- 1Departments of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
- 3Departments of Pathology, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, South Korea
- 4Departments of Obstetrics and Gynecology, College of Medicine, Konyang University, Daejeon, South Korea
Background: In a previous study, a proteomic panel consisting of BCL-2, HER2, CD133, CAIX, and ERCC1 significantly predicted survival in patients with locally advanced cervical cancer. However, the prognostic significance of these proteins has not been assessed in early cervical cancer. The present study investigated the clinical significance and chemoradioresistance prediction power of these proteins in patients with early-stage cervical cancer.
Materials and Methods: BCL-2, HER2, CD133, CAIX, and ERCC1 expression was determined by the immunohistochemical staining of 336 cervical cancer tissue microarrays. The associations of these proteins with clinicopathologic characteristics and disease progression were assessed.
Results: There was a trend of low CAIX expression (p=0.082) and high ERCC1 expression (p=0.059) in patients with a favorable response to adjuvant radiation. High HER2 expression was significantly associated with shorter disease-free survival (DFS) in the total group (5-year DFS of 80.1% vs. 92.2%, p=0.004). A prognostic significance remained in multivariate analysis (Hazard ratio, HR=2.10, p=0.029). In the adjuvant radiation group, low CAIX and high ERCC1 expression indicated significantly unfavorable DFS (75.0% vs. 89.0%, p=0.026 and 76.8% vs. 88.6%, p=0.022, respectively). Low CAIX expression remained an independent prognostic marker in multivariate analysis (HR=0.45, p=0.037). The combined molecular-clinical model using random survival forest method predicted DFS with improved power compared with that of the clinical variable model (C-index 0.77 vs. 0.71, p=0.006).
Conclusion: HER2, CAIX, and ERCC1 expression can be predictive protein markers for clinical outcomes in early cervical cancer patients treated primarily with radical surgery with or without adjuvant radiation.
Introduction
Cervical cancer is the fourth most common malignancy and the leading cause of cancer-related death in women in developing countries (1). In the early stage, radical hysterectomy and adjuvant radiation with or without chemotherapy is the primary treatment and performed if there are risk factors. Although the prognosis is generally good, once disease recurs, there are limited options. The choice of adjuvant treatment depends not only on the side effects but also on the efficacy of the treatment. Each patient has a varied response to adjuvant radiation and/or chemotherapy; therefore, the prediction of the response to each treatment is important.
Several clinical factors that can predict the response to radiotherapy in cervical cancer have been investigated. Larger tumor size, regional metastases, and histologic subtypes are associated with a poor response to radiation (2, 3). Also, high tumor vascularity (4) and tumor hypoxia (5) contribute to radiation therapy resistance and are related to poor survival rates. Hyperthermia during radiotherapy can also make cancer cells more sensitive and affect the outcome of radiotherapy (6). However, these factors alone cannot accurately predict chemoradiosensitivity. Therefore, new markers with molecular approaches using genes or proteins are needed to predict the clinical outcomes of cancer patients more accurately.
Kitahara et al. identified a set of 62 genes that might be of great benefit for diagnosing the radiosensitivity of individual cervical squamous cell carcinomas (7). The expression of several apoptotic regulators, such as the B cell lymphoma-2 (BCL-2) family of proteins (BCL-2, BCL-XL, and BAX) and p53, may correspond to cervical cancer cell radiosensitivity (8, 9). In addition, the expression level of cyclooxygenases (COXs) (10, 11), epidermal growth factor receptor (EGFR), and vascular endothelial growth factor (VEGF) (12, 13) have been associated with radiosensitivity in several experimental studies. Clinicians have based methods of treatment on clinical factors; however, it is necessary to develop and identify biomarkers in an era of personalized therapy.
Previously, we have identified protein markers predicting survival using reverse-phase protein array (RPPA) in locally advanced cervical cancer. We used 181 locally advanced cervical cancer tissues to assess the expression levels of 22 selected protein markers using well-based RPPAs. The expression signals in well-based RPPA were correlated with data from western blot and immunohistochemistry (IHC). We also found that a panel of proteins, BCL-2, HER2, CD133, CAIX, and ERCC1, can be predictors of overall survival in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy (CCRT) by calculating risk scores, which were the sum of estimated coefficients from age, cancer stage, and the protein panel (14).
In this study, because a correlation existed between the protein panel and cancer prognosis or chemoradiation resistance in locally advanced cervical cancer, we assessed if the protein panel could predict disease prognosis or treatment response in patients with early-stage cervical cancer who had been treated using radical hysterectomy with or without adjuvant radiation. We analyzed the prognostic significance of these markers using IHC in tissue microarrays.
Materials and Methods
Patients and Tumor Samples
In this study, we retrieved the data of a total of 336 early-stage cervical cancer patients who were treated in the Department of Gynecologic Oncology, Samsung Medical Center (Seoul, South Korea), Sungkyunkwan University School of Medicine between 2002 and 2009. Tissue samples and medical records were obtained from patients who had signed an informed consent form, which was approved by the Institutional Review Board of Samsung Medical Center (IRB no. SMC 2009-09-002 and 2015-07-122; Seoul, South of Korea).
For the primary treatment, all patients underwent radical hysterectomy with or without pelvic/para-aortic lymph node dissection. In addition, patients received adjuvant radiotherapy with or without CCRT if the following risk factors were found; larger tumor size (more than 4 cm), lymphovascular invasion, deep stromal invasion (more than half), positive resection margin, parametrial invasion, or pelvic/para-aortic lymph node metastasis. After primary treatment, all patients received adequate follow-up treatment. During this period, patients underwent physical examination, Pap smears, and tumor marker measurements every 3 months for the first 2 years, and every 6 months for the next 3 years. HPV test was performed in not all patients but in those decided by the clinicians. HPV typing was done by using HPV type-specific primers. Cancer staging was classified by international federation of gynecology and obstetrics (FIGO) staging 2008. Imaging studies, such as chest radiography and abdominopelvic and/or chest computed tomography (CT), were conducted every 3–6 months for the first 2 years and then 6–12 months for the next 3 years.
Disease-free survival (DFS) was defined as the time interval from treatment to the first evidence of recurrence or the last follow-up. To examine the association between protein expression and chemoradiotherapy resistance, we defined ‘resistant response’ as recurrence within 3 years from adjuvant therapy and ‘sensitive response’ as no recurrence over three years from adjuvant therapy (15).
Tissue Microarray and Immunohistochemistry
Tissue microarrays (TMAs) were constructed from tissue blocks used for routine pathologic evaluation. In each case, areas with the most representative histology were selected, and three 0.6 mm cylindrical tissue cores were taken from formalin-fixed paraffin-embedded (FFPE) tissue blocks and extruded into the recipient paraffin block. To check the adequacy of tissue sampling, sections from each microarray were stained with hematoxylin and eosin and examined by light microscopy.
Immunohistochemical staining of BCL-2, HER2, CD133, CAIX, and ERCC1 was performed on 4 μm sections of TMAs and was performed using a standard streptavidin–peroxidase method as described previously (16). In order to prevent possible antigenicity loss during slide ageing (delay between cutting section and IHC staining), we used fresh-cut sections from original TMA blocks. After deparaffinization by using xylene and dehydration with graded ethanol, heat-induced antigen retrieval was performed for 20 minutes in an antigen retrieval buffer (Dako, Carpinteria, CA) of pH 6.0 (for BCL-2, HER2, CD133, and CAIX) and pH 8.0 (for ERCC1) in a pressure cooker (Pascal, Dako). Endogenous peroxidase activity was blocked with 3% H2O2 for 10 minutes at room temperature. The sections were incubated with primary antibodies. A detailed list of antibodies and adequate dilutions are provided in Supplementary Table 1. The primary antibodies were applied to test sections and positive-control sections for an adequate incubation time. Also, negative control slides were incubated by omitting the primary antibodies and no detectable staining was observed. The antigen–antibody reaction was detected with Dako EnVision+ Dual Link System-HRP (Dako) and DAB+ (3,3′-diaminobenzidine; Dako). Tissue sections were lightly counterstained with hematoxylin and then examined by light microscopy.
