- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 2Department of Pathology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 4Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 5Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
Background: Gallbladder cancer (GBC) has a poor prognosis. Although complete surgical resection is the only successful approach for improving survival, additional therapeutic modalities are required for recurrent or surgically unresectable GBCs.
Materials and Methods: To determine the expression status of HER2 and the mismatch repair (MMR) proteins MLH1, MSH2, MSH6, and PMS2, immunohistochemical staining of MMR proteins and HER2 was carried out in 216 surgically resected GBCs. HER2 labeling was scored by adopting a scoring system for gastric carcinomas. Tissues scoring 0 to 2+ were defined as HER2 negative, whereas those scoring 3+ were regarded as HER2-positive. In addition, silver in situ hybridization and microsatellite instability (MSI) analysis were conducted to confirm HER2 amplification and MSI, respectively.
Results: Three of 216 GBCs (1.3%) showed MMR protein deficiency. All three observed MSI cases exhibited dual loss of MSH2 and MSH6 protein expression. However, no cases showed loss of either MLH1 or PMS2 expression. No association was observed between MMR protein deficiency and other clinicopathological factors. HER2 amplification was noted in 30 (13.9%) GBCs and associated with Crohn-like lymphoid reaction (P = 0.023). No survival difference was observed based on HER2 overexpression or HER2 amplification status.
Conclusion: MMR protein deficiency and HER2 overexpression were observed in a small subset (1.3% and 13.9%, respectively) of GBCs without simultaneous occurrence of deficient MMR protein expression and HER2 overexpression. The presence of Crohn-like lymphoid reaction may help identify cases with HER2 amplification, by using hematoxylin-stained slides. Although the proportion of MMR protein-deficient- and HER2-overexpressing GBCs was small, applying immunotherapy to MMR protein-deficient GBCs and herceptin to HER2-overexpressing GBCs may provide alternative treatment options for patients with GBC.
Introduction
Gallbladder cancer (GBC) is the most common malignancy of the biliary tract and is characterized by dismal prognosis (1, 2). The prevalence of GBC is relatively high in several countries, including Korea, Chile, Poland, India, Pakistan, Ecuador, and Japan, whereas being low in many Western countries (3). GBC and cholangiocarcinoma, is the seventh most common cancer in Korea (4). Currently, surgical resection is the only promising therapeutic option for cure. The median survival time of patients with GBC is 75 months, with a 5-year survival rate of about 50% after surgical resection (5). However, surgical resection is applicable only to up to 25% of all GBC patients exhibiting localized disease (6, 7). Conversely, systemic chemotherapies are required for patients with recurrent or metastatic GBC. Although gemcitabine and cisplatin are currently being used as the backbone of chemotherapeutic regimens for patients with GBC, the median overall survival for GBC patients treated with gemcitabine and cisplatin is less than 1 year (8, 9). Therefore, to improve the treatment response rate, a better understanding of the molecular mechanisms of GBC is pivotal.
Microsatellite instability (MSI), caused by abrogation of functional mismatch repair mechanisms, alters tumor biology by causing the inactivation of genes containing repeat sequences. For the cure of MSI-high metastatic colon cancer, the use of immune checkpoint inhibitors, such as pembrolizumab, was approved by the Food and Drug Administration (FDA) (10, 11). Subsequently, the FDA approvals for checkpoint inhibitors were extended to the treatment of all MSI-high solid tumors. Human epidermal growth factor 2 (HER2), a member of the receptor tyrosine kinase family, promotes cell proliferation thorough various signaling pathways (12). Therefore, blocking these HER2-dependent pathways has been considered a promising therapeutic strategy for patients harboring HER2-overexpressing or -amplified cancers; for instance, trastuzumab has been approved for the management of HER2-overexpressing breast and stomach cancers (13, 14). In addition, targeted exome sequencing of gallbladder cancer was recently introduced in clinical oncology for identifying druggable targets (15). Therefore, patients with gallbladder cancers with either MSI-high or HER2-overexpression or amplification could be used specific target agent. To date, several studies have examined the prevalence of HER2 overexpression (15–24) and DNA mismatch repair deficiency (25–31) in GBC. However, marked variations exist among these studies because of their relatively small sample size, varying experimental methods, and differing inclusion criteria. Therefore, to better understand the molecular biology and pathogenesis of GBC, systemic data analysis with a sufficient sample size is necessary.
The aim of this study was to investigate the expression status of MMR proteins and HER2 in patients with GBC, to provide a rationale for the application of immunotherapy and HER2-targeted therapy to GBC cases with advanced or metastatic setting.
Materials And Methods
Case Selection
This study was performed after approval from the institutional review board (approval number: 2019-0142) with a waiver of informed consent. A total of 216 surgically resected primary GBC patients were selected from a surgical pathology database. Clinical data, including patient’s age, sex, type of surgical resection, tumor location, survival time, and survival status, were reviewed in electronic medical records. Patients who had received neoadjuvant radiation and/or chemotherapy were excluded.
Histopathologic Evaluation
Pathologic features, including tumor size, differentiation, histologic subtype, Crohn-like lymphoid reaction, and lymphovascular and perineural invasion, were reviewed by two pathologists (SMH and YNS). Histologic subtypes were classified according to the fifth edition of the World Health Organization (WHO) classification (32). T- and N-categories and stage grouping were evaluated according to the eighth American Joint Committee on Cancer (AJCC) staging system (33). Crohn-like lymphoid reaction was defined as discrete lymphoid aggregates with occasional germinal centers at the advanced edge of the tumor (34).
Tissue Microarray Construction
Tissue microarrays (TMAs) were constructed from all 216 formalin-fixed, paraffin-embedded GBC tissue blocks by using a manual tissue micro-arrayer (Uni TMA Co, Ltd, Seoul, Korea). Areas occupied for >75% by tumor cells characterized by major histological differentiation and without accompanying tumor necrosis were selected, and four representative tumor tissue cores and one core from a matched normal gallbladder mucosal tissue were punched out from a donor block and transferred into a new recipient block with a punch of a 2-mm diameter.
Immunohistochemical Staining of HER2 and MMR Proteins
Immunohistochemical (IHC) labeling was performed at the IHC laboratory of the Department of Pathology. Briefly, 4-μm-thick tissue sections from TMAs and whole-section slides of cases showing MMR protein deficiency in TMAs were deparaffinized and hydrated in xylene and serially diluted alcohol solutions. Endogenous peroxidase was blocked by incubation in 3% H2O2 for 10 min; next, heat-induced antigen retrieval was performed. Primary antibodies with BenchMark auto-stainers (Ventana Medical Systems, Tucson, AZ, USA) were used following the manufacturer’s protocol. Primary antibodies for HER2 (4B5, mouse monoclonal, 1:8, Ventana), MLH1 (clone M1, mouse monoclonal, prediluted, Roche, Basel, Switzerland), MSH2 (clone G219-1129, mouse monoclonal, 1:1000, Cell Marque, Rocklin, CA, USA), MSH6 (clone 44, mouse monoclonal, 1:200, Cell Marque), and PMS2 (clone EP51, rabbit monoclonal, 1:100, Dako, Glostrup, Denmark) were used. After the evaluation of IHC-labeled TMA slides, additional immunolabeling was performed on sections on the whole-section slides if samples showed any loss of MMR proteins or an HER2 immunolabeling score of 2+ or 3+ on TMA slides.
