- 1Clinical Oncology Center, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
- 2Department of Clinical Oncology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong
- 3Department of Radiation Oncology, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou, China
- 4Department of Radiation Oncology, University of Toronto, Princess Margaret Cancer Centre, Toronto, ON, Canada
This study aims to identify prognostic factors in nasopharyngeal carcinoma (NPC) to improve the current 8th edition TNM classification. A systematic review of the literature reported between 2013 and 2019 in PubMed, Embase, and Scopus was conducted. Studies were included if (1) original clinical studies, (2) ≥50 NPC patients, and (3) analyses on the association between prognostic factors and overall survival. The data elements of eligible studies were abstracted and analyzed. A level of evidence was synthesized for each suggested change to the TNM staging and prognostic factors. Of 5,595 studies screened, 108 studies (44 studies on anatomical criteria and 64 on non-anatomical factors) were selected. Proposed changes/factors with strong evidence included the upstaging paranasal sinus to T4, defining parotid lymph node as N3, upstaging N-category based on presence of lymph node necrosis, as well as the incorporation of non-TNM factors including EBV-DNA level, primary gross tumor volume (GTV), nodal GTV, neutrophil-lymphocyte ratio, lactate dehydrogenase, C-reactive protein/albumin ratio, platelet count, SUVmax of the primary tumor, and total lesion glycolysis. This systematic review provides a useful summary of suggestions and prognostic factors that potentially improve the current staging system. Further validation studies are warranted to confirm their significance.
Introduction
Nasopharyngeal carcinoma (NPC) is an important global health burden with approximately 130,000 new cases diagnosed and more than 70,000 deaths in 2018 (1). It is a unique disease with distinctive natural behavior, epidemiology, and histopathology that differs from other head and neck cancers. Estimation of prognosis is a fundamental step in patient management. Among the various prognostic factors, the tumor–node–metastasis (TNM) staging, which has been jointly adopted by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC), remains the most robust factor for global application. The TNM 5th Edition issued in 1997, which introduced a customized staging system for NPC by merging the strengths of the AJCC/UICC 4th edition and Ho’s system, is a historic milestone with worldwide acceptance. Subsequent revisions refined the staging system based on diagnostic and therapeutic advances (2, 3); the current 8th Edition, released in 2017, is another milestone with the unification of the TNM and the Chinese staging systems (4).
In addition to the refinement of TNM parameters, there is a growing interest in the incorporation of non-anatomical prognostic factors that reflect biological tumor behavior. These factors are potentially useful for providing biomarkers on personalized risk stratification, especially with regard to metastatic risk, for tailoring the treatment intensity. There is increasing evidence that incorporation of these factors/biomarkers with TNM staging system could further improve risk stratification (5, 6).
To provide the best available evidence for the upcoming TNM 9th Edition and associated prognostic grouping, a comprehensive systematic review was carried out to identify potentially important suggestions on anatomic and non-anatomic prognostic factors. These suggestions will then be confirmed by a multicenter validation study before the final recommendation to UICC and AJCC for consideration. The current paper is our summary of suggested prognostic factors that warrant further validation.
Materials and Methods
Study Protocol
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline (7). A systematic search of PubMed, Scopus, and Embase for relevant literature published from January 1, 2013, to September 13, 2019, was performed. This timeframe was selected because the construction of TNM 8th Edition was based on literature reviews up to December 31, 2012. Both English and Chinese literatures were accepted, although unpublished studies were not included in the search. The search terms (Supplementary Table 1) were as follows: (“staging” or “TNM” or “prognostic”) and (“nasopharyngeal carcinoma” or “nasopharyngeal cancer” or “nasopharyngeal neoplasm”).
Inclusion Process and Criteria
From the literature identified in the initial search, the following studies were excluded after screening their titles and citations: duplicated studies, conference abstracts, reviews, letters, editorials, case reports, book chapters, and basic science studies. The remaining studies were further assessed to determine eligibility, which included original clinical studies, either prospective or retrospective, with a sample size of at least 50 NPC patients, treated with intensity modulated radiotherapy (IMRT) or equivalent, and showing a significant association between prognostic factors and overall survival (OS). Novel prognostic markers with limited potential for global applicability (e.g., radiomics, micro-RNA, circulating tumor cells, and genetic signatures) were excluded from this review. In cases of multiple studies from one institution, the study with the largest number of patients and the most recently published study was prioritized.
Two independent teams (University of Hong Kong–Shenzhen Hospital and Fujian Cancer Hospital) performed the first review to exclude the ineligible studies. Three independent reviewers (AL, W-TN, and C-LC) further assessed papers that generated disagreements based on the inclusion/exclusion before a final decision was made on the list of studies to be selected for inclusion in this review.
Data Extraction and Analyses
The primary data from the articles were extracted. The primary endpoint for the assessment of prognostic value in this review was OS; the secondary endpoints of distant-metastasis-free survival (DMFS) and local-relapse-free survival (LRFS) were included if they were reported by the original study.
We used the QUality In Prognosis Studies (QUIPS) tool to assess the risk of bias within individual studies (8). The QUIPS tool was originally designed to assess bias in studies of prognostic factors. The tool originally comprised six domains—Study Participation, Prognostic Factor Measurement, Outcome Measurement, Statistical Analysis and Reporting, Study Confounding, and Study Attrition—each of which is guided by three to seven prompting items. Based on the risk of bias, the overall quality of each study was determined as high (score 5–6), moderate (score 3–4), or low (score 0–2); low-quality studies were excluded from this review.
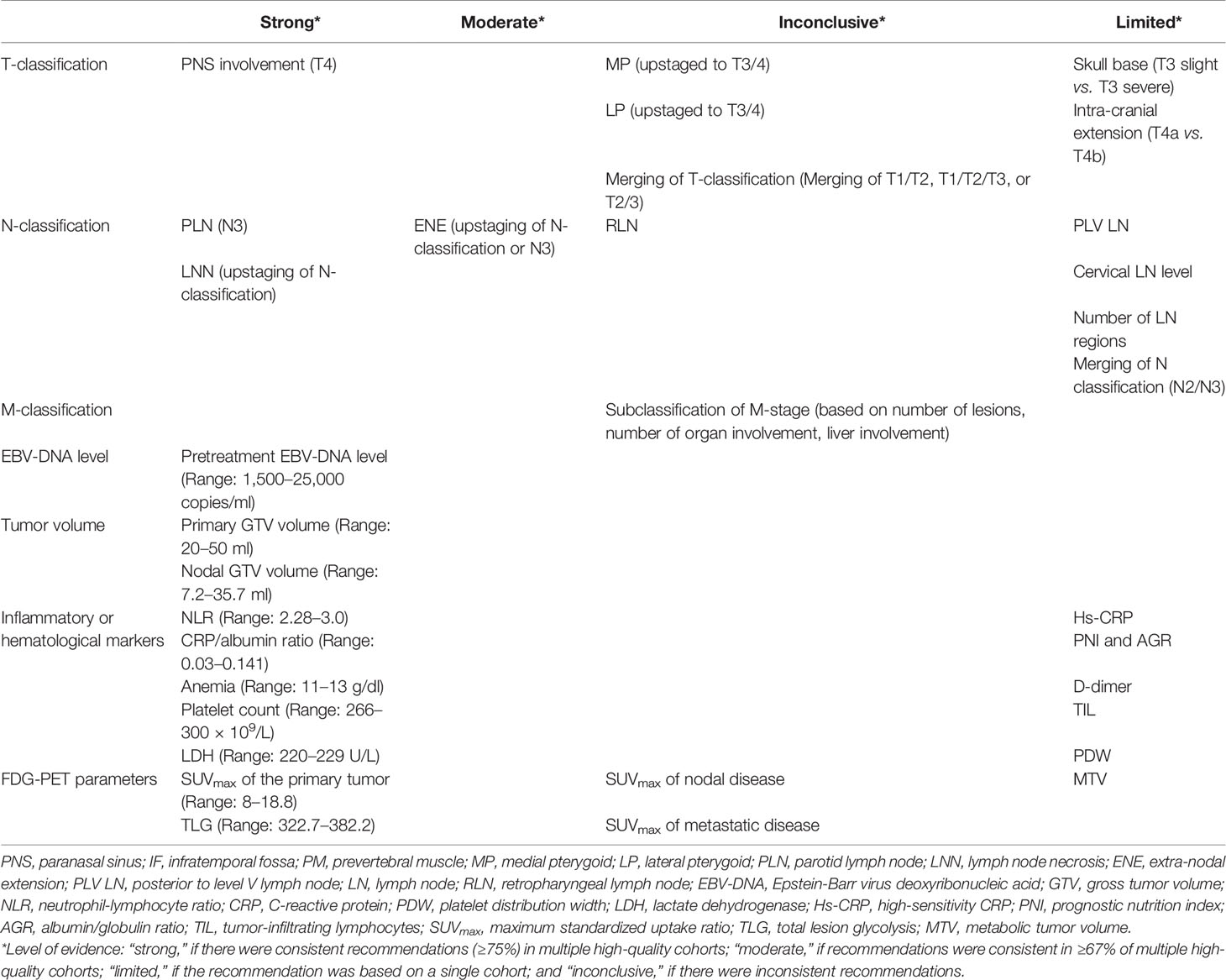
The criteria adopted in this systematic review were designed to synthesize the level of evidence (9), which was defined as “strong,” if there were consistent recommendations (≥75%) in multiple high-quality cohorts; “moderate,” if recommendations were consistent in ≥67% of multiple high-quality cohorts; “limited,” if the recommendation was based on a single cohort; and “inconclusive,” if there were inconsistent recommendations.
Results
Study Selection
An initial search of the three databases identified 5,595 studies that fit the search terms. Following the exclusion of ineligible articles (based on the predefined study eligibility criteria), two independent teams were constituted to identify new suggestions for improving the current TNM 8th Edition. Among the 2,200 studies evaluated, 34 original studies were selected for inclusion by both teams, whereas 198 studies were selected by only one team. The studies with a discrepancy in agreement were further reviewed by three independent reviewers, and 74 were accepted for inclusion. Thus, a total of 108 original studies were included in this in-depth systematic review.
