- 1Department of Medical Oncology, West German Cancer Center, University Hospital Essen, Essen, Germany
- 2Institute of Pathology, West German Cancer Center, University Hospital Essen, Essen, Germany
- 3Department of Otorhinolaryngology, West German Cancer Center, University Hospital Essen, Essen, Germany
- 4Department of Radiation Oncology, West German Cancer Center, University Hospital Essen, Essen, Germany
- 5German Cancer Consortium (DKTK), Essen, Partner Site University Hospital Essen, Essen, Germany
- 6Department of Urology, West German Cancer Center, University Hospital Essen, Essen, Germany
Cetuximab-based chemoimmunotherapy has been the standard of care for recurrent or metastatic squamous cell carcinoma of the head and neck (r/m SCCHN) for more than a decade. To date, no predictive or prognostic biomarkers have been established to further guide the systemic treatment with cetuximab-based chemoimmunotherapy in r/m SCCHN. Against this background, we retrospectively analyzed clinical and blood-based parameters from 218 r/m SCCHN patients treated with chemoimmunotherapy including cetuximab. Multivariate Cox-regression models were used to assess their prognostic or predictive value. Eastern Co-operative Oncology Group (ECOG) performance status (≥2), older age (≥61.8 years), anemia (hemoglobin <11.80), and increased neutrophil-to-lymphocyte ratio (NLR ≥5.73) were independently and strongly associated with inferior overall survival (OS). To group patients according to risk profiles we established a prognostic clinical score (PCS) that can easily be used in clinical practice. The PCS stratified the cohort into low, intermediate, poor or very poor risk subgroups with median OS times of 23.4, 12.1, 7.5, and 4.0 months, respectively. Patients with low risk PCS had a prolonged progression-free survival (PFS) and increased overall response rate (ORR) under first-line cetuximab-based therapy. Interestingly, only patients with low and intermediate risk benefitted from the more intensive first-line cisplatin/cetuximab combination compared to carboplatin/cetuximab therapy, whereas the intensity of first-line treatment had no impact in the poor and very poor risk subgroups. Following external validation, particularly in the context of newly established first-line options, the PCS may guide clinical decision making and serve for stratification of patients with r/m SCCHN in future clinical trials.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most common cancer worldwide with more than 600,000 death annually. Platinum-based systemic chemotherapy in combination with the monoclonal antibody cetuximab, targeting the epidermal growth factor receptor (EGFR) has been the standard of care for recurrent or metastatic (r/m) SCCHN for over a decade (1, 2). Recently, the immune checkpoint inhibitors (CPIs) nivolumab and pembrolizumab have changed the therapeutic landscape of patients with r/m SCCHN. Nivolumab is considered as therapeutic standard after failure of platinum-based therapy based on the results of the phase III Checkmate-141 trial (3). In addition, pembrolizumab has become the new standard in the first-line setting in combination with platinum/fluorouracil chemotherapy or as monotherapy for patients with programmed cell death ligand 1 (PD-L1) positive tumors based on the data from the phase III Keynote-048 trial (4). However, response rate and progression-free survival upon CPI therapy in patients with r/m SCCHN are still disappointing and most patients will receive cetuximab and/or cytotoxic drugs in further lines (5). So far, no prognostic or predictive biomarkers have been established to stratify the systemic treatment of patients with r/m SCCHN and to identify patients with the highest likelihood of response to cetuximab (6). Ideal biomarkers are easily accessible, cost-effective and can reproducibly discriminate patients with different risks. Clinical and laboratory routine parameters could help to establish nomograms or prognostic clinical scores (PCS) to identify patients with higher chance of response or higher risk of progression upon different therapies. For example, the Eastern Co-operative Oncology Group performance status (ECOG) or the age has been correlated with a poor prognosis in a variety of cancer entities (7–9). In addition, systemic inflammatory response (SIR) parameters like the C-reactive protein (CRP), the neutrophil–lymphocyte ratio (NLR), anemia and others have been correlated with the risk of recurrence and overall survival in curative treated malignancies (9–11). The prognostic impact of these blood based SIR markers was also validated in patient receiving palliative systemic chemo- and immunotherapy (12, 13).
Against this background, we tested whether clinical and blood-based parameters had prognostic and predictive value in patients with r/m SCCHN treated with cetuximab-based therapy. The identified prognostic markers were combined to develop a new prognostic clinical score (PCS), which stratifies patients into four different risk groups. In addition, the PCS characterizes a group of patients with a higher likelihood for response and prolonged survival due to cisplatin/cetuximab-based therapy.
Patients and Methods
Study Design
Patients with r/m SCCHN treated with cetuximab-based systemic therapy between October 2006 and February 2018 at the West German Cancer Center (WTZ), University Hospital Essen, were retrospectively enrolled into this study. Follow-up was routinely assessed and documented in the electronic health record (EHR). Clinical and routinely assessed blood-based parameters, administered therapies and radiological response parameters were also retrieved from the EHR. The data cut-off for follow-up was October 1, 2019. Statistical and correlative analyses were performed using SPSS Statistics (V26, IBM, Armonk, NY, USA) and MS Excel 2010 (VS 14.0, Microsoft, Richmond, WA, USA). The study was approved by the local Ethics Committee of the Medical Faculty of the University Duisburg-Essen (Az 13-5486-BO).
Assessments and Statistical Analysis
Routine staging procedures included computed tomography (CT) scan and/or magnetic resonance imaging (MRI) of the head and neck, a thoracic CT and a CT of the abdomen and/or abdominal ultrasound. Initial staging was performed according to the seventh edition (2010) of the International Union Against Cancer (UICC) TNM classification. In the recurrent or metastatic setting patients were radiologically examined routinely every 6–8 weeks during the palliative therapy according to the institutional guidelines of the West German Cancer Center. Overall response rate was evaluated according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) (14, 15). Response assessment was feasible if at least one baseline CT or MRI and one follow-up imaging was available. Overall survival was defined as time from first administration of systemic treatment to death from any cause. Patients were censored at the time of last follow-up, if time point of death was not evaluable. Progression-free survival (PFS) was defined as time from start of therapy to date of radiologic or clinical progression or death. For survival analyses Kaplan–Meier method and log-rank test were used. For univariate and multivariate analyses of blood-based and clinical parameters a Cox proportional-hazard model with Hazard ratios (HR) and 95% confidence intervals (CI) were applied. Differences in overall response were dissected using the chi-square test. Overall, P-values ≤0.05 were regarded statistically significant.
Clinical- and Blood-Based Parameters
Based on a review of the literature, the following five blood-based parameters including markers of systemic-inflammatory response (SIR) were assessed: C-reactive protein (CRP), neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), platelet–lymphocyte ratio (PLR) and anemia (hemoglobin—HB). The three analyzed clinical markers included age, ECOG performance status and the extent of disease (local disease only vs metastatic disease). All parameters were assessed up to five days before the administration of the first palliative chemotherapy. For explorative analyses, we choose the median as cut-off for all blood-based parameters. We also used the median as cut-off for the clinical parameter age. For ECOG we distinguished between ECOG PS 0–1 and ≥2 and for the extent of disease we differentiated between patients with local disease only or metastatic disease.
