- 1Department of Hematology, Fujian Institute of Hematology, Fujian Provincial Key Laboratory on Hematology, Fujian Medical University Union Hospital, Fuzhou, China
- 2Department of Pediatrics, Southern Medical University/Nanfang Hospital, Guangzhou, China
- 3Nanfang-Chunfu Children’s Institute of Hematology & Oncology, TaiXin Hospital, Dongguan, China
- 4Hematology and Oncology, Hunan Children’s Hospital, Changsha, China
- 5Department of Pediatric Hematology & Oncology, Sun Yat-sen Memorial Hospital, Guangzhou, China
- 6Department of Pediatric Hematology/Oncology, Guangzhou Women and Children’s Medical Center, Guangzhou, China
- 7Pediatrics, People’s Hospital of Hunan Province, Changsha, China
Background: A high ecotropic viral integration site 1 (EVI1) expression (EVI1high) is an independent prognostic factor in adult acute myeloid leukemia (AML). However, little is known of the prognostic value of EVI1high in pediatric AML. This study aimed to examine the biological and prognostic significance of EVI1high in uniformly treated pediatric patients with AML from a large cohort of seven centers in China.
Methods: A diagnostic assay was developed to determine the relative EVI1 expression using a single real-time quantitative polymerase chain reaction in 421 newly diagnosed pediatric AML patients younger than 14 years from seven centers in southern China. All patients were treated with a uniform protocol, but only 383 patients were evaluated for their treatment response. The survival data were included in the subsequent analysis (n = 35 for EVI1high, n = 348 for EVI1low).
Results: EVI1high was found in 9.0% of all 421 pediatric patients with de novo AML. EVI1high was predominantly found in acute megakaryoblastic leukemia (FAB M7), MLL rearrangements, and unfavorable cytogenetic aberrance, whereas it was mutually exclusive with t (8; 21), inv (16)/t (16; 16), CEBPA, NPM1, or C-KIT mutations. In the univariate Cox regression analysis, EVI1high had a significantly adverse 5-year event-free survival (EFS) and overall survival (OS) [hazard ratio (HR) = 1.821 and 2.401, p = 0.036 and 0.005, respectively]. In the multivariate Cox regression analysis, EVI1high was an independent prognostic factor for the OS (HR = 2.447, p = 0.015) but not EFS (HR = 1.556, p = 0.174). Furthermore, EVI1high was an independent adverse predictor of the OS and EFS of patients with MLL rearrangements (univariate analysis: HR = 9.921 and 7.253, both p < 0.001; multivariate analysis: HR = 7.186 and 7.315, p = 0.005 and 0.001, respectively). Hematopoietic stem cell transplantation (HSCT) in first complete remission (CR1) provided EVI1high patients with a tendential survival benefit when compared with chemotherapy as a consolidation (5-year EFS: 68.4% vs. 50.8%, p = 0.26; 5-year OS: 65.9% vs. 54.8%, p = 0.45).
Conclusion: It could be concluded that EVI1high can be detected in approximately 10% of pediatric AML cases. It is predominantly present in unfavorable cytogenetic subtypes and predicts adverse outcomes. Whether pediatric patients with EVI1high AML can benefit from HSCT in CR1 needs to be researched further.
1 Introduction
Acute myeloid leukemia (AML) accounts for approximately 20% of pediatric leukemia diagnoses, and its long-term survival rate has dramatically increased from less than 20% to approximately 70% in the past 50 years (1). One of the most important reasons for these dramatic improvements is the accurate prediction of the prognosis to initiate the appropriate therapy regimens. Cytogenetic abnormalities and gene mutations are the classical and most important framework for risk stratification (2, 3). In addition to genetic alterations, aberrant overexpression of specific genes may also serve as biomarkers to evaluate the risk of treatment failure or relapse; ecotropic viral integration site 1 (EVI1) is a representative of this group (4, 5).
The EVI1 gene encodes a zinc-finger protein that functions as a transcription factor essential for hematopoietic stem cell (HSC) proliferation and differentiation, and is located on chromosome 3q26 (6). Aberrantly high EVI1 expression (EVI1high) plays an important role in the pathogenesis of hematological malignancies, including AML, chronic myeloid leukemia, and myelodysplastic syndrome (MDS) (7). In adult AML, EVI1high is frequently associated with cytogenetic abnormalities of 3q, especially 3q26, whereas in pediatric AML, it is rarely correlated with the cytogenetic rearrangements of this locus (8). In pediatric AML, EVI1high is commonly found together with mixed lineage leukemia (MLL) rearrangements, which indicates that the pathogenetic and prognostic significance of EVI1high may be different between adult and pediatric patients with AML (9, 10).
In the past decade, several studies have shown that EVI1high is a poor independent prognostic predictor for the complete remission (CR), overall survival (OS), relapse-free (RFS), and event-free survival (EFS) in adult AML, irrespective of the cytogenetic abnormalities of 3q (8). However, few studies have examined how EVI1high affects the prognosis in pediatric AML, and some of its effects are contradictory to those of adult AML. Using multivariate analysis, Balgobind et al. (10) and Ho et al. (9) reported that EVI1high had a significantly lower EFS but was not independently associated with an inferior OS and EFS in pediatric AML. In consideration of the inconsistent conclusions, the aim of our study was to examine the biological and prognostic significance of EVI1high in uniformly treated pediatric AML patients from a large cohort of seven centers in China.
2 Material and Methods
2.1 Patients and Treatment
A total of 421 newly diagnosed pediatric patients with AML (≤14 years) were enrolled in this retrospective study. Patients with acute promyelocytic leukemia, secondary AML, constitutional trisomy 21, or antecedent MDS were excluded. These patients were consecutively diagnosed at seven centers in southern China between January 2015 and December 2020. Morphological, flow cytometric, cytogenetic, and molecular analyses were performed on all patients at diagnosis, and the results were available for all patients included in this study. AML was diagnosed and classified according to the World Health Organization (2016) classification (11).
All patients were treated using the C-HUANAN-AML15 protocol. In the C-HUANAN-AML15 protocol, two tandem courses of the FLAG-IDA or DAE regimen were applied as induction chemotherapy. One course of homoharringtonine cytarabine/etoposide and one course of mitoxantrone/cytarabine in consolidation chemotherapy were uniformly administered to both groups. Intermediate-risk patients who had human leukocyte antigen (HLA) matched donors and high-risk patients were advised to undergo HSC transplantation (HSCT) in CR1. Details of the treatment protocols are provided in the Supplementary Data section. This study was approved by the ethics committee of all seven centers. All patients and volunteers provided a written informed consent, in accordance with the Declaration of Helsinki, to participate in the present study.
2.2 Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from nucleated cells of the bone marrow using the Trizol Reagent (Invitrogen, Carlsbad, CA, USA), and subsequent reverse transcription was performed on 1 μg of the total diagnostic RNA using the standard protocol (Invitrogen, Carlsbad, CA, USA). EVI1 expression was measured by qRT-PCR using the TaqMan Universal PCR Master Mix and TaqMan EVI1 Gene Expression Assay (Applied Biosystems, Foster City, CA) with a primer/probe set designed to hybridize within a region spanning exons 2 and 3, as follows:
Forward primer: 5’-GTACTTGAGCCAGCTTCCAACA-3’ (in exon 3)
Reverse primer: 5’-CTTCTTGACTAAAGCCCTTGGA-3’ (in exon 2)
Probe: 5’-FAM-TCTTAGACGAATTTTACAATGTGAAGTTCTGCATAGATG-TAMRA-3’ (in exon 3).
Abelson murine leukemia viral oncogene homolog (ABL) was used as a control gene, and the corresponding primers and probes were based on a report from the Europe Against Cancer Program (12). The primer of EVI1 can detect the expression of the total EVI1 (including EVI1-1A, 1B, 1C, and 1D), and the MDS1 and EVI1 complex fusion transcript. A total of 15 bone marrow samples from healthy donors were processed as calibrators for quantification. EVI1 transcript levels were calculated as the percentage of the target transcript copies/ABL copies. The mean value of EVI1 in the 15 normal controls was considered as the baseline. Relative quantification was performed using the 2-△△Ct method (13). The relative expression level of EVI1 is expressed as a percentage. The percentile of the expression level in all EVI1 test results in the present study was used as a percentage. EVI1 expression levels were dichotomized based on a cutoff value of 75% (approximately 10 times the baseline), according to Santamaría et al. (14). A total of 38 patients were defined as having EVI1high and the remainder as EVI1low.
