patient
Collision was manually assessed for 38 couch- and gantry-angle configurations of the two different linear accelerators based on approximately 400 measurements from different couch coordinates. An Alderson Radiation Therapy (ART) phantom immobilized with a vacuum bag was used to test the model collision estimation. The couch heights were chosen to be at the lowest reachable position, 10 cm below the isocenter, and 20 cm below the isocenter; these positions span the heights at which most treatments are performed. The couch angles ranged from 0 to 90 degrees counterclockwise and 270 degrees clockwise. The gantry angles were tested up to the points at which couch-gantry or gantry-phantom collisions were encountered within the 3-cm safety zone or when the “Collision” alarm was displayed. The couch rotation angles were measured in increments of 10 degrees from 0 to 350 degrees. For the Varian TrueBeam™ STx, the official “LaserGuard Protection Zones” rules were applied for reference. DICOM images of one breast cancer patient, who underwent postlumpectomy adjuvant radiotherapy in the prone position and encountered a real gantry-patient collision problem on the first treatment day, were used to verify the validity of our models. The frequency of inaccurate predictions was counted by difference in every specific gantry-couch combination between prediction and on-site measurement. False negative (FN) was defined as no collision was estimated but a collision was warned in the on-site measurement. The false negative rate and false positive (FP) rate could be adjusted by expanding or shirking the safety zone.
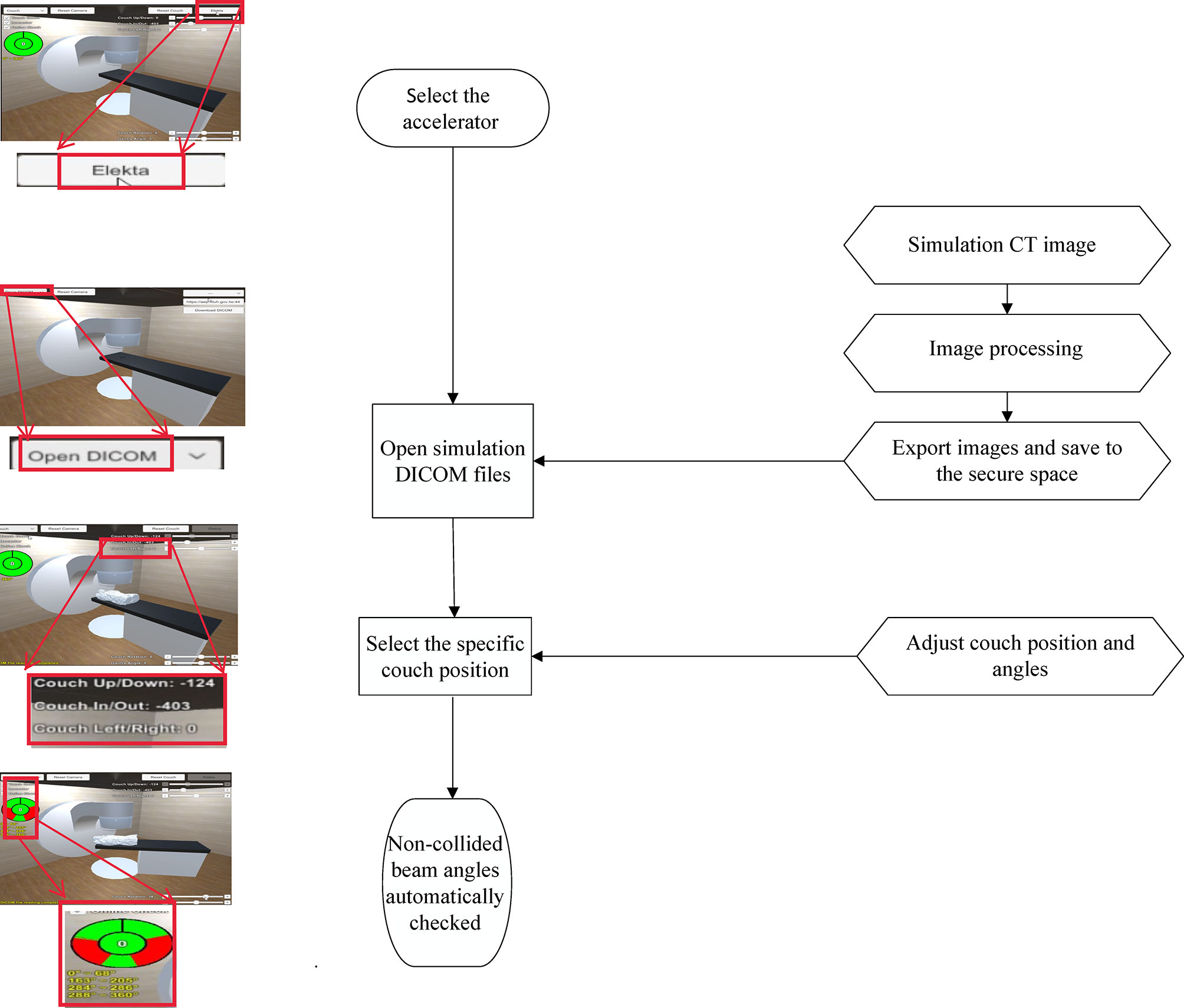
The workflow of the software was shown as Figure 1. The study was approved by the Institutional Review Board (201811050RINB).
