- 1Yale Comprehensive Cancer Center, Yale School of Medicine, New Haven, CT, United States
- 2Dept. of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk, Poland
- 3Access Centre of Excellence, Global Access, F. Hoffmann-La Roche, Basel, Switzerland
- 4Quantics Biostatistics, Edinburgh, United Kingdom
- 5York Health Economics Consortium Ltd, University of York, York, United Kingdom
- 6Sandra and Edward Meyer Cancer Center, Weill-Cornell Medicine, New York, NY, United States
- 7Division of Thoracic Oncology, European Institute of Oncology, Milan, Italy
In the absence of head-to-head trials of first-line treatments for metastatic non-small cell lung cancer (NSCLC), synthesis of available evidence is needed. We conducted a systematic literature review and network meta-analysis of randomized controlled trials in patients with stage IV NSCLC and high programmed death-ligand 1 (PD-L1) expression. Patients with other-stage NSCLC or without PD-L1 expression and populations with < 80% stage IV NSCLC were excluded. Outcomes included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events. English records from MEDLINE and Embase published through October 2020 were eligible, supplemented by hand searches of other sources. Three evidence networks were constructed based on histology (mixed, squamous, non-squamous). OS and PFS results were analyzed applying Bayesian fractional polynomial random-effects models. Hazard ratios over time with 95% credible intervals (CrIs) and expected differences in OS and PFS between each cancer immunotherapy regimen and the chemotherapy common comparator were generated. Seventeen clinical trials were included after screening 32,527 records. Heterogeneity and risk of bias were generally low across trials. In the mixed-histology network of PD-L1–high patients, expected OS was significantly longer with atezolizumab (estimated difference: 10.4 months [95% CrI: 1.9, 18.2]), pembrolizumab (7.2 [2.2, 12.3]), and cemiplimab (13.0 [4.2, 21.0]) versus chemotherapy but not with nivolumab (3.5 [−2.5, 10.6]) or nivolumab plus ipilimumab (6.7 [−0.5, 14.2]) versus chemotherapy. OS improvements were not significant compared with chemotherapy for any regimen in the squamous and non-squamous networks, except pembrolizumab plus chemotherapy in the non-squamous network. All regimens showed significantly longer expected PFS versus chemotherapy in the non-squamous network, whereas the increases were not significant in the mixed or squamous networks. ORR was significantly higher with pembrolizumab and cemiplimab versus chemotherapy in the mixed-histology network, with sintilimab in the non-squamous network, and with combination regimens, including pembrolizumab or atezolizumab, in the squamous and non-squamous networks, except with atezolizumab plus carboplatin, paclitaxel, and bevacizumab. Survival and safety versus chemotherapy were generally similar across cancer immunotherapies and histology networks. These findings may support treatment decisions for patients with high PD-L1 status receiving first-line treatment for NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) constitutes approximately 85% of all lung cancers and is the leading cause of cancer-related death (1, 2). The advent of cancer immunotherapy (CIT) and targeted treatments has introduced effective first-line treatment options for metastatic and advanced disease (3, 4). Targeted therapies, such as the anti-vascular endothelial growth factor monoclonal antibody bevacizumab, are recommended in certain combination regimens regardless of programmed death-ligand 1 (PD-L1) status (3). Immunohistochemistry testing is recommended to determine the suitability for first-line CIT based on the level of PD-L1 expression, the patient’s health status, and clinical circumstances (3, 4).
Single-agent and combination first-line CIT has been investigated in several phase III clinical trials, and clinical practice recommendations factor in levels of PD-L1 expression and squamous or non-squamous histology. The anti–programmed death-1 inhibitors pembrolizumab and nivolumab, including nivolumab in combination with the CTLA-4 inhibitor ipilimumab, and the PD-L1 inhibitor atezolizumab are all recommended for first-line use in different clinical scenarios (3–8). CIT regimens may be used as monotherapy or in combination with chemotherapy or other regimens based on treatment history, genetic alterations, histology, and level of PD-L1 expression (3, 4).
The pace of cancer treatment research and the complexity of yet-evolving biomarker testing make clinical and policy decision making challenging—and the process is further complicated by the lack of head-to-head comparisons among standards of care and emerging treatment options. Several attempts have been made to provide meaningful indirect comparisons, but these either have been limited in scope or have not included data from all relevant CIT trials (9–11). Against this background, and to accommodate CIT-specific considerations with appropriate statistical methodology and the most recent clinical trial findings, we conducted a network meta-analysis (NMA) of CIT monotherapy and combination regimens for patients with metastatic NSCLC.
Methods
We conducted a systematic literature review (SLR) of randomized controlled trials (phase II, III, or IV) including adults (≥ 18 years) with stage IV squamous or non-squamous NSCLC and no prior chemotherapy for stage IV disease. This analysis focused on patients whose tumors had high PD-L1 expression (defined as a tumor proportion score ≥ 50% or as either ≥ 50% of tumor cells [TC; TC3] or ≥ 10% of tumor-infiltrating immune cells [IC; IC3]). Trials that were not limited to patients with stage IV NSCLC were eligible if > 80% of the population had stage IV disease. Interventions of interest included monotherapy or combination therapy with CIT (atezolizumab, pembrolizumab, nivolumab, ipilimumab, durvalumab), bevacizumab, tremelimumab, or chemotherapy (carboplatin, cisplatin, docetaxel, etoposide, gemcitabine, nab-paclitaxel, paclitaxel, pemetrexed, or vinorelbine).
The systematic literature search was carried out in September and October 2020 in a variety of databases, including Embase (including MEDLINE), PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), and the Health Technology Assessment database (HTA). The search strategy comprised two main concepts: “NSCLC” AND “RCTs”. Full search terms are provided in Table S1, and the details of the databases searched are noted in Table S2. Additional sources included reference lists from relevant publications and congress abstracts (2012–2020) from the American Society of Clinical Oncology, the European Society for Medical Oncology, the International Association for the Study of Lung Cancer, the World Conference on Lung Cancer, the European Lung Cancer Congress, and the British Thoracic Oncology Group. Clinical trial registries, including ClinicalTrials.gov, the International Clinical Trials Registry Platform, and the European Union Clinical Trials Registry, were searched. We also searched the following health technology assessment websites: the European Medicines Agency, the National Institute for Health and Care Excellence, the Canadian Agency for Drugs and Technologies in Health (including the pan-Canadian Oncology Drug Review), the Independent Institute for Quality and Efficiency in Health Care (IQWiG), and the United States Food and Drug Administration (accesstodata.fda.gov). Two independent reviewers screened titles and abstracts of retrieved records and then the full texts of potentially eligible records, with discrepancies adjudicated by a third reviewer. Detailed data extraction and risk of bias assessment were carried out by two independent reviewers, with discrepancies adjudicated by a third reviewer (Table S3).