2.3 Statistical Analysis
Continuous variables of patient characteristics were compared using the Wilcoxon rank-sum test (abnormal distribution) or Mann–Whitney U test (normal distribution), while categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test when data were sparse. A total of 38 children (3 children with EVI1high and 35 children with EVI1low), including those that were lost during the follow-up (n = 21), discontinued the treatment (n = 12), or were transferred (n = 5) before completing two courses of chemotherapy or were excluded from the survival analysis. The cutoff date for follow-up was February 28, 2021. OS endpoints were death (failure) and being alive at the last follow-up (censored), measured from the onset to the start of chemotherapy. EFS endpoints were disease relapse or death from any cause, measured from the onset to the start of chemotherapy. Distribution estimations and survival distributions of the OS and EFS were calculated using the Kaplan-Meier method and log-rank test, respectively. Univariate analyses were performed using the unadjusted Cox proportional hazards model to calculate the hazard ratios (HRs). Variables that were significant in the univariate analyses were included in the multivariate analyses. Multivariate analyses were performed using the Cox proportional hazards model to identify the independent prognostic factors. All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Software Environment R, version 4.0.4.
3 Results
3.1 Clinical Characteristics of Pediatric Patients With Acute Myeloid Leukemia (AML) and a High Ecotropic Viral Integration Site 1 Expression (EVI1high)
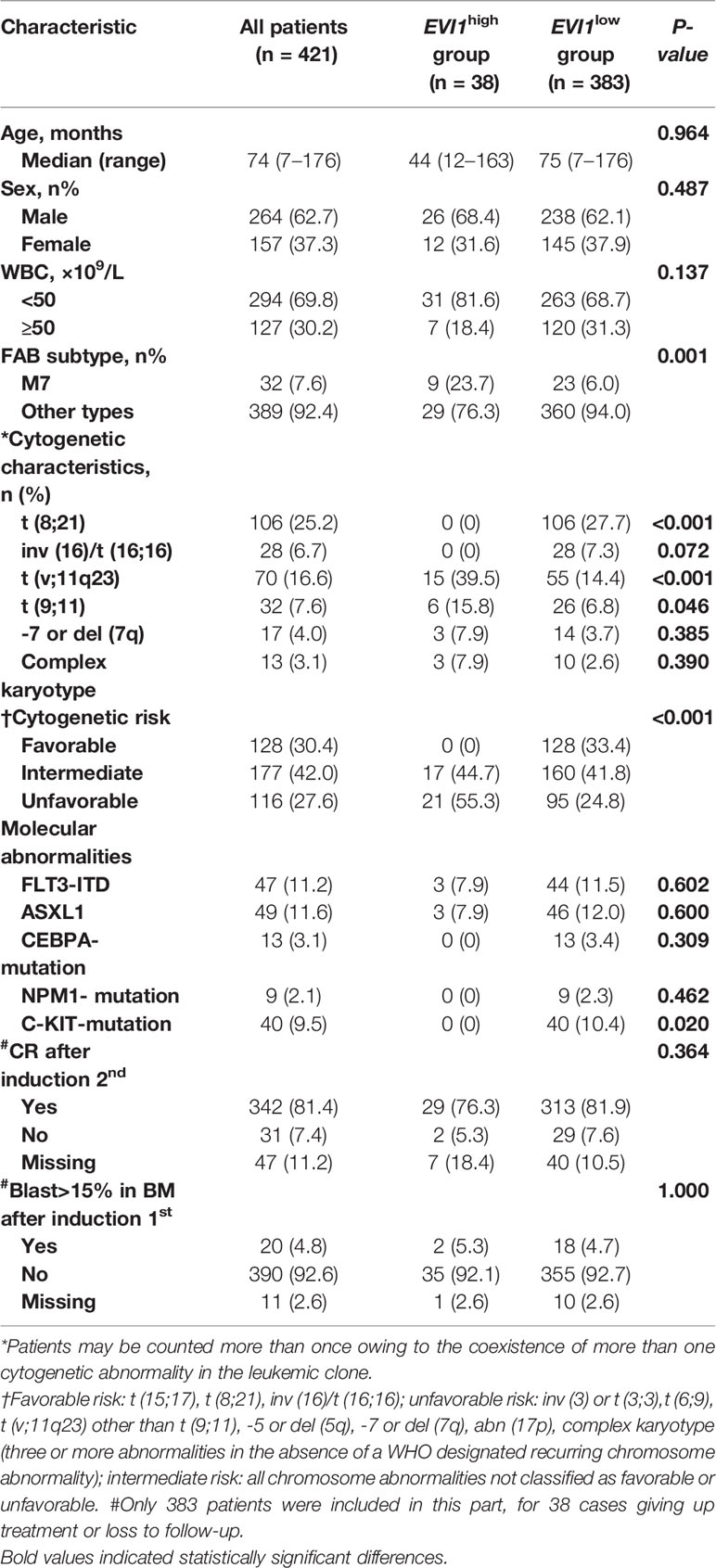
The clinical features of all 421 patients are summarized in Table 1. The median age was 74 months (range, 7–176 months), and 12 patients had infant AML. The most common cytogenetic changes included t (8; 21) and 11q23 chromosome abnormality. In 47/421 (11.2%) patients, FLT3-ITD mutations were detected, while ASXL1 mutations occurred in 11.6%. EVI1high, which was found in 9.0% (38/421) of all the patients, was not detected in infant patients. EVI1high was not correlated with age, sex, or white blood cells (WBC), whereas patients with EVI1high had a significantly higher frequency of (1) acute megakaryoblastic leukemia (FAB-M7) (23.7% vs. 6.0%, p = 0.001) (2), MLL rearrangements (39.5% vs. 14.4%, p < 0.0001), especially MLL-AF9 (15.8% vs. 6.8%, p = 0.046) (3); unfavorable cytogenetic aberrance (55.3% vs. 24.8%, p < 0.0001), but only one patient harbored a 3q26 rearrangement. EVI1high was not found in the favorable subtypes, including t (8;21) and inv (16). We also studied EVI1high in relation to five common single-gene mutations. Three patients with EVI1high had an internal tandem duplication of FMS-like tyrosine kinase 3 (FLT3-ITD), and three patients with EVI1high had an ASXL1 mutation; this association was not statistically significant compared to patients with EVI1low. EVI1high was not found in patients with NPM1, C-KIT, or CEBPA-biallelic mutations.
3.2 Survival Analysis of Whole Pediatric Acute Myeloid Leukemia (AML)
An evaluation of the treatment response and the survival data were only available for 383 patients, including 35 patients with EVI1high, because some of them discontinued treatment or were lost to follow-up. The median follow-up time for survival was 32.5 (range, 2.6–134.1) months. Of the 383 cases, the CR rate was 85.6% (328/383) and 91.7% (342/373) after the first and second courses of induction, respectively; the relapse rate was 19.5% (64/328) and the chemotherapy-related mortality was 4.7% (18/383). The 5-year EFS and OS were 66.7% and 75.2%, respectively.
3.3 Survival Analysis of Pediatric Acute Myeloid Leukemia (AML) With a High Ecotropic Viral Integration Site 1 Expression (EVI1high)
For the 35 patients with EVI1high, 32 patients (91.4%) achieved CR and 26 (74.3%) patients achieved MRD negative after the first course of induction, whereas 27 patients (93.1%) achieved CR and 25 (86.2%) patients achieved MRD negative after the second course of induction, respectively; the relapse rate was 20% (7/35) and the chemotherapy-related mortality was 2.9% (1/35).
The proportion of patients with bone marrow blasts >15% after the first course of induction, and CR rate after the second course of induction was not statistically different between the EVI1high and EVI1low subgroups. Patients with EVI1high had a significantly worse 5-year EFS and OS than those with EVI1low (EFS: 51.7% vs. 68.1%, p = 0.041; OS: 53.1% vs. 77.0%, p = 0.041) (Figure 1).