Study Characteristics
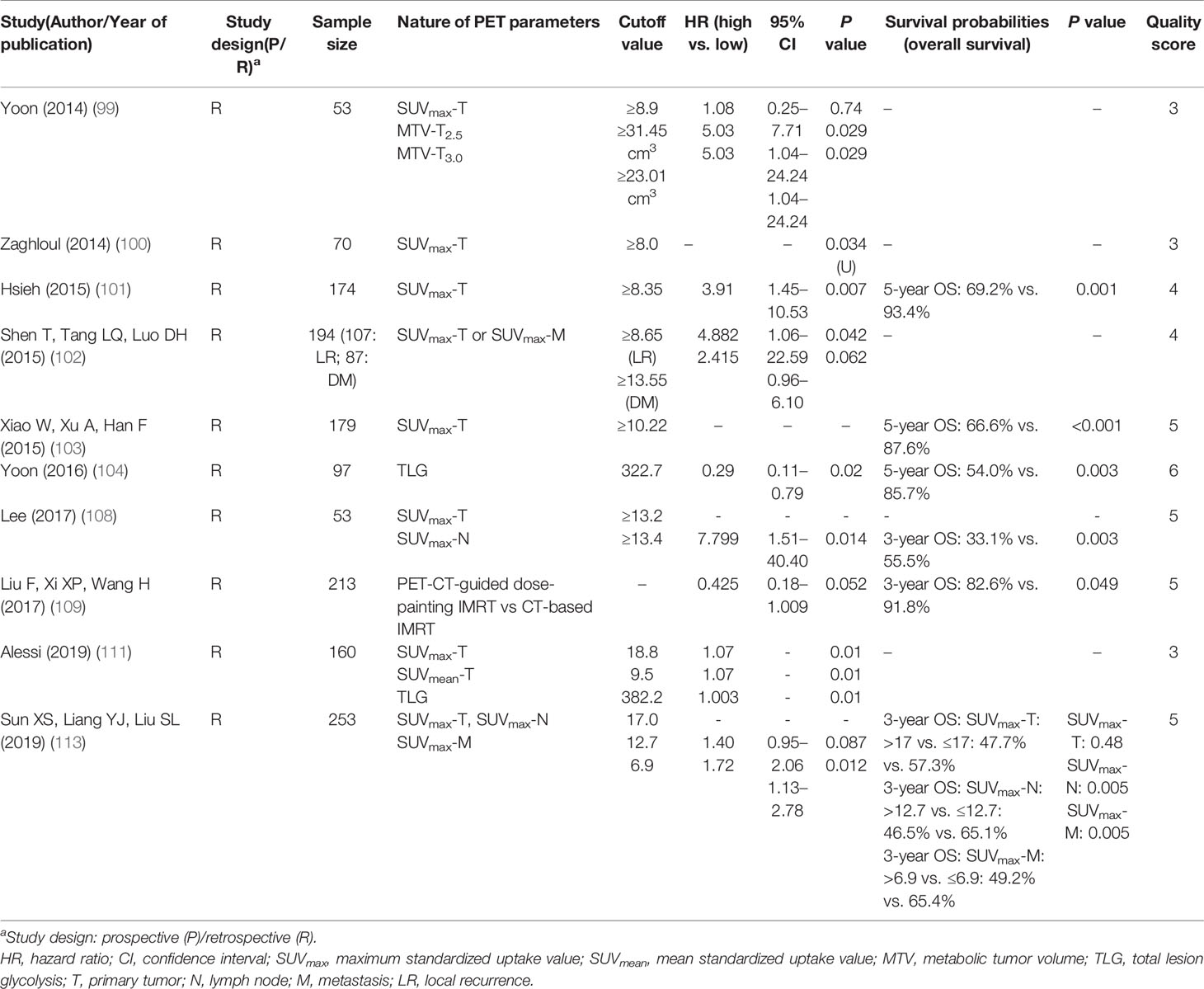
The characteristics of the 108 studies are presented in Supplementary Tables 2–6. Only six studies are prospective analyses, while the rest (n = 102) were retrospective. The majority of studies (n = 101) included only patients without distant metastasis. Forty-four studies focused on anatomical criteria: 22 studies on primary tumor (T-classification) (6, 10–30), 20 on nodal disease (N-classification) (14, 22, 23, 31–47), 5 studies on metastatic disease (M-classification) (48–52), and 3 studies included more than one category. In the 64 studies that evaluated non-anatomical factors, 22 studies focused on plasma Epstein-Barr virus (EBV) deoxyribonucleic acid (DNA) level (53–71), 12 studies on tumor volume (63, 65, 72–81), 18 studies on inflammatory/hematological factors (54, 82–98), and 15 studies on the parameters of fluorodeoxyglucose (FDG) positron emission tomography (PET) (99–113). Three studies had more than one non-anatomical category.
Risks of Bias
The assessment on study quality using the QUIPS tool showed that 62 (57.4%) of the included articles were classified as high quality and 46 (42.6%) as moderate quality. Supplementary Figure 1 presents an algorithm of the study selection process, and Supplementary Tables 2–6 list the QUIPS scores of the included studies. Suggestions from well-conducted studies with large sample sizes or with evidence supported by multiple studies were identified for inclusion in this review.
Proposed Changes and Prognostic Factors
Summary of the level of evidence on the recommendations and studied prognostic factors is summarized in Table 1. Among the 44 reports on TNM parameters (Table 2), 13 proposed changes to current TNM-8 were identified: six on T-category, eight on N-category, and one on M-category. The recommendations that were considered to have a strong level of evidence included the involvement of the paranasal sinus (PNS) as T4 disease (16, 18–20), parotid lymph node (PLN) as N3 disease (34, 35), and the upstaging of N-classification in the presence of lymph node necrosis (LNN) (39, 40, 42).
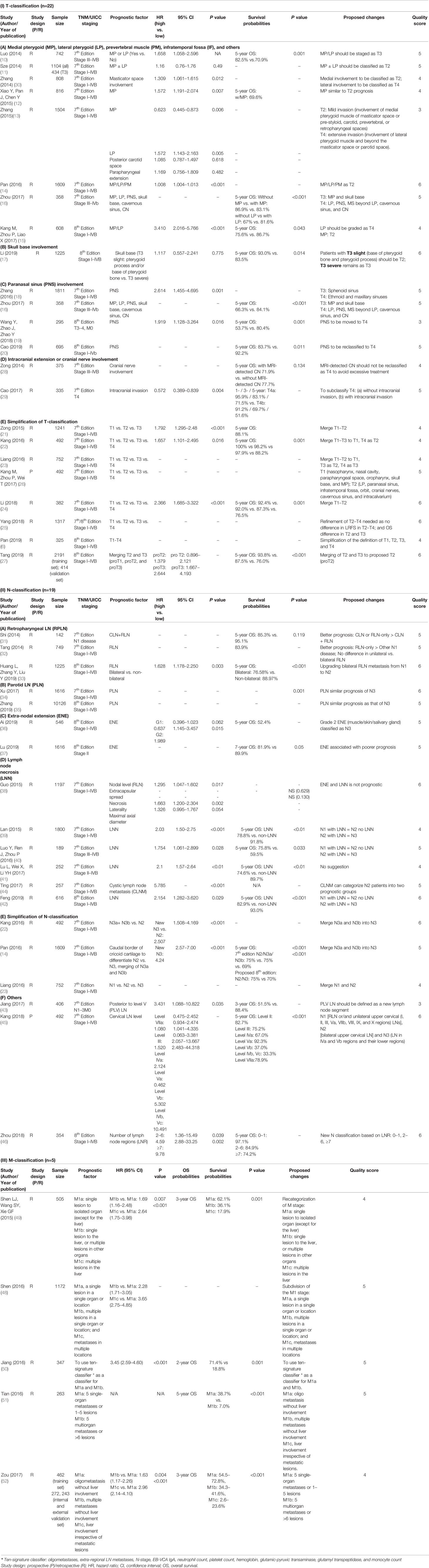
Table 2 Characteristics of studies of T-, N-, and M-classification prognostic factors and survival (n=44).
Among the 64 studies on non-TNM factors, 18 proposed parameters were identified. Prognostic factors with consistent support from multiple studies included EBV-DNA level (Table 3), primary gross tumor volume (GTV) (63, 72–74, 76, 78–81), nodal GTV (Table 4) (74, 75, 77, 81), neutrophil-lymphocyte ratio (NLR) (Table 5) (83, 85, 91, 92, 97), C-reactive protein (CRP)/albumin ratio (83, 89, 98), anemia (84, 87, 96), platelet count (82, 86), lactate dehydrogenase (LDH) (88, 95), and SUVmax of the primary tumor (99–101, 103, 108, 111, 113) and total lesion glycolysis (TLG) (Table 6) (104, 111).
Discussion
To our understanding, this systematic review that evaluated the prognostic factors for NPC patients in 108 articles published from 2013 to 2019 is the most comprehensive review on this topic. The TNM 8th Edition, based entirely on the anatomical tumor extent, is the most widely used prognostic tool for NPC and remains the most robust factor for guiding treatment decisions, evaluating treatment results, and comparing outcomes between institutions worldwide. However, continuous improvement is necessary in view of the advances in investigations and treatments. Furthermore, refinement of prognostic tools by the incorporation of novel proposals based on functional imaging, plasma biomarkers, and molecular tumor characteristics is desirable in the current era of personalized oncology. For tumors with disease sites such as the prostate, breast, and skin (i.e., melanoma), non-anatomical factors have been successfully incorporated while still maintaining essential anatomical information. For NPC, considerable progress on both anatomical and non-anatomical prognostic factors have been made since the publication of the TNM 8th Edition. This systematic review reviewed the latest evidence to facilitate the formulation of a comprehensive proposal for designing the upcoming TNM 9th Edition.
T-Classification
A major change in the TNM 8th Edition was the replacement of the ambiguous terms IF/masseter space involvement with a clear specification of extensive soft tissue infiltration beyond the lateral surface of LP as T4, and the downstaging of MP/LP/PM to T2. This change was supported by two studies (10, 13, 14). However, five studies showed that MP and/or LP involvement was associated with a worse prognosis than T2 and should be upstaged; suggestions included categorizing MP as T3 and LP as T4 disease (n = 1) (16), MP as T2 and LP as T4 (n = 3) (13, 15, 30), and both MP and LP as T3 disease (n = 1) (10). Thus, further validation of the prognostic significance of MP/LP is recommended.
Three studies, comprising a total of 1,348 patients, showed that PNS involvement should be upstaged from current T3 to T4 disease given its poorer outcomes (5-year OS rate of 53.7–83.7%) (16, 19, 20). Of note, Zhang et al. reported worse prognoses among patients with ethmoid sinus or maxillary sinus involvement as T4 disease, but better prognosis in those with sphenoid sinus invasion alone as T3 disease (18); further studies on the relapse risks of various PNS are warranted.
The widespread use of magnetic resonance imaging (MRI) has improved the accuracy of detection of the extent of involvement of the skull base and of intracranial extension. With better disease characterization, Li et al. proposed the subdivision of skull base involvement into T3-slight (pterygoid process and/or base of the pterygoid bone only) and T3-severe (others) (24); similarly, Cao et al. suggested the subdivision of T4 into T4a (without intracranial extension) and T4b (with intracranial extension) based on the presence of intracranial extension (29). Further studies are needed to validate these findings.
With the technological advances in both diagnostics and treatment, the differences in survival and local control in the T-category has diminished. Eight of the included studies proposed the simplification of the T-category (6, 21–27); these included three studies that suggested the merging of T1 and T2 disease (21, 23, 24), one suggested combining of T1, T2, and T3 disease (22), and one proposed a merging of T2 and T3 (27). Other studies proposed simplification of the definition of T-classification, refinement of T2–T4 disease, and reclassification as T1 and T2 only (6, 25, 26).
Level of Evidence:
Strong: PNS involvement (T4 disease)
Moderate: Nil
Inconclusive: MP (upstaged to T3/4), LP (upstaged to T3/4), and merging of T-classification (T1–T2, T1, T2, and T3, or T2–T3)
Limited: Skull base (T3 slight vs. T3 severe) and intracranial extension (T4a vs. T4b).
N-Classification
Despite the rarity of PLN metastasis (0.4–2.8%), consistent findings were noted on its adverse prognostic outcome, which was similar to those with N3 disease, as demonstrated in two studies that included a total of 11,742 patients. Both reports recommended PLN involvement as the criteria for N3 classification (34, 35). Also, suspicion of PLN metastasis, especially in patients with advanced nodal diseases, should be raised on pretreatment imaging, and biopsy is indicated in the suspected case.
Furthermore, in five studies, there was consensus that LNN was an adverse prognostic factor (hazard ratio [HR]: 1.75–5.79) (38–42). In the largest study by Lan et al., patients with LNN had worse OS and DMFS (OS, 78.8 vs. 91.8%; DMFS, 78.4 vs. 91.6%, both p < 0.001); the authors proposed that patients with LNN should be upstaged in their respective N-category (39).
