- 1Department of Gynecologic Oncology, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
- 2Department of Chemotherapy, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
- 3Department of Pathology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China
Background: PARP inhibitor (PARPi) is an important progress in ovarian cancer treatment. The available evidence suggests that BRCA mutation and homologous recombination deficiency (HRD) are effective biological markers for PARPi. Here we investigated the relationship between adverse events (AEs) and efficacy of PARPi in ovarian cancer patients.
Methods: Seventy-eight patients with ovarian cancer patients underwent Olaparib and Niraparib from July 2018 to July 2020 were analyzed. AEs were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v5.0. Chi-square test or fisher exact tests was performed to observe the association between categorical variables. Logistic regression analysis was conducted to investigate the independent variables for disease control response (DCR). Progression-free survival (PFS) was compared between AEs variables by log-rank test.
Results: Patients with AEs in the first one week had a higher DCR compared with those after one week (86.11% versus 60.98%, p=0.013). Patients with serious AEs (SAEs) had a significantly higher DCR (81.40% versus 60.60%, p=0.045). There were associations between anemia and DCR in both occurrence (79.63% versus 56.52%, p=0.037) and grade (100% versus 73.17%, p=0.048). The median PFS of patients with hematological toxicity was longer than that of patients with no-hematological toxicity (30 versus 20 weeks, p=0.047). Patients with hematological toxicity within four weeks had prolonged median PFS than those with hematological toxicity after four weeks (40 versus 22 weeks, p=0.003).
Conclusions: The early presence of AEs and SAEs in hematological toxicity of PARPi were related to the antitumor efficacy, which might be a valid and easily measurable clinical marker in ovarian cancer patients.
Introduction
Ovarian cancer remains the first leading cause of cancer death in gynecological malignancy (1). Seventy percent of ovarian cancer patients can benefit from the traditional standard first-line treatment including cytoreductive surgery followed by platinum-based chemotherapy (2). However, about 80% patients will develop disease recurrence after traditional initial treatment and ultimately progress to platinum-resistance ovarian cancer (3). Recently, PARPi have transformed the treatment landscape of patients with ovarian cancer (4–11).
DNA damage in cells manifests mainly as single-strand breaks (SSBs), double strand breaks (DSBs) or replication fork stalling (12). PARP1 and PARP2 enzymes play an important role in the repair of SSBs in DNA, and they can recognize and bind to the DNA fracture site, and mediate DNA single-strand damage repair in tumor cells. HRD-positive tumor cells (cells with BRCA mutation or other mutations in homologous recombination repair (HRR) pathway genes such as RAD51 and ATM) cannot repair DNA single-strand damage, forming the synthetic lethal effect (13). Therefore, BRCAmt or HRD-positive tumor cells are more sensitive to PARPi in terms of molecular mechanisms (14).
PARPi are recommended as maintenance treatment and multi-line treatment in ovarian cancer patients according to National Comprehensive Cancer Network (NCCN) guidelines. The five-year follow-up data of SOLO1 showed that nearly 50% of patients harbored BRCA mutation have not progress with olaparib as first-line maintenance treatment, compared with 20% of patients in placebo group in 2020 ESMO meeting (15). Furthermore, olaparib as second-line maintenance treatment significantly increased progression-free survival (PFS) and overall survival (OS) for patients with BRCA mutation in SOLO2 study (16, 17). Both NOVA and PRIMA studies demonstrated that patients with HRD positive could get more benefit from niraparib as maintenance treatment (4, 5). On the other hand, olaparib could be used as single-agent therapy for multi-line treatment in ovarian cancer patients harbored BRCA mutation (18). QUADRA study demonstrated that women with heavily pretreated ovarian cancer, especially in patients with HRD positive platinum-sensitive disease, which included not only patients with BRCA mutation but also population with BRCA wild-type could benefit from niraparib (19). Previous clinical trials showed that ovarian cancer patients with BRCA mutation or HRD positive were more likely to benefit from PARPi. It was confirmed that BRCA mutation or HRD positive was an effective predictive biomarker for efficacy of PARPi from both molecular mechanisms and clinical practice.
However, there were no early clinical biomarkers to predict the efficacy. We observed that most patients suffered different PARPi-related adverse events (PrAEs) that might correlate with prognosis in our previous real-world studies (20). Based on these observations, we conducted this study to investigate the association of PrAEs with clinical outcomes in ovarian cancer patients.
Materials and Methods
Study Population
Between July 2018 to July 2020, seventy-eight advanced ovarian cancer/fallopian tube cancer/peritoneal cancer patients treated with PARPi, including olaparib with initial dose as 300 mg twice-daily and niraparib with initial dose as 200mg once-daily that was based on the baseline weight or platelets were enrolled in Jiangsu Cancer Hospital. If the patient experienced SAEs (Grade 3-4), the dose reduction and interruption would be done according to drug instruction of olaparib or niraparib. Treatment discontinued until the occurrence of radiological progression, as defined by Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1), unacceptable adverse events or death. Basic characteristics were collected from these patients. Platinum-sensitive ovarian cancer was defined as patients who relapsed more than or equal to 6 months after initial treatment and platinum-resistant ovarian cancer was considered as patients who progressed during initial treatment, or relapsed less than 6 months after initial treatment.