Results
Linear Accelerator Delineation
The linear accelerator models were divided into different parts for size and shape delineation, including the accelerator, the gantry, the touch guard mounted on the gantry, the couch plate, and the patient support. The couch rotator and base were modeled as a polygonal piece and a cuboid, respectively. The couch plate was a cuboid, with a black sphere representing the isocenter. Three parts formed the gantry from each vendor, namely, the gantry gun, gantry arm, and gantry wall. The gantry was able to rotate with a 360-degree range. The couch plate could be freely adjusted in the up-down, right-left, and in-out directions, and the couch rotator could be freely adjusted for angles. The on/off switch for the collision detector was designed as shown in Figure 2.
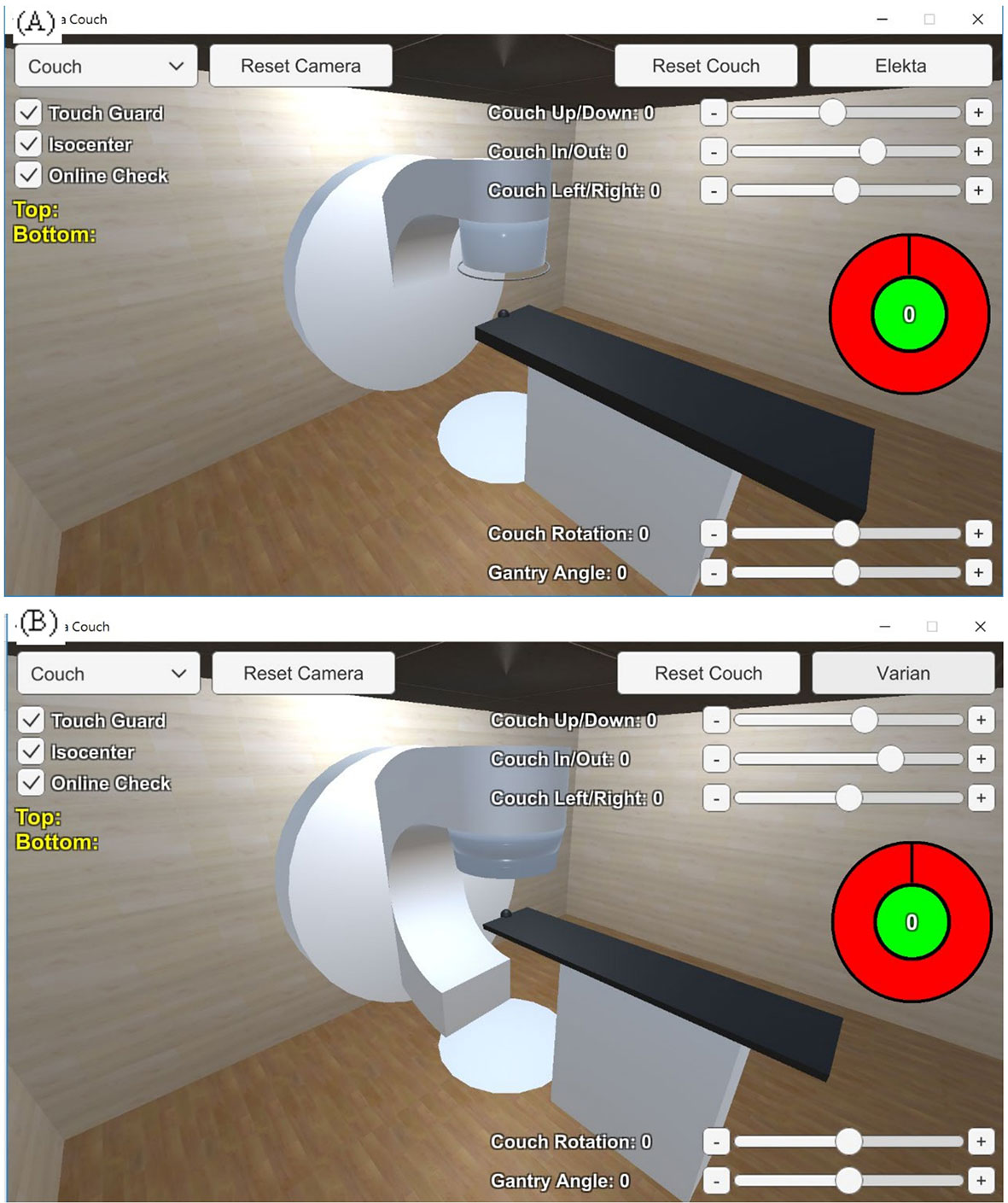
Figure 2 Linear accelerator models of (A) the Elekta Synergy® and (B) the Varian TrueBeam™ STx delineated with different polygonal pieces using Unity™ 3D.
Surface Contours And Image Transfer
The external surface of the phantom or patient was first automatically contoured based on the simulation CT images. A platform application was established showing the current slice number, the total number of slices, the intensity transform values, the plate height, and the position of the isocenter. The user could adjust the intensity transform values to achieve CT contouring and apply computer vision processing to obtain outline images. The images were stored in DICOM format and could be uploaded to the cloud or downloaded from the cloud to the software.