In addition to the proposals identified in the current literature search, extra-nodal extension (ENE) was recently advocated as a new criterion for N3-classification in the TNM 8th Edition for other head and neck cancers, but not for NPC. Specifically, Ai et al. proposed the categorization, as N3 disease, of ENE with infiltration into the adjacent muscle/skin/salivary gland (36). Lu et al. showed that ENE was a poor prognostic factor for NPC and proposed to categorize ENE as G0: lymph nodes without ENE; G1: tumor infiltration beyond the individual nodal capsule(s) into the surrounding fat plane; G2: coalescent nodal mass with unequivocal evidence of ENE; G3: tumor infiltration beyond the nodal capsule into adjacent structures (37). Only G2/G3 ENE, but not G1, was independently prognostic of death; the authors hence proposed a refined N-classification: New-N1: N1/N2 without G2-/G3-ENE; New-N2: N1 with G2-ENE; New-N3: N2 with G2-ENE, N1/N2 with G3-rENE, or N3. On the contrary, Guo et al. suggested that ENE was not a poor prognostic factor; but the definition of ENE was not mentioned in their study (38).
The current TNM 8th Edition categorizes retropharyngeal lymph node involvement (≤6 cm) as N1 disease, regardless of its unilateral or bilateral involvement. Tang et al. supported the current classification (32), but Study by Huang et al. on 1,225 patients (33) suggested upstaging bilateral retropharyngeal lymph node involvement as N2 disease, as they have worse 5-year OS (89.4 vs. 82.6%) and DMFS (91.5 vs. 82.9%).
Furthermore, four studies proposed the simplification of the N-classification and supported the current N3 disease with merging of the previous N3a and N3b (14, 23, 45, 47). Other studies on PLV LN, cervical LN level, and the number of LN regions had limited evidence (22, 43, 44, 46).
Level of Evidence:
Strong: PLN (N3 disease), LNN (Upstaging of N-classification)
Moderate: ENE (Upstaging of N-classification or N3)
Inconclusive: RLN involvement
Limited: PLV LN, cervical LN level, number of LN regions, merging of N2 and N3
M-Classification
Several suggestions have been made on the subcategorization of de novo oligo-metastatic disease based on the number of metastatic lesions and the site(s) of involvement (48–52). However, given the diversity of definition and management of patients with oligo-metastasis, no conclusive recommendation could be made. Most studies have shown that the number of metastatic lesions and the number of organ involvements were independent poor prognostic factors. Furthermore, both Shen et al. and Zou et al. reported that single (or oligo-) metastatic lesions without liver involvement had better prognoses compared with lesions with liver involvement (49, 52). In a multicenter study of 977 patients that was reported by Zou et al., liver metastases represented a worse prognostic factor regardless of the number of metastatic lesions with a 3-year OS rate of 34.3–72.8% vs. 22.6–23.6% (52).
Level of Evidence:
Inconclusive: Subclassification of M-category
Plasma EBV-DNA Level
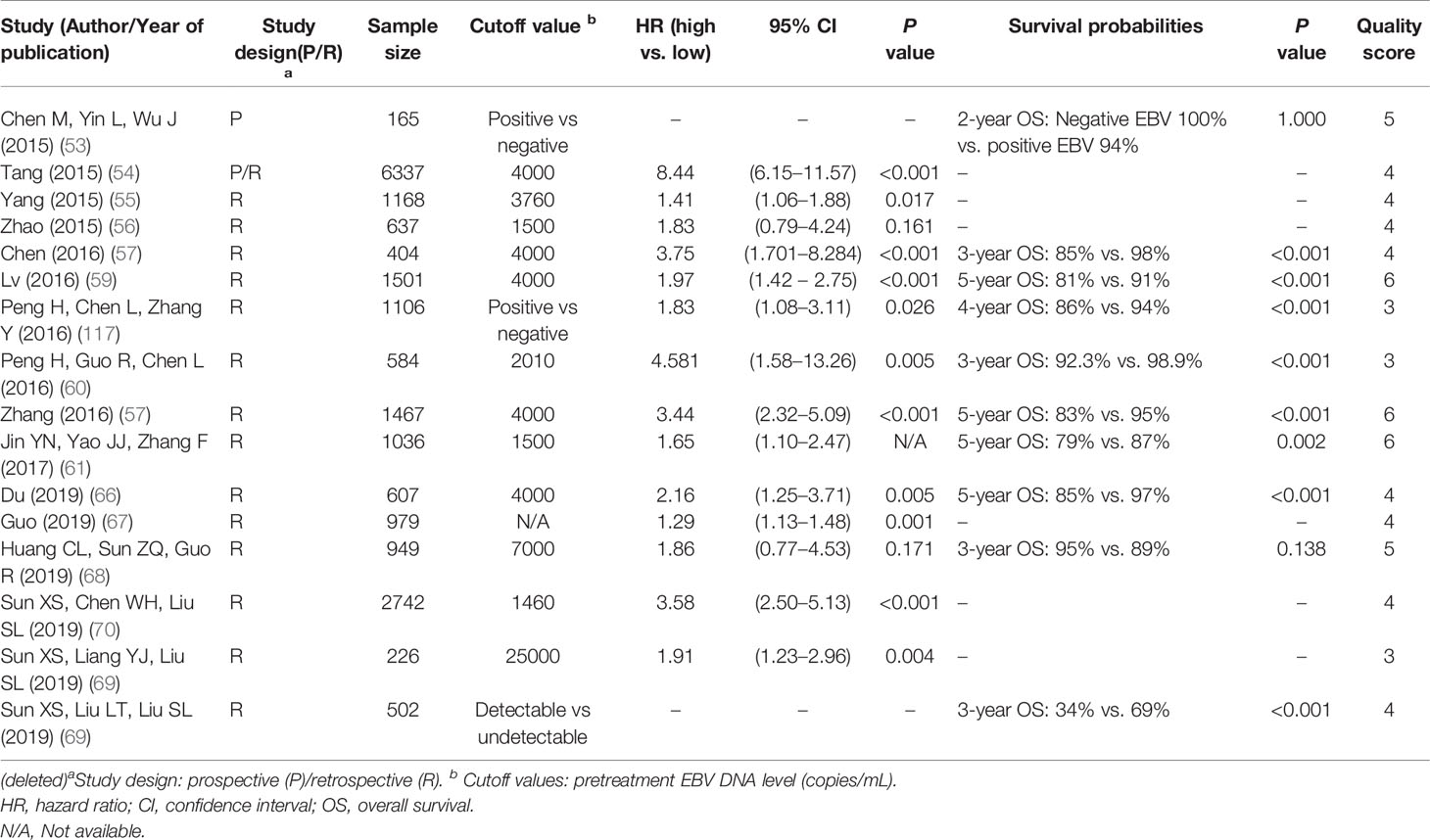
The measurement of EBV-DNA concentration is widely used in diagnosis, prognostication, treatment monitoring, and the surveillance of recurrence. In concordance with previous meta-analyses (115–117), we found that the pretreatment plasma EBV-DNA level was a prognostic factor; the risk of mortality, local failure, and metastases was 1.3- to 8.4-fold, 1.1- to 3.1-fold, and 1.4- to 8.1-fold higher, respectively, for patients with high EBV-DNA levels compared to patients with low EBV-DNA levels (53–71, 114).
Several studies have highlighted the important role of EBV-DNA to refine the prognosis of patients with similar TNM stage groups. In a study of 385 patients with Stage II (TNM 7th edition) disease, the 3-year PFS, LRFS, and DMFS rates for the detectable and undetectable EBV-DNA groups were 89.1 vs. 96.4%, 94.3 vs. 98.2%, and 94.2 vs. 98.6%, respectively (p = 0.005, 0.039, and 0.017, respectively) (63). For locally advanced disease, Zhang et al. revealed that patients with stage II–III (TNM 7th Edition) and a high EBV-DNA level had worse survival than those with stage IVa–b and a low EBV-DNA level (5-year OS: 82.7 vs. 92.9%, PFS: 70.7 vs. 89%) (57). Similarly, Jin et al. showed that the prognosis of patients with stage IVa–b (TNM 7th Edition) and low EBV-DNA level was similar to that of patients with Stage III disease and high EBV-DNA level (61).
Furthermore, two studies demonstrated that recursive partitioning analysis (RPA), which integrated stage groups and the plasma EBV-DNA level, had better survival predictive ability compared to the TNM 8th Edition (67, 71). Guo et al. proposed the following RPA classes: Stage RI (T1N0), RIIA (T2–T3N0 or T1–T3N1, EBV-DNA ≤2,000 copies/ml), Stage RIIB (T2–T3N0 or T1–T3N1, EBV-DNA >2,000 copies/ml; T1–T3N2, EBV-DNA ≤2,000 copies/ml), Stage RIII (T1–T3N2, EBV-DNA >2,000 copies/ml; T4N0–N2), and Stage RIVA (any T and N3) (67). The 5-year PFS rate was 100, 87.9, 76.7, 68.7, and 50.4% for the proposed stages RI, RIIA, RIIB, RIII, and RIV, respectively (p < 0.001). In a similar study by Lee VH et al., RPA derived four new stages: RPA-I (T1–T4, N0–N2, and EBV-DNA <500 copies/ml), RPA-II (T1–T4, N0–N2, and EBV-DNA ≥500 copies/ml), RPA-III (T1–T2 and N3), and RPA-IVA (T3–T4 and N3) (71).
The EBV-DNA concentration could provide biological information of tumors beyond the anatomical factors and thereby improve the prognostic performance of the staging system. Nonetheless, the heterogeneity of cutoff values has hindered the wide application of EBV-DNA in NPC staging. The EBV-DNA cutoff values varied markedly among our included studies (1,500–25,000 copies/ml), with 4,000 copies/ml being the most frequently used cutoff value (54, 57, 59, 66). Plasma EBV-DNA is a laboratory-developed test with heterogeneity based on different DNA extraction, purification, and stabilization methods; different instruments used; different primers and probes that target a different part of the EBV genome; and different quantification controls (118). An earlier study showed that different PCR assays using primer/probe sets for latent membrane protein-2 (LMP-2) and BamHI-W might yield slightly different plasma EBV-DNA concentrations from that in the same sample (119). Also, the low sensitivity of EBV-DNA assays in patients with low-volume NPC is another concern (120). Thus, further international efforts are encouraged to harmonize the assay and validate it in large prospective cohorts to ensure that plasma EBV-DNA can unleash its full potential and be incorporated into the staging system.
