- 1Department of Medical Oncology, Campus Bio-Medico University of Rome, Rome, Italy
- 2Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 3Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) Dino Amadori, Meldola, Italy
- 4Onco-ematological Department, IRCCS Ospedale Policlinico San Martino, Genova, Italy
- 5Medical Oncology Unit 1, Department of Oncology, Istituto Oncologico Veneto IOV IRCCS, Padua, Italy
- 6Department of Surgical, Medical and Molecular Pathology and Critical Area Medicine, University of Pisa, Pisa, Italy
- 7Oncologia, Università del Molise, Campobasso, Italy
- 8Oncology Unit, Camposampiero General Hospital, Padova, Italy
- 9Division of Medical Oncology, Istituto Tumori Bari Giovanni Paolo II—IRCCS, Bari, Italy
- 10Medical Oncology Unit, University Hospital of Parma, Parma, Italy
- 11Department of Oncology, San Bortolo General Hospital, Vicenza, Italy
- 12AO Ordine Mauriziano, SCDU Oncologia, Turin, Italy
- 13U.O. Medicina Oncologica, Ospedale Ramazzini, Carpi-AUSL Modena, Carpi, Italy
- 14Azienda Ospedaliero-Universitario “Mater Domini”, Policlinico of Catanzaro, Catanzaro, Italy
- 15Department of Health Sciences, University of Florence, Firenze, Italy
- 16Department of Urology and Gynecology, Istituto Nazionale Tumori IRCCS Fondazione “G. Pascale”, Napoli, Italy
Background: Immune-Oncology (IO) improves Overall Survival (OS) in metastatic Renal Cell Carcinoma (mRCC). The prognostic impact of previous Cytoreductive Nephrectomy (CN) and radical nephrectomy (RN), with curative intent, in patients treated with IO is not well defined. The aim of our paper is to evaluate the impact of previous nephrectomy on outcome of mRCC patients treated with IO.
Methods: 287 eligible patients were retrospectively collected from 16 Italian referral centers adhering to the MeetUro association. Patients treated with IO as second and third line were included, whereas patients treated with IO as first line were excluded. Kaplan–Meier method and log-rank test were performed to compare Progression Free Survival (PFS) and OS between groups. In our analysis, both CN and RN were included. The association between nephrectomy and other variables was analyzed in univariate and multivariate setting using the Cox proportional hazard model.
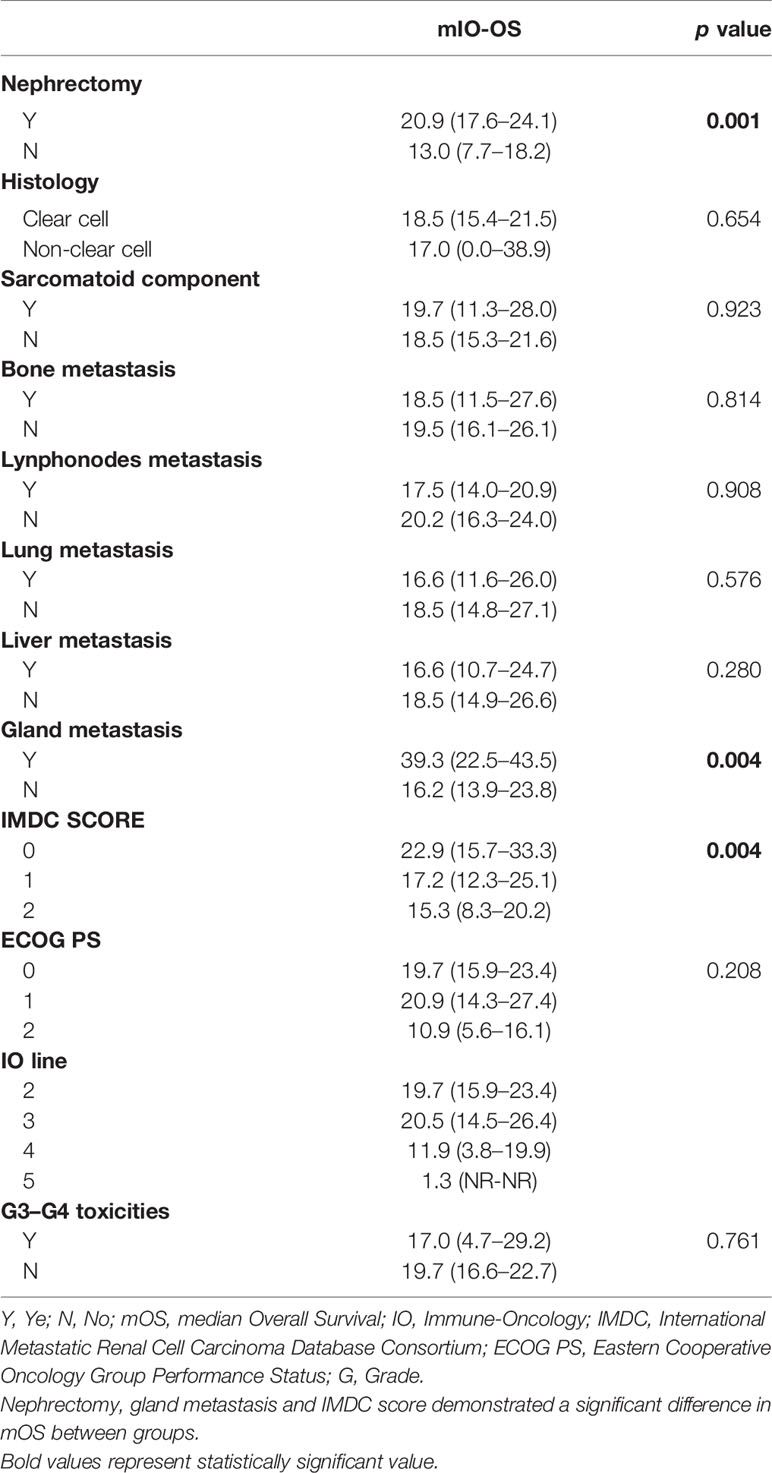
Results: 246/287 (85.7%) patients had nephrectomy before IO treatment. Median PFS in patients who underwent nephrectomy (246/287) was 4.8 months (95%CI 3.9–5.7) vs 3.7 months (95%CI 1.9–5.5) in patients who did not it (HR log rank 0.78; 95%CI 0.53 to 1.15; p = 0.186). Median OS in patients who had previous nephrectomy (246/287) was 20.9 months (95%CI 17.6–24.1) vs 13 months (95%CI 7.7–18.2) in patients who did not it (HR log rank 0.504; 95%CI 0.337 to 0.755; p = 0.001). In the multivariate model, nephrectomy showed a significant association with OS (HR log rank 0.638; 95%CI 0.416 to 0.980), whereas gland metastases were still associated with better outcome in terms of both OS (HR log rank 0.487; 95%CI 0.279 to 0.852) and PFS (HR log rank 0.646; 95%CI 0.435 to 0.958).