Software Validation for Collisions With An Empty Couch
Figure 3 shows the confusion matrices based on software predictions and on-site measurements at 19 couch angles (every 10 degrees of rotation on either side) for the two linear accelerators without either a phantom or a patient on the couch. A total of 13,680 measurements were performed to verify the predicted collisions against the true collisions. The frequency of inaccurate predictions for the TrueBeam accelerator reached a maximum at a couch angle of 330 degrees and gantry angles of 220 degrees and 310 degrees, which were the positions with the smallest distance between couch and gantry. Similarly, for the Synergy accelerator, the worst predictions were found for a couch angle of 330 degrees and gantry angles of 310 degrees and 220 degrees. Among all the assessable couch and gantry angles, the software models achieved accuracies of 98.7% and 97.3% for the Synergy and TrueBeam accelerators, respectively. The true positive rates (TPRs) for the Synergy and TrueBeam accelerators were 97.8% and 96.9%, respectively, and the true negative rates (TNRs) were 99.8% and 97.9%, respectively.
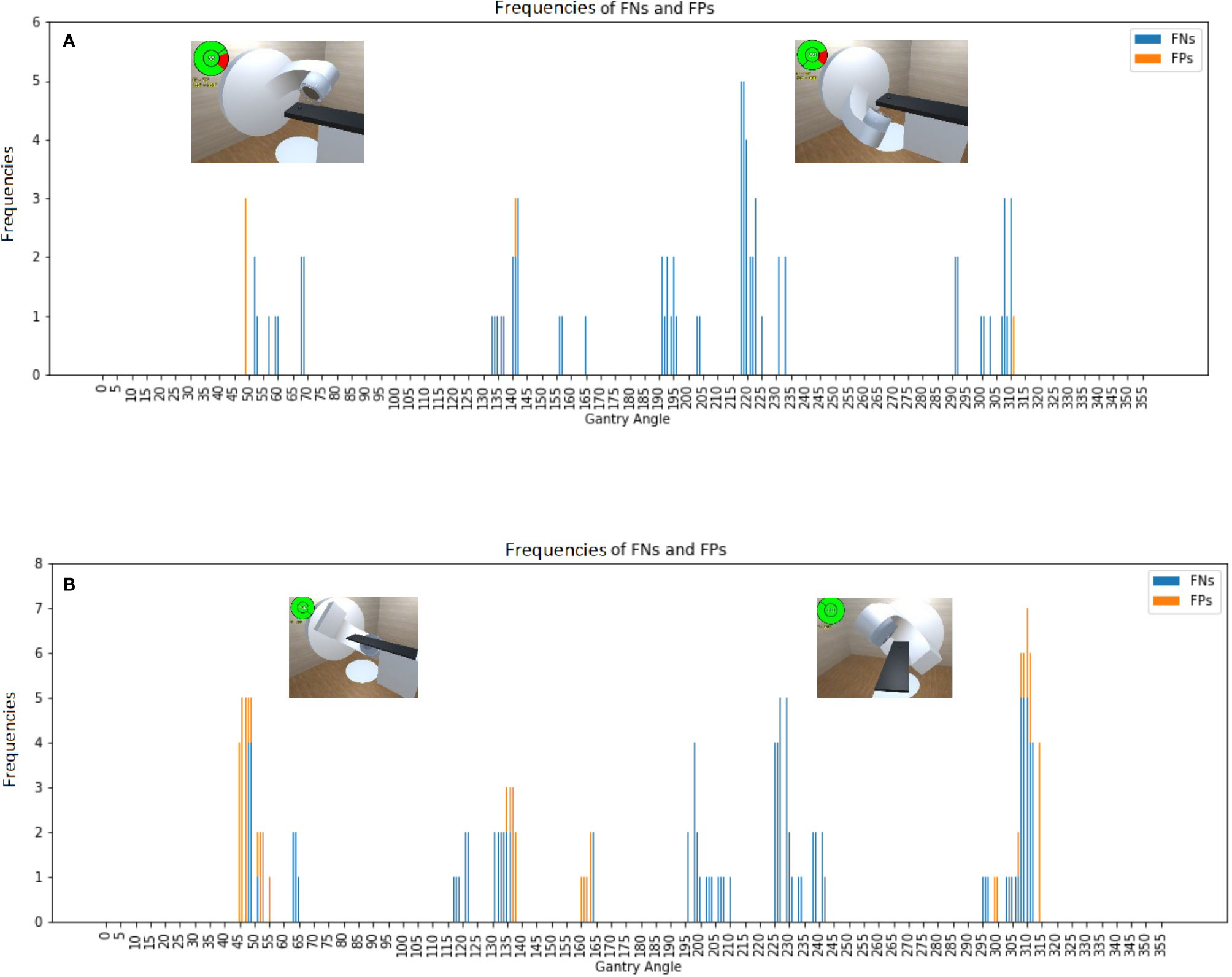
Figure 3 Frequencies of false negative predictions (FNs) and false positive predictions (FPs) for an empty couch for the (A) Synergy and (B) TrueBeam accelerators, with the representative collision conditions at gantry angles of 55°, 130°, 220°, and 310° illustrated.
Software Validation for Collisions With A Phantom
The software was also validated for collisions with an ART phantom immobilized with a vacuum bag on the couch for both accelerators (Figure 4). The software models achieved accuracies of 96.8% and 97.3% for the Synergy and TrueBeam accelerators, respectively. The TPRs for the Synergy and TrueBeam accelerators were 95.0% and 96.5%, respectively, and the TNRs were 99.2% and 98.6%, respectively. Collision predictions in matrix with an empty couch for (A) Elekta Synergy and (B) Varian TrueBeam accelerators, and with an Alderson Radiation Therapy phantom on the couch for (C) Elekta Synergy and (D) Varian TrueBeam accelerators were shown in Figure 5. Above operating process was described in our supplementary video file (Supplementary Video 1).