Level of Evidence:
Strong: Pretreatment EBV-DNA level
Tumor Volume
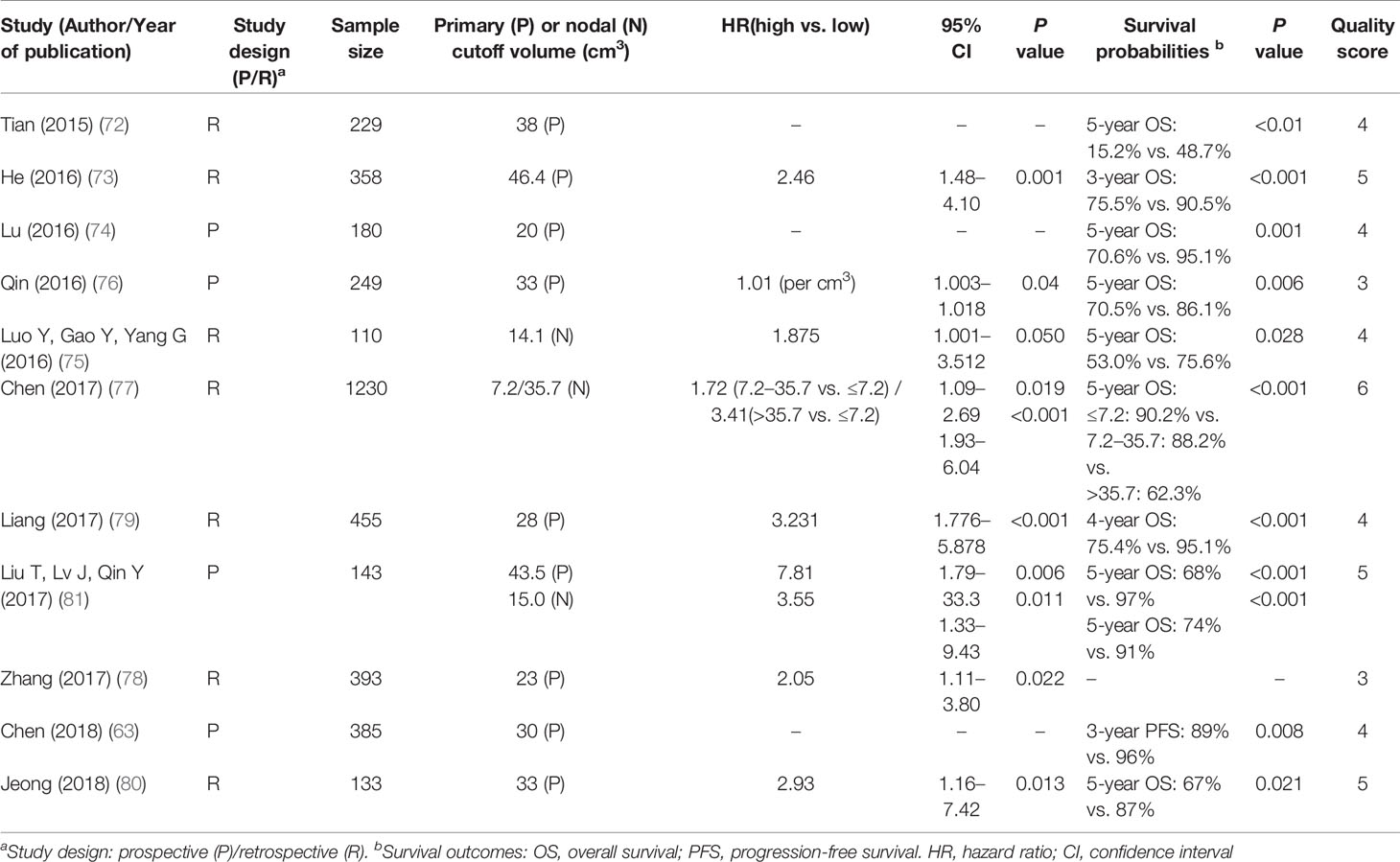
There were 12 studies with 8,403 patients in the current systematic review that evaluated the significance of tumor volumes (GTV-P and/or GTV-N). Seven papers focused on the primary tumor volume (GTV-P; n = 7) (63, 72, 73, 76, 78–80), two on the nodal tumor volume (GTV-N; n = 2) (75, 77), and two on the total tumor volume including primary and node (GTV-P and GTV-N; n = 2) (74, 81). One study did not include a cutoff for GTV-N and GTV-P (65).
The findings suggested that large GTV-P was an independent predictor of OS (HR 1.56–3.23) (63, 72–74, 76, 78–80), DMFS (HR 1.01–3.23) (63, 65, 77–81), and LRFS (HR 1.01–2.79) (63, 73, 76, 78–81). Similarly, large GTV-N was an adverse prognostic factor for OS (HR 1.56–3.41) (75, 77) and DMFS (HR 2.72–6.33) (75, 81). However, the proposed cutoff values varied widely among the studies included in this review (Table 4): GTV-P ranged from 20 to 50 ml (median 33 ml), and GTV-N ranged from 7.2 to 35.7 ml (median 15 ml).
The current T- and N-classifications of the staging system are primarily based on the extent of tumor invasion and the maximum diameter of the LN, respectively. Tumor volume might correlate better with the number of clonogenic tumor cells, leading to a more accurate prediction of the chance of cure (121). Volumetric stratification has been demonstrated to improve the prognostic ability of the TNM staging system. Jeong et al. divided stage II–IV (TNM 8th Edition) into the volume subgroup and found that the 5-year OS was significantly better in participants with GTV-P ≤33 ml compared to those with GTV-P >33 ml (87.3 vs. 66.7%) (80); Chen et al. showed that among 385 TNM-8th Edition classified Stage II patients, those with a total GTV <30 cm3 was associated with a better prognosis than those with a total GTV ≥30 cm3 (63).
Despite the growing body of evidence, tumor volume is yet to be used for cancer staging in routine clinical practice for several reasons. Firstly, there are significant intra- and inter-observer variations in volume delineation. Secondly, the malignant tumor often grows into irregular shapes, and accurate measurement of tumor volume is hard to achieve with conventional imaging. Furthermore, the cutoff value of the tumor volume is difficult to define due to the differences in assessment software, measurement timing, and methods of statistical analysis (122, 123). Future efforts are needed to overcome these challenges before tumor volume can be used as a widely applied prognostic marker.
Level of Evidence:
Strong: Primary GTV volume and nodal GTV volume
Blood Inflammatory/Hematological Markers
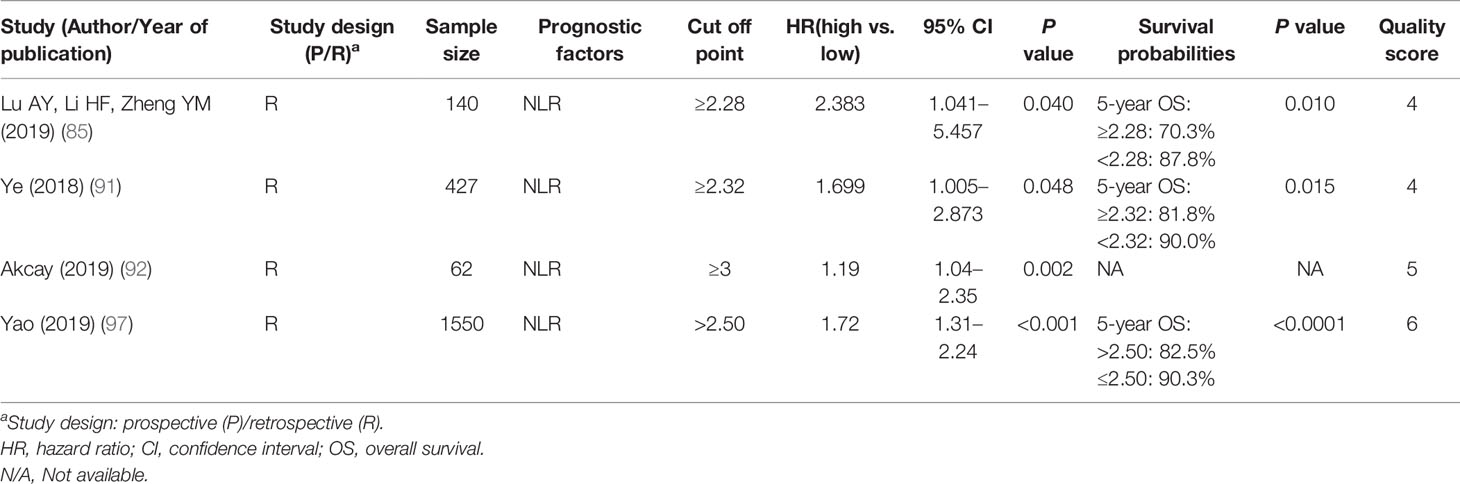
In the 18 studies that were included, nine inflammatory/hematological markers were evaluated: the most frequently proposed marker (n = 5) is NLR (83, 85, 91, 92, 97), followed by anemia (n = 3) (84, 87, 96), LDH (n = 2) (88, 95), platelet count (n = 2) (82, 86), and the CRP/albumin ratio (n = 3) (83, 89, 98). Other proposals with limited supporting evidence included high-sensitivity CRP (hs-CRP; n = 1) (54), platelet distribution width (PDW) (86), prognostic nutrition index (PNI) and albumin/globulin ratio (AGR) (n = 1) (93), D-dimer (n = 1) (94), and tumor-infiltrating lymphocytes (TIL; n = 1) (90).
The results of 2,225 NPC patients in five studies showed that elevated pretreatment NLR was consistently associated with worse OS (HR 1.19–2.38), DMFS (HR 1.45), and LRFS (HR 1.35) (Supplementary Table 5) (83, 85, 91, 92, 97). Evidence suggested that proinflammatory tumor microenvironments are closely related to cancer development and progression. Lymphocytes are immune cells that exhibit an antitumor function, while neutrophils are inflammatory cells that influence the cytotoxic activity of the immune system. Therefore, an increased NLR, with an elevated neutrophil count and/or reduced lymphocyte count, is a biomarker that reflects an imbalance in pro- and antitumor activities in the host’s immune system. Various cutoff values of NLR have been suggested (range 2.28–3.00, median 2.32), and the analysis suggested that NLR was a reliable prognostic marker regardless of the cutoff value (124).
Other hematological markers such as hemoglobin, platelet count, LDH, and CRP have the advantages of easy accessibility, inexpensive measurement, and high reproducibility and therefore possess a promising potential for integration into the international prognostic system. In particular, the significance of LDH and CRP have long been recognized (125–127), and these parameters had been incorporated in various recently published prognostic nomograms of NPC (128–130). Accordingly, further validations of these findings are encouraged.
Level of Evidence:
Strong: NLR, CRP/albumin ratio, anemia, PDW and platelet count, and LDH
Limited: Hs-CRP, PNI and AGR, D-dimer, and TIL
FDG-PET Parameters
Among the 15 studies on FDG-PET included in the current review, most evaluated the maximum SUV (SUVmax), either alone (n = 11) (100–103, 105–108, 110, 112, 113); some also proposed other metabolic parameters, such as metabolic tumor volume (MTV; n = 1) (99) or TLG (n = 2) (104, 111) (Supplementary Table 6). A single study further evaluated the difference in prognosis between PET-CT-guided dose-painting intensity-modulated radiation therapy (IMRT) and CT-based IMRT (109).
Four studies consistently showed that the high SUVmax of the primary tumor was associated with poor OS (HR 1.07–4.88) (99, 101, 102, 111); however, conflicting results were shown with regard to the high SUVmax of nodal and metastatic disease (102, 113). High TLG was associated with inferior OS in two studies, and MTV was a poor prognostic factor in one study (Supplementary Table 6) (99, 104, 111). Therefore, we recommend further validation of the role of the high SUVmax of the primary tumor and high TLG.
The metabolic information of FDG-PET could predict tumor aggressiveness and be correlated with patient survival (131). The majority of FDG-PET studies evaluated the prognostic role of the SUVmax of the tumor mass; however, the SUVmax was limited by representing only the maximum uptake within the volume of interest (VOI) instead of within the entire mass. Emerging metabolic parameters such as TLG and MTV have been proposed to overcome these limitations: MTV is measured by contouring margins defined by thresholds, whereas TLG is calculated by multiplying the MTV by the mean SUV. Additional studies are encouraged to define the prognostic role of the abovementioned factor. However, the diverse range of cutoff values of these PET parameters used in different studies are attributable to several reasons. First, variables such as tumor delineation and definition of VOI may affect the MTV and TLG values; second, the cutoff values are established by the statistical parameters of each institution without cross-validation. Based on the evidence in the current literature, we cannot recommend a concrete cutoff value for further validation as the wide range of values has limited its reproducibility and global applicability.