Results
Patients’ Characteristics
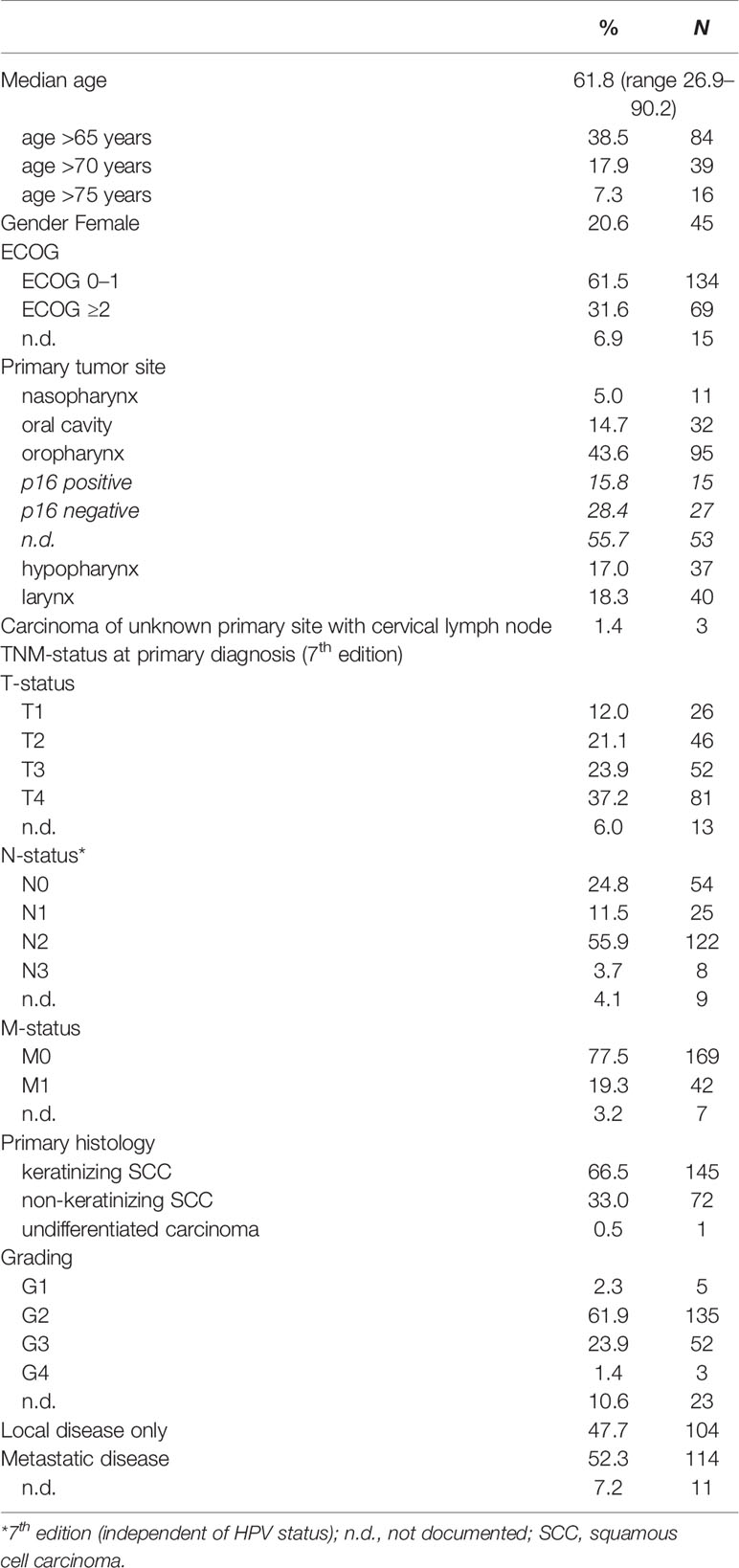
A total of 218 patients with r/m SCCHN treated with cetuximab-based chemoimmunotherapy were enrolled into this study. Baseline clinical characteristics are listed in Table 1. The median age was 61.8 years (range 26.9–90.2). Notably, 39 patients (17.9%) were older than 70 years at start of the palliative therapy and 69 patients (31.6%) had an ECOG performance status of 2 or higher. Most patients were male (N = 173, 79.4%). One patient had an undifferentiated carcinoma (0.5%), all other had a keratinizing (N = 145, 66.5%) or non-keratinizing squamous cell carcinoma (N = 72, 33.0%). The main primary tumor site was the oropharynx (N = 95, 43.6%) followed by larynx (N = 40, 18.3%), hypopharynx (N = 37, 17.0%) and oral cavity (N = 32, 14.7%), respectively. In total, 42 patients (19.3%) presented with metastases, mostly in the lung at time point of initial diagnosis. At start of palliative therapy, 114 patients (52.3%) had distant metastasis and 104 (47.7%) had local recurrence only.
Therapy
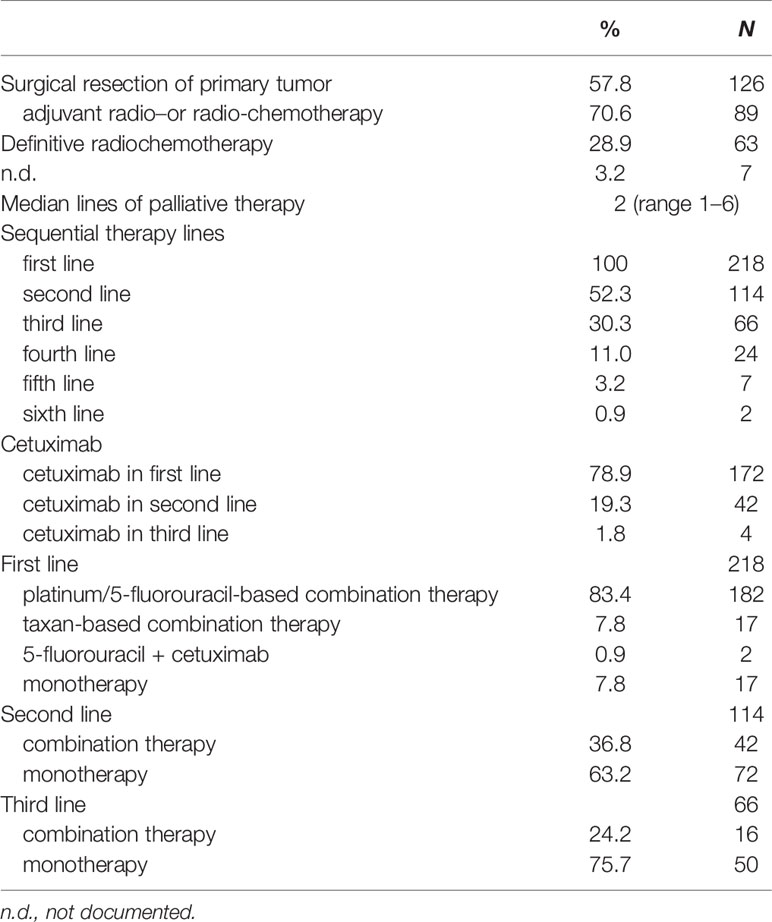
The primary tumor was resected in 126 patients (57.8%) at time point of initial diagnosis, 89 patients of these (70.6%) received an adjuvant or additive radio- or radio-chemotherapy (Table 2). In total, 63 patients (28.9%) underwent definitive radio-chemotherapy. In the recurrent or metastatic setting all patients were treated with a palliative systemic therapy. The majority of patients (N = 114, 52.3%) received second-line systemic therapy and a substantial number of patients (N = 66, 30.3%) underwent at least third- or further line therapies. Cetuximab-based therapy was administered in 172 patients (78.9%) in the first-line setting and in 42 patients (19.3%) in second-line. Nearly all patients (N = 199, 91.3%) received platinum-based therapies (cisplatin or carboplatin) in combination with 5-fluorouracil (N = 182, 83.4%) or a taxane (N = 17, 7.8%) in first-line.
Outcome
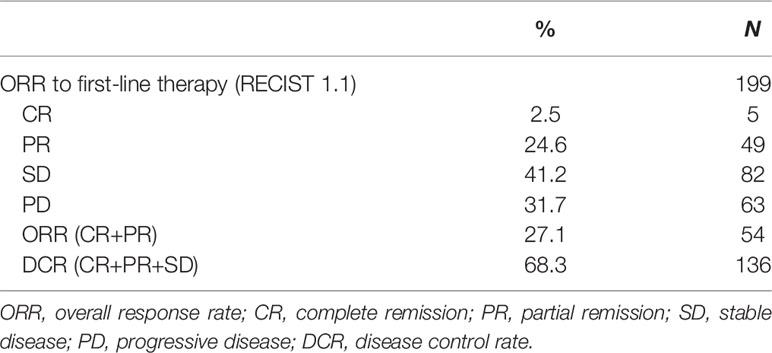
Median overall survival (OS) from start of first-line therapy was 11.5 month (95% CI 9.9–13.2) (Figure 1). Interestingly, there was no statistically significant difference in OS for patients who received cetuximab in first line compared with those patients who received cetuximab in second or third line (11.0 months vs 12.1 month, HR 1.06, 95% CI 0.73–1.53, P = 0.557) (Figure 2). In total 199 patients (91.3%) were evaluable for response according to RECIST 1.1. Overall response rate (ORR) was 27.1% under first-line therapy, including five patients (2.5%) with complete remission (CR) and the disease control rate (DCR) was 68.3% (Table 3). The first-line progression-free survival (PFS) was 2.6 month (95% CI 2.3–2.9).
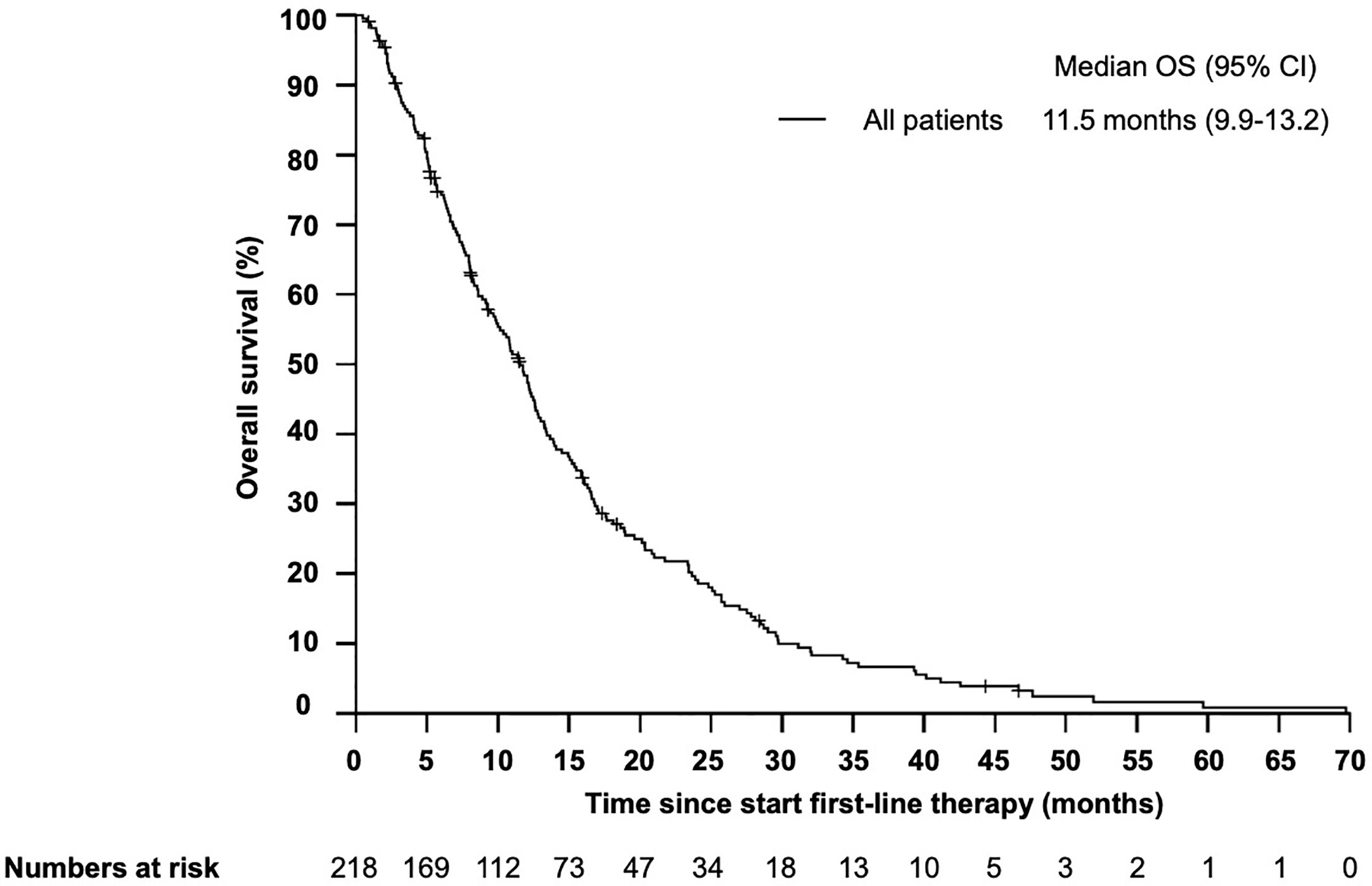
Figure 1 Kaplan–Meier plot for overall survival (OS) in patients with r/m SCCHN from start of first-line therapy. The median overall survival was 11.5 months (95% CI 9.9–13.2 months).