Included trials were evaluated for the reporting of allocation sequence and concealment, blinding, handling of incomplete outcomes data, selective reporting, and other potential sources of bias. A feasibility assessment was conducted to evaluate the comparability of the patient populations, the outcome measure definitions, and the timing of outcome measures across trials. Heterogeneity was assessed for all treatment comparisons that included two or more studies by visual inspection of the Kaplan-Meier curves, forest plots, and I2 statistic for summary measures.
Systematic Literature Review Results
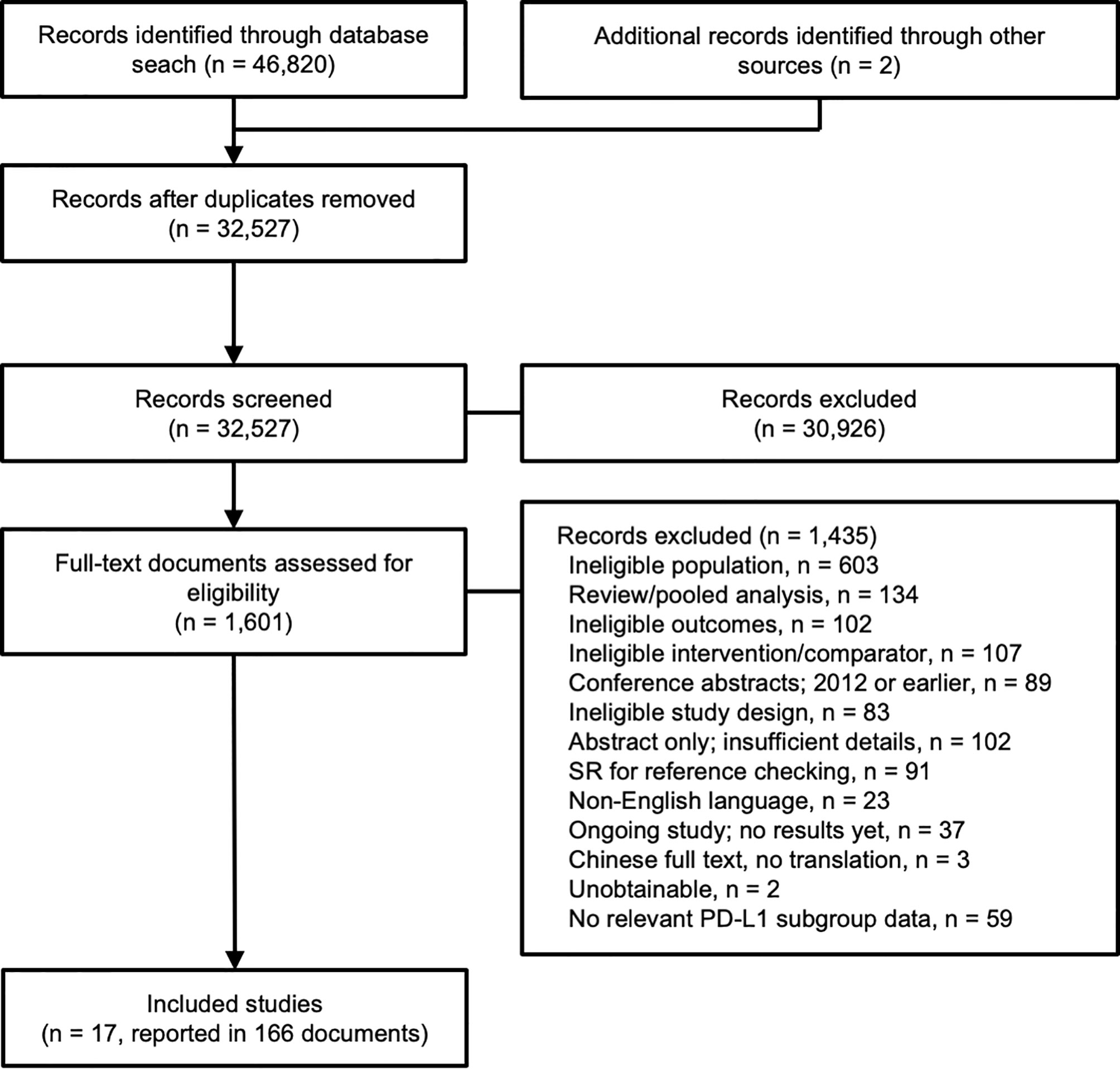
A total of 46,820 records were identified through the searches, and an additional two records were identified from other sources (supplied by the study sponsor, F. Hoffmann-La Roche Ltd). Following de-duplication, 32,527 records were screened according to their titles and abstracts, and 1,601 (5%) records underwent full-text review (Figure 1). Seventeen clinical trials reported in 166 publications were included in the evidence networks.
All trials except for KEYNOTE-189, KEYNOTE-407, and ORIENT-11 were open label. None of the trials were designed as crossover trials. However, in nine trials, patients were permitted to receive subsequent CIT or other treatments beyond the trial interventions following disease progression or according to other criteria: CHECKMATE-026—58% of patients in the chemotherapy group received nivolumab at disease progression by the investigator and confirmed by an independent radiologist (12); EMPOWER-LUNG 1—74% of patients who progressed on chemotherapy received subsequent cemiplimab (13); IMpower130—41% of patients in the carboplatin plus nab-paclitaxel group received atezolizumab as monotherapy upon disease progression as assessed by the investigator; a protocol amendment in June 2016 removed this option; KEYNOTE-021, KEYNOTE-024, KEYNOTE-189, and KEYNOTE-407—32%, 44%, 33%, and 27% of patients in these trials, respectively, received pembrolizumab after radiological disease progression or disease progression verified by blinded independent radiological review (5, 14–20); ORIENT-11—27% of patients in the placebo plus chemotherapy group received sintilimab monotherapy during the study after confirmed disease progression, and 5% had received immunotherapy outside the study, which resulted in an effective treatment change rate of 31% (21); and RATIONALE 304—patients in the comparator arm were eligible to receive the intervention after disease progression (22).
Evidence Networks
Three histology-based evidence networks were constructed: a mixed-histology network, a squamous NSCLC network, and a non-squamous NSCLC network. The mixed-histology network included atezolizumab monotherapy (IMpower110), pembrolizumab monotherapy (KEYNOTE-024, KEYNOTE-042), nivolumab and nivolumab plus ipilimumab (CheckMate-026, CheckMate-227), durvalumab and durvalumab plus tremelimumab (MYSTIC), and cemiplimab (EMPOWER-LUNG 1). We were unable to determine the proportion of patients in the CheckMate-9LA trial with stage IV disease from the available publications; therefore, CheckMate-9LA could not be included in the evidence network. The squamous NSCLC network included atezolizumab monotherapy and combination therapy (IMpower110 subgroup, IMpower131) and pembrolizumab monotherapy and combination therapy (KEYNOTE-024 subgroup, KEYNOTE-042 subgroup, KEYNOTE-407). The non-squamous NSCLC network included atezolizumab monotherapy and combination therapy (IMpower110 subgroup, IMpower130, IMpower132, IMpower150), pembrolizumab monotherapy and combination therapy (KEYNOTE-021, KEYNOTE-024 subgroup, KEYNOTE-042 subgroup, KEYNOTE-189), sintilimab plus chemotherapy (ORIENT-11), and tislelizumab plus chemotherapy (RATIONALE 304).