Conclusions: IO treatment, in patients who had previously undergone nephrectomy, was associated with a better outcome in terms of OS. Further prospective trials would assess this issue in order to guide clinicians in real word practice.
Introduction
Kidney cancer represents 5% of estimated new cases of cancer in men and 3% in women, being the 13th most commonly diagnosed solid malignancy (1, 2).
Approximately 20% of patients will develop metastases after nephrectomy, while 15% patients have already developed synchronous metastatic disease at the time of diagnosis (2, 3).
Radical Nephrectomy (RN) and Partial Nephrectomy (PR) with curative intent can be considered standard of care in patients with localized disease (4–7). In the management of metastatic renal cell carcinoma (mRCC) patients, randomized data from the “interferon era” demonstrated that Cytoreductive Nephrectomy (CN) improves survival, decreasing the risk of death (8). Despite the retrospective data from IMDC by Heng et al. and the prospective results from CARMENA and SURTIME trial (9, 10), CN remains controversial in patients treated with VEGF-targeted therapy. Recently, Immune-Oncology (IO), alone or in combination, has changed the standard of care in mRCC due to the high rate of survival in pretreated and treatment-naïve patients (11–13). In CHECKMATE214, Overall Survival (OS) favored nivolumab plus ipilimumab over sunitinib, also in patients who had previous nephrectomy. Updated analysis confirms that median OS was longer among those randomized to nivolumab-ipilimumab with target kidney lesion (14). Nevertheless, in patients treated with IO, the role of previous CN or RN has not been defined. It is unclear if previous nephrectomy affects outcome in patients treated with IO. Data about response of primary renal tumor to IO are partial and prospective trials evaluating the effect of nephrectomy in patients treated with IO are lacking.
Previous reports demonstrated some changes in the immune system after nephrectomy, but data are not conclusive (15). Therefore, the aim of our study was to retrospectively evaluate the impact of previous nephrectomy on mRCC patients treated with IO.
Patients and Methods
We retrospectively collected data of mRCC patients treated with IO in 16 Italian referral centers adhering to the Meet-Uro group, between February 2017 and January 2020.
Inclusion criteria were at least 18 years old at the time of enrollment, histological diagnosis of RCC and radiological diagnosis of metastatic disease.
Patients treated with IO, as single agent or in combination, were considered eligible. Patients enrolled in the expanded access program of nivolumab or nivolumab–ipilimumab were excluded. Patients treated with first line IO were excluded to homogenize our population, whereas patients treated with IO as second and third line were included.
Baseline characteristics were collected at the start of immunotherapy. Outcome data, including PFS and toxicities, were collected too. Data included site of metastatic disease, duration of first line and subsequent IO therapy, previous CN or RN. Glandular metastasis included metastasis in glandular organs such as thyroid, pancreas and adrenal gland.
The International Metastatic RCC Database Consortium (IMDC) prognostic risk group was computed at the index date based on the presence of six individual risk factors including time from diagnosis to systemic treatment <1 year, hemoglobin < lower limit of normal, calcium >10 mg/dl, platelet > upper limit of normal, neutrophil > upper limit of normal, Performance Status (PS) <80% (Karnofsky) (16).
Primary endpoint was to evaluate difference in IO-OS between patients who previously received nephrectomy and patients who did not it. IO-OS was defined as the time from the start of IO to death.
Secondary endpoints were to evaluate difference in PFS between the twogroups of patients. PFS was defined as the time from the start of IO to radiological or clinical progression.
Patients with no evidence of death were censored at the date of last tumor assessment.
Real-world physician-assessed progression and response was based on clinical criteria or radiographic criteria using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (17), with imaging assessments occurring at clinically variable time points.
Baseline demographic and clinical characteristics have been described using frequencies and percentages for categorical variables.
Descriptive analysis was made using median values and ranges. Kaplan–Meier method and Mantel–Haenszel log-rank test were performed to compare differences in OS and PFS between groups. The association between nephrectomy and other variables was analyzed in univariate and multivariable setting using the Cox proportional hazard model. Variables to be included in multivariate analysis were selected according to the levels of significance in cox regression univariate analysis. P-values <0.05 were considered significant. All statistical analyses were performed using SPSS software (version 19.00, SPSS, Chicago).
Written informed consent for patient information to be published was provided by the patients or a legally authorized representative. All participating centers received local ethics approval for data collections. The study was conducted in accordance with good clinical practice and the Declaration of Helsinki.
Results
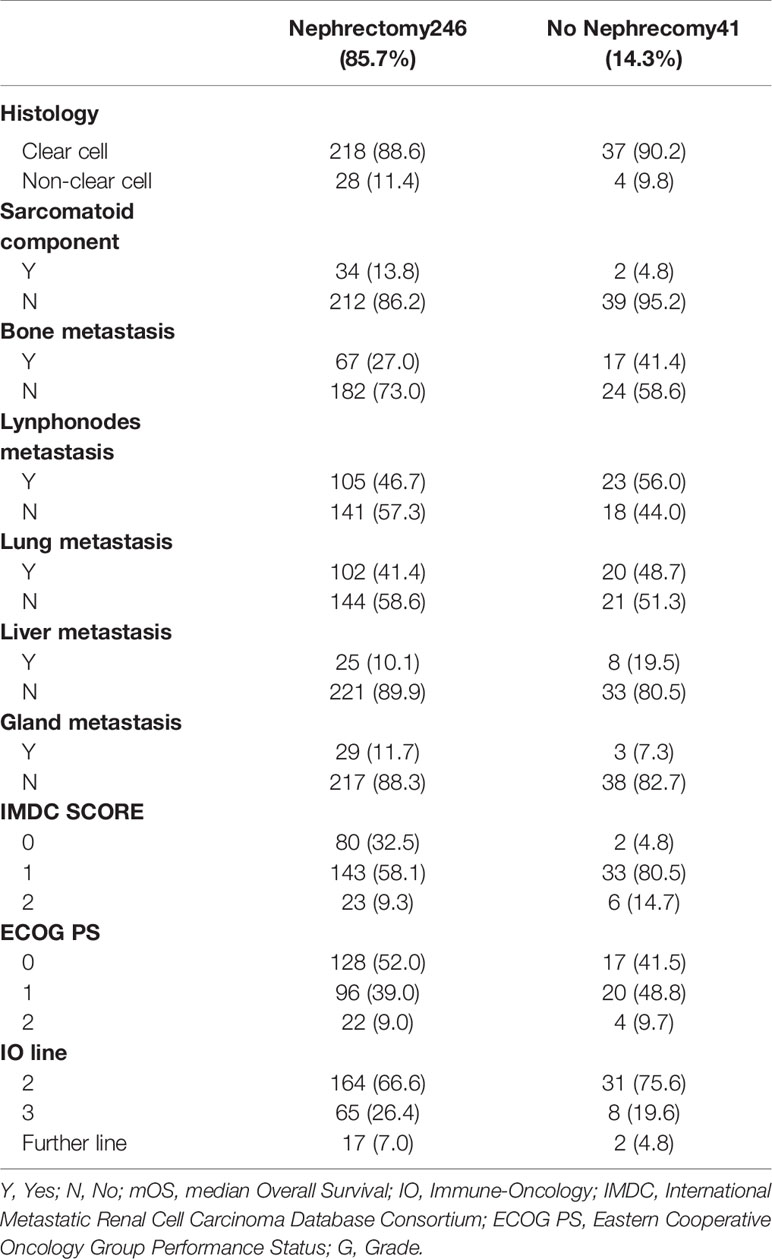
287 patients were considered eligible. Characteristics of patients are described in Table 1. All patients received nivolumab as IO.
