- 1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Epidemiology and Biostatistics, School of Public Health and Management, Wenzhou Medical University, Wenzhou, China
- 3Department of Hepatobiliary Surgery, Shandong Provincial Hospital, Jinan, China
- 4Department of Hepatobiliary Surgery, Qilu Hospital of Shandong University, Jinan, China
- 5Department of Clinical Medicine, The Second School of Medicine, Wenzhou Medical University, Wenzhou, China
Background: Surgical resection is the only widely accepted curative method for intrahepatic cholangiocarcinoma (ICC). However, little is known about the efficacy of laparoscopic liver resection for ICC, especially in patients with early-stage disease. The aim of this study was to compare the short-term and long-term effects of laparoscopy and open surgery for the treatment of ICC.
Methods: Data from 1,084 patients treated at three hospitals from January 2011 to December 2018 were selected and analyzed. Propensity score matching was performed to compare the long-term outcomes (overall survival and recurrence-free survival) and short-term outcomes (perioperative outcomes) of all-stage and early-stage patients.
Results: After matching, 244 patients (122 vs. 122) in the all-stage group and 65 patients (27 vs. 38) in the early-stage group were included. The baseline of the two groups was balanced, and no significant differences were found in sex or age. The short-term results of the laparoscopic group were better than those of the open group, including less blood loss [blood loss ≥400 ml 27 (22.1%) vs. 6 (4.92%), p<0.001 for all-stage, 12 (31.6%) vs. 2 (7.41%), p=0.042 for early stage), shorter surgery [200 (141; 249) min vs. 125 (115; 222) min, p=0.025 for early stage] and shorter hospital stay [11.0 (9.00; 16.0) days vs. 9.00 (7.00; 12.0) days, p=0.001 for all stage, 11.0 (8.50; 17.8) days vs. 9.00 (6.50; 11.0) days, p=0.011 for early stage]. Regarding long-term outcomes, no significant differences were found for all-stage patients, while there were significant differences observed for the early-stage group (p=0.013 for OS, p=0.014 for RFS). For the early-stage patients, the 1-, 3-, and 5-year OS rates of the OLR group were 84.2, 65.8, and 41.1%, respectively, and those of the LLR group were 100, 90.9, and 90.9%, respectively. The RFS rates of the OLR group were 84.2, 66.7, and 41.7%, respectively, and those of the LLR group were and 92.3, 92.3, and 92.3%, respectively.
Conclusion: Patients treated with laparoscopy seemed to have better short-term outcomes, such as less blood loss, shorter operation duration, and shorter hospital stay, than patients undergoing open surgery. Based on the long-term results, laparoscopic treatment for early ICC may have certain advantages.
Introduction
Laparoscopic liver resection (LLR) has become a widely accepted surgical method (1–3) with equivalent safety and effectiveness to open liver resection (OLR) (3–6). Both minor and “difficult” major LLR procedures performed in large hepatobiliary centers have acceptable short-term and long-term outcomes (2, 7, 8). However, this conclusion is supported by studies related to hepatocellular carcinoma (HCC), benign tumors, or colorectal liver metastases (5, 9–11). Whether intrahepatic cholangiocarcinoma (ICC) is suitable for laparoscopic resection and the oncological outcome of laparoscopic resection for ICC are still unclear.
Randomized controlled trials (RCTs) are the gold standard for clinical studies, but they are difficult to implement in cancer-related surgical research due to uncontrollable factors such as tumor staging and differentiation. Propensity score (PS) analysis is a well-performed approach to estimate the causal treatment effects of clinical problems found in observational studies. The data generated from a large observational cohort (12, 13) can be used to evaluate important clinical problems when randomized trials are limited or non-existent.
Some articles have compared the short-term and long-term outcomes of LLR and OLR for the treatment of ICC (14–17), but no articles have explored the effect of laparoscopic hepatectomy for early-stage intrahepatic cholangiocarcinoma. The purpose of this study was to compare the short-term and long-term outcomes of LLR and OLR for the treatment of ICC, especially early-stage patients, to fully investigate the advantages and disadvantages of LLR and OLR among different patient populations.