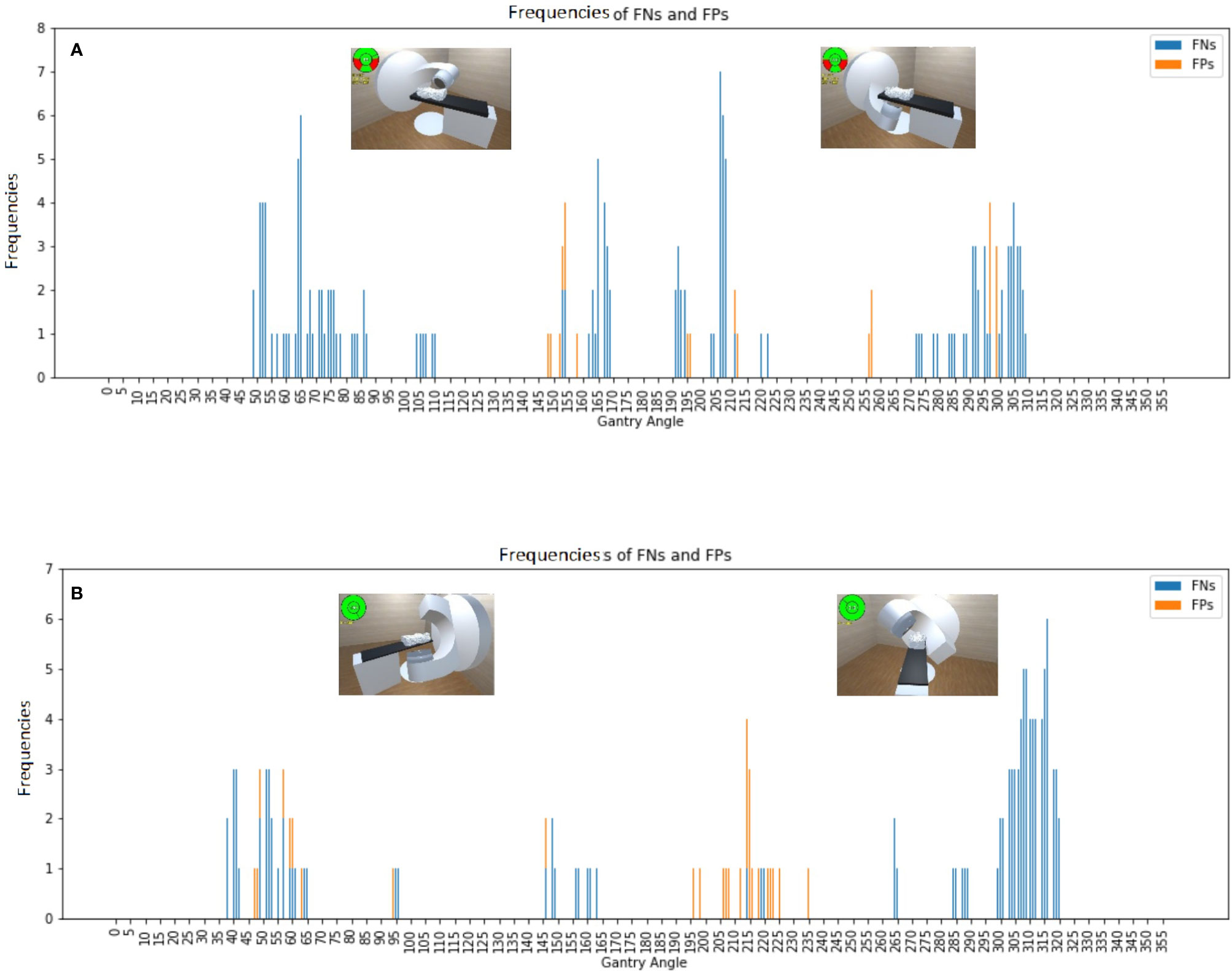
Figure 4 Frequencies of false negative predictions (FNs) and false positive predictions (FPs) with an Alderson Radiation Therapy phantom on the couch for the (A) Synergy and (B) TrueBeam accelerators, with the representative collision conditions at gantry angles of 65°, 150°, 210°, and 310° illustrated.