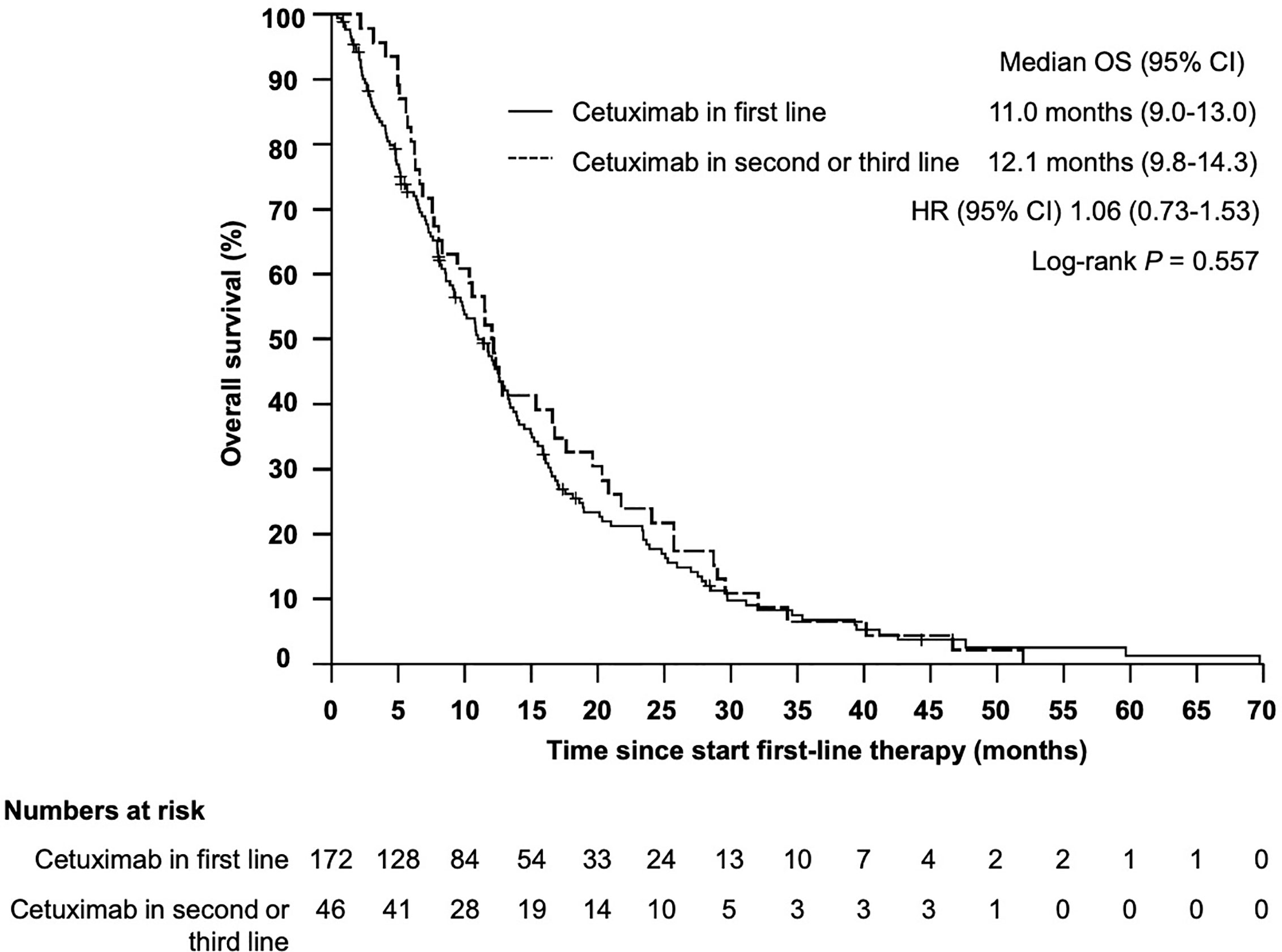
Figure 2 Kaplan–Meier plot for overall survival (OS) from start of first-line therapy for patients receiving cetuximab in first vs cetuximab in second or third line. The median overall survival was 11.0 months (95% CI 9.0–13.0 months) vs 12.1 months (95% CI 9.8–14.3), P = 0.557.
Explorative Prognostic Clinical- and Blood-Based Parameters
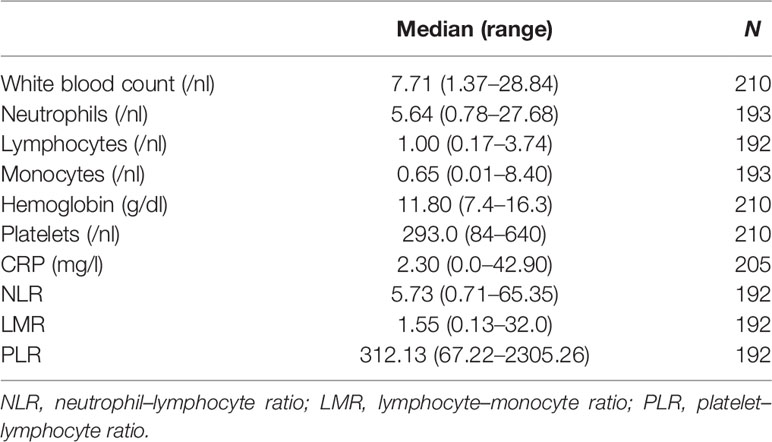
Based on a review of the literature, eight parameters were evaluated as potential prognostic markers of survival outcomes: the blood-based parameters C-reactive protein (CRP), neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), platelet–lymphocyte ratio (PLR) and anemia (hemoglobin—HB) and the clinical factors age, ECOG performance status and the extent of disease (local disease only vs metastatic disease). For all blood-based parameters, the median was chosen as cut-off, as listed in Table 4.
First, the impact of all eight parameters on OS were analyzed in exploratory univariate analyses (Table 5). Here, we identified anemia, CRP, NLR, LMR and ECOG as prognostic factors for overall survival. Afterwards, we performed a multivariate Cox proportional-hazard analysis including all eight parameters to verify the independence of the identified prognostic markers. In this multivariate analysis, only anemia, NLR, age and ECOG status significantly correlated with OS (P < 0.05) and were therefore considered as independent prognostic markers (Figure 3).

Table 5 Median overall survival from start of first-line therapy and hazard ratio (univariate analysis).