Methods
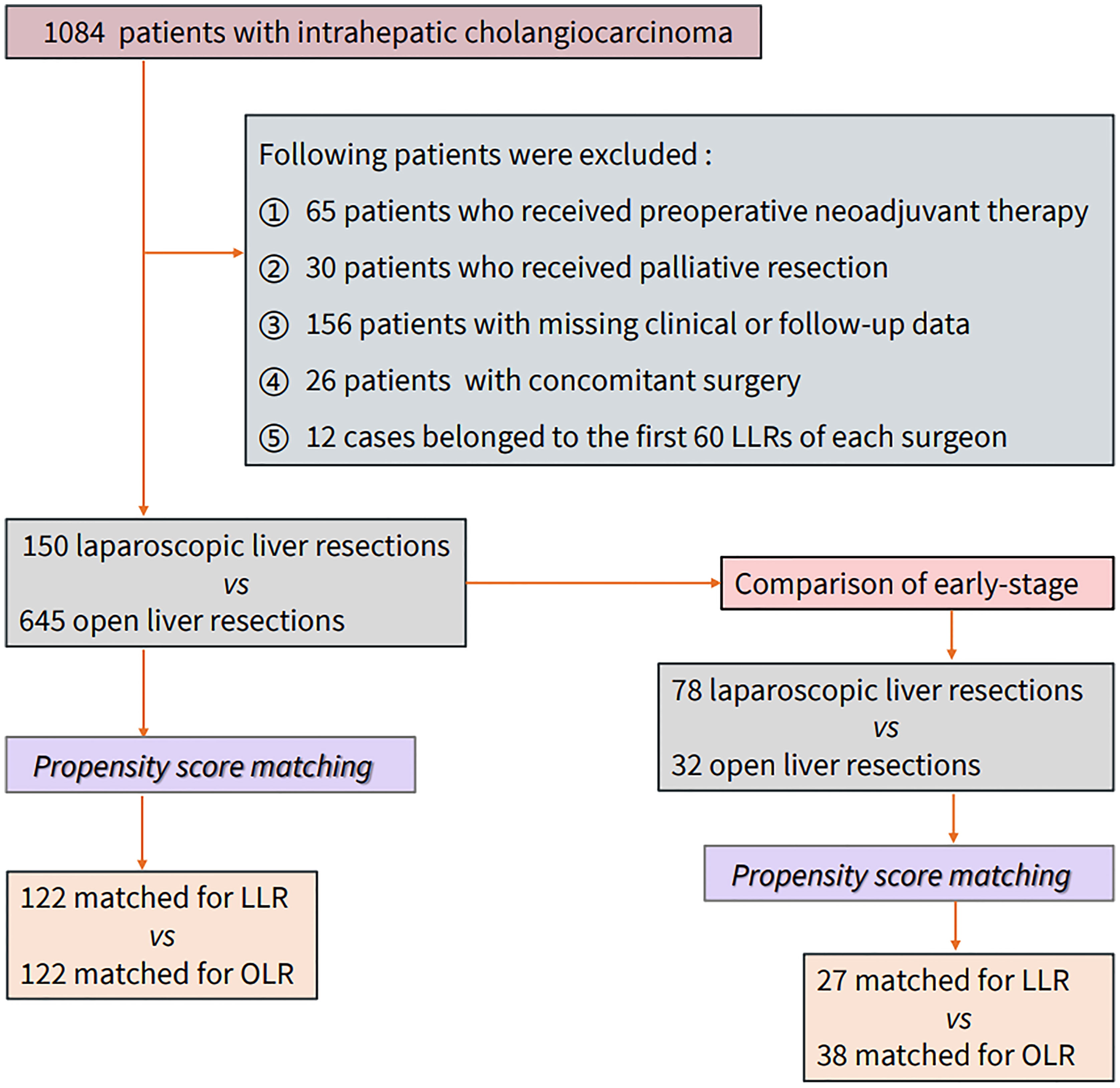
Patients diagnosed with ICC at 12 hepatobiliary surgical wards across three large hepatobiliary centers in southern and northern China from January 2011 to December 2018 were selected. Patients who underwent preoperative neoadjuvant therapy, palliative resection, or concomitant surgery and those with missing clinical or follow-up data along with cases of laparoscopic surgery early on in the mastery of the learning curve were excluded. According to different surgical methods, cases were divided into an open liver resection (OLR) group and a laparoscopic liver resection (LLR) group. Allocation to the LLR group was based on treatment intent. All operations selected were performed by senior hepatobiliary surgeons after mastering the learning curve (with at least 5 years of experience and ≥60 cases of LLR). Propensity score matching was used to reduce confounding factors and to promote a balance in the baseline characteristics. The matching factors were stratified according to the literature and clinical experience, including basic demographic information (sex, age, BMI, and smoking and drinking status), tumor pathology information (tumor size, tumor number, TNM stage, differentiation, lymphatic invasion, vascular invasion, and nerve invasion), and other important clinical factors (HBV infection, hepatolithiasis, diabetes, cirrhosis, previous abdominal surgery, Child–Pugh classification, resection range, Charlson Comorbidity Index score, and anatomical resection). Anatomical resection (AR) is defined as resection of the tumor together with the portal veins draining the tumor and the corresponding hepatic territory, as determined by dye injection into the feeding portal vein. Non-anatomical resection (NAR) is defined as resection of a lesion regardless of the anatomical segment or section of the lobar anatomy and includes limited resection or enucleation. According to the accepted conferences in the literature (5, 9), minor LLR was regarded as a procedure in which ≤2 Couinaud segments located in the anterolateral part of the liver (II, III, IVb, V, VI) are removed. Major LLR was regarded as a procedure in which ≥3 segments are removed or involving the posterior superior segments (I, IVA, VII, VIII) regardless of the number of Couinaud segments removed. The long-term outcomes were overall survival (OS) and recurrence-free survival (RFS). The overall survival time was calculated from the day of operation to the time of