246/287 (85.7%) patients had nephrectomy, whereas 41 (14.3%) patients did not it. 95 (33.1%) patients had CN and 151 (52.6%) patients had RN with curative intent. Nephrectomy was performed before IO treatment.
136/287 patients (47.4%) had synchronous metastatic disease, whereas 151/287 patients (52.6%) had metachronous disease.
G3–G4 immune-related Adverse Events (irAEs) were reported in 24/287 patients (8.3%).
At a median follow up of 24.7 months, 114/287 patients (56.4%) received target therapy (TT), such as mTOR inhibitors and VEGFR inhibitors at progression to IO, whereas 68/287 patients (25.4%) did not receive further treatment for clinical deterioration. 68/287 patients (23.7%) continued IO beyond progression.
52/287 patients (18.2%) were still in treatment at the time of analysis.
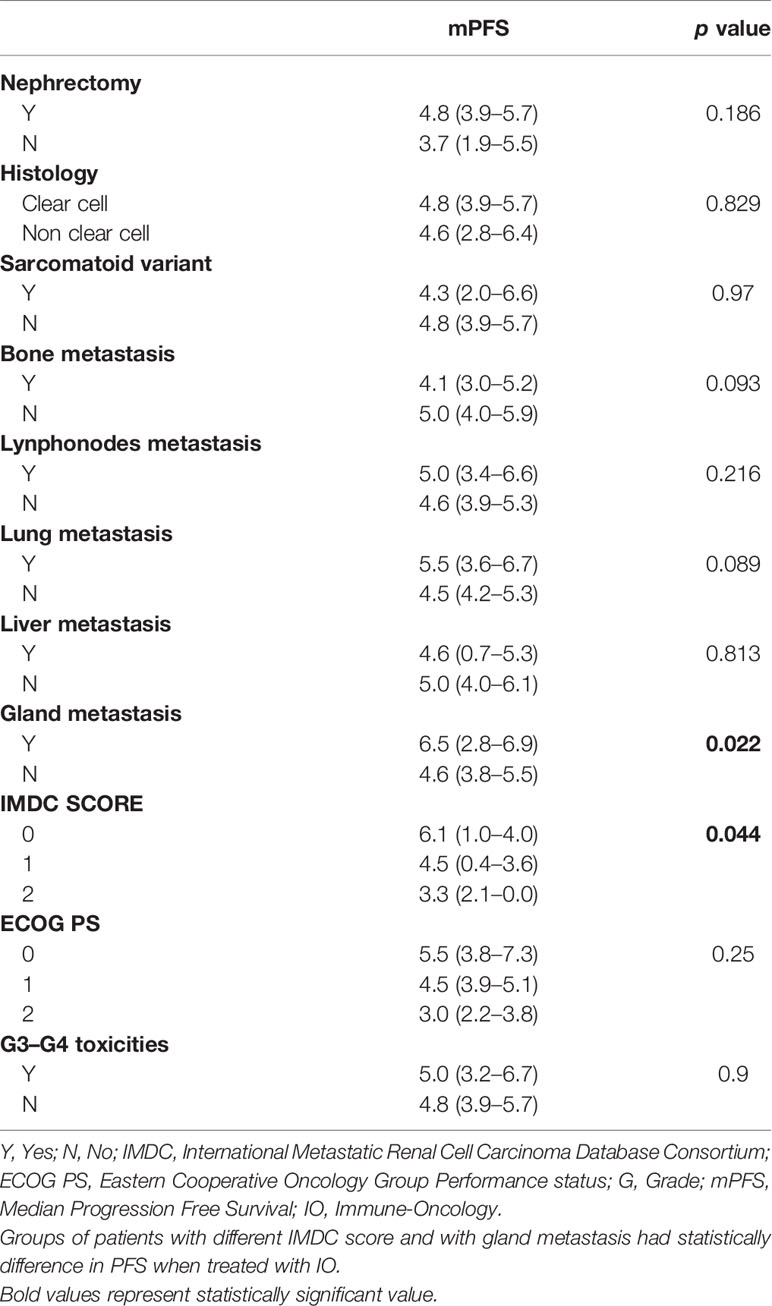
Median IO-PFS was 4.6 months (95%CI 3.85–5.42). Median PFS in patients who underwent nephrectomy (246/287) was 4.8 months (95%CI 3.9–5.7) vs 3.7 months (95%CI 1.9–5.5) in patients who did not it (HR log rank 0.78; 95%CI 0.53 to 1.15; p = 0.186) (Figure 1) (Table 2).
