- 1Surgical Research Center, Institute of Urology, Medical School of Southeast University, Nanjing, China
- 2Department of Urology, Zhongda Hospital Affiliated to Southeast University, Nanjing, China
- 3Department of Urology, Binhai County People’s Hospital, Yancheng, China
- 4Department of Urology, Zhongda Hospital Affiliated to Southeast University Lishui Branch, Nanjing, China
Background: Neoadjuvant chemotherapy has been accepted as an effective curative treatment for muscle-invasive bladder cancer patients and has resulted in better survival outcomes than radical cystectomy or a cisplatin-based regimen. In the present study, we aimed to compare the two most commonly used cisplatin-based neoadjuvant chemotherapies, gemcitabine plus cisplatin and methotrexate plus vinblastine plus doxorubicin plus cisplatin, by summarizing and analyzing clinical data and outcomes of published research.
Methods: We searched for qualified studies that compared these two types of neoadjuvant chemotherapy, including 4 randomized controlled trials and 14 retrospective studies. Data and information on pathological responses and long-term survival studies were extracted and analyzed separately.
Results: A total of 18 studies with 3116 patients were selected from 1188 studies, which contained data on pathological complete response, pathological partial response, and overall survival. In contrast to the results of previous studies, there was no significant difference in pathological complete response (odds ratio, 0.97; 95% confidence interval, 0.81-1.15), pathological partial response (odds ratio, 0.85; 95% confidence interval, 0.72-1.14), and overall survival (hazard ratio, 0.99; 95% confidence interval, 0.83-1.17) between GC and MVAC in this meta-analysis.
Conclusion: No significant differences were observed between GC and MVAC in the muscle-invasive bladder cancer treatment due to the similar curative effect and parallel long survival outcomes due to the similar curative effect and parallel long survival outcomes. The priority selection of GC or MVAC in the clinic should be guided by further investigation, and the clinical standard strategy still counts on the results of more randomized controlled trials in the future.
1 Introduction
Bladder cancer is the second most common carcinoma in the urinary tract, and its occurrence rate continues to increase annually worldwide. Muscle-invasive bladder cancer (MIBC), an aggressive type of bladder cancer with a high risk of peripheral and distant metastasis, has been widely recognized as one of the primary causes of tumor-related death currently (1). Many randomized controlled trials (RCTs) have reported that cisplatin-based neoadjuvant chemotherapy (NCT) has superior curative effects to either radical cystectomy or locoregional treatment alone and could improve long-term survival outcomes. Recent reports also supported the ability of NCT to effectively decrease the morbidity and mortality of MIBC when combined with radical cystectomy, making NCT a clinically preferable option for treating MIBC (2).
However, the preferred regimen of cisplatin-based NCT for MIBC remains debatable. Considering the outcomes of the significant SWOG-8710 RCT, methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) were established as the most effective regimen in the NCT setting (3). In contrast, another RCT showed that gemcitabine plus cisplatin (GC) had an advantage in terms of survival outcomes, and therefore, this regimen has been increasingly applied in the NCT setting over MVAC (4).
In 2016, a two-step meta-analysis claimed that MVAC is superior in terms of overall survival of GC and might have the same treatment response rate as the latter, suggesting that MVAC should be the standard care in MIBC (5). On the contrary, another systematic review reporting comparative outcomes of the two chemotherapies two years later argued that GC ought to be used as the first-line chemotherapeutics during the treatment, as it showed better clinical efficacy than MVAC (6). However, results of both studies were not significant. In addition, the references cited in these studies might include repetitive samples. In the present study, we attempted to provide an independent and distinct conclusion with minimum possible bias. It could help to update the relevant information and offer clinical guidance for the future studies.
2 Materials and Methods
2.1 Documents Retrieval and Search Strategy