The inclusion criteria for all patients included histologically confirmed advanced ovarian cancer, fallopian tube cancer, peritoneal cancer, taking PARPi for more than four weeks, at least one measurable lesion as defined by RECIST 1.1, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and acceptable hematologic, hepatic, and renal function. Patients were excluded if they received platelet or red blood cell infusion within 4 weeks before taking the drug and had other malignant diseases within 2 years. All methods were performed in accordance with the relevant guidelines and regulations by the ethics committee of Jiangsu Cancer Hospital (2020- science-040).
Assessments
Patient demographics, adverse events and treatment efficacy were available and collected from all enrolled subjects. Efficacy assessments were performed based on computed tomography at baseline, after two and three cycles, and every 8 weeks thereafter until disease progression. The baseline of serum CA125 and a following monthly examination of CA125 were also conducted. The efficacy was assessed as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) by RECIST 1.1. Disease control rate (DCR) was defined as the proportion of patients achieving CR, PR or SD for at least 12 weeks. PFS was assessed from the first day of treatment with PARPi to disease progression or death from any cause. Treatment-related AEs were graded according to NCI CTCAE 5.0.
Statistical Analysis
Data were statistically analyzed using SPSS version 19.0 professional statistical software and all the count data were expressed as a percentage (%). Baseline characteristics and AEs were compared using t tests for continuous variables and fisher’s exact or chi-squared tests for categorical variables. Logistic regression analysis was conducted to investigate the association between independent variables and DCR. PFS was assessed using Kaplan–Meier method and compared between AEs variables by log-rank test. Single factors with p < 0.10 were defined as independent variable. Multivariate cox regression analysis was conducted to investigate the association between independent variables and PFS. A two-sided p-value less than 0.05 was considered statistically significant.
Results
Patient Characteristics
The demographic and baseline characteristics of the seventy-eight patients were summarized in Table S1, of whom seventy-four patients were ovary cancer and four patients were fallopian tube cancer. The median age of patients was 56 years (range 30–80 years). The median follow-up time was 22 weeks (range 12–88 weeks), and median PFS was 28 weeks with 95% confidence interval (CI) of 21.6–34.4%. Among overall population, the overall DCR was 72.7% (95% CI: 62.6–82.9%) and ORR was 14.3% (95% CI: 6.3–22.3%).