Quantitative Evaluation of Immunostaining
The evaluation of immunohistochemical staining was scored independently by two investigators (SJB and CHC) without knowledge of the clinicopathological findings. The intensity of staining was categorized as 0, 1+, 2+, and 3+ according to the distribution pattern across cores. The overall protein expression was measured as the mean value of histoscores, which is a result of multiplying the intensity score (0–3) and the percentage of stained cells, with a maximum of 300. For the survival analysis, expression values were dichotomized (high vs. low) with the cut-off values showing the most discriminative power (histoscore of 1 for BCL-2, 1 for HER2, 1 for CD133, 6 for CAIX, and 50 for ERCC1).
In Silico Analysis Using GSE44001
To examine the correlation between each protein expressions and corresponding mRNA expressions, data from the Gene Expression Omnibus (GEO) were analyzed as described previously (17). We downloaded the GES44001 dataset from the GEO website (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44001) and the samples of 300 patients were available. The analysis was carried out in the patients included in both studies.
Statistical Analysis
We performed statistical analysis using R 3.3.2 (Vienna, Austria; http://www.R-project.org). The expression levels of the proteins according to the clinicopathological characteristics were analyzed using Student’s t-test or Mann–Whitney U-test. Analysis of the Spearman’s rho coefficient was used to assess the correlation between proteins and mRNA expression. Analyses for survival distributions were performed by the Kaplan–Meier method and comparison between survival and each parameter was done with the log-rank test. We used the Cox proportional hazards model to evaluate the prognostic predictors of DFS.
To identify the predictive power of integrating the molecular data with clinical variables, we modified the random survival forest (RSF) method to include both clinical and molecular data (18). We used clinical data (FIGO stage, lymph node metastasis, tumor histology, tumor size, and parametrial invasion) to build the clinical RSF model and combined the molecular-level features with the clinical variables to build a new RSF model. A concordance index (C-index), which is a nonparametric measure to quantify the discriminatory power of a predictive model, was calculated and compared between the clinical and combined models using the Wilcoxon signed-rank sum test (19). All p-values were two-sided, and we considered p-values of less than 0.05 as statistically significant.
Results
Clinicopathological Characteristics of Patients
The clinicopathological characteristics of 336 patients are summarized in Table 1. The mean age of the patients was 49 years and 45 patients (13.4%) with IB2 or IIB were included because they were primarily treated with radical surgery and adjuvant radiotherapy with or without chemotherapy. In total, 291 (86.6%) patients were stage IIA or less and 256 (76.2%) patients had squamous cell carcinoma (76.2%). In165 patients, a HPV infection test was performed and 128 (77.6%) had high-risk types of HPV. Lymph node metastasis was found in 80 (23.8%) patients, parametrial invasion in 31 (9.2%), and positive resection margin in 13 (3.9%). Overall, 165 patients (49.1%) were treated with adjuvant radiation after radical surgery.
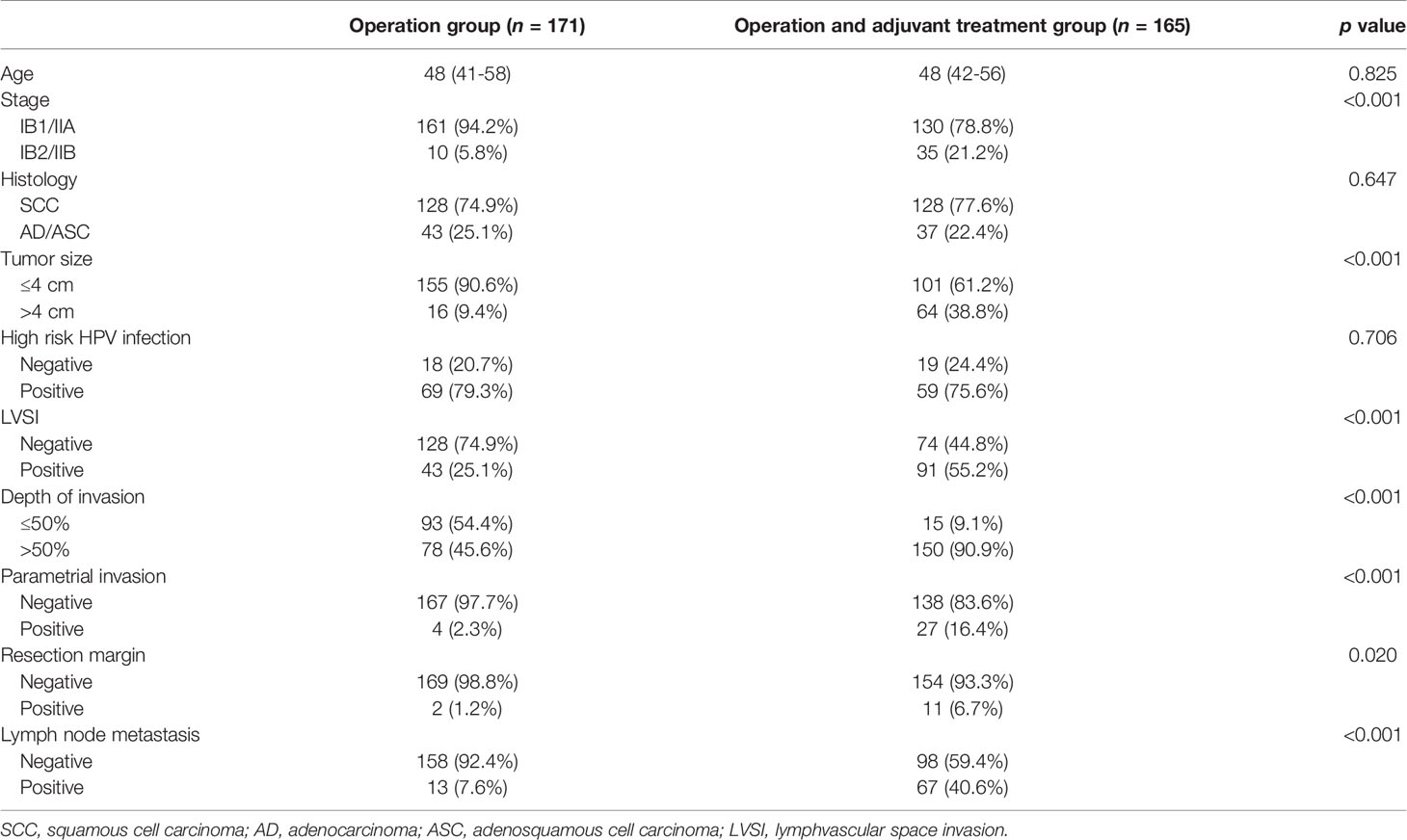
Table 1 Clinicopathological characteristics of 336 early cervical cancer patients according to adjuvant treatment.
Of the patients treated with adjuvant radiation, 113 patients (85.0%) were classified as chemoradiosensitive and 20 patients (15.0%) were classified as chemoradioresistant. The clinicopathological characteristics of patients with adjuvant treatment are shown in Supplementary Table 2. In total, 76 patients (57.1%) had CCRT and 57 (42.9%) patients had radiotherapy. Squamous cell carcinoma was more sensitive to radiation than adenocarcinoma or adenosquamous carcinoma (p=0.001).
BCL-2, HER2, CD133, CAIX, and ERCC1 Protein Expression
To examine the expression level of the proteins, we assessed cervical cancer tissues using IHC. BCL-2 and CD133 proteins were observed mainly in the cytoplasm, HER2 and CAIX mainly in the cell membrane, and ERCC1 mainly in the nucleus. Representative IHC images of these proteins are shown in Figure 1. We used histoscore to compare the extent of overall protein expression. CAIX and ERCC1 were expressed more than the other three proteins in early cervical cancer tissues. To examine the mRNA expression of the five proteins, we analyzed the GEO database (GSE44001), which contains the results of a DASL assay for RNA profiling with paraffin tissue from 300 patients with cervical cancer. We examined the correlation between each protein and mRNA. CD133 and CAIX protein expression was correlated weakly with mRNA expression (r=0.155; p=0.021 and r=0.190; p=0.005). Although other proteins had no statistical significance, the correlation had a positive trend (Supplementary Figure 1).