The standards of retrieved document were in line with the principle of participants, interventions, comparators, outcomes, and study design (PICOS),which were shown as follows: Participants, patients diagnosed as MIBC who were willing to undergo radical cystectomy and patients who underwent systemic neoadjuvant chemotherapy; Interventions, MIBC patients who underwent systemic NCT using MVAC or dense dose MVAC (ddMVAC); Comparators, MIBC patients who underwent systemic NCT using GC; Outcomes, comparison of pathologic (pathologic complete response (PCR), pathologic complete response (PPR) and prognostic outcomes [Overall Survival (OS)]; and Study design, both prospective RCTs and retrospective observational studies could be included for analyses.
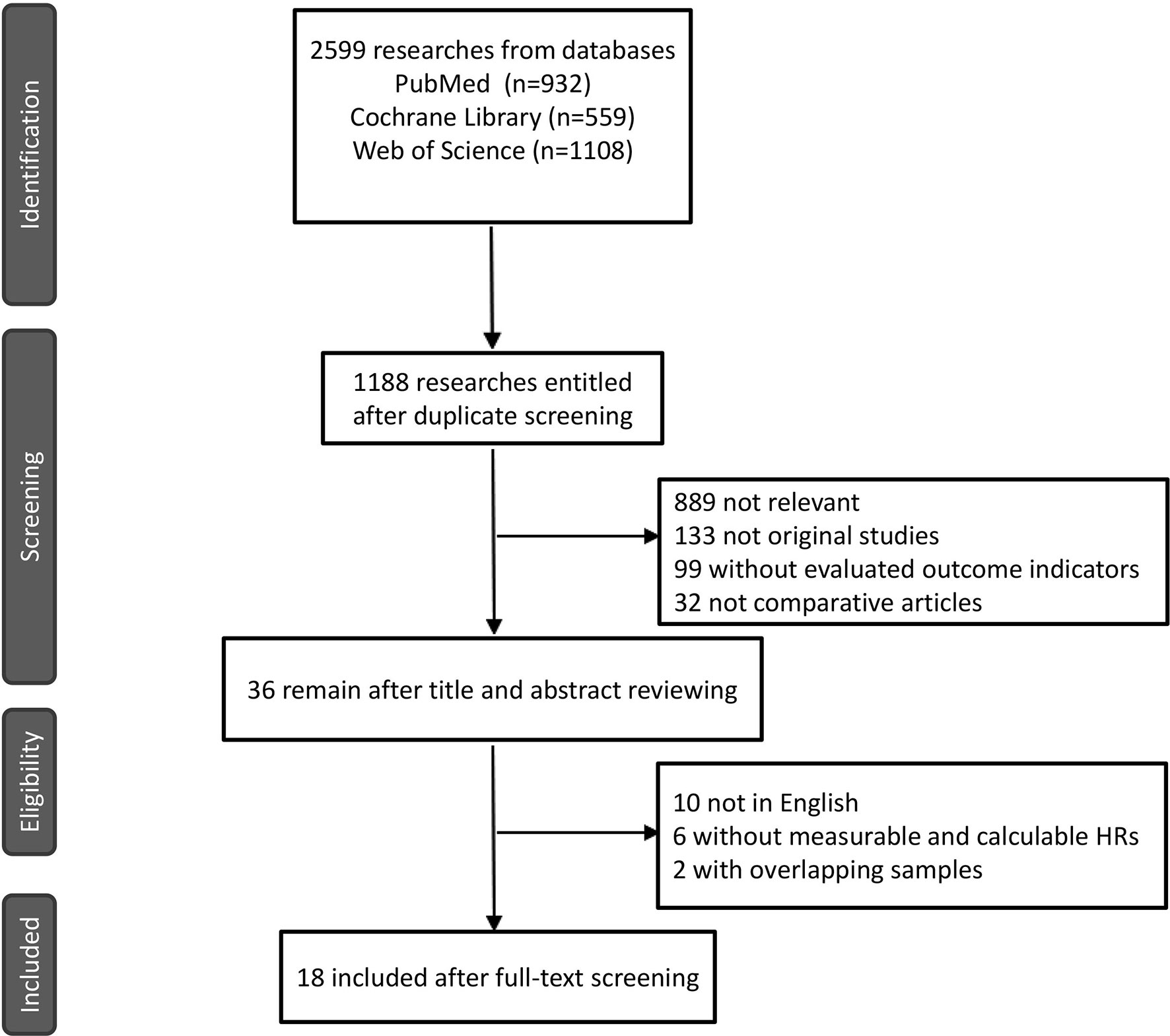
We extensively searched PubMed, the Cochrane Central Search Library, and the Web of Science for related clinical trials and retrospective studies ranging from January 1, 2005, to September 30, 2021. The search terms used included bladder cancer, gemcitabine, cisplatin, methotrexate, vinblastine, and doxorubicin. The formula was as follows: (“GC” OR “gemcitabine and cisplatin/carboplatin” OR “gemcitabine/cisplatin” OR “gemcitabine plus cisplatin” OR “gemcitabine and cisplatin” OR “gemcitabine PLUS carboplatin” OR “gemcitabine/carboplatin”) AND (“MVAC” OR “Methotrexate, vinblastine, doxorubicin, and cisplatin” OR “Methotrexate/vinblastine/doxorubicin/cisplatin” OR “Methotrexate plus vinblastine plus doxorubicin plus cisplatin”) AND (“bladder cancer” OR “MIBC” OR “bladder tumor” OR “carcinoma” OR “tumor” OR “neoplasm”). The qualified studies were identified using the following inclusion criteria: A) Patients diagnosed with MIBC in accordance with the biopsy results. B) Patients voluntarily willing to undergo cisplatin-based neoadjuvant chemotherapy. C) Research included meaningful comparative results of GC versus MVAC, such as pathological complete response, pathological partial response, and overall survival outcomes. D) No overlapping samples represented in different studies.
2.2 Data Extraction
All data were extracted in the NoteExpress form and included the original title, data source, author, research objects, test and control measures, measurement and evaluation, statistical analysis, and conclusion derivation. We contacted the authors for the missing content wherever feasible. All objective data were extracted from the qualified publications by two investigators after an independent assessment. Controversial articles were re-evaluated by a third researcher. Odds ratios (ORs) were used to describe quantitative outcome indicators, including PCR and PPR. Hazard ratios (HRs) belonging to the survival analysis were used to evaluate OS. If the HRs were unavailable from the corresponding authors, we used Engauge Digitizer v10.8 to extract the Kaplan-Meier curves provided by articles to calculate HRs, lower confidence intervals, and upper confidence intervals (7).
2.3 Statistical Analysis
The meta-analysis was performed to summarize and analyze clinical outcomes from the literature, including PCR, PPR, and OS in both GC and MVAC groups. The Cochran Q test was applied to evaluate heterogeneity among studies with a significance level of p<0.05. When I^2 < 50% and p≥0.1, we performed the analysis using a fixed-effect model. If I^2≥50%, a random-effects model was used instead. Funnel plots were used to assess probable publication bias. All applicable data were analyzed with Review Manager 5.4 and Stata SE 12.0, to perform a meta-analysis. The analyses of PCR and PPR were performed with Review Manager version 5.4 (Cochrane, Oxford, U.K.), while the survival analysis was performed with Stata SE 12.0. All p-values were two-sided and set as p <0.05.
3 Results
3.1 Researches Screening and Risk Bias Assessment
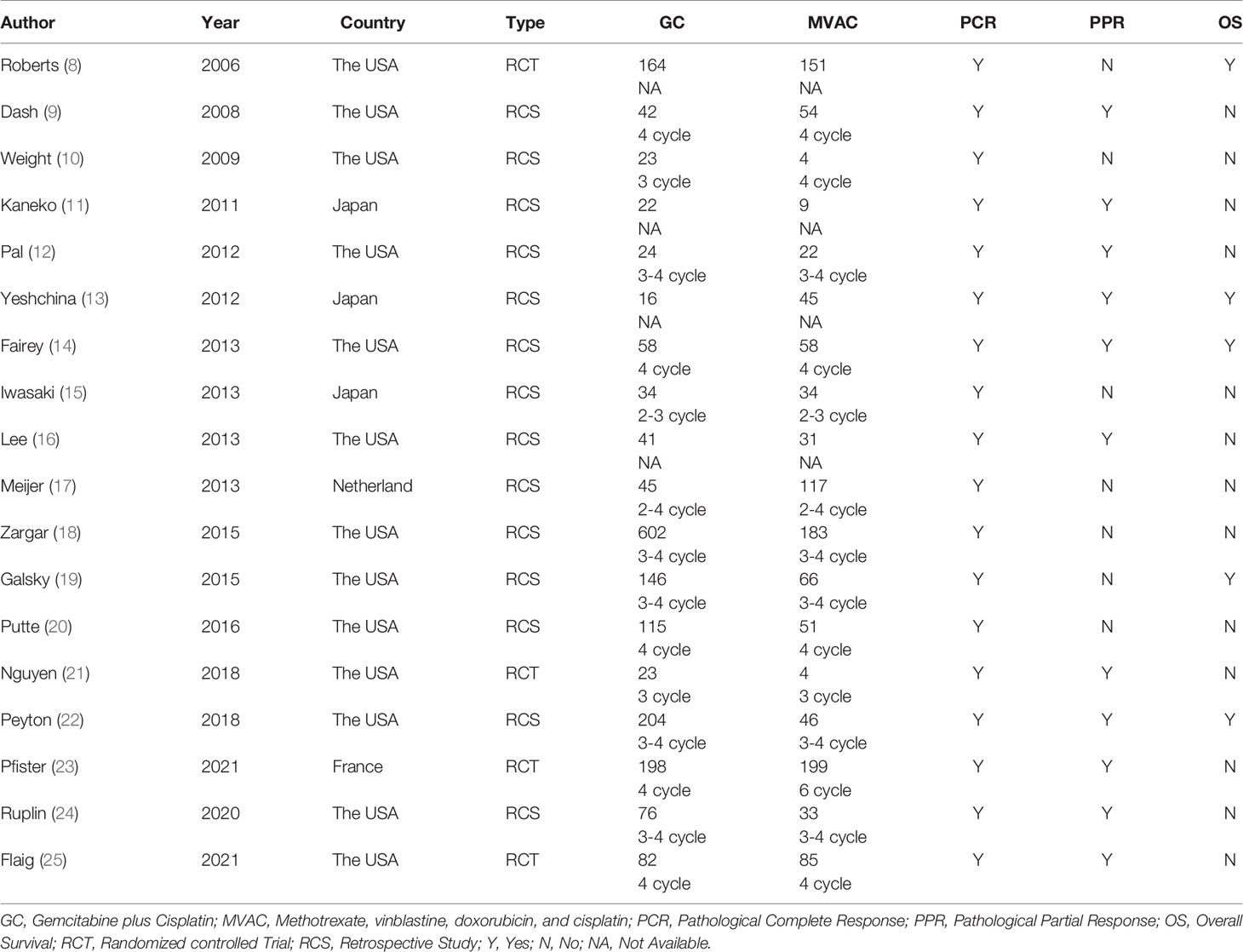
A total of 18 reports of 3116 patients receiving neoadjuvant chemotherapy were included, comprising 4 RCTs and 14 retrospective studies (8–25). All 18 articles provided comparative outcomes of PCR, in which seven studies had PPR outcomes and four studies conducted survival analyses. The risk of bias item presented in Figure 1 showed a generally high quality of included articles, and the process of selecting qualified studies is displayed in Figure 2. Baseline characteristics are listed in Table 1. The quality of included articles was assessed by two reviewers with the use of Cochrane Risk-of-Bias Tool and Jadad for RCTs and Newcastle-Ottawa Scale (NOS) for case-control study. The items were separately shown in Figures 2A, B and Appendix Tables 1, 2 (Supplementary Material).