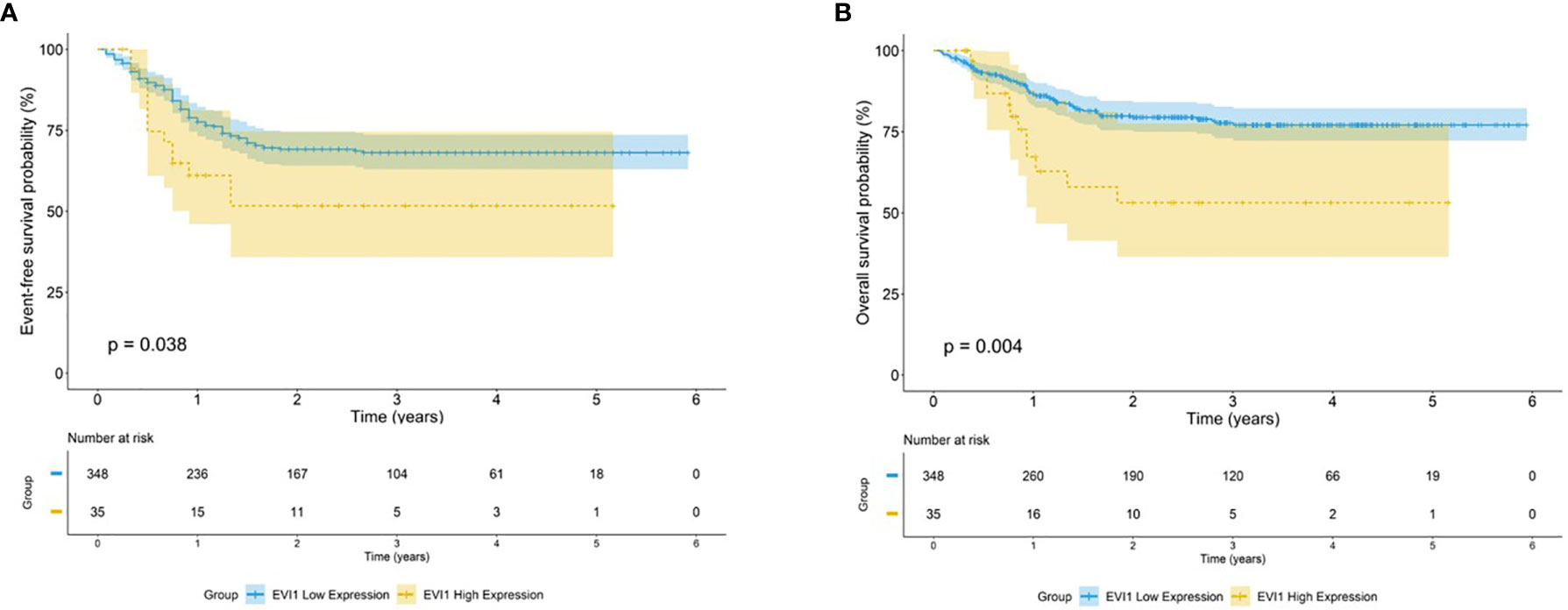
Figure 1 Survival outcome for high EVI1 expression in pediatric AML. Kaplan–Meier curve estimates for the (A) EFS and (B) OS in the total cohort between EVI1high and EVI1low patients.
Risk factors, including age, sex, WBC, FAB type, risk category, and genetic abnormalities, were evaluated using a univariate Cox analysis (Figure 2). EVI1high was a significant factor for the decreased EFS and OS (HR = 1.821 and 2.401, p = 0.036 and 0.005, respectively). In addition, poor prognostic predictors also included WBC ≥ 50 × 109/L, risk category, FLT3-ITD mutation, failure to achieve CR after the second course of induction, and the proportion of bone marrow blasts higher than 15% after the first course of induction (all HR > 1 and p < 0.05). In contrast, an AML1-ETO as a favorable predictor of improved the EFS and OS (HR = 0.442 and 0.465, p = 0.002 and 0.015, respectively).
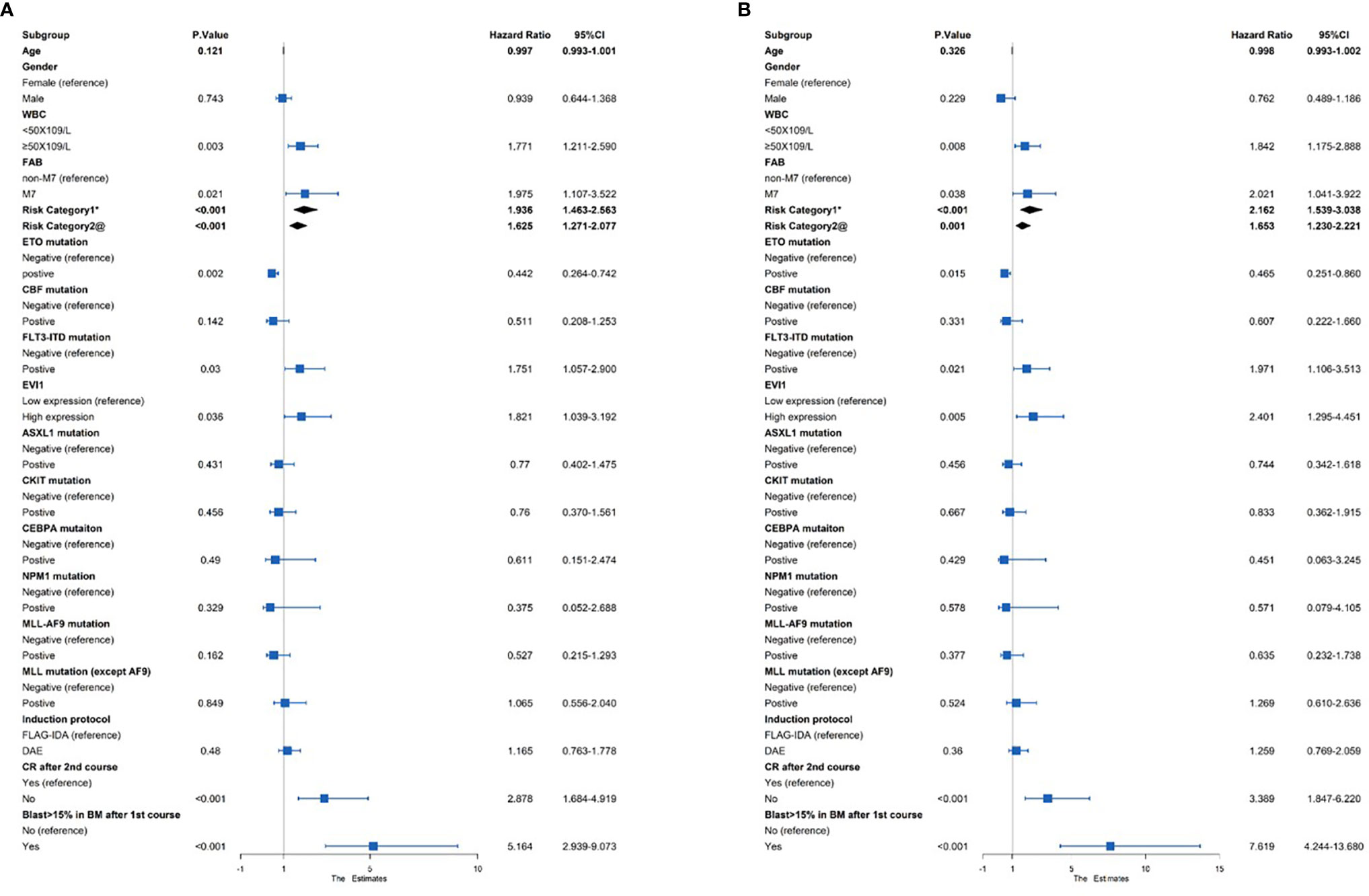
Figure 2 Univariate Cox regression analysis of the (A) EFS and (B) OS among 383 pediatric AML patients. *Risk category based on treatment regimens. Refer to Supplementary Table S1. @Risk category based on cytogenetic stratification of ELN 2017.