death or the last follow-up. Recurrence-free survival was calculated from the day of surgery to the day of tumor recurrence or the last follow-up. The short-term outcomes included perioperative indicators, including blood loss, duration of surgery, intraoperative blood transfusion, postoperative blood transfusion, complications, duration of hospital stay, hospitalization expenses, and postoperative mortality. Complications were defined according to the Clavien–Dindo classification. Grade 1–2 complications were defined as minor complications and included wound infection (bedside), nausea, vomiting, and elevated blood pressure; grade 3 or higher complications were defined as major complications and included postoperative pleural effusion (excluding reactive pleural effusion in patients undergoing right liver resection), bile leakage, postoperative bleeding, liver failure, and death. Postoperative bile leakage and hepatocyte failure were defined by the international research group on liver surgery (18, 19). Postoperative mortality was defined as death occurring within 90 days after hepatectomy. The indications and contraindications of LLR are the same as those of OLR. This study was approved by the ethics committees of two centers. Early stage was defined as a unifocal lesion with a diameter of ≤3 cm and no vascular invasion, namely, Tis or T1a (≤3 cm) stage according to the AJCC eighth edition Cancer Staging system. Figure 1 shows the study design.
Statistical Analysis
Numerical variables are expressed as the mean ± SD or median (quartile range). χ2 or Fisher’s exact test was used to compare categorical variables, whereas the T test (normal distribution) and Wilcoxon rank sum test (nonnormal distribution) were used for continuous variables. The Kaplan–Meier method was used to obtain the cumulative survival rate. The log rank test was used to compare survival curves between the two groups. A two-tailed P<0.05 was considered to indicate significance. The nearest-neighbor matching method was used for the following matches. For the all-stage groups, the matching ratio was 1:1, and the caliper was 0.1. For the early-stage groups, the ratio was 1:2, and the caliper was 0.2. No samples with replacement was used. A stepwise backward Cox multivariable regression analysis including all available clinically relevant prognostic variables was used to identify prognostic factors for OS and RFS. Statistical analysis was carried out using R version 4.0.2 software for Windows.