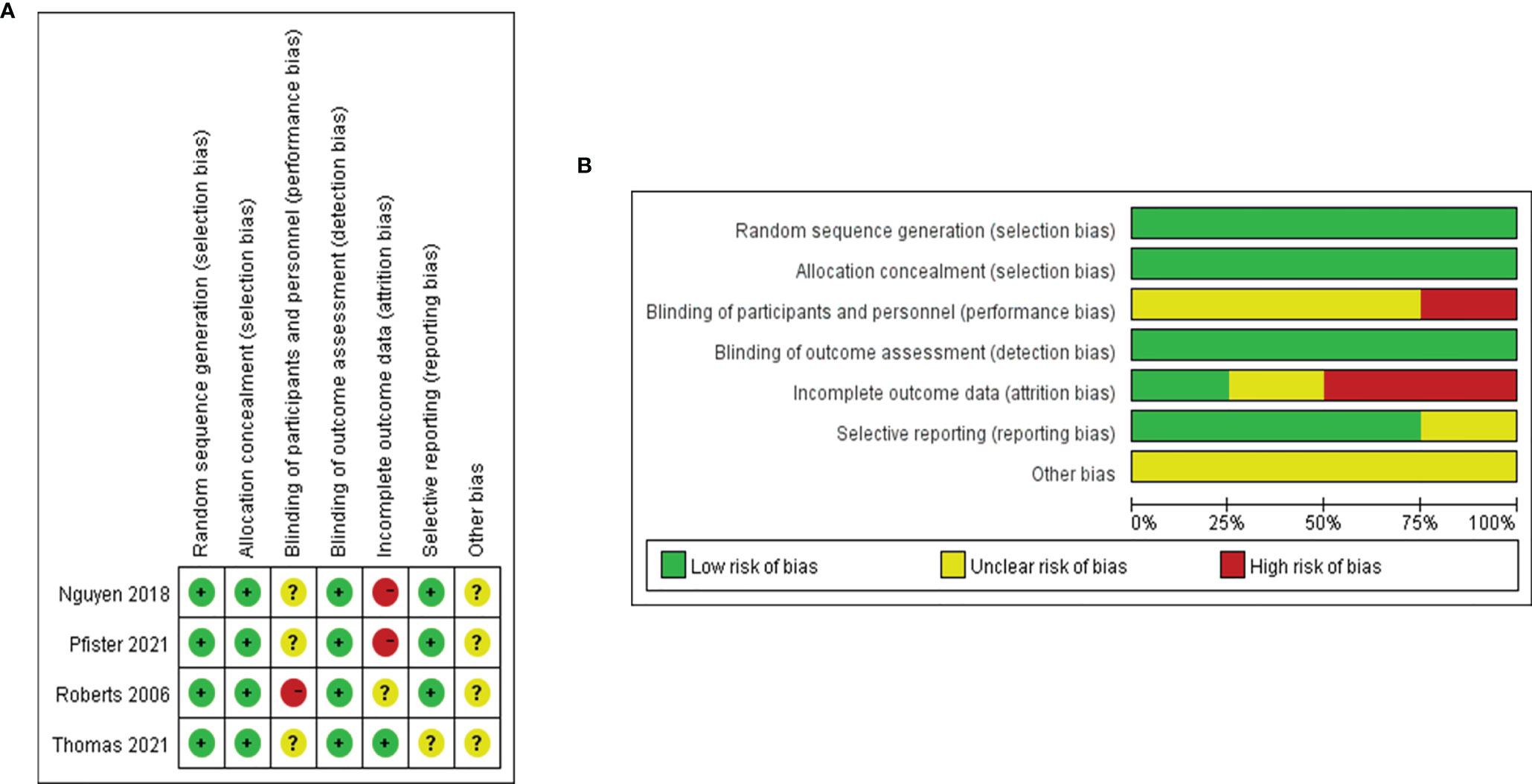
Figure 2 (A) Risk of bias graph based on Cochrane Risk-of-Bias Tool: review authors’ judgements about each risk of bias item presented as percentages across all included RCTs. (B) Risk of bias graph based on Cochrane Risk-of-Bias Tool: review authors’ judgements about each risk of bias item for each included RCT.
3.2 Treatment Response of GC vs. MVAC
3.2.1 Pathological Complete Response (pT0)
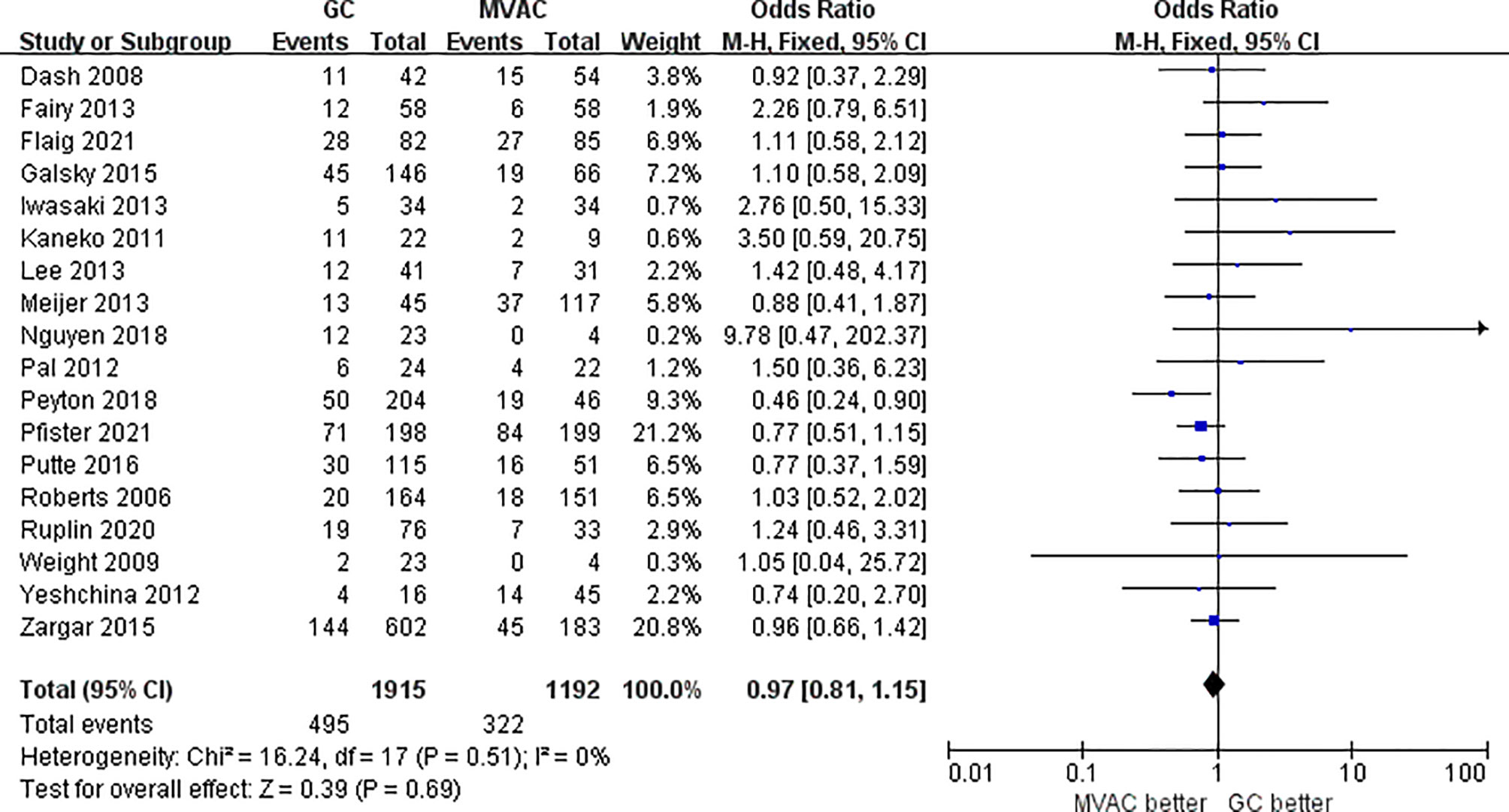
All 18 studies with 3116 patients compared the PCRs between GC and MVAC. The difference between GC and MVAC for PCR was insignificant (OR, 0.97; 95% confidence interval (CI), 