- 1Unit of Radiation Oncology, Ospedale del Mare, Naples, Italy
- 2Medical Oncology Unit, Grand Metropolitan Hospital “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy
- 3Biostatistical Unit, National Cancer Institute “Regina Elena”, IRCCS, Rome, Italy
- 4Section of Radiation Oncology, Medical School, University of Siena, Siena, Italy
- 5Unit of Intensive Care Medicine and Anesthesia, Grande Ospedale Metropolitano Bianchi Melacrino Morelli, Reggio Calabria, Italy
- 6Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples, Italy
- 7Laboratory of Precision and Molecular Oncology, Institute of Genetic Research, Biogem Scarl, Ariano Irpino, Italy
- 8Sbarro Institute for Cancer Research and Molecular Medicine and Center of Biotechnology, College of Science and Technology, Temple University, Philadelphia, PA, United States
- 9Department of Medical Biotechnology, University of Siena, Siena, Italy
Peripheral-immune-checkpoint blockade (P-ICB) with mAbs to PD-1 (nivolumab and pembrolizumab) or PD-L1 (atezolizumab, durvalumab, avelumab) alone or combination with chemotherapy represents a novel active treatment for mNSCLC patients. However, this therapy can be associated to immune-related adverse events (irAEs) and high cost. Therefore, finding reliable biomarkers of response and irAEs is strongly encouraged to accurately select patients who may potentially benefit from the immuno-oncological treatment. This is a retrospective multi-institutional analysis performed on ninety-five mNSCLC patients who received real-world salvage therapy with nivolumab or atezolizumab between December 2015 and April 2020. The outcome of these patients in term of PFS and OS was evaluated in comparison with different serum levels of C-reactive protein (CRP), Erythrocyte Sedimention Rate (ESR) and Procalcitonin (PCT) by performing Kaplan–Meier and Log-rank test and multivariate analysis. We found that high baseline levels of CRP, ESR, and PCT were strongly predictive of poor outcome (P <0.05) with the worse prognosis detected in those patients with a baseline levels of both ESR and PCT over the pre-established cut off (median OS recorded in patients with no marker over the cut off vs. those with just one marker over the cut off vs. those with both markers over the cut off: 40 ± 59 vs. 15.5 ± 5.5 vs. 5.5 ± 1.6 months, respectively; P <0.0001). Our results suggest the predictive value of systemic inflammation and suggest a potential role of PCT in predicting a poor outcome in mNSCLC receiving PD-1/PD-L1 blocking mAbs. This finding also suggests a potential role of subclinical bacterial infections in defining the response to PD-1/PD-L1 blocking mAbs that deserves further and more specific investigations.
Introduction
Peripheral immune-check point blockade (P-ICB) with mAbs to the programmed cell death receptor-1 (PD-1) (nivolumab and pembrolizumab) and PD-Ligand (PD-L1) (atezolizumab, avelumab and durvalumab) alone or in combination with chemotherapy is a promising treatment option for metastatic non-small cell lung cancer (mNSCLC) (1, 2).
These innovative immune-oncological strategies are often very effective in the management of mNSCLC patients; however, they may be hampered by frequent more or less severe immuno-related adverse events (irAEs) and rising costs. Additionally, their fast-track evaluation in controlled trials has allowed their introduction in the clinical practice leaving a large amount of questions to be addressed (3, 4).
This treatment has substantial differences by other anticancer strategies like chemotherapy, radiotherapy and molecular target therapy that exert a direct cytotoxic/cytostatic effect on the tumor. On the other hand, PD-1-ICB does not act on the tumor cells but they rather rescue the antitumor activity of tumor infiltrating cytotoxic T cells (CTLs) mostly attenuated as consequence of PD-1 binding to PD-L1/2 in the tumor (1, 5, 6).
In this context, micro-environmental conditions related to chronic inflammation and/or relapsing infections might greatly affect the efficacy of these immune-effectors at several levels. These conditions may induce CTL exhaustion or promote the synthesis of pro-inflammatory cytokines like Interleukin (IL-6) and chemokines that, in turn, can have the following effects: i) to hamper the CTL-mediated response; ii) to enhance the production of immunosuppressive cell lineages (Treg, MSDC, M2 macrophages etc.) and iii) to promote the activation of multiple peripheral and central immune-checkpoints including those related to the hypoxic induced factor (HIF) and the adenosine receptor pathway (7–10).
This events might be of critical interest in mNSCLC patients who often present coexisting subclinical inflammatory and/or infectious conditions eventually related to the smoking habit, to chronic pulmonary obstructive disease symptoms and to the presence of relapsing infections within the low airways (11–13).
On these bases, we believe that the study of both inflammatory and infection markers in mNSCLC patients addressed to receive immune-checkpoint blocking mAbs could have a key role in understanding their effective interference in CTL activation and consequently on the outcome of these patients. At the present, the majority of the studies in the literature rely on the prognostic role of unspecific inflammatory markers such as white cell counts, NLR, CRP, ESR, LDH or more sophisticated techniques including tumor immune-profiling or microbiology studies (14–17).
Therefore, we have hypothesized that the presence of inflammatory markers mostly associated to bacterial infections of the low airways such as procalcitonin (PCT), might be easily correlated to the clinical outcome of mNSCLC patients subjected to immuno-oncological treatments. PCT, is a 116 amino acid peptide physiologically synthesized by thyroid parafollicular C cells that contribute to maintain the calcium homeostasis once converted to the calcitonin hormone and released in the blood stream (18–20). In the presence of a bacterial infection provoking a systemic inflammatory response, PCT synthesis may be induced in nearly all the involved tissues leading to a massive release of the peptide in the blood stream. On these bases, it is recommended as a reliable marker of typical bacterial infections, a feature not shared by other common inflammatory markers (18, 20). Systemic PCT production is triggered by bacterial toxins (endotoxin) and by pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin-1-β (IL-1β), and interleukin-6 (IL-6) as an immunological danger signal able to alert the host of a possible bacterial infection (18–24). Serum PCT is undetectable in healthy persons while it is greatly risen in patients bearing clinical or subclinical bacterial infections (20). On the other hand, PCT synthesis is not induced in most viral infections due to their ability to trigger the release of cytokines such as interferon (IFN)-γ that, in turn, inhibits the production of PTC inducers such as TNF-α (25–30).
At the same time, preliminary reports show that PCT in NSCLC could be correlated to survival, with a worse survival in patients with a PCT >0.1 ng/ml (31–33).
On the light of all these considerations, we carried out a retrospective study aimed to investigate whether the blood levels of PCT compared with conventional inflammatory markers such as CRP and ESR may predict the outcome of mNSCLC patients receiving PD-1/PD-L1 immune-checkpoint inhibitor mAbs.
Materials and Methods
Study Design and Patients
This work is part of a retrospective real-world evidence (RWE) multi-institutional database study including 95 chemo-refractory mNSCLC patients consecutively enrolled to receive salvage therapy with anti-PD-1 (nivolumab) or anti-PD-L1 (atezolizumab) mAb at the OU-RC, and ROU-SI between September 2015 and April 2020 with a median follow-up time of 28 months (34–36).