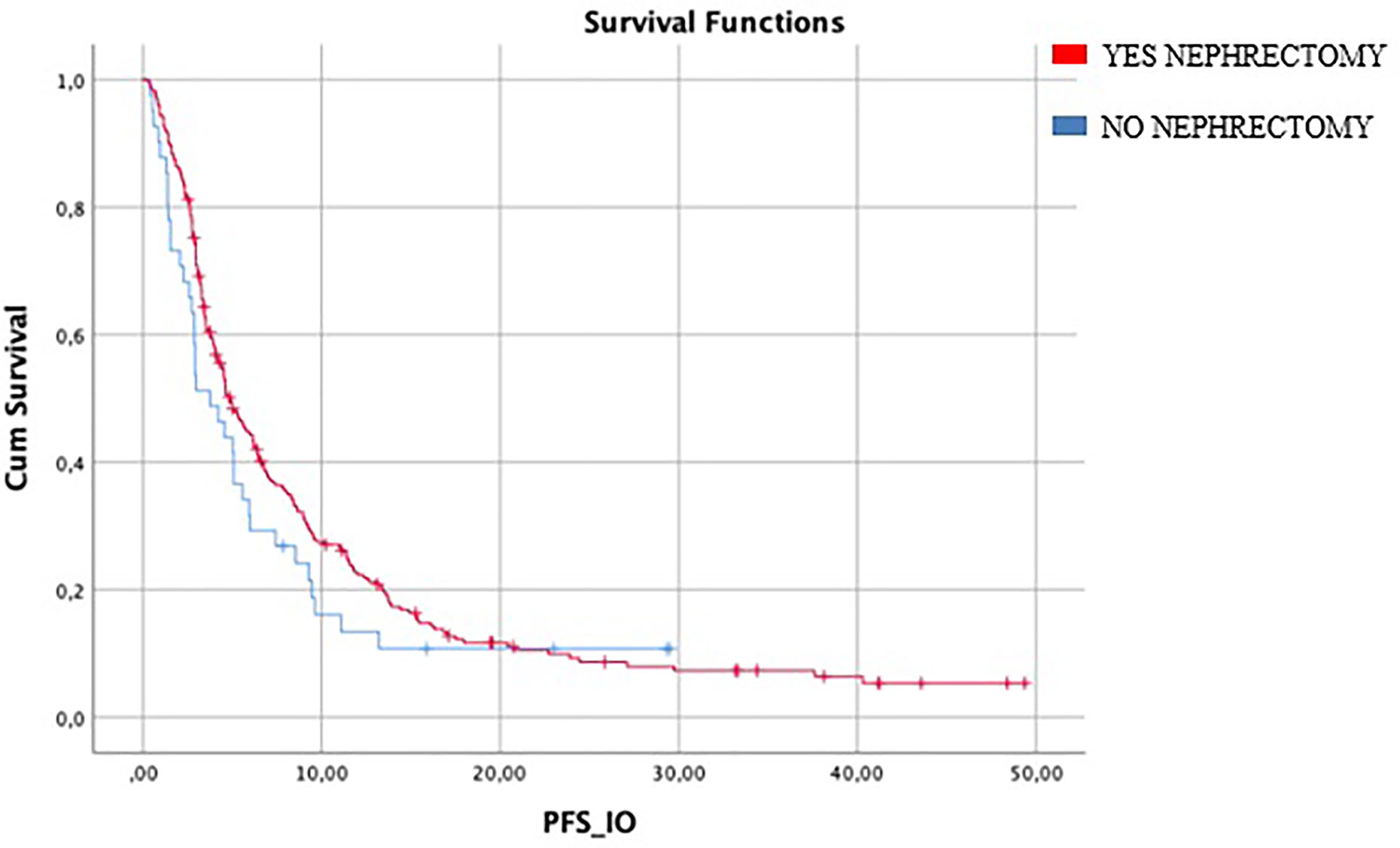
Figure 1 Median mIO-PFS in patients who underwent nephrectomy (246/287) was 4.8 months vs 3.7 months in patients who did not (HR log rank 0.78; 95%CI 0.53 to 1.15; p = 0.186). mIO-PFS (mPFS in patient treated with IO).
Median IO-OS in the entire population was 18.5 months (95%CI 15.5–21.4). In patients with metastasis to glandular organs (37/287), mOS was 39.3 months (95%CI 22.5–43.5) compared to 16.2 months (95%CI 13.9–23.8) in patients without gland metastasis (250/287) (Figure 2).
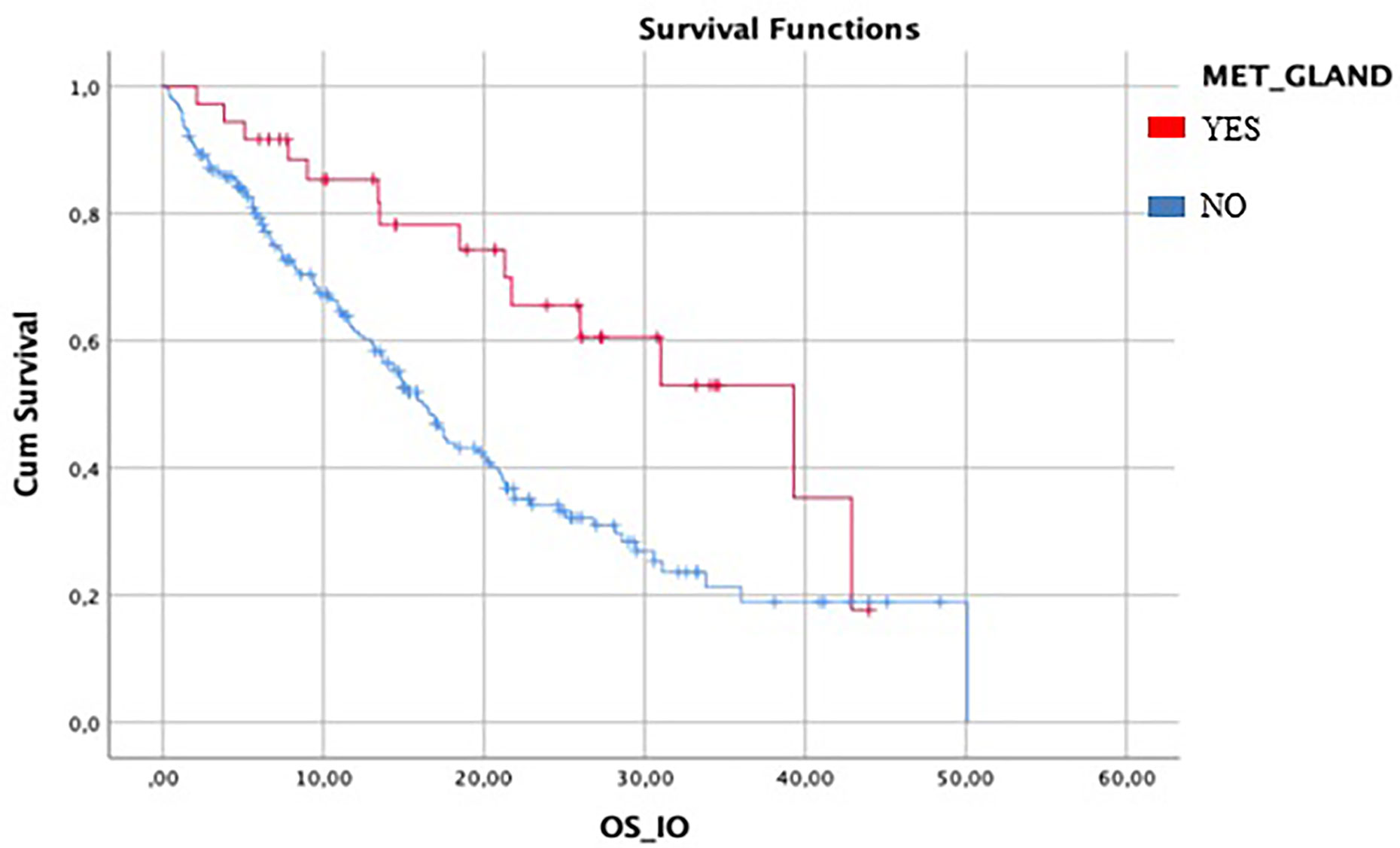
Figure 2 Difference between mIO-OS between patient with gland metastasis and patient without gland metastasis. Median IO-OS was longer in patients with gland metastasis.