0.81-1.15, p=0.69). No significant heterogeneity was found among the studies (p= 0.51, I^2 = 0%), and a fixed-effects model was used in the analysis (Figure 3). Four studies with relatively small sample sizes (< 50) were excluded for the sensitivity analysis to reduce possible bias. The analysis of the remaining 12 studies showed no significant difference (OR, 0.93; 95% CI, 0.78-1.11, p=0.42). No significant heterogeneity was found among the studies (p= 0.58, I^2 = 0%) (Figure 4).
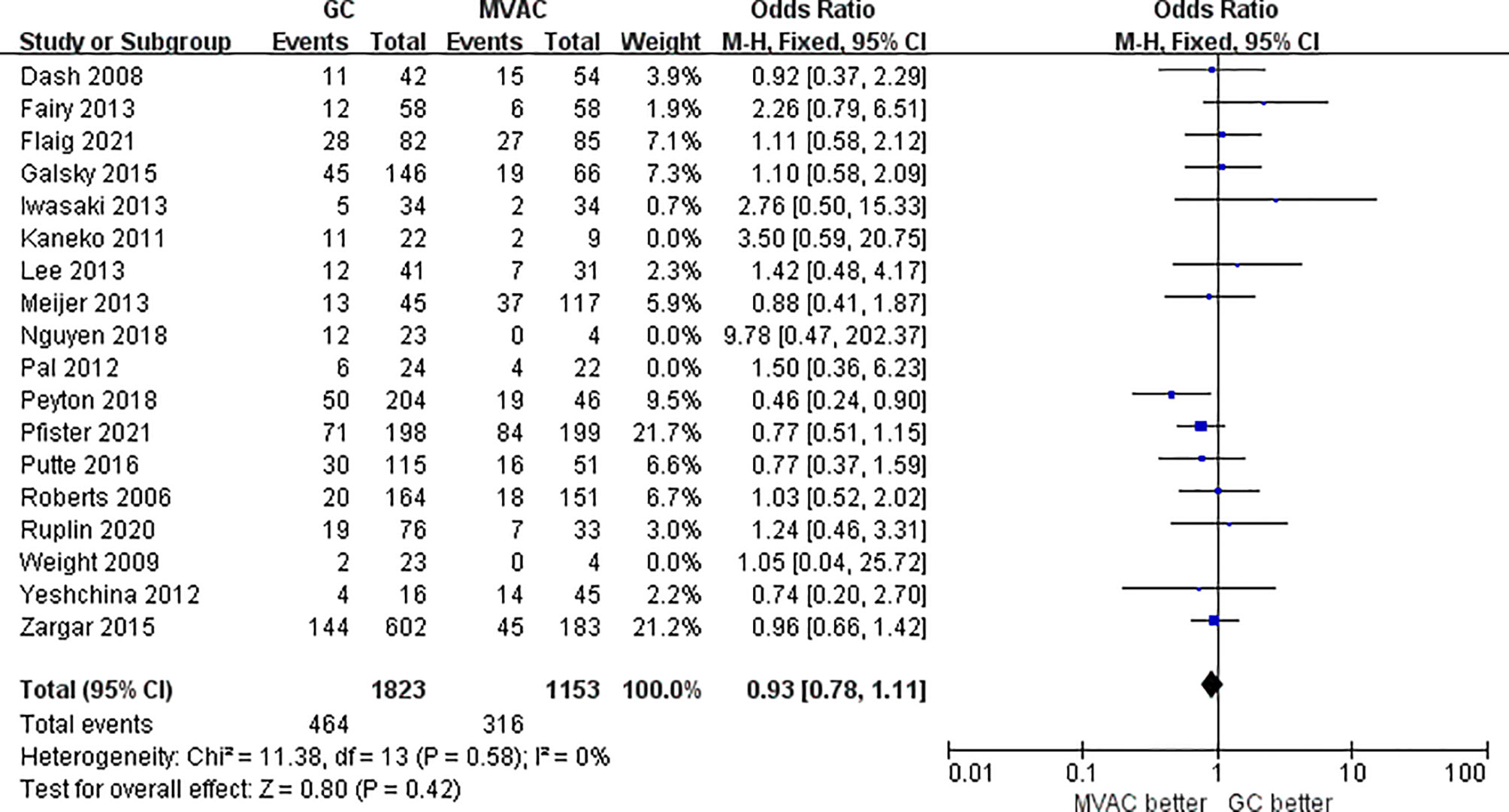
Figure 4 Forest plot of pathological complete response (PCR) to GC versus MVAC after removing studies with small sample sizes.