Statistical Analysis
Outcomes of interest were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and safety outcomes, including any treatment-related adverse events (TRAEs). Descriptive statistics were provided for the study and patient characteristics. Individual patient data were available from IMpower110, IMpower130, and IMpower150; summary and subgroup data were available from other included trials. All chemotherapy arms across trials were assumed to be exchangeable (such as the combination of chemotherapy and bevacizumab) and utilized as a single node in the evidence networks (cisplatin and carboplatin were assumed to be equivalent, and studies with and without pemetrexed maintenance therapy were also assumed to be equivalent). Traditional meta-analytic methods require an assumption of proportional hazards, which does not account for the delayed onset or duration of treatment effect observed with CITs (23, 24). Our NMA for time-to-event data (OS and PFS) in the PD-L1–high subgroup used non-proportional hazards fractional polynomial (FP) models within a Bayesian framework (24) using informative priors for the between-study variance of treatment effects (25), allowing the HRs to change over time. Evaluation of the best model for analysis included inspection of predicted survival and observed Kaplan-Meier curves and Deviance Information Criterion measure of model fit. Inspection of the log cumulative hazard plots suggested that the non-proportionality of hazards assumption was upheld. In general, the curves crossed early (maximum, 6 months) compared with the entire observation period. OS and PFS were also analyzed in an NMA using hazard ratios (HRs) assuming proportional hazards to examine the consistency of findings.
We conducted NMAs assuming binomial distribution and logit link for ORR and safety outcomes, in line with the recommendations of the National Institute for Health and Care Excellence (NICE) Decision Support Unit (26). Informative priors were used for the heterogeneity of treatment effects (25). Safety was only analyzed in a mixed-histology network because of the availability of data. Chemotherapy was used as the reference treatment for network comparisons. Results of FP NMAs are presented as HRs over time with 95% credible intervals (CrIs) and expected difference in OS or PFS (60-month time horizons). For the survival outcomes, results from first-order FP random effects models with p1 = 0 (Weibull) are presented based on statistical criteria of goodness-of-fit and clinical plausibility of the survival extrapolations. Results of proportional hazards-based NMAs are presented as HRs and associated 95% CrI. Estimated differences and ratios are considered significant if the 95% Crl lie completely below or above 0 and 1, respectively. All analyses were conducted using R version 3.6.1 in combination with rjags and JAGS version 4.3.0.
Results
Study and Patient Characteristics
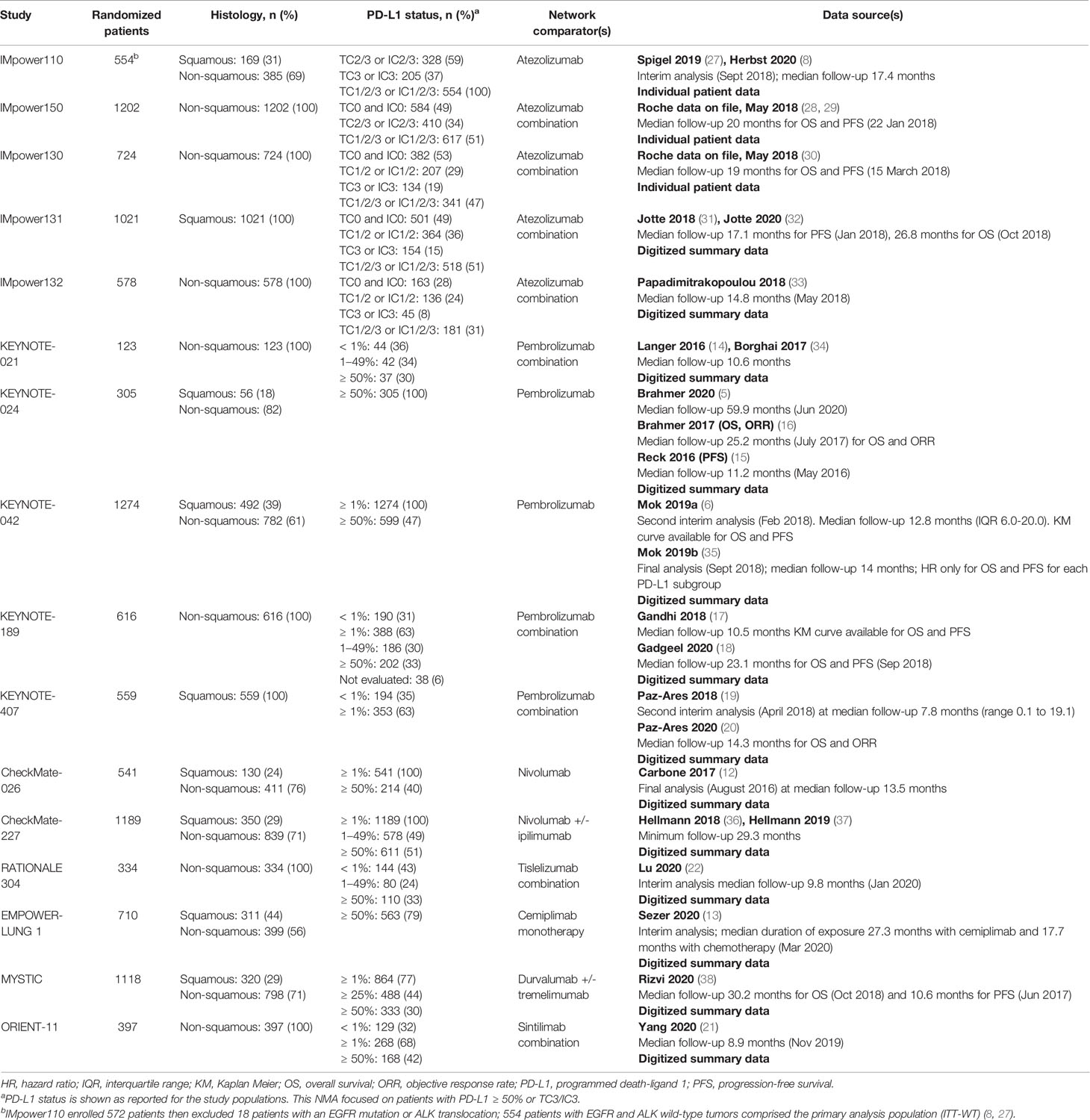
The characteristics of the 17 studies and inclusive treatments in the analyses for each evidence network are presented in Table 1. All included trials had similar eligibility requirements in terms of age, Eastern Cooperative Oncology Group (ECOG) performance status, disease stage, availability of PD-L1 status, and prior chemotherapy for metastatic disease. The analyzed populations of the CheckMate trials, EMPOWER-LUNG 1, IMpower110, IMpower132, KEYNOTE trials, MYSTIC, ORIENT-11, and RATIONALE 304 were restricted to patients with an absence of EGFR mutations or ALK translocations. IMpower130, IMpower131, and IMpower150 included patients with EGFR mutations or ALK translocations but required these patients to have progressed on or after appropriate tyrosine kinase inhibitor or ALK inhibitor treatment. IMpower130 and IMpower150 reported pre-specified analyses in populations that excluded patients with these mutations; we used the relevant subgroups of patients with PD-L1 ≥ 50% from these populations in our analyses. In the mixed-histology network, the MYSTIC trial only provided a hazard ratio for OS and so could only be included in the proportional hazards HR analysis for that outcome. In the non-squamous network, tislelizumab plus chemotherapy could only be evaluated in the proportional hazards HR analysis of PFS, and sintilimab could be evaluated only in the PFS and ORR analyses.