A multivariate Cox analysis was then performed to evaluate the independent prognostic factors (Figure 3). The results showed that EVI1high significantly affected the OS (HR = 2.447, p = 0.015) but not EFS (HR = 1.556, p = 0.174). Similarly, the risk category based on the treatment program was a poor independent prognostic predictor for the OS (HR = 1.759, p = 0.033) and tendentially predicted a worse EFS (HR = 1.569, p = 0.058). A WBC count ≥ 50 × 109/L and a proportion of bone marrow blasts greater than 15% after the first course were independent risk predictors for the OS and EFS (all HR > 1 and p < 0.05). However, failure to achieve CR after two courses of induction treatment was an independent prognostic factor for an inferior EFS (HR = 1.915, p = 0.047), excluding the OS (HR = 1.591, p = 0.25).
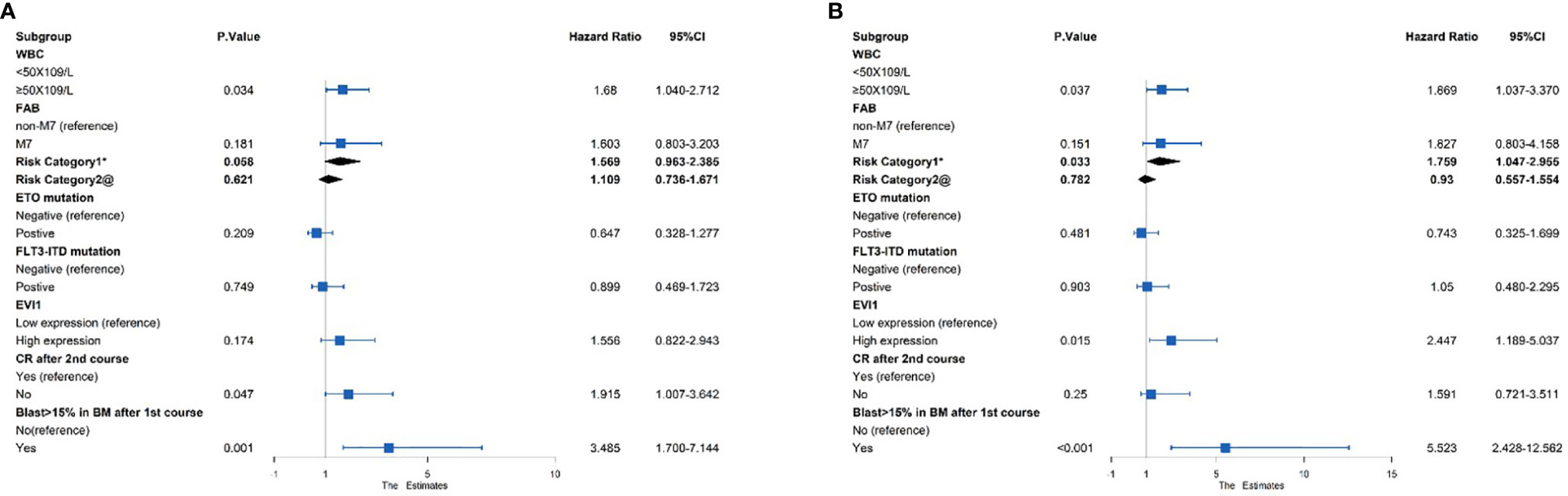
Figure 3 Multivariate Cox regression analysis of the (A) EFS and (B) OS among 383 pediatric AML patients. *Risk category based on treatment regimens. Refer to Supplementary Table S1. @Risk category based on cytogenetic stratification of ELN 2017.
3.4 Characteristics and Prognostic Value of High Ecotropic Viral Integration Site 1 Expression (EVI1high) in Patients With MLL Rearrangements
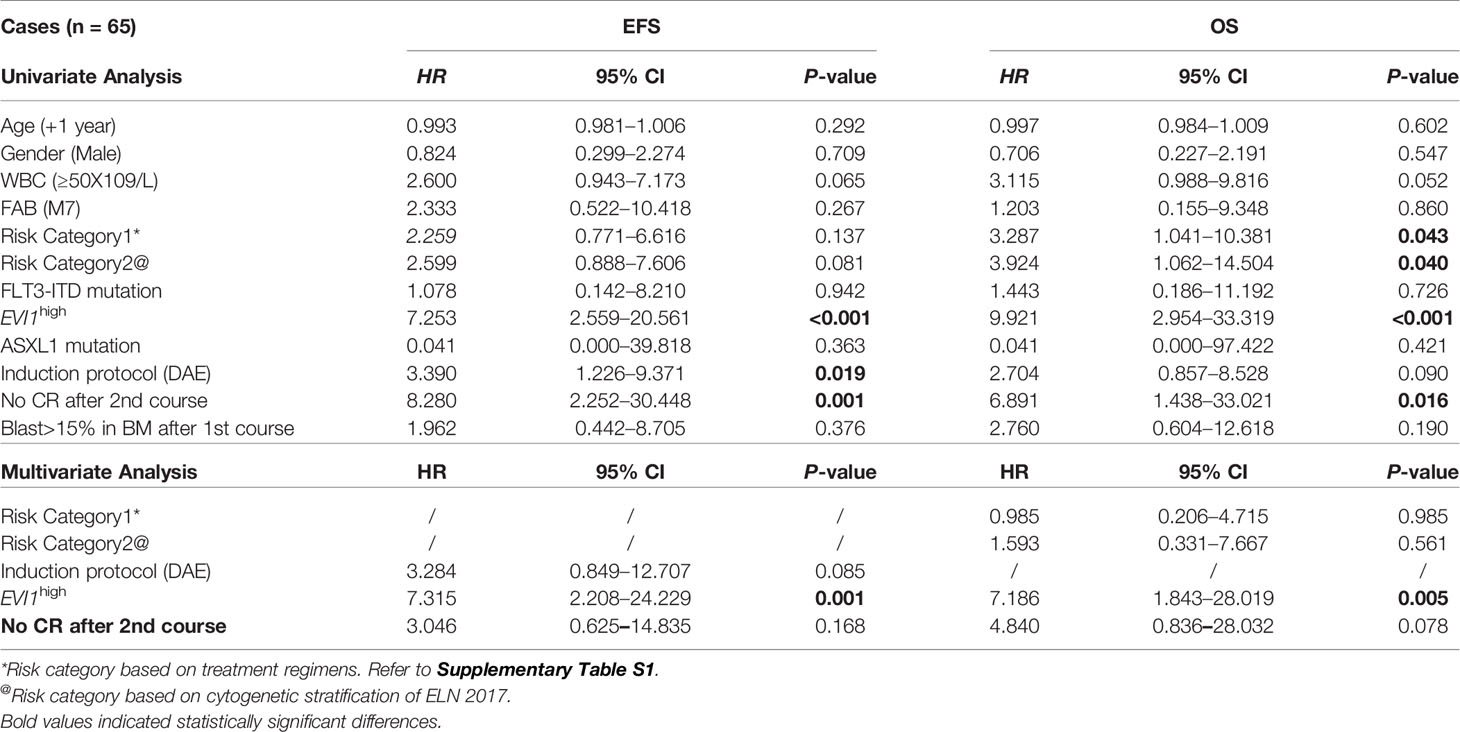
As noted above, EVI1high was associated with MLL rearrangement. EVI1high was detected in 21.4% (15/70) of all patients with MLL rearrangements and 18.8% (6/32) of patients with MLL-AF9. The characteristics of patients with MLL rearrangements categorized according to the EVI1 status are shown in Supplementary Table S2. EVI1high was significantly correlated to an unfavorable cytogenetic aberrance (73.3% vs. 38.2%, both p < 0.02). EVI1high had a significantly adverse 5-year EFS and OS in all patients with MLL rearrangements (EFS: 34.5% vs. 84.5%, OS: 34.8% vs. 89.7%, both p < 0.0001) (Figure 4). The same conclusion was found in patients with MLL-AF9 (EFS: 50.0% vs. 87.7%, p = 0.013; OS: 50.0% vs. 93.7%, p = 0.0035) (Figure 4).