3.2.2 Pathological Partial Response (<T2)
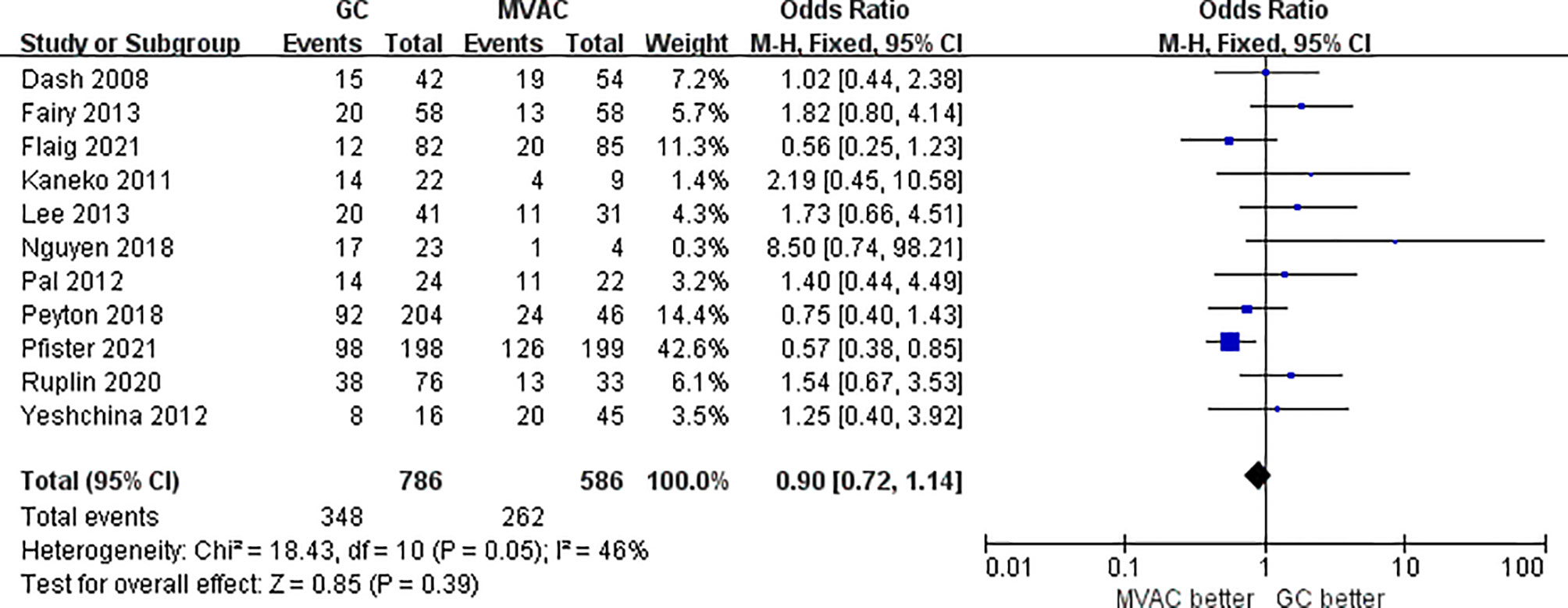
11 studies with 1372 patients reported the results of PPR to GC and MVAC regimens. The difference between GC and MVAC for PPR was insignificant (OR, 0.90; 95% CI, 0.72-1.14, p=0.39). No significant heterogeneity was found among the studies (p= 0.05, I^2 = 46%), and a fixed-effects model was used in the analysis (Figure 5). However, after removing 3 studies with small sample sizes under 50 to reduce inherent bias, the result generally appeared the same (OR, 0.84; 95% CI, 0.66-1.08, p=0.17). Significant heterogeneity was found among the studies (p= 0.07, I^2 = 47%) (Figure 6).
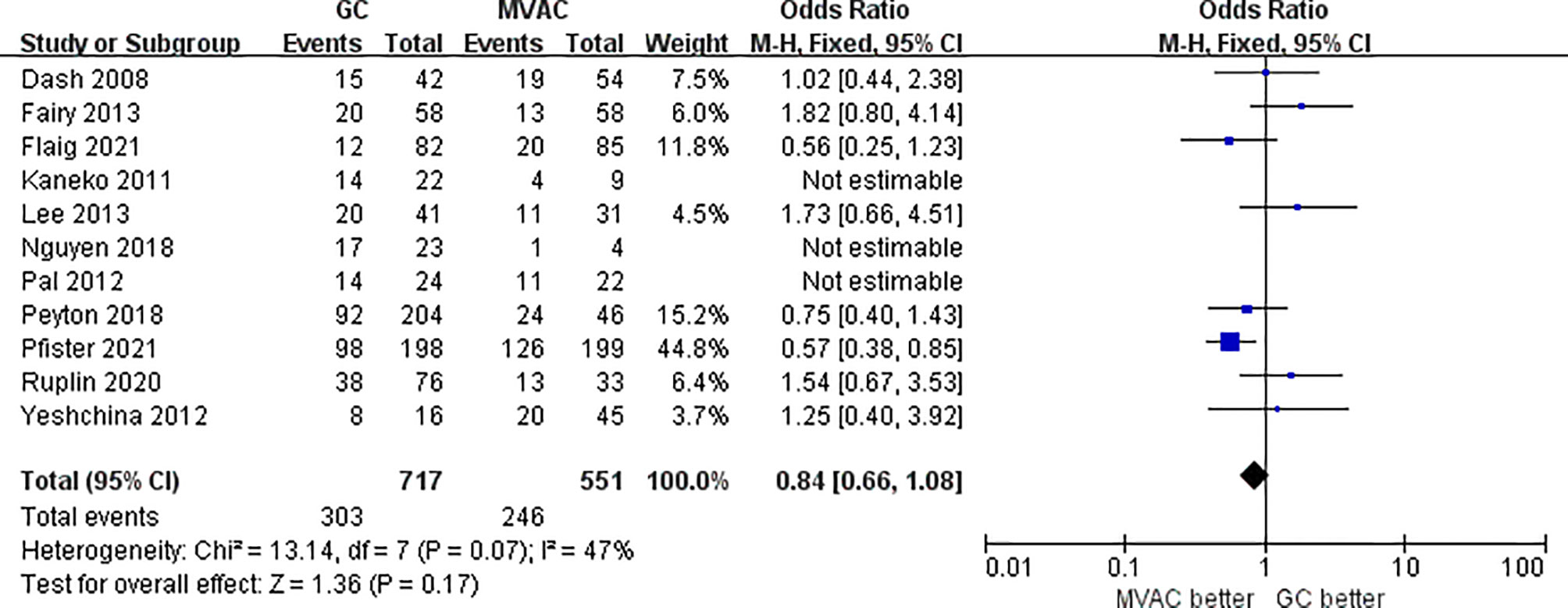
Figure 6 Forest plot of pathological partial response (PPR) to GC versus MVAC after removing studies with small sample sizes.