All the patients gave an informed consent for the anonymous use of their clinical data for the research aim. All procedures were undertaken in compliance with the ethical statements of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association and respect of their privacy. All patients received PD-1/PD-L1 blockade in real world setting as recommended by the international guidelines and regulatory agencies following the standard procedures of administration for each drug. All patients according to their specific disease received: nivolumab (intravenous infusion of 3 mg/kg every two weeks) (84 patients) or atezolizumab (intravenous infusion of 1,200 mg every three weeks) (33 patients) until disease progression or occurrence of severe adverse events. All patients were fit for treatment with no heart, kidney, and liver failure, no alterations in the blood cell counts and no clear sign of infection. All of the patients aimed to receive the treatment presented a good performance status ≤1 according to the Eastern Cooperative Oncology Group (ECOG). A complete physical examination report, histological sampling, hematologic, biochemical, immune-biological, radiological, and instrumental monitoring were available at baseline. Clinical history, physical examination, and record of adverse events were reported prior to each treatment cycle. A CT scan was performed at baseline and repeated every 3 months or in any case of suspected progressive disease (PD). CT scans were evaluated according to the immune Response Evaluation Criteria in Solid Tumors (iRECIST 1.1) (37).
All patients were monitored for blood cell counts, biochemistry, CRP, ESR, and PCT before each treatment course and were also monitored for their adrenal hormone profile, ACTH, TSH, thyroid hormones, anti-thyroid auto-antibodies (AAbs), extractable nuclear antigen antibodies (ENA), anti-nucleus antibodies (ANA), anti-smooth cells antibodies (ASMA), and c/p- anti-neutrophil cytoplasmic antibodies (ANCA) each month from the beginning of treatment as reported in previous study (38–40). Only pre-therapy (before the start of immunotherapy) parameters PCR, CRP and ESR were considered for the present analysis.
Statistical Analysis
In order to perform a statistical correlation among continuous parameters and outcomes, we determined different cut-off for survival analysis (Kaplan–Meier analysis), on the overall population.
The PCT threshold chosen (0.1 ng/ml) was determined on the recent analysis from Kajikawa et al. (33), as it included only NSCLC patients and the same threshold was confirmed on multivariate analysis. For the other biomarkers (CRP, ESR), since a consensus in literature is lacking, we chose the median value as a cut-off (respectively CRP 1.6 mg/dl and ESR 40 mm/h).
Time to events was analyzed with the Kaplan–Meier method and statistics was performed by the log-rank test. Median survival and 95% confidence intervals were reported. Median follow-up was estimated with the reverse method. Hazard ratios (HRs) and their 95% confidence intervals were estimated through the Cox regression proportional model.
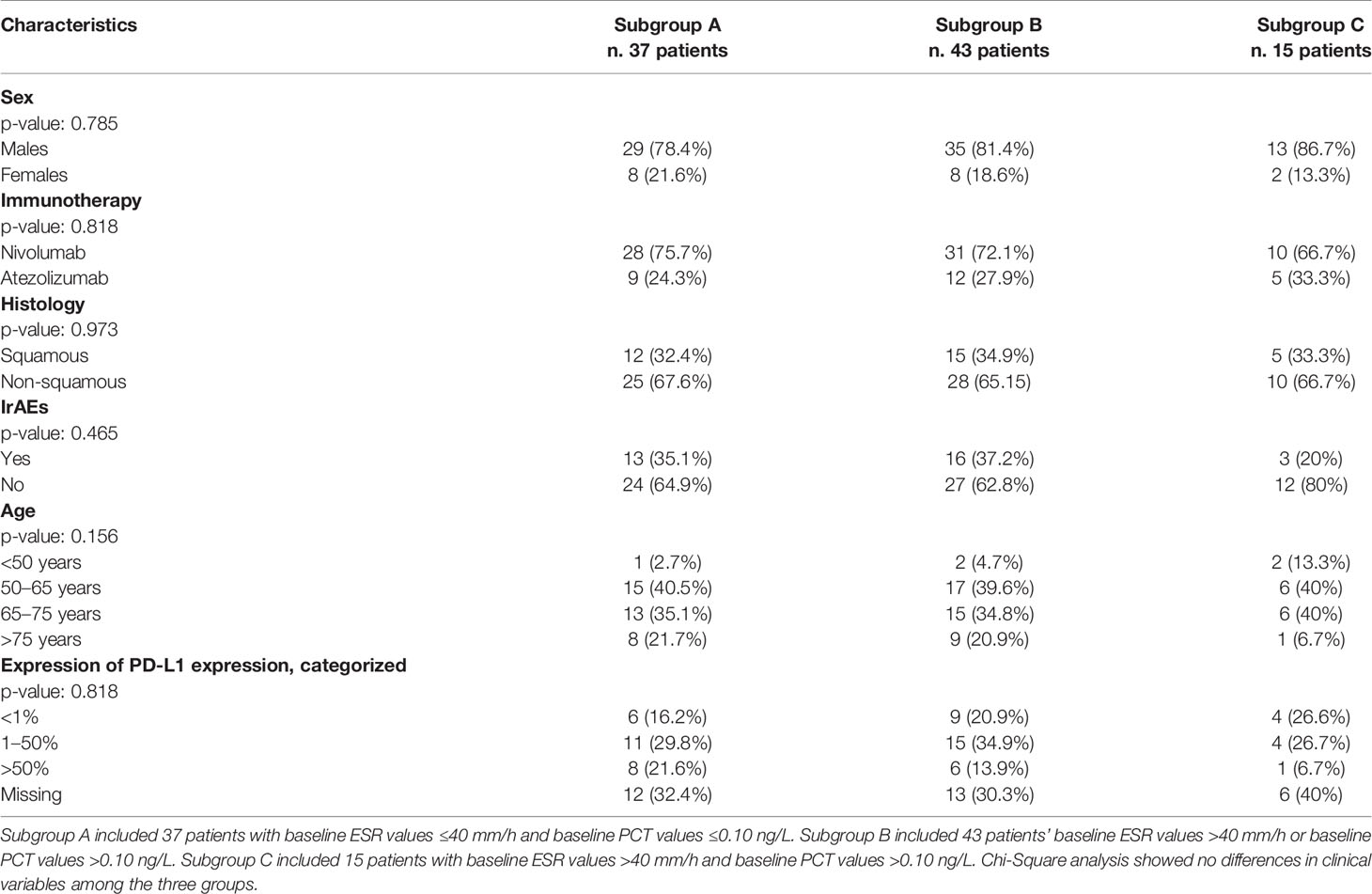
In the multivariate approach, a forward stepwise procedure was used and the enter and remove limit set to 0.05 and 0.10, respectively. Significant parameters at multivariate analysis were used to build a final model of survival, identifying different subgroups of patients. Chi-Square analysis was used to test the differences among the subgroup identified in terms of clinical variables.
Statistics were performed by the SPSS software 23.0 (International Business Machines Corp., New York, NY, USA).