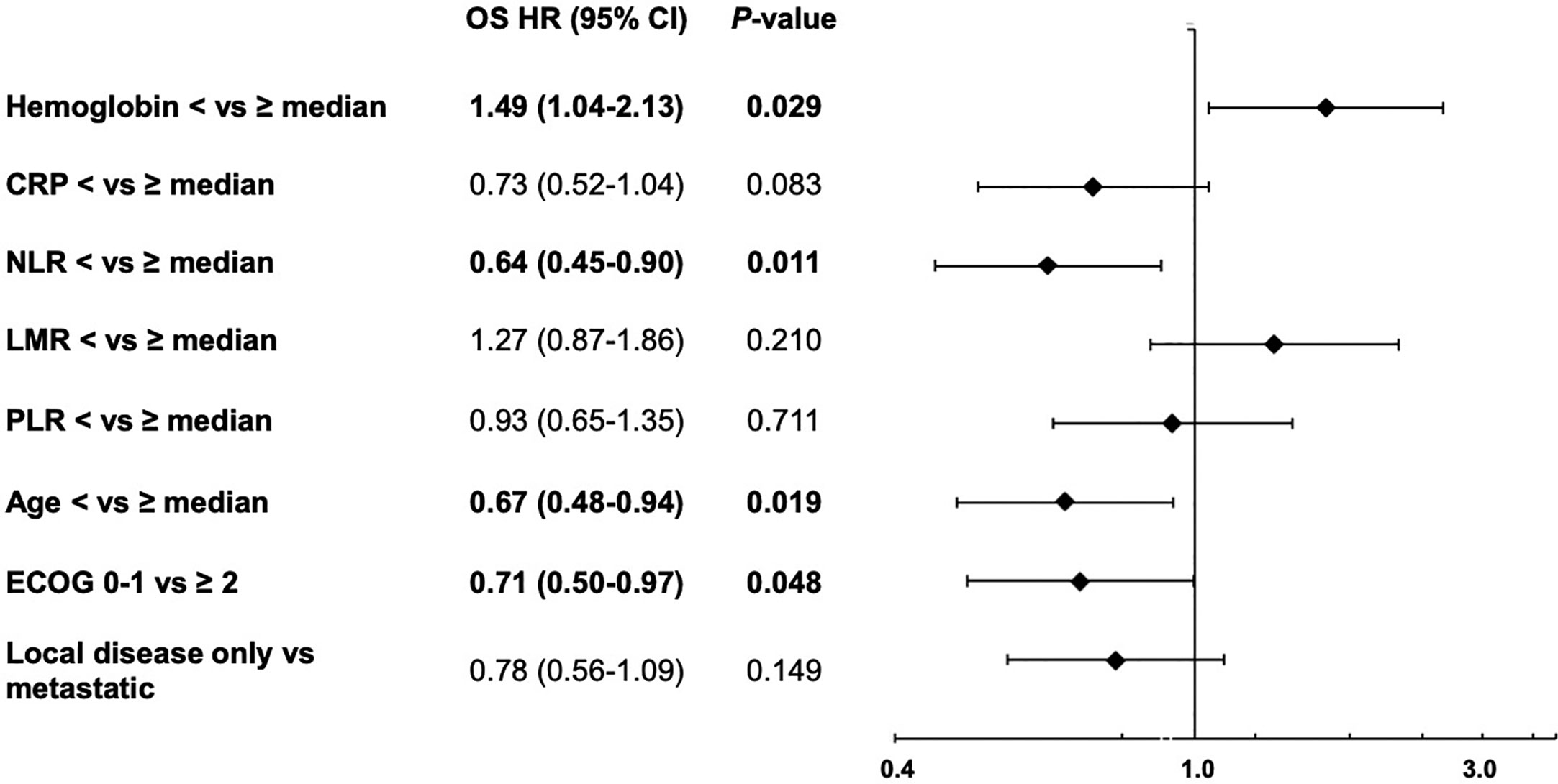
Figure 3 Forest plot for the multivariate analysis of overall survival from start of first-line therapy, including hemoglobin, C-reactive protein (CRP), neutrophil/lymphocyte ratio (NLR), lymphocyte/monocyte ratio (LMR), platelets/lymphocyte ratio (PLR), age, ECOG performance status and extend of disease (local disease only vs metastatic).
Prognostic Clinical Score
Based on the four identified independent prognostic markers, we establish a prognostic model (prognostic clinical score—PCS) for survival upon chemoimmunotherapy including cetuximab for r/m SCCHN. Due to a comparable effect size for each marker, one point was allocated for each positive factor. A marker was considered positive if the following condition was met: NLR ≥5.73; hemoglobin <11.80; age ≥61.8; ECOG ≥2. Next, we stratified patients into four groups (0, 1–2, 3, and 4 points) and correlated the different groups with the OS (Figure 4). Thereby, we identified patients with a favorable prognosis (PCS = 0, median OS 23.4 months, 95% CI 12.0 - 34.9), an intermediate prognosis (PCS = 1–2, median OS 12.1 months, 95% CI 10.3–13.8), a poor prognosis (PCS = 3, median OS 7.5 months, 95% CI 6.1–8.9) and a very poor prognosis (PCS = 4, median OS 4.0 months, 95% CI 2.0–6.1 months), P < 0.001. Each risk group hat a statistically significant different prognosis: HR for low vs intermediate risk was 0.434 (95% CI 0.246–0.765; P = 0.004); HR for intermediate vs poor risk was 0.552 (95% CI 0.365–0.834; P = 0.005); and the HR for poor vs very poor risk was 0.471 (95% CI 0.219–0.998; P = 0.046). Next, to exclude potential bias, we evaluated the PCS in the homogenous subgroup of patients which received cetuximab in first line and for whom all clinical-and blood-based parameters where available (N = 146). In this subgroup the prognostic value of the PCS could be confirmed (Figure 5, P < 0.001).
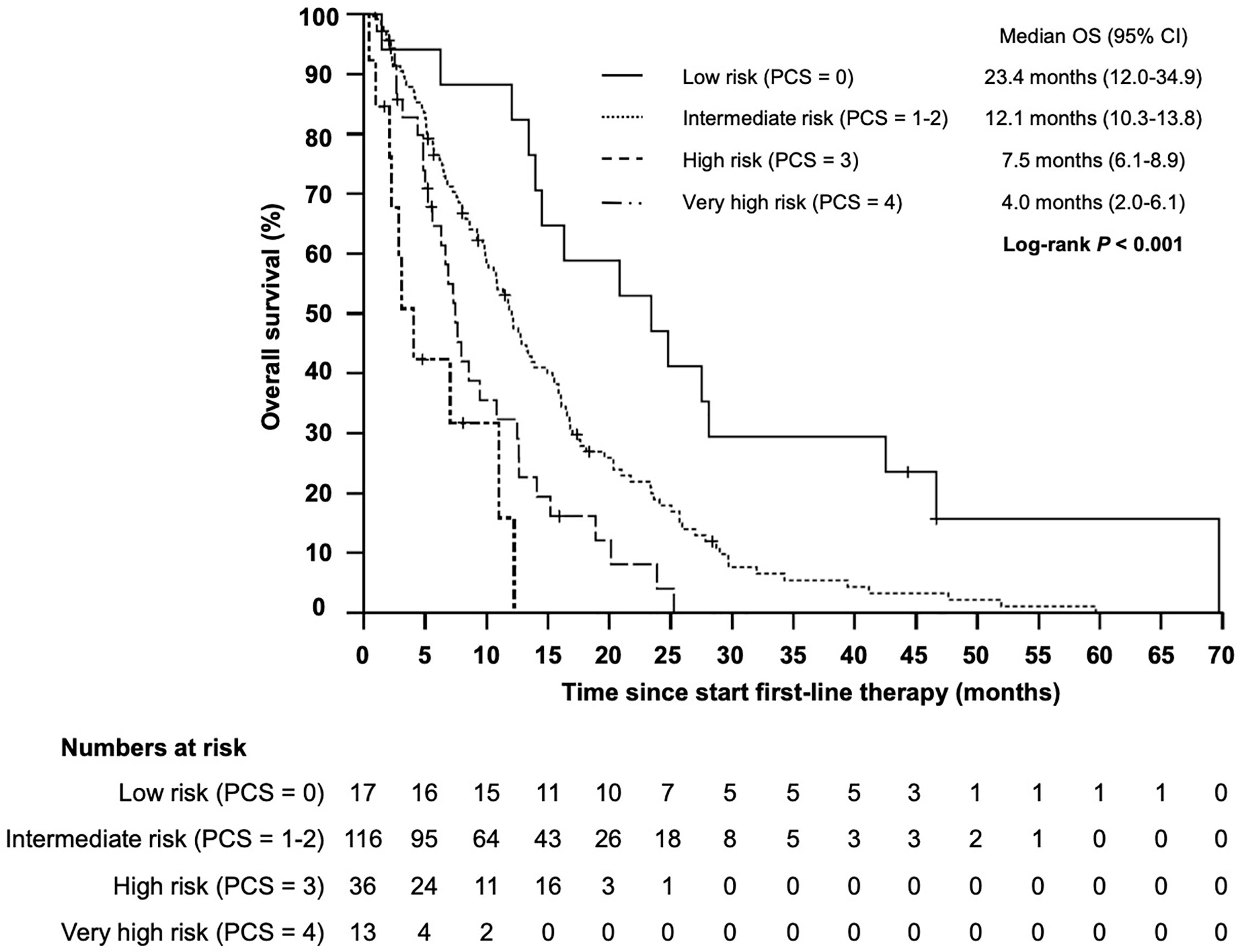
Figure 4 Kaplan–Meier plot for overall survival (OS) according to the prognostic clinical score (PCS) from start of first-line therapy for low (PCS = 0 points), intermediate (PCS = 1–2 points), high (PCS = 3 points), and very high risk (PCS = 4 points) groups (P < 0.001).