Of those, forty-eight patients (61.5%) treated with olaparib and the remaining thirty patients (38.5%) treated with niraparib. During olaparib treatment, a total of thirty-seven patients experienced anemia, twelve of whom were diagnosed with grade 3-4 anemia. Thrombocytopenia occurred in seven patients, two of whom were grade 3-4. In patients treated with niraparib, seventeen patients had mild (grade 1–2) anemia except for one case with grade 4 anemia. Thrombocytopenia developed in eighteen patients, six of whom had grade 3-4 thrombocytopenia. In addition, there were 83.1% patients suffered fatigue, 66.2% patients had nausea, and 62.3% patients experienced decreased appetite in total subjects.
AEs and DCR
This cohort analysis showed that early presence of AEs (within one week), SAEs, residual disease at initial surgery, and ECOG ps were associated with DCR. Patients with AEs in the first one week had a higher DCR compared with after one week (86.11% versus 60.98%, p=0.013). Also patients with SAEs had a significantly higher DCR (81.40% versus 60.60%, p=0.045). Besides, the DCR among patients with R0 resection was higher (83.33% versus 54.84%, p=0.008) than those with R1 resection. The same results were also observed in patients with ECOG 0 (83.33% versus 60.00%, p=0.038) compared with those with ECOG1 (Table 1).
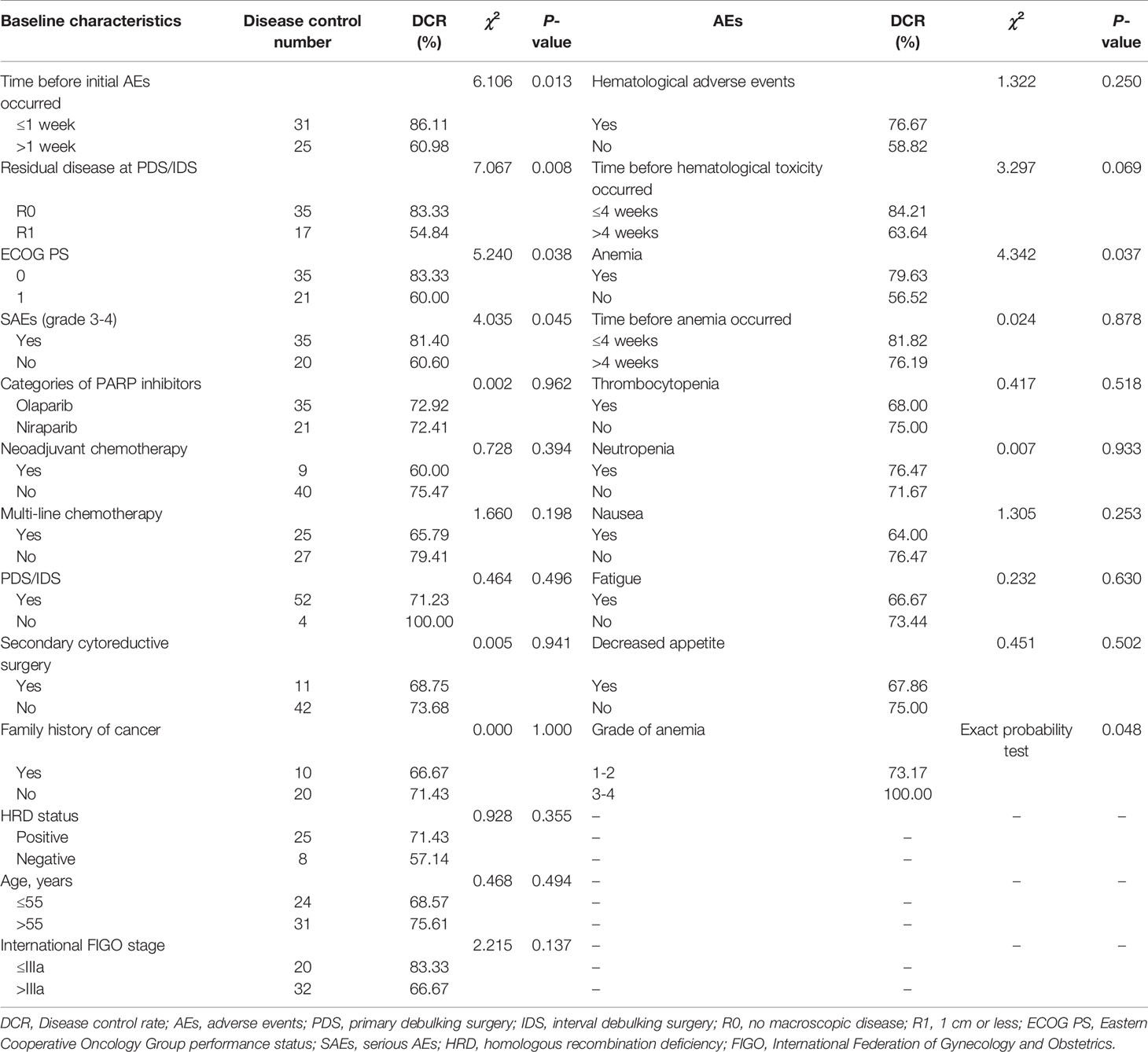
Table 1 Disease control rate of patients with different baseline characteristics and adverse events.
In the further analysis of each AE, it was found that there were relationships between anemia and DCR in both occurrence (79.63% versus 56.52%, p=0.037) and grade (100% versus 73.17%, p=0.048) (Table 1). Similarly, DCR among patients treated with olaparib was association with occurrence of anemia (83.78% versus 36.36%, p=0.007). However, patients treated with niraparib had a higher DCR in those experienced thrombocytopenia within four weeks than after four weeks (86.67% versus 40.00%, p=0.044) (Table S2). Baseline characteristics between the occurrence of AEs were not significantly different (Table S3).
A multivariable logistic regression model was constructed to predict DCR in the study population. It showed that the occurrence of AEs (odds ratio (OR): 0.162; 95% CI: 0.041-0.643, p = 0.010), ECOG score (OR: 0.188; 95% CI: 0.051-0.684, p=0.011) and residual disease at initial surgery (OR: 0.275; 95% CI: 0.084-0.903, p=0.033) were statistically significant for predicting DCR (Table 2). After internal verification of the existing population by logistic model, it was found that the accuracy rate of three factors including occurrence of AEs, ECOG score and residual disease at initial surgery for DCR was 96.1% and the total accuracy for DCR or PD was 76.4% (Table S4).
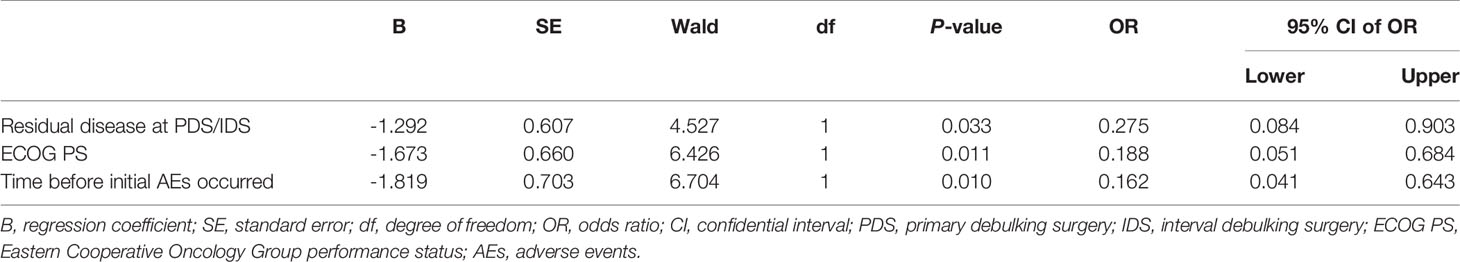
Table 2 Logistic regression analysis (Forward: LR) of multi-factor for predicting disease control rate.