The median age of patients was similar across trials, ranging from 60 to 66 years. The proportion of males in each treatment arm varied substantially both within and across the trials, ranging from 37% (KEYNOTE-021) to 85% (EMPOWER-LUNG 1). In the trials that reported ethnicity, ≥ 80% of the patients in each treatment arm were White (IMpower150, IMpower130, IMpower131, KEYNOTE-021, CheckMate-026) except in MYSTIC and IMpower132 (66–71% White) and RATIONALE 304 (100% Asian). Six trials did not report the ethnic breakdown of patients (CheckMate-227, IMpower110, KEYNOTE-024, KEYNOTE-042, KEYNOTE-189, KEYNOTE-407). All trials required patients to be chemotherapy naive for metastatic stage IV NSCLC. The proportion of patients with prior adjuvant or neoadjuvant chemotherapy was low: 0% to 11% in the trials reported this information (CheckMate-026, EMPOWER-LUNG 1, KEYNOTE-021, KEYNOTE-024, KEYNOTE-189, KEYNOTE-402, KEYNOTE-407, IMpower130, IMpower150). Approximately 11% to 23% of patients had liver metastases across the trials in which this was reported (CheckMate-026, CheckMate-227, IMpower110, IMpower130, IMpower131, IMpower132, IMpower150, RATIONALE 304).
Similar definitions of OS were used across trials. For PFS and ORR, all trials used RECIST 1.1. Atezolizumab trials and the 60-month follow-up report from KEYNOTE-024 used investigator-assessed PFS and ORR, whereas all other trials used independent review for both outcomes. For the purposes of this analysis, PFS and ORR definitions were assumed to be comparable. Risk of bias was generally low across trials, although all but three were open label (KEYNOTE-189, KEYNOTE-407, ORIENT-11). Blinding of the outcome assessor was unclear for the atezolizumab trials (Table S4).
NMA Results: Overall Survival
The FP NMA models for OS fit the data reasonably well for all treatments and studies. In the mixed-histology network of PD-L1–high patients, the expected OS was significantly longer with atezolizumab (10.4 months [95% CrI: 1.9, 18.2]), pembrolizumab (7.2 months [2.2, 12.3]), and cemiplimab (13.0 [4.2, 21.0]) versus chemotherapy but not with nivolumab or nivolumab plus ipilimumab versus chemotherapy (Figure 2). The OS HRs over time illustrated the delayed onset of treatment effect observed with CIT, where all regimens, except nivolumab, appeared to show superiority versus chemotherapy (Figure 3) from approximately 9 months onward. Results were consistent in the NMA based on HRs with proportional hazards assumptions, where atezolizumab (HR, 0.59 [95% CrI: 0.37, 0.95]), pembrolizumab (0.66 [0.52, 0.85]), and cemiplimab (0.57 [0.38, 0.85]) showed significantly improved survival compared with chemotherapy; the durvalumab and nivolumab regimens showed lower HRs but were not significant compared with chemotherapy (Figure S2).

Figure 2 Expected OS difference with chemotherapy versus cancer immunotherapy comparators, mixed histology (60-month time horizon). Median posterior estimate and 95% posterior CrI. ATZ, atezolizumab; CEMIP, cemiplimab; chemo, chemotherapy; Diff., difference; NIV, nivolumab; NIV+IPI, nivolumab plus ipilimumab; OS, overall survival; PD-L1, programmed death-ligand 1; PEMB, pembrolizumab; FP, fractional polynomial; P1=0, Weibull; RE, random effects.
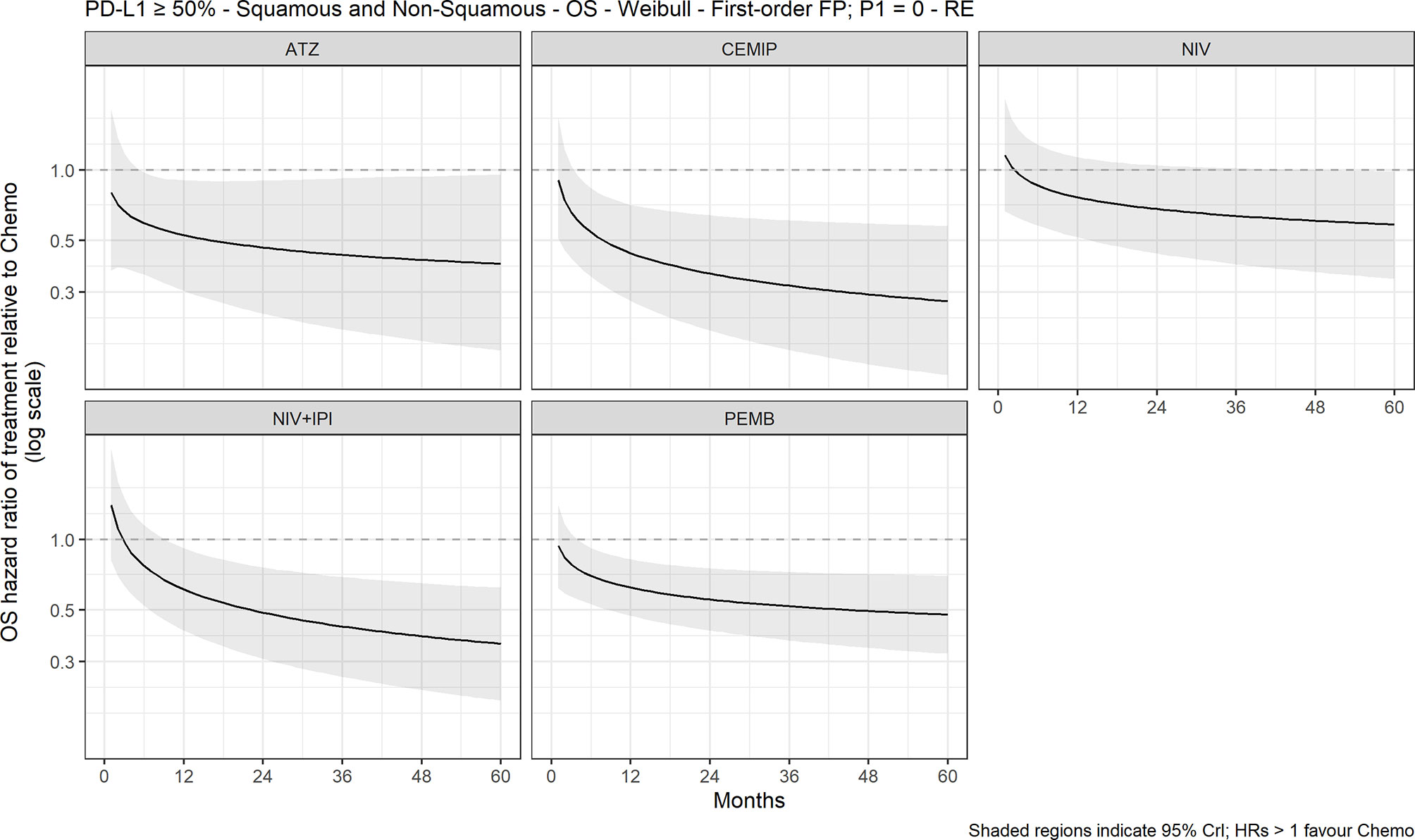
Figure 3 Expected OS HR over time for cancer immunotherapy versus chemotherapy with 95% CrI, mixed histology (60-month time horizon). ATZ, atezolizumab; CEMIP, cemiplimab; chemo, chemotherapy; Diff., difference; HR, hazard ratio; NIV, nivolumab; NIV+IPI, nivolumab plus ipilimumab; OS, overall survival; PD-L1, programmed death-ligand 1; PEMB, pembrolizumab; FP, fractional polynomial, P1=0, Weibull. RE, random effects.