Level of Evidence:
Strong: High SUVmax of the primary tumor and TLG
Limited: MTV
Inconclusive: High SUVmax of nodal disease and SUVmax of metastatic disease
Limitations
The limitations of this research merit discussion. Firstly, despite the exclusion of poor-quality studies, most of the included studies had a retrospective observational design, which is prone to biases. Secondly, the majority of the included studies that evaluated the non-anatomical markers used dichotomous variables to determine the prognostic value. The cutoff value of parameters varied among different studies, as it was calculated statistically in each study to achieve the most significant prognostic effect; therefore, the generalizability of the findings is uncertain. Thirdly, due to the heterogeneity of study designs, study populations, measurement techniques, and cutoff values, we were unable to perform a meta-analysis to estimate a pooled value reliably. Also, some of the studies of plasma EBV-DNA in early years were not included in the present analysis; however, our conclusion remains consistent with the previous findings (115–117). Lastly, some of the novel markers, such as radiomics, micro-RNA, circulating tumor cells, and genetic signatures, were not included in this review due to their limited global applicability at present.
Summary Remarks
This systematic review has identified a comprehensive list of prognostic factors and suggestions that could contribute toward more accurate risk stratification for designing personalized treatment for NPC. Further studies for the validation of these factors are needed to confirm reproducibility and define the optimal cutoff criterion, to formulate the recommendations for designing the upcoming 9th Edition of the TNM staging system.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
WN, JP, and AL: conception and design. CC, QG, TM, ZX, HC, and JL: collection and assembly of data. CC, WN, HC, JP, and AL: data analysis and interpretation. CC, WN, and AL: manuscript writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
CC: Consulting or Advisory Role: AstraZeneca, Eiasi; Research funding: AstraZeneca, Merck Kgga.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.703995/full#supplementary-material
References
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, et al. Global Cancer Observatory: Cancer Today (2018). International Agency for Research on Cancer. Available at: https://gco.iarc.fr/today (Accessed 25 February, 2020).
2. AJCC Cancer Staging Manual. sixth edition. Greene PL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. New York: Springer (2002).
3. AJCC Cancer Staging Handbook. Seventh edition. Edge S, Byrd DR, Compton CC, Greene FL, Trotti A. New York: Springer-Verlag (2010).
4. Chinese Committee for Staging of Nasopharyngeal Carcinoma. Report on Revision of the Chinese 1992 Staging System for Nasopharyngeal Carcinoma. J Radiat Oncol (2013) 2:233–40. doi: 10.1007/s13566-013-0088-5
5. Guo R, Mao YP, Tang LL, Chen L, Sun Y, Ma J. The Evolution of Nasopharyngeal Carcinoma Staging. Br J Radiol (2019) 92(1102):20190244. doi: 10.1259/bjr.20190244
6. Pan XX, Tong LH, Chen YF, Li FL, Tang WB, Liu YJ, et al. A Simplified T Classification Based on the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma. Cancer Manag Res (2019) 11:3163–9. doi: 10.2147/CMAR.S185860
7. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
8. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing Bias in Studies of Prognostic Factors. Ann Intern Med (2013) 158(4):280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
9. Bosma SE, Ayu O, Fiocco M, Gelderblom H, Dijkstra PDS. Prognostic Factors for Survival in Ewing Sarcoma: A Systematic Review. Surg Oncol (2018) 27(4):603–10. doi: 10.1016/j.suronc.2018.07.016
10. Luo DH, Yang J, Qiu HZ, Shen T, Chen QY, Huang PY, et al. A New T Classification Based on Masticator Space Involvement in Nasopharyngeal Carcinoma: A Study of 742 Cases With Magnetic Resonance Imaging. BMC Cancer (2014) 14:653. doi: 10.1186/1471-2407-14-653
11. Sze H, Chan LL, Ng WT, Hung AW, Lee MC, Chang AT, et al. Should All Nasopharyngeal Carcinoma With Masticator Space Involvement be Staged as T4? Oral Oncol (2014) 50(12):1188–95. doi: 10.1016/j.oraloncology.2014.09.001
12. Xiao Y, Pan J, Chen Y, Lin S, Zong J, Chen Y, et al. The Prognosis of Nasopharyngeal Carcinoma Involving Masticatory Muscles: A Retrospective Analysis for Revising T Subclassifications. Med (Baltimore) (2015) 94(4):e420. doi: 10.1097/MD.0000000000000420
13. Zhang GY, Huang Y, Hu XF, Chen XP, Xu T, Liu LZ, et al. Prognostic Value of Classifying Parapharyngeal Extension in Nasopharyngeal Carcinoma Based on Magnetic Resonance Imaging. BioMed Res Int (2015) 2015:749515. doi: 10.1155/2015/749515
14. Pan JJ, Ng WT, Zong JF, Chan LL, O'Sullivan B, Lin SJ, et al. Proposal for the 8th Edition of the AJCC/UICC Staging System for Nasopharyngeal Cancer in the Era of Intensity-Modulated Radiotherapy. Cancer (2016) 122(4):546–58. doi: 10.1002/cncr.29795
15. Kang M, Zhou P, Liao X, Xu M, Wang R. Prognostic Value of Masticatory Muscle Involvement in Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiation Therapy. Oral Oncol (2017) 75:100–5. doi: 10.1016/j.oraloncology.2017.11.002
16. Zhou Q, He Y, Zhao Y, Wang Y, Kuang W, Shen L. A Study of 358 Cases of Locally Advanced Nasopharyngeal Carcinoma Receiving Intensity-Modulated Radiation Therapy: Improving the Seventh Edition of the American Joint Committee on Cancer T-Staging System. BioMed Res Int (2017) 2017:1419676. doi: 10.1155/2017/1419676
17. Li HJ, Hu YY, Huang L, Zhou J, Li JJ, Xie CB, et al. Subclassification of Skull-Base Invasion for Nasopharyngeal Carcinoma Using Cluster, Network and Survival Analyses: A Double-Center Retrospective Investigation. Radiother Oncol (2019) 134:37–43. doi: 10.1016/j.radonc.2019.01.021
18. Zhang Y, Peng H, Guo R, Li WF, Chen L, Liu X, et al. Should All Nasopharyngeal Carcinoma With Paranasal Sinus Invasion be Staged as T3 in the Intensity-Modulated Radiotherapy Era? A Study of 1811 Cases. J Cancer (2016) 7(10):1353–9. doi: 10.7150/jca.15141
19. Wang Y, Zhao J, Zhao Y, Yang Z, Lei M, Li Z, et al. Impact of Paranasal Sinus Invasion on Advanced Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiation Therapy: The Validity of Advanced T Stage of AJCC/UICC Eighth Edition Staging System. Cancer Med (2018) 7(7):2826–36. doi: 10.1002/cam4.1506
20. Cao C, Jiang F, Jin Q, Jin T, Huang S, Hu Q, et al. Paranasal Sinus Invasion in Nasopharyngeal Carcinoma After Intensity-Modulated Radiotherapy. Cancer Res Treat (2019) 51(1):73–9. doi: 10.4143/crt.2017.607
21. Zong J, Lin S, Lin J, Tang L, Chen B, Zhang M, et al. Impact of Intensity-Modulated Radiotherapy on Nasopharyngeal Carcinoma: Validation of the 7th Edition AJCC Staging System. Oral Oncol (2015) 51(3):254–9. doi: 10.1016/j.oraloncology.2014.10.012
22. Kang M, Long J, Li G, Yan H, Feng G, Liu M, et al. A New Staging System for Nasopharyngeal Carcinoma Based on Intensity-Modulated Radiation Therapy: Results of a Prospective Multicentric Clinical Study. Oncotarget (2016) 7(12):15252–61. doi: 10.18632/oncotarget.7553
23. Liang ZG, Chen XQ, Niu ZJ, Chen KH, Li L, Qu S, et al. Recommendations for Updating T and N Staging Systems for Nasopharyngeal Carcinoma in the Era of Intensity-Modulated Radiotherapy. PloS One (2016) 11(12):e0168470. doi: 10.1371/journal.pone.0168470
24. Li Y, Ou X, Hu C. Validation and Suggestion of Eighth T Classifications of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma Patients: A Retrospective Analysis. Jpn J Clin Oncol (2018) 48(10):927–33. doi: 10.1093/jjco/hyy109
25. Yang XL, Wang Y, Liang SB, He SS, Chen DM, Chen HY, et al. Comparison of the Seventh and Eighth Editions of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma: Analysis of 1317 Patients Treated With Intensity-Modulated Radiotherapy at Two Centers. BMC Cancer (2018) 18(1):606. doi: 10.1186/s12885-018-4419-1
26. Kang M, Zhou P, Wei T, Zhao T, Long J, Li G, et al. A New T Staging System for Nasopharyngeal Carcinoma Based on Intensity-Modulated Radiation Therapy: Results From a Prospective Multicentric Clinical Study. Am J Cancer Res (2017) 7(2):346–56. doi: 10.1016/j.ijrobp.2016.06.1489
27. Tang LL, Liang SB, Huang CL, Zhang F, Xu C, Mao YP, et al. The Development and External Validation of Simplified T Category Classification for Nasopharyngeal Carcinoma to Improve the Prognostic Value in the Intensity-Modulated Radiotherapy Era. Cancer Med (2019) 8(5):2213–22. doi: 10.1002/cam4.2131
28. Zong J, Lin S, Chen Y, Wang B, Xiao Y, Lin J, et al. Does MRI-Detected Cranial Nerve Involvement Affect the Prognosis of Locally Advanced Nasopharyngeal Carcinoma Treated With Intensity Modulated Radiotherapy? PloS One (2014) 9(6):e100571. doi: 10.1371/journal.pone.0100571
29. Cao C, Luo J, Gao L, Yi J, Huang X, Li S, et al. Magnetic Resonance Imaging-Detected Intracranial Extension in the T4 Classification Nasopharyngeal Carcinoma With Intensity-Modulated Radiotherapy. Cancer Res Treat (2017) 49(2):518–25. doi: 10.4143/crt.2016.299
30. Zhang GY, Huang Y, Cai XY, Chen XP, Xu T, Wu J, et al. Prognostic Value of Grading Masticator Space Involvement in Nasopharyngeal Carcinoma According to MR Imaging Findings. Radiology (2014) 273(1):136–43. doi: 10.1148/radiol.14132745
31. Shi Q, Shen C, Kong L, Wang X, Ding J, Gao Y, et al. Involvement of Both Cervical Lymph Nodes and Retropharyngeal Lymph Nodes has Prognostic Value for N1 Patients With Nasopharyngeal Carcinoma. Radiat Oncol (2014) 9:7. doi: 10.1186/1748-717X-9-7
32. Tang LL, Guo R, Zhou G, Sun Y, Liu LZ, Lin AH, et al. Prognostic Value and Staging Classification of Retropharyngeal Lymph Node Metastasis in Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiotherapy. PloS One (2014) 9(10):e108375. doi: 10.1371/journal.pone.0108375
33. Huang L, Zhang Y, Liu Y, Li H, Wang S, Liang S, et al. Prognostic Value of Retropharyngeal Lymph Node Metastasis Laterality in Nasopharyngeal Carcinoma and a Proposed Modification to the UICC/AJCC N Staging System. Radiother Oncol (2019) 140:90–7. doi: 10.1016/j.radonc.2019.04.024
34. Xu Y, Chen X, Zhang M, Xiao Y, Zong J, Guo Q, et al. Prognostic Effect of Parotid Area Lymph Node Metastases After Preliminary Diagnosis of Nasopharyngeal Carcinoma: A Propensity Score Matching Study. Cancer Med (2017) 6(10):2213–21. doi: 10.1002/cam4.1154
35. Zhang Y, Zhang ZC, Li WF, Liu X, Liu Q, Ma J. Prognosis and Staging of Parotid Lymph Node Metastasis in Nasopharyngeal Carcinoma: An Analysis in 10,126 Patients. Oral Oncol (2019) 95:150–6. doi: 10.1016/j.oraloncology.2019.06.013
36. Ai QY, King AD, Poon DMC, Mo FKF, Hui EP, Tong M, et al. Extranodal Extension is a Criterion for Poor Outcome in Patients With Metastatic Nodes From Cancer of the Nasopharynx. Oral Oncol (2019) 88:124–30. doi: 10.1016/j.oraloncology.2018.11.007
37. Lu T, Hu Y, Xiao Y, Guo Q, Huang SH, O'Sullivan B, et al. Prognostic Value of Radiologic Extranodal Extension and its Potential Role in Future N Classification for Nasopharyngeal Carcinoma. Oral Oncol (2019) 99:104438. doi: 10.1016/j.oraloncology.2019.09.030
38. Guo Q, Pan J, Zong J, Zheng W, Zhang C, Tang L, et al. Suggestions for Lymph Node Classification of UICC/AJCC Staging System: A Retrospective Study Based on 1197 Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiation Therapy. Med (Baltimore) (2015) 94(20):e808. doi: 10.1097/MD.0000000000000808
39. Lan M, Huang Y, Chen CY, Han F, Wu SX, Tian L, et al. Prognostic Value of Cervical Nodal Necrosis in Nasopharyngeal Carcinoma: Analysis of 1800 Patients With Positive Cervical Nodal Metastasis at MR Imaging. Radiology (2015) 276(2):619. doi: 10.1148/radiol.15154020
40. Luo Y, Ren J, Zhou P, Gao Y, Yang G, Lang J. Cervical Nodal Necrosis is an Independent Survival Predictor in Nasopharyngeal Carcinoma: An Observational Cohort Study. Onco Targets Ther (2016) 9:6775–83. doi: 10.2147/OTT.S110558
41. Lu L, Wei X, Li YH, Li WB. Sentinel Node Necrosis is a Negative Prognostic Factor in Patients With Nasopharyngeal Carcinoma: A Magnetic Resonance Imaging Study of 252 Patients. Curr Oncol (2017) 24(3):e220–5. doi: 10.3747/co.24.3168
42. Feng Y, Cao C, Hu Q, Chen X. Prognostic Value and Staging Classification of Lymph Nodal Necrosis in Nasopharyngeal Carcinoma After Intensity-Modulated Radiotherapy. Cancer Res Treat (2019) 51(3):1222–30. doi: 10.4143/crt.2018.595
43. Jiang C, Zhang T, Gao H, Zhang L. Prognosis of Cervical and Posterior to Level V Lymph Node Metastasis in 406 Cases of Nasopharyngeal Carcinoma. Chin J Clin Onco (2017) 44:1019–23.