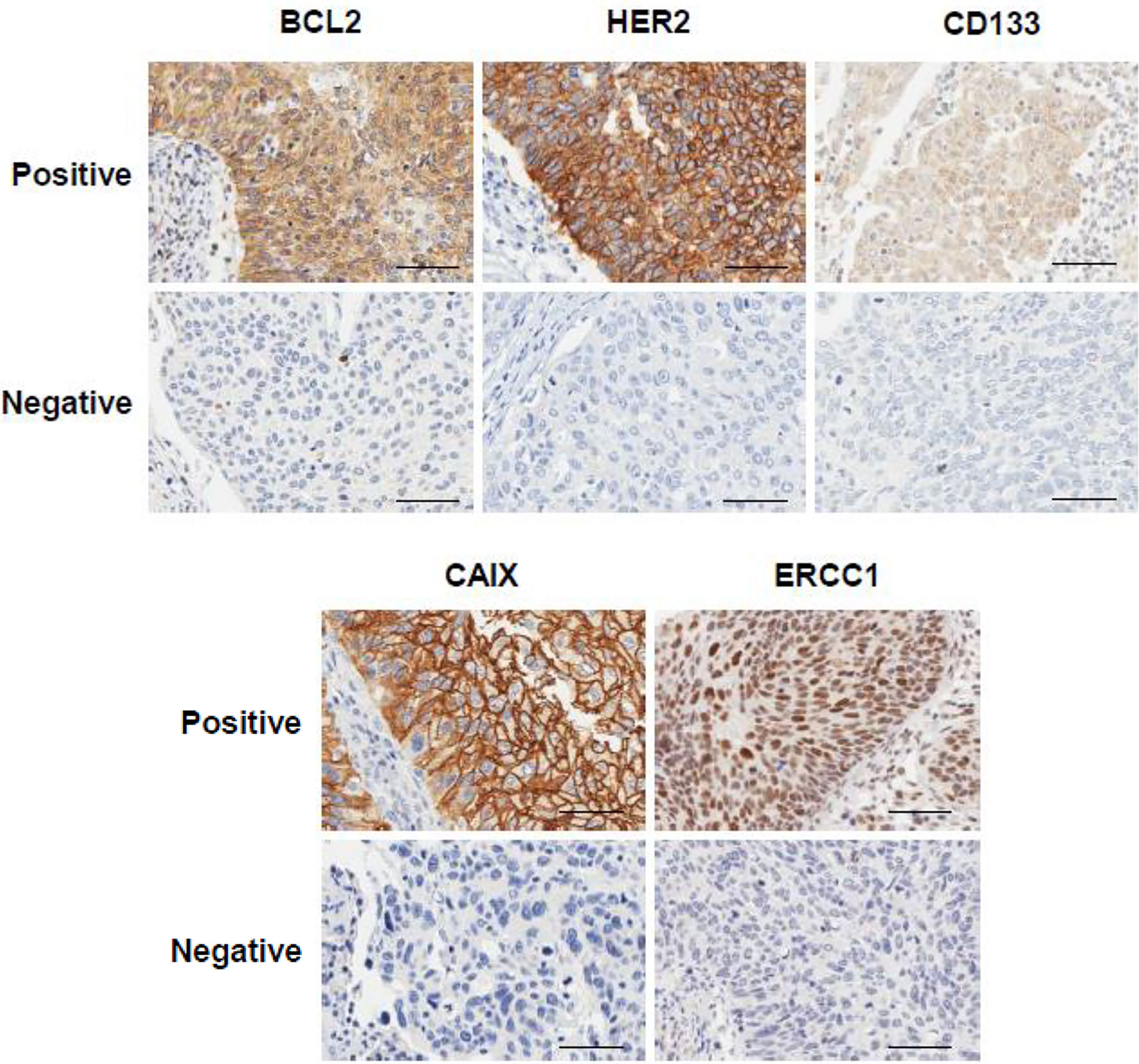
Figure 1 Expression of BCL-2, HER2, CD133, CAIX, and ERCC1 in early cervical cancer patients. Representative immunohistochemical images of positive and negative expression of each protein. The scale bar represents 50 μm.
CD133 and CAIX expression was cell type-dependent; CD133 and CAIX were more highly expressed in adeno/adenosquamous carcinoma (p=0.030 and p=0.003, respectively) (Table 2). These results suggest that CD133 and CAIX have different roles in cervical cancer according to cell type. In addition, the higher expression of ERCC1 was negatively correlated with the depth of invasion (p=0.013) and higher expression of CD133 was negatively correlated with high-risk type HPV infection (p=0.034). When protein expression level was analyzed in the adjuvant treatment group, CAIX positive was more frequent in the chemoradiosensitive group (58.9% vs. 35.0%, p=0.082), and ERCC1 positive was more frequent in the chemoradioresistant group (68.4% vs. 42.0%, p=0.059), though statistically not significant (Table 3). The two proteins should further be investigated as markers of chemoradiosensitivity in early cervical cancers.
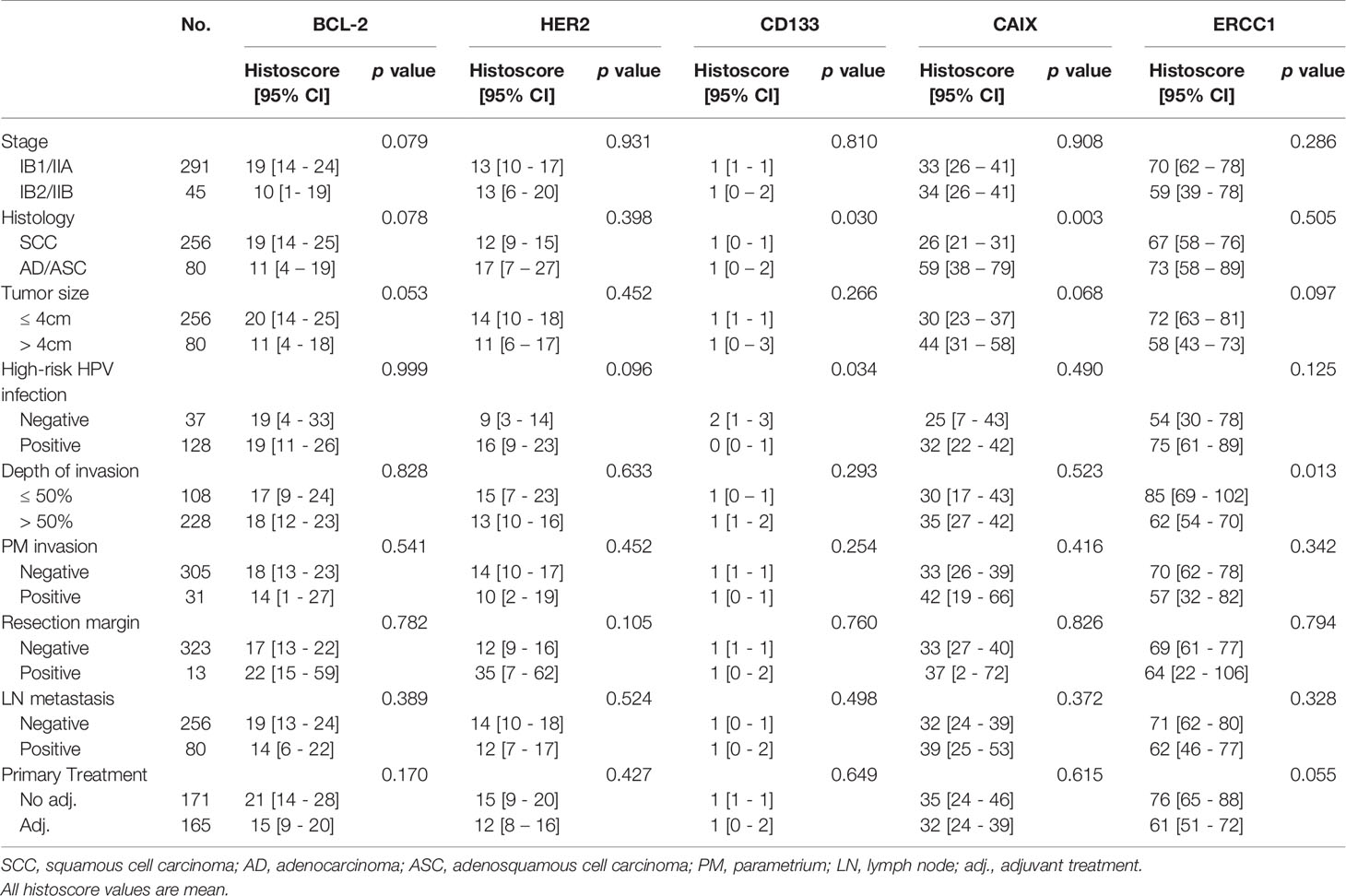
Table 2 The correlation between expression of potential protein markers predicting chemoradioresistance with clinicopathologic. characteristics of early cervical cancer.