HER2 IHC Scoring
HER2 IHC-sections were assigned a score from 0 to 3+ based on the intensity and pattern of membranous labeling, according to the scoring system recommended by the consensus panel on HER2 scoring for gastric cancer (35). Briefly, a HER2 IHC score of 0 (negative) was assigned for no or <10% reactivity in tumor tissue at 40× magnification; the score 1+ (negative) for faint/slight or partial (≥10%) membrane reactivity in tumor tissue at 40× magnification; the score 2+ (equivocal) for weak to moderate, complete or basolateral (≥10%) reactivity in tumor tissue at 10× to 20× magnification; and the score 3+ (positive) for moderate to strong, complete or basolateral (≥10%) reactivity in tumor tissue at 5× magnification [15]. Gastric cancer tissue with strong HER2 positivity (3+), confirmed to be effective in anti HER2 therapy, was used a positive control. Negative controls were performed by substituting a nonimmune purified mouse monoclonal antibody for the primary HER2 antibody. Tissues scoring from 0 to 2+ were defined as HER2 negative, whereas those with a score of 3+ were regarded as HER2 positive (Figure 1).
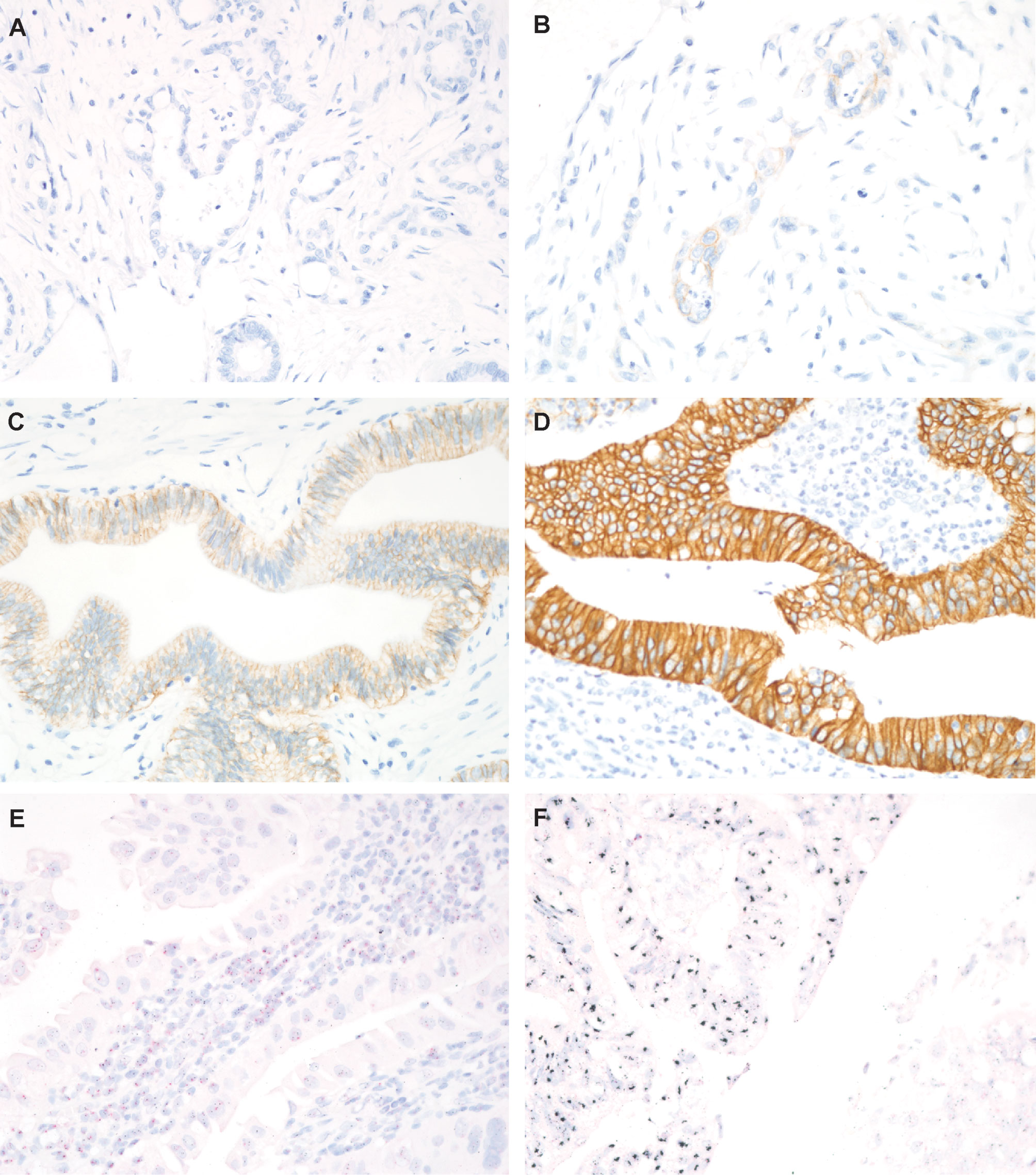
Figure 1 Representative images of HER2 immunohistochemical (IHC) staining and HER2 silver in situ hybridization (SISH). (A) HER2 IHC score 0, no expression (magnification, 400×); (B) HER2 IHC score 1+, barely visible (400×); (C) HER2 IHC score 2+, weak to moderate staining (400×); (D) HER2 IHC score 3+, intense staining (400×); (E) Absence of HER2 amplification in cases with HER2 IHC score 0; (F) HER2 amplification in cases with HER2 IHC score 3+. Red dots correspond to CEP17, and black dots correspond to HER2 (magnification, 400×).
HER2 Silver In Situ Hybridization and Scoring Criteria
Silver in situ hybridization (SISH) was conducted to confirm HER2 amplification in all cases with a HER2 immunolabeling score of 2+ or 3+. Unstained slides were processed with an automated system following the manufacturer’s protocols to hybridize HER2 and chromosome 17 (CEP17) probes (Ventana) (36). Both HER2 and CEP17 probes were sequentially hybridized on the same slide. The HER2 gene was visualized as a black dot and CEP17 as a red dot. The specimen was then counterstained with Harris hematoxylin.
The HER2 amplification status was evaluated by counting HER2 and CEP17 signals in nuclei of 20 consecutive tumor cells, according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines (37). In brief, specimens were defined negative for HER2 amplification if they displayed an HER2/CEP17 ratio of <2 and an average HER2 copy number of <4.0 signals/cell; equivocal if they exhibited an HER2/CEP17 ratio of <2 and an average HER2 copy number of ≥4.0 and <6.0 signals/cell; and positive if they showed an HER2/CEP17 ratio of ≥2 (37). Areas with overlapping nuclei, high nonspecific background staining, or weak signal were excluded from the evaluation (Figure 1).
Analysis of MMR Protein Immunolabeling
Samples showing complete loss of nuclear staining for each MMR protein were defined as deficient for MMR proteins. Adjacent stromal cells and inflammatory cells with intact nuclear staining served as positive controls. For cases displaying loss of any MMR protein on TMA slides, MMR IHC staining was repeated on whole sections for histopathologic confirmation.
