- 1Department of Hepatobiliary Surgery and Fujian Institute of Hepatobiliary Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 2Key Laboratory of The Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China
- 3Shengli Clinical Medical College of Fujian Medical University, Fujian Medical University, Fujian Provincial Hospital, Fuzhou, China
- 4Fujian Provincial Key Laboratory on Hematology, Fujian Institute of Hematology, Fujian Medical University Union Hospital, Fuzhou, China
- 5Pancreatic Disease Center, Department of General Surgery, Ruijin Hospital, Research Institute of Pancreatic Diseases, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background/Aims: Pancreatic ductal adenocarcinoma (PDAC) can occur in different parts of the pancreas. This study aimed to identify clinicopathological characteristics independently correlated with the prognosis of PDAC of the pancreatic head/uncinate (PHC) or body-tail (PBTC), and to develop novel nomograms for predicting cancer-specific survival (CSS) according to different primary cancer locations.
Methods: 1160 PDAC patients were retrospectively enrolled and assigned to training and test sets with each set divided into PHC and PBTC groups. Comparative analysis of clinicopathologic characteristics, survival analysis, and multivariate analysis were performed. Independent factors were identified and used for constructing nomograms. The performance of the nomograms was validated in the test set.
Results: Primary tumor location was an independent risk factor for prognosis of PDAC after surgery. Specially, gender, fasting blood glucose, and preoperative cancer antigen 19-9 were significantly associated with prognosis of PHC, whereas age, body mass index, and lymph nodes were significantly correlated with the prognosis of PBTC. A significant difference in prognosis was found between PHC and PBTC in stage Ia and stage III. Three nomograms were established for predicting the prognosis for PDAC, PHC, and PBTC. Notably, these nomograms were calibrated modestly (c-indexes of 0.690 for PDAC, 0.669 for PHC, and 0.704 for PBTC), presented better accuracy and reliability than the 8th AJCC staging system, and achieved clinical validity.
Conclusions: PHC and PBTC share the differential clinical-pathological characteristics and survival. The nomograms show good performance for predicting prognosis in PHC and PBTC. Therefore, these nomograms hold potential as novel approaches for predicting survival of PHC and PBTC patients after surgery.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), a predominate type of pancreatic cancer (PC), is among the leading causes of cancer-related death, accounting for approximately 260,000 deaths worldwide annually (1). It has been recognized in recent decades that PDAC has an extremely poor prognosis with a 5-year survival rate of less than 10%. For PDAC patients eligible for surgical treatment, curative-intent surgical resection followed by adjuvant chemotherapy is considered the only curative treatment option (2). Although substantial progress has been made in the diagnosis and treatment of PDAC, early relapse after pancreatectomy commonly occurs in PDAC patients. Thus, an accurate prognostic method is urgently required for the precise stratification of patients to guide appropriate clinical management and follow-up plans.
Currently, the stratification of patients mainly relies on the American Joint Committee on Cancer (AJCC) staging system, in which tumor size and the histological characteristics are considered as the major factors for evaluation. However, many significant factors, such as cancer antigen 19-9 (CA19-9) level, tumor differentiation, histological grade, and genomic analysis, have been proposed to be potential determinants of survival but have not been included in the AJCC staging system. Moreover, PDAC can occur in various parts of the pancreas (head, body or tail) of the pancreas, and the risk factors influencing the prognosis of PDAC according to different primary locations have not been thoroughly investigated.
A number of previous studies have indicated that tumor location is closely related to the prognosis of PC, with primary tumor location at the body/tail of the pancreas (PBTC) tending to have a poorer prognosis compared with that at the head of the pancreas (3–6). Additionally, resected PBTC has shown more aggressive tumor biology than PDAC at the head of the pancreas. On the contrary, some previous studies demonstrated that PBTC had a better outcome than PDAC at the head of the pancreas for patients at the early stage (7), and Winer et al. (8) found that patients with pancreatic head cancer had worse overall survival (OS) than patients with PC at either the body or tail locations for all stages when analyzing the National Cancer Database. Nevertheless, van Erning et al. (9) indicated that OS for PDAC at different tumor locations does not differ significantly according to the database in the Netherlands. Thus, the findings regarding the prognosis of PDAC at different primary tumor locations remain inconsistent and even conflicting. Use of the TNM staging system for stratifying PDAC patients in order to determine the precise prognosis is questionable (10). Until now, research of the survival difference for PDAC at different locations after curative-intent surgical resection has been rare.
In the present study, we aimed to identify clinicopathological characteristics independently correlated with the prognosis of PDAC at the pancreatic head/uncinate (PHC) versus the PBTC and to develop and validate novel nomograms for predicting the cancer-specific survival (CSS) of PDAC cases according to different primary cancer locations after curative-intent resection. The findings of this study may provide a novel prognostic tool for managing PDAC cases with different primary tumor locations.
Methods
Patients and Study Design
A total of 1160 PDAC patients who underwent curative-intent pancreatic resection at multiple centers, including Fujian Medical University Union Hospital, Fujian Provincial Hospital, and Shanghai Ruijin Hospital, spanning the period between January 2014 and March 2017 were retrospectively enrolled in this study. PDAC was histopathologically diagnosed and confirmed. Of 1160 enrolled patients, 813 enrolled from Fujian Medical University Union Hospital and Fujian Provincial Hospital were assigned to the training group, while 347 from Shanghai Ruijin Hospital were assigned to the test group for external validation. During enrollment, the following inclusion criteria were used: (1) histologically confirmed PDAC; (2) no prior receipt of other curative treatment including radiotherapy, immunogene and target therapy; (3) curatively resectable PDAC as preoperatively assessed by imaging, even with peripancreatic invasion or artery (hepatic, superior mesenteric and celiac artery) or vein (portal or superior and inferior mesenteric vein) that could be completely resected and constructed; (4) negative for intraoperative frozen section analysis; (5) only the single metastatic lesion in liver for patients with stage IV disease after 8-12 times paclitaxel-albumin or gemcitabine chemotherapy. PDAC patients with the following conditions were excluded from this study: (1) absent or incomplete information for important clinical characteristics needed for this study; (2) unresected tumors, based on bypass surgery, exploratory operation, or microscopic residual tumor in the resection margin; (3) death within 30 days after the surgery; and (4) causes of death other than PDAC and its complications.
This study was approved by the ethics committees of the institutional review boards of the participating hospitals, Fujian Medical University Union Hospital, Fujian Provincial Hospital, and Shanghai Ruijin Hospital. The need for written consent was waived due to the retrospective nature of this study.
Follow-Up
The patients were followed up by the operating surgeons at 1 month after surgery and every 3 months thereafter. The last follow-up was conducted in March 2020.
Statistical Analysis
Categorical variables were compared between groups using the chi square test. Continuous variables were analyzed using the independent-samples T test. To assess an association between various prognostic predictors and CSS, univariate and multivariate analyses were conducted using the Cox regression model, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Goodness of fit was maximized using the log-likelihood, while information loss was minimized with the Akaike information criterion (AIC) (11). Based on the AIC of the Cox proportional hazards model, variables were selected in a backward stepwise manner. Nomograms were constructed on the basis of the independent variables identified by the multivariate analysis in the training cohort.
Nomogram performance was assessed using the Harrell’s concordance index (c-index). The maximum c-index value of 1.0 represents a perfect discrimination. whereas 0.5 indicates no discriminative capacity. Calibration was made to graphically evaluate the performance of the model by comparing the means of predicted survival with those of actual survival. To reduce potential bias, 200-sample bootstrap validation was performed for internal validation. The values of c-indexes were compared using the compare C package (12). The precision of the 1-, 2-, and 3-year survival rates predicted by the nomograms was evaluated with time-dependent receiver operating characteristic (ROC) curve analysis using the time ROC package (13).
The ranges of threshold probabilities were finalized by decision curve analysis (DCA) (14) to assess the clinical validity of the nomograms. The Kaplan–Meier survival curve was utilized for comparing the nomograms with the latest edition of the 8th AJCC staging system (revised in 2018) by risk classification and stratification (15). For risk stratification, the accumulated nomogram scores were ranked by deciles to develop 10 risk groups, which composed the new nomo stages. Accordingly, each 8thAJCC substage was divided by nomo stages to derive three prognostic strata: low-, median-, and high-risk.