Median OS in patients who had previous nephrectomy (246/287) was 20.9 months (95%CI 17.6–24.1) vs 13 months (95%CI 7.7–08.2) in patients who did not it (HR log rank 0.504; 95%CI 0.337 to 0.755; p = 0.001) (Figure 3).
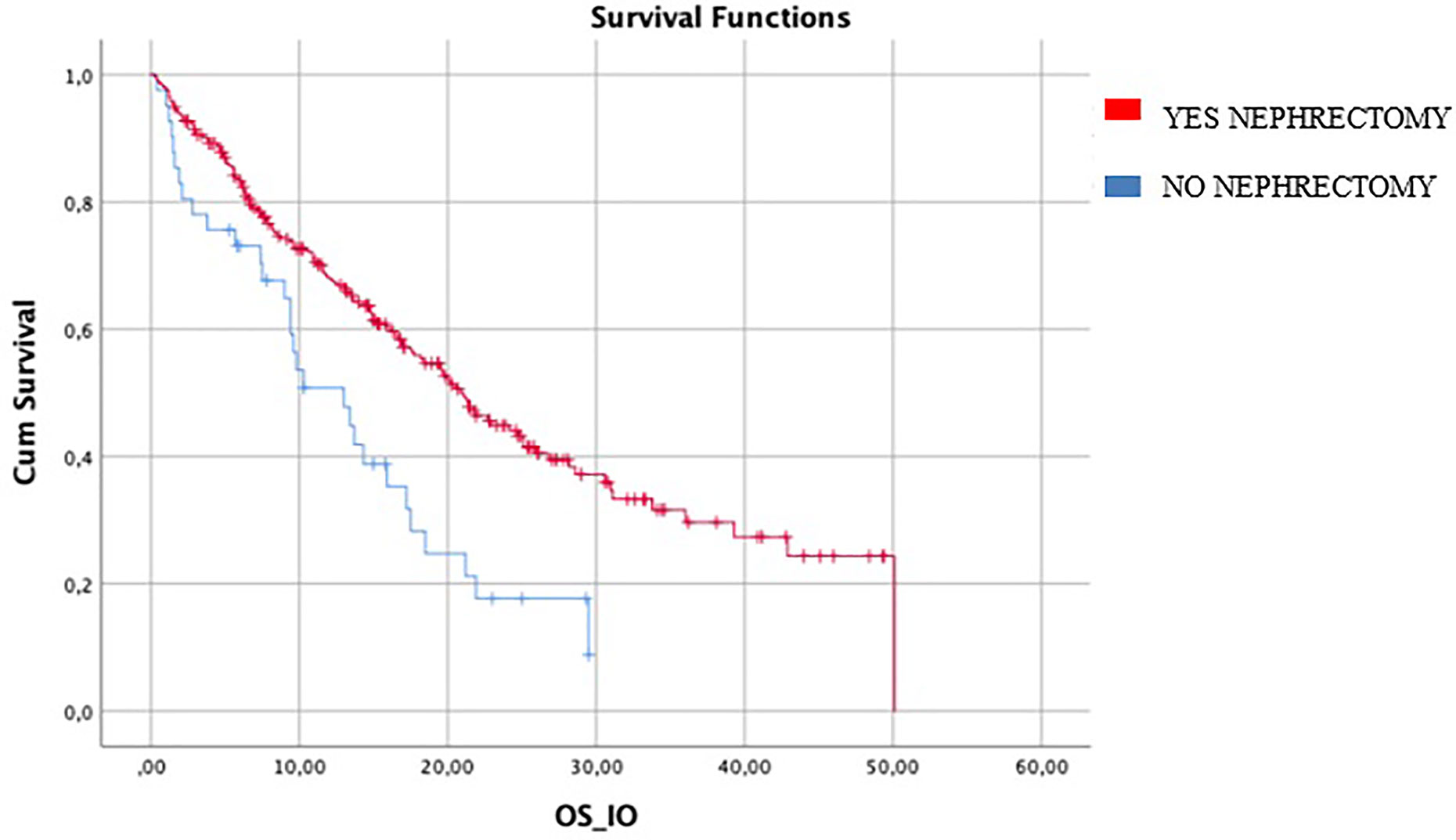
Figure 3 difference in mIO-OS between who underwent nephrectomy was 20.9 (95%CI 17.6–24.1) vs 13.0 (95%CI 7.7–18.2) in patients who did not. IO-OS (median OS in patients treated with IO).
In patients with synchronous metastatic disease (136/287), mOS was 20.5 months for those who underwent CN, compared to 13 months in patients who did not it (HR log rank 0.51; 95%CI 0.305 to 0.855; p = 0.0024). On the other hand, mPFS was 4.6 months in patients who underwent CN vs 3.7 months in patients who did not it (HR log rank 0.83; 95%CI 0.554 to 1.247; p = 0.34) (Table 3).
In the multivariate model, including gland metastasis and IMDC score, nephrectomy showed significant association with OS (HR log rank 0.638; 95%CI 0.416 to 0.980), whereas gland metastases were still associated with better outcome in terms of both OS (HR log rank 0.487; 95%CI 0.279 to 0.852) and PFS (HR log rank 0.646; 95%CI 0.435 to 0.958). However, IMDC score showed significant association with both OS (HR log rank 1.352; 95%CI 1.020 to 1.791) and PFS (HR log rank 1.27; 95%CI 1.016 to 1.587).