MSI Analysis
Only in cases showing deficiency for MMR proteins on whole-sectioned slides, additional PCR was done to confirm the suspected MSI. Five quasi-monomorphic mononucleotide repeats, namely BAT25, BAT26, NR21, NR25, and NR27, were amplified in a single multiplex PCR reaction (38, 39). The PCR products were analyzed by capillary electrophoresis with an ABI310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Samples with MSI at ≥2 mononucleotide loci were considered MSI high, those with instability at a single mononucleotide locus MSI low, and those with no instability at any of the loci tested microsatellite stable (MSS), in accordance with National Cancer Institute (NCI) guidelines (40).
Statistical Analysis
The R software (version 4.0.2, Vienna, Austria) was used to perform statistical analyses. The unpaired Student’s t-test was used to compare the means. Associations between HER2 expression and MSI status or other clinicopathologic factors were tested using the χ2 and/or Fisher exact tests. The overall survival rate was calculated using the Kaplan–Meier method, and significance was evaluated with the log-rank test. P<0.05 was considered to denote statistically significant differences.
Results
Clinicopathological Characteristics
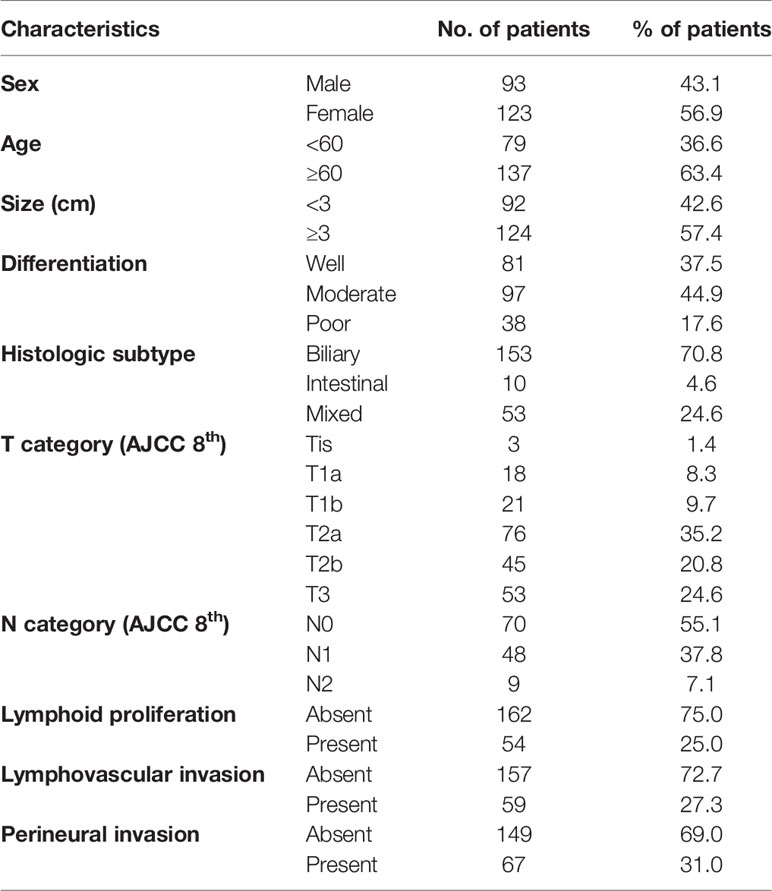
The clinicopathological characteristics of the patients are summarized in Table 1. The mean age of the patients was 62.8 ± 10.1 years (range, 36–89 years) with a male-to-female ratio of 0.8. The mean tumor size was 3.4 ± 1.8 cm. One hundred and fifty-three cases (70.8%) were of biliary histologic subtype tumors, 10 cases (4.6%) were of intestinal subtype tumors, and 53 cases (24.6%) were of a mixed subtype. Ninety-seven (44.9%) cases consisted of moderately differentiated tumors. Based on the eighth edition of the AJCC cancer staging scheme, three carcinomas were in situ (Tis, 1.5%), 18 T1a (8.3%), 21 T1b (9.7%), 76 T2a (35.2%), 45 T2b (20.8%), and 53 T3 (24.6%). However, no T4 tumor was observed because all samples were obtained from surgically resected GBCs. Among the 127 evaluated cases with at least one examined lymph node, 70 were N0 (55.1%), 48 N1 (37.8%), and nine N2 (7.1%) tumors, according to the N category. Crohn-like lymphoid reaction was observed in 54 (25.0%) cases. Lymphovascular and perineural invasion was identified in 59 (27.3%) and 67 (31.0%) cases, respectively.
HER2 Immunolabeling and HER2 SISH
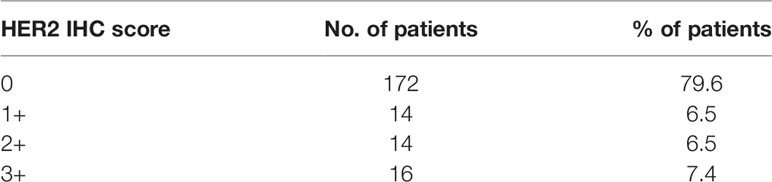
An IHC score of 0, 1+, 2+, and 3+ was observed in 172 (79.6%), 14 (6.5%), 14 (6.5%), and 16 (7.4%) cases, respectively (Table 2). Thirty GBC cases with IHC scores of 2+ or 3+ were evaluated by HER2 SISH, and all exhibited HER2 amplification. All cases with IHC score of 0 and 1+ showed no HER2 amplification (Figure 1E). Diffuse HER2 immunolabeling was noted in 67% (14 of 21) of HER2 IHC 2+ cases, whereas focal HER2 immunolabeling, characterized by a strong intensity of HER2 overexpression in association with HER2-negative areas, denoting heterogeneous HER2 expression, was observed in 33% (seven of 21) of GBC cases with an HER IHC score of 2+ on TMA slides (Figure 2). Similarly, diffuse HER2 immunolabeling was observed in 56% (9 of 16) of HER2 IHC 3+ cases; in contrast, 44% (seven of 16) of HER2 IHC 3+ tissues showed heterogeneous HER2 IHC labeling (focal HER2 IHC 3+). The results of correlation analysis between HER2 amplification and other clinicopathologic factors are summarized in Table 3. Notably, there was no significant association between HER2 amplification and clinicopathologic factors in patients with GBC.

Figure 2 Representative images of matched HER2 immunohistochemical (IHC) staining and HER2 silver in situ hybridization (SISH) from the same case. (A, B) Weak to moderate HER2 IHC staining (A, left panel, 400×) corresponding to HER2 amplification as shown by silver in situ hybridization (SISH; B, left panel, 400×). (C, D) Intense HER2 IHC staining at low magnification (C, left panel, 400×) corresponding to HER2 amplification as shown by SISH (D, left panel, 400×). Red dots correspond to CEP17, and black dots correspond to HER2.

Table 3 HER2 amplification and microsatellite status in association with clinicopathologic characteristics in gallbladder cancer.
Correlation Between HER2 Protein Expression, HER2 Gene Amplification, and Clinical Outcomes
The overall 5-year survival rate of GBC patients expressing HER2 (IHC score, 3) was 62.8%, whereas that of patients not expressing HER2 (IHC score, 0 to 2) was 45.6%. Nevertheless, there was no significant difference in survival of GBC patients according to the HER expression status (P = 0.19; Figure 3A).