Results
Baseline Demographic and Clinical Characteristics of PDAC Patients
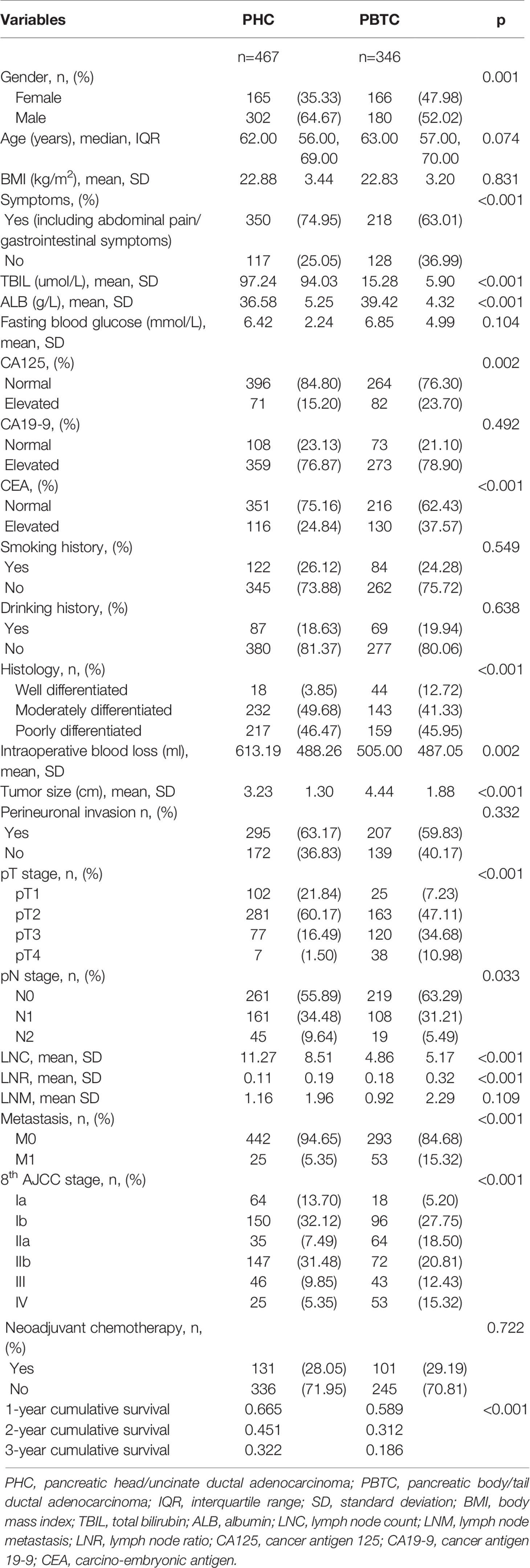
A total of 1160 patients with PDAC were enrolled from three participating hospitals, of which 813 (467 PHC cases and 346 PBTC cases) recruited from Fujian Medical University Union Hospital and Fujian Provincial Hospital were assigned to the training set. The baseline demographic and clinical characteristics of the PHC and PBTC patients are summarized in Table 1. The age and gender of patients were comparable between the training and test cohorts in both PHC and PBTC groups (Suppl. Tables 1, 2). It was noted that a majority of PDAC patients (PHC and PBTC groups) were men, with a greater male predominance in the PHC group (64.67% males and 35.33% females) than in the PBTC group (p=0.001). Comparison of clinical features revealed that patients in the PHC group showed a higher proportion of symptoms (e.g., jaundice, abdominal pain; p<0.001), thus leading to more timely medical intervention for PHC patients than PBTC patients. It was of note that PHC patients presented the earlier T and AJCC stage and less hepatic metastasis compared with PBTC patients (p<0.001). In addition, PHC patients exhibited a higher number of harvested lymph nodes confirmed by postoperative pathology, which described as lymph node count (LNC) afterward, than PBTC patients (11.27 and 4.86 for PHC and PBTC, respectively). The number of lymph nodes dissected during operation might be enough, but many of them were confirmed as adipose tissue by postoperative pathology, which could be the reason for that LNC in this study was lower than the number of 8th AJCC or ISGPS (International Study Group of Pancreatic Surgery) recommend. Lymph node metastasis occurred more frequently in the PHC group compared with the PBTC group (261 vs 219 in N0, 161 vs 108 in N1, 45 vs 19 in N2, p<0.001). Notably, the PBTC group had better tumor differentiation, less intraoperative blood loss, and higher values for lymph node ratio (LNR), albumin, carcino-embryonic antigen (CEA) and cancer antigen 125 (CA125) compared with the PHC group. The demographic and clinical characteristics were comparable among the patients in the training and test groups.
The median survival was 20 months (range, 1–74 months) and the 1-, 2-, and 3-year survival rates were 66.5%, 45.1%, and 32.2% in the PHC group, respectively. For patients with PBTC, the median survival was 14 months (range, 1–74 months), and the 1-, 2-, and 3-year survival rates were 58.9%, 31.2%, and 18.6%, respectively. Notably, PBTC patients had a significantly worse CSS compared with PHC patients (p<0.001).
Survival Analysis for Patients With PHC and PBTC
Survival rates were compared between the PHC and PBTC groups according to the AJCC stages (Figure 1). As a result, significant differences in survival were found in stage Ia and stage III, and patients in the PHC group had poorer clinical outcomes than those in the PBTC group (p=0.007 and <0.001 in stage Ia and stage III, respectively). The differences in clinical characteristics between the PHC and PBTC groups in stage Ia and stage III are summarized in Suppl. Tables 3, 4. In stage Ia, patients with PBTC showed significantly worse tumor differentiation (p=0.012) and a lower LNC (p=0.001) compared to patients with PHC. In stage III, patients in the PBTC group had a significantly larger tumor size (p=0.004) and later T stage (p<0.001) than patients in the PHC group, while the PHC group showed significantly more lymph nodes metastasis (p=0.015) and later N stage (p<0.001), with a higher LNC (p<0.001) than the PBTC group.
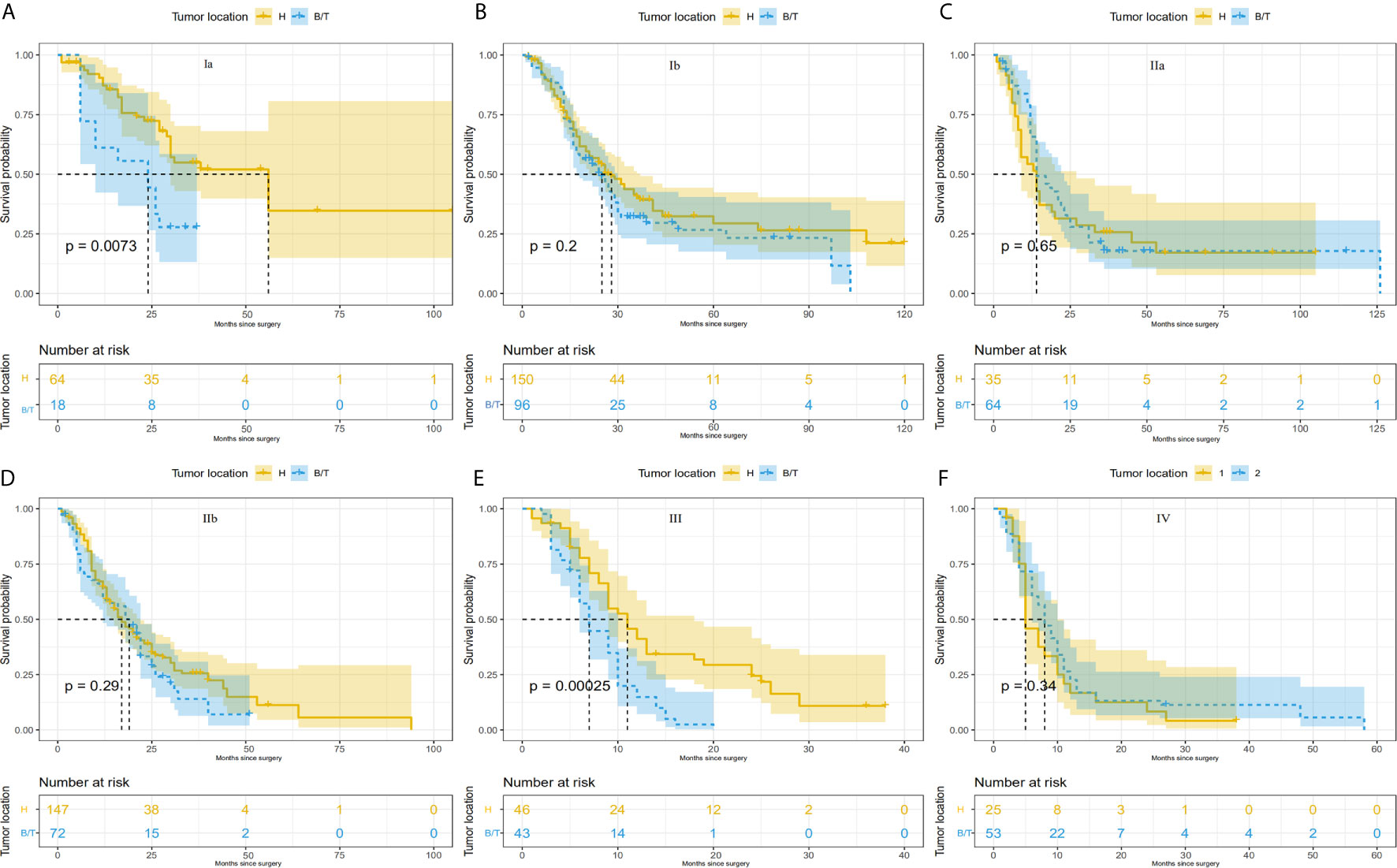
Figure 1 Comparison of survival differences between PHC and PBTC according to the 8thAJCC system. Comparison between PHC and PBTC in (A) stage Ia; (B) stage Ib; (C) stage IIa; (D) stage IIb; (E) stage III; and (F) stage IV. Significant differences were observed in Ia and III (p=0.0073 and 0.00025, respectively).
Identification of Independent Prognostic Factors for PHC and PBTC After Curative-Intent Surgical Resection
Univariate and multivariate analyses were conducted to identify prognostic factors that correlate with different primary cancer locations of PDAC, including PHC and PBTC, and detailed results are listed in Tables 2–4. In all enrolled PDAC patients with any primary cancer location, tumor location, gender, age, BMI, histological grade, symptoms, fasting blood glucose, tumor size, perineuronal invasion, T category, N category, hepatic metastasis, LNR, lymph node metastasis (LNM), and preoperative levels of CA19-9, CA125, and CEA were identified to be significantly associated with survival (Table 2). Further, multivariate analysis revealed that primary tumor location was an independent prognostic factor (PBTC: hazard ratio [HR] 1.443, 95% CI, 1.225–1.699, p<0.0001). In addition, gender, BMI, histological grade, symptoms, fasting blood glucose, tumor size, perineuronal invasion, M category, LNR, LNM, and preoperative CA19-9 and CEA levels were also independent prognostic factors for PDAC (Table 2).