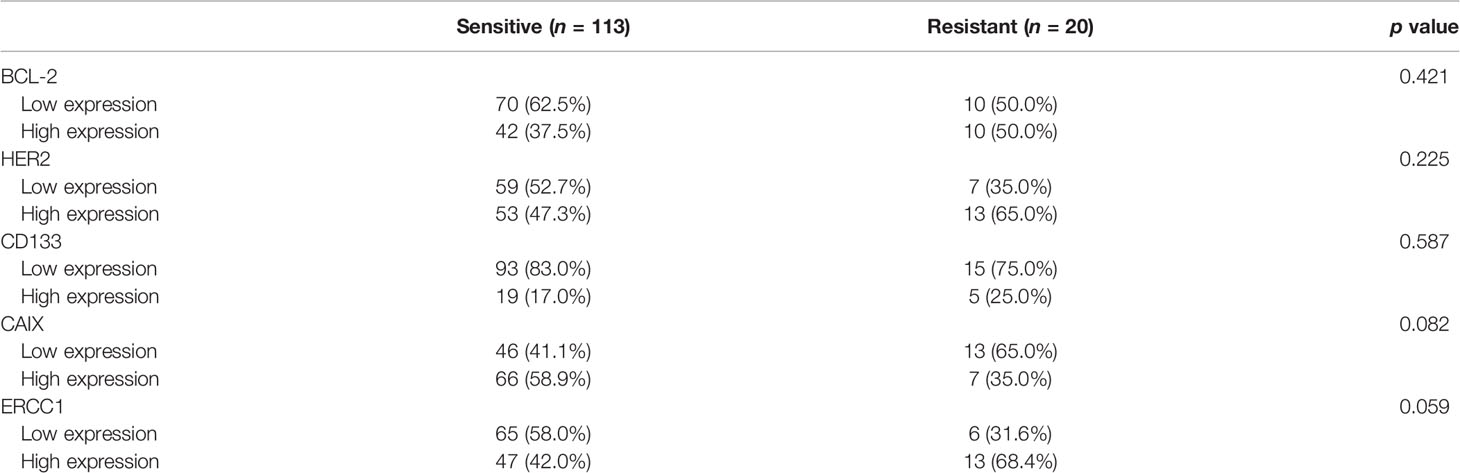
Table 3 Expression of protein markers according to chemoradioresistance in 133 early cervical cancer patients treated with operation and adjuvant treatment.
Prognostic Significance of BCL-2, HER2, CD133, CAIX, and ERCC1 Expressions in Early Cervical Cancer
In total, there was a median follow-up period of 66 months (range 1–143) and a 5-year DFS of 87% (95% CI, 83–91). Patients with higher HER2 expression had significantly poor DFS compared to the total group (80.1% vs. 92.2%, p=0.004; Figure 2). In subgroup analysis, high HER2 expression was also associated with poor DFS (p=0.012) and low ERCC1 expression tended to be associated with inferior DFS in the non-adjuvant treatment group (p=0.058; Figure 2). In the adjuvant radiation group, patients with higher expression of CAIX had significantly better DFS (89.0% vs. 75.0%, p=0.026, Figure 2). Interestingly, ERCC1 expression was associated with poorer DFS (76.1% vs. 88.9%, p=0.022) in the adjuvant radiation group, though it was a favorable marker in the surgery only group. In high-risk group, such as lymph node metastasis or large tumor size, the gap in DFS became wider with higher protein expression, especially HER2 and ERCC1 (Supplementary Figure 2). This indicated that the protein expression level could be a more unfavorable factor in cervical cancer patients with high-risk factors. The infection of HPV and its two viral oncoproteins, E6 and E7 that cause tumorigenic transformation of cervical epithelium was also analyzed. In Supplementary Table 2, it has been shown that high risk HPV infected patients are more likely to be radiosensitive. In Kaplan-Meier curve, HPV infection has a trend of better DFS in adjuvant radiation group, however, it was not statistically significant (see Supplementary Figure 3).
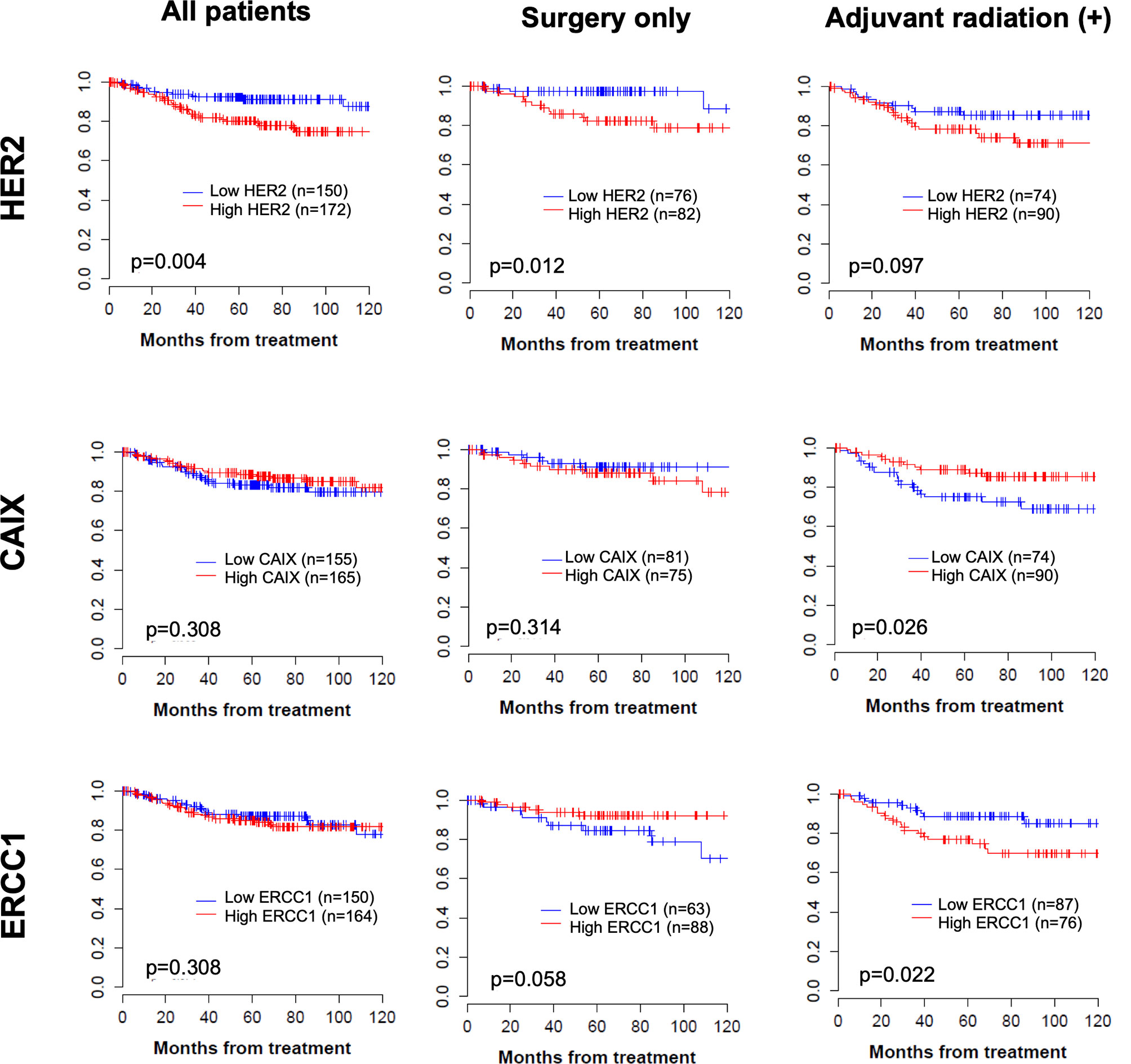
Figure 2 Kaplan–Meier curve of disease-free survival according to each protein expression in total group, operation only group, and operation followed by adjuvant treatment group.