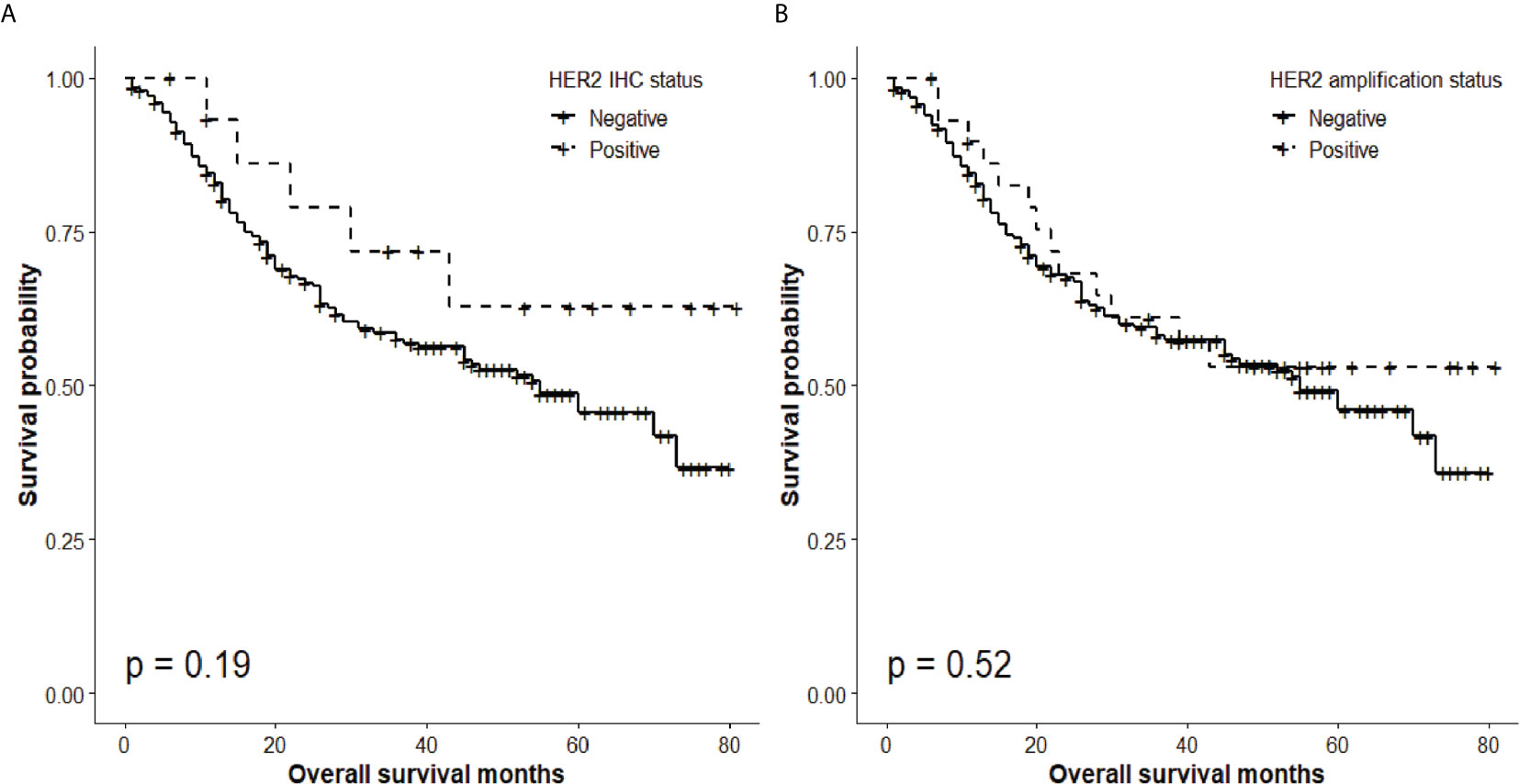
Figure 3 Overall survival according to HER2 status. (A) Absence of significant differences in overall 5-year survival rate according to HER2 IHC status (HER2 IHC 3+ tumors, 62.8%; HER2-negative (0, 1, 2+) tumors, 45.6%; P = 0.19). (B) Absence of significant differences in 5-year survival rate according to HER2 amplification status (HER2 SISH+, 53.1%; HER2 SISH−, 45.9%; P = 0.52).
Similarly, although the overall 5-year survival rate of GBC patients with HER2 gene amplification was 53.1%, whereas that of patients without HER2 amplification was 45.9%, there was no significant difference in survival of patients with different HER2 amplification status (P = 0.52; Figure 3B).
MSI of GBC
Fourteen (6.5%) out of the 216 analyzed GBCs showed loss of at least one MMR protein on TMA slides. Validation of MMR IHC on sections on the whole-section slides confirmed six of these 14 cases. Conversely, heterogeneous MMR protein expression was observed in the sections on the whole-section slides of the other eight cases (Figure 4). Finally, three (1.3%) GBC cases were confirmed as microsatellite unstable (two MSI high and one MSI low), and exhibited dual loss of MSH2 and MSH6. Furthermore, three cases displaying only MLH1 loss (case 6) or MSH6 loss (cases 11 and 14) in whole sections were MSS according to MSI PCR.
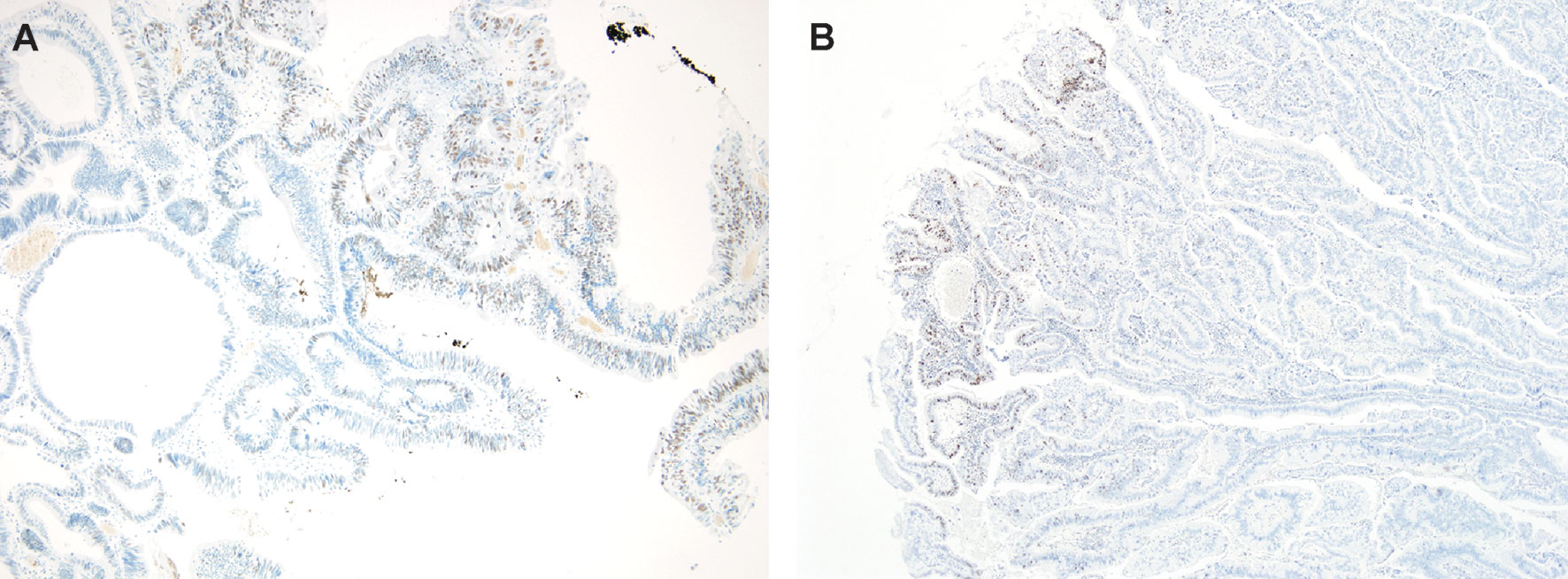
Figure 4 Heterogeneity of MMR protein immunolabeling. Loss of (A) MSH2 protein (left panel, 100×), and (B) MSH6 protein (right panel, 100×) with intact MMR proteins in another area of the same slide.