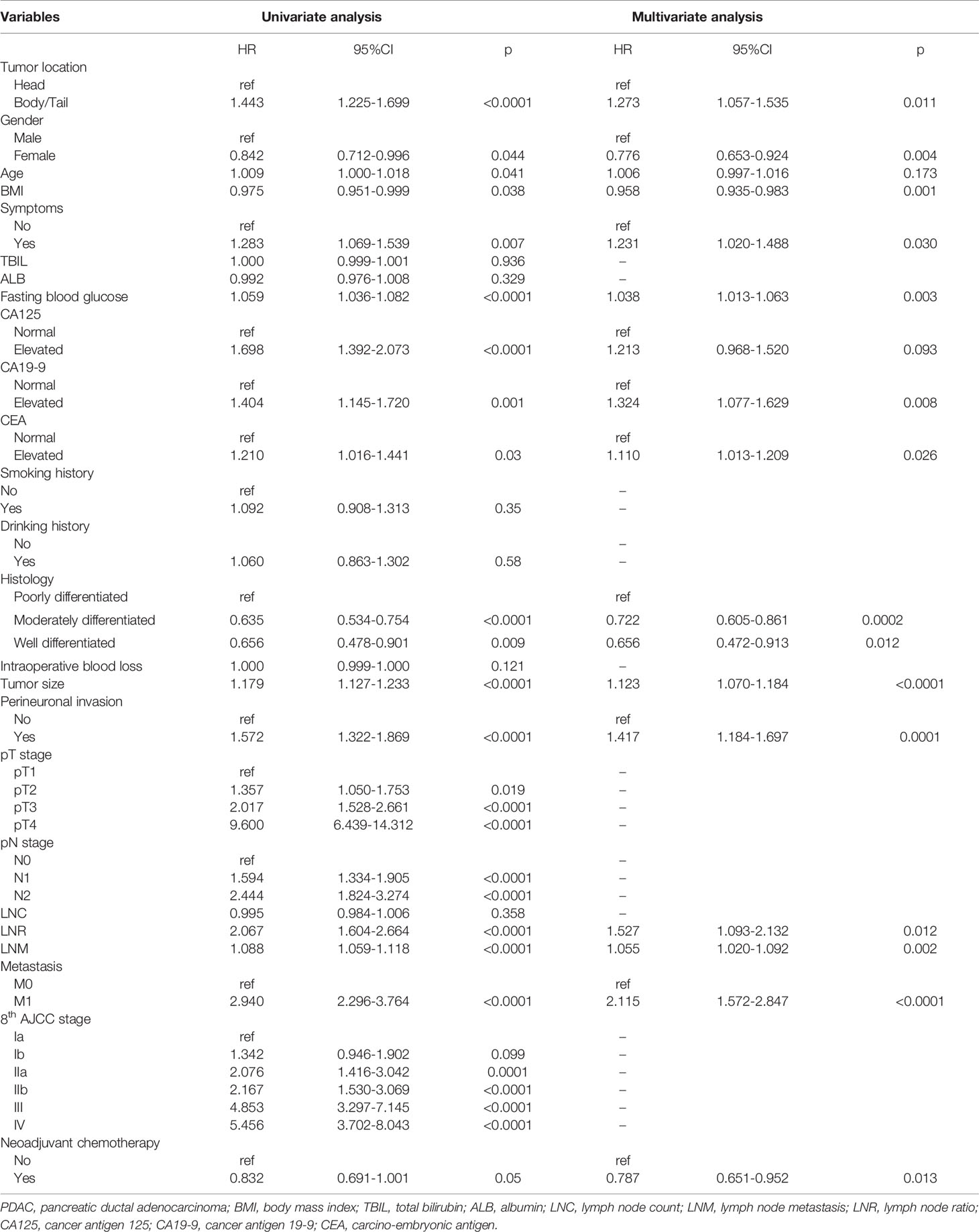
Table 2 Univariate and multivariate Cox regression analyses of prognostic factors in PDAC patients with curative-intent surgical resection.
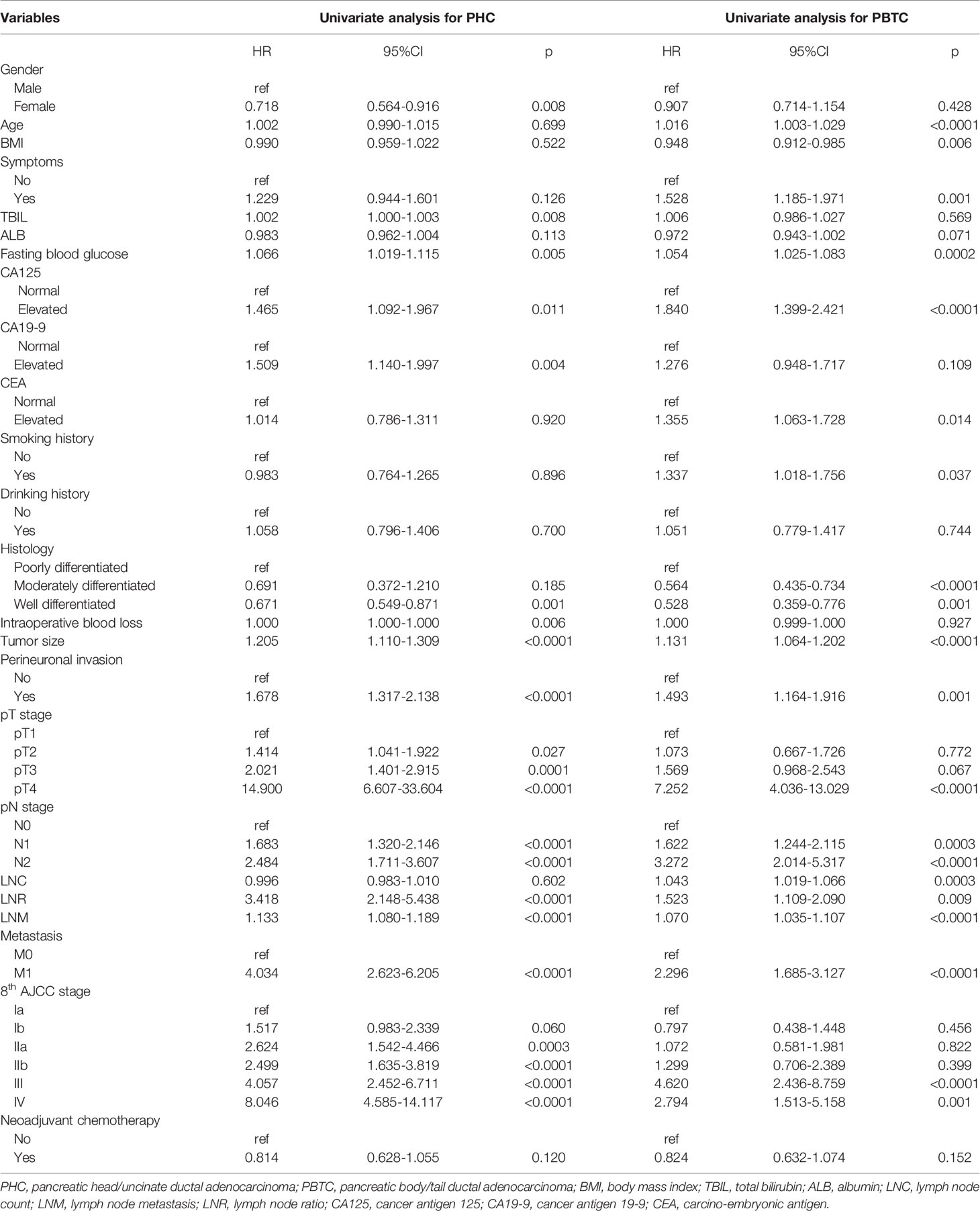
Table 3 Univariate Cox regression analysis of prognostic factors in PHC and PBTC patients with curative-intent surgical resection.

Table 4 Multivariate Cox regression analysis of prognostic factors for PHC and PBTC patients with curative-intent surgical resection.
The PDAC at different locations (PHC and PBTC) shared common independent prognostic factors: histological grade, tumor size, LNR, perineuronal invasion, M category and symptoms (Table 4). Notably, gender, fasting blood glucose, and preoperative CA19-9 level were significantly associated with the prognosis of PHC only, whereas age, BMI, and LNC were significantly correlated with the prognosis of PBTC only (Table 4), reflecting differences in independent prognostic factors between PHC and PBTC.
Construction and Validation of Prognostic Nomograms for PHC and PBTC
The identified independent risk factors influencing the prognosis of PHC and PBTC were used to construct prognostic nomograms for PHC and PBTC. As shown in Figure 2, covariates were selected on the basis of the AIC and likelihood rather than statistical significance (p value) to balance model complexity and performance. Points in the nomogram could be summed to calculate the probability of individual survival. The labels and points in the nomogram are presented in detail in Suppl. Tables 5, 6.
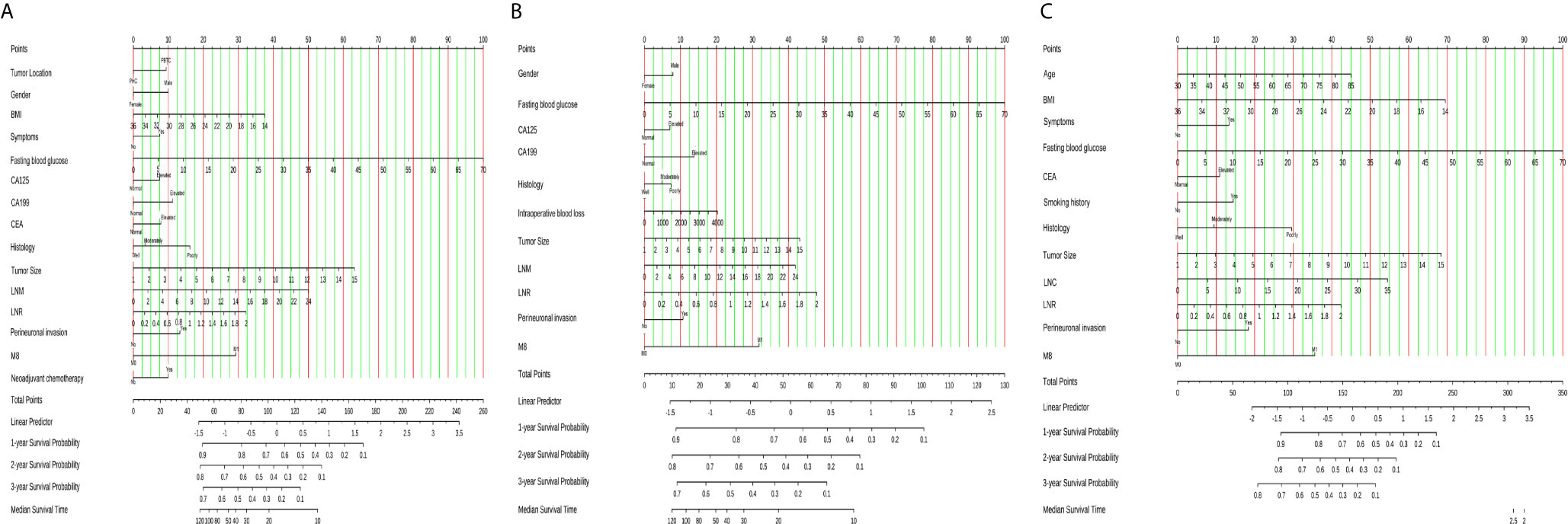
Figure 2 Nomograms for survival prediction in PDAC, PHC and PBTC patients after surgery. (A) 1-, 2-, and 3-year CSS of PDAC; (B) 1-, 2-, and 3-year CSS of PHC; (C) 1-, 2-, and 3-year CSS of PBTC. The total points for the nomograms were calculated according to the points for each covariate. A higher total number of points represented a higher possibility of unfavorable expected survival. CSS, cancer- specific survival.