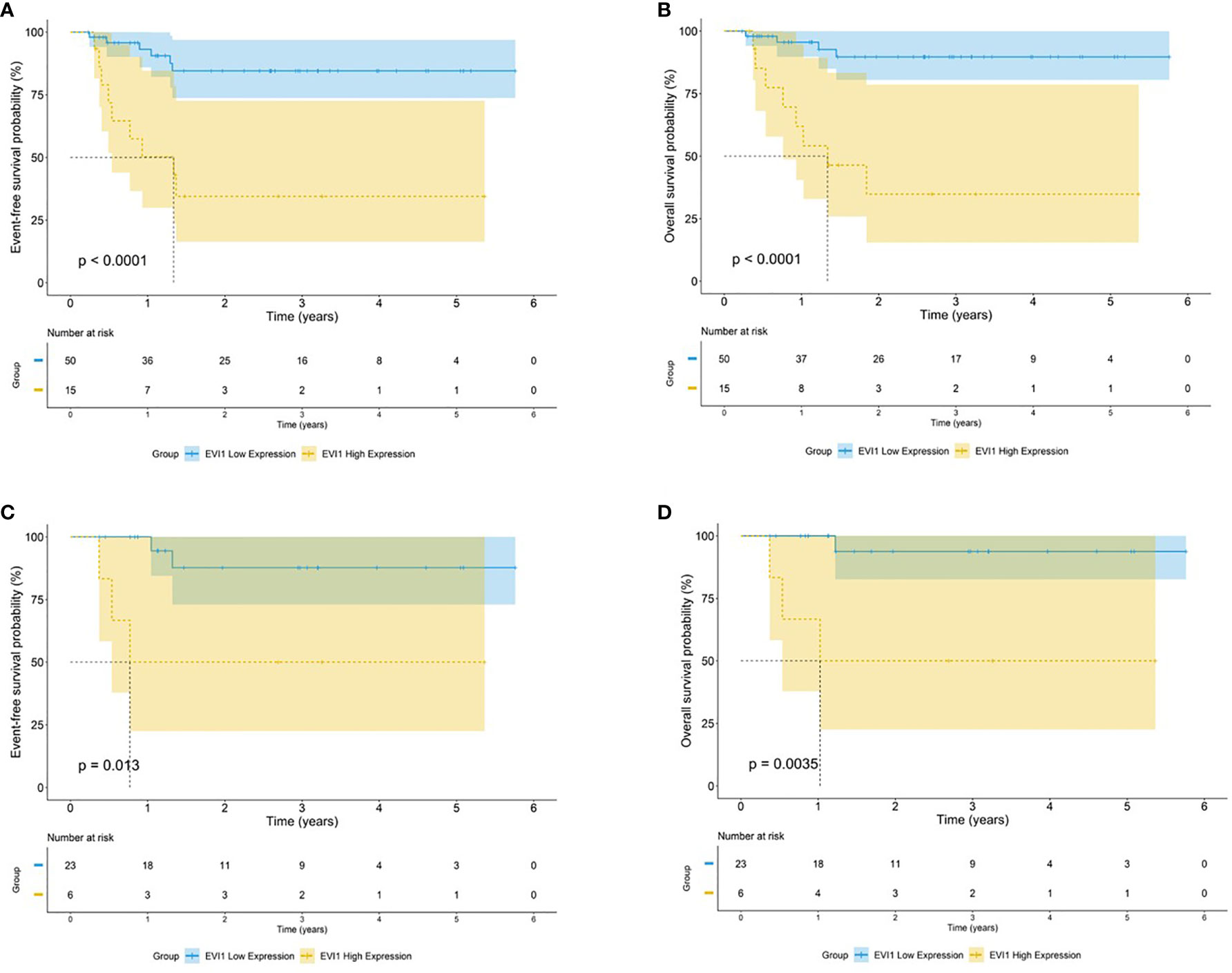
Figure 4 Survival outcomes by EVI1 expression among patients carrying t(v;11). Kaplan–Meier curve estimates for the (A) EFS and (B) OS in the cohort of MLL rearranged AML between EVI1high and EVI1low patients. Kaplan–Meier curve estimates for the (C) EFS and (D) OS in the cohort of MLL-AF9 rearranged AML between EVI1high and EVI1low patients.
A Cox regression analysis was performed. Only 65 patients with MLL rearrangements were included. The results revealed that EVI1high was an independent adverse predictor of the OS and EFS in patients with MLL rearrangements (univariate analysis: HR = 9.921 and 7.253, both p < 0.001; multivariate analysis: HR = 7.186 and 7.315, p = 0.005 and 0.001, respectively) (Table 2). Among the AML patients with MLL-AF9, significant differences in the OS and EFS were observed in the univariate analysis (HR = 13.349 and 7.112, p = 0.025 and 0.032, respectively); however, multivariate analysis did not identify EVI1high as an independent prognostic factor for the OS and EFS (HR = 13.056 and 10.091, p = 0.060 and 0.066, respectively; Table S2).
3.5 The Impact of a Different Induction Regimen and Effect of Hematopoietic Stem Cell Transplantation (HSCT) after First Complete Remission (CR1) in Patients With a High Ecotropic Viral Integration Site 1 Expression (EVI1high)
Of the 35 patients with EVI1high who underwent an evaluation of the treatment response and survival data, the CR rate after the first course of chemotherapy of the 26 patients who received FLAG-IDA induction was significantly higher than that of the remaining 9 patients who received DAE induction (100% vs. 66%, p = 0.013). The Kaplan-Meier analysis showed that the FLAG-IDA group had a trend for a better 5-year EFS and OS, without a significant statistical difference (EFS: 62.7% vs. 41.7%, p = 0.37; OS: 65.7% vs. 38.9%, p = 0.19). A total of 14 patients with EVI1high who underwent HSCT after CR1 had a better 5-year EFS and OS, but this difference was not statistically different (EFS: 68.4% vs. 50.8%, p = 0.26; OS: 65.9% vs. 54.8%, p = 0.45) (Figure 5).
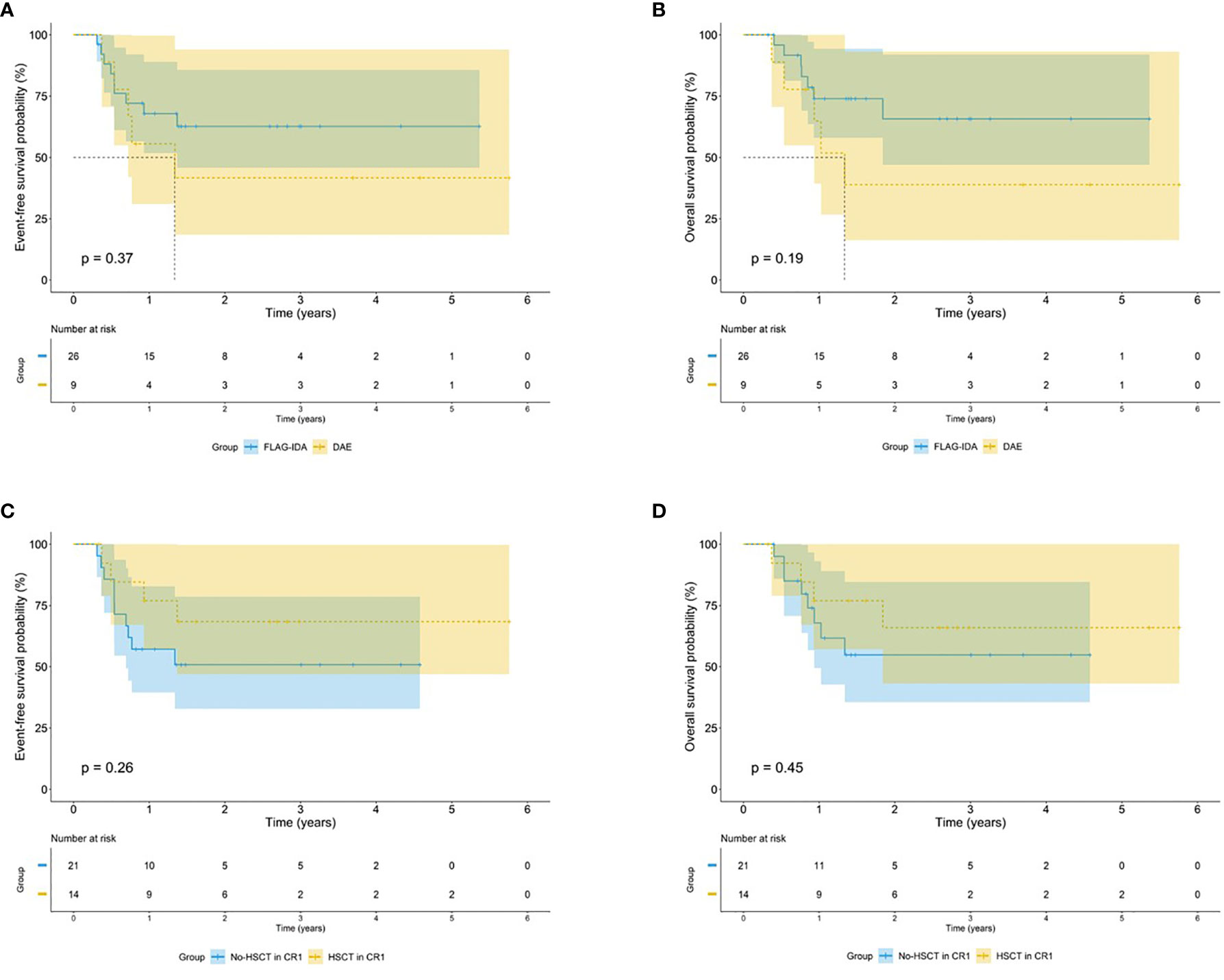
Figure 5 Survival outcomes by treatment regimens among EVI1high patients. Kaplan–Meier curve estimates the impact of different induction regimens on the (A) EFS and (B) OS in the cohort of EVI1high patients. Kaplan–Meier curve estimates the impact of HSCT after CR1 on the (C) EFS and (D) OS in the cohort of EVI1high patients.