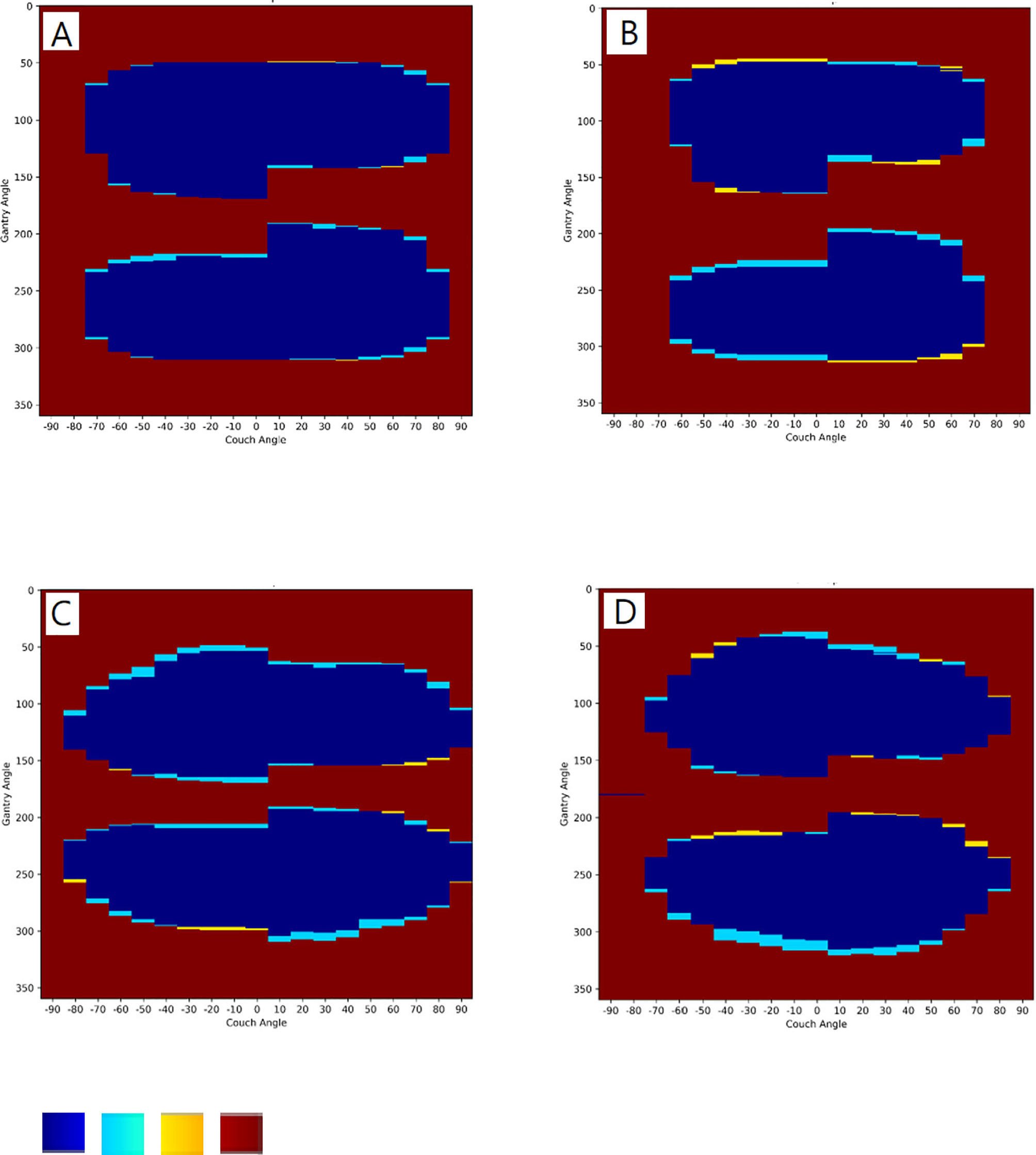
Figure 5 Collision predictions in matrix with an empty couch for (A) Elekta Synergy and (B) Varian TrueBeam accelerators, and with an Alderson Radiation Therapy phantom on the couch for (C) Elekta Synergy and (D) Varian TrueBeam accelerators. Color in blue, true positive (TP) collision; light blue, false positive (FP) collision; yellow, false negative (FN) collision; brown: true negative (TN) collision.
Software Validation for A Breast Cancer Patient With A Collision Problem
Data from a breast cancer patient who encountered a gantry collision problem in the initial setup position in the Bionix prone breast system (Bionix, Toledo, OH, USA) were selected to validate the predictive power of the software. The external surface of the patient and the prone base were automatically contoured and exported to the software. On the first day of treatment, a collision between the gantry and couch was encountered in the high setup position with the prone base. This collision was accurately predicted by the software (Figure 6A), and the downward shift of the isocenter necessary to avoid collision was also effectively estimated by the software (Figure 6B).
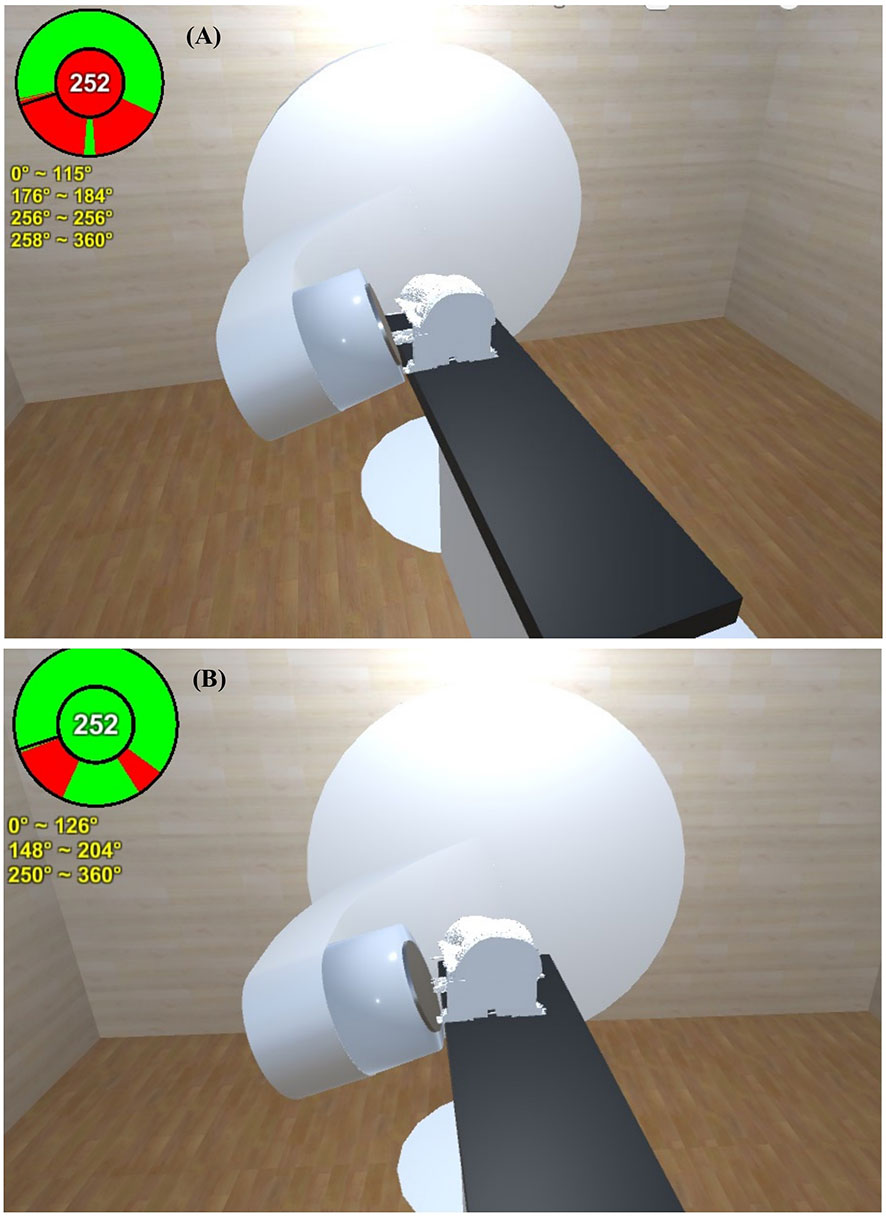
Figure 6 A collision between the gantry and couch of a representative breast cancer patient was encountered in the high setup position with the prone base. (A) This collision was accurately predicted by the software. (B) The downward shift of the isocenter necessary to avoid collision was also effectively estimated by the software.
Discussion
We designed an automatic, user-friendly, and vendor-independent software tool that can produce accurate predictions of mechanical collision. The software performance is satisfactory, with all gantry-angle discrepancies being less than 5 degrees. With this convenient and effective tool, radiation oncologists and physicists will be able to adapt their procedures to complex body shapes and more efficiently adopt noncoplanar beam designs during the treatment planning process.
Several methods have been proposed in the attempt to solve the problem of possible collisions in clinical settings. Nguyen et al. (11) used supplemental live-view cameras to reduce blind spots. Cardan et al. (5) adapted a set of consumer depth cameras to create a polygon mesh of each object and achieved a high TNR for predictions. In addition, 4π radiotherapy is a useful therapeutic approach for ensuring target-dose conformity while sparing organs at risk (OARs), in which collision avoidance can be achieved through the use of 3D cameras (2). However, all of these solutions require additional devices. To the best of our knowledge, our software tool is the first to be able to simply use simulation CT images to predict mechanical collisions for various accelerators.