Calibration plots were generated for the probabilities of 1-, 2-, and 3-year CSS of PDAC, PHC, and PBTC, and favorable consistency was illustrated by the survival predicted by the nomograms and the corresponding Kaplan–Meier estimates in both the training and test cohorts (Figures 3, 4), indicating that the established nomograms were reliable for predicting survival.
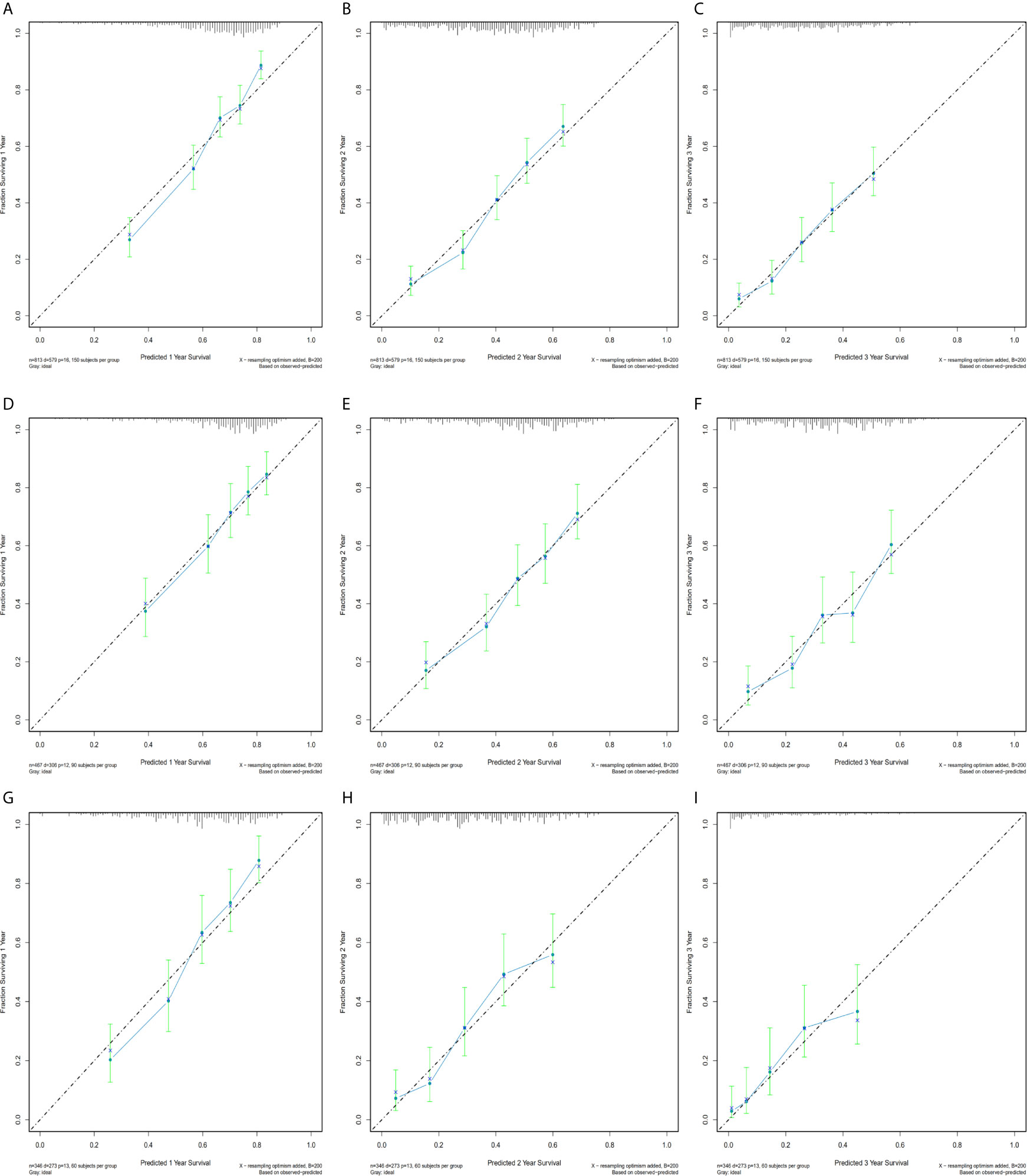
Figure 3 Bootstrap calibrations of the nomograms in the training cohorts. Bootstrap calibrations of the nomograms for predicting (A) 1-year CSS, (B) 2-year CSS, and (C) 3-year CSS in PDAC group; (D) 1-year CSS, (E) 2-year CSS, and (F) 3-year CSS in the PHC group; and (G) 1-year CSS, (H) 2-year CSS, and (I) 3-year CSS in the PBTC group. The predictions were well correlated with the actual survival probabilities.
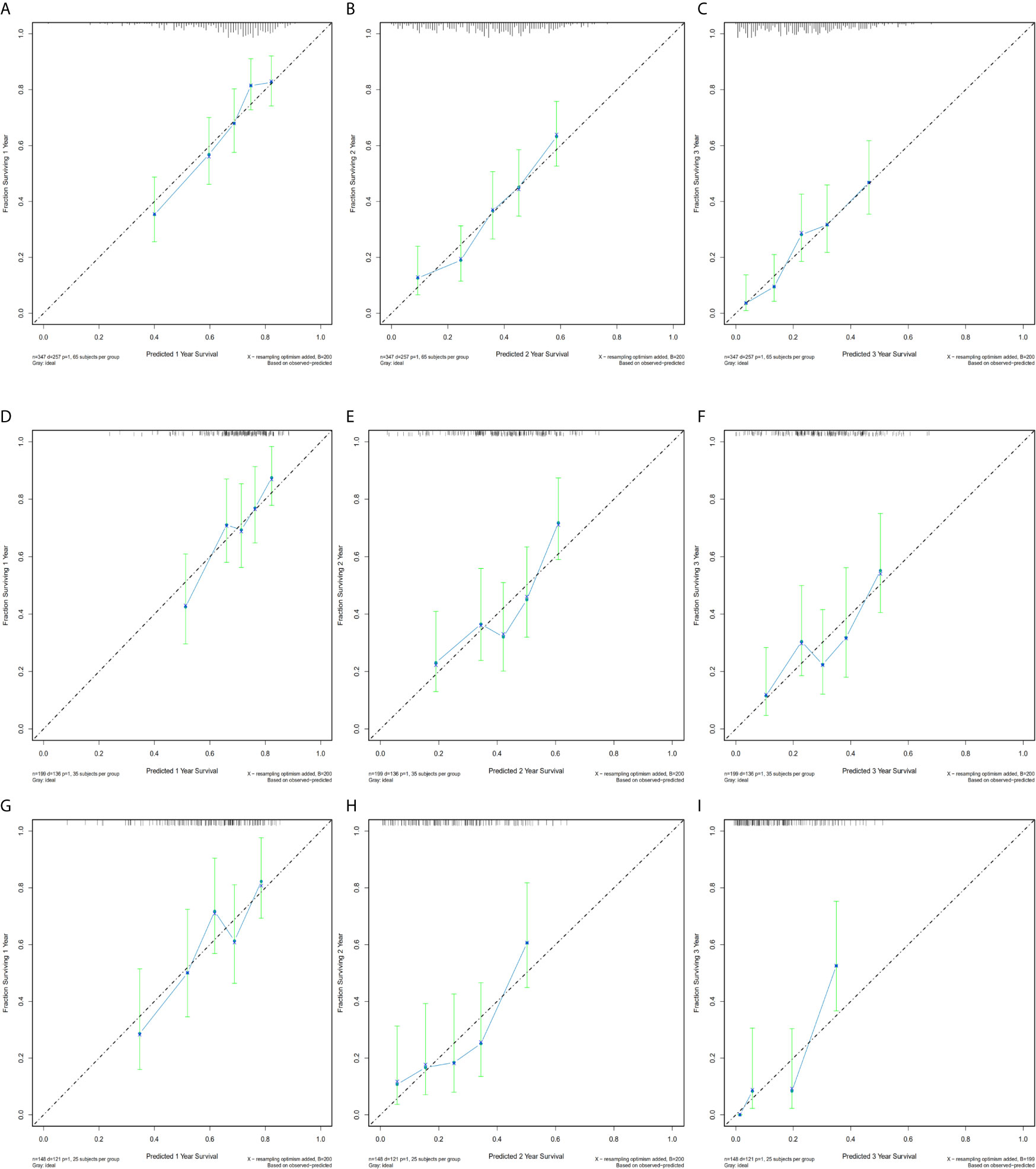
Figure 4 Bootstrap calibration of nomograms in the test cohorts. The nomograms were externally validated in the test cohorts by predicting (A) 1-year CSS, (B) 2-year CSS, and (C) 3-year CSS in the PDAC group; (D) 1-year CSS, (E) 2-year CSS, and (F) 3-year CSS in the PHC group; and (G) 1-year CSS, (H) 2-year CSS, and (I) 3-year CSS in the PBTC group. All results showed good validation.