4 Discussion
In the present study, we retrospectively analyzed the characteristics and prognostic value of EVI1high in pediatric patients with AML. Our study enrolled pediatric patients aged 7–176 months in a large cohort of 421 pediatric patients with AML who received uniform treatments from multiple centers, with the inclusion of the respective FAB subtypes, cytogenetic characteristics, and molecular genetic characteristics, which are broadly representative. This study showed that EVI1high significantly correlated with unfavorable types, including MLL rearrangements, FAB-M7, and a complex karyotype, but was mutually exclusive for favorable types, including t (8;21), inv (16), an NPM1-mutation, and CEBPA-biallelic mutations. Furthermore, our results demonstrate that EVI1high is a poor independent prognostic factor for the survival in pediatric AML, especially in patients with MLL rearrangements. In view of its significant correlation with the survival, EVI1high is expected to be an excellent prognostic marker.
In this cohort, EVI1high was detected in 9.0% of cases, which was consistent with a previous pediatric study by Balgobind et al. (10), and several adult studies that indicated a percentage of 6%–11% (15, 16). However, the prevalence of EVI1high in our study was lower than the 28% reported by Ho et al. (9) (58/206) and 16% reported by Jo et al. (17) (21/130). This difference may reflect the distinct definitions in the studies, including the detection method, the cutoff value selection method, and the control gene used for normalization. In the study of Balgobind et al. (10), the cumulative relative expression of EVI1-1A, -1B, and -3L to a GAPDH above 1.5% is consistent with our definition of EVI1high, showing the highest correlation with EVI1high cases based on the gene expression profiling; all normal bone marrow samples were below this threshold. In the study of Ho et al. (9), beta glucuronidase was quantified as an internal control, and EVI1high was defined as the cumulative relative expression of the total EVI1 (including EVI1-1A, 1B, 1C, and 1D), and the MDS1 and EVI1 complex fusion transcript, which was >1.0-fold increase compared to the normal peripheral blood controls. In the study of Jo et al. (17), patients with an EVI1/ABL1 ratio higher than 0.1 were defined as EVI1high. In our study, to avoid the inclusion of false-positive cases, patients were defined as EVI1high with an expression level of 10 or higher compared to a pool of 15 healthy bone marrow controls. The definition of EVI1high is still debatable, as the AML patient groups selected by the different definitions vary in size and may have an inferior prognosis. Thus, the studies may not be directly comparable, and standardization of EVI1 transcript testing and reporting is required.
Previous studies have indicated that EVI1high is strongly associated with specific genetic and morphological subtypes in both pediatric and adult AML (9, 10, 15, 17), and the similarities and differences regarding EVI1high in pediatric vs. adult AML are shown in Supplementary Table S4. EVI1high in adult AML is frequently associated with and presumed to directly result from alterations in 3q26. In contrast, we detected only one patient with chromosomal rearrangements of 3q26 in our study. In pediatric AML, 3q26 abnormalities are rare. In the study of Balgobind et al., no 3q26 abnormalities were identified in pediatric AML patients with EVI1 overexpression (10). A similar result was shown by the research of Jo et al., and the 3q26 abnormality was not detected either at the level of conventional cytogenetics or cryptically in the whole genome and transcriptome sequencing data (17). The mechanisms of EVI1 overexpression in pediatric AML appear to be different from that of adult patients. We speculate that EVI1 overexpression may not be the driving factor in pediatric AML, but a secondary event after leukemogenesis.
All patients with EVI1high in this study belonged to the intermediate- or high-risk cytogenetic/molecular aberrance group based on the current risk stratification (3, 18), as they showed MLL rearrangements and complex karyotypes. Furthermore, consistent with previous pediatric studies (9, 10, 17), EVI1high was also significantly associated with a FAB class M7 unrelated to trisomy 21, which has been reported to confer a poor prognosis in pediatric AML (19, 20), while EVI1high had a higher incidence of FAB-M5 in adult AML (21). Moreover, EVI1high was mutually exclusive with t (8;21), inv (16), NPM1-mutations, and CEBPA-biallelic mutations, representing favorable types of pediatric AML.
Although almost all relevant studies have shown an adverse impact of EVI1high on the therapeutic response and prognosis in adult AML (8, 15, 16), this was not completely consistent in pediatric AML. The reasons for these differences may lie with the therapy and prognosis between adults and children, but also in the different definitions of EVI1high and smaller sample sizes. In previous pediatric studies (9, 10, 17), EVI1high had significantly lower rates of EFS and OS but had no independent prognostic value for pediatric AML. In the present study, 421 pediatric patients with de novo AML were included, and patients with EVI1high had a significantly lower 5-year OS and EFS rates. Furthermore, EVI1high was also a significant predictor of a decreased OS and EFS in a separate univariate model and an independent prognostic factor for the OS but not EFS in a multivariate model including EVI1high and the aforementioned risk groups. To our knowledge, this study is the first to indicate that EVI1high has an independent prognostic value for pediatric AML.
MLL rearrangements are present in 15%–20% of pediatric patients with de novo AML and are associated with a poor prognosis (22). However, MLL rearrangements comprise a biologically and clinically heterogeneous group (23). A large international study of pediatric AML with MLL rearrangements identified specific translocations with prognostic associations (24). Previous studies have indicated that EVI1 is a transcriptional target of MLL oncoproteins in hematopoietic stem cells and plays a critical role in tumor growth in a subset of MLL-r AML (25, 26). In a report of adult AML, EVI1high was the sole prognostic factor for the inferior OS, RFS, and EFS in both patients with MLL-r AML and patients with MLL-AF9 (27). However, there have been inconsistent conclusions in the pediatric studies. Ho et al. (9) showed that EVI1high could not determine its prognostic value in pediatric AML with MLL rearrangements. Jo et al. (17) revealed that EVI1high was mainly detected and had a prognostic significance in myelomonocytic-lineage leukemia with MLL rearrangements. Matsuo et al. (28) also showed that EVI1high was an independent poor prognostic factor for the EFS but not OS in children with MLL-r AML. In the present study, EVI1high was associated with adverse EFS and OS in both the MLL-r and MLL-AF9 subgroups. A multivariate analysis identified EVI1high as an independent prognostic factor predicting a poor EFS and OS in the total cohort of MLL-r AML, but not in the MLL-AF9 subgroup, which may be caused by the smaller sample size.
At present, chemotherapy remains the front-line treatment for newly diagnosed pediatric AML, but the regimen for patients with EVI1high is not unified. Based on the medical research council AML15 trial (29), we have considered the FLAG-IDA or DAE regimen as induction chemotherapy. In the present study, the excellent CR rate of the FLAG-IDA regimen was significantly higher than that of the DAE regimen, which may indicate that the FLAG-IDA regimen is more suitable for induction in children with EVI1high. Studies on whether pediatric AML patients with EVI1high need to undergo HSCT in CR1 are few. However, EVI1high is predominantly present in unfavorable cytogenetic subtypes (high or intermediate risk) and predicts adverse outcomes for all AML patients (also for the MLL-r subtype). Furthermore, previous research has shown that HSCT significantly improved the prognosis of adult AML patients with EVI1high and those with the MLL-r subtype as well (15, 27). Moreover, patients with EVI1high who underwent HSCT after CR1 had a higher OS and EFS than those who only received chemotherapy. Therefore, we suggest that pediatric AML patients (also in MLL-r subtype) with EVI1high should undergo HSCT in the first CR. However, it is still essential to conduct prospective multicenter clinical studies including more patients to confirm whether HSCT could improve the long-term survival of pediatric patients with AML and EVI1high.