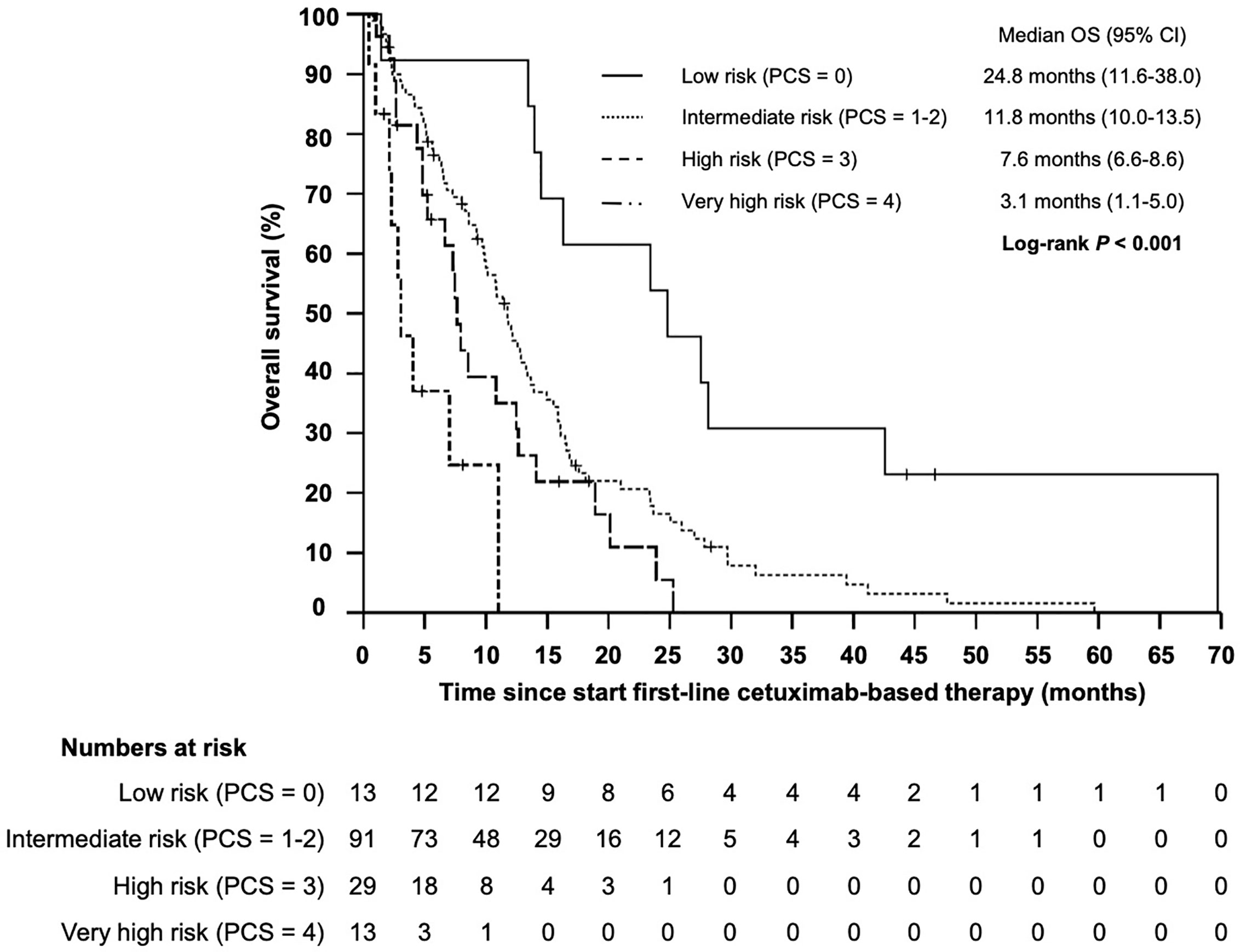
Figure 5 Kaplan–Meier plot for overall survival (OS) according to the prognostic clinical score (PCS) from start of cetuximab-based first-line therapy for low (PCS = 0 points), intermediate (PCS = 1–2 points), high (PCS = 3 points) and very high risk (PCS = 4 points) groups (P < 0.001).
Impact of Clinical- and Blood-Based Parameters on PFS and ORR Upon Cetuximab
The median progression-free survival (PFS) in patients who received cetuximab-based first-line therapy was 3.5 months (95% CI 2.8–4.2) (Figure 6). Comparable to the OS, median PFS differed significantly between patients with different PCS. Patients in the low risk group had a median PFS of 5.6 months, whereas patients in the very high risk group only had a median PFS of 0.7 months (Figure 7, P = 0.01). In line, patients with low risk PCS had a favorable overall response rate (ORR) of 69.2% and a disease control rate (DCR) of 100%, whereas no objective response was observed in the very high risk group (Figure 8).
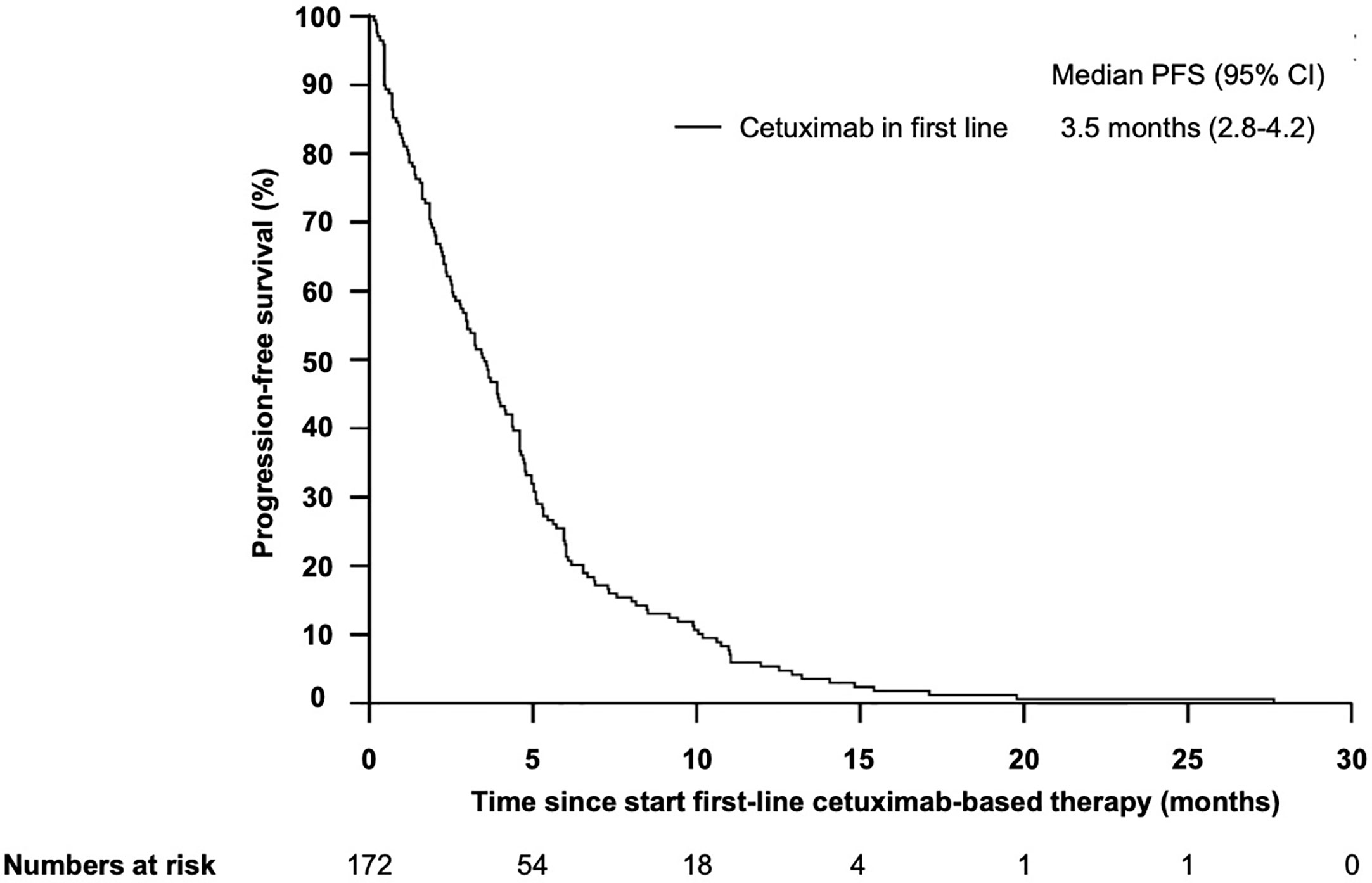
Figure 6 Kaplan–Meier plot for progression-free survival (PFS) for patients with r/m SCCHN from start of first-line cetuximab-based therapy. The median PFS was 3.5 months (95%CI 2.8–4.2).