AEs and PFS
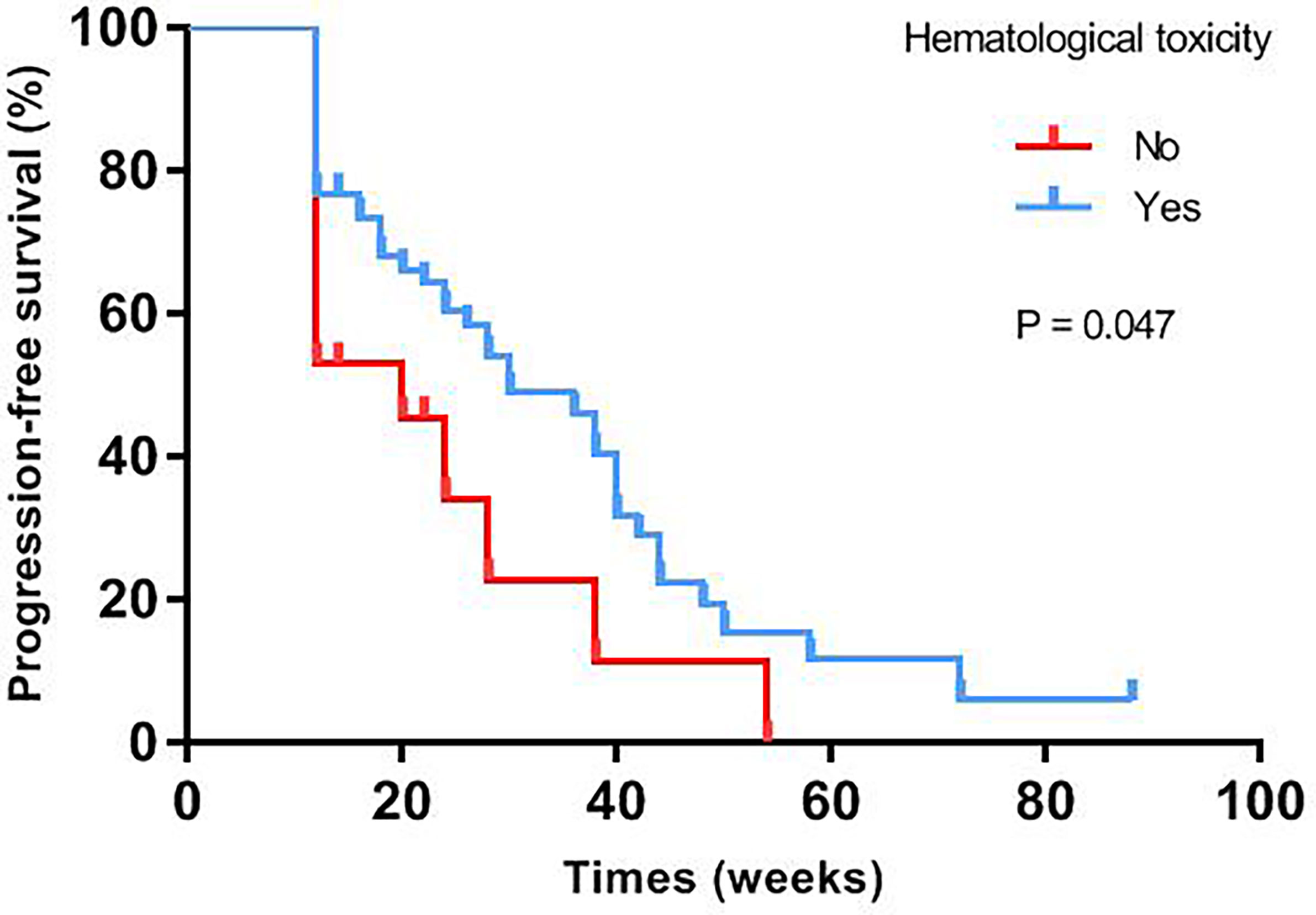
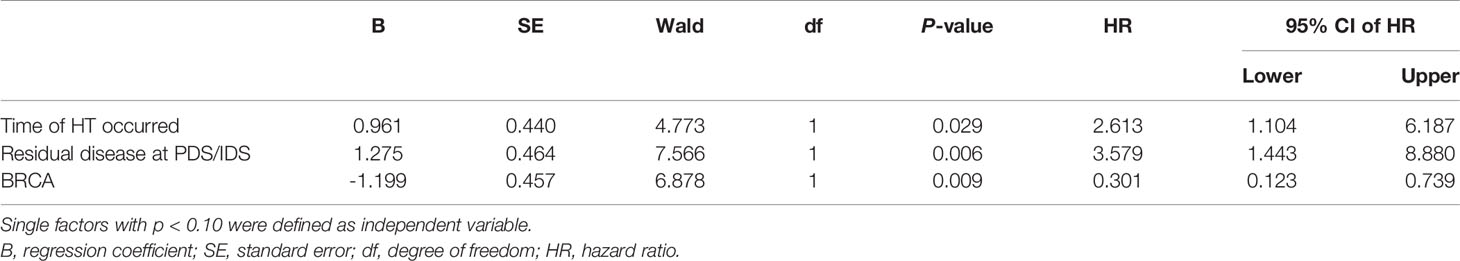
Median progression-free survival of patients with different baseline characteristics and adverse events were presented in Table 3. Univariate log-rank test analysis showed that the PFS among patients with hematological toxicity was longer (median: 30 weeks [95% CI: 20.78, 39.22]) than with no-hematological toxicity patients (median: 20 weeks [95% CI: 13.61, 26.39], p=0.047) (Figure 1). Patients with hematological toxicity within four weeks had prolonged median PFS than who with hematological toxicity after four weeks (40 versus 22 weeks, p=0.003) (Figure 2). Multivariate Cox regression analysis showed that hematological toxicity after four weeks (HR: 2.613; 95% CI:1.104-6.187, p=0.029), residual disease at PDS/IDS(R1/R2) (HR: 3.579; 95% CI:1.443-8.880, p=0.006) and BRCAmt (HR:0.301; 95% CI:0.123-0.739, p=0.009) were the independent factors (Table 4). Further interaction analysis with these independent factors found that there was no interaction between BRCAmt and hematological toxicity within four weeks [(relative excess risk due to interaction, (RERI): -1.246; 95% CI: -4.255-1.763], residual disease at PDS/IDS and hematological toxicity within four weeks (RERI: 2.134; 95% CI: - 3.270-7.538).