Our 3D software platform is based on Unity™ 3D (not Windows) mainly because Unity is well developed for real-time 3D projects for games, animation, film, architecture, engineering, and construction. It can be easily used for rendering 3D images and with the function to help collision detection conventionally used in computer gaming.
The performance indicators of our software, including the accuracy, TPR, and TNR, were all higher than 95% for two different accelerators and were comparable to those reported by Cardan et al. (5). Cardan et al. built a model with an average accuracy and TNR of 97.3% and 96.9%, respectively. Mann et al. achieved 94.2% accuracy (17). TNR in our study was less than their previously reports. However, the unavailability of the size and shape details of some small components of both accelerators made it difficult to achieve higher predictive power for collisions using our virtual models.
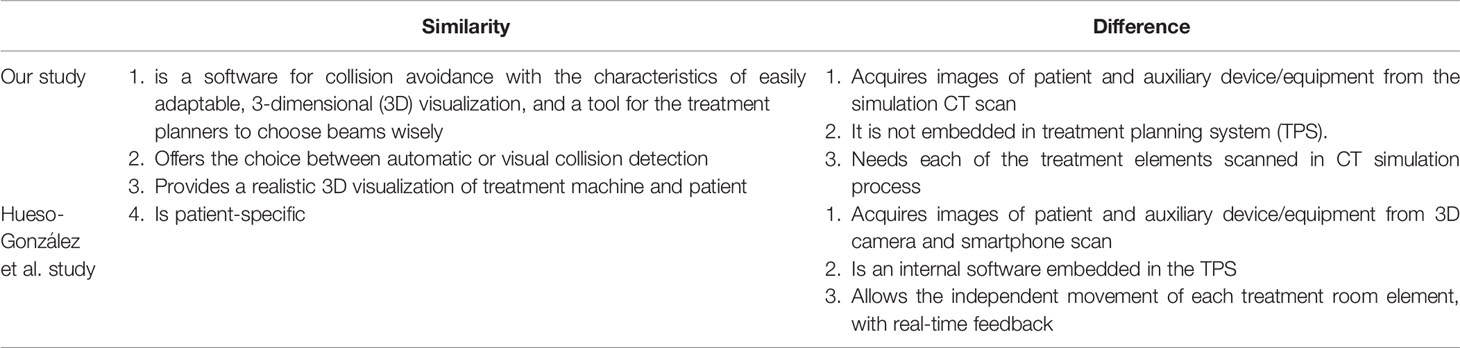
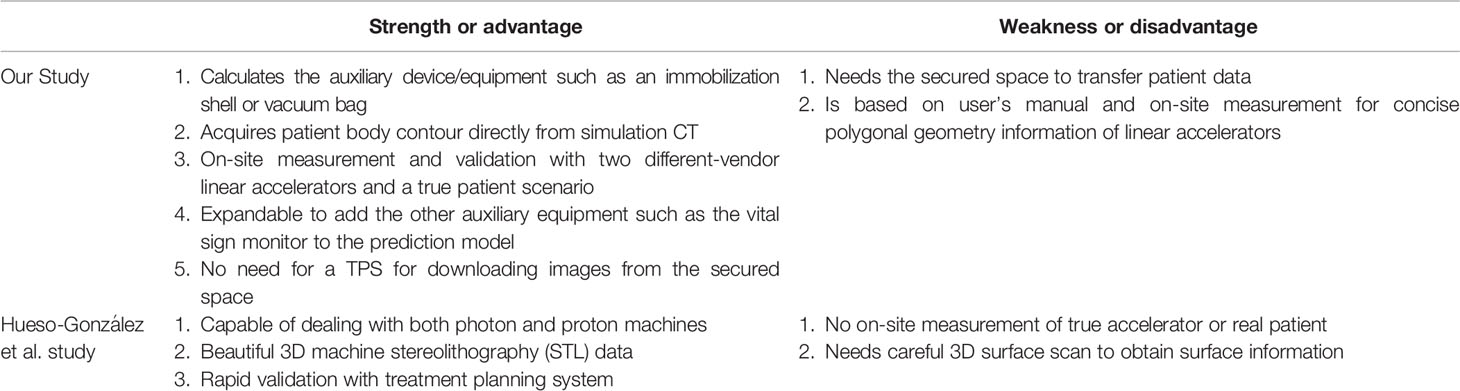
The F. Hueso-González et al. (18) offered an similar innovation with both photon and proton machine simulation data by beautiful machine stereolithography (STL) data with treatment planning system (TPS) system validation. However, our research with the strength as follows: 1. With auxiliary equipment such as a shell or a vacuum bag 2. Getting patient body contour information from simulation CT directly. 3. Validation by on-site two different brands linear accelerators measurements and a true patient scenario. The comparison between two studies listed in Tables 1 and 2.
The determined value of the confusion matrix was made based on the comparison between the model prediction and on-site measurement. The model built-up was not perfect due to doing polygonal geometry simulation for some machine parts with technical difficulty and some uncertainties from real world machine assembly processing. The accuracy may improve if a finer mesh grid was used with much more computational complexity (19, 20). Therefore, the touch guard and safe zone were offered by the two linear accelerators respectively to ensure the patient safety. In our model, we set-up 5-degree difference tolerance and adjust our polygonal geometry simulation. False negative rate in our study with empty couch was 2.2% and 3.1% respectively, and it could be close to almost zero if we sacrificed 5 