Using the Cox proportional analysis, the association between prognostic values and disease recurrence was analyzed in all the patients (Table 4). Clinicopathologic factors, histology, and lymph node metastasis were independent predictors of DFS (hazard ratio, HR=3.52, 95% CI 1.96–6.32, p<0.001; HR=3.69, 95% CI 1.95–6.99, p=0.001) in the multivariate analysis. High expression of HER2 was an independent prognostic factor for DFS (HR=2.10, 95% CI 1.08–4.07, p=0.029), which persisted as a prognostic marker in the non-adjuvant treatment group. In the adjuvant radiation group, low expression of CAIX was an independent prognostic value for DFS (HR=0.45, 95% CI 0.21–0.95, p=0.037).
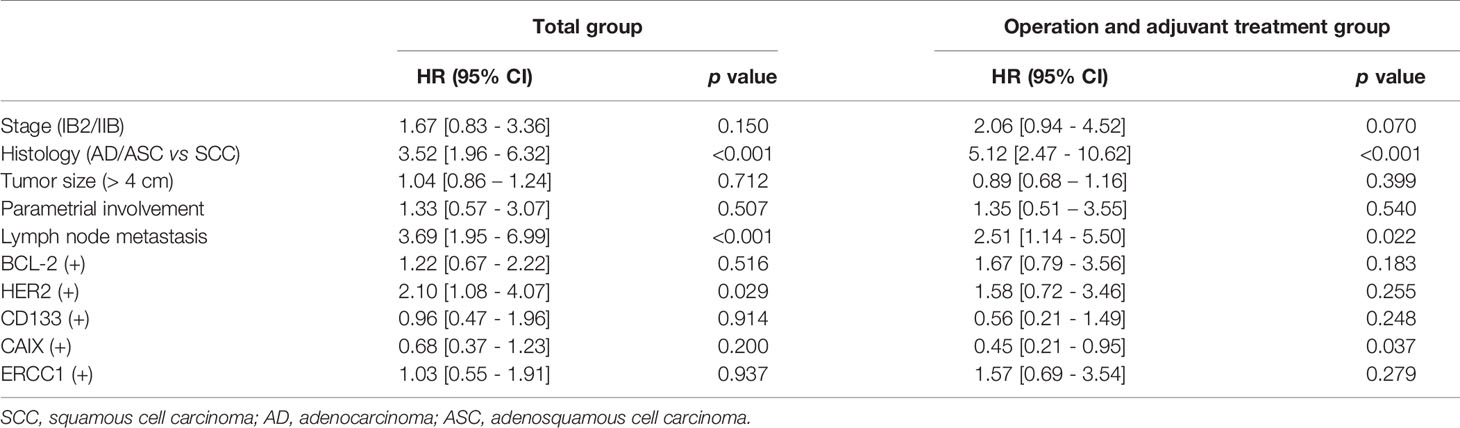
Table 4 Multivariate analysis of the association between prognostic variables and disease-free survival in cervical cancer patients according to adjuvant treatment.
With data of previous article, we compared the results of univariate analysis whether each of the markers are independently significant and have similar effect in both early and advanced stage (Supplementary Table 3). High expression of HER2 was an independent prognostic factor of disease recurrence and overall survival in early cervical cancer. However, the significance of HER2 in disease recurrence had decreased in locally advanced cervical cancer. Also, of early cervical cancer patients, low expression of CAIX was an independent prognostic value for DFS in adjuvant radiation group. Patients with locally advanced cervical cancer had almost received adjuvant radiation, and low CAIX expression tended to be associated with disease recurrence (HR=0.73, 95% CI 0.51–1.04, p=0.078).
Assessment of the Prognostic Power of the Combined Clinical–Molecular Model
To examine whether the data associated with the five proteins enhanced the prognostic power of the clinical data, we compared the C-index between the clinical model and the combined clinical–molecular model to predict disease recurrence. Importantly, the combined clinical–molecular model predicted recurrence (mean C-index, 0.77; range, 0.50–0.93) with significantly improved power compared to the clinical model (mean C-index, 0.71; range, 0.48–0.81) (p=0.006, Figure 3).
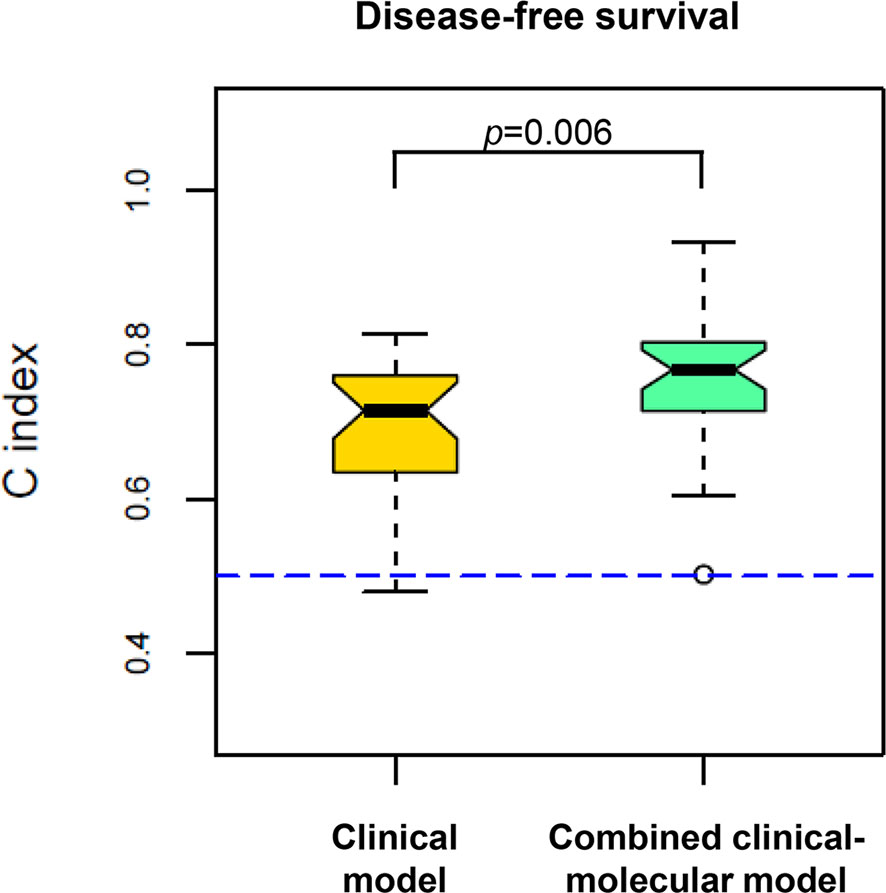
Figure 3 Comparison of predictive power in disease recurrence between the clinical model (yellow, left) and the combined clinical–molecular model (green, right). The plots show the distribution of 100 C-indexes and are compared by using the Wilcoxon signed-rank test. The combined clinical–molecular model had better performance in predicting disease recurrence (median C-index 0.77) compared to the clinical model (median C-index 0.71; p=0.006). The dashed line indicates the C-index equivalent to a random guess (C-index=0.50).