In the squamous-histology network, OS was not significantly longer with any of the treatment regimens versus chemotherapy, including pembrolizumab with carboplatin and paclitaxel (CP; 2.5 months [−4.7, 17.0]), atezolizumab (14.5 months [−1.4, 29.4]), or atezolizumab with CP (7.2 months [−1.3, 18.6]; Figure S3). In the proportional hazards analysis, pembrolizumab showed significantly improved survival versus chemotherapy (HR, 0.58 [0.39, 0.89]; Figure S3).
In the non–squamous histology network, the pembrolizumab plus platinum-based chemotherapy followed by maintenance pemetrexed regimen significantly improved survival compared with chemotherapy in both the FP NMA (8.9 months [0.4, 18.3]) and the proportional hazards HR analysis (HR, 0.59 [0.35, 0.99]; Figure S4). Pembrolizumab monotherapy also significantly improved survival compared with chemotherapy in the proportional hazards HR analysis (HR, 0.71 [0.51, 0.97]).
Progression-Free Survival
The FP NMA models for PFS fit the data reasonably well for all treatments and studies. There is a marginally poorer fit to the atezolizumab data where PFS is slightly overestimated at the start of the follow-up period and to the KEYNOTE-024 data where PFS is overestimated at first then underestimated later in the follow-up period in both arms. In the mixed-histology evidence network, PFS increase was not significant with any of the CIT regimens versus chemotherapy in both the FP NMA (Figure 4) and proportional hazards HR analysis (Figure S5). The analysis of HRs over time showed that the improvement in PFS was not significant over 60 months with atezolizumab, while for pembrolizumab, cemiplimab, nivolumab plus ipilimumab, and nivolumab monotherapy, it became significant over time (Figure 5).
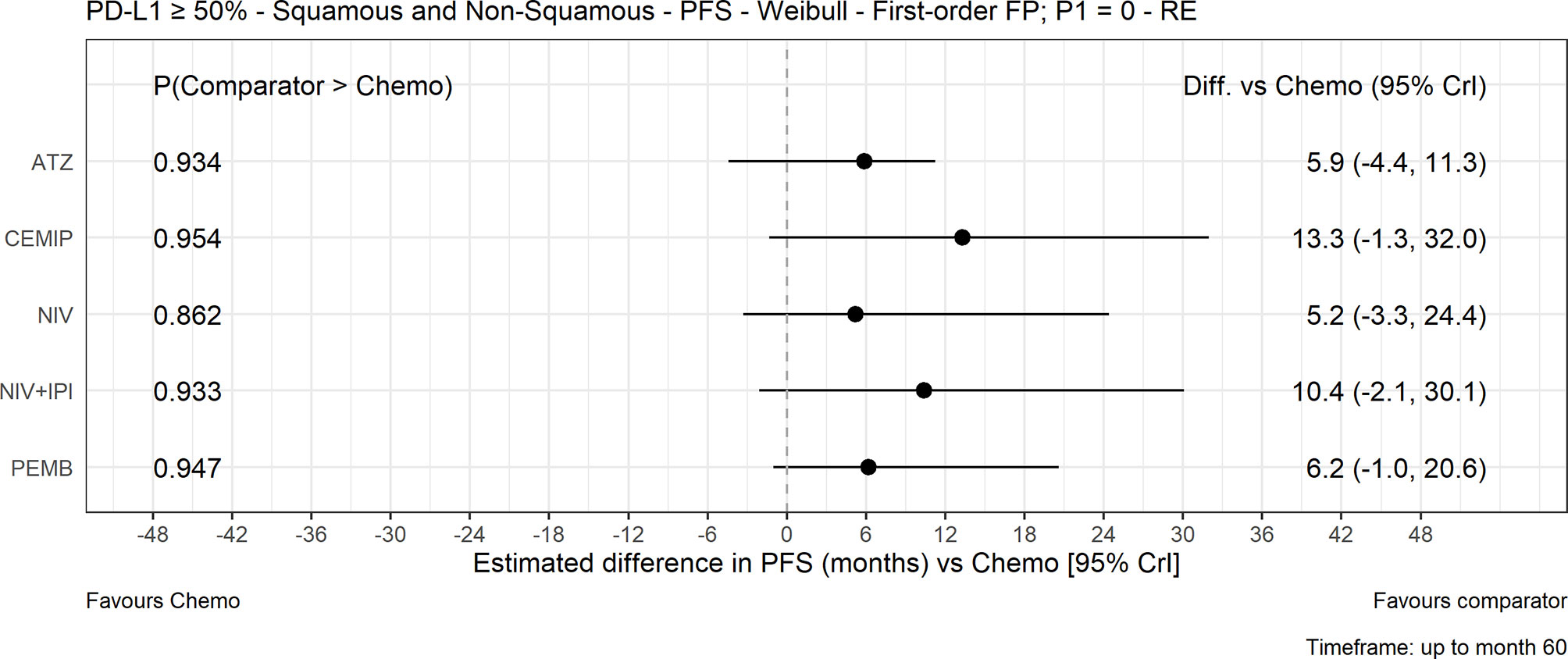
Figure 4 Expected PFS difference with chemotherapy versus cancer immunotherapy comparators, mixed histology (60-month time horizon). Median posterior estimate and 95% posterior CrI. ATZ, atezolizumab; CEMIP, cemiplimab; chemo, chemotherapy; Diff., difference; NIV, nivolumab; NIV+IPI, nivolumab plus ipilimumab; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PEMB, pembrolizumab; FP, fractional polynomial; P1=0, Weibull; RE, random effects.