Association Between HER2 and MMR Protein Expression Status and Other Clinicopathologic Factors
Cases with HER2 amplification were associated with Crohn-like lymphoid reaction (P = 0.023, Figure 5). On the other hand, although all three MSI-H GBC cases showed Crohn-like lymphoid reaction, there was no statistically significant association between these two factors because of the small number of MSI cases (three cases). In addition, there was no other association between MSI or HER2 amplification status and other clinicopathologic factors (Table 3).
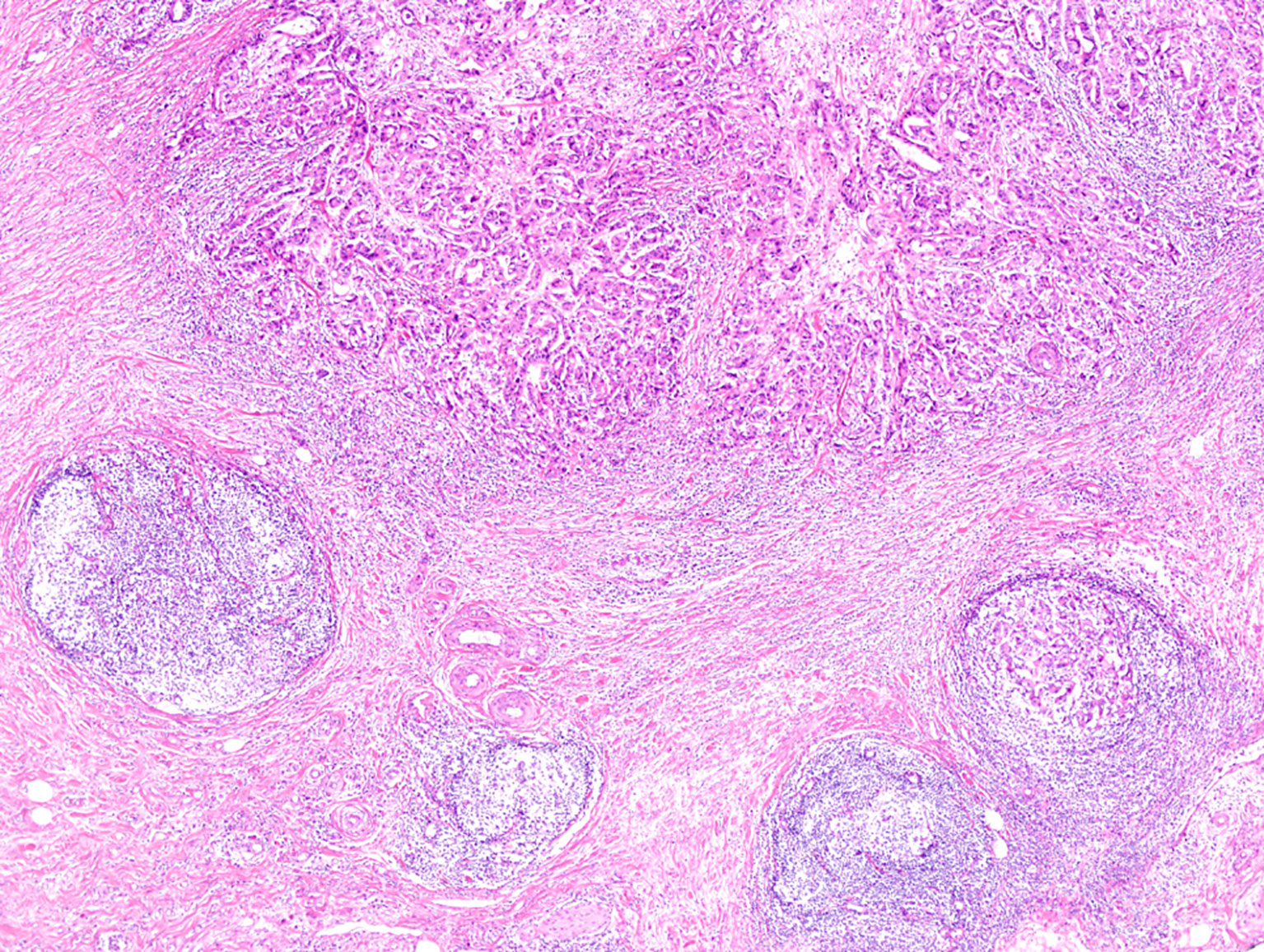
Figure 5 Representative image of Crohn-like lymphoid reaction observed at an advanced tumor edge (magnification, 40×).
Discussion
The present study, conducted in a large cohort, showed that about 14% of patients with GBC exhibited HER2 amplification, and only 1% of them had high MSI. In particular, HER2 amplification was observed in all the 30 cases that displayed an HER2 IHC score of 2+ or 3+.