Discussion
IMDC and Memorial Sloan-Kettering Cancer Center (MSKCC) score are currently the gold standard for predicting survival in patients with mRCC but metastatic sites influence prognosis too. Indeed, gland metastasis, such as pancreatic metastasis, are related to more favorable prognostic features, long response to TTs and prolonged survival (18). Lung and nodes metastasis, instead, are related to higher complete response rate in patients treated with nivolumab–ipilimumab (19). Bone metastasis has been identified as an independent prognostic variable associated with poor survival in patients with mRCC (20) and brain metastasis seem to influence prognosis when they are more than 4 (21, 22). Even though nephrectomy has not been reported as a prognostic factor or in prognostic scores, patients who underwent nephrectomy are usually patients with metachronous disease or more indolent disease, compared to patients with synchronous disease.
The first evidence regarding the role of nephrectomy in mRCC refers to patients treated with cytokines (IL-2, IFN-a). CN demonstrated improved survival rate in them (8, 23). Later, Heng et al. retrospectively reported data from IMDC. CN was related to OS and PFS benefit in patients treated with TT, compared to patients who did not it. Patients with four or more IMDC prognostic criteria did not benefit from CN, as well as patients with reduced life expectancy (24).
Recently, the phase III CARMENA trial has investigated the role of immediate CN followed by sunitinib versus sunitinib alone and the phase III SURTIME trial has compared immediate CN followed by sunitinib therapy, versus treatment with three cycles of sunitinib followed by CN. Results from both trials have showed that patients with intermediate and poor risk, according to MSKCC and IMDC criteria, should be more appropriately treated with systemic therapy, deferring upfront CN, whereas CN might be considered in good risk patients with low burden disease (9, 10).
Retrospective data from 1,541 patients included in an international, multicenter, prospective database, confirm that deferred CN in mRCC patients treated with upfront sunitinib is associated with improved OS, in appropriately selected patients, whereas upfront CN followed by sunitinib is associated to a lower probability of OS. Authors suggest that initial course of systemic treatment might be a way to identify patients with more aggressive biology, already destined to low survival and who consequently would not benefit from CN (25).
Nowadays, it is difficult to put into practice data about CN, because the influence on outcome of previous nephrectomy in mRCC patients treated with IO is not well defined.
Most of the patients included in the CHECKMATE025 (88%) underwent nephrectomy, so that a subgroup analysis was not performed and data about outcome of patients treated with IO stratified by nephrectomy are not available (11, 12).
Nevertheless, CN after receipt of IO currently remains limited to case reports (26–29), whereas results from CHECKMATE214 showed OS benefit for patients, who had previous nephrectomy (CN or RN), receiving Nivolumab–Ipilimumab versus Sunitinib.
Recently, update results from Javelin Renal 101 favor avelumab plus axitinib over sunitinib across prespecified subgroups, including prior nephrectomy (30). Post hoc analysis of the same trial showed that almost 20% of patients who did not undergo prior nephrectomy and 34.5% of patients, treated with avelumab–axitinib, had 30% or greater shrinkage of primary renal tumor from baseline compared to 9.7% in sunitinib arm (31). These results demonstrate that IO-Tyrosine Kinase Inhibitors (TKIs) combination is active on primary disease, even if in a small number of patients.
The above-mentioned reports seem to suggest an interplay between IO, immune system and primary tumor that need to be researched further.
Our study reported difference in IO-OS between patients who had previous nephrectomy and patients who did not it. Previous nephrectomy seems to extend OS in mRCC patients treated with immunotherapy. Analyzing patients with synchronous metastatic disease, who underwent CN, the benefit in OS was confirmed, compared to patients who did not have CN. This result confirms the findings of Bakouny et al. reported at the ASCO GU 2020. The authors, in a propensity score-based analysis, found that CN was associated with a significant OS benefit in patients treated with IO and TT both. 198 patients treated with IO were included in the analysis (32).
In our multivariate analysis, nephrectomy retained a significant association with OS irrespective of the gland metastases and IMDC score.
Reported PFS’ results do not demonstrate difference between patients who underwent nephrectomy and patients who did not it (4.8 vs 3.7 p 0.186). This result confirms that PFS is not a surrogate for OS in patients treated with IO and confirms the delayed benefit in PFS with nivolumab, as previously reported in CHECKMATE025.
Furthermore, our study demonstrates that gland metastases are related to better prognosis and outcome, as demonstrated in univariate and multivariate analysis. Biological and immunological effects of the primary tumor on IO, which are mostly unknown, might explain the different outcome between patients who underwent nephrectomy and patients who did not it.
Previous report from Wald et al. analyzed how RN could influence immune response, collecting the immune signature in subjects with RCC before and after nephrectomy. Authors reported that the removal of the tumor produced few changes in the cellular immune response at 1 month post-nephrectomy, for example the level of circulating BTLA(B and T lymphocyte attenuator)-expressing CD8+ T cells decreased significantly, suggesting a reversal of T-cell exhaustion and dysfunction (15).
Finally, it is noteworthy to mention the retrospective study by Pignot et al. regarding patients who underwent delayed nephrectomy following IO. Patients who received IO and who experienced complete response on metastatic sites, underwent nephrectomy to achieve complete response. At a median follow up of 15 months, 73% of patients were free from progression, but inflammatory infiltration after long exposure to IO resulted in challenging surgery (33).
Our study covered a well-balanced population and represented all risk categories according to IMDC (Table 4).
Specifically, it included 30% of patients with bone metastasis, already known poor prognostic factor in mRCC, which is consistent with frequency of bone metastasis in RCC.
We analyzed patients who received IO mostly as second or third therapy, to homogenize our population and minimize the selection bias between patients with metachronous disease, or patients with low burden disease referred to CN, and patients with synchronous disease or with higher burden of disease referred to upfront systemic therapy. Indeed, recently, Donskov et al. demonstrated that, in patients treated with first line TKI, synchronous disease was associated with poorer OS and shorter Time to Treatment Failure (TTF) (34).
The purpose of our trial was to suggest that the persistence of primary renal cell tumor could influence the efficacy of Immunotherapy because it could influence the immune system. This is the reason why patients who had CN or RN were not divided into two groups.
In spite of the novel treatments available for renal cell carcinoma, our paper remains relevant because TKI monotherapy can still be considered as a standard of care for many patients. Indeed, IO-TKI failed to show an OS advantage over sunitinib in favorable risk patients according to IMDC and MSKCC score. Moreover, we included patients treated with IO as second line treatment since in Europe IO and IO-TKI has not been available for long time.