Discussion
In the present study, we investigated the prognostic significance of BCL-2, HER2, CD133, CAIX, and ERCC1 expression in early-stage cervical cancer because they were prognostic factors in locally advanced cervical cancer. We identified that each protein had a different implication in early cervical cancer. There was a trend of low expression of CAIX and high expression of ERCC1 in patients with a resistant response to adjuvant treatment. Furthermore, high HER2 expression predicted an unfavorable oncologic outcome, and low CAIX and high ERCC1 expression predicted an unfavorable response to adjuvant treatment in patients with early cervical cancer. In addition, we demonstrate for the first time that ERCC1 had a different association with DFS depending on whether patients received adjuvant treatment; low expression with poor DFS in the group that received no adjuvant and high expression with poor DFS in the adjuvant radiation group. Based on these results, we suggest that this is the first study to validate the prognostic significance of proteins, which are important in locally advanced cancer, in a cohort with early cervical cancer. HER2, CAIX, and ERCC1 may be useful as predictive markers in chemoradioresistance and prognostic markers in the recurrence of cervical cancer.
Carbonic anhydrase IX (CAIX) is a transmembrane protein that catalyzes the reversible hydration of carbon dioxide to carbonic acid, regulating intracellular pH and maintaining a normal pH in tumor cells under hypoxic conditions (20). Therefore, it is a useful endogenous marker of tumor hypoxia and a predictor of radiation-resistant hypoxic cells. Other studies refute the relationship between CAIX expression and hypoxia (21, 22). Similarly, the prognostic significance of CAIX is controversial. Some studies have found significant associations between CAIX and poor prognosis in locally advanced cervical cancer (23, 24). However, other studies have shown no significant association (21, 25) or that the high expression of CAIX is related to better survival (26). Our study showed that CAIX expression is associated with RT response and that the low expression of CAIX is related with a poor response to radiation in early cervical cancer patients. The discrepancy between CAIX expression and RT susceptibility in the current study suggests that other factors may be associated with hypoxia, rather than CAIX expression.
In the locally advanced stage or early stage of cervical cancer with risk factors, patients receive radiotherapy or CCRT with cisplatin. The main cytotoxic activity of cisplatin is based on the formation of DNA adducts, which trigger a series of intracellular events that ultimately result in cancer cell death (27, 28). Excision repair cross-complementing 1 (ERCC1) is a key protein in the nucleotide excision repair (NER) pathway, which recognizes and removes cisplatin-induced DNA adducts, decreasing the cell response to cisplatin (29). In our study, high ERCC1 expression was associated with poor DFS in patients with adjuvant radiotherapy or CCRT with cisplatin. This is because higher ERCC1 expression increases the repair of DNA adducts induced by cisplatin, which results in a poor response to treatment and unfavorable oncologic outcomes. A significant correlation between ERCC1 mRNA expression levels and cisplatin resistance has been demonstrated in cervical cancer cell lines (30) and several studies in patients with locally advanced cervical cancer have arrived at similar results (31, 32). In normal cells, impaired DNA repair may lead to cell toxicity or genomic instability, a critical step in cancer pathogenesis. Therefore, the levels of ERCC1 were significantly lower in cancer patients than in normal controls (33). In addition, the International Adjuvant Lung Cancer Trial (IALT) showed that those with ERCC1-positive tumors survived longer than those with ERCC1-negative tumors among patients who did not receive platinum-based chemotherapy (34). This can explain why the low expression of ERCC1 is associated with poor DFS in patients who were only treated with surgery in our study.
HER2 is one of the EGFR family and its expression in cervical cancer ranges from 1 to 12%. Several studies have found that HER2 expression is an independent predictor of poor prognosis in cervical cancer (13, 35). Our study also showed that high HER2 expression was significantly associated with poor survival in patients with early cervical cancer. However, other studies have revealed that there was no association with unfavorable outcome (36, 37).
CD133 is probably one of the most studied markers in cancer stem cells. High expression of CD133 expression has been correlated with poor prognostic features and chemoresistance (38). In the locally advanced stage of cervical cancer, patients expressing a high level of CD133 demonstrated a better response to CCRT (14). BCL-2 is an anti-apoptotic molecule and the expression of BCL-2 and BAX might correspond to disease stage progression or cell radiosensitivity in cervical cancer (39). However, there was no association with the expression of CD133 or BCL-2 and disease recurrence or chemoradioresistance in this study.
RPPA can identify proteomic profiling of clinical samples and the quantitative detection of signaling proteins by detecting three-dimensional epitope structure in fresh frozen samples (40). This is a powerful approach for identifying and validating targets, classifying tumor subsets, assessing pharmacodynamics, and identifying prognostic and predictive markers, adaptive responses, and rational drug combinations in model systems and patient samples (41). However, the long-term preservation of high-quality specimens such as frozen tissue is not practical in a routine clinical care environment. The tissues of patients are usually FFPE because this is the most common tissue preparation method for diagnostic histopathology and can be stored for archival purposes. IHC is well-established and commonly used in histopathology for diagnosis, prognosis, and biomarker identification. In this context, we examined the possibility that potential protein markers predict chemoradioresistance in early cervical cancer. We identified each protein expression by IHC and the results was that BCL-2 and CD133 were stained in mainly cytoplasm, HER2 and CAIX in cell membrane, and ERCC1 in nucleus and the expression level was measured with ‘histoscore’ in various range. Further studies using fresh frozen and FFPE paired tissues using both IHC and RPPA are needed to translate these findings into clinical applications.
There are a few limitations in this study. First, we used conventional IHC methods for the quantification of these 5 markers. Despite its increasing role in the clinic, IHC still presents with challenges such as inter-assay variability of antibody clones, intra-tumor heterogeneity, and lack of optimal scoring systems and standard cut-off values for positivity. To account for such variability, commercially available clones of all 5 markers, which were previously validated in locally advanced cervical cancer specimens (Supplementary Figure 4), were used under the supervision of experienced pathologists, and the Cox model of disease-free survival using R software was adopted to determine the optimal cut-off values for each marker. Further studies are needed to develop standardization for clinical utility. Second, these proteins were not identified continuously, and the expression level was almost negative, especially in CD133, making it difficult to apply the predictive model which was identified in Choi et al. (14). Third, the study design was retrospective and included a relatively small population treated with adjuvant radiotherapy at a single institution. Further studies with prospective design and a larger multicenter cohort are necessary to validate the association of the factors and chemoradioresistance.
HPV is the most common cause of cervical cancer. Among them, the E6 and E7 oncoproteins are thought to be mainly responsible for malignant conversion by inducing disruptions in transmembrane signaling, regulation of the cell cycle, which consequently result in the transformation of established cell lines, immortalization of primary cell lines, and disregulation of chromosomal stability. These interactions occur with the inactivation of tumor suppressors p53 and/or pRB (42, 43). Studies of proteomics related to HPV oncoproteins have been continued to identify potential therapeutic approaches in cervical cancer and some proteins in phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathways have been found to be closely related with HPV oncoproteins (44, 45). The results of the present study along with those from our previous ones (14), BCL2, HER-2, CD133, CAIX and ERCC1 were revealed to have significant associations with cervical cancer prognosis. These findings warrant future studies to further identify any potential influences of HPV oncoproteins in disease development.