Figure 5 Expected PFS HR over time for cancer immunotherapy versus chemotherapy with 95% CrI, mixed histology (60-month time horizon). ATZ, atezolizumab; CEMIP, cemiplimab; HR, hazard ratio; NIV, nivolumab; NIV+IPI, nivolumab plus ipilimumab; PD-L1, programmed death-ligand 1; PEMB, pembrolizumab; PFS, progression-free survival; FP, fractional polynomial; P1=0, Weibull; RE, random effects.
PFS increase was not significant with any of the CIT treatment regimens versus chemotherapy in the FP NMA or proportional hazards HR squamous-histology networks (Figure S6). All CIT regimens included in the non-squamous network significantly improved PFS compared with chemotherapy in the FP NMA, including atezolizumab monotherapy, atezolizumab plus CP, atezolizumab plus CP and bevacizumab (CPB), pembrolizumab plus C followed by maintenance pemetrexed, and sintilimab plus pemetrexed and platinum-based chemotherapy (Figure S7). All CIT regimens, including tislelizumab plus chemotherapy, significantly improved PFS compared with chemotherapy in the non-squamous proportional hazards model analysis, with the exception of atezolizumab monotherapy, for which the upper CrI lies just above 1.0 (HR, 0.55 [0.30, 1.01]; Figure S7).
Objective Response Rate
In the mixed-histology network NMA analysis of ORR, pembrolizumab monotherapy (odds ratio [OR], 1.55 [1.10, 2.29]) and cemiplimab monotherapy (OR, 2.55 [1.52, 4.24]) significantly improved ORR compared with chemotherapy (Figure S8). In the squamous network, CP combination regimens with atezolizumab (OR, 3.12 [1.08, 9.15]) and pembrolizumab (3.07 [1.24, 8.18]), except with atezolizumab plus CP and bevacizumab, significantly improved ORR compared with chemotherapy, as did atezolizumab plus CP (OR, 2.41 [1.17, 5.02]), pembrolizumab plus platinum-based chemotherapy followed by maintenance pemetrexed (OR, 5.85 [3.05, 11.90]), and sintilimab (OR, 3.45 [1.61, 7.25]) in the non-squamous network (Figure S8).
Safety
In the mixed-histology network, the CIT regimens with evaluable safety data (atezolizumab and pembrolizumab) had significantly lower odds of any TRAE than chemotherapy (Figure S9), but there was no significant difference between the CIT regimens and chemotherapy.
Discussion
This NMA synthesized all available direct and indirect evidence of CIT regimens versus chemotherapy for first-line NSCLC treatment. In the mixed-histology network FP NMA, OS estimates were significantly longer with the atezolizumab and pembrolizumab monotherapy regimens—but not with the nivolumab or nivolumab plus ipilimumab regimens—compared with chemotherapy. None of the CIT regimens were significantly different from chemotherapy in either the squamous or non-squamous NMAs for OS, with the exception of pembrolizumab plus platinum-based chemotherapy followed by maintenance pemetrexed for non-squamous NSCLC. The analysis of OS HRs over time illustrated the delayed treatment effect of CIT compared with chemotherapy. None of the CIT regimens showed significant advantages in PFS versus chemotherapy for the 60-month time horizon FP NMA analysis in the mixed- or squamous-histology networks. Expected PFS differences were greater with the CIT regimens in the non-squamous network, which included atezolizumab and sintilimab monotherapy regimens, and atezolizumab or pembrolizumab combination regimens. ORR was significantly better with pembrolizumab and cemiplimab monotherapy regimens versus chemotherapy in the mixed-histology network and with carboplatin and paclitaxel combinations with atezolizumab or pembrolizumab in the squamous-histology network. ORR was also significantly better with sintilimab, atezolizumab, or pembrolizumab combination regimens in the non-squamous FP NMA network. Chemotherapy showed greater odds of any TRAE compared with the CIT regimens with evaluable safety data. Our findings suggest that monotherapy or combination regimens with atezolizumab or pembrolizumab offer greater OS and ORR benefits with less risk of side effects than a traditional chemotherapy regimen alone. Our findings are confirmatory of the individual trial findings for CIT versus chemotherapy and are consistent with NCCN and ESMO recommendations for first-line treatment of patients with NSCLC and high PD-L1 expression.
NMA is a useful approach to synthesize direct and indirect evidence of available treatments when patient populations and outcome measures may be appropriately aggregated. Our NMA of CIT and chemotherapy used the most recent clinical trial findings and the most appropriate statistical methods accounting for CIT-specific considerations. An indirect comparison analysis was required because of the absence of direct comparison trials. Liu (9) compared atezolizumab- and pembrolizumab-containing CIT regimens across histology and PD-L1 expression subgroups using the IMpower and KEYNOTE trial results available at the time (9). Our findings were generally consistent with those of Liu et al. (9), who reported favorable results with CIT-containing regimens, with some differences—such as improved outcomes when bevacizumab was combined with atezolizumab and chemotherapy. Tun (10) showed improved OS, PFS, and ORR when adding CIT to chemotherapy for first-line NSCLC treatment, even among patients with EGFR alterations and ALK translocations, with incremental increase in adverse events (10). Meta-analyses by Addeo (39) and Chen (11) suggested improved OS, PFS, and ORR with CIT with or without chemotherapy versus chemotherapy but did not use a Bayesian FP NMA approach and did not include the IMpower110 trial findings. Their results were more favorable for subgroups with high PD-L1 expression (11, 39), which was the focus of our FP NMA.
This NMA should be interpreted in the context of certain strengths and limitations. Individual patient data were only available for the atezolizumab trials; digitized summary data extracted from publications were used for other trials. Findings were consistent across histology subgroups and sensitivity analyses that included different contributing clinical trials for each of the CIT interventions. In general, heterogeneity was low within the evidence networks where evidence was available for assessment. One exception was noted with the PFS comparison of pembrolizumab and chemotherapy in the squamous and non-squamous networks (KEYNOTE-024, KEYNOTE-042). Many treatment comparisons were informed by a single study, limiting the means to quantitatively evaluate between-study heterogeneity. As such, informative priors for the between-study variance were used to fit random effects models. All chemotherapy treatment arms across trials were considered exchangeable and assigned to the same network node. This may have introduced heterogeneity in the analysis, resulting in undetected biases when estimating the different treatment effects. There were no closed loops in the networks, and inconsistency could not be evaluated. PFS data from the atezolizumab studies were based on investigator assessment, whereas all other studies used independent review committee assessments. We assumed these to be equivalent, but this may have been a source of inherent bias. Sub-populations for each study for every possible histology group in the PD-L1-high population were analyzed. The individual studies were not necessarily powered to inform these comparisons, and in some instances, these subgroups included few patients (e.g.., the squamous NSCLC PD-L1-high IMpower110 population included 50 patients across the two treatment groups). Length of follow-up was relatively short for some studies, which may be a partial reflection of the poor prognosis for advanced NSCLC and introduce uncertainty regarding longer-term effects. For some trials, updated findings based on longer follow-up times were not available for this report, as some data were from reports of interim analyses. Estimated quantities from the FP analysis, such as expected OS, expected PFS, hazard ratios, and survival functions over time, were presented for a period of 5 years maximum and should be interpreted with caution, in particular for the later time points. Heterogeneity of PD-L1 assay methods used across trials remains a point of concern when conducting indirect comparisons across CIT treatment options. Finally, although it was not an objective of this analysis, further work is needed to better understand the relative effectiveness of CIT for other PD-L1 expression subgroups.