Moreover, novel therapeutic strategies effective for pediatric patients with EVI1high are also constantly being explored. Saito et al. (30) found that CD52 was highly expressed in most EVI1high leukemia cells, and humanized anti-CD52 monoclonal antibody CAMPATH-1H could inhibit cell growth and induce the apoptosis of EVI1high leukemia cells. These suggest that CAMPATH-1H may be effective in treating myeloid leukemia with EVI1high. However, the correlation between CD52 and EVI1high in AML patients still needs to be verified because CD52 is not tested routinely during flow cytometry for establishing a diagnosis. Furthermore, whether CAMPATH-1H is effective in treating myeloid leukemia with EVI1high also needs to be clarified in clinical studies. Mittal et al. (31) reported that EVI1-induced hypermethylation and downregulation of miR-9 play an important role in leukemogenesis in pediatric patients with AML and EVI1high, indicating that hypomethylating agents may be a potential therapeutic strategy for these patients. Nguyen et al. (32) showed that EVI1 plays an important role in the key properties of AML leukemic stem cells, and all-trans retinoic acid (ATRA) enhances the effects of EVI1 on AML stemness, thus, raising the possibility of using RAR antagonists in the therapy of EVI1high AML. However, Steinmetz et al. (33) and Verhagen et al. (34) demonstrated that primary AML cells with EVI1high were sensitive to ATRA, indicating that ATRA may be a candidate for patients with AML and EVI1high. As noted above, EVI1high was associated with MLL rearrangement. As a highly effective inhibitor targeting MLL, Menin reduced leukemia burden significantly and prolonged survival in in vivo experiments, which indicated that Menin may be effective in treating myeloid leukemia with EVI1high (35, 36).
The limitation of this study is that it was a retrospective study and only 35 patients expressed EVI1high, and it will be necessary to explore more effective treatments through prospective multicenter studies to improve long-term outcomes of pediatric patients with AML and EVI1high.
In conclusion, EVI1high is significantly associated with specific unfavorable cytogenetic (MLL rearrangements and complex karyotypes) and morphologic (FAB-M7) subtypes in pediatric AML. Furthermore, this study is the first to indicate that EVI1high is an independent adverse prognosis predictor for survival, especially in MLL-r AML. These results may be conducive to risk stratification and therapy decisions in pediatric patients with AML, and EVI1 transcript levels should be routinely assessed at diagnosis for risk stratification once a standard laboratory protocol is established and the cutoff value is determined.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committees from Fujian Medical University Union Hospital, Nanfang Hospital, TaiXin Hospital, Hunan Children’s Hospital, Sun Yat-sen Memorial Hospital, Guangzhou Women and Children’s Medical Center, and People’s Hospital of Hunan Province. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
YZ, JL, and JH conceived and designed the experiments. YZ performed the experiments. YZ, SL, HZ, XHu, ZC, XF, CL, MZ, HX, YHe, and XHe collected the clinical data. YZ and YHu analyzed and interpreted the data. YZ and YHu wrote the manuscript. JH critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Construction Project of the Fujian Medical Center of Hematology (Min201704), Startup fund of scientific research, Fujian medical university (2019QH1022, 2019QH1032).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our deepest gratitude to patients who donated samples for research purpose. We would like to express our sincere thanks to the doctors of the cooperative units for providing the clinical data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.712747/full#supplementary-material
Supplementary Figure 1 | C-HUANAN-AML15 protocol workflow. FLAG-IDA: Fludarabine 30 mg/m2/d d2-6, cytarabine 2g/m2/d d2-6, Idarubicin 8 mg/m2/d d4-6, Granulocyte Colony Stimulating Factor (G-CSF) 5μg/kg/d d1-7. DAE (3 + 10+5): Daunorubicin 50 mg/m2/d d1,3,5; Cytarabine 100mg/m2 q12h d1-10, Etoposide 100mg/m2/d d1-5. DAE (3 + 8+5): Daunorubicin 50 mg/m2/d d1,3,5; Cytarabine 100mg/m2 q12h d1-8, Etoposide 100mg/m2/d d1-5. HAE: Homoharringtonine 3mg/m2/d d1-5, Cytarabine 100mg/m2 q12h d1-7, Etoposide 100mg/m2/d d1-5. HHA: Homoharringtonine 3mg/m2/d d1-7, Cytarabine 2g/m2 q12h d1-3. MidAc: Mitoxantrone 10mg/m2/d d1-5, Cytarabine 1g/m2 q12h d1-3.
References
1. Kolb EA, Meshinchi S. Acute Myeloid Leukemia in Children and Adolescents: Identification of New Molecular Targets Brings Promise of New Therapies. Hematol Am Soc Hematol Educ Program (2015) 2015(1):507–13. doi: 10.1182/asheducation-2015.1.507
2. Taga T, Tomizawa D, Takahashi H, Adachi S. Acute Myeloid Leukemia in Children: Current Status and Future Directions. Pediatr Int (2016) 2:71–80. doi: 10.1111/ped.12865
3. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations From an International Expert Panel. Blood (2017) 4:424–47. doi: 10.1182/blood-2016-08-733196
4. Mawad R, Estey EH. Acute Myeloid Leukemia With Normal Cytogenetics. Curr Oncol Rep (2012) 5:359–68. doi: 10.1007/s11912-012-0252-x
5. Qin YZ, Zhao T, Zhu HH, Wang J, Jia JS, Lai YY, et al. High EVI1 Expression Predicts Poor Outcomes in Adult Acute Myeloid Leukemia Patients With Intermediate Cytogenetic Risk Receiving Chemotherapy. Med Sci Monit (2018) 24:758–67. doi: 10.12659/msm.905903
6. Kataoka K, Kurokawa M. Ecotropic Viral Integration Site 1, Stem Cell Self-Renewal and Leukemogenesis. Cancer Sci (2012) 8:1371–7. doi: 10.1111/j.1349-7006.2012.02303.x
7. Goyama S, Kurokawa M. Pathogenetic Significance of Ecotropic Viral Integration Site-1 in Hematological Malignancies. Cancer Sci (2009) 6:990–5. doi: 10.1111/j.1349-7006.2009.01152.x
8. Hinai AA, Valk PJ. Review: Aberrant EVI1 Expression in Acute Myeloid Leukaemia. Br J Haematol (2016) 6:870–8. doi: 10.1111/bjh.13898
9. Ho PA, Alonzo TA, Gerbing RB, Pollard JA, Hirsch B, Raimondi SC, et al. High EVI1 Expression is Associated With MLL Rearrangements and Predicts Decreased Survival in Paediatric Acute Myeloid Leukaemia: A Report From the Children's Oncology Group. Br J Haematol (2013) 5:670–7. doi: 10.1111/bjh.12444
10. Balgobind BV, Lugthart S, Hollink IH, Arentsen-Peters ST, van Wering ER, de Graaf SS, et al. EVI1 Overexpression in Distinct Subtypes of Pediatric Acute Myeloid Leukemia. Leukemia (2010) 5:942–9. doi: 10.1038/leu.2010.47
11. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood (2016) 20:2391–405. doi: 10.1182/blood-2016-03-643544
12. Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of Candidate Control Genes for Diagnosis and Residual Disease Detection in Leukemic Patients Using 'Real-Time' Quantitative Reverse-Transcriptase Polymerase Chain Reaction (RQ-PCR) - a Europe Against Cancer Program. Leukemia (2003) 12:2474–86. doi: 10.1038/sj.leu.2403136