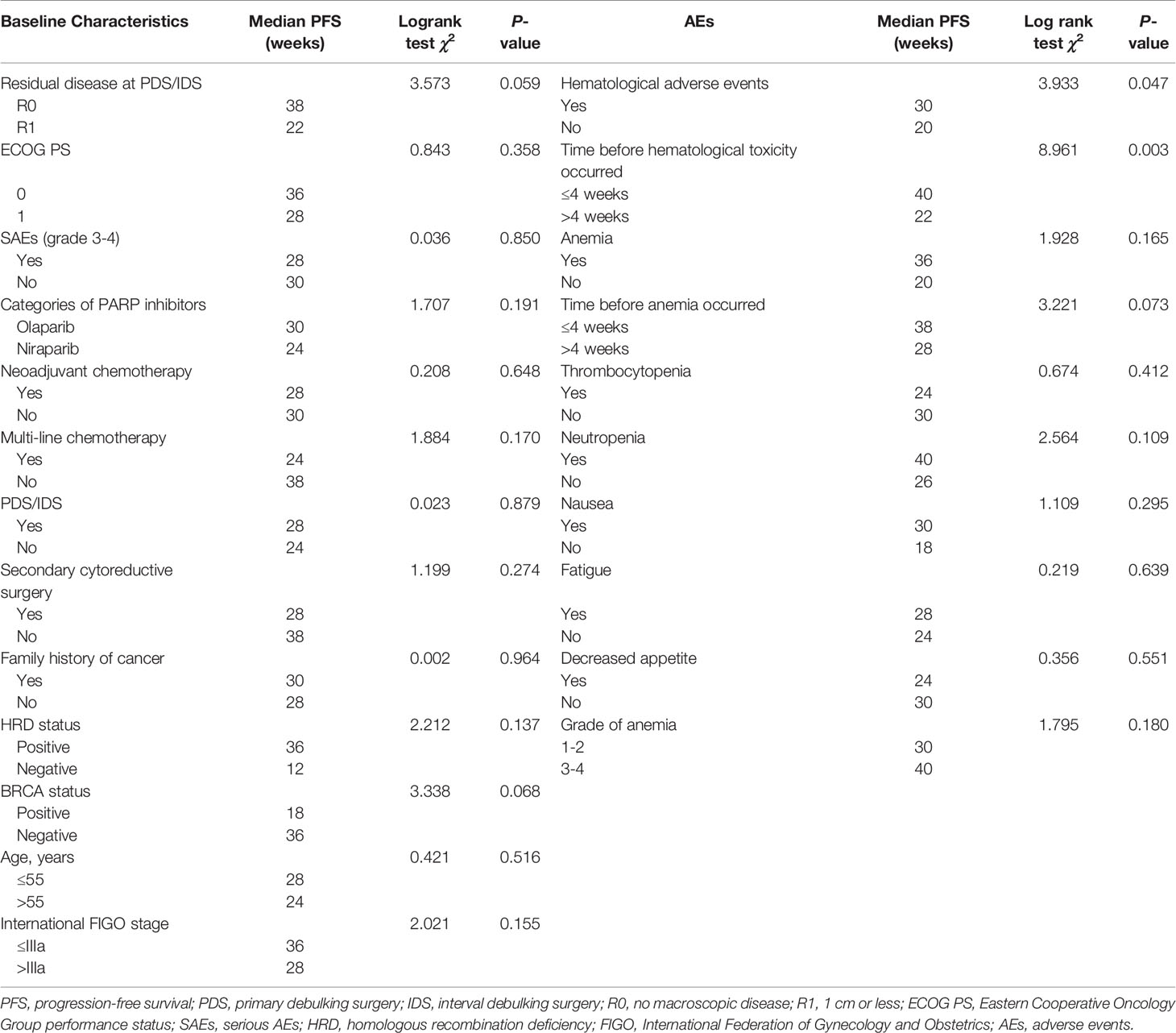
Table 3 Median progression-free survival of patients with different baseline characteristics and adverse events.