Limits of our study include the retrospective collection of data, the small sample size and the lack of central radiological review. As basis for further considerations, there is significant residual confounding in the analysis of nephrectomy versus no nephrectomy, particularly in the metachronous group. Those patients, who are not offered curative intent surgery, are likely more unwell and less fit and thus, perhaps, destined to do poorly regardless of type of systemic therapy received at the time of metastatic diagnosis.
Furthermore, patients who had nephrectomy were more likely to be favorable risk and this might bias results of multivariate analysis.
Our assumption can be made that resection of primary tumor could have an effect on immune system and IO response, even if IO treatment is administered long after surgical intervention. Data are still unclear and further prospective trials would assess this issue.
Conclusion
In our real-world experience in mRCC patients treated with IO, previous nephrectomy, including CN, seems to be associated with a better outcome, in terms of OS, with all the limitations of a retrospective collection. The benefit of previous nephrectomy persisted also in multivariate analysis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Campus Bio-Medico University of Rome. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MSt and DS provided study conception and design. GP made critical revisions. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.682449/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
3. Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal Cell Carcinoma Recurrences and Metastases in Primary Non-Metastatic Patients: A Population-Based Study. World J Urol (2016) 34(8):1081–6. doi: 10.1007/s00345-016-1773-y
4. Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A Prospective, Randomised EORTC Intergroup Phase 3 Study Comparing the Oncologic Outcome of Elective Nephron-Sparing Surgery and Radical Nephrectomy for Low-Stage Renal Cell Carcinoma. Eur Urol (2011) 59(4):543–52. doi: 10.1016/j.eururo.2010.12.013
5. Lee JH, You CH, Min GE, Park JS, Lee SB, Ahn H, et al. Comparison of the Surgical Outcome and Renal Function Between Radical and Nephron-Sparing Surgery for Renal Cell Carcinomas. Korean J Urol (2007) 48(7):671–6. doi: 10.4111/kju.2007.48.7.671
6. Gratzke C, Seitz M, Bayrle F, Schlenker B, Bastian PJ, Haseke N, et al. Quality of Life and Perioperative Outcomes After Retroperitoneoscopic Radical Nephrectomy (RN), Open RN and Nephron-Sparing Surgery in Patients With Renal Cell Carcinoma. BJU Int (2009) 104(4):470–5. doi: 10.1111/j.1464-410X.2009.08439.x
7. Butler BP, Novick AC, Miller DP, Campbell SA, Licht MR. Management of Small Unilateral Renal Cell Carcinomas: Radical Versus Nephron-Sparing Surgery. Urology (1995) 45(1):34–40. doi: 10.1016/S0090-4295(95)96306-5
8. Mickisch GHJ, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical Nephrectomy Plus Interferon-Alfa-Based Immunotherapy Compared With Interferon Alfa Alone in Metastatic Renal-Cell Carcinoma: A Randomised Trial. Lancet (2001) 358(9286):966–70. doi: 10.1016/S0140-6736(01)06103-7
9. Méjean A, Ravaud A, Thezenas S, Colas S, Beauval J-B, Bensalah K, et al. Sunitinib Alone or After Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med (2018) 379(5):417–27. doi: 10.1056/NEJMoa1803675
10. Bex A, Mulders P, Jewett M, Wagstaff J, van Thienen JV, Blank CU, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol (2019) 5(2):164–70. doi: 10.1001/jamaoncol.2018.5543
11. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab Versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
12. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
13. Santini D, Stellato M, De Giorgi U, Pantano F, De Lisi D, Casadei C, et al. Clinical Outcomes of Metastatic Renal Carcinoma Following Disease Progression to Programmed Death (PD)-1 or PD-L1 Inhibitors (Io): A Meet-Uro Group Real World Study (Meet-Uro 7). Am J Clin Oncol (2021) 44(3):121–5. doi: 10.1097/COC.0000000000000791
14. Albiges L, Tannir N, Burotto M, McDermott DF, Plimack ER, Barthélémy P, et al. 711p - Nivolumab + Ipilimumab (N+I) vs Sunitinib (s) for First-Line Treatment of Advanced Renal Cell Carcinoma (aRCC) in CheckMate 214: 4-Year Follow-Up and Subgroup Analysis of Patients (Pts) Without Nephrectomy. Ann Oncol (2020) 31:S550–0. doi: 10.1016/j.annonc.2020.08.783
15. Wald G, Barnes KT, Bing MT, Kresowik TP, Tomanek-Chalkley A, Kucaba TA, et al. Minimal Changes in the Systemic Immune Response After Nephrectomy of Localized Renal Masses. Urol Oncol Semin Orig Investig (2014) 32(5):589–600. doi: 10.1016/j.urolonc.2014.01.023
16. Heng DYC, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External Validation and Comparison With Other Models of the International Metastatic Renal-Cell Carcinoma Database Consortium Prognostic Model: A Population-Based Study. Lancet Oncol (2013) 14(2):141–8. doi: 10.1016/S1470-2045(12)70559-4
17. Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. New Response Evaluation Criteria in Solid Tumours - Revised Recist Guideline (Version 1.1). Japanese J Cancer Chemotherapy (2009) 36:2495–501. doi: 10.1016/j.ejca.2008.10.026
18. Grassi P, Doucet L, Giglione P, Grünwald V, Melichar B, Galli L, et al. Clinical Impact of Pancreatic Metastases From Renal Cell Carcinoma: A Multicenter Retrospective Analysis. PloS One (2016) 11(4):e0151662. doi: 10.1371/journal.pone.0151662
19. Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in First-Line Treatment for Advanced Renal Cell Carcinoma: Extended Follow-Up of Efficacy and Safety Results From a Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/S1470-2045(19)30413-9