44. Ting Y, Chee J, Charn TC, Loh KS, Choong CC, Ting E, et al. Prognostic Significance of Cystic Lymph Nodal Metastasis in Nasopharyngeal Carcinoma. Head Neck (2017) 39(9):1832–9. doi: 10.1002/hed.24844
45. Kang M, Zhou P, Wei T, Zhao T, Long J, Li G, et al. A Novel N Staging System for NPC Based on IMRT and RTOG Guidelines for Lymph Node Levels: Results of a Prospective Multicentric Clinical Study. Oncol Lett (2018) 16(1):308–16. doi: 10.3892/ol.2018.8676
46. Zhou X, Ou X, Yang Y, Xu T, Shen C, Ding J, et al. Quantitative Metastatic Lymph Node Regions on Magnetic Resonance Imaging are Superior to AJCC N Classification for the Prognosis of Nasopharyngeal Ccarcinoma. J Oncol (2018) 2018:9172585. doi: 10.1155/2018/9172585
47. Yue D, Xu YF, Zhang F, Lin L, Mao YP, Li WF, et al. Is Replacement of the Supraclavicular Fossa With the Lower Level Classification Based on Magnetic Resonance Imaging Beneficial in Nasopharyngeal Carcinoma? Radiother Oncol (2014) 113(1):108–14. doi: 10.1016/j.radonc.2014.08.036
48. Shen L, Li W, Wang S, Xie G, Zeng Q, Chen C, et al. Image-Based Multilevel Subdivision of M1 Category in TNM Staging System for Metastatic Nasopharyngeal Carcinoma. Radiology (2016) 280(3):805–14. doi: 10.1148/radiol.2016151344
49. Shen LJ, Wang SY, Xie GF, Zeng Q, Chen C, Dong AN, et al. Subdivision of M Category for Nasopharyngeal Carcinoma With Synchronous Metastasis: Time to Expand the M Categorization System. Chin J Cancer (2015) 34(10):450–8. doi: 10.1186/s40880-015-0031-9
50. Jiang R, You R, Pei XQ, Zou X, Zhang MX, Wang TM, et al. Development of a Ten-Signature Classifier Using a Support Vector Machine Integrated Approach to Subdivide the M1 Stage Into M1a and M1b Stages of Nasopharyngeal Carcinoma With Synchronous Metastases to Better Predict Patients’ Survival. Oncotarget (2016) 7(3):3645–57. doi: 10.18632/oncotarget.6436
51. Tian YH, Zou WH, Xiao WW, Zeng L, Yuan X, Bai L, et al. Oligometastases in AJCC Stage IVc Nasopharyngeal Carcinoma: A Subset With Better Overall Survival. Head Neck (2016) 38(8):1152–7. doi: 10.1002/hed.24345
52. Zou X, You R, Liu H, He YX, Xie GF, Xie ZH, et al. Establishment and Validation of M1 Stage Subdivisions for De Novo Metastatic Nasopharyngeal Carcinoma to Better Predict Prognosis and Guide Treatment. Eur J Cancer (2017) 77:117–26. doi: 10.1016/j.ejca.2017.02.029
53. Chen M, Yin L, Wu J, Gu JJ, Jiang XS, Wang DJ, et al. Impact of Plasma Epstein-Barr Virus-DNA and Tumor Volume on Prognosis of Locally Advanced Nasopharyngeal Carcinoma. BioMed Res Int (2015) 2015:617949. doi: 10.1155/2015/617949
54. Tang LQ, Li CF, Chen QY, Zhang L, Lai XP, He Y, et al. High-Sensitivity C-Reactive Protein Complements Plasma Epstein-Barr Virus Deoxyribonucleic Acid Prognostication in Nasopharyngeal Carcinoma: A Large-Scale Retrospective and Prospective Cohort Study. Int J Radiat Oncol Biol Phys (2015) 91(2):325–36. doi: 10.1016/j.ijrobp.2014.10.005
55. Yang L, Hong S, Wang Y, Chen H, Liang S, Peng P, et al. Development and External Validation of Nomograms for Predicting Survival in Nasopharyngeal Carcinoma Patients After Definitive Radiotherapy. Sci Rep (2015) 5:15638. doi: 10.1038/srep15638
56. Zhao FP, Liu X, Chen XM, Lu J, Yu BL, Tian WD, et al. Levels of Plasma Epstein-Barr Virus DNA Prior and Subsequent to Treatment Predicts the Prognosis of Nasopharyngeal Carcinoma. Oncol Lett (2015) 10(5):2888–94. doi: 10.3892/ol.2015.3628
57. Chen WH, Tang LQ, Guo SS, Chen QY, Zhang L, Liu LT, et al. Prognostic Value of Plasma Epstein-Barr Virus DNA for Local and Regionally Advanced Nasopharyngeal Carcinoma Treated With Cisplatin- Based Concurrent Chemoradiotherapy in Intensity-Modulated Radiotherapy Era. Med (Baltimore) (2016) 95(5):e2642. doi: 10.1097/MD.0000000000002642
58. Du XJ, Tang LL, Mao YP, Guo R, Sun Y, Lin AH, et al. Circulating EBV DNA, Globulin and Nodal Size Predict Distant Metastasis After Intensity-Modulated Radiotherapy in Stage II Nasopharyngeal Carcinoma. J Cancer (2016) 7(6):664–70. doi: 10.7150/jca.14183
59. Lv JW, Chen YP, Zhou GQ, Tang LL, Mao YP, Li WF, et al. Cigarette Smoking Complements the Prognostic Value of Baseline Plasma Epstein-Barr Virus Deoxyribonucleic Acid in Patients With Nasopharyngeal Carcinoma Undergoing Intensity-Modulated Radiation Therapy: A Large-Scale Retrospective Cohort Study. Oncotarget (2016) 7(13):16806–17. doi: 10.18632/oncotarget.7609
60. Peng H, Guo R, Chen L, Zhang Y, Li WF, Mao YP, et al. Prognostic Impact of Plasma Epstein-Barr Virus DNA in Patients With Nasopharyngeal Carcinoma Treated Using Intensity-Modulated Radiation Therapy. Sci Rep (2016) 6:22000. doi: 10.1038/srep22000
61. Jin YN, Yao JJ, Zhang F, Wang SY, Zhang WJ, Zhou GQ, et al. Is Pretreatment Epstein-Barr Virus DNA Still Associated With 6-Year Survival Outcomes in Locoregionally Advanced Nasopharyngeal Carcinoma? J Cancer (2017) 8(6):976–82. doi: 10.7150/jca.18124
62. Yao JJ, Zhou GQ, Wang YQ, Wang SY, Zhang WJ, Jin YN, et al. Prognostic Values of the Integrated Model Incorporating the Volume of Metastatic Regional Cervical Lymph Node and Pretreatment Serum Epstein-Barr Virus DNA Copy Number in Predicting Distant Metastasis in Patients With N1 Nasopharyngeal Carcinoma. Chin J Cancer (2017) 36(1):98. doi: 10.1186/s40880-017-0264-x
63. Chen QY, Guo SY, Tang LQ, Lu TY, Chen BL, Zhong QY, et al. Combination of Tumor Volume and Epstein-Barr Virus DNA Improved Prognostic Stratification of Stage II Nasopharyngeal Carcinoma in the Intensity Modulated Radiotherapy Era: A Large-Scale Cohort Study. Cancer Res Treat (2018) 50(3):861–71. doi: 10.4143/crt.2017.237
64. He SS, Wang Y, Bao Y, Cai XY, Yang XL, Chen DM, et al. Dynamic Changes in Plasma Epstein-Barr Virus DNA Load During Treatment Have Prognostic Value in Nasopharyngeal Carcinoma: A Retrospective Study. Cancer Med (2018) 7(4):1110–7. doi: 10.1002/cam4.1381
65. Peng L, Yang Y, Guo R, Mao YP, Xu C, Chen YP, et al. Relationship Between Pretreatment Concentration of Plasma Epstein-Barr Virus DNA and Tumor Burden in Nasopharyngeal Carcinoma: An Updated Interpretation. Cancer Med (2018) 7(12):5988–98. doi: 10.1002/cam4.1858
66. Du YY, Luo DH, Sun XS, Tang LQ, Mai HQ, Chen QY, et al. Combining Pretreatment Plasma Epstein-Barr Virus DNA Level and Cervical Node Necrosis Improves Prognostic Stratification in Patients With Nasopharyngeal Carcinoma: A Cohort Study. Cancer Med (2019) 8(16):6841–52. doi: 10.1002/cam4.2481
67. Guo R, Tang LL, Mao YP, Du XJ, Chen L, Zhang ZC, et al. Proposed Modifications and Incorporation of Plasma Epstein-Barr Virus DNA Improve the TNM Staging System for Epstein-Barr Virus-Related Nasopharyngeal Carcinoma. Cancer (2019) 125(1):79–89. doi: 10.1002/cncr.31741
68. Huang CL, Sun ZQ, Guo R, Liu X, Mao YP, Peng H, et al. Plasma Epstein- Barr Virus DNA Load After Induction Chemotherapy Predicts Outcome in Locoregionally Advanced Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2019) 104(2):355–61. doi: 10.1016/j.ijrobp.2019.01.007
69. Sun XS, Liu LT, Liu SL, Guo SS, Wen YF, Xie HJ, et al. Identifying Optimal Candidates for Local Treatment of the Primary Tumor Among Patients With De Novo Metastatic Nasopharyngeal Carcinoma: A Retrospective Cohort Study Based on Epstein-Barr Virus DNA Level and Tumor Response to Palliative Chemotherapy. BMC Cancer (2019) 19(1):92. doi: 10.1186/s12885-019-5281-5
70. Sun XS, Chen WH, Liu SL, Liang YJ, Chen QY, Guo SS, et al. Individualized Concurrent Chemotherapy by Pretreatment Plasma Epstein-Barr Viral DNA in II-III Stage Nasopharyngeal Carcinoma: A Propensity Score Matching Analysis Using a Large Cohort. Cancer Med (2019) 8(9):4214–25. doi: 10.1002/cam4.2343
71. Lee VH, Kwong DL, Leung TW, Choi CW, O'Sullivan B, Lam KO, et al. The Addition of Pretreatment Plasma Epstein-Barr Virus DNA Into the Eighth Edition of Nasopharyngeal Cancer TNM Stage Classification. Int J Cancer (2019) 144(7):1713–22. doi: 10.1002/ijc.31856
72. Tian YM, Xiao WW, Bai L, Liu XW, Zhao C, Lu TX, et al. Impact of Primary Tumor Volume and Location on the Prognosis of Patients With Locally Recurrent Nasopharyngeal Carcinoma. Chin J Cancer (2015) 34(6):247–53. doi: 10.1186/s40880-015-0019-5
73. He YX, Wang Y, Cao PF, Shen L, Zhao YJ, Zhang ZJ, et al. Prognostic Value and Predictive Threshold of Tumor Volume for Patients With Locally Advanced Nasopharyngeal Carcinoma Receiving Intensity-Modulated Radiotherapy. Chin J Cancer (2016) 35(1):96. doi: 10.1186/s40880-016-0159-2