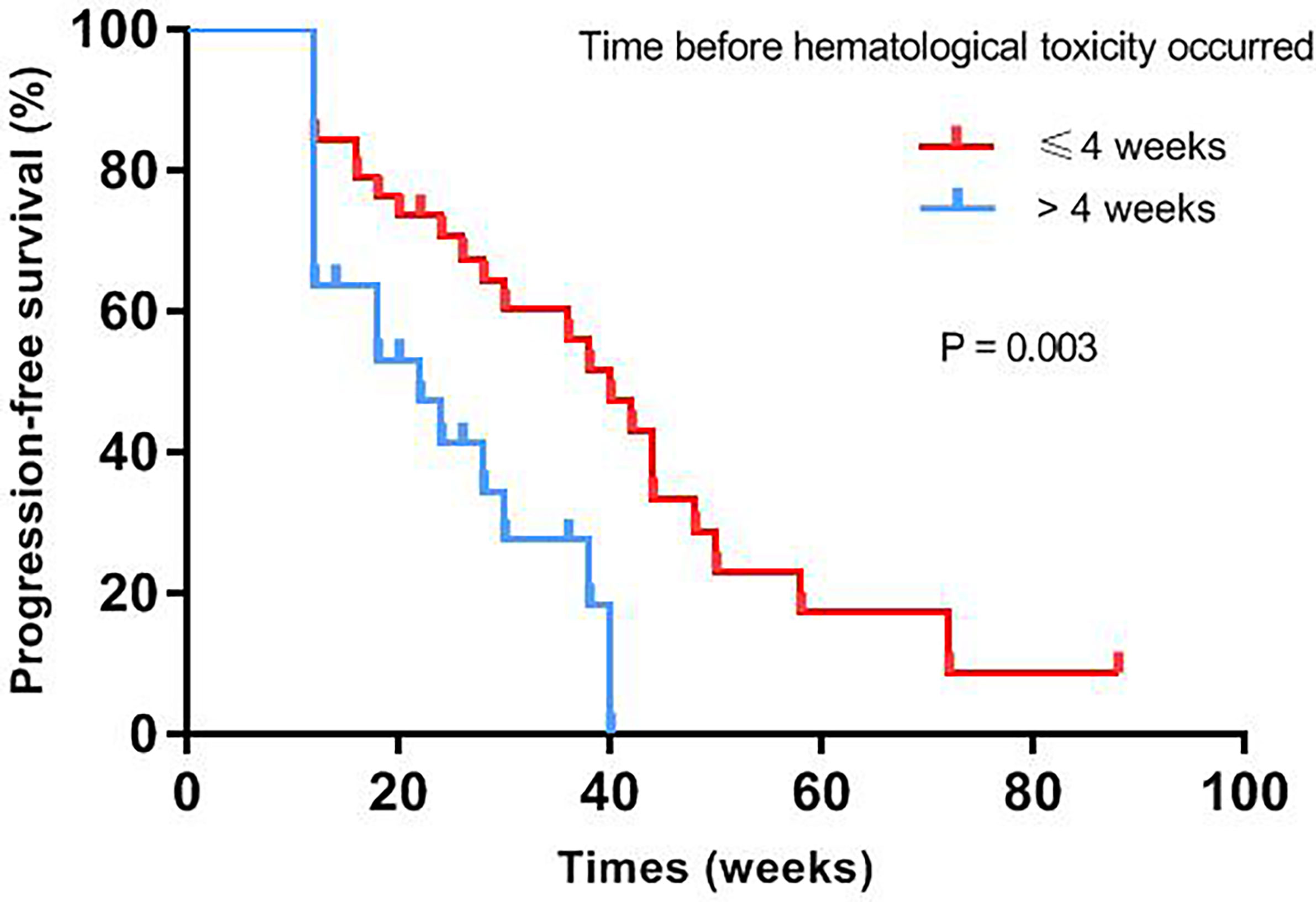
Figure 2 PFS was compared between patients with hematological toxicity within 4 weeks or after 4 weeks.
Discussion
PARPi is an important milestone in ovarian cancer treatment. Clinical studies showed that most patients experienced different degrees of AEs after taking PARPi. And mild or moderate AEs, namely CTCAE grade 1-2 is more common, including hematologic toxicity, gastrointestinal reactions and fatigue. Most of the hematologic laboratory abnormalities occurred within the first three months. The incidence of grade 3 or 4 anemia, thrombocytopenia and neutropenia was the main reason of dose reduction, interruption and even discontinuation. 10-15% of patients terminated their medication due to adverse reactions, and most patients could be treated with long-term medication (4, 5).
Similar adverse events were also observed in our previous real-world studies as well as in the population of this study. Interesting, it was observed that AEs of PARP inhibitors were highly similar to the traditional cytotoxic drugs that might be related to the distribution of PARP in various tissues of the body (20, 21). And molecular mechanism of PARP inhibitors is different from traditional targeted drugs which are targeted at a known oncogenic site, whether a protein molecule or a gene fragment. PARP inhibitors have high therapeutic index and low off-target effect based on the mechanism (22). Therefore, we combined with our clinical observation and mechanism of PARPi to further speculated that the AEs of PARP inhibitors might be related to the efficacy.
Some studies have found that there is a correlation between AEs of apatinib and the efficacy in treatment of gastric cancer, non-small cell lung cancer, colorectal cancer and liver cancer that may be due to the simultaneous expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors in tumor tissues and normal tissues (23–27). Recent studies reported that patients who experienced immune-related adverse events demonstrated marked improvements in survival and response rate compared to those lacking toxicity, which might be triggered by antigens that were common to both tumor and inflamed organ (28–30). Similar to the mechanism of immune-related adverse events, the correlation in PARP inhibitors is likely to be related to the widespread distribution of PARP in the cells. At present, there are no studies on the efficacy and AEs of PARPi.
In this study, we found that early presence of AEs (within one week), SAEs, residual disease at initial surgery, and ECOG score were correlated with the short efficacy of PARPi. But multivariable analysis showed that early presence of AEs, ECOG score and residual disease at initial surgery were statistically significant for DCR. The accuracy rate of these three factors for DCR was 96.1% and the total accuracy for DCR or PD was 76.4% through internal verification of the existing population which needed to be further performed by external validation. DCR among patients treated with olaparib and niraparib were association with anemia and thrombocytopenia, respectively. However, all patients who experienced anemia had a higher DCR that might be attributed to more enrolled patients taking olaparib. In addition, the prolonged PFS was observed among patients with hematological toxicity and hematological toxicity within four weeks, especially the latter. Further interaction analysis verified that hematological toxicity within four weeks, residual disease with R0 at PDS/IDS and BRCAmt were three independent factors for the efficacy of PARPi. The differences between PFS and DCR related factors were due to the small sample and short follow-up time.