Results
Patients’ Feature and Clinical Outcome
Our retrospective analysis was performed on a cohort of 95 patients with mNSCLC in our database who presented a parallel monitoring of serum CRP, ESR, and PCT values. In our series there were 77 males and 18 females who had been consecutively enrolled to receive salvage therapy with nivolumab (69 cases) or atezolizumab (26 cases) between November 2015 and April 2020 with a median follow of 28 months.
Patients’ demographic and clinical characteristics are summarized in Table 1.
All of the patients were fit for the immunological treatment and presenting an ECOG performance status in a range of 0–1; no clinical or radiological sign of active infection at baseline and no major impairment of heart, liver and kidney functions were recorded.
Mean age was 66 years ± 9.9 years, median age 67 years, range 32–85 years.
On the overall, we recorded a median OS of 17.9 (95%CI; 11.0–24.6) months, with no statistical differences correlated with the treatment (nivolumab vs. atezolizumab, p: 0.378) (Figure 1). In this patients’ series we recorded irAEs in 33.7% of the patients (32/95 patients, grades 1–3) and no other adverse events or major organ failures unrelated to the malignant disease progression.
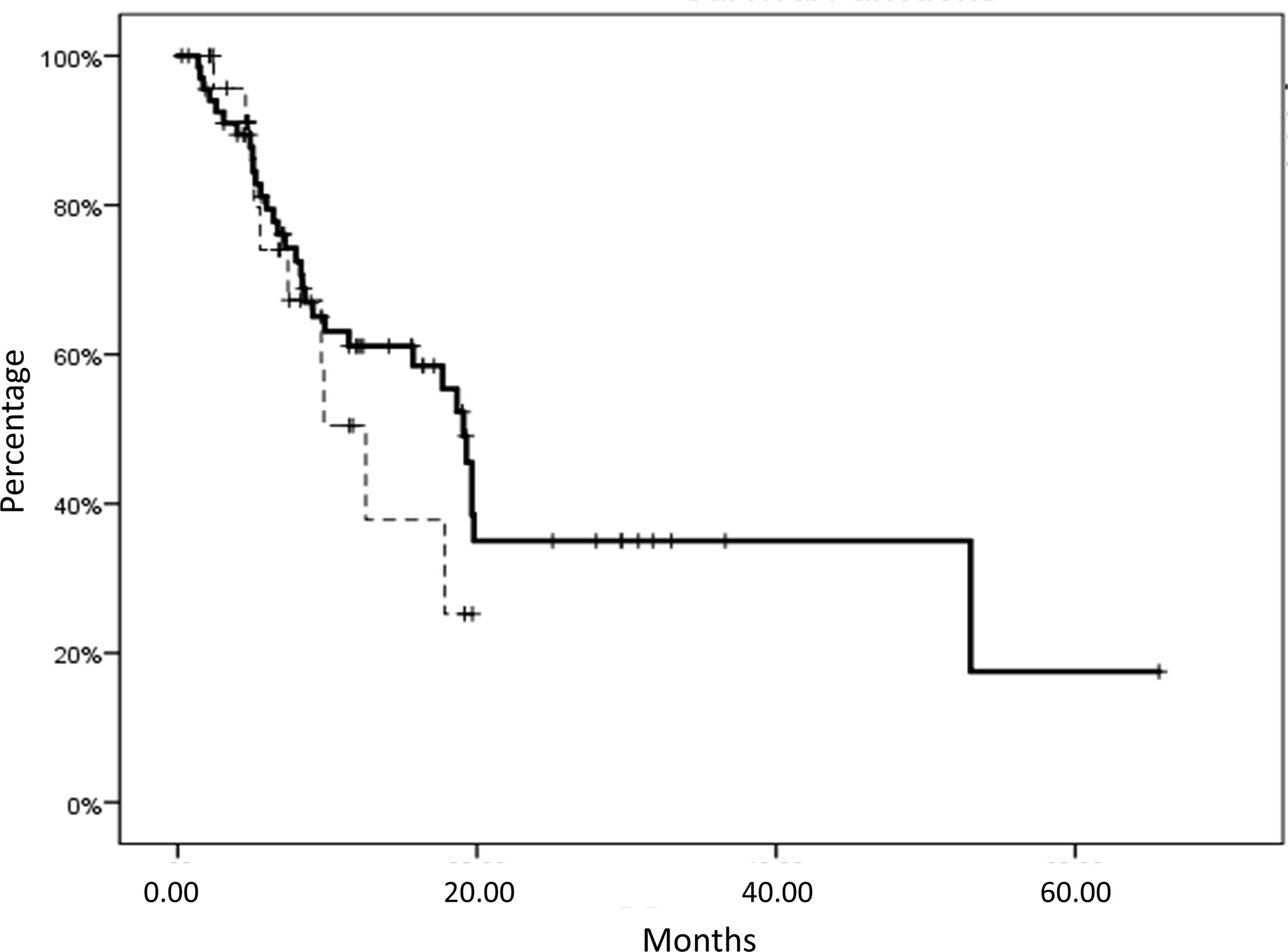
Figure 1 Overall survival (OS) of patients with metastatic non-small cell lung cancer (mNSCLC) subjected to nivolumab (solid line) or atezolizumab treatment (dashed line). No statistical differences in survival were correlated with the treatment (nivolumab vs. atezolizumab, p:0.378). Nivolumab subgroup median OS: 19 ± 0.8 months, mean OS: 27.9 ± 3.9 months, versus Atezolizumab subgroup median OS: 12.5 ± 2.1 months, mean OS: 12.1 ± 1.6 months.
Haematological parameters mean value and standard deviations were as follow: PCT 0.21 ± 0.50 ng/ml, ESR 44.2 ± 31.4 mm/H, CRP 3.3 ± 5 mg/dl. All the evaluated parameters are reported in Supplementary Materials.
Univariate Analysis of Survival
We carried out a statistical analysis aimed to compare the outcome of the patients presenting CRP, ESR and PCT baseline values below or over the respective pre-established cut-off value. The overall survival was obtained for each specific marker (Figures 2A–C). In details, patients presenting baseline values of CRP≤ vs. >16 mg/L showed a median OS of 19.3 ± 0.6 (mean: 30.5 ± 4.7) vs. 9.7 ± 1.4 (mean: 15.9 ± 2.7) months, respectively, with a p-value of 0.033. Additionally, patients presenting baseline values of ESR≤ vs. >40 mm/h showed a median OS of 19.8 ± 1.5 (mean: 36.5 ± 5.4) vs. 9.8 ± 1.9 (mean: 17.7 ± 3.5) months, respectively, with a p-value of 0.01. Finally, patients presenting baseline values of PCT ≤ 0.1 vs. >0.1 mg/L showed a median OS of 19.6 ± 0.4 (mean: 31.0 ± 4.3) vs. 7.3 ± 0.6 (mean: 10.1 ± 1.4) months, respectively, with a p-value of 0.002.