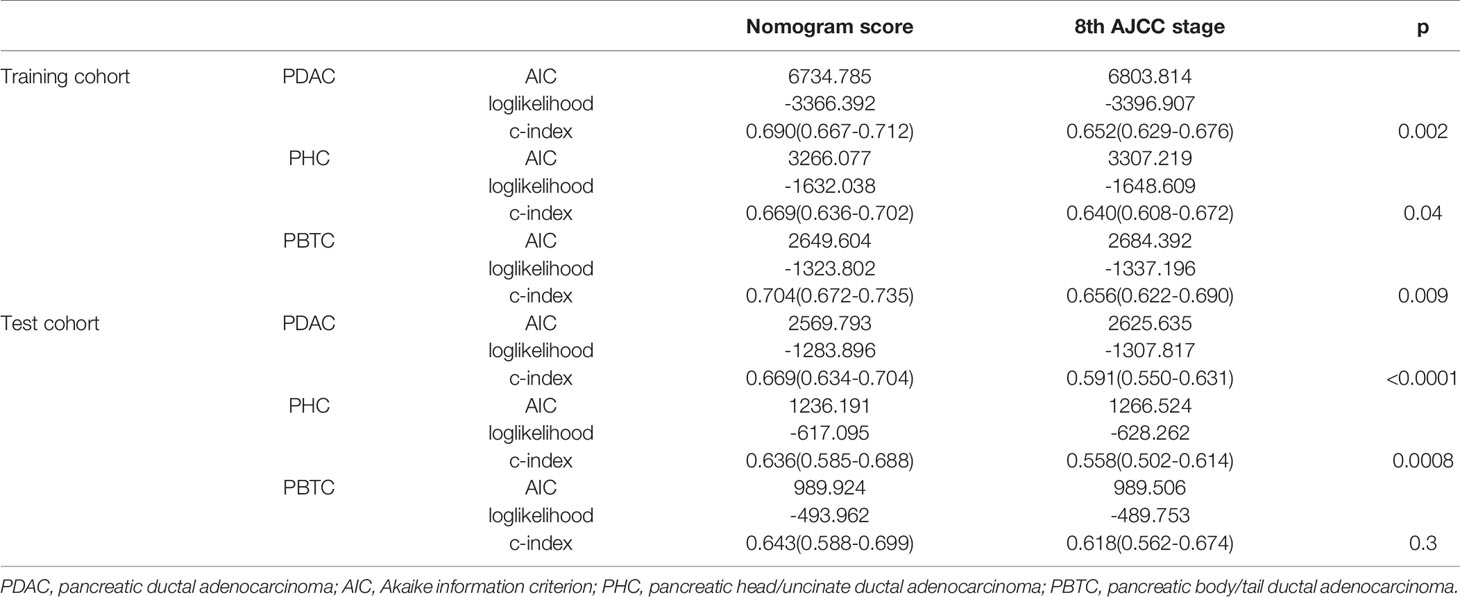
The bootstrap-corrected c-indexes in the training cohort were 0.690 (95% CI 0.667–0.712) for PDCA, 0.669 (95% CI 0.636–0.702) for PHC, and 0.704 (95% CI 0.672–0.735) for PBTC. In the test cohort, the c-indexes were 0.669 (95% CI 0.634–0.704) for PDCA, 0.636 (95% CI 0.585–0.688) for PHC, and 0.643 (95% CI 0.588–0.699) for PBTC (Table 5).
Performance Comparison Between the Nomograms and 8th Edition TNM Staging Systems
In comparison to the AJCC 8TH staging system, the nomograms showed greater log-likelihoods and c-indexes, together with smaller values of AIC for CSS in the PDAC, PHC, and PBTC groups (Table 5), indicating that the newly established nomograms were more robust for survival prediction than the AJCC stages. Additionally, instead of the six stages classified by 8th AJCC system, the new models stratified patients into 10 nomo stages, providing better discriminative ability (Figure 5). As shown in Suppl. Table 7, the HRs for the Nomo stages also confirmed the classification ability of the nomograms. Further analysis (Figure 6) showed a good ability for risk stratification using the nomograms by stratifying the AJCC 8th stages into the low-, medium-, and high-risk groups. The mosaic plots intuitively demonstrated the dramatic survival heterogeneity between the 8th edition AJCC stages and the nomo stages (Figure 7). Finally, the ROC curve showed the superior sensitivity and specificity of nomograms compared with the 8th edition AJCC stages (Suppl. Figure 1), and DCA demonstrated that the net benefit was consistently enhanced in all cohorts of the nomograms with wide ranges of threshold probabilities compared with the TNM stages, suggesting the favorable clinical applicability of the nomograms for predicting survival (Figure 8).
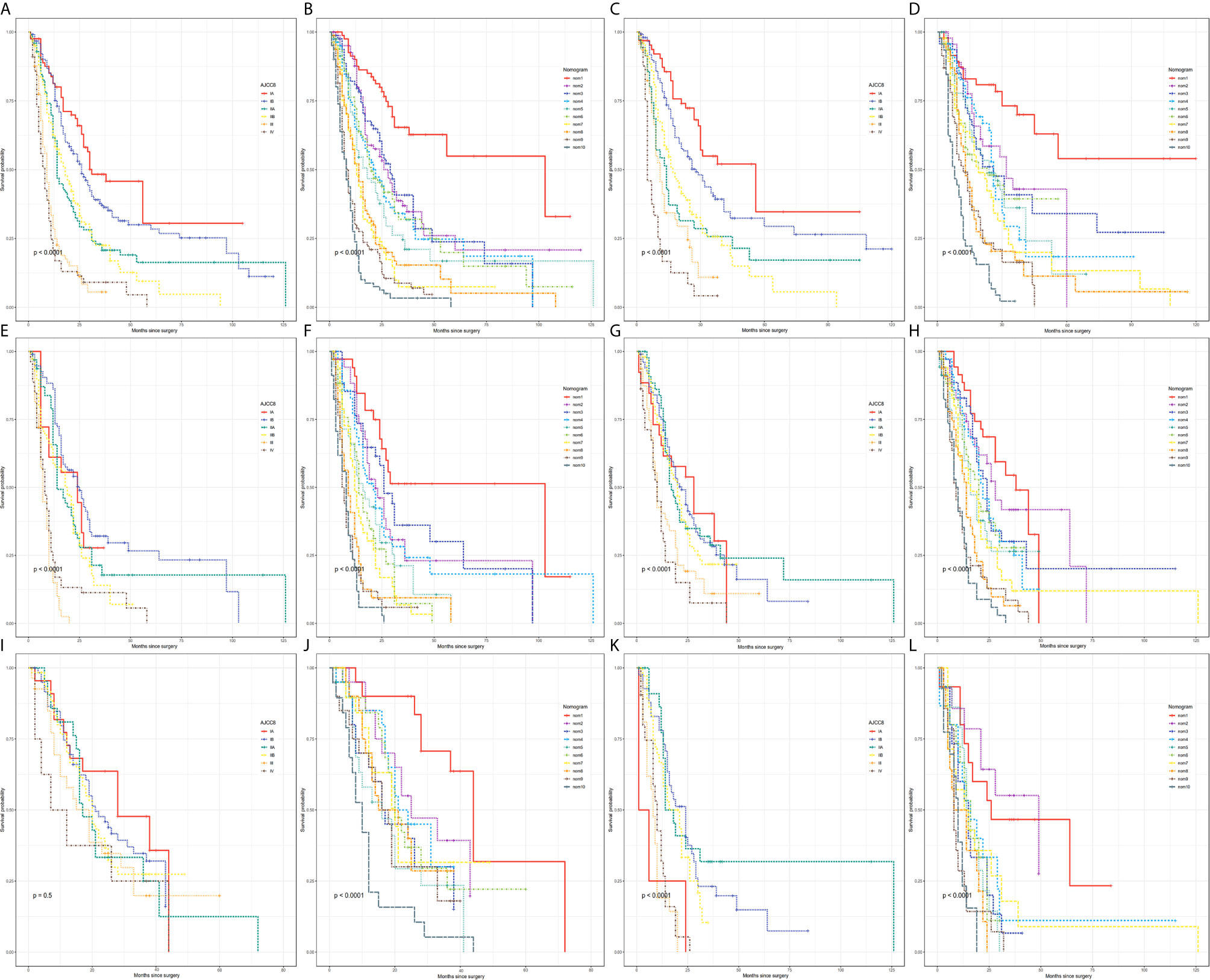
Figure 5 Kaplan–Meier curve analysis of risk classification. Risk classification of the (A, B) PDAC group, (C, D) PHC group, and (E, F) PBTC group in the training cohort. Risk classification of the (G, H) PDAC group, (I, J) PHC group, and (K, L) PBTC group in the test cohort. All log-rank p values for trends were <0.0001, except p=0.5 for (I).
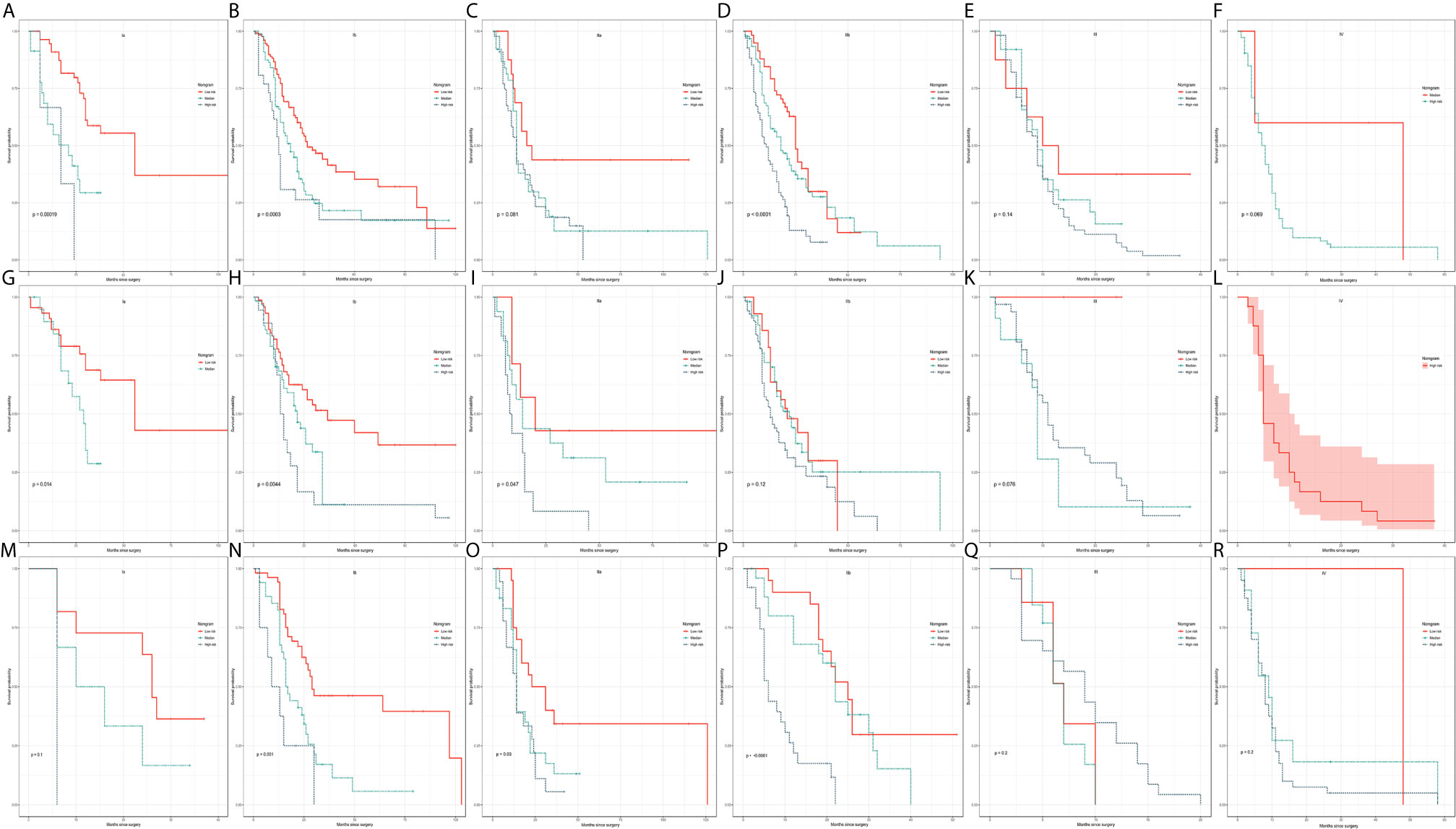
Figure 6 Kaplan–Meier curve analysis of risk stratification. Risk stratification in the training cohort for each 8th AJCC substage in the (A–F) PDAC group, (G–L) PHC group, and (M–R) PBTC group. The log-rank p values were <0.05 for (A, B, D, G, H, I, N–P).