Small sample size and diverse cohort is the most critical limitation in our single-center analysis that may affect parts of results to demonstrate statistically significant differences. The data of overall survival were lacking in our study because PARPi was approved in China not long ago. The level of evidence for our retrospective study was insufficient. In clinical practice, it may only be used in the process of patients using PARP inhibitor to roughly evaluate the immediate or short-term efficacy. RECIST 1.1 is still the evaluation standard of curative effect. And further randomized studies should be performed to evaluate the role of PrAEs as a potential prognostic marker in advanced ovarian cancer patients treated with PARPi. Therefore, we recently initiated a prospective study to that intended to confirm the results of this retrospective study, and to further explore other possible clinical markers and the possibility of establishing a comprehensive evaluation model for the efficacy of PARP inhibitors (Clinical trial information: NCT04582552).
In conclusion, we firstly found that the early presence of AEs, and SAEs in hematological toxicity of PARPi were related to the antitumor efficacy, which might be a valid and easily measurable clinical marker in ovarian cancer patients.
Data Availability Statement
The datasets presented in this article are not readily available because we would not share the data and material used in this article, because we need them for further research. Although it is available from the corresponding author on reasonable request. Requests to access the datasets should be directed to Y3h4eHhjeWRAZ21haWwuY29t.
Ethics Statement
The studies involving human participants were reviewed and approved by Jiangsu Cancer Hospital’s Ethical Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JN participated in the design of present study and drafted the manuscript. XianC and RZ carried out the cases recruit of present study. QZ and HG participated in the cases recruit of present study. WG carried out statistical analysis. XX and CC participated in the statistical analysis and drafted the manuscript. XiaoC designed of the study, performed the statistical analysis and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81472441, 81501205), Jiangsu Provincial Scientific research and Health Project for Women and Children (No. F202004), Natural Science Foundation of Youth Fund Projects of Jiangsu Province (SBK2021040731), Institute level project of Jiangsu Cancer Hospital(No. ZM201804) and Beijing Kanghua Foundation for the Development of Traditional Chinese and Western Medicine -Le Fund (KH-2020-LJJ-021, KH-2021-LLZX-058).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank Yanlin Tang and Yang Ye for their technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.724620/full#supplementary-material
Abbreviations
PARP, Poly ADP-ribose polymerase; PARPi, Poly ADP-ribose polymerase inhibitor; BRCAmt, BRCA mutation type; HRD, Homologous recombination deficiency; AEs, Adverse events; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; DCR, Disease control response; PFS, Progression-free survival; SAEs, Serious AEs; SSBs, Single-strand breaks; DSBs, Double strand breaks; HRR, Homologous recombination repair; PrAEs, PARPi-related adverse events; RECIST 1.1, Response Evaluation Criteria in Solid Tumors 1.1; ECOG PS, Eastern Cooperative Oncology Group performance status; CR, Complete response; PR, Partial response; SD, Stable disease; PD, progressive disease; OS, Overall survival.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Ghirardi V, Moruzzi MC, Bizzarri N, Vargiu V, D'Indinosante M, Garganese G, et al. Residual Disease at Primary Debulking Surgery Versus Complete Tumor Resection at Interval Debulking Surgery in Advanced Epithelial Ovarian Cancer: A Survival Analysis. Gynecol Oncol (2020) 157(1):209–13. doi: 10.1016/j.ygyno.2020.01.010
3. Coleridge SL, Bryant A, Lyons TJ, Goodall RJ, Kehoe S, Morrison J. Chemotherapy Versus Surgery for Initial Treatment in Advanced Ovarian Epithelial Cancer. Cochrane Database System Rev (2019) 2019(10):CD005343. doi: 10.1002/14651858.CD005343.pub4
4. Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in Patients With Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med (2019) 381(25):2391–402. doi: 10.1056/NEJMoa1910962
5. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med (2016) 375(22):2154–64. doi: 10.1056/NEJMoa1611310
6. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N Engl J Med (2012) 366(15):1382–92. doi: 10.1056/NEJMoa1105535
7. Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib Plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med (2019) 381(25):2416–28. doi: 10.1056/NEJMoa1911361
8. Marchetti C, D’Indinosante M, Bottoni C, Di Ilio C, Di Berardino S, Costantini B, et al. NLR and BRCA Mutational Status in Patients With High Grade Serous Advanced Ovarian Cancer. Sci Rep (2021) 11(1):11125. doi: 10.1038/s41598-021-90361-w
9. Marchetti C, Minucci A, D’Indinosante M, Ergasti R, Arcieri M, Capoluongo ED, et al. Feasibility of Tumor Testing for BRCA Status in High-Grade Serous Ovarian Cancer Using Fresh-Frozen Tissue Based Approach. Gynecol Oncol (2020) 158(3):740–6. doi: 10.1016/j.ygyno.2020.06.479