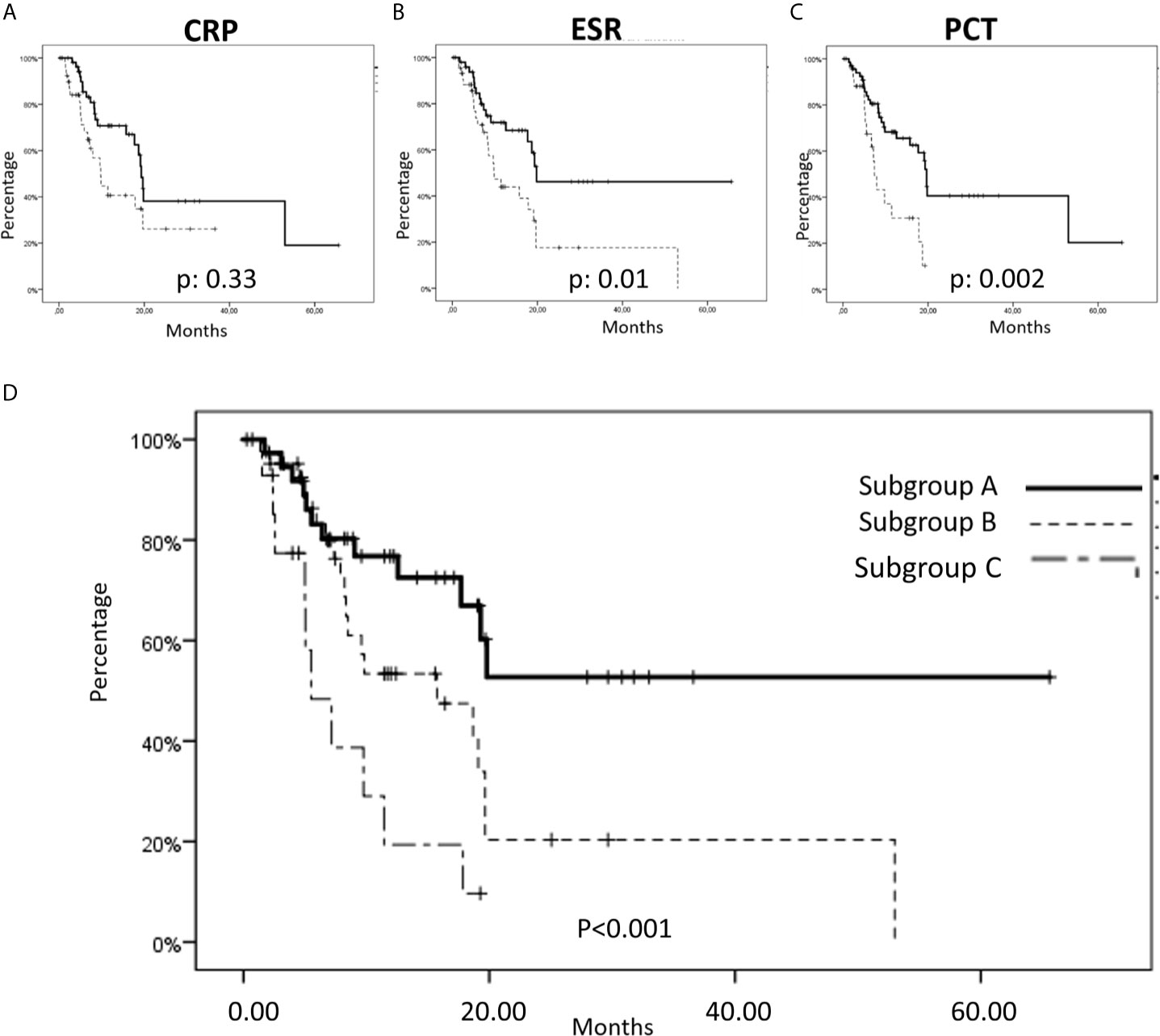
Figure 2 Overall survival (OS) considering inflammation status markers in metastatic non-small cell lung cancer (mNSCLC) patients. (A–C) In our series, we found a significant cut-off of the baseline expression of CRP, ESR and PCT that was strongly correlated to a worse outcome in terms of OS; (A) Specifically, patients with a CRP ≤16 showed a median OS of 19.3 ± 0.6 months, mean: 30.5 ± 4.7 months, versus a median OS of 9.7 ± 1.4 months, mean: 15.9 ± 27 months in patients with a CRP >16, with a p-value of 0.033; (B) Patients with ESR ≤40 showed a median OS of 19.8 ± 1.5 months, mean: 36.5 ± 5.4 months, versus a median OS of 9.8 ± 1.9 months, mean: 17.7 ± 3.5 months in patients with an ESR >40, with a p-value of 0.01; (C) Finally, patients with a PCT ≤0.1 showed a median OS of 19.6 ± 0.4 months, mean: 31.0 ± 4.3 months, versus a median OS of 7.3 ± 0.6 months, mean: 10.1 ± 1.4 months, in patients with a PCT >0.10, with a p-value of 0.002. (D) OS in tree subgroups of patients: Subgroup A (Baseline ESR values ≤40 mm/h and baseline PCT values ≤0.10 mg/L, were detected in 37/95 patients) showed a median OS not reached, mean 40 ± 5.9 months; Subgroup B (baseline ESR values >40 mm/h or baseline PCT values >0.10 mg/L were detected in 43/95 patients) showed a mean OS of 15.5 ± 5.5 months, mean: 20.1 ± 4.0 months; Sugbroup C (baseline ESR values >40 mm/h and baseline PCT values >0.10 mg/L were detected in 15/95 patients) showed a median OS of 5.5 ± 1.6 months, mean OS: 8.3 ± 1.7 months.
Multivariate Analysis of Survival
Cox regression analysis of OS showed that only ESR (HR 2.11; 95% CI: 1.12–3.99; p = 0.02) and PCT (HR 2.64, 95% CI: 1.34–5.18; p = 0.005) were statistically significant. ESR and PCT were used to build a model of OS, dividing the cohort in three subgroups with the relative characteristics summarized in Table 2. Chi-Square analysis showed no significant difference among the three groups in terms of clinical variables (see Table 2). In the subgroup A were included patients (37 cases; 38.9%) with ESR and PCT baseline values equal to or below the respective cut-offs, who showed a prolonged OS (median OS not reached with a mean of 40 ± 5.9 months); in the subgroup B were included patients (43 cases; 45.3%) presenting just one of ESR or PCT baseline values over the respective cut off, who showed a median OS of 15.5 ± 5.5 (mean: 20.1 ± 4.0 months) months; finally, in the subgroup C were included patients (15 cases; 15.8%) with both ESR and PCT baseline values over the cut-off who showing the worst median OS of 5.5 ± 1.6 (mean: 8.3 ± 1.7) months. The differences among the three subgroups were statistically significant with a model p-value <0.001, HR 2.3, 95% CI 1.5–3.5 (Figure 2D).
Discussion
Our retrospective multi-institutional analysis performed in real world setting and aimed to evaluate the effects of either inflammatory and/or infection markers in mNSCLC receiving PD-1/PD-L1 blocking mAbs, showed a direct correlation of CRP, ESR and PCT baseline values with a poor outcome in terms of survival. The PCT threshold chosen was determined on the recent analysis from Kajikawa et al. (33), as it included only NSCLC patients and the same threshold was confirmed on multivariate analysis. Conversely, other analyses included generally patients with lung cancer (both SCLC and NSCLC) and concluded that PCT is elevated in patients with lung cancer with neuroendocrine component or with metastases (31, 32). For the other biomarkers (CRP and ESR), since a consensus in literature is lacking, we chose the median value as a cut-off. The arbitrary choice of the cut-off must be recognized as a limitation of our retrospective study.