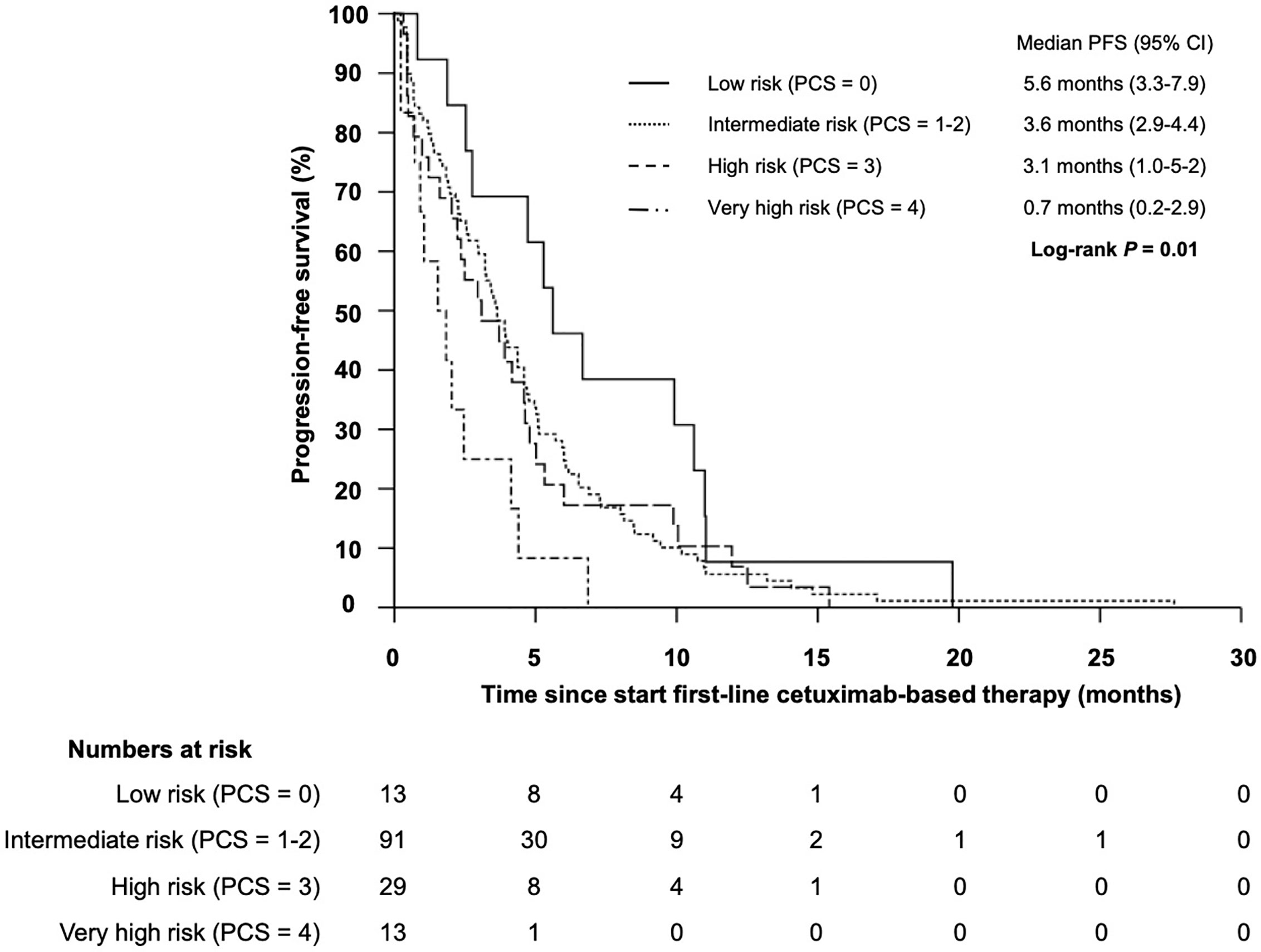
Figure 7 Kaplan–Meier plot for progression-free survival (PFS) for patients with r/m SCCHN from start of first-line cetuximab-based therapy according to the prognostic clinical score for low (PCS = 0 points), intermediate (PCS = 1–2 points), high (PCS = 3 points) and very high risk (PCS = 4 points) groups (log-rank P = 0.01).
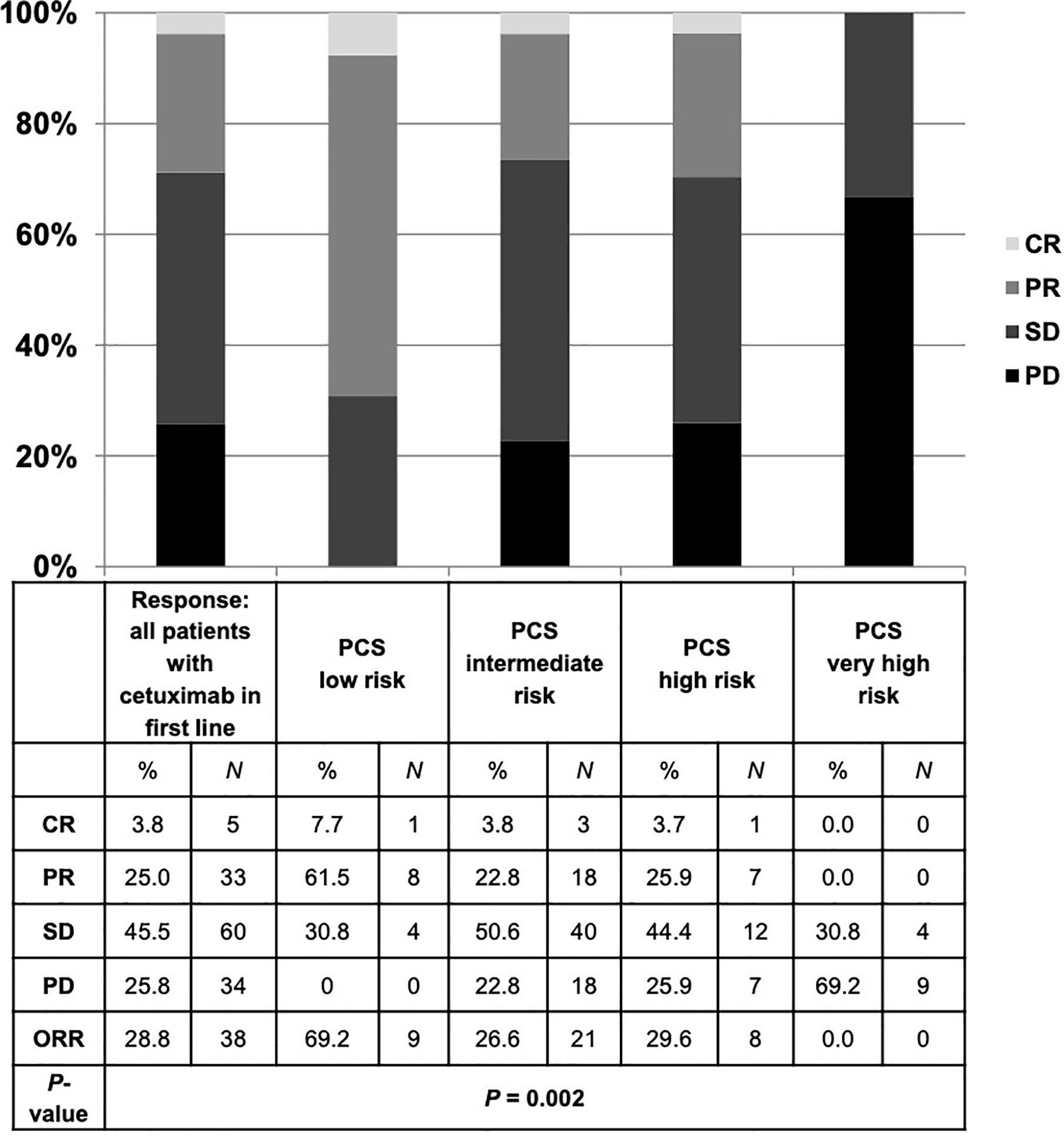
Figure 8 Best response to cetuximab-based first-line therapy for all patients and in each risk group of the prognostic clinical score (PCS). Patients with low risk PCS had a favorable overall response rate (ORR) of 69.2% and disease control rate (DCR) of 100%, whereas no objective response was detected in the very high risk group. CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Impact of Cisplatin- or Carboplatin-Based First-Line Therapy
To address the question, if the poor prognosis of patients with a high PCS could be overcome by a more intensive chemotherapy, we performed a subgroup analysis in patients which received cisplatin/cetuximab or carboplatin/cetuximab in first line. Patients who received cisplatin/cetuximab-based therapy had a significant prolonged OS compared to patients treated with carboplatin/cetuximab (13.7 months vs 9.5 months, HR 0.487, 95% CI 0.329–0.720, P < 0.001). However, only patients in the good and intermediate prognosis group (PCS 0-2) benefitted from the more intensive cisplatin-based therapy (28.1 vs 16.3 months for PCS 0, P = 0.034 and 13.7 vs 10.2 months for PCS 1-2, P = 0.005). In contrast, patients in the poor and very poor prognosis group had a dramatically shorter median OS irrespectively of cisplatin- or carboplatin-based first-line therapy (Figure 9).