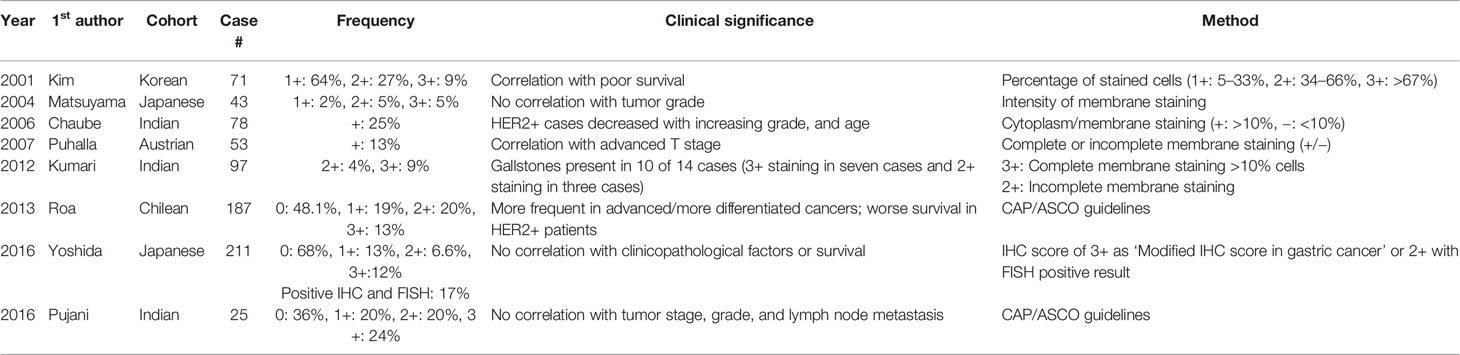
The frequency of HER2 amplification in the Korean cohort examined in the present study was about 14%. However, several previous studies with diverse ethnic backgrounds reported various frequencies of HER2 expression or HER2 amplification in GBCs (Table 4) (16). In particular, the frequency of HER2 expression (IHC score >2+) in the previous studies ranged from 9.4% to 44% (16–21, 23, 24). Nevertheless, because most studies evaluated less than 100 GBC cases and applied different criteria for evaluating HER2 IHC results, direct comparison of the prevalence of HER2 expression was difficult. Only one recent study performed by Yoshida et al. (23) investigated HER2 expression within a large cohort (211 GBC patients) with the same HER2 IHC scoring system that we used in the present study; these authors found a frequency of HER2 ICH 2+ and 3+ GBCs of 6.6% (14/211) and 11.8% (25/211), respectively. Although the frequency of HER2 IHC 2+ tumors in the study by Yoshida et al. was similar to that of our study (6.5%), the proportion of GBCs with an HER2 IHC score of 3+ was slightly higher than that of the present study (7.4%). In addition, when investigating HER2 amplification by HER2 FISH, these authors found a quite different result from that of our study. Indeed, in our study, HER2 amplification was observed in all tested cases scoring 2+ and 3+ for HER2 IHC. In contrast, a few GBC cases showed no HER2 amplification (HER2 IHC score 2+: 2/12; IHC score 3+: 1/25) in the study by Yoshida et al. (23). Although both SISH and FISH are good methods to determine the amplification status, SISH is believed to be a better analytic method than FISH, because observation in bright fields enables more precise diagnosis, and lower bleaching risk allows longer-term storage of SISH samples (35). This could explain the difference in the observed amplification rate between the study of Yoshida et al. and the present study. Additionally, we observed an association between HER2 amplification and Crohn-like lymphoid reaction. Similarly, tertiary lymphoid structures were more frequently observed in HER2-positive breast cancers (41). Moreover, an association between Crohn-like lymphoid reaction and HER2 amplification status has been reported in urothelial carcinoma (42), and is probably related to HER2-dependent activation of inflammatory pathways, especially those connected with IL-1α and IL-6 (43, 44).
In the present study, we observed heterogeneous HER2 immunolabeling in 33% of HER2 IHC 2+ and 44% of HER2 IHC 3+ GBCs, which showed focal HER2 immunolabeling with locally strong HER2 labeling within a HER2-negative area. This result suggests that IHC examination of HER2 in samples from patients with GBC, by small endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNAB) could lead to false-negative results, especially for tumors with unresectable or metastatic setting. Thus, to prevent possible HER2 IHC false negative results, it is required to get a larger number of cancer cells from metastatic or unresectable GBC lesions.
Most of the previous studies on HER2 expression included only IHC experiments (16–21, 24). In contrast, our present study confirmed the HER2 IHC score of 2+ in 14 cases and HER2 amplification in all 14 cases by SISH.
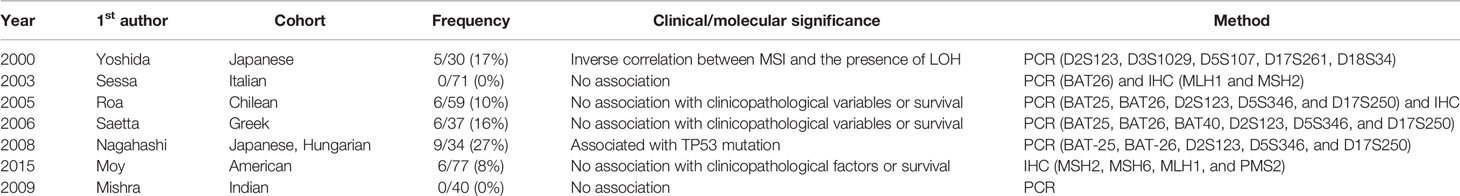
MSI testing could be considered as a diagnostic approach for patients with GBC, because patients with MSI-high GBC stand a chance to benefit from immune checkpoint inhibitors (45). However, the proportion of MSI-high GBCs was found to be only 1.3% in the present study. The prevalence of various MSI conditions and their association with other clinicopathologic factors in GBC reported by previous studies are summarized in Table 5. In these studies, the prevalence of MSI in GBC varied widely from 0% to 26.5%, and no significant association with any clinicopathologic factor was identified. Although a few studies reported the association between MSI status and LOH or p53 mutation (25, 29), the number of cases was too small to draw a solid conclusion. We evaluated a relatively large number of surgically resected GBCs and observed only a small subset of MSI-high GBC cases (1.3%), with no obvious clinicopathologic association. Several previous papers evaluated PD-1 expression in prognostic aspect of gallbladder cancers (46–51), and one of the study reported association of MSI-H and PD-L1 expression (49). For precise evaluation of tumor infiltrating lymphocytes (TILs) and PD-L1 expression, TIL and PD-L1 expression should be evaluated mainly at the tumor-normal interphase, whole-section slides are required. However, the limitation of performing additional PD-L1 expression is that our present study is a TMA-based study. Therefore, further studies with large number of cases with whole sectioned slides are required for proper evaluation of MSI-H and association with PD-L1 expression.
We observed heterogeneity of MMR protein expression, leading to a high false-negative rate in the evaluation of MSI status of GBCs, during the validation of IHC results of sections on the whole-section slides by PCR. Similar to the consequences of heterogeneous HER2 IHC staining, false-negative or false-positive results could be obtained during the evaluation of MMR protein expression or MSI status by EUS-FNAB on small samples from patients with GBC, especially for tumors with unresectable or metastatic setting.
The current findings indicate that subsets of patients with GBC may benefit from HER2-targeted therapy and anti-PD-1 therapy. Multiple anti-HER2 agents, including trastuzumab, HER2-based drug combinations, and HER2-directed antibody-drug conjugates, are now under investigation for treating patients with HER2-overexpressing biliary tract cancer, based on their promising efficacy in prior small retrospective studies (52). As pembrolizumab, an anti-PD-1 agent, was recently approved for the management of patients harboring MSI-high tumors, regardless of cancer type (53–55), a greater effort should be made to identify MSI-high GBC patients, considering the limited therapeutic options and the poor prognosis of recurring or metastatic GBC cases.