In conclusion, the present study used immunohistochemical staining to validate how the expression of BCL-2, HER2, CD133, CAIX, and ERCC1 could predict chemoradioresistance and disease recurrence in patients with early cervical cancer. CAIX and ERCC1 showed a trend in expression level according to the response to adjuvant radiotherapy or chemoradiotherapy. A lower expression of CAIX and overexpression of ERCC1 were independently poor prognostic factors of recurrence in patients with adjuvant treatment. Overexpression of HER2 was also associated with unfavorable disease prognosis in early cervical cancer. Each protein had a different association with disease recurrence in early-stage cervical cancer and this result was not similar to that found in locally advanced cervical cancer. This information could improve our understanding of the necessity of applying predictive factors adequately according to patient clinical factors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB no. SMC 2009-09-002 and 2015-07-122; Seoul, South of Korea). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SJ: Conceptualization, Formal analysis, Data curation, Writing- original draft. J-YC: Methodology, Investigation, Writing- review & editing. S-JB: Methodology, Investigation. CK, Y-YL, T-JK, J-WL, B-GK, YC, and SO: Resources. CC: Formal analysis, Investigation, Resources, Data curation, Writing-review & editing, Supervision. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by funding from the National Research Foundation of Korea (NRF-2017R1D1A1B05035844).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.665595/full#supplementary-material
Supplementary Figure 1 | Correlations between each protein and messenger RNA (mRNA) expression. mRNA expression level was measured by microarray gene expression profiling, whereas the protein expression was assessed by immunohistochemistry. CD133 and CAIX protein expression was correlated with mRNA expression (Spearman’s rho (r)=0.155; p=0.021, and r=0.190; p=0.005, respectively). Red color represent recurrence, and triangular shape represent adjuvant radiation.
Supplementary Figure 2 | Kaplan-Meier curve of disease-free survival according to each protein expression by status of (A) lymph node metastasis and (B) tumor size.
Supplementary Figure 3 | Kaplan-Meier curve of disease-free survival according to status of (A) HPV infection (B) high risk HPV infection in total, operation, and operation and adjuvant treatment group.
Supplementary Figure 4 | (A) Western blot data from locally advanced cervical cancer patient specimens (B) Correlations between IHC score and Western blotting of each proteins.
References
1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob Health (2020) 8:e191–203. doi: 10.1016/s2214-109x(19)30482-6
2. Eifel PJ, Jhingran A, Levenback CF, Tucker S. Predictive Value of a Proposed Subclassification of Stages I and II Cervical Cancer Based on Clinical Tumor Diameter. Int J Gynecol Cancer (2009) 19:2–7. doi: 10.1111/IGC.0b013e318197f185
3. Monk BJ, Tian C, Rose PG, Lanciano R. Which Clinical/Pathologic Factors Matter in the Era of Chemoradiation as Treatment for Locally Advanced Cervical Carcinoma? Analysis of Two Gynecologic Oncology Group (GOG) Trials. Gynecol Oncol (2007) 105:427–33. doi: 10.1016/j.ygyno.2006.12.027
4. Cooper RA, West CM, Wilks DP, Logue JP, Davidson SE, Roberts SA, et al. Tumour Vascularity Is a Significant Prognostic Factor for Cervix Carcinoma Treated With Radiotherapy: Independence From Tumour Radiosensitivity. Br J Cancer (1999) 81:354–8. doi: 10.1038/sj.bjc.6690700
5. Vaupel P, Briest S, Höckel M. Hypoxia in Breast Cancer: Pathogenesis, Characterization and Biological/Therapeutic Implications. Wien Med Wochenschr (2002) 152:334–42. doi: 10.1046/j.1563-258x.2002.02032.x
6. McDaniel JR, Dewhirst MW, Chilkoti A. Actively Targeting Solid Tumours With Thermoresponsive Drug Delivery Systems That Respond to Mild Hyperthermia. Int J Hyperthermia (2013) 29:501–10. doi: 10.3109/02656736.2013.819999
7. Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y. Classification of Sensitivity or Resistance of Cervical Cancers to Ionizing Radiation According to Expression Profiles of 62 Genes Selected by cDNA Microarray Analysis. Neoplasia (2002) 4:295–303. doi: 10.1038/sj.neo.7900251
8. Harima Y, Harima K, Shikata N, Oka A, Ohnishi T, Tanaka Y. Bax and Bcl-2 Expressions Predict Response to Radiotherapy in Human Cervical Cancer. J Cancer Res Clin Oncol (1998) 124:503–10. doi: 10.1007/s004320050206
9. Hara T, Omura-Minamisawa M, Kang Y, Cheng C, Inoue T. Flavopiridol Potentiates the Cytotoxic Effects of Radiation in Radioresistant Tumor Cells in Which P53 is Mutated or Bcl-2 is Overexpressed. Int J Radiat Oncol Biol Phys (2008) 71:1485–95. doi: 10.1016/j.ijrobp.2008.03.039
10. Jeon YT, Song YC, Kim SH, Wu HG, Kim IH, Park IA, et al. Influences of Cyclooxygenase-1 and -2 Expression on the Radiosensitivities of Human Cervical Cancer Cell Lines. Cancer Lett (2007) 256:33–8. doi: 10.1016/j.canlet.2007.05.008
11. Wang AH, Tian XY, Yu JJ, Mi JQ, Liu H, Wang RF. Celecoxib Radiosensitizes the Human Cervical Cancer HeLa Cell Line via a Mechanism Dependent on Reduced Cyclo-Oxygenase-2 and Vascular Endothelial Growth Factor C Expression. J Int Med Res (2012) 40:56–66. doi: 10.1177/147323001204000106
12. Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor (VEGF) Negatively Affect Overall Survival in Carcinoma of the Cervix Treated With Radiotherapy. Int J Radiat Oncol Biol Phys (2003) 56:922–8. doi: 10.1016/s0360-3016(03)00209-8
13. Pérez-Regadera J, Sánchez-Muñoz A, De-la-Cruz J, Ballestín C, Lora D, García-Martín R, et al. Impact of Epidermal Growth Factor Receptor Expression on Disease-Free Survival and Rate of Pelvic Relapse in Patients With Advanced Cancer of the Cervix Treated With Chemoradiotherapy. Am J Clin Oncol (2011) 34:395–400. doi: 10.1097/COC.0b013e3181e84634
14. Choi CH, Chung JY, Kang JH, Paik ES, Lee YY, Park W, et al. Chemoradiotherapy Response Prediction Model by Proteomic Expressional Profiling in Patients With Locally Advanced Cervical Cancer. Gynecol Oncol (2020) 157:437–43. doi: 10.1016/j.ygyno.2020.02.017
15. Kim T-J, Lee J-W, Song SY, Choi J-J, Choi CH, Kim B-G, et al. Increased Expression of pAKT Is Associated With Radiation Resistance in Cervical Cancer. Br J Cancer (2006) 94:1678–82. doi: 10.1038/sj.bjc.6603180
16. Choi CH, Chung JY, Park HS, Jun M, Lee YY, Kim BG, et al. Pancreatic Adenocarcinoma Up-Regulated Factor Expression Is Associated With Disease-Specific Survival in Cervical Cancer Patients. Hum Pathol (2015) 46:884–93. doi: 10.1016/j.humpath.2015.02.016
17. Lee YY, Kim TJ, Kim JY, Choi CH, Do IG, Song SY, et al. Genetic Profiling to Predict Recurrence of Early Cervical Cancer. Gynecol Oncol (2013) 131:650–4. doi: 10.1016/j.ygyno.2013.10.003
18. Ishwaran H, Kogalur UB. Consistency of Random Survival Forests. Stat Probab Lett (2010) 80:1056–64. doi: 10.1016/j.spl.2010.02.020
19. Dutta S, Datta S. A Rank-Sum Test for Clustered Data When the Number of Subjects in a Group Within a Cluster Is Informative. Biometrics (2016) 72:432–40. doi: 10.1111/biom.12447
20. Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, et al. Down-Regulation of Transmembrane Carbonic Anhydrases in Renal Cell Carcinoma Cell Lines by Wild-Type Von Hippel-Lindau Transgenes. Proc Natl Acad Sci USA (1998) 95:12596–601. doi: 10.1073/pnas.95.21.12596
21. Hedley D, Pintilie M, Woo J, Morrison A, Birle D, Fyles A, et al. Carbonic Anhydrase IX Expression, Hypoxia, and Prognosis in Patients With Uterine Cervical Carcinomas. Clin Cancer Res (2003) 9:5666–74.