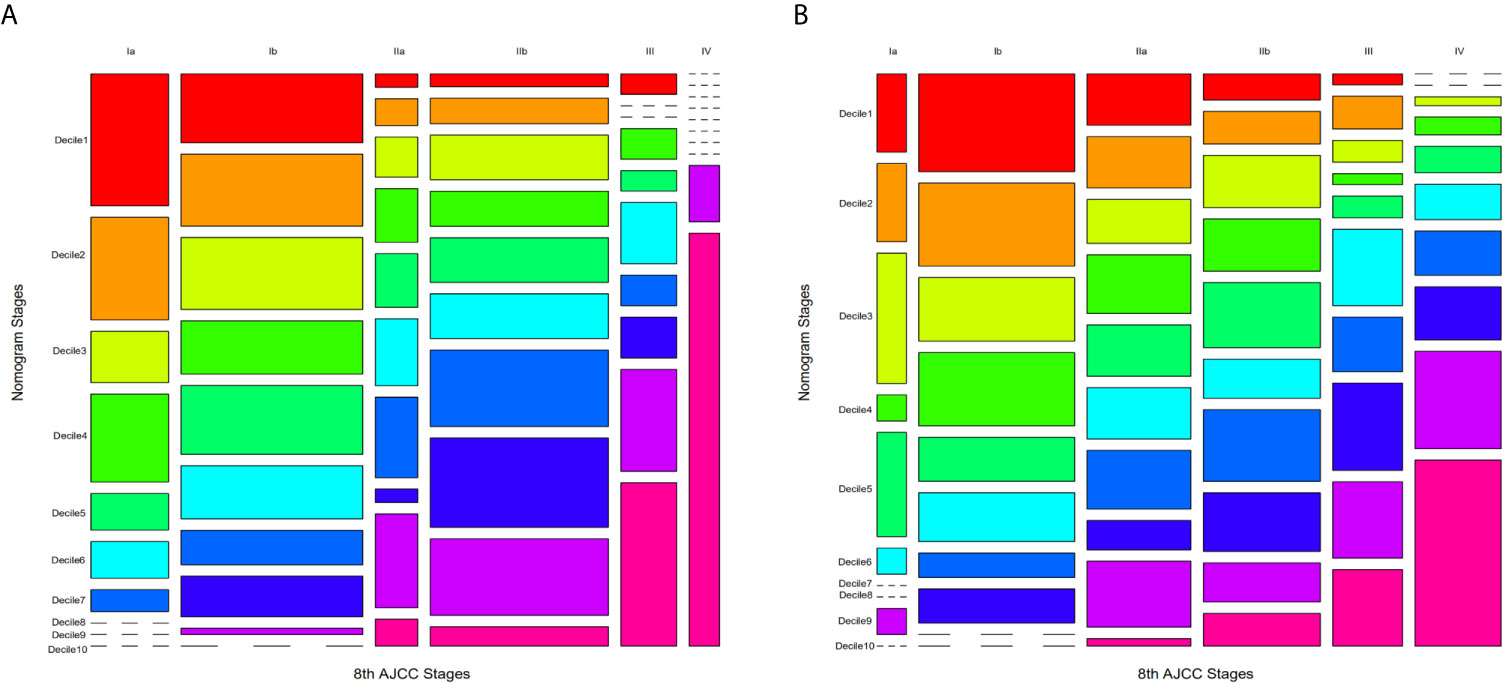
Figure 7 Mosaic plots using the training cohort. (A) Mosaic plots for PHC and (B) PBTC in which each of the 10 deciles was represented by 1 of 10 consecutive rainbow colors. The area of the individual mosaics represents the represents the proportions of corresponding patients. The short-segmented lines indicate a frequency of zero.
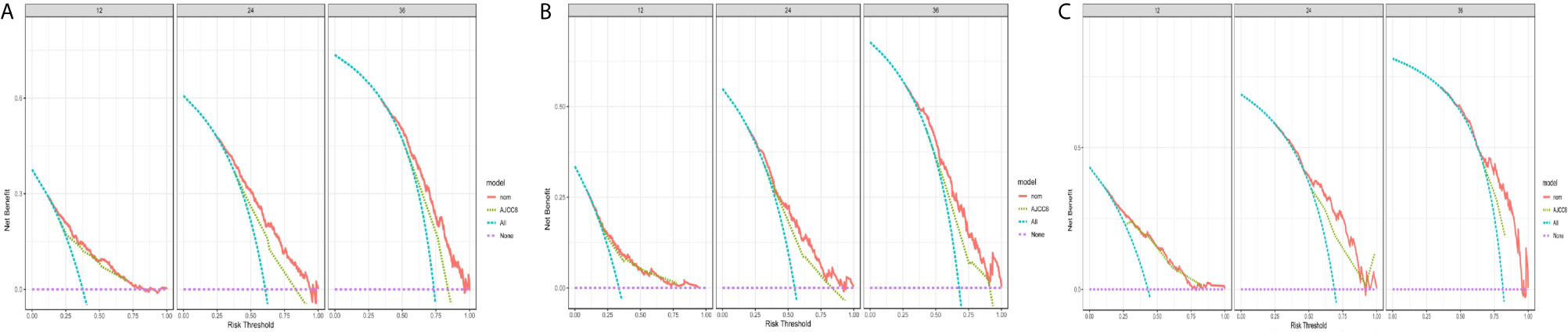
Figure 8 Decision curve analysis and comparison of the nomograms with the 8thAJCC stages. Decision curve analysis (DCA) of the nomograms for predicting (A) 1-, 2-, and 3-year survival in the PDAC group; (B) 1-, 2-, and 3-year survival in the PHC; and (C) 1-, 2-, and 3-year survival in the PBTC group.
Comparative Analysis of the Predictive Performance Among Three Nomograms
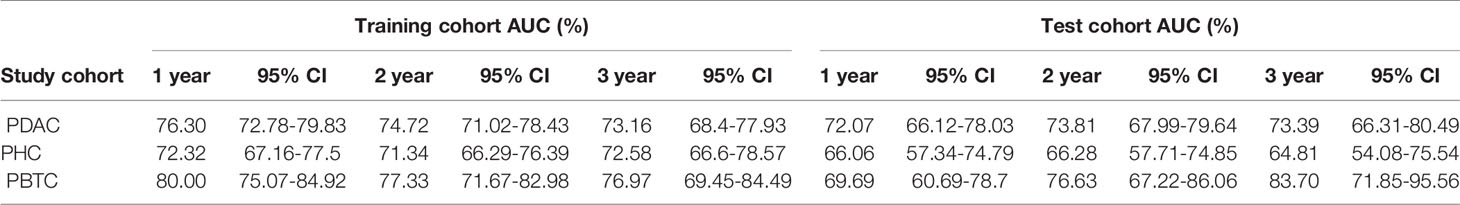
The PBTC nomogram had optimal AUCs in both the training cohort and test cohort, while the AUCs for the PDAC nomogram were higher than those of the PHC nomogram (Table 6). The aforementioned criteria (c-index) were consistent with the results of ROC curves (Table 5), indicating that the nomogram for PBTC performed best and the nomogram for PDAC was more robust for survival prediction compared with that for PHC.
Discussion
PDAC can occur in different regions of the pancreas, and the influence of primary cancer location on the prognosis of PDAC has not been fully elucidated. Several nomograms had been established before to demonstrated their superiority over 8thAJCC system, some for the PBTC (16, 17), others for the PHC (18). However, this is the first study, to the best of our knowledge, developed three nomograms simultaneously based on the heterogeneous clinicopathological characteristics identified between PHC and PBTC. The novel major findings of this study are summarized as follows: (1) the primary cancer location was an independent factor for prognosis of patients with PDAC after surgery; (2) differential independent risk factors according to different primary tumor locations were identified to be significantly correlated with a poor prognosis; (3) three nomograms for the prediction of prognosis in PDAC, PHC, and PBTC were constructed on the basis of the identified independent prognosis factors; (4) these nomograms performed and calibrated well, with c-indexes of 0.690 (95% CI 0.667–0.712) for PDCA, 0.669 (95% CI 0.636–0.702) for PHC, and 0.704 (95% CI 0.672–0.735) for PBTC; and (5) performance comparison suggested that the newly established nomograms offer greater clinical net benefits than the 8th edition AJCC staging system. As such, these nomograms have the potential to be novel and better approaches for predicting survival of PHC and PBTC patients after surgery.
In the present study, we identified that tumor location was an independent risk factor for poor prognosis in PDAC. The prognosis of patients with PHC was better than that of patients with PBTC. Previous studies have shown the primary tumor location may have a significant impact on prognosis in colorectal and gastric cancer (19, 20), whereas its influence in PDAC remains controversial. Some previous studies have demonstrated that differences existed in the biological and oncological behavior and prognosis between head/uncinate and body/tail PC (6, 7, 21–25), while other studies (9, 26, 27) identified no significant correlation between primary tumor location and OS. Huang et al. (27) analyzed 11,837 patients with chemotherapy and surgical resection from five different countries, indicating that tumor location had no influence on survival. Nevertheless, they recruited the patients of stage I and II from 2003 to 2014, and the AJCC staging system was less accurate in early years. In contrast, we included patients from all stages based on their resectability, and the differences of prognosis between PHC and PBTC mainly occurred in stage Ia and III. It has been recognized that the head of the pancreas and the tail of pancreas arise from different embryonic anlagen, with the anterior domain of the pancreatic head together with the body and tail of pancreas derived from the dorsal primordia,while the ventral primordia formed inferior portions of the pancreatic head and uncinate process. Due to their differential embryological origins and differences in histology and cytology (28, 29), Dreyer et al. (24) reported that tumors in body and tail more likely were of the squamous subtype and were enriched for gene programs associated with tumor invasion and poor antitumor immune response. Hence, worse survival was observed with tumors in PBTC, consistent with the findings of some other studies (3, 18, 30). However, other studies proposed conclusion contrarily (8, 31).