3.3 Overall Survival
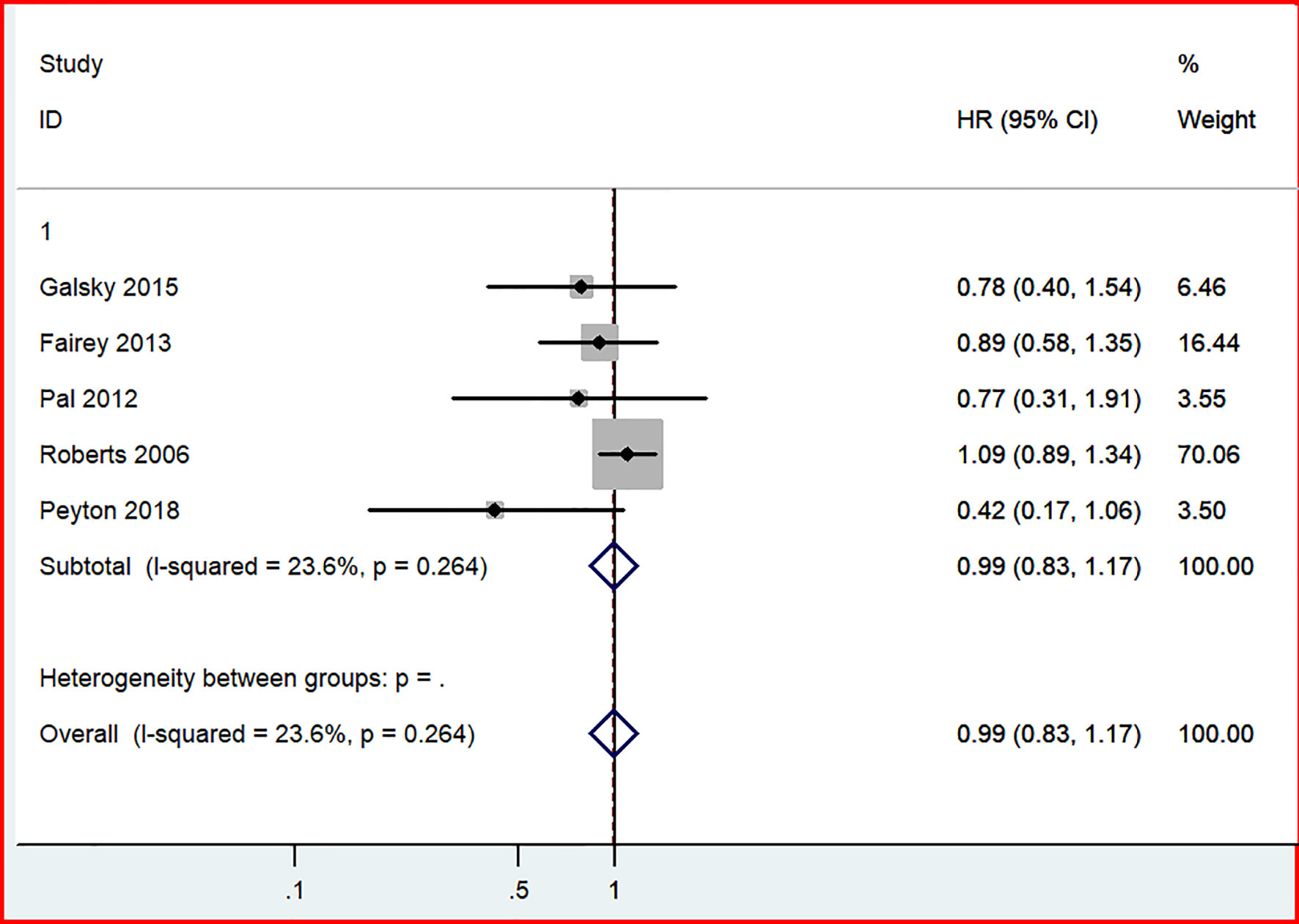
In our analysis of long-term outcomes from four studies, including 689 patients, no significant difference in OS was discovered between the GC and MVAC groups (HR, 0.99; 95% CI, 0.83-1.17, p=0.868). No significant heterogeneity was not observed in this analysis (p= 0.264, I^2 = 23.6%) (Figure 7).
3.4 Publication Bias
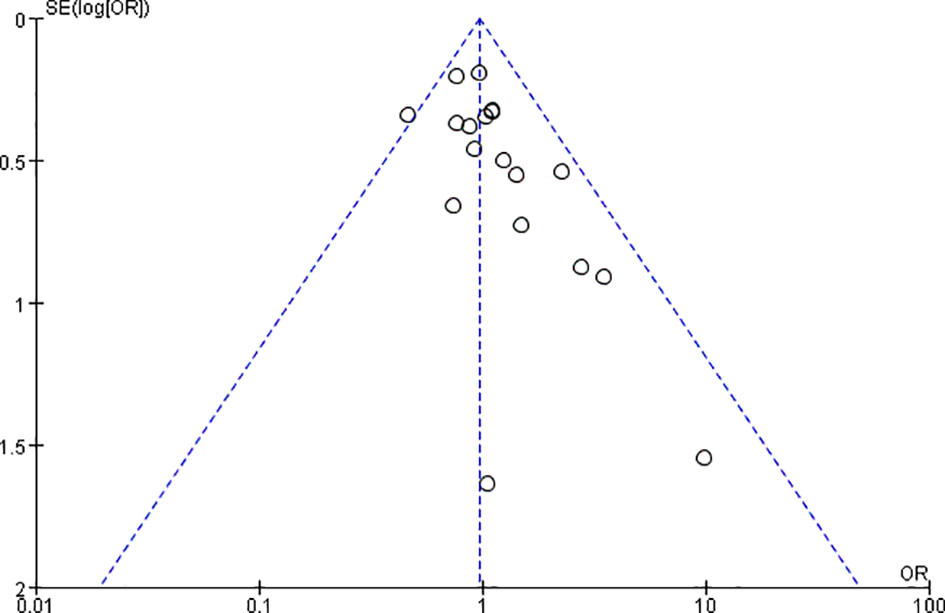
A funnel plot was established to evaluate publication bias among all 18 studies (Figure 8). Publication bias was not detected in the funnel plot or Egger’s test (data not shown).
4 Discussion
Since the 1980s, treatment of locally advanced and metastatic bladder cancer has been cisplatin-based (26). Several clinical trials have suggested MVAC as the standard therapy for almost 10 years (27). Although a survival advantage was seen with MVAC compared with cisplatin alone, MVAC still showed a high risk of nonnegligible toxicity. It was worth noting that ddMVAC showed better tolerability compared with classical dose of MVAC in previous researches, but there was no significant difference in OS outcome between the two regimens. Therefore, we did not treat them differently, since our analyses were partially focused on the prognosis of OS. The ddMVAC was more recommended in the regimen for the subgroup of patients pursuing persistent disease control, as was referred to in the review published by Vaibhav G Patel, et al. in 2020 (28). Thus, a less toxic but identical curative regimen is required. In 1995, GC was developed and found to be effective in many types of cancers, including bladder carcinoma. Since then it has been officially utilized for bladder cancer treatment as an alternative to MVAC (29, 30). However, clinical prioritization of individual regimen is still debatable.
According to the first quantitative analysis in 2016 comparing GC and MVAC as the two most commonly used chemotherapies in neoadjuvant settings, MVAC was suggested as the preferable regimen to cure MIBC with better long-term survival outcomes and similar clinical efficacy compared with GC (5). However, two years later, another meta-analysis claimed that GC was the standard guidance in MIBC treatment, considering its better curative effect than MVAC (6). Consequently, there is a dispute regarding which one of the two regimens could be acknowledged as the authoritative neoadjuvant chemotherapy for MIBC. Despite the similar purpose of these two meta-analyses, the different studies they included leaded to opposite conclusions. Under these circumstances, we believe it is necessary to add new studies and proceed with the research on this topic to obtain an updated conclusion precisely.
This study completed a comprehensive analysis, the conclusion of which was different from the previous analyses. In the present study, PCR and PPR were considered as indicators reflecting the clinical efficacy of GC and MVAC (PCR: 25.8% vs. 27.0%; PPR: 43.3% vs. 46.0%) among the 18 independent studies. Both types of pathological responses exhibited insignificant differences between the two groups of regimens, even when we removed studies with small samples for sensitivity analyses (PCR: 25.5% vs. 27.4%; PPR: 42.3% vs. 44.6%). These results further indicated the stability and reliability. The comparative result of PCR was more persuasive than that of PPR, in that the PCR analysis covered a larger number of patients who participated in RCTs. Despite the relatively better pathological responses to MVAC, a definite choice between the two therapies could still not be made because the difference was not statistically significant. As for the long-term survival outcome in 6 studies, no significant difference was observed in OS between the two groups of interest, though the MVAC also manifested a tendency of better prognosis. The HRs applied were directly extracted from the articles or identified from visualized Kaplan-Meier curves by Engauge Digitizer v10.8, which were more accurately calculated than other similar studies, which could be considered as an innovation.