13. Schmittgen TD, Livak KJ. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat Protoc (2008) 6:1101–8. doi: 10.1038/nprot.2008.73
14. Santamaría CM, Chillón MC, García-Sanz R, Pérez C, Caballero MD, Ramos F, et al. Molecular Stratification Model for Prognosis in Cytogenetically Normal Acute Myeloid Leukemia. Blood (2009) 1:148–52. doi: 10.1182/blood-2008-11-187724
15. Gröschel S, Lugthart S, Schlenk RF, Valk PJ, Eiwen K, Goudswaard C, et al. High EVI1 Expression Predicts Outcome in Younger Adult Patients With Acute Myeloid Leukemia and is Associated With Distinct Cytogenetic Abnormalities. J Clin Oncol (2010) 12:2101–7. doi: 10.1200/jco.2009.26.0646
16. Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck CA, Valk PJ, et al. High EVI1 Levels Predict Adverse Outcome in Acute Myeloid Leukemia: Prevalence of EVI1 Overexpression and Chromosome 3q26 Abnormalities Underestimated. Blood (2008) 8:4329–37. doi: 10.1182/blood-2007-10-119230
17. Jo A, Mitani S, Shiba N, Hayashi Y, Hara Y, Takahashi H, et al. High Expression of EVI1 and MEL1 is a Compelling Poor Prognostic Marker of Pediatric AML. Leukemia (2015) 5:1076–83. doi: 10.1038/leu.2015.5
18. Rau RE, Loh ML. Using Genomics to Define Pediatric Blood Cancers and Inform Practice. Hematol Am Soc Hematol Educ Program (2018) 1:286–300. doi: 10.1182/asheducation-2018.1.286
19. Teyssier AC, Lapillonne H, Pasquet M, Ballerini P, Baruchel A, Ducassou S, et al. Acute Megakaryoblastic Leukemia (Excluding Down Syndrome) Remains an Acute Myeloid Subgroup With Inferior Outcome in the French ELAM02 Trial. Pediatr Hematol Oncol (2017) 8:425–7. doi: 10.1080/08880018.2017.1414905
20. Schweitzer J, Zimmermann M, Rasche M, von Neuhoff C, Creutzig U, Dworzak M, et al. Improved Outcome of Pediatric Patients With Acute Megakaryoblastic Leukemia in the AML-BFM 04 Trial. Ann Hematol (2015) 8:1327–36. doi: 10.1007/s00277-015-2383-2
21. He X, Wang Q, Cen J, Qiu H, Sun A, Chen S, et al. Predictive Value of High EVI1 Expression in AML Patients Undergoing Myeloablative Allogeneic Hematopoietic Stem Cell Transplantation in First CR. Bone Marrow Transplant (2016) 7:921–7. doi: 10.1038/bmt.2016.71
22. Conneely S, Rau R. The Genomics of Acute Myeloid Leukemia in Children. Cancer Metastasis Rev (2020) 1:189–209. doi: 10.1007/s10555-020-09846-1
23. Winters A, Bernt K. MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches. Front Pediatr (2017) 4:4. doi: 10.3389/fped.2017.00004
24. Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel Prognostic Subgroups in Childhood 11q23/MLL-Rearranged Acute Myeloid Leukemia: Results of an International Retrospective Study. Blood (2009) 12:2489–96. doi: 10.1182/blood-2009-04-215152
25. Arai S, Yoshimi A, Shimabe M, Ichikawa M, Nakagawa M, Imai Y, et al. Evi-1 Is a Transcriptional Target of Mixed-Lineage Leukemia Oncoproteins in Hematopoietic Stem Cells. Blood (2011) 23:6304–14. doi: 10.1182/blood-2009-07-234310
26. Bindels EM, Havermans M, Lugthart S, Erpelinck C, Wocjtowicz E, Krivtsov AV, et al. EVI1 is Critical for the Pathogenesis of a Subset of MLL-AF9-Rearranged AMLs. Blood (2012) 24:5838–49. doi: 10.1182/blood-2011-11-393827
27. Gröschel S, Schlenk RF, Engelmann J, Rockova V, Teleanu V, Kühn MW, et al. Deregulated Expression of EVI1 Defines a Poor Prognostic Subset of MLL-Rearranged Acute Myeloid Leukemias: A Study of the German-Austrian Acute Myeloid Leukemia Study Group and the Dutch-Belgian-Swiss HOVON/SAKK Cooperative Group. J Clin Oncol (2013) 1:95–103. doi: 10.1200/jco.2011.41.5505
28. Matsuo H, Kajihara M, Tomizawa D, Watanabe T, Saito AM, Fujimoto J, et al. EVI1 Overexpression is a Poor Prognostic Factor in Pediatric Patients With Mixed Lineage Leukemia-AF9 Rearranged Acute Myeloid Leukemia. Haematologica (2014) 11:e225–7. doi: 10.3324/haematol.2014.107128
29. Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of Chemotherapy for Younger Patients With Acute Myeloid Leukemia: Results of the Medical Research Council AML15 Trial. J Clin Oncol (2013) 27:3360–8. doi: 10.1200/jco.2012.47.4874
30. Saito Y, Nakahata S, Yamakawa N, Kaneda K, Ichihara E, Suekane A, et al. CD52 as a Molecular Target for Immunotherapy to Treat Acute Myeloid Leukemia With High EVI1 Expression. Leukemia (2011) 6:921–31. doi: 10.1038/leu.2011.36
31. Mittal N, Li L, Sheng Y, Hu C, Li F, Zhu T, et al. A Critical Role of Epigenetic Inactivation of miR-9 in EVI1(high) Pediatric AML. Mol Cancer (2019) 1:30. doi: 10.1186/s12943-019-0952-z
32. Nguyen CH, Bauer K, Hackl H, Schlerka A, Koller E, Hladik A, et al. All-Trans Retinoic Acid Enhances, and a Pan-RAR Antagonist Counteracts, the Stem Cell Promoting Activity of EVI1 in Acute Myeloid Leukemia. Cell Death Dis (2019) 12:944. doi: 10.1038/s41419-019-2172-2
33. Steinmetz B, Hackl H, Slabáková E, Schwarzinger I, Smějová M, Spittler A, et al. The Oncogene EVI1 Enhances Transcriptional and Biological Responses of Human Myeloid Cells to All-Trans Retinoic Acid. Cell Cycle (2014) 18:2931–43. doi: 10.4161/15384101.2014.946869
34. Verhagen HJ, Smit MA, Rutten A, Denkers F, Poddighe PJ, Merle PA, et al. Primary Acute Myeloid Leukemia Cells With Overexpression of EVI-1 are Sensitive to All-Trans Retinoic Acid. Blood (2016) 4:458–63. doi: 10.1182/blood-2015-07-653840
35. Dzama MM, Steiner M, Rausch J, Sasca D, Schönfeld J, Kunz K, et al. Synergistic Targeting of FLT3 Mutations in AML via Combined Menin-MLL and FLT3 Inhibition. Blood (2020) 21:2442–56. doi: 10.1182/blood.2020005037
Keywords: EVI1, prognostic factor, acute myeloid leukemia, pediatric, adverse outcome, transplantation
Citation: Zheng Y, Huang Y, Le S, Zheng H, Hua X, Chen Z, Feng X, Li C, Zheng M, Xu H, He Y, He X, Li J and Hu J (2021) High EVI1 Expression Predicts Adverse Outcomes in Children With De Novo Acute Myeloid Leukemia. Front. Oncol. 11:712747. doi: 10.3389/fonc.2021.712747
Received: 21 May 2021; Accepted: 09 August 2021;
Published: 13 September 2021.
Edited by:
Gurvinder Kaur, All India Institute of Medical Sciences, IndiaReviewed by:
Michael Diamantidis, University Hospital of Larissa, GreeceBranko Cuglievan, University of Texas MD Anderson Cancer Center, United States
Barbara McClure, South Australian Health and Medical Research Institute (SAHMRI), Australia
Copyright © 2021 Zheng, Huang, Le, Zheng, Hua, Chen, Feng, Li, Zheng, Xu, He, He, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianda Hu, ZHJqaWFuZGFodUAxNjMuY29t; Jian Li, MTM1NDExMzcyM0BxcS5jb20g
†These authors have contributed equally to this work
 Yongzhi Zheng1†
Yongzhi Zheng1† Yan Huang
Yan Huang Xiaoqin Feng
Xiaoqin Feng Honggui Xu
Honggui Xu Yingyi He
Yingyi He Jianda Hu
Jianda Hu