74. Lu L, Li J, Zhao C, Xue W, Han F, Tao T, et al. Prognostic Efficacy of Combining Tumor Volume With Epstein-Barr Virus DNA in Patients Treated With Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. Oral Oncol (2016) 60:18–24. doi: 10.1016/j.oraloncology.2016.06.013
75. Luo Y, Gao Y, Yang G, Lang J. Clinical Outcome and Prognostic Factors of Intensity-Modulated Radiotherapy for T4 Stage Nasopharyngeal Carcinoma. BioMed Res Int (2016) 2016:4398498. doi: 10.1155/2016/4398498
76. Qin L, Wu F, Lu H, Wei B, Li G, Wang R. Tumor Volume Predicts Survival Rate of Advanced Nasopharyngeal Carcinoma Treated With Concurrent Chemoradiotherapy. Otolaryngol Head Neck Surg (2016) 155(4):598–605. doi: 10.1177/0194599816644408
77. Chen FP, Zhou GQ, Qi ZY, Lin L, Hu J, Wang XJ, et al. Prognostic Value of Cervical Nodal Tumor Volume in Nasopharyngeal Carcinoma: Analysis of 1230 Patients With Positive Cervical Nodal Metastasis. PloS One (2017) 12(5):e0176995. doi: 10.1371/journal.pone.0176995
78. Zang J, Li C, Zhao LN, Wang JH, Xu M, Luo SQ, et al. Prognostic Model of Death and Distant Metastasis for Nasopharyngeal Carcinoma Patients Receiving 3DCRT/IMRT in Nonendemic Area of China. Med (Baltimore) (2016) 95(21):e3794. doi: 10.1097/MD.0000000000003794
79. Liang SB, Teng JJ, Hu XF, Yang XL, Luo M, Fang XN, et al. Prognostic Value of Total Tumor Volume in Patients With Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy. BMC Cancer (2017) 17(1):506. doi: 10.1186/s12885-017-3480-5
80. Jeong Y, Lee SW. Tumor Volume/Metabolic Information can Improve the Prognostication of Anatomy Based Staging System for Nasopharyngeal Cancer? Evaluation of the 8th Edition of the AJCC/UICC Staging System for Nasopharyngeal Cancer. Radiat Oncol J (2018) 36(4):295–303. doi: 10.3857/roj.2018.00430
81. Liu T, Lv J, Qin Y. Standardized Tumor Volume: An Independent Prognostic Factor in Advanced Nasopharyngeal Carcinoma. Oncotarget (2017) 8(41):70299–309. doi: 10.18632/oncotarget.20313
82. Chen YP, Zhao BC, Chen C, Shen LJ, Gao J, Mai ZY, et al. Pretreatment Platelet Count Improves the Prognostic Performance of the TNM Staging System and Aids in Planning Therapeutic Regimens for Nasopharyngeal Carcinoma: A Single-Institutional Study of 2,626 Patients. Chin J Cancer (2015) 34(3):137–46. doi: 10.1186/s40880-015-0006-x
83. Li JP, Chen SL, Liu XM, He X, Xing S, Liu YJ, et al. A Novel Inflammation-Based Stage (I Stage) Predicts Overall Survival of Patients With Nasopharyngeal Carcinoma. Int J Mol Sci (2016) 17(11):1900. doi: 10.3390/ijms17111900
84. Li X, Chang H, Tao Y, Wang X, Gao J, Zhang W, et al. Revalidation of a Prognostic Score Model Based on Complete Blood Count for Nasopharyngeal Carcinoma Through a Prospective Study. Chin J Cancer Res (2016) 28(5):467–77. doi: 10.21147/j.issn.1000-9604.2016.05.01
85. Lu AY, Li HF, Zheng YM, Tang MZ, Li J, Wu HH, et al. Prognostic Significance of Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio, and Platelet to Lymphocyte Ratio in Patients With Nasopharyngeal Carcinoma. BioMed Res Int (2017) 2017:3047802. doi: 10.1155/2017/3047802
86. Xie X, Zeng X, Cao S, Hu X, Shi Q, Li D, et al. Elevated Pretreatment Platelet Distribution Width and Platelet Count Predict Poor Prognosis in Nasopharyngeal Carcinoma. Oncotarget (2017) 8(62):106089–97. doi: 10.18632/oncotarget.22528
87. Zhang LL, Zhou GQ, Li YY, Tang LL, Mao YP, Lin AH, et al. Combined Prognostic Value of Pretreatment Anemia and Cervical Node Necrosis in Patients With Nasopharyngeal Carcinoma Receiving Intensity-Modulated Radiotherapy: A Large-Scale Retrospective Study. Cancer Med (2017) 6(12):2822–31. doi: 10.1002/cam4.1233
88. Zhou JY, Chen T, Li W, Ye K, ZB W. Effect of Pretreatment Serum LDH and ALP Levels on the Prognosis of Patients With Nasopharyngeal Carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2017) 31:1069–73. doi: 10.13201/j.issn.1001-1781.2017.14.004
89. Wang Y, Yang L, Xia L, Chen Y. High C-Reactive Protein/Albumin Ratio Predicts Unfavorable Distant Metastasis-Free Survival in Nasopharyngeal Carcinoma: A Propensity Score-Matched Analysis. Cancer Manag Res (2018) 10:371–81. doi: 10.2147/CMAR.S155604
90. Wang YQ, Chen YP, Zhang Y, Jiang W, Liu N, Yun JP, et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes in Nondisseminated Nasopharyngeal Carcinoma: A Large-Scale Cohort Study. Int J Cancer (2018) 142(12):2558–66. doi: 10.1002/ijc.31279
91. Ye L, Oei RW, Kong F, Xu T, Shen C, Wang X, et al. Prognostic Values of Hematological Biomarkers in Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiotherapy. Eur Arch Otorhinolaryngol (2018) 275(5):1309–17. doi: 10.1007/s00405-018-4956-x
92. Akcay M, Etiz D, Ozen A, Saylisoy S. Neutrophil/lymphocyte Ratio and Prognosis in Patients With non-Metastatic Nasopharyngeal Cancer: A Single-Center Experience. Turk Oncol Derg (2019) 34(2):92–9. doi: 10.5505/tjo.2019.1845
93. Gundog M, Basaran H. Pretreatment Low Prognostic Nutritional Index and Low Albumin-Globulin Ratio are Predictive for Overall Survival in Nasopharyngeal Cancer. Eur Arch Oto-Rhino-L (2019) 276(11):3221–30. doi: 10.1007/s00405-019-05595-2
94. He SS, Wang Y, Wang CT, Zhu MY, Yang XL, Chen DM, et al. A Combined Marker Based on Plasma D-Dimer and Serum Albumin Levels in Patients With Nasopharyngeal Carcinoma is Associated With Poor Survival Outcomes in a Retrospective Cohort Study. J Cancer (2019) 10(16):3691–7. doi: 10.7150/jca.32387
95. Long GX, Tang WH, Fu XG, Liu DB, Zhang LL, Hu GY, et al. Pre-Treatment Serum Lactate Dehydrogenase Predicts Distant Metastasis and Poor Survival in Nasopharyngeal Carcinoma. J Cancer (2019) 10(16):3657–64. doi: 10.7150/jca.32716
96. Topkan E, Ekici NY, Ozdemir Y, Besen AA, Yildirim BA, Mertsoylu H, et al. Baseline Hemoglobin <11.0 G/dL has Stronger Prognostic Value Than Anemia Status in Nasopharynx Cancers Treated With Chemoradiotherapy. Int J Biol Marker (2019) 34:139–47. doi: 10.1177/1724600818821688
97. Yao JJ, Zhu FT, Dong J, Liang ZB, Yang LW, Chen SY, et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Advanced Nasopharyngeal Carcinoma: A Large Institution-Based Cohort Study From an Endemic Area. BMC Cancer (2019) 19:37. doi: 10.1186/s12885-018-5236-2
98. Tao CJ, Chen YY, Jiang F, Feng XL, Jin QF, Jin T, et al. The C-Reactive Protein/Albumin Ratio is an Independent Prognostic Factor for Overall Survival in Patients With Nasopharyngeal Carcinoma Receiving Intensity-Modulated Radiotherapy. J Cancer (2016) 7(14):2005–11. doi: 10.7150/jca.16210
99. Yoon YH, Lee SH, Hong SL, Kim SJ, Roh HJ, Cho KS. Prognostic Value of Metabolic Tumor Volume as Measured by Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Nasopharyngeal Carcinoma. Int Forum Allergy Rhinol (2014) 4(10):845–50. doi: 10.1002/alr.21363
100. Zaghloul HA, Khedr GA, Rostom Y, Refaat T. The Predictive Value of Pretreatment (18)-F-FDG-PET-CT in Locally Advanced Nasopharyngeal Cancer Patients Treated Definitively With Induction Chemotherapy Followed by Concurrent Chemo-Radiotherapy. J Nucl Med Radiat Ther (2014) 5(1):166. doi: 10.4172/2155-9619.1000166
101. Hsieh TC, Hsieh CY, Yang TY, Chen TT, Lin CY, Lin CC, et al. [18F]-Fluorodeoxyglucose Positron Emission Tomography Standardized Uptake Value as a Predictor of Adjuvant Chemotherapy Benefits in Patients With Nasopharyngeal Carcinoma. Oncologist (2015) 20(5):539–45. doi: 10.1634/theoncologist.2014-0291
102. Shen T, Tang LQ, Luo DH, Chen QY, Li PJ, Mai DM, et al. Different Prognostic Values of Plasma Epstein-Barr Virus DNA and Maximal Standardized Uptake Value of 18F-FDG PET/CT for Nasopharyngeal Carcinoma Patients With Recurrence. PloS One (2015) 10(4):e0122756. doi: 10.1371/journal.pone.0122756
103. Xiao W, Xu A, Han F, Lin X, Lu L, Shen G, et al. Positron Emission Tomography-Computed Tomography Before Treatment is Highly Prognostic of Distant Metastasis in Nasopharyngeal Carcinoma Patients After Intensity-Modulated Radiotherapy Treatment: A Prospective Study With Long-Term Follow-Up. Oral Oncol (2015) 51(4):363–9. doi: 10.1016/j.oraloncology.2015.01.009
104. Yoon HI, Kim KH, Lee J, Roh YH, Yun M, Cho BC, et al. The Clinical Usefulness of (18)F-Fluorodeoxyglucose Positron Emission Tomography (PET) to Predict Oncologic Outcomes and PET-Based Radiotherapeutic Considerations in Locally Advanced Nasopharyngeal Carcinoma. Cancer Res Treat (2016) 48(3):928–41. doi: 10.4143/crt.2015.275