What is more, it was debatable when it comes to the standard cycle number of treatments. 3 or 4 cycles have been recommended as the optimal manner for GC receiver, which could provide the adequate efficacy and avoid missing the best opportunity for radical cystectomy (31). However, disputes remained in choosing an appropriate treatment period of MVAC regimen. Although large-scale multicenter randomized trials were included in our study for quantitative synthesis and analyses, the numbers of MVAC cycles were different, ranging from 2 to 6 cycles. Among the studies included in our research, the PCR and PPR were respectively 5.9%-41.3% and 22.4%-62.2% for the numbers of cycles, which were less than or equal to 4, and they were respectively 42.2% and 63.3% for 6 two-week cycles. Consequently, 6 cycles of MVAC might have an edge over 2~4 cycles on pathological response for better operative opportunity but could lead to a postponed checkpoint that delays the surgery.
Some limitations could not be ignored in our updated research. First, double-blinding was difficult to achieve because of the nature of the intervention. Second, although we included 4 prospective RCTs, the sample size for the meta-analysis was still relatively small, given the main component was retrospective analyses pooled in our research, so the prospective data were still insufficient. Nevertheless, the retrospective studies were of generally high quality when rated with NOS. Moreover, the difference of effectiveness between GC and MVAC is vague, based on our updated conclusion. Therefore, though our research puts forward a different perspective from before, we were not able to propose instructive conclusions for regimen choice. When we looked up and attempted to explore the better option considering the side effects of toxicity, two small-scale RCTs (32, 33) comparing the drug toxicity were found, published in the beginning of 21st Century. However, in 2021, Pfister C. et al. performed the latest VESPER randomized phase III trial with a large sample size and prospectively demonstrated the superiority of ddMVAC (25), which was taken as strong evidence in our meta-analysis. The results of this large-scale clinical trial showed that although GC was more manageable with slighter asthenia and gastrointestinal side effects than ddMVAC, the latter had a higher local control rate and showed prominent advantage in pathological response. Due to this conflict, we anticipated more clinical trials concerning toxicity to provide rationales for medication in the future.
4.1 Conclusion
There is no significant difference between GC and MVAC, regarding the use of neoadjuvant chemotherapy to treat MIBC. Clinicians should develop treatment schemes counting on other factors, such as drug side effects, interaction with surgeries and treatment conditions. However, the conclusions drawn from our study should be interpreted cautiously. Large-scale and multicenter RCTs integrated with drug toxicity and cycle number comparison are demanded before final clinical guidance can be officially recommended as standards.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
TW, MC, and HL designed the study. BX and YW conducted the study and maintained the data. SC, JW, and WZ analyzed the data and made the figures. All authors drafted and revised the paper. TW and YW reanalyzed the data and completed the final version of the manuscript and figures. All authors approved the final version of the manuscript.
Funding
This study was funded by The National Natural Science Foundation of China (No. 81872089, 81370849, 81672551, 81300472, 81070592, 81202268, 81202034), Natural Science Foundation of Jiangsu Province (BK20161434, BL2013032, BK20150642 and BK2012336), Six talent peaks project in Jiangsu Province, Jiangsu Provincial Medical Innovation Team (CXTDA2017025), The National Key Research and Development Program of China (SQ2017YFSF090096), Jiangsu Provincial Key Research and Development Program(BE2019751), Innovative Team of Jiangsu Provincial (2017ZXKJQWO7), and Jiangsu Provincial Medical Talent (ZDRCA2016080).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Editage Author Services (http://editage.com/frontiers/) for English language editing and review services.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.678896/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
2. Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2013 Guidelines. Eur Urol (2014) 65(4):778–92. doi: 10.1016/j.eururo.2013.11.046
3. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant Chemotherapy Plus Cystectomy Compared With Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med (2003) 349(9):859–66. doi: 10.1056/NEJMoa022148
4. Porter MP, Kerrigan MC, Donato BM, Ramsey SD. Patterns of Use of Systemic Chemotherapy for Medicare Beneficiaries With Urothelial Bladder Cancer. Urol Oncol (2011) 29(3):252–8. doi: 10.1016/j.urolonc.2009.03.021
5. Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist (2016) 21(6):708–15. doi: 10.1634/theoncologist.2015-0440
6. Yu C, Hequn C, Jinbo C, Feng Z, Xiongbing Z, Jian D, et al. Gemcitabine/cisplatin Versus Methotrexate/Vinblastine/Doxorubicin/Cisplatin for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J Cancer Res Ther (2018) 14(6):1260–5. doi: 10.4103/0973-1482.188434
7. Ma G, Wang Q, Lv C, Qiang F, Hua Q, Chu H, et al. The Prognostic Significance of HOTAIR for Predicting Clinical Outcome in Patients With Digestive System Tumors. J Cancer Res Clin Oncol (2015) 141(12):2139–45. doi: 10.1007/s00432-015-1980-8
8. Roberts JT, von der Maase H, Sengeløv L, Conte PF, Dogliotti L, Oliver T, et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine/Cisplatin and Methotrexate/Vinblastine/Doxorubicin/Cisplatin in Patients With Locally Advanced and Metastatic Bladder Cancer. Ann Oncol (2006) 22(11):118–22. doi: 10.1093/annonc/mdj965
9. Dash A, Pettus JA 4th, Herr HW, Bochner BH, Dalbagni G, Donat SM, et al. A Role for Neoadjuvant Gemcitabine Plus Cisplatin in Muscle-Invasive Urothelial Carcinoma of the Bladder: A Retrospective Experience. Cancer (2008) 113(9):2471–7. doi: 10.1002/cncr.23848
10. Weight CJ, Garcia JA, Hansel DE, Fergany AF, Campbell SC, Gong MC, et al. Lack of Pathologic Down-Staging With Neoadjuvant Chemotherapy for Muscle-Invasive Urothelial Carcinoma of the Bladder: A Contemporary Series. Cancer (2009) 115(4):792–9. doi: 10.1002/cncr.24106
11. Kaneko G, Kikuchi E, Matsumoto K, Obata J, Nakamura S, Miyajima A, et al. Neoadjuvant Gemcitabine Plus Cisplatin for Muscle-Invasive Bladder Cancer. Jpn J Clin Oncol (2011) 41(7):908–14. doi: 10.1093/jjco/hyr068