The number of metastatic lymph nodes was not an independent risk factor as compared with other important clinical indicators such as tumor size and LNR. The 8th AJCC staging system overestimated the weight of lymph nodes, and it was inappropriate to classify all N2 stage cases as stage III (32, 33). We further compared the risk factors for PHC and PBTC and obtained some interesting findings. First, PHC and PBTC were found to have some unique independent risk factors, which indicated that their clinical-pathological behaviors might be different. Secondly, tumor size, LNR, tumor differentiation degree, nerve invasion, and distant metastasis were all independent prognostic factors both for PHC and PBTC, which was consistent with previous reports (34–37). Third, the LNR in both groups exhibited independent predictive significance while LNM not. Some studies (38, 39) showed that the LNR had the strongest prediction ability compared with LNC and the 8th N stage. He et al. (40) identified the LNR as an independent predictive factor. Shi et al. (41) and Slidell et al. (42) found that LNC was as important as LNR, especially in node-negative disease. Similarly, in our study, negative lymph nodes were found more often in patients with PBTC, which might explain the strong correlation between LNC and the prognosis of PBTC. Fourth, hyperglycemia was found to an independent risk factor for PHC but not PBTC. Previous research (43–47) had shown that hyperglycemia is associated with worse survival of PC, but only a few studies focused on whether hyperglycemia affects the postoperative prognosis of PDAC. Raghavan et al. (44) reported that the prognosis of PDAC patients with hyperglycemia after surgery is poor, and the mechanism may be related to the Warburg effect. Li et al. (45) suggested that hyperglycemia might correlate with EMT. To date, there has been no report on whether hyperglycemia has distinctive impacts on pancreatic tumors in different locations. The above results demonstrated that the factors for prognosis of PDAC in different regions were heterogeneous, and the ability of 8th AJCC staging system to predict the outcome of PDAC remained mediocre as it defines PHC and PBTC as the same tumor.
We established nomograms on the basis of the differences in independent risk factors for PDAC, PHC and PBTC, and they showed high accuracy and reliability in the prognostic prediction of PHC and PBTC. Notably, our results support that the performance of these nomograms was superior to the latest edition 8th AJCC staging system. Furthermore, the newly established nomograms were able to stratify PDAC into 10 nomo stages in comparison with only three prognostic subgroups by each 8th AJCC stage, achieving more robust risk classification and stratification. Although there were many stages, clinicians only need the scores of patients according to nomograms, and the scores had one-to-one correspondence with the corresponding stage. Given that the better classification and stratification abilities can classify patients more precisely, the nomograms developed in this study may better help clinicians to identify high-risk patients and thereby promote personalized treatment planning. In addition, DCA verified the favorable clinical validity of the nomograms with consistently enhanced net benefits compared with the latest AJCC staging system. Among the three new models, the nomogram for PBTC had the best performance, as evidenced by the highest c-index and AUC value, while the nomogram for PDAC was slightly better than that for PHC. Therefore, we suggest that the nomogram for PDAC can be used in PHC patients to achieve more accurate survival prediction.
The present study has several potential limitations. First, PDAC patients were retrospectively recruited from three medical centers in China, the information of some impactful predictors such as cancer recurrence, neoadjuvant and adjuvant chemotherapy was incomplete, and differences in surgical procedure and postoperative pathological examinations may have existed, these might be the reasons for the moderate c-indexes, and thus, a prospective study is needed to validate the performance of the nomograms. Second, the enrolled patients included mainly individuals of Chinese ethnic population; thus, the nomograms established in this study will need to be verified in other ethnic populations. Third, this study enrolled patients with M1 stage; however, these were the patients with hepatic metastasis who showed a survival benefit following hepatic metastasis resection for PDAC, as reported by two small single-center series (48, 49). Fourth, genetic factors were not integrated into the analysis of risk factors for prognosis, as they might influence the prognosis.
In summary, the present study shows the differential clinical-pathological characteristics and after-surgery outcomes between PHC and PBTC, and demonstrates the prognosis of them should be evaluated by different staging systems, which have been successfully constructed in this study. The results show that these nomograms perform well and are well calibrated, and therefore, they hold potential to be used as novel and improved tools for the prediction of survival among PHC and PBTC patients after surgical treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Fujian Medical University Union Hospital, Fujian Provincial Hospital, Ruijin Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GL, C-YL and J-ZC: carried out the concepts, design, definition of intellectual content, literature search, data acquisition, data analysis and manuscript preparation. LH, CY, and Y-FT: helped perform the analysis with constructive discussions. QD and Y-TW: provided assistance for data acquisition, data analysis and statistical analysis. SC, QZ, and Y-LC: contributed to the conception of the study and performed manuscript review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the high-level hospital foster grants from Fujian Provincial Hospital (#2019HSJJ13 to SC), the Natural Science Foundation for Distinguished Young Scholars of Fujian Province (#2018J06020 to SC), the Education and Scientific Research Foundation of Fujian Province (#2060402 to SC), the joint Funds for the innovation of science and technology, Fujian Province (#2018Y9098 to SC), the Fujian Provincial Health and Family Planning Research Medical Innovation Project (2019-cx-3 to SC), the Natural Science Foundation of Fujian Province (#2017J01206 to QD), the Startup Fund for scientific research, Fujian Medical University (#2019QH1036 to GL) and Fujian Province Educational Research Project for Youths (#JAT190185 to GL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.646082/full#supplementary-material
Supplementary Figure 1 | ROC curve analysis and comparison of the nomograms with the 8thAJCC stages. ROC curve analysis of the nomograms for predicting (A) 1-, 2-, and 3-year survival in the PDAC group; (B) 1-, 2-, and 3-year survival in the PHC; and (C) 1-, 2-, and 3-year survival in the PBTC group.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of Adjuvant Gemcitabine and Capecitabine With Gemcitabine Monotherapy in Patients With Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet (London England) (2017) 389:1011–24. doi: 10.1016/s0140-6736(16)32409-6
3. Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The Anatomic Location of Pancreatic Cancer is a Prognostic Factor for Survival. HPB (Oxford) (2008) 10:371–6. doi: 10.1080/13651820802291233
4. Brennan MF, Moccia RD, Klimstra D. Management of Adenocarcinoma of the Body and Tail of the Pancreas. Ann Surg (1996) 223:506–11; discussion 11-2. doi: 10.1097/00000658-199605000-00006
5. Watanabe I, Sasaki S, Konishi M, Nakagohri T, Inoue K, Oda T, et al. Onset Symptoms and Tumor Locations as Prognostic Factors of Pancreatic Cancer. Pancreas (2004) 28:160–5. doi: 10.1097/00006676-200403000-00007
6. Lau MK, Davila JA, Shaib YH. Incidence and Survival of Pancreatic Head and Body and Tail Cancers: A Population-Based Study in the United States. Pancreas (2010) 39:458–62. doi: 10.1097/MPA.0b013e3181bd6489
7. Zheng Z, Wang M, Tan C, Chen Y, Ping J, Wang R, et al. Disparities in Survival by Stage After Surgery Between Pancreatic Head and Body/Tail in Patients With Nonmetastatic Pancreatic Cancer. PloS One (2019) 14:e0226726. doi: 10.1371/journal.pone.0226726
8. Winer LK, Dhar VK, Wima K, Morris MC, Lee TC, Shah SA, et al. The Impact of Tumor Location on Resection and Survival for Pancreatic Ductal Adenocarcinoma. J Surg Res (2019) 239:60–6. doi: 10.1016/j.jss.2019.01.061
9. van Erning FN, Mackay TM, van der Geest LGM, Groot Koerkamp B, van Laarhoven HWM, Bonsing BA, et al. Association of the Location of Pancreatic Ductal Adenocarcinoma (Head, Body, Tail) With Tumor Stage, Treatment, and Survival: A Population-Based Analysis. Acta Oncol (Stockholm Sweden) (2018) 57:1655–62. doi: 10.1080/0284186x.2018.1518593
10. Li G, Chen JZ, Chen S, Lin SZ, Pan W, Meng ZW, et al. Development and Validation of Novel Nomograms for Predicting the Survival of Patients After Surgical Resection of Pancreatic Ductal Adenocarcinoma. Cancer Med (2020) 9:3353–70. doi: 10.1002/cam4.2959
11. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Verlag New York: Springer (2002).