20. Kankuri M, Pelliniemi T-T, Pyrhönen S, Nikkanen V, Helenius H, Salminen E. Feasibility of Prolonged Use of Interferon-α in Metastatic Kidney Carcinoma. Cancer (2001) 92(4):761–7. doi: 10.1002/1097-0142(20010815)92:4<761::AID-CNCR1380>3.0.CO;2-#
21. Vickers MM, Al-Harbi H, Choueiri TK, Kollmannsberger C, North S, MacKenzie M, et al. Prognostic Factors of Survival for Patients With Metastatic Renal Cell Carcinoma With Brain Metastases Treated With Targeted Therapy: Results From the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer (2013) 11(3):311–5. doi: 10.1016/j.clgc.2013.04.012
22. Peverelli G, Raimondi A, Ratta R, Verzoni E, Bregni M, Cortesi E, et al. Cabozantinib in Renal Cell Carcinoma With Brain Metastases: Safety and Efficacy in a Real-World Population. Clin Genitourin Cancer (2019) 17(4):291–8. doi: 10.1016/j.clgc.2019.05.002
23. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive Nephrectomy in Patients With Metastatic Renal Cancer: A Combined Analysis. J Urol (2004) 171(3):1071–6. doi: 10.1097/01.ju.0000110610.61545.ae
24. Heng DYC, Wells JC, Rini BI, Beuselinck B, Lee J-L, Knox JJ, et al. Cytoreductive Nephrectomy in Patients With Synchronous Metastases From Renal Cell Carcinoma: Results From the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol (2014) 66(4):704–10. doi: 10.1016/j.eururo.2014.05.034
25. Bhindi B, Graham J, Wells JC, Bakouny Z, Donskov F, Fraccon A, et al. Deferred Cytoreductive Nephrectomy in Patients With Newly Diagnosed Metastatic Renal Cell Carcinoma. Eur Urol (2020) 78(4):615–23. doi: 10.1016/j.eururo.2020.04.038
26. Woldu SL, Brugarolas J, Kapur P, Margulis V. What Is the Role of Nephrectomy Following Complete Response to Checkpoint Inhibitors? Urol Case Rep (2018) 18:60–3. doi: 10.1016/j.eucr.2018.02.016
27. Singla N, Elias R, Ghandour R, Freifeld YN, Bowman AI, Woldu SL, et al. Safety and Feasibility of Nephrectomy After Receipt of Immune Checkpoint Inhibitors for Renal Cell Carcinoma. J Clin Oncol (2019) 37(7_suppl):619. doi: 10.1200/JCO.2019.37.7_suppl.619
28. Ikarashi D, Kato Y, Katagiri H, Takahara T, Uesugi N, Shiomi E, et al. Case of Complete Response to Neoadjuvant Therapy Using Nivolumab in a Patient With Metastatic Renal Cell Carcinoma. Int J Urol (2018) 25(6):630–2. doi: 10.1111/iju.13590
29. Labbate C, Hatogai K, Werntz R, Stadler WM, Steinberg GD, Eggener S, et al. Complete Response of Renal Cell Carcinoma Vena Cava Tumor Thrombus to Neoadjuvant Immunotherapy. J Immunother Cancer (2019) 7(1):1–6. doi: 10.1186/s40425-019-0546-8
30. Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated Efficacy Results From the JAVELIN Renal 101 Trial: First-Line Avelumab Plus Axitinib Versus Sunitinib in Patients With Advanced Renal Cell Carcinoma. Ann Oncol [Internet] (2020) 31(8):1030–9. doi: 10.1016/j.annonc.2020.04.010
31. Albiges L, Rini BI, Haanen JBAG, Motzer RJ, Kollmannsberger CK, Negrier S, et al. Primary Renal Tumour Shrinkage in Patients (Pts) Who did Not Undergo Upfront Cytoreductive Nephrectomy (uCN): Subgroup Analysis From the Phase 3 JAVELIN Renal 101 Trial of First-Line Avelumab + Axitinib (a + Ax) vs Sunitinib (s) for Advanced Renal Cell Ca. Ann Oncol (2019) 30:v356–402. doi: 10.1093/annonc/mdz249.007
32. Bakouny Z, Xie W, Dudani S, Wells C, Gan CL, Donskov F, et al. Cytoreductive Nephrectomy (CN) for Metastatic Renal Cell Carcinoma (mRCC) Treated With Immune Checkpoint Inhibitors (ICI) or Targeted Therapy (TT): A Propensity Score-Based Analysis. J Clin Oncol (2020) 38(6_suppl):608. doi: 10.1200/JCO.2020.38.6_suppl.608
33. Pignot G, Thiery-Vuillemin A, Walz J, Lang H, Bigot P, Werle P, et al. Nephrectomy After Complete Response to Immune Checkpoint Inhibitors for Metastatic Renal Cell Carcinoma: A New Surgical Challenge? Eur Urol (2020) 77(6):761–3. doi: 10.1016/j.eururo.2019.12.018
34. Donskov F, Xie W, Overby A, Wells JC, Fraccon AP, Sacco CS, et al. Synchronous Versus Metachronous Metastatic Disease: Impact of Time to Metastasis on Patient Outcome—Results From the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol Oncol (2020) 3(4):530–9. doi: 10.1016/j.euo.2020.01.001
Keywords: nephrectomy, immune-oncology, metastatic renal cell carcinoma, immunotherapy, nivolumab
Citation: Stellato M, Santini D, Verzoni E, De Giorgi U, Pantano F, Casadei C, Fornarini G, Maruzzo M, Sbrana A, Di Lorenzo G, Soraru M, Naglieri E, Buti S, De Vivo R, Napolitano A, Vignani F, Mucciarini C, Grillone F, Roviello G, Di Napoli M and Procopio G (2021) Impact of Previous Nephrectomy on Clinical Outcome of Metastatic Renal Carcinoma Treated With Immune-Oncology: A Real-World Study on Behalf of Meet-URO Group (MeetUro-7b). Front. Oncol. 11:682449. doi: 10.3389/fonc.2021.682449
Received: 18 March 2021; Accepted: 11 May 2021;
Published: 08 June 2021.
Edited by:
Antonio Augusto Ornellas, National Cancer Institute (INCA), BrazilReviewed by:
Amin Nassar, Brigham and Women’s Hospital and Harvard Medical School, United StatesAhmet Murat Aydin, Moffitt Cancer Center, United States
Copyright © 2021 Stellato, Santini, Verzoni, De Giorgi, Pantano, Casadei, Fornarini, Maruzzo, Sbrana, Di Lorenzo, Soraru, Naglieri, Buti, De Vivo, Napolitano, Vignani, Mucciarini, Grillone, Roviello, Di Napoli and Procopio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Stellato, bS5zdGVsbGF0b0B1bmljYW1wdXMuaXQ=
 Marco Stellato
Marco Stellato Daniele Santini
Daniele Santini Elena Verzoni
Elena Verzoni Ugo De Giorgi
Ugo De Giorgi Francesco Pantano
Francesco Pantano Chiara Casadei
Chiara Casadei Giuseppe Fornarini4
Giuseppe Fornarini4 Giuseppe Di Lorenzo
Giuseppe Di Lorenzo Mariella Soraru
Mariella Soraru Emanuele Naglieri
Emanuele Naglieri Sebastiano Buti
Sebastiano Buti Andrea Napolitano
Andrea Napolitano Francesco Grillone
Francesco Grillone Marilena Di Napoli
Marilena Di Napoli Giuseppe Procopio
Giuseppe Procopio