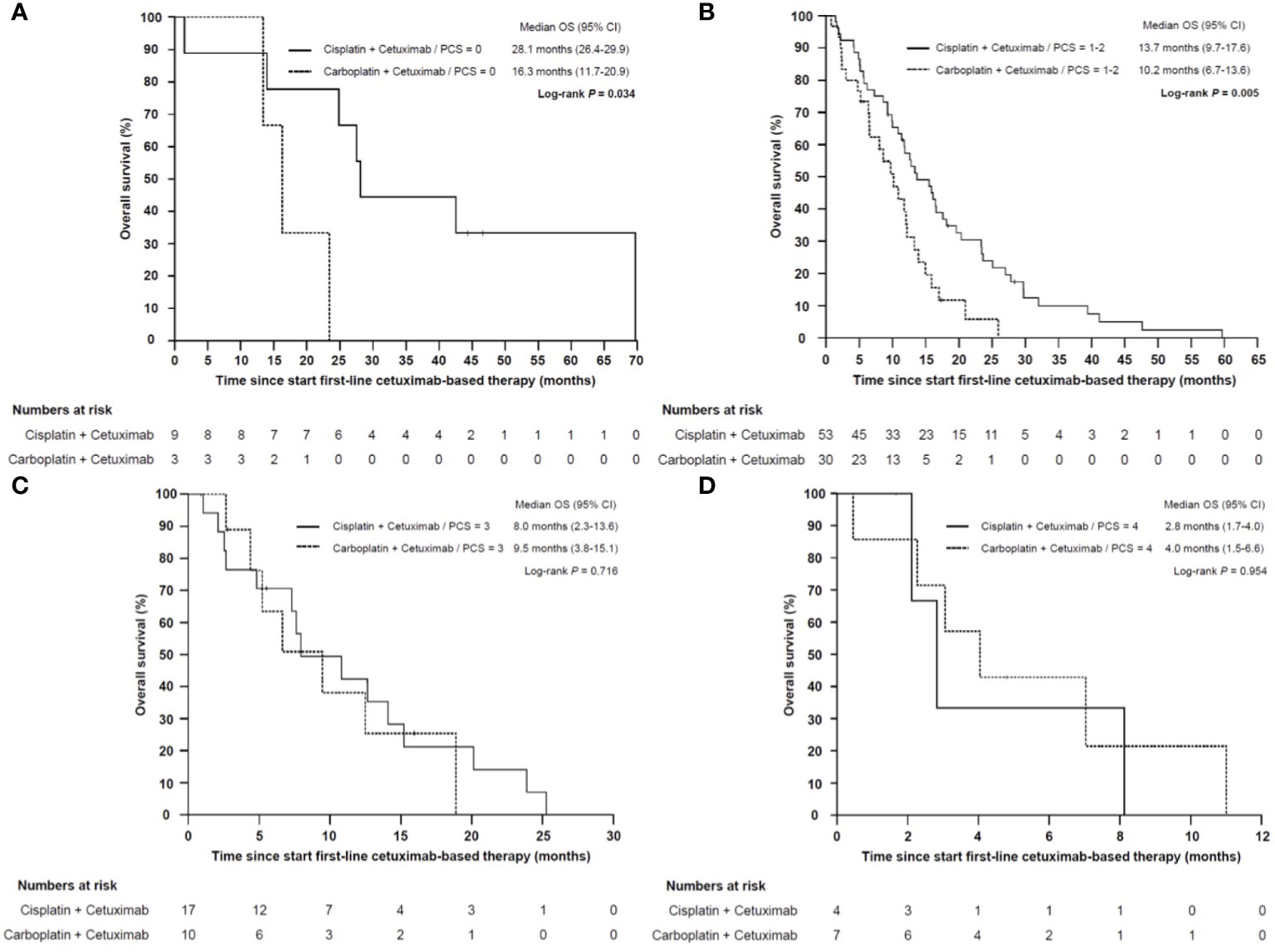
Figure 9 Kaplan–Meier plot for overall survival (OS) from start of cisplatin/cetuximab- vs carboplatin/cetuximab-based first-line therapy in low (A), intermediate (B), high (C) and very high risk (D) sub-groups. (A) Patients with low (PCS = 0) or (B) intermediate risk (PCS = 1–2) who were treated with cisplatin/cetuximab had a statistically significant prolonged overall survival compared to carboplatin/cetuximab (P = 0.034 and P = 0.005, respectively). (C) In high risk (PCS = 3) and (D) very high risk subgroups (PCS = 4) the prognosis remained poor regardless whether cisplatin or carboplatin was administered (P = 0.716 and P = 0.954, respectively).
Discussion
Cetuximab-based chemotherapy has been the standard first-line therapy for patients with r/m SCCHN for the last decade and it is still a standard option for patients who progressed on or are not candidates for immunotherapy with checkpoint inhibitors (1). However, prognostic or predictive biomarkers for patients treated with cetuximab are still missing. In this study, we aimed to identify clinical and routinely assessed blood-based and clinical biomarkers for the efficacy of cetuximab-based chemoimmunotherapies in recurrent or metastatic disease. We focused on markers on inflammation and immune response, which are two essential hallmarks of cancer (3, 4, 16, 17). In our retrospective study, 218 patients with r/m SCCHN were included who received at least one cycle of palliative chemotherapy in combination with cetuximab, irrespectively of ECOG performance status, comorbidities, age, abnormal blood values or previous therapy. The median OS in our unselected patient population was 11.5 months which were highly comparable with the results of the pivotal EXTREME trial (median OS 10.1 months) or the patients population treated with the EXTREME regimen in the recently published KEYNOTE-048 study (median OS 10.7 months) (1, 4). Although our cohort represents a real-world population with nearly 20% of patients being older than 70 years and one third of patients with an ECOG performance status ≥2, the median OS was markedly longer than those reported in comparable retrospective studies like the GLANCE H&N (5). This finding could possibly be explained by the relative high number of patients receiving second and further lines therapies at our center.
Absolute neutrophil, monocyte and platelet counts, CRP, and anemia are easily accessible blood-based biomarkers to assess the systemic inflammation. In addition, lower lymphocyte count may be associated with immunodeficiency and could have an impact on the efficacy of immunomodulating agents like the monoclonal antibody cetuximab, which has the capability to mediate antibody dependent cellular cytotoxicity (ADCC) (18). By using ratios of these different blood parameters like the neutrophil–lymphocyte ratio (NLR) or the lymphocyte–monocyte ratio (LMR) the extent of the systemic inflammation and the competence of the immune system could be easily assessed. The NLR has been previously linked to a higher risk of recurrence after curative resection in locally SCCHN (19). Moreover, it has been described as a prognostic marker for a wide variety of other malignancies treated with palliative immunotherapy or chemotherapy (12, 13). In our patient population, a higher NLR was an independent prognostic marker for OS in the recurrent or metastatic disease upon palliative treatment. Here, we chose the median NLR of 5.73 as a cut-off. In previous published studies in patients with early and advanced stage SCCHN, cut-offs between 1.29 and 6.0 have been proposed (20). We additionally tested NLR cut-offs of 4, 5 and 6, which all discriminated patients with good and poor prognosis (data not shown). This is in line with a large meta-analysis published by Cho et al., 2018 (20). Thus, the NLR seems to be a continues instead of a simple dichotomous biomarker (21).
Cancer-related anemia is common in a wide variety of malignancies. Up to 60% of SCCHN patients present with reduced hemoglobin levels at initial diagnosis (22). Cytokine-mediated systemic inflammation with increased interleukin-1 and -6 levels and nutritional and metabolic alterations contribute to this multifactorial paraneoplastic syndrome (23). The prognostic significance of pre-treatment cancer related anemia has been demonstrated for curative treated SCCHN patients (9, 24, 25). In our cohort of palliative treated r/m SCCHN patients, we chose the median hemoglobin level (11.8 mg/dl) as cut-off for further analyses. We could clearly demonstrate that anemia was independently associated with a reduced OS in our cohort. This is in contrast to a previous study published by Magnes et al. in a smaller cohort of r/m SCCHN patients (26). However, a different cut-off and heterogeneous patient’s characteristics or therapeutic strategies could have affected the results.
Recently, pembrolizumab has been approved as monotherapy and in combination with platinum-based chemotherapy in first-line treatment of PD-L1 positive r/m SCCHN (4). Moreover, the TPEx protocol with cisplatin, docetaxel and cetuximab has emerged as a highly effective and less toxic regiment than the EXTREME protocol (27). These new options result in a broader therapeutic armamentarium in the first-line setting. However, predictive or prognostic biomarkers for the first-line treatment in r/m SCCHN are still missing and are urgently needed to identify the best therapeutic option for each patient. To this end, we developed an easy-to-use prognostic clinical scoring (PCS) system based on our identified independent clinical- and blood-based biomarkers. In the EXTREME trial, sub-group analysis suggested a benefit in median OS only for cetuximab in combination with cisplatin, but not for the combination with carboplatin (1). In line, we found a significant higher OS in patients treated with cisplatin/cetuximab compared to carboplatin/cetuximab in our real-world cohort. Interestingly, only patients in our new established good and intermediate PCS benefited from a cisplatin/cetuximab-based therapy compared to carboplatin/cetuximab. In contrast, patients with a poor (PCS = 3) and very poor prognosis (PCS = 4) had a dramatic reduced OS irrespectively of the type of first-line therapy.
Our analysis has some important limitations. The data were collected retrospectively and all patients were treated at a single institution. The decision of first- and further line therapies were made individually by the clinicians and/or patients and not according to a pre-specified protocol. This real-world scenario resulted in a more heterogeneous treatment selection with cetuximab given not always in first-line and not always in combination with platin/5-fluorouracil. We adapted to this specific limitation by testing the established PCS in the subset of patients who received cetuximab in the first-line setting.
In conclusion, we identified age, ECOG, NLR and anemia as independent prognostic factors for patients with r/m SCCHN treated with cetuximab-based therapy. By combining these four independent parameters we established a new prognostic clinical score (PCS) which could discriminate patients in groups with good (PCS = 0), intermediate (PCS = 1–2), poor (PCS = 3) and very poor (PCS = 4) prognosis. With the PCS we characterize a subgroup of patients who were most likely to respond to cetuximab-based first-line therapy, and a subgroup of patients without any response to this chemoimmunotherapy in the first-line setting. Furthermore, the PCS enables to distinguish between patients who benefit from an intensive cisplatin/cetuximab-based first-line therapy (patients with good and intermediate PCS) and those patients with poor outcome irrespectively of the selected first-line therapy (patients with poor and very poor PCS). Based on this findings, the PCS will help to guide treatment selection for patients with PD-L1 negative r/m SCCHN, patients with contraindications for CPI and patients who progressed on CPI. Cisplatin/cetuximab-based therapy should be considered as the standard first-line option for patients with a PCS of 0 or 1–2, for patients with a PCS of 3 carboplatin/cetuximab is an adequate option due to a better toxicity profile and comparable efficacy in this subgroup, and best supportive care should be considered in patients with a PCS of 4.