Conclusions
This systematic literature review and NMA suggested superiority of CIT regimens versus chemotherapy as first-line treatment of NSCLC in terms of survival, objective response, and safety. These findings may support evidence evaluations and decisions in the clinic and for health technology assessments applied to population health policies.
Author Contributions
All authors contributed to the interpretation of results and development of the manuscript, including approval for submission. SA, DJ, RM, and RB designed the study. RM and DJ collected and analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This study is sponsored by F. Hoffmann-La Roche, Ltd.
Conflict of Interest
RH reports non-financial support from Genentech/Roche during the conduct of the study; personal fees from Abbvie Pharmaceuticals, personal fees from ARMO Biosciences, grants and personal fees from AstraZeneca, personal fees from Biodesix, personal fees from Bolt Biotherapeutics, personal fees from Bristol Myers Squibb, personal fees from Cybrexa Therapeutics, personal fees from eFFECTOR Therapeutics, Inc., grants and personal fees from Eli Lilly and Company, personal fees from EMD Serono, grants and personal fees from Genentech/Roche, personal fees from Genmab, personal fees from Halozyme Therapeutics, personal fees from Heat Biologics, personal fees from I-Mab Biopharma, personal fees from Immunocore, personal fees from Infinity Pharmaceuticals, personal fees from Junshi Pharmaceuticals, personal fees from Loxo Oncology, grants and personal fees from Merck and Company, personal fees from Mirati Therapeutics, personal fees from Nektar, personal fees from Neon Therapeutics, personal fees from NextCure, personal fees from Novartis, personal fees from Oncternal Therapeutics, personal fees from Pfizer, personal fees from Sanofi, personal fees from Seattle Genetics, personal fees from Shire PLC, personal fees from Spectrum Pharmaceuticals, personal fees from STCube Pharmaceuticals, Inc, personal fees from Symphogen, personal fees from Takeda, personal fees from Tesaro, personal fees from Tocagen, personal fees from WindMIL Therapeutics, personal fees from Xencor, Inc, outside the submitted work. JJ reports personal fees and non-financial support from Roche during the conduct of the study; personal fees from AstraZeneca, personal fees from Pfizer, personal fees from BMS, personal fees from MSD outside the submitted work. SA and RB are employees of F. Hoffmann-La Roche, Ltd, the study sponsor. DJ is an employee of Quantics Biostatistics, and RM is an employee of the York Health Economics Consortium, Ltd, both of which received funding from the study sponsor for this work. FM reports fees as advisor/speaker from AstraZeneca, BMS, Roche/Genentech, Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from F. Hoffmann-La Roche, Ltd. The funder was involved in the study design, collection and analysis of the data.
Acknowledgments
Medical writing assistance for this manuscript was provided by Jeff Frimpter, MPH, of Health Interactions and funded by F. Hoffmann-La Roche, Ltd. Katy Wilson of York Health Economics Consortium assisted with data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.676732/full#supplementary-material
References
1. World Health Organization, International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=lung (Accessed July 16, 2018).
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108.
3. National Comprehensive Care Network. Non-Small Cell Lung Cancer. Version 8.2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed October 19, 2020).
4. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2018) 29(Suppl 4):iv192–237.
5. Brahmer J, Rodriguez-Abreu D, Robinson AG, Hui R, Csõszi T, Fülöp A, et al. KEYNOTE-024 5-Year OS Update: First-Line (1L) Pembrolizumab (Pembro) vs Platinum-Based Chemotherapy (Chemo) in Patients (Pts) With Metastatic NSCLC and PD-L1 Tumour Proportion Score (TPS) ≥50%. Ann Oncol (2020) 31(Suppl 4):S1142–1215.
6. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Final Analysis of the Phase III Keynote-042 Study: Pembrolizumab (Pembro) Versus Platinum-Based Chemotherapy (Chemo) as First-Line Therapy for Patients (Pts) With PD-L1-Positive Locally Advanced/Metastatic NSCLC. Ann Oncol (2019) 30(Suppl 2):ii38.
7. Reck M, Ciuleanu T-E, Dols MC, Schenker M, Zurawski B, Menezes J, et al. Nivolumab (NIVO) + Ipilimumab (IPI) + 2 Cycles of Platinum-Doublet Chemotherapy (Chemo) vs 4 Cycles Chemo as First-Line (1L) Treatment (Tx) for Stage IV/recurrent Non-Small Cell Lung Cancer (NSCLC): CheckMate 9la. Ann Oncol (2020) 38(Suppl 15):9501–1.
8. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients With NSCLC. N Engl J Med (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
9. Liu J, Chengming L, Seery S, Yu J, Meng X. Identifying Optimal First-Line Interventions for Advanced Non-Small Cell Lung Carcinoma According to PD-L1 Expression: A Systematic Review and Network Meta-Analysis. Oncoimmunol (2020) 9(1):e1746112. doi: 10.1080/2162402X.2020.1746112
10. Tun AM, Thein KZ, Thein WL, Guevara E. Checkpoint Inhibitors Plus Chemotherapy for First-Line Treatment of Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Future Sci OA (2019) 5(9):FSO421. doi: 10.2144/fsoa-2019-0081
11. Chen Y, Zhou Y, Tang L, Peng X, Jiang H, Wang G, et al. Immune-Checkpoint Inhibitors as the First Line Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. J Cancer (2019) 10(25):6261–8. doi: 10.7150/jca.34677
12. Carbone D, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med (2017) 376(25):2415–26. doi: 10.1056/NEJMoa1613493
13. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. EMPOWER-Lung 1: Phase III First-Line (1L) Cemiplimab Monotherapy vs Platinum-Doublet Chemotherapy (Chemo) in Advanced Non-Small Cell Lung Cancer (NSCLC) With Programmed Cell Death-Ligand 1 (PD-L1) ≥50%. Ann Oncol (2020) 31(Suppl 4):S1182–3.
14. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and Pemetrexed With or Without Pembrolizumab for Advanced, Non-Squamous Non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. Lancet Oncol (2016) 17(11):1497–508.