predictive gantry degrees based on our validation results or expand the safety zone to reach the 99.3% TNR of TrueBeam accelerators but sacrifice the TPR down to 96.0%. It deserves attention that the indeterminations may be associated with different phases of simulation for our software. The input uncertainties include the precise geometry modeling of the accelerator before simulation, the relative position of auxiliary device to the patient in simulation, and body shape change from weight gain/loss after simulation (21).
The currently designed software has a few limitations. Some clinical collision scenarios may arise due to auxiliary equipment, such as the vital sign monitor, the breath-control device, or the image-acquisition guidance system. These devices may not be shown on simulation CT images and thus will not be considered by the software for collision estimation. We are making ongoing efforts to improve the functionality of the software to enable the incorporation of other in-room systems after CT simulation. Finer mesh grid may need to be established if the couch motion scale of the linear accelerator could be less than one degree. Notably, our system performs only collision prediction; it is not capable of spatially optimized beam selection for organ-sparing dosimetric purposes. In future work, we intend to integrate the target/OAR contours into the calculation process of the software with overlap metrics to generate noncolliding beam configurations for the spatial separation of OARs from targets and enhance advantageous dosimetric plan optimization.
In summary, our software is a convenient, vendor-independent, and high-performance tool for predicting gantry-couch and gantry-patient collisions using simulation CT images. It may help practitioners select noncolliding beam configurations and prevent unexpected mechanical collisions and treatment delays.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the National Taiwan University Hospital Research Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Y-JW was responsible for drafting the article and for analysis and interpretation of the data. J-SY was involved in model building, data acquisition, and analysis of the data. J-SY, FL, and JC were involved in the interpretation of the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.617007/full#supplementary-material
Supplementary Video 1 | Operating process including step 1. select the accelerator, step 2. open simulation DICOM files, step 3. select the specific couch position, and step 4. non-collided beam angles automatically checked was described.