In summary, MMR protein deficiency and HER2 overexpression were observed in a small subset (1.3% and 13.9%, respectively) of GBCs. There was no simultaneous occurrence of deficient MMR protein expression and HER2 overexpression. The occurrence of Crohn-like lymphoid reaction could help identify GBC cases with HER2 amplification on hematoxylin-stained slides. Although the proportion of MMR protein-deficient and HER2-overexpressing tumors was small, applying immunotherapy to MMR protein-deficient GBCs and trastuzumab to HER2-overexpressing GBCs may provide additional treatment options for patients with surgically resected GBC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Asan Medical Center (approval number: 2019-0142). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization, Y-NS and S-MH. Methodology, Y-NS and S-MH. Software, Y-NS. Investigation, Y-NS, S-YJ, and S-MH. Data curation and formal analysis, SK. JL, DH, SL, and S-MH. Resources, DH, SH, and S-MH. Writing—original draft preparation, Y-NS, S-YJ. Writing—review and editing, Y-NS, CY, K-PK, and S-MH. Visualization, Y-NS. Supervision, S-MH. Project administration, Y-NS. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was presented, in part, at the 69th annual fall meeting of the Korean Society of Pathologists, taking place from October 31st to November 2nd, 2017 in Seoul, Republic of Korea.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.658564/full#supplementary-material
References
1. Lai CH, Lau WY. Gallbladder Cancer–a Comprehensive Review. Surgeon (2008) 6:101–10. doi: 10.1016/S1479-666X(08)80073-X
2. Hundal R, Shaffer EA. Gallbladder Cancer: Epidemiology and Outcome. Clin Epidemiol (2014) 6:99–109. doi: 10.2147/CLEP.S37357
3. Vijayakumar A, Patil V, Mallikarjuna MN, Shivaswamy BS. Early Diagnosis of Gallbladder Carcinoma: An Algorithm Approach. ISRN Radiol (2013) 2013:239424. doi: 10.5402/2013/239424
4. Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. Prediction of Cancer Incidence and Mortality in Korea, 2020. Cancer Res Treat (2020) 52:351–8. doi: 10.4143/crt.2020.203
5. Sung YN, Song M, Lee JH, Song KB, Hwang DW, Ahn CS, et al. Validation of the 8th Edition of the American Joint Committee on Cancer Staging System for Gallbladder Cancer and Implications for the Follow-Up of Patients Without Node Dissection. Cancer Res Treat (2020) 52:455–68. doi: 10.4143/crt.2019.271
6. Zhu AX, Hong TS, Hezel AF, Kooby DA. Current Management of Gallbladder Carcinoma. Oncologist (2010) 15:168–81. doi: 10.1634/theoncologist.2009-0302
7. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res Treat (2020) 52:335–50. doi: 10.4143/crt.2020.206
8. Kim BJ, Hyung J, Yoo C, Kim KP, Park SJ, Lee SS, et al. Prognostic Factors in Patients With Advanced Biliary Tract Cancer Treated With First-Line Gemcitabine Plus Cisplatin: Retrospective Analysis of 740 Patients. Cancer Chemother Pharmacol (2017) 80:209–15. doi: 10.1007/s00280-017-3353-2
9. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin Plus Gemcitabine Versus Gemcitabine for Biliary Tract Cancer. N Engl J Med (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
10. Arora SP, Mahalingam D. Immunotherapy in Colorectal Cancer: For the Select Few or All? J Gastrointest Oncol (2018) 9:170–9. doi: 10.21037/jgo.2017.06.10
11. Song W, Shen L, Wang Y, Liu Q, Goodwin TJ, Li J, et al. Synergistic and Low Adverse Effect Cancer Immunotherapy by Immunogenic Chemotherapy and Locally Expressed PD-L1 Trap. Nat Commun (2018) 9:2237. doi: 10.1038/s41467-018-04605-x
12. Moasser MM. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene (2007) 26:6469–87. doi: 10.1038/sj.onc.1210477
13. English DP, Roque DM, Santin AD. HER2 Expression Beyond Breast Cancer: Therapeutic Implications for Gynecologic Malignancies. Mol Diagn Ther (2013) 17:85–99. doi: 10.1007/s40291-013-0024-9
14. Scholl S, Beuzeboc P, Pouillart P. Targeting HER2 in Other Tumor Types. Ann Oncol (2001) 12(Suppl 1):S81–7. doi: 10.1093/annonc/12.suppl_1.S81
15. Chae H, Kim D, Yoo C, Kim KP, Jeong JH, Chang HM, et al. Therapeutic Relevance of Targeted Sequencing in Management of Patients With Advanced Biliary Tract Cancer: DNA Damage Repair Gene Mutations as a Predictive Biomarker. Eur J Cancer (2019) 120:31–9. doi: 10.1016/j.ejca.2019.07.022
16. Kim YW, Huh SH, Park YK, Yoon TY, Lee SM, Hong SH. Expression of the C-Erb-B2 and P53 Protein in Gallbladder Carcinomas. Oncol Rep (2001) 8:1127–32. doi: 10.3892/or.8.5.1127
17. Matsuyama S, Kitajima Y, Sumi K, Mori D, Satoh T, Miyazaki K. Gallbladder Cancers Rarely Overexpress HER-2/Neu, Demonstrated by Hercep Test. Oncol Rep (2004) 11:815–9. doi: 10.3892/or.11.4.815
18. Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary Study of P53 and c-erbB-2 Expression in Gallbladder Cancer in Indian Patients Manuscript Id: 8962091628764582. BMC Cancer (2006) 6:126. doi: 10.1186/1471-2407-6-126
19. Puhalla H, Wrba F, Kandioler D, Lehnert M, Huynh A, Gruenberger T, et al. Expression of P21(Wafl/Cip1), P57(Kip2) and HER2/neu in Patients With Gallbladder Cancer. Anticancer Res (2007) 27:1679–84.
20. Kumari N, Kapoor VK, Krishnani N, Kumar K, Baitha DK. Role of C-Erbb2 Expression in Gallbladder Cancer. Indian J Pathol Microbiol (2012) 55:75–9. doi: 10.4103/0377-4929.94862
21. Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients With Advanced Gallbladder Cancer. Gastrointest Cancer Res (2014) 7:42–8.
22. Javle M, Churi C, Kang HC, Shroff R, Janku F, Surapaneni R, et al. HER2/neu-Directed Therapy for Biliary Tract Cancer. J Hematol Oncol (2015) 8:58. doi: 10.1186/s13045-015-0155-z
23. Yoshida H, Shimada K, Kosuge T, Hiraoka N. A Significant Subgroup of Resectable Gallbladder Cancer Patients has an HER2 Positive Status. Virchows Arch (2016) 468:431–9. doi: 10.1007/s00428-015-1898-1
24. Pujani M, Makker I, Makker A, Jetley S, Goel MM, Jetley S. Expression of Human Epidermal Growth Factor Receptor (Her 2/Neu) and Proliferative Marker Ki-67: Association With Clinicopathological Parameters in Gallbladder Carcinoma. Asian Pac J Cancer Prev (2016) 17:3903–9.
25. Yoshida T, Sugai T, Habano W, Nakamura S, Uesugi N, Funato O, et al. Microsatellite Instability in Gallbladder Carcinoma: Two Independent Genetic Pathways of Gallbladder Carcinogenesis. J Gastroenterol (2000) 35:768–74. doi: 10.1007/s005350070036
26. Sessa F, Furlan D, Genasetti A, Billo P, Feltri M, Capella C. Microsatellite Instability and P53 Expression in Gallbladder Carcinomas. Diagn Mol Pathol (2003) 12:96–102. doi: 10.1097/00019606-200306000-00005
27. Roa JC, Roa I, Correa P, Vo Q, Araya JC, Villaseca M, et al. Microsatellite Instability in Preneoplastic and Neoplastic Lesions of the Gallbladder. J Gastroenterol (2005) 40:79–86. doi: 10.1007/s00535-004-1497-4
28. Saetta AA, Gigelou F, Papanastasiou PI, Koilakou SV, Kalekou-Greca H, Miliaras D, et al. High-Level Microsatellite Instability Is Not Involved in Gallbladder Carcinogenesis. Exp Mol Pathol (2006) 80:67–71. doi: 10.1016/j.yexmp.2005.04.001
29. Nagahashi M, Ajioka Y, Lang I, Szentirmay Z, Kasler M, Nakadaira H, et al. Genetic Changes of P53, K-Ras, and Microsatellite Instability in Gallbladder Carcinoma in High-Incidence Areas of Japan and Hungary. World J Gastroenterol (2008) 14:70–5. doi: 10.3748/wjg.14.70
30. Moy AP, Shahid M, Ferrone CR, Borger DR, Zhu AX, Ting D, et al. Microsatellite Instability in Gallbladder Carcinoma. Virchows Arch (2015) 466:393–402. doi: 10.1007/s00428-015-1720-0
31. Mishra PK, Jatawa SK, Raghuram GV, Pathak N, Jain A, Tiwari A, et al. Correlation of Aberrant Expression of P53, Rad50, and Cyclin-E Proteins With Microsatellite Instability in Gallbladder Adenocarcinomas. Genet Mol Res (2009) 8:1202–10. doi: 10.4238/vol8-4gmr653
32. Lokuhetty D, White VA, Watanabe R, Cree IA. WHO Classification of Tumours Editorial Board. 5th ed. Lyon (France: IARC (2019).
33. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK. AJCC Cancer Staging Manual. 8th edition. New York: Springer (2017).
34. Graham DM, Appelman HD. Crohn's-Like Lymphoid Reaction and Colorectal Carcinoma: A Potential Histologic Prognosticator. Mod Pathol (1990) 3:332–5.
35. Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 Testing in Gastric Cancer: A Practical Approach. Mod Pathol (2012) 25:637–50. doi: 10.1038/modpathol.2011.198
36. Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, Murillo AE, Gaire F, Farrell M, et al. Development of Automated Brightfield Double in Situ Hybridization (BDISH) Application for HER2 Gene and Chromosome 17 Centromere (CEN 17) for Breast Carcinomas and an Assay Performance Comparison to Manual Dual Color HER2 Fluorescence in Situ Hybridization (FISH). Diagn Pathol (2008) 3:41. doi: 10.1186/1746-1596-3-41
37. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Arch Pathol Lab Med (2014) 138:241–56. doi: 10.5858/arpa.2013-0953-SA
38. Nardon E, Glavac D, Benhattar J, Groenen PJ, Hofler G, Hofler H, et al. A Multicenter Study to Validate the Reproducibility of MSI Testing With a Panel of 5 Quasimonomorphic Mononucleotide Repeats. Diagn Mol Pathol (2010) 19:236–42. doi: 10.1097/PDM.0b013e3181db67af
39. Jun SY, Lee EJ, Kim MJ, Chun SM, Bae YK, Hong SU, et al. Lynch Syndrome-Related Small Intestinal Adenocarcinomas. Oncotarget (2017) 8:21483–500. doi: 10.18632/oncotarget.15277
40. Mojtahed A, Schrijver I, Ford JM, Longacre TA, Pai RK. A Two-Antibody Mismatch Repair Protein Immunohistochemistry Screening Approach for Colorectal Carcinomas, Skin Sebaceous Tumors, and Gynecologic Tract Carcinomas. Mod Pathol (2011) 24:1004–14. doi: 10.1038/modpathol.2011.55
41. Liu X, Tsang JYS, Hlaing T, Hu J, Ni YB, Chan SK, et al. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist (2017) 22:1316–24. doi: 10.1634/theoncologist.2017-0029
42. Tschui J, Vassella E, Bandi N, Baumgartner U, Genitsch V, Rotzer D, et al. Morphological and Molecular Characteristics of HER2 Amplified Urothelial Bladder Cancer. Virchows Arch (2015) 466:703–10. doi: 10.1007/s00428-015-1729-4
43. Liu S, Lee JS, Jie C, Park MH, Iwakura Y, Patel Y, et al. HER2 Overexpression Triggers an IL1alpha Proinflammatory Circuit to Drive Tumorigenesis and Promote Chemotherapy Resistance. Cancer Res (2018) 78:2040–51. doi: 10.1158/0008-5472.CAN-17-2761
44. Hartman ZC, Yang XY, Glass O, Lei G, Osada T, Dave SS, et al. HER2 Overexpression Elicits a Proinflammatory IL-6 Autocrine Signaling Loop That Is Critical for Tumorigenesis. Cancer Res (2011) 71:4380–91. doi: 10.1158/0008-5472.CAN-11-0308
45. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12:252–64. doi: 10.1038/nrc3239
46. Kim H, Kim J, Byeon S, Jang KT, Hong JY, Lee J, et al. Programmed Death Ligand 1 Expression as a Prognostic Marker in Patients With Advanced Biliary Tract Cancer. Oncology (2021) 2021:1–8. doi: 10.1159/000514404
47. Albrecht T, Brinkmann F, Albrecht M, Lonsdorf AS, Mehrabi A, Hoffmann K, et al. Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer. Cancers (Basel) (2021) 13:1682. doi: 10.3390/cancers13071682
48. Patil PA, Lombardo K, Cao W. Immune Microenvironment in Gallbladder Adenocarcinomas. Appl Immunohistochem Mol Morphol (2021). doi: 10.1097/PAI.0000000000000922
49. Kim JH, Kim K, Kim M, Kim YM, Suh JH, Cha HJ, et al. Programmed Death-Ligand 1 Expression and its Correlation With Clinicopathological Parameters in Gallbladder Cancer. J Pathol Transl Med (2020) 54:154–64. doi: 10.4132/jptm.2019.11.13
50. Lin J, Long J, Wan X, Chen J, Bai Y, Wang A, et al. Classification of Gallbladder Cancer by Assessment of CD8(+) TIL and PD-L1 Expression. BMC Cancer (2018) 18:766. doi: 10.1186/s12885-018-4651-8
51. Neyaz A, Husain N, Kumari S, Gupta S, Shukla S, Arshad S, et al. Clinical Relevance of PD-L1 Expression in Gallbladder Cancer: A Potential Target for Therapy. Histopathology (2018) 73:622–33. doi: 10.1111/his.13669
52. Mondaca S, Razavi P, Xu C, Offin M, Myers M, Scaltriti M, et al. Genomic Characterization of ERBB2-Driven Biliary Cancer and a Case of Response to Ado-Trastuzumab Emtansine. JCO Precis Oncol (2019) 3:PO.19.00223. doi: 10.1200/PO.19.00223
53. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin Cancer Res (2019) 25:3753–8. doi: 10.1158/1078-0432.CCR-18-4070
54. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
55. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol (2020) 38:1–10. doi: 10.1200/JCO.19.02105
Keywords: gallbladder, cancer, mismatch repair proteins, microsatellite Instability, HER2
Citation: Sung Y-N, Kim SJ, Jun S-Y, Yoo C, Kim K-P, Lee JH, Hwang DW, Hwang S, Lee SS and Hong S-M (2021) Expression of HER2 and Mismatch Repair Proteins in Surgically Resected Gallbladder Adenocarcinoma. Front. Oncol. 11:658564. doi: 10.3389/fonc.2021.658564
Received: 26 January 2021; Accepted: 29 June 2021;
Published: 22 July 2021.
Edited by:
Giuseppe Giaccone, Cornell University, United StatesReviewed by:
Gurjeet Kaur, Universiti Sains Malaysia (USM), MalaysiaZhi Sheng, Virginia Tech, United States
Copyright © 2021 Sung, Kim, Jun, Yoo, Kim, Lee, Hwang, Hwang, Lee and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung-Mo Hong, c21ob25nMjhAZ21haWwuY29t
 You-Na Sung
You-Na Sung Sung Joo Kim1
Sung Joo Kim1 Sun-Young Jun
Sun-Young Jun Changhoon Yoo
Changhoon Yoo Seung-Mo Hong
Seung-Mo Hong