22. Mayer A, Höckel M, Vaupel P. Carbonic Anhydrase IX Expression and Tumor Oxygenation Status do Not Correlate at the Microregional Level in Locally Advanced Cancers of the Uterine Cervix. Clin Cancer Res (2005) 11:7220–5. doi: 10.1158/1078-0432.Ccr-05-0869
23. Kim JY, Shin HJ, Kim TH, Cho KH, Shin KH, Kim BK, et al. Tumor-Associated Carbonic Anhydrases Are Linked to Metastases in Primary Cervical Cancer. J Cancer Res Clin Oncol (2006) 132:302–8. doi: 10.1007/s00432-005-0068-2
24. Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic Anhydrase (CA IX) Expression, a Potential New Intrinsic Marker of Hypoxia: Correlations With Tumor Oxygen Measurements and Prognosis in Locally Advanced Carcinoma of the Cervix. Cancer Res (2001) 61:6394–9.
25. Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, et al. Pimonidazole Binding and Tumor Vascularity Predict for Treatment Outcome in Head and Neck Cancer. Cancer Res (2002) 62:7066–74.
26. Patard JJ, Fergelot P, Karakiewicz PI, Klatte T, Trinh QD, Rioux-Leclercq N, et al. Low CAIX Expression and Absence of VHL Gene Mutation are Associated With Tumor Aggressiveness and Poor Survival of Clear Cell Renal Cell Carcinoma. Int J Cancer (2008) 123:395–400. doi: 10.1002/ijc.23496
27. Gossage L, Madhusudan S. Current Status of Excision Repair Cross Complementing-Group 1 (ERCC1) in Cancer. Cancer Treat Rev (2007) 33:565–77. doi: 10.1016/j.ctrv.2007.07.001
28. Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 Expression as a Predictive Marker of Squamous Cell Carcinoma of the Head and Neck Treated With Cisplatin-Based Concurrent Chemoradiation. Br J Cancer (2008) 99:167–72. doi: 10.1038/sj.bjc.6604464
29. Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, et al. ERCC1-XPF Endonuclease Facilitates DNA Double-Strand Break Repair. Mol Cell Biol (2008) 28:5082–92. doi: 10.1128/mcb.00293-08
30. Britten RA, Liu D, Tessier A, Hutchison MJ, Murray D. ERCC1 Expression as a Molecular Marker of Cisplatin Resistance in Human Cervical Tumor Cells. Int J Cancer (2000) 89:453–7. doi: 10.1002/1097-0215(20000920)89:5<453::AID-IJC9>3.0.CO;2-E
31. Park JS, Jeon EK, Chun SH, Won HS, Lee A, Hur SY, et al. ERCC1 (Excision Repair Cross-Complementation Group 1) Expression as a Predictor for Response of Neoadjuvant Chemotherapy for FIGO Stage 2B Uterine Cervix Cancer. Gynecol Oncol (2011) 120:275–9. doi: 10.1016/j.ygyno.2010.10.034
32. Hasegawa K, Kato R, Torii Y, Ichikawa R, Oe S, Udagawa Y. The Relationship Between ERCC1 Expression and Clinical Outcome in Patients With FIGO Stage I to Stage II Uterine Cervical Adenocarcinoma. Int J Gynecol Cancer (2011) 21:1479–85. doi: 10.1097/IGC.0b013e31822265e7
33. Yang M, Kim WH, Choi Y, Lee SH, Kim KR, Lee HS, et al. Effects of ERCC1 Expression in Peripheral Blood on the Risk of Head and Neck Cancer. Eur J Cancer Prev (2006) 15:269–73. doi: 10.1097/01.cej.0000195709.79696.0c
34. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-Based Adjuvant Chemotherapy in Patients With Completely Resected non-Small-Cell Lung Cancer. N Engl J Med (2004) 350:351–60. doi: 10.1056/NEJMoa031644
35. Pérez-Regadera J, Sánchez-Muñoz A, De-la-Cruz J, Ballestín C, Lora D, García-Martín R, et al. Negative Prognostic Impact of the Coexpression of Epidermal Growth Factor Receptor and c-erbB-2 in Locally Advanced Cervical Cancer. Oncology (2009) 76:133–41. doi: 10.1159/000195539
36. Leung TW, Cheung AN, Cheng DK, Wong LC, Ngan HY. Expressions of c-erbB-2, Epidermal Growth Factor Receptor and Pan-Ras Proto-Oncogenes in Adenocarcinoma of the Cervix: Correlation With Clinical Prognosis. Oncol Rep (2001) 8:1159–64. doi: 10.3892/or.8.5.1159
37. Kim JW, Kim YT, Kim DK, Song CH, Lee JW. Expression of Epidermal Growth Factor Receptor in Carcinoma of the Cervix. Gynecol Oncol (1996) 60:283–7. doi: 10.1006/gyno.1996.0039
38. Artells R, Moreno I, Díaz T, Martínez F, Gel B, Navarro A, et al. Tumour CD133 mRNA Expression and Clinical Outcome in Surgically Resected Colorectal Cancer Patients. Eur J Cancer (2010) 46:642–9. doi: 10.1016/j.ejca.2009.11.003
39. Crawford RA, Caldwell C, Iles RK, Lowe D, Shepherd JH, Chard T. Prognostic Significance of the Bcl-2 Apoptotic Family of Proteins in Primary and Recurrent Cervical Cancer. Br J Cancer (1998) 78:210–4. doi: 10.1038/bjc.1998.466
40. Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, et al. Requirement of Fas for the Development of Autoimmune Diabetes in Nonobese Diabetic Mice. J Exp Med (1997) 186:613–8. doi: 10.1084/jem.186.4.613
41. Lu Y, Ling S, Hegde AM, Byers LA, Coombes K, Mills GB, et al. Using Reverse-Phase Protein Arrays as Pharmacodynamic Assays for Functional Proteomics, Biomarker Discovery, and Drug Development in Cancer. Semin Oncol (2016) 43:476–83. doi: 10.1053/j.seminoncol.2016.06.005
42. Yim EK, Park JS. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res Treat (2005) 37:319–24. doi: 10.4143/crt.2005.37.6.319
43. Gupta S, Kumar P, Das BC. HPV: Molecular Pathways and Targets. Curr Probl Cancer (2018) 42:161–74. doi: 10.1016/j.currproblcancer.2018.03.003
44. Pal A, Kundu R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front Microbiol (2020) 10:3116. doi: 10.3389/fmicb.2019.03116
Keywords: HER2, CAIX, ERCC1, immunohistochemistry, chemoradioresistance, uterine cervical neoplasm
Citation: Jeong SY, Chung J-Y, Byeon S-J, Kim CJ, Lee Y-Y, Kim T-J, Lee J-W, Kim B-G, Chae YL, Oh SY and Choi CH (2021) Validation of Potential Protein Markers Predicting Chemoradioresistance in Early Cervical Cancer by Immunohistochemistry. Front. Oncol. 11:665595. doi: 10.3389/fonc.2021.665595
Received: 08 February 2021; Accepted: 05 July 2021;
Published: 19 July 2021.
Edited by:
Anindita Chakrabarty, Shiv Nadar University, IndiaReviewed by:
Bhudev Chandra Das, Amity University, IndiaAasma Nalwa, All India Institute of Medical Sciences Jodhpur, India
Binayak Kumar, ICMR-National Institute of Cancer Prevention and Research, India
Tasaduq H. Wani, University of Oxford, United Kingdom
Copyright © 2021 Jeong, Chung, Byeon, Kim, Lee, Kim, Lee, Kim, Chae, Oh and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chel Hun Choi, Y2hlbGh1bi5jaG9pQHNhbXN1bmcuY29t
†These authors have contributed equally to this work and share first authorship
 Soo Young Jeong
Soo Young Jeong Joon-Yong Chung
Joon-Yong Chung Sun-Ju Byeon
Sun-Ju Byeon Chul Jung Kim4
Chul Jung Kim4 Yoo-Young Lee
Yoo-Young Lee Tae-Joong Kim
Tae-Joong Kim Chel Hun Choi
Chel Hun Choi