12. Pal SK, Ruel NH, Wilson TG, Yuh BE. Retrospective Analysis of Clinical Outcomes With Neoadjuvant Cisplatin-Based Regimens for Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer (2012) 10(4):246–50. doi: 10.1016/j.clgc.2012.08.004
13. Yeshchina O, Badalato GM, Wosnitzer MS, Hruby G, RoyChoudhury A, Benson MC, et al. Relative Efficacy of Perioperative Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Adriamycin, and Cisplatin in the Management of Locally Advanced Urothelial Carcinoma of the Bladder. Urology (2012) 79(2):384–90. doi: 10.1016/j.urology.2011.10.050
14. Fairey AS, Daneshmand S, Quinn D, Dorff T, Dorin R, Lieskovsky G, et al. Neoadjuvant Chemotherapy With Gemcitabine/Cisplatin vs. Methotrexate/Vinblastine/Doxorubicin/Cisplatin for Muscle-Invasive Urothelial Carcinoma of the Bladder: A Retrospective Analysis From the University of Southern California. Urol Oncol (2013) 31(8):1737–43. doi: 10.1016/j.urolonc.2012.07.005
15. Iwasaki K, Obara W, Kato Y, Takata R, Tanji S, Fujioka T, et al. Neoadjuvant Gemcitabine Plus Carboplatin for Locally Advanced Bladder Cancer. Jpn J Clin Oncol (2013) 43(2):193–9. doi: 10.1093/jjco/hys213
16. Lee FC, Harris W, Cheng HH, Shenoi J, Zhao S, Wang J, et al. Pathologic Response Rates of Gemcitabine/Cisplatin Versus Methotrexate/Vinblastine/Adriamycin/Cisplatin Neoadjuvant Chemotherapy for Muscle Invasive Urothelial Bladder Cancer. Adv Urol (2013) 2013:317190. doi: 10.1155/2013/317190
17. Meijer RP, Nieuwenhuijzen JA, Meinhardt W, Bex A, van der Poel HG, van Rhijn BW, et al. Response to Induction Chemotherapy and Surgery in non-Organ Confined Bladder Cancer: A Single Institution Experience. Eur J Surg Oncol (2013) 39(4):365–71. doi: 10.1016/j.ejso.2013.01.003
18. Zargar H, Espiritu PN, Fairey AS, Mertens LS, Dinney CP, Mir MC, et al. Multicenter Assessment of Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Eur Urol (2015) 67(2):241–9. doi: 10.1016/j.eururo.2014.09.007
19. Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative Effectiveness of Gemcitabine Plus Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin as Neoadjuvant Therapy for Muscle-Invasive Bladder Cancer. Cancer (2015) 121(15):2586–93. doi: 10.1002/cncr.29387
20. van de Putte EE, Mertens LS, Meijer RP, van der Heijden MS, Bex A, van der Poel HG, et al. Neoadjuvant Induction Dose-Dense MVAC for Muscle Invasive Bladder Cancer: Efficacy and Safety Compared With Classic MVAC and Gemcitabine/Cisplatin. World J Urol (2016) 34(2):157–62. doi: 10.1007/s00345-015-1636-y
21. Nguyen TT, Huillard O, Dabi Y, Anract J, Sibony M, Zerbib M, et al. Neoadjuvant Chemotherapy in Patients With Muscle-Invasive Bladder Cancer and Its Impact on Surgical Morbidity and Oncological Outcomes: A Real-World Experience. Front Surg (2018) 5(58). doi: 10.3389/fsurg.2018.00058
22. Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and Survival Outcomes Associated With Neoadjuvant Chemotherapy Regimens Among Patients Treated With Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol (2018) 4(11):1535–42. doi: 10.1001/jamaoncol.2018.3542
23. Pfister C, Gravis G, Fléchon A, Soulié M, Guy L, Laguerre B, et al. Randomized Phase III Trial of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Muscle-Invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur Urol (2021) 79(2):214–21. doi: 10.1016/j.eururo.2020.08.024
24. Ruplin AT, Spengler AM, Montgomery RB, Wright JL. Downstaging of Muscle-Invasive Bladder Cancer Using Neoadjuvant Gemcitabine and Cisplatin or Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin as Single Regimens or as Switch Therapy Modalities. Clin Genitourin Cancer (2020) 18(5):e557–62. doi: 10.1016/j.clgc.2020.02.010
25. Flaig TW, Tangen CM, Daneshmand S, Alva A, Lerner SP, Lucia MS, et al. A Randomized Phase II Study of Coexpression Extrapolation (COXEN) With Neoadjuvant Chemotherapy for Bladder Cancer (SWOG S1314; Nct02177695). Clin Cancer Res (2021) 27(9):2435–41. doi: 10.1158/1078-0432.CCR-20-2409
26. Loehrer PS Sr, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A Randomized Comparison of Cisplatin Alone or in Combination With Methotrexate, Vinblastine, and Doxorubicin in Patients With Metastatic Urothelial Carcinoma: A Cooperative Group Study. J Clin Oncol (1992) 10(7):1066–73. doi: 10.1200/JCO.1992.10.7.1066
27. Logothetis CJ, Dexeus FH, Finn L, Sella A, Amato RJ, Ayala AG, et al. A Prospective Randomized Trial Comparing MVAC and CISCA Chemotherapy for Patients With Metastatic Urothelial Tumors. J Clin Oncol (1990) 8(6):1050–5. doi: 10.1200/JCO.1990.8.6.1050
28. Patel VG, Oh WK, Galsky MD. Treatment of Muscle-Invasive and Advanced Bladder Cancer in 2020. CA Cancer J Clin (2020) 70(5):404–23. doi: 10.3322/caac.21631
29. Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ, et al. Interaction Between Cisplatin and Gemcitabine In Vitro and In Vivo. Semin Oncol (1995) 22(4 Suppl 11):72–9.
30. May M, Bastian PJ, Burger M, Bolenz C, Trojan L, Herrmann E, et al. Multicenter Evaluation of the Prognostic Value of Pt0 Stage After Radical Cystectomy Due to Urothelial Carcinoma of the Bladder. BJU Int (2011) 108(8 Pt 2):E278–83. doi: 10.1111/j.1464-410X.2011.10189.x
31. Ramakrishnan VM, Eswara JR. The Timing of Radical Cystectomy Following Neoadjuvant Chemotherapy. Transl Androl Urol (2018) 7(S6):S758–59. doi: 10.21037/tau.2018.08.07
32. Hussain SA, Stocken DD, Riley P, Palmer DH, Peake DR, Geh JI, et al. A Phase I/II Study of Gemcitabine and Fractionated Cisplatin in an Outpatient Setting Using a 21-Day Schedule in Patients With Advanced and Metastatic Bladder Cancer. Br J Cancer (2004) 91(5):844–9. doi: 10.1038/sj.bjc.6602112
Keywords: bladder cancer, neoadjuvant chemotherapy, gemcitabine plus cisplatin, methotrexate plus vinblastine plus doxorubicin plus cisplatin, pathological response
Citation: Wu T, Wu Y, Chen S, Wu J, Zhu W, Liu H, Chen M and Xu B (2021) Curative Effect and Survival Assessment Comparing Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin and Cisplatin as Neoadjuvant Therapy for Bladder Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 11:678896. doi: 10.3389/fonc.2021.678896
Received: 10 March 2021; Accepted: 08 November 2021;
Published: 25 November 2021.
Edited by:
Monica Montopoli, University of Padua, ItalyReviewed by:
Rasha Cosman, St Vincent’s Hospital Sydney, AustraliaMatthew Zibelman, Fox Chase Cancer Center, United States
Copyright © 2021 Wu, Wu, Chen, Wu, Zhu, Liu, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Chen, bWluZ2NoZW5zZXVAMTI2LmNvbQ==; Hui Liu, MzA3NTgxODQwMUBxcS5jb20=; Bin Xu, bmp4YjE5ODJAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Tiange Wu
Tiange Wu Yuqing Wu1,2†
Yuqing Wu1,2† Ming Chen
Ming Chen