These results, however, are in line with our previous results and with what reported by several authors concerning the negative influence of inflammation on the outcome of patients subjected to palliative chemotherapy, radiotherapy and/or immunomodulating treatments for mNSCLC. On the basis of these data, it can be hypothesized that a coexisting chronic inflammation in mNSCLC patients may affect the immune-balance between the immune-system and cancer growth affecting the anti-tumor activity of T cells and promoting the mechanisms of cancer immune-escape (39–41).
At the same time, additional analysis on the correlation between inflammation parameters and outcomes in the specific setting of immunotherapy is needed, in order to investigate whether these parameters are simply prognostic biomarkers (i.e.: they are correlated to the prognosis of lung cancer patients independently from the choice of the systemic therapies) or predictive biomarkers of response to immunotherapy.
It has already been shown that chronic inflammation is commonly associated to the production of cytokines/chemokines that can hamper the efficient CTL response; to the rise of immune-suppressive cell lineages (including Tregs and MDSCs) and to the triggering of the activation of multiple immune-checkpoints and immune-suppressive adenosine receptors (42, 43).
The presence of inflammatory processes in mNSCLC patients may be related to the presence of malignancy itself, to concomitant smoke-associated bronchopulmonary chronic disease and to the presence of relapsing bacterial infections related to incomplete integrity the airways (44–49).
In this light, there are several evidences on the impairment of the existing balance between immune cell-mediated destruction and growth of cancer cells during a long-lasting inflammatory status in cancer patients. This can indeed result in an accelerated progression of the disease and a worse prognosis. In this context, our results highlighting the ability of PCT, an inflammatory marker associated to gram-positive bacterial infection, to predict the outcome of mNSCLC patients receiving immunotherapy, are in line with the results of other authors who recently showed that high baseline levels of PCT are predictive of a poor prognosis in mNSCLC (31, 33, 50).
It is noteworthy to underline that in the setting of mNSCLC an increase of infection biomarkers, such as PCT, CRP and ESR, cannot be considered very specific for active infections, as these biomarkers may be related to the malignancy itself or to concomitant smoke-associated bronchopulmonary chronic disease (32), although PCT can be considered more specific and aid in the differential diagnosis between infectious fever and tumor fever (50, 51).
However, in our series we observed, in a multivariate analysis, that patients presenting at the same time ESR and PCT baseline values over the cut-off showed the worse outcome (mean OS = 8.3 ± 1.7 months) compared with the outcome recorded in patients with just one of the two marker values over the cut-off (mean OS = 20.1 ± 4.0 months) or in patients with both marker values below the cut-off (mean OS = 40 ± 5.9 months). This finding suggests that both inflammation and infection have an additive and independent detrimental effect on the outcome and on the treatment response to the immuno-oncological treatment with anti PD-1 and PD-L1 blocking mAbs.
PCT is considered a serum biomarker able to distinguish bacterial infection from other causes of infection or inflammation. This could be of particular interest in patients with infections of the lower respiratory tract where PCT may help in resolving diagnostic uncertainty and guiding to antibiotic therapy; in details, antibiotics’ discontinuation in patients with pneumonia, is commonly recommended on defined PCT thresholds (below 0.25 or 0.5 ng/ml or decrease by ≥80% from peak if initial value is >5 ng/ml) in combination with clinical judgment (19, 52, 53). It is conceivable that this recommendation might be still valid in patients bearing a malignant disease and need to be investigated in appropriate trials.
It should be stated that not all bacterial infections cause similar PCT increase. The most common infections by typical bacteria such as Streptococcus pneumonia and Haemophilus influentia, are commonly associate to higher PCT levels (median blood values of 2.5 ng/ml) compared with atypical bacteria (like Mycoplasma, Legionella, Chlamydia, Mycobacterium tubercolosis, etc.), or other eukaryotic parasites (alike Candida and Pneumocystis species), (median blood values of 0.20 ng/ml) and viruses (median blood values of 0.09 ng/ml) (19, 52). On the other hand, systemic inflammation not associated with pathogens, including cancer (with the exception of neuroendocrine malignancies), shock, injuries and chronic kidney disease and more severe autoimmune diseases have a limited effect on PCT levels.
However, unspecific PCT rises have been sporadically reported upon treatment with immunomodulatory treatments such as T-cell antibodies, alemtuzumab, IL-2, and granulocyte transfusions even though in our setting we were unable to show any significant increase in CRP, ESR and PCT related to the use of mAbs to PD-1 or PD-L1 (54–56).
Serum PCT blood levels commonly rise within few hours after the microbiological insult and roughly correlate with the severity of infection declining at a predictable fast rate with complete resolution. However, it should be taken in consideration that PCT production continues maintaining a plateau level when the infection/inflammatory stimulus is not completely solved (57–59). On these bases of the results derived from multiple observational studies, it has been hypothesized that PCT levels over the thresholds, reflect the existence of an active bacterial infection even though other clinical signs and symptoms are missing. Additionally, there is also evidence that the speed of rising in PCT serum levels correlates with the severity of the infection, a fact which allow the physicians to rely on this marker to monitor the systemic evolution of the ongoing infectious disease and the efficacy of the antibiotic therapy in elderly and cancer patients where the morbidity from bacterial infections is unpredictable and often lethal (60–64).
As an additional consideration, it is important to take in consideration the in mNSCLC patients receiving immuno-oncological treatments the role of the specific broncho-pulmonary microbiota that in analogy with what reported for the intestinal resident bacteria, may affect the immunological response of the host to the tumor and consequently the efficacy of immune-checkpoint inhibitors. In this context, it has been recently shown that the unspecific use of antibiotics prior to PD-1 blockade is correlated with a poor response to the treatment and it is commonly not advised, although some concerns of bias related to the selection of patients (65–71). In this regard, the use of infectious biomarkers such as PCT could help to properly select patients with active infections requiring antibiotics and to spare the unspecific use of these drugs avoiding their detrimental effects on specific microbiota.
Also, we did not find any correlation between the inflammation biomarkers and the development of irAEs. At this regard, we can speculate that basal parameters cannot predict the immune-related side effects, unfortunately, as immunotherapy has not started yet. We believe that the dynamic evaluation of these biomarkers during the course of immunotherapy (i.e.: in different time points, before each cycle of immunotherapy) could help in this prediction. A proper investigation of this phenomenon is needed in the next future with a dedicated prospective trial.
Our work recognizes the limitation of a monocentric retrospective study that deserves validation in external datasets and/or prospective validation. Presently, external validation was not possible, as PCT is not included in the routinary clinical management of mNSCLC.