15. Reck M, Rodríguez-Abreu D, Robinson A, Hui R, Csoszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33.
16. Brahmer J, Rodriguez-Abreu D, Robinson A, Hui R, Csoszi T, Fulop A, et al. Updated Analysis of Keynote-024: Pembrolizumab vs Platinum-Based Chemotherapy for Advanced NSCLC With PD-L1 TPS ≥50%. J Thorac Oncol (2017) 12(11 Suppl 2):S1793–S94.
17. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378:2078–92.
18. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38(14):1505–7. doi: 10.1200/JCO.19.03136
19. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
20. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Parra HS, Mazieres J, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous Non-Small-Cell Lung Cancer: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol (2020) 15(10):1657–69. doi: 10.1016/j.jtho.2020.06.015
21. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: A Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT Anti-PD-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.09.028
22. Lu S, Yu Y, Yu X, Hu Y, Ma Z, Li X, et al. Tislelizumab + Chemotherapy vs Chemotherapy Alone as First-Line Treatment for Locally Advanced/Metastatic Nonsquamous NSCLC. Ann Oncol (2020) 31(Suppl 4):S816–7. doi: 10.1016/j.annonc.2020.08.1577
23. Chu P, Watkins CL. Fractional Polynomial Modelling in Network Meta-Analyses of Cancer Immunotherapies in Advanced Non-Small Cell Lung Cancer (NSCLC). In: Presented at: 20th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research, Glasgow, Scotland, 4-8 November 2017, abstract PRM150.
24. Jansen JP. Network Meta-Analysis of Survival Data With Fractional Polynomials. BMC Med Res Methodol (2011) 11:61. doi: 10.1186/1471-2288-11-61
25. Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JPT. Predictive Distributions for Between-Study Heterogeneity and Simple Methods for Their Application in Bayesian Meta-Analysis. Stat Med (2015) 34(6):984–98. doi: 10.1002/sim.6381
26. Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Available at: https://www.ncbi.nlm.nih.gov/books/NBK310366/pdf/Bookshelf_NBK310366.pdf (Accessed October 27, 2020).
27. Spigel D, De Marinis F, Giaccone G, Reinmuth N, Vergnenegre A, Barrios C, et al. IMpower110: Interim Overall Survival (OS) Analysis of a Phase III Study of Atezolizumab (Atezo) vs Platinum-Based Chemotherapy (Chemo) as First-Line (1L) Treatment (Tx) in PD-L1–Selected Nsclc. Ann Oncol (2019) 30(Suppl 5):v915. doi: 10.1093/annonc/mdz293
28. F. Hoffmann-La Roche Ltd, Reck M. Primary CSR Study GO29436 (IMpower150) a Phase III, Open-Label, Randomized Study of MPDL3280A (Anti-PDL-1 Antibody) in Combination With Carboplatin + Paclitaxel With or Without Bevacizumab Compared With Carboplatin + Paclitaxel + Bevacizumab in Chemotherapy-Naive Patients With Stage IV non-Squamous Non-Small Cell Lung Cancer. Grosshansdorf, Germany: F. Hoffmann-La Roche Ltd (2018). Report No. 1077726.
29. F. Hoffmann-La Roche Ltd, Reck M. Update CSR Study GO29436 (IMpower150) a Phase III, Open-Label, Randomized Study of MPDL3280A (anti-PDL-1 Antibody) in Combination With Carboplatin + Paclitaxel With or Without Bevacizumab Compared With Carboplatin + Paclitaxel + Bevacizumab in Chemotherapy-Naive Patients With Stage IV Non-Squamous Non-Small Cell Lung Cancer. Grosshansdorf, Germany: F. Hoffmann-La Roche Ltd (2018). Report No. 1085182.
30. F. Hoffmann-La Roche Ltd, Capuzzo F. Primary CSR Study GO29537 (IMpower130) Phase III, Multicenter, Randomized, Open-Label Study Evaluating the Efficacy and Safety of Atezolizumab (MPDL3280A, anti-PD-L1 Antibody) in Combination With Carboplatin+Nab-Paclitaxel for Chemotherapy-Naïve Patients With Stage IV Non-Squamous Non-Small Cell Lung Cancer. Grosshansdorf, Germany: F. Hoffmann-La Roche Ltd (2018). Report No. 1080283.
31. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu D, Hussein M, et al. IMpower131: Primary PFS and Safety Analysis of a Randomized Phase III Study of Atezolizumab + Carboplatin + Paclitaxel or Nab-Paclitaxel vs Carboplatin + Nab-Paclitaxel as 1L Therapy in Advanced Squamous NSCLC. J Clin Oncol (2018) 36(18 Suppl):LBA9000. doi: 10.1200/JCO.2018.36.18_suppl.LBA9000
32. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous Non-Small-Cell Lung Cancer (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
33. Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G, et al. IMpower132: PFS and Safety Results With 1L Atezolizumab + Carboplatin/Cisplatin + Pemetrexed in Stage IV non-Squamous NSCLC. J Thorac Oncol (2018) 13(10):S332–S33. doi: 10.1016/j.jtho.2018.08.262
34. Borghaei H, Langer C, Gadgeel S, Papadimitrakopoulou V, Patnaik A, Powell S, et al. Updated Results From KEYNOTE-021 Cohort G: A Randomized, Phase 2 Study of Pemetrexed and Carboplatin (PC) With or Without Pembrolizumab (Pembro) as First-Line Therapy for Advanced Nonsquamous NSCLC. Ann Oncol (2017) 28(Suppl 5):v636–v37. doi: 10.1093/annonc/mdx440.052
35. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393(10183):1819–30.
36. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
37. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
38. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-Line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol (2020) 6(5):661–74.
Keywords: non-small cell lung cancer, immunotherapy, atezolizumab, pembrolizumab, nivolumab, ipilimumab, meta-analysis
Citation: Herbst R, Jassem J, Abogunrin S, James D, McCool R, Belleli R, Giaccone G and De Marinis F (2021) A Network Meta-Analysis of Cancer Immunotherapies Versus Chemotherapy for First-Line Treatment of Patients With Non-Small Cell Lung Cancer and High Programmed Death-Ligand 1 Expression. Front. Oncol. 11:676732. doi: 10.3389/fonc.2021.676732
Received: 05 March 2021; Accepted: 04 June 2021;
Published: 09 July 2021.
Edited by:
Idris Bahce, Academic Medical Center, NetherlandsReviewed by:
Santiago Viteri, Instituto Oncológico Dr Rosell, SpainChristopher Gerard Azzoli, Brown University, United States
Copyright © 2021 Herbst, Jassem, Abogunrin, James, McCool, Belleli, Giaccone and De Marinis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roy Herbst, cm95LmhlcmJzdEB5YWxlLmVkdQ==
 Roy Herbst
Roy Herbst Jacek Jassem2
Jacek Jassem2 Seye Abogunrin
Seye Abogunrin Rossella Belleli
Rossella Belleli Giuseppe Giaccone
Giuseppe Giaccone Filippo De Marinis
Filippo De Marinis