105. Zhang Y, Li WF, Mao YP, Zhou GQ, Peng H, Sun Y, et al. Establishment of an Integrated Model Incorporating Standardised Uptake Value and N-Classification for Predicting Metastasis in Nasopharyngeal Carcinoma. Oncotarget (2016) 7(12):13612–20. doi: 10.18632/oncotarget.7253
106. Jeong Y, Baek S, Park JW, Joo JH, Kim JS, Lee SW. Lymph Node Standardized Uptake Values at Pre-Treatment (18)F-Fluorodeoxyglucose Positron Emission Tomography as a Valuable Prognostic Factor for Distant Metastasis in Nasopharyngeal Carcinoma. Br J Radiol (2017) 90(1071):20160239. doi: 10.1259/bjr.20160239
107. Jin YN, Yao JJ, Wang SY, Zhang WJ, Zhou GQ, Zhang F, et al. Prognostic Value of Primary Gross Tumor Volume and Standardized Uptake Value of (18)F-FDG in PET/CT for Distant Metastasis in Locoregionally Advanced Nasopharyngeal Carcinoma. Tumour Biol (2017) 39(7):1010428317717843. doi: 10.1177/1010428317717843
108. Lee SJ, Kay CS, Kim YS, Son SH, Kim M, Lee SW, et al. Prognostic Value of Nodal SUVmax of 18F-FDG PET/CT in Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy. Radiat Oncol J (2017) 35(4):306–16. doi: 10.3857/roj.2017.00115
109. Liu F, Xi XP, Wang H, Han YQ, Xiao F, Hu Y, et al. PET/CT-Guided Dose-Painting Versus CT-Based Intensity Modulated Radiation Therapy in Locoregional Advanced Nasopharyngeal Carcinoma. Radiat Oncol (2017) 12(1):15. doi: 10.1186/s13014-016-0739-y
110. Zhong L, Li C, Ren Y, Wu D. Prognostic Value of (18)F-Fluorodeoxyglucose PET Parameters and Inflammation in Patients With Nasopharyngeal Carcinoma. Oncol Lett (2017) 14(4):5004–12. doi: 10.3892/ol.2017.6816
111. Alessi A, Lorenzoni A, Cavallo A, Padovano B, Iacovelli NA, Bossi P, et al. Role of Pretreatment 18F-FDG PET/CT Parameters in Predicting Outcome of non-Endemic EBV DNA-Related Nasopharyngeal Cancer (NPC) Patients Treated With IMRT and Chemotherapy. Radiol Med (2019) 124(5):414–21. doi: 10.1007/s11547-018-0980-6
112. Fei Z, Chen C, Huang Y, Qiu X, Li Y, Li L, et al. Metabolic Tumor Volume and Conformal Radiotherapy Based on Prognostic PET/CT for Treatment of Nasopharyngeal Carcinoma. Med (Baltimore) (2019) 98(28):e16327. doi: 10.1097/MD.0000000000016327
113. Sun XS, Liang YJ, Liu SL, Chen QY, Guo SS, Wen YF, et al. Maximal Standard Uptake Values of (18)F-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography Compared With Epstein-Barr Virus DNA as Prognostic Indicators in De Novo Metastatic Nasopharyngeal Carcinoma Patients. BMC Cancer (2019) 19(1):908. doi: 10.1186/s12885-019-6106-2
114. Peng H, Chen L, Zhang Y, Guo R, Li WF, Mao YP, et al. Survival Analysis of Patients With Advanced-Stage Nasopharyngeal Carcinoma According to the Epstein-Barr Virus Status. Oncotarget (2016) 7(17):24208–16. doi: 10.18632/oncotarget.8144
115. Zhang J, Shu C, Song YL, Li QF, Huang JW, Ma XL. Epstein-Barr Virus DNA Level as a Novel Prognostic Factor in Nasopharyngeal Carcinoma: A Meta-Analysis. Medicine (2016) 95(40):e5130. doi: 10.1097/MD.0000000000005130
116. Zhang WN, Chen YP, Chen L, Guo R, Zhou GQ, Tang LL, et al. The Clinical Utility of Plasma Epstein-Barr Virus DNA Assays in Nasopharyngeal Carcinoma: The Dawn of a New Era? A Systematic Review and Meta-Analysis of 7836 Cases. Medicine (2015) 94(20):e845. doi: 10.1097/MD.0000000000000845
117. Qu H, Huang Y, Zhao S, Zhou Y, Lv W. Prognostic Value of Epstein-Barr Virus DNA Level for Nasopharyngeal Carcinoma: A Meta-Analysis of 8128 Cases. Eur Arch Otorhinolaryngol (2020) 277(1):9–18. doi: 10.1007/s00405-019-05699-9
118. Kim KY, Le Q-T, Yom SS, Pinsky BA, Bratman SV, Ng RHW. Current State of PCR-Based Epstein- Barr Virus DNA Testing for Nasopharyngeal Cancer. J Natl Cancer Inst (2017) 109(4). doi: 10.1093/jnci/djx007
119. Le QT, Zhang Q, Cao H, Cheng AJ, Pinsky BA, Hong RL, et al. An International Collaboration to Harmonize the Quantitative Plasma Epstein-Barr Virus DNA Assay for Future Biomarker-Guided Trials in Nasopharyngeal Carcinoma. Clin Cancer Res (2013) 19(8):2208–15. doi: 10.1158/1078-0432.CCR-12-3702
120. Nicholls JM, Lee VH, Chan SK, Tsang KC, Choi CW, Kwong DL, et al. Negative Plasma Epstein-Barr Virus DNA Nasopharyngeal Carcinoma in an Endemic Region and Its Influence on Liquid Biopsy Screening Programmes. Br J Cancer (2019) 121(8):690–8. doi: 10.1038/s41416-019-0575-6
121. Wu Z, Zeng RF, Su Y, Gu MF, Huang SM. Prognostic Significance of Tumor Volume in Patients With Nasopharyngeal Carcinoma Undergoing Intensity-Modulated Radiation Therapy. Head Neck (2013) 35(5):689–94. doi: 10.1002/hed.23010
122. Arens AI, Troost EG, Hoeben BA, Grootjans W, Lee JA, Gregoire V, et al. Semiautomatic Methods for Segmentation of the Proliferative Tumour Volume on Sequential FLT PET/CT Images in Head and Neck Carcinomas and Their Relation to Clinical Outcome. Eur J Nucl Med Mol Imaging (2014) 41(5):915–24. doi: 10.1007/s00259-013-2651-0
123. Bhatia KS, King AD, Yu KH, Vlantis AC, Tse GM, Mo FK, et al. Does Primary Tumour Volumetry Performed Early in the Course of Definitive Concomitant Chemoradiotherapy for Head and Neck Squamous Cell Carcinoma Improve Prediction of Primary Site Outcome? Br J Radiol (2010) 83(995):964–70. doi: 10.1259/bjr/27631720
124. Yin J, Qin Y, Luo YK, Feng M, Lang JY. Prognostic Value of Neutrophil-to-Lymphocyte Ratio for Nasopharyngeal Carcinoma: A Meta-Analysis. Med (Baltimore) (2017) 96(29):e7577. doi: 10.1097/MD.0000000000007577
125. Zhou GQ, Tang LL, Mao YP, Chen L, Li WF, Sun Y, et al. Baseline Serum Lactate Dehydrogenase Levels for Patients Treated With Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma: A Predictor of Poor Prognosis and Subsequent Liver Metastasis. Int J Radiat Oncol Biol Phys (2012) 82(3):e359–65.
126. Wan XB, Wei L, Li H, Dong M, Lin Q, Ma XK, et al. High Pretreatment Serum Lactate Dehydrogenase Level Correlates With Disease Relapse and Predicts an Inferior Outcome in Locally Advanced Nasopharyngeal Carcinoma. Eur J Cancer (2013) 49(10):2356–64. doi: 10.1016/j.ejca.2013.03.008
127. Turen S, Ozyar E, Altundag K, Gullu I, Atahan IL. Serum Lactate Dehydrogenase Level is a Prognostic Factor in Patients With Locoregionally Advanced Nasopharyngeal Carcinoma Treated With Chemoradiotherapy. Cancer Invest (2007) 25(5):315–21. doi: 10.1080/07357900701209103
128. Tang XR, Li YQ, Liang SB, Jiang W, Liu F, Ge WX, et al. Development and Validation of a Gene Expression-Based Signature to Predict Distant Metastasis in Locoregionally Advanced Nasopharyngeal Carcinoma: A Retrospective, Multicentre, Cohort Study. Lancet Oncol (2018) 19(3):382–93. doi: 10.1016/S1470-2045(18)30080-9
129. Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX, et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst (2016) 108(1):djv291. doi: 10.1093/jnci/djv291
130. Xia WX, Zhang HB, Shi JL, Lu X, Wang L, Ye YF, et al. A Prognostic Model Predicts the Risk of Distant Metastasis and Death for Patients With Nasopharyngeal Carcinoma Based on Pre-Treatment Serum C-Reactive Protein and N-Classification. Eur J Cancer (2013) 49(9):2152–60. doi: 10.1016/j.ejca.2013.03.003
Keywords: nasopharyngeal carcinoma, prognostic factors, AJCC/UICC staging system, TMN classification, systematic review, anatomical criteria
Citation: Chiang CL, Guo Q, Ng WT, Lin S, Ma TSW, Xu Z, Xiao Y, Li J, Lu T, Choi HCW, Chen W, Chau ESC, Luk PHY, Huang SH, O’Sullivan B, Pan J and Lee AWM (2021) Prognostic Factors for Overall Survival in Nasopharyngeal Cancer and Implication for TNM Staging by UICC: A Systematic Review of the Literature. Front. Oncol. 11:703995. doi: 10.3389/fonc.2021.703995
Received: 01 May 2021; Accepted: 29 July 2021;
Published: 02 September 2021.
Edited by:
Yong Yin, Shandong Cancer Hospital, ChinaReviewed by:
Maria Grazia Ghi, Veneto Institute of Oncology (IRCCS), ItalyQiqi Xie, Affiliated Hospital of Qinghai University, China
Copyright © 2021 Chiang, Guo, Ng, Lin, Ma, Xu, Xiao, Li, Lu, Choi, Chen, Chau, Luk, Huang, O’Sullivan, Pan and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Leung Chiang, Y2hpYW5nY2xAaGt1Lmhr; Anne Wing Mui Lee, YXdtbGVlQGhrdS5oaw==
 Chi Leung Chiang
Chi Leung Chiang Qiaojuan Guo3
Qiaojuan Guo3 Wai Tong Ng
Wai Tong Ng Shaojun Lin
Shaojun Lin Youping Xiao
Youping Xiao Tianzhu Lu
Tianzhu Lu Horace Cheuk Wai Choi
Horace Cheuk Wai Choi Peter Ho Yin Luk
Peter Ho Yin Luk Shao Hui Huang
Shao Hui Huang Brian O’Sullivan
Brian O’Sullivan Jianji Pan
Jianji Pan Anne Wing Mui Lee
Anne Wing Mui Lee