In the present study we describe the detrimental effect of systemic inflammation and infection in mNSCLC receiving PD-1/PD-L1 blockade immunotherapy and suggest a perspective investigation of CRP, ESR and PCT as potential biomarker of response to the immuno-oncological treatment. The results of this study also open a new research scenario potentially played by bacterial influence and eventually on the preventive use of antibiotics in patients with high baseline levels of ESR and PCT, aimed to receive immune-checkpoint blockade.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VN, DG, MC, and PC conceived and designed the study and wrote the manuscript. RG, GB, PT, PP, AF, SM, and PC acquired the clinical data. DG performed statistical analysis. VN, LM, LP, AG and PC followed the patients, including planning clinical visits, blood sample collection and follow-up. AL, MC, PC and SZ revised the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.684110/full#supplementary-material
References
1. Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res (2019) 25(15):4592–602. doi: 10.1158/1078-0432.CCR-18-1538
2. Dafni U, Tsourti Z, Vervita K, Peters S. Immune Checkpoint Inhibitors, Alone or in Combination With Chemotherapy, as First-Line Treatment for Advanced Non-Small Cell Lung Cancer. A Systematic Review and Network Meta-Analysis. Lung Cancer (2019) 134:127–40. doi: 10.1016/j.lungcan.2019.05.029
3. Huang Z, Su W, Lu T, Wang Y, Dong Y, Qin Y, et al. First-Line Immune-Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Current Landscape and Future Progress. Front Pharmacol (2020) 11:578091. doi: 10.3389/fphar.2020.578091
4. Camidge DR, Doebele RC, Kerr KM. Comparing and Contrasting Predictive Biomarkers for Immunotherapy and Targeted Therapy of NSCLC. Nat Rev Clin Oncol (2019) 16(6):341–55. doi: 10.1038/s41571-019-0173-9
5. Philips GK, Atkins M. Therapeutic Uses of Anti-PD-1 and Anti-PD-L1 Antibodies. Int Immunol (2015) 27(1):39–46. doi: 10.1093/intimm/dxu095
6. Urwyler P, Earnshaw I, Bermudez M, Perucha E, Wu W, Ryan S, et al. Mechanisms of Checkpoint Inhibition-Induced Adverse Events. Clin Exp Immunol (2020) 200(2):141–54. doi: 10.1111/cei.13421
7. Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, et al. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu Rev Med (2018) 69:301–18. doi: 10.1146/annurev-med-012017-043208
8. Saigi M, Alburquerque-Bejar JJ, Sanchez-Cespedes M. Determinants of Immunological Evasion and Immunocheckpoint Inhibition Response in Non-Small Cell Lung Cancer: The Genetic Front. Oncogene (2019) 38(31):5921–32. doi: 10.1038/s41388-019-0855-x
9. Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J (2018) 24(1):47–53. doi: 10.1097/PPO.0000000000000303
10. Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, et al. Macrophage Polarity in Cancer: A Review. J Cell Biochem (2019) 120(3):2756–65. doi: 10.1002/jcb.27646
11. Seda G, Stafford CC, Parrish JS, Praske SP, Daheshia M. Chronic Obstructive Pulmonary Disease and Vascular Disease Delay Timeliness of Early Stage Lung Cancer Resectional Surgery. COPD (2013) 10(2):133–7. doi: 10.3109/15412555.2012.728260
12. McMullen S, Hess LM, Kim ES, Levy B, Mohamed M, Waterhouse D, et al. Treatment Decisions for Advanced Non-Squamous Non-Small Cell Lung Cancer: Patient and Physician Perspectives on Maintenance Therapy. Patient (2019) 12(2):223–33. doi: 10.1007/s40271-018-0327-3
13. Brown T, Pilkington G, Bagust A, Boland A, Oyee J, Tudur-Smith C, et al. Clinical Effectiveness and Cost-Effectiveness of First-Line Chemotherapy for Adult Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer: A Systematic Review and Economic Evaluation. Health Technol Assess (2013) 17(31):1–278. doi: 10.3310/hta17310
14. Wojas-Krawczyk K, Kalinka E, Grenda A, Krawczyk P, Milanowski J. Beyond PD-L1 Markers for Lung Cancer Immunotherapy. Int J Mol Sci (2019) 20(8):1915. doi: 10.3390/ijms20081915
15. Ni X-F, Wu J, Ji M, Shao Y-J, Xu B, Jiang J-T, et al. Effect of C-reactive Protein/Albumin Ratio on Prognosis in Advanced Non-Small-Cell Lung Cancer. Asia Pac J Clin Oncol (2018) 14(6):402–9. doi: 10.1111/ajco.13055
16. Thompson JC, Hwang W-T, Davis C, Deshpande C, Jeffries S, Rajpurohit Y, et al. Gene Signatures of Tumor Inflammation and Epithelial-to-Mesenchymal Transition (EMT) Predict Responses to Immune Checkpoint Blockade in Lung Cancer With High Accuracy. Lung Cancer (2020) 139:1–8. doi: 10.1016/j.lungcan.2019.10.012
17. Akamine T, Takada K, Toyokawa G, Kinoshita F, Matsubara T, Kozuma Y, et al. Association of Preoperative Serum CRP With PD-L1 Expression in 508 Patients With Non-Small Cell Lung Cancer: A Comprehensive Analysis of Systemic Inflammatory Markers. Surg Oncol (2018) 27(1):88–94. doi: 10.1016/j.suronc.2018.01.002
18. Becker KL, Nylén ES, White JC, Müller B, Snider RHJ. Clinical Review 167: Procalcitonin and the Calcitonin Gene Family of Peptides in Inflammation, Infection, and Sepsis: A Journey From Calcitonin Back to Its Precursors. J Clin Endocrinol Metab (2004) 89(4):1512–25. doi: 10.1210/jc.2002-021444
19. Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of Procalcitonin-Guided Treatment on Antibiotic Use and Outcome in Lower Respiratory Tract Infections: Cluster-Randomised, Single-Blinded Intervention Trial. Lancet (Lond Engl) (2004) 363(9409):600–7. doi: 10.1016/S0140-6736(04)15591-8