10. Marchetti C, De Leo R, Musella A, D’Indinosante M, Capoluongo E, Minucci A, et al. BRCA Mutation Status to Personalize Management of Recurrent Ovarian Cancer: A Multicenter Study. Ann Surg Oncol (2018) 25(12):3701–8. doi: 10.1245/s10434-018-6700-6
11. Gallotta V, Conte C, D’Indinosante M, Capoluongo E, Minucci A, De Rose AM, et al. Prognostic Factors Value of Germline and Somatic brca in Patients Undergoing Surgery for Recurrent Ovarian Cancer With Liver Metastases. Eur J Surg Oncol (2019) 45(11):2096–102. doi: 10.1016/j.ejso.2019.06.023
12. Lord CJ, Ashworth A. The DNA Damage Response and Cancer Therapy. Nature (2012) 481(7381):287–94. doi: 10.1038/nature10760
13. Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res (2012) 72(21):5588–99. doi: 10.1158/0008-5472.CAN-12-2753
14. Tangutoori S, Baldwin P, Sridhar S. PARP Inhibitors: A New Era of Targeted Therapy. Maturitas (2015) 81(1):5–9. doi: 10.1016/j.maturitas.2015.01.015
15. Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oakninet A, et al. 811mo Maintenance Olaparib for Patients (Pts) With Newly Diagnosed, Advanced Ovarian Cancer (OC) and a BRCA Mutation (BRCAm): 5-Year (Y) Follow-Up (F/U) From SOLO1. Ann Oncol (2020) 31:S613. doi: 10.1016/j.annonc.2020.08.950
16. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib Tablets as Maintenance Therapy in Patients With Platinum-Sensitive, Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2017) 18(9):1274–84. doi: 10.1016/S1470-2045(17)30469-2
17. Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib Tablets as Maintenance Therapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Final Analysis of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2021) 22(5):620–31. doi: 10.1016/S1470-2045(21)00073-5
18. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. J Clin Oncol: Off J Am Soc Clin Oncol (2015) 33(3):244–50. doi: 10.1200/JCO.2014.56.2728
19. Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib Monotherapy for Late-Line Treatment of Ovarian Cancer (QUADRA): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol (2019) 20(5):636–48. doi: 10.1016/S1470-2045(19)30029-4
20. Ni J, Cheng X, Zhou R, Xu X, Guo W, Chen X. Olaparib in the Therapy of Advanced Ovarian Cancer: First Real World Experiences in Safety and Efficacy From China. J Ovarian Res (2019) 12(1):117. doi: 10.1186/s13048-019-0594-1
21. Peralta-Leal A, Rodriguez-Vargas JM, Aguilar-Quesada R, Rodriguez MI, Linares JL, de Almodovar MR, et al. PARP Inhibitors: New Partners in the Therapy of Cancer and Inflammatory Diseases. Free Radical Biol Med (2009) 47(1):13–26. doi: 10.1016/j.freeradbiomed.2009.04.008
22. Pilie PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin Cancer Res: An Off J Am Assoc Cancer Res (2019) 25(13):3759–71. doi: 10.1158/1078-0432.CCR-18-0968
23. Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai Y, et al. Early Presence of Anti-Angiogenesis-Related Adverse Events as a Potential Biomarker of Antitumor Efficacy in Metastatic Gastric Cancer Patients Treated With Apatinib: A Cohort Study. J Hematol Oncol (2017) 10(1):153. doi: 10.1186/s13045-017-0521-0
24. Yang X, Hou Z, Zhu K, Zhang S, Gu X, Wang Z, et al. Drug-Related Hypertension Associated With the Efficacy of Apatinib on Hepatocellular Carcinoma. Cancer Manage Res (2020) 12:3163–73. doi: 10.2147/CMAR.S240394
25. Osterlund P, Soveri LM, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and Overall Survival in Metastatic Colorectal Cancer Patients Treated With Bevacizumab-Containing Chemotherapy. Br J Cancer (2011) 104(4):599–604. doi: 10.1038/bjc.2011.2
26. Fang SC, Huang W, Zhang YM, Zhang HT, Xie WP. Hypertension as a Predictive Biomarker in Patients With Advanced non-Small-Cell Lung Cancer Treated With Apatinib. OncoTargets Ther (2019) 12:985–92. doi: 10.2147/OTT.S189984
27. Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, et al. Ten Years of Sorafenib in Hepatocellular Carcinoma: Are There Any Predictive and/or Prognostic Markers? World J Gastroenterol (2018) 24(36):4152–63. doi: 10.3748/wjg.v24.i36.4152
28. Das S, Johnson DB. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J Immunother Cancer (2019) 7(1):306. doi: 10.1186/s40425-019-0805-8
29. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis With Combination Immune Checkpoint Blockade. N Engl J Med (2016) 375(18):1749–55. doi: 10.1056/NEJMoa1609214
Keywords: PARP inhibitor, ovarian cancer, efficacy, clinical marker, adverse events
Citation: Ni J, Cheng X, Zhou R, Zhao Q, Xu X, Guo W, Gu H, Chen C and Chen X (2021) Adverse Events as a Potential Clinical Marker of Antitumor Efficacy in Ovarian Cancer Patients Treated With Poly ADP-Ribose Polymerase Inhibitor. Front. Oncol. 11:724620. doi: 10.3389/fonc.2021.724620
Received: 13 June 2021; Accepted: 19 August 2021;
Published: 06 September 2021.
Edited by:
Stergios Boussios, King’s College London, United KingdomReviewed by:
Neil Phippen, Brooke Army Medical Center, United StatesMarco D’Indinosante, Catholic University of the Sacred Heart, Italy
Copyright © 2021 Ni, Cheng, Zhou, Zhao, Xu, Guo, Gu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxiang Chen, Y3h4eHhjeWRAZ21haWwuY29t
†These authors have contributed equally to this work
 Jing Ni
Jing Ni Xianzhong Cheng
Xianzhong Cheng Rui Zhou
Rui Zhou Qian Zhao
Qian Zhao Xia Xu
Xia Xu Wenwen Guo
Wenwen Guo Hongyuan Gu
Hongyuan Gu Chen Chen
Chen Chen Xiaoxiang Chen
Xiaoxiang Chen