12. Kang L, Chen W, Petrick NA, Gallas BD. Comparing Two Correlated C Indices With Right-Censored Survival Outcome: A One-Shot Nonparametric Approach. Stat Med (2015) 34:685–703. doi: 10.1002/sim.6370
13. Hung H CC. Estimation Methods for Time-Dependent AUC Models With Survival Data. Can J Stat (2010) 38:8–26. doi: 10.2307/27805213
14. Vickers AJ, Elkin EB. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med Decis Making (2006) 26:565–74. doi: 10.1177/0272989x06295361
15. Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-Institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg (2017) 265:185–91. doi: 10.1097/sla.0000000000001763
16. He C, Sun S, Zhang Y, Lin X, Li S. Score for the Overall Survival Probability of Patients With Pancreatic Adenocarcinoma of the Body and Tail After Surgery: A Novel Nomogram-Based Risk Assessment. Front Oncol (2020) 10:590. doi: 10.3389/fonc.2020.00590
17. Zou Y, Shi N, Ruan S, Jin L, Yin Z, Hang H, et al. Development of a Nomogram to Predict Diseasespecific Survival for Patients After Resection of a non-Metastatic Adenocarcinoma of the Pancreatic Body and Tail. Front Oncol (2020) 10:526602. doi: 10.3389/fonc.2020.526602
18. Li H-b, Zhou J, Zhao F-q. A Prognostic Nomogram for Disease-Specific Survival in Patients With Pancreatic Ductal Adenocarcinoma of the Head of the Pancreas Following Pancreaticoduodenectomy. Med Sci Monit (2018) 24:6313–21. doi: 10.12659/MSM.909649
19. Petrelli F, Ghidini M, Barni S, Steccanella F, Sgroi G, Passalacqua R, et al. Prognostic Role of Primary Tumor Location in non-Metastatic Gastric Cancer: A Systematic Review and Meta-Analysis of 50 Studies. Ann Surg Oncol (2017) 24:2655–68. doi: 10.1245/s10434-017-5832-4
20. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-Analysis. JAMA Oncol (2017) 3:211–9. doi: 10.1001/jamaoncol.2016.4227
21. Birnbaum DJ, Bertucci F, Finetti P, Birnbaum D, Mamessier E. Head and Body/Tail Pancreatic Carcinomas are Not the Same Tumors. Cancers (2019) 11:497. doi: 10.3390/cancers11040497
22. Mackay TM, van Erning FN, van der Geest LGM, de Groot JWB, Haj Mohammad N, Lemmens VE, et al. Association Between Primary Origin (Head, Body and Tail) of Metastasised Pancreatic Ductal Adenocarcinoma and Oncologic Outcome: A Population-Based Analysis. Eur J Cancer (Oxford Engl 1990) (2019) 106:99–105. doi: 10.1016/j.ejca.2018.10.008
23. Yin L, Xiao L, Gao Y, Wang G, Gao H, Peng Y, et al. Comparative Bioinformatical Analysis of Pancreatic Head Cancer and Pancreatic Body/Tail Cancer. Med Oncol (Northwood London England) (2020) 37:46. doi: 10.1007/s12032-020-01370-0
24. Dreyer SB, Jamieson NB, Upstill-Goddard R, Bailey PJ, McKay CJ, Biankin AV, et al. Defining the Molecular Pathology of Pancreatic Body and Tail Adenocarcinoma. Br J Surg (2018) 105:e183–e91. doi: 10.1002/bjs.10772
25. Lee M, Kwon W, Kim H, Byun Y, Han Y, Kang JS, et al. The Role of Location of Tumor in the Prognosis of the Pancreatic Cancer. Cancers (2020) 12:2036. doi: 10.3390/cancers12082036
26. Ruess DA, Makowiec F, Chikhladze S, Sick O, Riediger H, Hopt UT, et al. The Prognostic Influence of Intrapancreatic Tumor Location on Survival After Resection of Pancreatic Ductal Adenocarcinoma. BMC Surg (2015) 15:123. doi: 10.1186/s12893-015-0110-5
27. Huang L, Balavarca Y, van der Geest L, Lemmens V, Van Eycken L, De Schutter H, et al. Development and Validation of a Prognostic Model to Predict the Prognosis of Patients Who Underwent Chemotherapy and Resection of Pancreaticadenocarcinoma: A Large International Population-Based Cohort Study. BMC Med (2019) 17:66. doi: 10.1186/s12916-019-1304-y
28. Radi M, Gaubert J, Cristol-Gaubert R, Baecker V, Travo P, Prudhomme M, et al. A 3D Reconstruction of Pancreas Development in the Human Embryos During Embryonic Period (Carnegie Stages 15-23). Surg Radiol Anat (2010) 32:11–5. doi: 10.1007/s00276-009-0533-8
29. Tadokoro H, Kozu T, Toki F, Kobayashi M, Hayashi N. Embryological Fusion Between the Ducts of the Ventral and Dorsal Primordia of the Pancreas Occurs in Two Manners. Pancreas (1997) 14:407–14. doi: 10.1097/00006676-199705000-00012
30. Sheng W, Dong M, Wang G, Shi X, Gao W, Wang K, et al. The Diversity Between Curatively Resected Pancreatic Head and Body-Tail Cancers Based on the 8th Edition of AJCC Staging System: A Multicenter Cohort Study. BMC Cancer (2019) 19:981. doi: 10.1186/s12885-019-6178-z
31. Meng Z, Cao M, Zhang Y, Liu Z, Wu S, Wu H. Tumor Location as an Indicator of Survival in T1 Resectable Pancreatic Ductal Adenocarcinoma: A Propensity Score-Matched Analysis. BMC Gastroenterol (2019) 19:59. doi: 10.1186/s12876-019-0975-3
32. Shin DW, Lee JC, Kim J, Woo SM, Lee WJ, Han SS, et al. Validation of the American Joint Committee on Cancer 8th Edition Staging System for the Pancreatic Ductal Adenocarcinoma. Eur J Surg Oncol (2019) 45:2159–65. doi: 10.1016/j.ejso.2019.06.002
33. Song M, Yoon SB, Lee IS, Hong TH, Choi HJ, Choi MH, et al. Evaluation of the Prognostic Value of the New AJCC 8th Edition Staging System for Patients With Pancreatic Adenocarcinoma; A Need to Subclassify Stage III? Eur J Cancer (Oxford Engl 1990) (2018) 104:62–9. doi: 10.1016/j.ejca.2018.08.027
34. Yu FJ, Shih HY, Wu CY, Chuang YS, Lee JY, Li HP, et al. Enteral Nutrition and Quality of Life in Patients Undergoing Chemoradiotherapy for Esophageal Carcinoma: A Comparison of Nasogastric Tube, Esophageal Stent, and Ostomy Tube Feeding. Gastrointest Endosc (2018) 88:21–31.e4. doi: 10.1016/j.gie.2017.11.030
35. Pindak D, Tomas M, Dolnik J, Duchon R, Pavlendova J. Morbidity, Mortality and Long Term Survival in Patients With Vascular Resection in Pancreatic Cancer - Single Center Experience. Neoplasma (2017) 64:460–3. doi: 10.4149/neo_2017_318
36. Yamamoto T, Yagi S, Kinoshita H, Sakamoto Y, Okada K, Uryuhara K, et al. Long-Term Survival After Resection of Pancreatic Cancer: A Single-Center Retrospective Analysis. World J Gastroenterol (2015) 21:262–8. doi: 10.3748/wjg.v21.i1.262
37. Schorn S, Demir IE, Haller B, Scheufele F, Reyes CM, Tieftrunk E, et al. The Influence of Neural Invasion on Survival and Tumor Recurrence in Pancreatic Ductal Adenocarcinoma - A Systematic Review and Meta-Analysis. Surg Oncol (2017) 26:105–15. doi: 10.1016/j.suronc.2017.01.007
38. Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The Lymph Node Ratio is the Strongest Prognostic Factor After Resection of Pancreatic Cancer. J Gastrointest Surg (2009) 13:1337–44. doi: 10.1007/s11605-009-0919-2
39. Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic Relevance of Lymph Node Ratio Following Pancreaticoduodenectomy for Pancreatic Cancer. Surgery (2007) 141:610–8. doi: 10.1016/j.surg.2006.12.013
40. He C, Zhang Y, Cai Z, Lin X, Li S. Overall Survival and Cancer-Specific Survival in Patients With Surgically Resected Pancreatic Head Adenocarcinoma: A Competing Risk Nomogram Analysis. J Cancer (2018) 9:3156–67. doi: 10.7150/jca.25494
41. Shi S, Hua J, Liang C, Meng Q, Liang D, Xu J, et al. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann Surg (2019) 269:944–50. doi: 10.1097/sla.0000000000002668
42. Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of Total Lymph Node Count and Lymph Node Ratio on Staging and Survival After Pancreatectomy for Pancreatic Adenocarcinoma: A Large, Population-Based Analysis. Ann Surg Oncol (2008) 15:165–74. doi: 10.1245/s10434-007-9587-1
43. Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood Glucose Concentration and Risk of Pancreatic Cancer: Systematic Review and Dose-Response Meta-Analysis. BMJ (Clinical Res ed) (2015) 350:g7371. doi: 10.1136/bmj.g7371
44. Raghavan SR, Ballehaninna UK, Chamberlain RS. The Impact of Perioperative Blood Glucose Levels on Pancreatic Cancer Prognosis and Surgical Outcomes: An Evidence-Based Review. Pancreas (2013) 42:1210–7. doi: 10.1097/MPA.0b013e3182a6db8e
45. Li W, Zhang L, Chen X, Jiang Z, Zong L, Ma Q. Hyperglycemia Promotes the Epithelial-Mesenchymal Transition of Pancreatic Cancer Via Hydrogen Peroxide. Oxid Med Cell Longev (2016) 2016:5190314. doi: 10.1155/2016/5190314
46. Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology (2018) 155:490–500.e2. doi: 10.1053/j.gastro.2018.04.025
47. Nagai M, Murakami Y, Tamakoshi A, Kiyohara Y, Yamada M, Ukawa S, et al. Fasting But Not Casual Blood Glucose is Associated With Pancreatic Cancer Mortality in Japanese: EPOCH-JAPAN. Cancer Causes Control (2017) 28:625–33. doi: 10.1007/s10552-017-0884-0
48. Shrikhande SV, Kleeff J, Reiser C, Weitz J, Hinz U, Esposito I, et al. Pancreatic Resection for M1 Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol (2007) 14:118–27. doi: 10.1245/s10434-006-9131-8
Keywords: pancreatic ductal adenocarcinoma, nomogram, cancer-specific survival (CSS), decision curve analysis, AJCC 8
Citation: Li G, Liao C-Y, Chen J-Z, Huang L, Yang C, Tian Y-F, Wang Y-T, Du Q, Zhan Q, Chen Y-L and Chen S (2021) Construction and Validation of Novel Nomograms for Predicting Prognosis of Pancreatic Ductal Adenocarcinoma After Surgery According to Different Primary Cancer Locations. Front. Oncol. 11:646082. doi: 10.3389/fonc.2021.646082
Received: 24 December 2020; Accepted: 06 April 2021;
Published: 23 April 2021.
Edited by:
Kanjoormana Aryan Manu, Amala Cancer Research Centre, IndiaReviewed by:
Benedict Kinny-Köster, Johns Hopkins Medicine, United StatesMing Cui, Peking Union Medical College Hospital (CAMS), China
Jishu Wei, Nanjing Medical University, China
Copyright © 2021 Li, Liao, Chen, Huang, Yang, Tian, Wang, Du, Zhan, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Chen, d2F3bGp3YWxqQGZqbXUuZWR1LmNu; Yan-Ling Chen, ZHJjaGVueWxAMTI2LmNvbQ==; Qian Zhan, emhhbnhpODBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ge Li
Ge Li Cheng-Yu Liao3†
Cheng-Yu Liao3† Shi Chen
Shi Chen