20. Maruna P, Nedelníková K, Gürlich R. Physiology and Genetics of Procalcitonin. Physiol Res (2000) 49(Suppl 1):S57–61.
21. BalcI C, Sungurtekin H, Gürses E, Sungurtekin U, Kaptanoglu B. Usefulness of Procalcitonin for Diagnosis of Sepsis in the Intensive Care Unit. Crit Care (2003) 7(1):85–90. doi: 10.1186/cc1843
22. Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin Increase After Endotoxin Injection in Normal Subjects. J Clin Endocrinol Metab (1994) 79(6):1605–8. doi: 10.1210/jcem.79.6.7989463
23. Reinhart K, Karzai W, Meisner M. Procalcitonin as a Marker of the Systemic Inflammatory Response to Infection. Intensive Care Med (2000) 26(9):1193–200. doi: 10.1007/s001340000624
24. Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guérin S, et al. Comparison of Procalcitonin With C-Reactive Protein, Interleukin 6 and Interferon-Alpha for Differentiation of Bacterial vs. Viral Infections. Pediatr Infect Dis J (1999) 18(10):875–81. doi: 10.1097/00006454-199910000-00008
25. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High Serum Procalcitonin Concentrations in Patients With Sepsis and Infection. Lancet (Lond Engl) (1993) 341(8844):515–8. doi: 10.1016/0140-6736(93)90277-N
26. Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic Value of Procalcitonin, Interleukin-6, and Interleukin-8 in Critically Ill Patients Admitted With Suspected Sepsis. Am J Respir Crit Care Med (2001) 164(3):396–402. doi: 10.1164/ajrccm.164.3.2009052
27. Hamade B, Huang DT. Procalcitonin: Where Are We Now? Crit Care Clin (2020) 36(1):23–40. doi: 10.1016/j.ccc.2019.08.003
28. Choi JJ, McCarthy MW. Novel Applications for Serum Procalcitonin Testing in Clinical Practice. Expert Rev Mol Diagn (2018) 18(1):27–34. doi: 10.1080/14737159.2018.1407244
29. Schuetz P, Bolliger R, Merker M, Christ-Crain M, Stolz D, Tamm M, et al. Procalcitonin-Guided Antibiotic Therapy Algorithms for Different Types of Acute Respiratory Infections Based on Previous Trials. Expert Rev Anti Infect Ther (2018) 16(7):555–64. doi: 10.1080/14787210.2018.1496331
30. Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, et al. Effect of Procalcitonin-Guided Antibiotic Treatment on Clinical Outcomes in Intensive Care Unit Patients With Infection and Sepsis Patients: A Patient-Level Meta-Analysis of Randomized Trials. Crit Care (2018) 22(1):191. doi: 10.1186/s13054-018-2125-7
31. Avrillon V, Locatelli-Sanchez M, Folliet L, Carbonnaux M, Perino E, Fossard G, et al. Lung Cancer may Increase Serum Procalcitonin Level. Infect Disord Drug Targets (2015) 15(1):57–63. doi: 10.2174/1871526515666150320162950
32. Patout M, Salaün M, Brunel V, Bota S, Cauliez B, Thiberville L. Diagnostic and Prognostic Value of Serum Procalcitonin Concentrations in Primary Lung Cancers. Clin Biochem (2014) 47(18):263–7. doi: 10.1016/j.clinbiochem.2014.09.002
33. Kajikawa S, Ohashi W, Kato Y, Fukami M, Yonezawa T, Sato M, et al. Prognostic Impact of Serum Procalcitonin in Non-Small Cell Lung Cancer. Tumori (2020) 20:300891620966647. doi: 10.1177/0300891620966647
34. Giannicola R, D’arrigo G, Botta C, Agostino R, Del Medico P, Falzea AC, et al. Early Blood Rise in Auto-Antibodies to Nuclear and Smooth Muscle Antigens Is Predictive of Prolonged Survival and Autoimmunity in Metastatic-Non-Small Cell Lung Cancer Patients Treated With PD-1 Immune-Check Point Blockade by Nivolumab. Mol Clin Oncol (2019) 11(1):81–90. doi: 10.3892/mco.2019.1859
35. Correale P, Saladino RE, Giannarelli D, Giannicola R, Agostino R, Staropoli N, et al. Distinctive Germline Expression of Class I Human Leukocyte Antigen (HLA) Alleles and DRB1 Heterozygosis Predict the Outcome of Patients With Non-Small Cell Lung Cancer Receiving PD-1/PD-L1 Immune Checkpoint Blockade. J Immunother Cancer (2020) 8:e000733. doi: 10.1136/jitc-2020-000733
36. Correale P, Saladino RE, Giannarelli D, Sergi A, Mazzei MA, Bianco G, et al. HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis. Cells (2020) 9(9):1964. doi: 10.3390/cells9091964
37. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol (2017) 18(3):e143–52. doi: 10.1016/S1470-2045(17)30074-8
38. Cusi MG, Botta C, Pastina P, Rossetti MG, Dreassi E, Guidelli GM, et al. Phase I Trial of Thymidylate Synthase Poly-Epitope Peptide (TSPP) Vaccine in Advanced Cancer Patients. Cancer Immunol Immunother (2015) 64(9):1159–73. doi: 10.1007/s00262-015-1711-7
39. Garner H, de Visser KE. Immune Crosstalk in Cancer Progression and Metastatic Spread: A Complex Conversation. Nat Rev Immunol (2020) 20(8):483–97. doi: 10.1038/s41577-019-0271-z
40. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the Tumor Microenvironment in PD-L1/PD-1-Mediated Tumor Immune Escape. Mol Cancer (2019) 18(1):10. doi: 10.1186/s12943-018-0928-4
41. Nardone V, Pastina P, Giannicola R, Agostino R, Croci S, Tini P, et al. How to Increase the Efficacy of Immunotherapy in NSCLC and HNSCC: Role of Radiation Therapy, Chemotherapy, and Other Strategies. Front Immunol (2018) 9:2941. doi: 10.3389/fimmu.2018.02941
42. Berraondo P, Minute L, Ajona D, Corrales L, Melero I, Pio R. Innate Immune Mediators in Cancer: Between Defense and Resistance. Immunol Rev (2016) 274(1):290–306. doi: 10.1111/imr.12464
43. Chen DS, Mellman I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
44. Nagy A, Müller V, Kolonics-Farkas AM, Eszes N, Vincze K, Horvath G. Worse Lung Cancer Outcome in Patients With Lower Respiratory Tract Infection Confirmed at Time of Diagnosis. Thorac Cancer (2019) 10(9):1819–26. doi: 10.1111/1759-7714.13153
45. Jin J, Li Y, Zhang X, Chen T, Wang Y, Wang Z. Evaluation of Both Free Radical Scavenging Capacity and Antioxidative Damage Effect of Polydatin. Adv Exp Med Biol (2016) 923:57–62. doi: 10.1007/978-3-319-38810-6_8
46. Liu H, Liu B, Zheng F, Chen X, Ye L, He Y. Distribution of Pathogenic Bacteria in Lower Respiratory Tract Infection in Lung Cancer Patients After Chemotherapy and Analysis of Integron Resistance Genes in Respiratory Tract Isolates of Uninfected Patients. J Thorac Dis (2020) 12(8):4216–23. doi: 10.21037/jtd-20-928
47. Nakhaee M, Rezaee A, Basiri R, Soleimanpour S, Ghazvini K. Relation Between Lower Respiratory Tract Microbiota and Type of Immune Response Against Tuberculosis. Microb Pathog (2018) 120:161–5. doi: 10.1016/j.micpath.2018.04.054
48. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, Incidence, and Risk Factors of Immune Checkpoint Inhibitor-Related Pneumonitis in Patients With non-Small Cell Lung Cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/j.lungcan.2018.09.015