As a next step, we plan an external and subsequently prospective validation of the PCS in an independent patient cohort. Finally, the new proposed PCS may help in the design of risk-adapted treatment strategies in future clinical trials.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Local Ethics Committee of the Medical Faculty of the University Duisburg-Essen (Az 13-5486-BO). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SK and MP were responsible for conception and design of this study. TH, MP, SK, TG, MSt, ST, BK, KS, SL, VG, and MSc collected the data. MP, TH, and SK performed the data extraction and statistical analyses. MP and SK wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by a grant of the “Stiftung Tumorforschung Kopf-Hals” to SK. The West German Cancer Center at University Hospital Essen is supported by the Oncology Center of Excellence Program of the German Cancer Aid (grant number 110534), and the German Federal and North Rhine-Westphalian State governments as partner site of the German Cancer Consortium (DKTK).
Conflict of Interest
MSc: Consultancy: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG), Lilly, Novartis; Honoraria for CME presentations: Alexion, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Lilly, Novartis; Research funding to institution: Boehringer Ingelheim, Bristol Myers-Squibb, Novartis; Other: Universität Duisburg-Essen (Patents). SK: Consultancy, Honoraria and Travel Support: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen, Merck Serono, Sanofi Aventis, Astra Zeneca, Janssen Pharmaceuticals, Celgene, Lilly, Servier. MP: Consultancy, Honoraria and Travel Support: Boehringer Ingelheim, Bristol Myers-Squibb, Merck Healthcare KGaA, Merck Sharp & Dohme. TG: Consultancy, Honoraria and Travel Support: Astra Zeneca, Bristol Myers-Squibb, Merck Healthcare KGaA, Merck Sharp & Dohme, Roche. VG: grants, personal fees and non-financial support from Astra Zeneca, Bristol-Myers Squibb, Ipsen, Pfizer; grants and personal fees from Novartis, personal fees from Eisai, EUSAPharm, Roche, Janssen-Cilag, Merck Serono, MSD Sharp & Dohme, Lilly, PharmaMar, Asklepios Clinic, Clinic of Oldenburg, Diakonie Clinic, Dortmund Hospital, and Onkowissen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.635096/full#supplementary-material
References
1. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-Based Chemotherapy Plus Cetuximab in Head and Neck Cancer. N Engl J Med (2008) 359(11):1116–27. doi: 10.1056/NEJMoa0802656
2. Taberna M, Oliva M, Mesia R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front Oncol (2019) 9:383. doi: 10.3389/fonc.2019.00383
3. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252
4. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab Alone or With Chemotherapy Versus Cetuximab With Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet (2019) 394(10212):1915–28. doi: 10.1016/S0140-6736(19)32591-7
5. Grunwald V, Chirovsky D, Cheung WY, Bertolini F, Ahn MJ, Yang MH, et al. Global Treatment Patterns and Outcomes Among Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results of the GLANCE H&N Study. Oral Oncol (2020) 102:104526. doi: 10.1016/j.oraloncology.2019.104526
6. Galot R, Le Tourneau C, Guigay J, Licitra L, Tinhofer I, Kong A, et al. Personalized Biomarker-Based Treatment Strategy for Patients With Squamous Cell Carcinoma of the Head and Neck: EORTC Position and Approach. Ann Oncol (2018) 29(12):2313–27. doi: 10.1093/annonc/mdy452
7. Polee MB, Hop WC, Kok TC, Eskens FA, van der Burg ME, Splinter TA, et al. Prognostic Factors for Survival in Patients With Advanced Oesophageal Cancer Treated With Cisplatin-Based Combination Chemotherapy. Br J Cancer (2003) 89(11):2045–50. doi: 10.1038/sj.bjc.6601364
8. Jang RW, Caraiscos VB, Swami N, Banerjee S, Mak E, Kaya E, et al. Simple Prognostic Model for Patients With Advanced Cancer Based on Performance Status. J Oncol Pract (2014) 10(5):e335–41. doi: 10.1200/JOP.2014.001457
9. Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal DI, Weber RS, Lambert L, et al. Development and Validation of Nomograms Predictive of Overall and Progression-Free Survival in Patients With Oropharyngeal Cancer. J Clin Oncol (2017) 35(36):4057–65. doi: 10.1200/JCO.2016.72.0748
10. Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K, et al. Preoperative Neutrophil:Lymphocyte and Platelet:Lymphocyte Ratios Predict Endometrial Cancer Survival. Br J Cancer (2015) 113(2):311–20. doi: 10.1038/bjc.2015.200
11. Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A Comparison of Inflammation-Based Prognostic Scores in Patients With Cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer (2011) 47(17):2633–41. doi: 10.1016/j.ejca.2011.03.028
12. Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, et al. The Prognostic and Predictive Impact of Inflammatory Biomarkers in Patients Who Have Advanced-Stage Cancer Treated With Immunotherapy. Cancer (2019) 125(1):127–34. doi: 10.1002/cncr.31778
13. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/Lymphocyte Ratio Predicts Chemotherapy Outcomes in Patients With Advanced Colorectal Cancer. Br J Cancer (2011) 104(8):1288–95. doi: 10.1038/bjc.2011.100
14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205
15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
16. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0
17. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
18. Kasper S, Breitenbuecher F, Reis H, Brandau S, Worm K, Kohler J, et al. Oncogenic RAS Simultaneously Protects Against anti-EGFR Antibody-Dependent Cellular Cytotoxicity and EGFR Signaling Blockade. Oncogene (2013) 32(23):2873–81. doi: 10.1038/onc.2012.302
19. Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic Inflammatory Markers as Independent Prognosticators of Head and Neck Squamous Cell Carcinoma. Head Neck (2015) 37(1):103–10. doi: 10.1002/hed.23567
20. Cho JK, Kim MW, Choi IS, Moon UY, Kim MJ, Sohn I, et al. Optimal Cutoff of Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Cancer Patients: A Meta-Analysis and Validation Study. BMC Cancer (2018) 18(1):969. doi: 10.1186/s12885-018-4876-6
21. Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, et al. Neutrophil Heterogeneity in Cancer: From Biology to Therapies. Front Immunol (2019) 10:2155. doi: 10.3389/fimmu.2019.02155
22. Maccio A, Madeddu C, Gramignano G, Mulas C, Tanca L, Cherchi MC, et al. The Role of Inflammation, Iron, and Nutritional Status in Cancer-Related Anemia: Results of a Large, Prospective, Observational Study. Haematologica (2015) 100(1):124–32. doi: 10.3324/haematol.2014.112813
23. Madeddu C, Gramignano G, Astara G, Demontis R, Sanna E, Atzeni V, et al. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front Physiol (2018) 9:1294. doi: 10.3389/fphys.2018.01294
24. Baumeister P, Canis M, Reiter M. Preoperative Anemia and Perioperative Blood Transfusion in Head and Neck Squamous Cell Carcinoma. PLoS One (2018) 13(10):e0205712. doi: 10.1371/journal.pone.0205712
25. Dubray B, Mosseri V, Brunin F, Jaulerry C, Poncet P, Rodriguez J, et al. Anemia is Associated With Lower Local-Regional Control and Survival After Radiation Therapy for Head and Neck Cancer: A Prospective Study. Radiology (1996) 201(2):553–8. doi: 10.1148/radiology.201.2.8888257
26. Magnes T, Melchardt T, Weiss L, Mittermair C, Neureiter D, Klieser E, et al. Prognostic Score in Patients With Recurrent or Metastatic Carcinoma of the Head and Neck Treated With Cetuximab and Chemotherapy. PLoS One (2017) 12(7):e0180995. doi: 10.1371/journal.pone.0180995PMCPMC5501656
27. Guigay J, Aupérin A, Fayette J, Saada-Bouzid E, Lafond C, Taberna M, et al. Cetuximab, Docetaxel, and Cisplatin Versus Platinum, Fluorouracil, and Cetuximab as First-Line Treatment in Patients With Recurrent or Metastatic Head and Neck Squamous-Cell Carcinoma (GORTEC 2014-01 TPExtreme): A Multicentre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol (2021) S1470-2045(20):30755–5. doi: 10.1016/S1470-2045(20)30755-5
Keywords: cetuximab, head and neck cancer, prognostic markers, predictive markers, systemic treatment, resistance mechanism, prognostic score
Citation: Pogorzelski M, Hilser T, Ting SC, Kansy B, Gauler TC, Stuschke M, Schmid KW, Lang S, Grünwald V, Schuler M and Kasper S (2021) Identification of a Prognostic Clinical Score for Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck Treated With Systemic Therapy Including Cetuximab. Front. Oncol. 11:635096. doi: 10.3389/fonc.2021.635096
Received: 29 November 2020; Accepted: 23 March 2021;
Published: 13 May 2021.
Edited by:
Panagiotis Balermpas, University Hospital Zürich, SwitzerlandReviewed by:
Markus Hecht, Friedrich-Alexander-Universität Erlangen-Nürnberg, GermanyOliver Riesterer, Aarau Cantonal Hospital, Germany
Copyright © 2021 Pogorzelski, Hilser, Ting, Kansy, Gauler, Stuschke, Schmid, Lang, Grünwald, Schuler and Kasper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Kasper, c3RlZmFuLmthc3Blci12aXJjaG93QHVrLWVzc2VuLmRl
 Michael Pogorzelski
Michael Pogorzelski Thomas Hilser
Thomas Hilser Saskia C. Ting2
Saskia C. Ting2 Benjamin Kansy
Benjamin Kansy Thomas C. Gauler
Thomas C. Gauler Kurt W. Schmid
Kurt W. Schmid Stephan Lang
Stephan Lang Viktor Grünwald
Viktor Grünwald