References
1. Yorke E, Gelblum D, Ford E. Patient safety in external beam radiation therapy. Am J Roentgenology (2011) 196:768–72. doi: 10.2214/AJR.10.6006
2. Dong P, Lee P, Ruan D, Long T, Romeijn E, Yang Y, et al. 4π non-coplanar liver SBRT: a novel delivery technique. Int J Radiat Oncology• Biol Phys (2013) 85:1360–6. doi: 10.1016/j.ijrobp.2012.09.028
3. Derycke S, Van Duyse B, De Gersem W, De Wagter C, De Neve W. Non-coplanar beam intensity modulation allows large dose escalation in stage III lung cancer. Radiotherapy Oncol (1997) 45:253–61. doi: 10.1016/S0167-8140(97)00132-1
4. Yu VY, Tran A, Nguyen D, Cao M, Ruan D, Low DA, et al. The development and verification of a highly accurate collision prediction model for automated noncoplanar plan delivery. Med Phys (2015) 42:6457–67. doi: 10.1118/1.4932631
5. Cardan RA, Popple RA, Fiveash J. A priori patient-specific collision avoidance in radiotherapy using consumer grade depth cameras. Med Phys (2017) 44:3430–6. doi: 10.1002/mp.12313
6. Felsenstein J. Cases in which parsimony or compatibility methods will be positively misleading. Systematic zoology (1978) 27:401–10. doi: 10.1093/sysbio/27.4.401
7. Becker SJ. Collision indicator charts for gantry-couch position combinations for Varian linacs. J Appl Clin Med Phys (2011) 12:16–22. doi: 10.1120/jacmp.v12i3.3405
8. Kessler ML, McShan DL, Fraass BA. A computer-controlled conformal radiotherapy system. III: Graphical simulation and monitoring of treatment delivery. Int J Radiat Oncology• Biology• Phys (1995) 33:1173–80. doi: 10.1016/0360-3016(95)02045-4
9. Nioutsikou E, Bedford JL, Webb S. Patient-specific planning for prevention of mechanical collisions during radiotherapy. Phys Med Biol (2003) 48:N313. doi: 10.1088/0031-9155/48/22/N02
10. Hamza-Lup FG, Sopin I, Zeidan O. Online external beam radiation treatment simulator. Int J Comput Assisted Radiol Surg (2008) 3:275–81. doi: 10.1007/s11548-008-0232-7
11. Nguyen SM, Chlebik AA, Olch AJ, Wong KK. Collision Risk Mitigation of Varian TrueBeam Linear Accelerator With Supplemental Live-View Cameras. Pract Radiat Oncol (2019) 9:e103–9. doi: 10.1016/j.prro.2018.07.001
12. Glaser S, Warfel B, Price J, Sinacore J, Albuquerque K. Effectiveness of virtual reality simulation software in radiotherapy treatment planning involving non-coplanar beams with partial breast irradiation as a model. Technol Cancer Res Treat (2012) 11:409–14. doi: 10.7785/tcrt.2012.500256
13. Suriyakumar VM, Xu R, Pinter C, Fichtinger G. Open-source software for collision detection in external beam radiation therapy, Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling. Int Soc Optics Photonics (2017) 101315:101351G. doi: 10.1117/12.2255644
14. Yu L, Bai J, Ni C. Real-time Perception of Patient Space for Collision Avoidance in Radiation Treatmet. In: 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES). Sarawak, Malaysia: IEEE, Conference (2018). p. 629–32. doi: 10.1109/IECBES.2018.8626739
15. Sunderland K, Woo B, Pinter C, Fichtinger G. Reconstruction of surfaces from planar contours through contour interpolation, Medical Imaging 2015: Image-Guided Procedures, Robotic Interventions, and Modeling. Int Soc Optics Photonics (2015) 9415:94151R. doi: 10.1117/12.2081436
16. Moustakas K, Tzovaras D, Strintzis MG. SQ-Map: Efficient layered collision detection and haptic rendering. IEEE Trans Visualization Comput Graphics (2006) 13:80–93. doi: 10.1109/TVCG.2007.20
17. Mann TD, Ploquin NP, Gill WR, Thind KS. Development and clinical implementation of eclipse scripting-based automated patient-specific collision avoidance software. Clin Med Physics (2019) 12–9. doi: 10.1002/acm2.12673
18. Hueso-González F, Wohlfahrt P, Craft D, Remillard K. An open-source platform for interactive collision prevention in photon and particle beam therapy treatment planning. Biomed Phys Eng Express (2020) 6:055013. doi: 10.1088/2057-1976/aba442
19. Boudier G, Gicquel L, Poinsot T. Effects of mesh resolution on large eddy simulation of reacting flows in complex geometry combustors. Combustion Flame (2008) 155:196–214. doi: 10.1016/j.combustflame.2008.04.013
20. Robertini N, Casas D, De Aguiar E, Theobalt C. Multi-view performance capture of surface details. Int J Comput Vision (2017) 124:96–113. doi: 10.1007/s11263-016-0979-1
Keywords: radiotherapy, collision, noncoplanar, beam angle, software
Citation: Wang Y-J, Yao J-S, Lai F and Cheng JC-H (2021) CT-Based Collision Prediction Software for External-Beam Radiation Therapy. Front. Oncol. 11:617007. doi: 10.3389/fonc.2021.617007
Received: 15 October 2020; Accepted: 26 January 2021;
Published: 11 March 2021.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Giuseppe Felici, Sordina IORT Technologies S.p.A., ItalySebastiaan Breedveld, Erasmus University Medical Center, Netherlands
Copyright © 2021 Wang, Yao, Lai and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jason Chia-Hsien Cheng, amFzb25jaGVuZ0BudHUuZWR1LnR3
 Yu-Jen Wang
Yu-Jen Wang Jia-Sheng Yao
Jia-Sheng Yao Feipei Lai
Feipei Lai Jason Chia-Hsien Cheng
Jason Chia-Hsien Cheng