49. Tini P, Nardone V, Pastina P, Pirtoli L, Correale P, Giordano A. The Effects of Radiotherapy on the Survival of Patients With Unresectable Non-Small Cell Lung Cancer. Expert Rev Anticancer Ther (2018) 18(6). doi: 10.1080/14737140.2018.1458615
50. Zhao Z, Li X, Zhao Y, Wang D, Li Y, Liu L, et al. Role of C-Reactive Protein and Procalcitonin in Discriminating Between Infectious Fever and Tumor Fever in Non-Neutropenic Lung Cancer Patients. Med (Baltimore) (2018) 97(33):e11930. doi: 10.1097/MD.0000000000011930
51. Tulek B, Koylu H, Kanat F, Arslan U, Ozer F. Serum C-Reactive Protein and Procalcitonin Levels in Non-Small Cell Lung Cancer Patients. Contemp Oncol (Poznan Poland) (2013) 17(1):68–72. doi: 10.5114/wo.2013.33777
52. Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of Procalcitonin-Based Guidelines vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections: The ProHOSP Randomized Controlled Trial. JAMA (2009) 302(10):1059–66. doi: 10.1001/jama.2009.1297
53. de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and Safety of Procalcitonin Guidance in Reducing the Duration of Antibiotic Treatment in Critically Ill Patients: A Randomised, Controlled, Open-Label Trial. Lancet Infect Dis (2016) 16(7):819–27. doi: 10.1016/S1473-3099(16)00053-0
54. Dornbusch HJ, Strenger V, Sovinz P, Lackner H, Schwinger W, Kerbl R, et al. Non-Infectious Causes of Elevated Procalcitonin and C-Reactive Protein Serum Levels in Pediatric Patients With Hematologic and Oncologic Disorders. Support Care Cancer (2008) 16(9):1035–40. doi: 10.1007/s00520-007-0381-1
55. Sabat R, Höflich C, Döcke WD, Oppert M, Kern F, Windrich B, et al. Massive Elevation of Procalcitonin Plasma Levels in the Absence of Infection in Kidney Transplant Patients Treated With pan-T-Cell Antibodies. Intensive Care Med (2001) 27(6):987–91. doi: 10.1007/s001340100949
56. Dornbusch HJ, Strenger V, Kerbl R, Lackner H, Schwinger W, Sovinz P, et al. Procalcitonin and C-Reactive Protein Do Not Discriminate Between Febrile Reaction to Anti-T-Lymphocyte Antibodies and Gram-Negative Sepsis. Bone Marrow Transplant (2003) 32(9):941–5. doi: 10.1038/sj.bmt.1704265
57. Meisner M. Update on Procalcitonin Measurements. Ann Lab Med (2014) 34(4):263–73. doi: 10.3343/alm.2014.34.4.263
58. Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E. Procalcitonin Decrease Over 72 Hours in US Critical Care Units Predicts Fatal Outcome in Sepsis Patients. Crit Care (2013) 17(3):R115. doi: 10.1186/cc12787
59. Vijayan AL, Vanimaya, Ravindran S, Saikant R, Lakshmi S, Kartik R, et al. Procalcitonin: A Promising Diagnostic Marker for Sepsis and Antibiotic Therapy. J Intensive Care (2017) 5:51. doi: 10.1186/s40560-017-0246-8
60. Bele N, Darmon M, Coquet I, Feugeas J-P, Legriel S, Adaoui N, et al. Diagnostic Accuracy of Procalcitonin in Critically Ill Immunocompromised Patients. BMC Infect Dis (2011) 11:224. doi: 10.1186/1471-2334-11-224
61. Schüttrumpf S, Binder L, Hagemann T, Berkovic D, Trümper L, Binder C. Procalcitonin: A Useful Discriminator Between Febrile Conditions of Different Origin in Hemato-Oncological Patients? Ann Hematol (2003) 82(2):98–103. doi: 10.1007/s00277-002-0584-y
62. Munsiff SS, Kambili C, Ahuja SD. Rifapentine for the Treatment of Pulmonary Tuberculosis. Clin Infect Dis (2006) 43(11):1468–75. doi: 10.1086/508278
63. Shomali W, Hachem R, Chaftari A-M, Jiang Y, Bahu R, Jabbour J, et al. Can Procalcitonin Distinguish Infectious Fever From Tumor-Related Fever in Non-Neutropenic Cancer Patients? Cancer (2012) 118(23):5823–9. doi: 10.1002/cncr.27602
64. El Haddad H, Chaftari A-M, Hachem R, Chaftari P, Raad II. Biomarkers of Sepsis and Bloodstream Infections: The Role of Procalcitonin and Proadrenomedullin With Emphasis in Patients With Cancer. Clin Infect Dis (2018) 67(6):971–7. doi: 10.1093/cid/ciy331
65. Banna GL, Passiglia F, Colonese F, Canova S, Menis J, Addeo A, et al. Immune-Checkpoint Inhibitors in Non-Small Cell Lung Cancer: A Tool to Improve Patients’ Selection. Crit Rev Oncol Hematol (2018) 129:27–39. doi: 10.1016/j.critrevonc.2018.06.016
66. Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients With Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann Oncol (2018) 29(6):1437–44. doi: 10.1093/annonc/mdy103
67. Pinato DJ, Gramenitskaya D, Altmann DM, Boyton RJ, Mullish BH, Marchesi JR, et al. Antibiotic Therapy and Outcome From Immune-Checkpoint Inhibitors. J Immunother Cancer (2019) 7(1):287. doi: 10.1186/s40425-019-0775-x
68. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy Against Epithelial Tumors. Science (2018) 359(6371):91–7. doi: 10.1126/science.aan3706
69. Nagasaka M, Sexton R, Alhasan R, Rahman S, Azmi AS, Sukari A. Gut Microbiome and Response to Checkpoint Inhibitors in Non-Small Cell Lung Cancer-A Review. Crit Rev Oncol Hematol (2020) 145:102841. doi: 10.1016/j.critrevonc.2019.102841
70. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol (2019) 5(12):1774–8. doi: 10.1001/jamaoncol.2019.2785
Keywords: bacterial infections, immune-check point blockade, programmed cell death ligand-1, real-world salvage therapy, cell death receptor, prognostic factors of response, procalcitonin, inflammatory markers
Citation: Nardone V, Giannicola R, Bianco G, Giannarelli D, Tini P, Pastina P, Falzea AC, Macheda S, Caraglia M, Luce A, Zappavigna S, Mutti L, Pirtoli L, Giordano A and Correale P (2021) Inflammatory Markers and Procalcitonin Predict the Outcome of Metastatic Non-Small-Cell-Lung-Cancer Patients Receiving PD-1/PD-L1 Immune-Checkpoint Blockade. Front. Oncol. 11:684110. doi: 10.3389/fonc.2021.684110
Received: 22 March 2021; Accepted: 24 May 2021;
Published: 14 June 2021.
Edited by:
Roberta Zappasodi, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Yina Hsing Huang, Dartmouth College, United StatesChengzhi Zhou, First Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2021 Nardone, Giannicola, Bianco, Giannarelli, Tini, Pastina, Falzea, Macheda, Caraglia, Luce, Zappavigna, Mutti, Pirtoli, Giordano and Correale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Caraglia, bWljaGVsZS5jYXJhZ2xpYUB1bmljYW1wYW5pYS5pdA==
 Valerio Nardone
Valerio Nardone Rocco Giannicola2
Rocco Giannicola2 Sebastiano Macheda
Sebastiano Macheda Michele Caraglia
Michele Caraglia Amalia Luce
Amalia Luce Silvia Zappavigna
Silvia Zappavigna Luciano Mutti
Luciano Mutti Antonio Giordano
Antonio Giordano Pierpaolo Correale
Pierpaolo Correale