Results
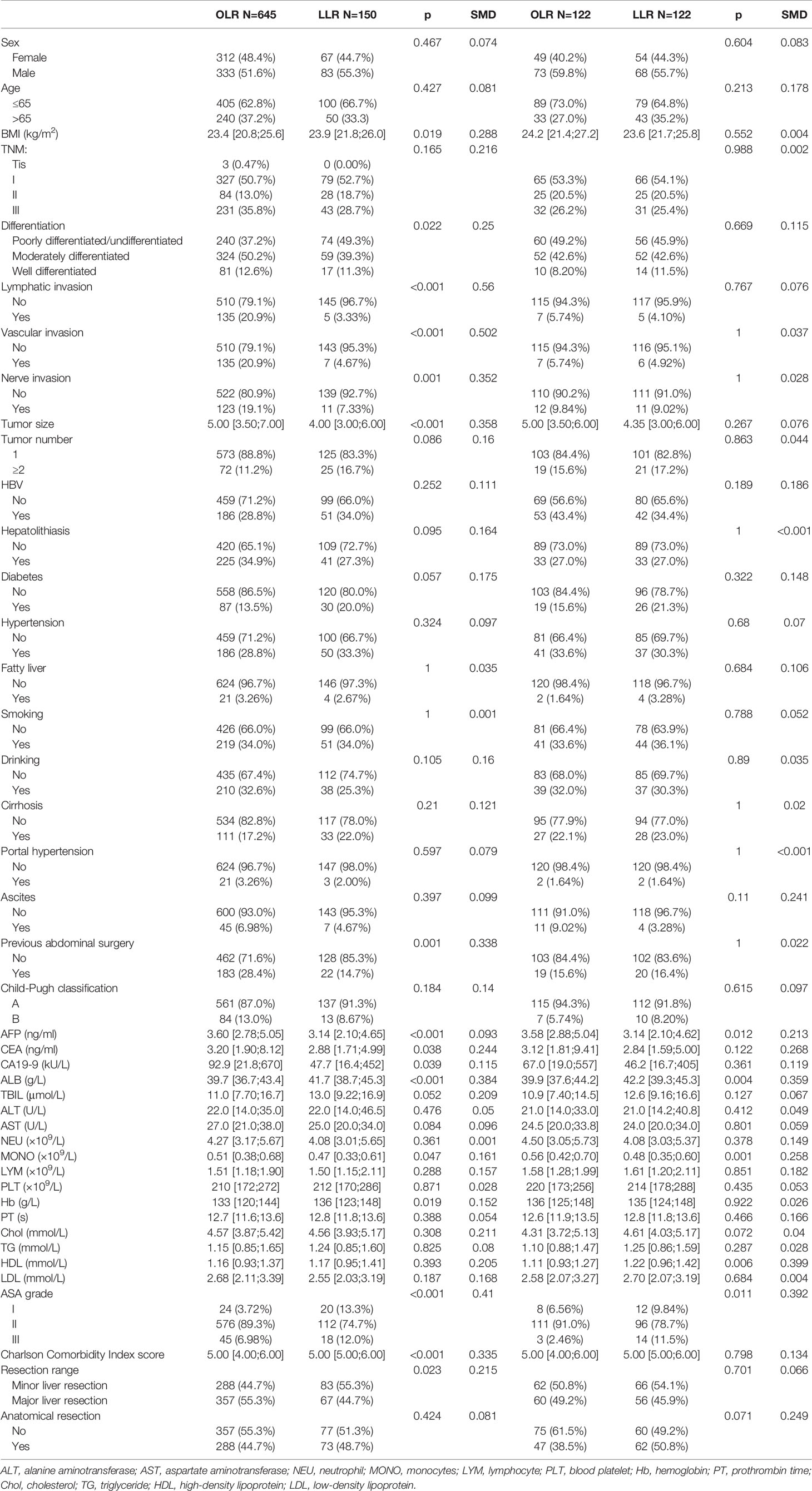
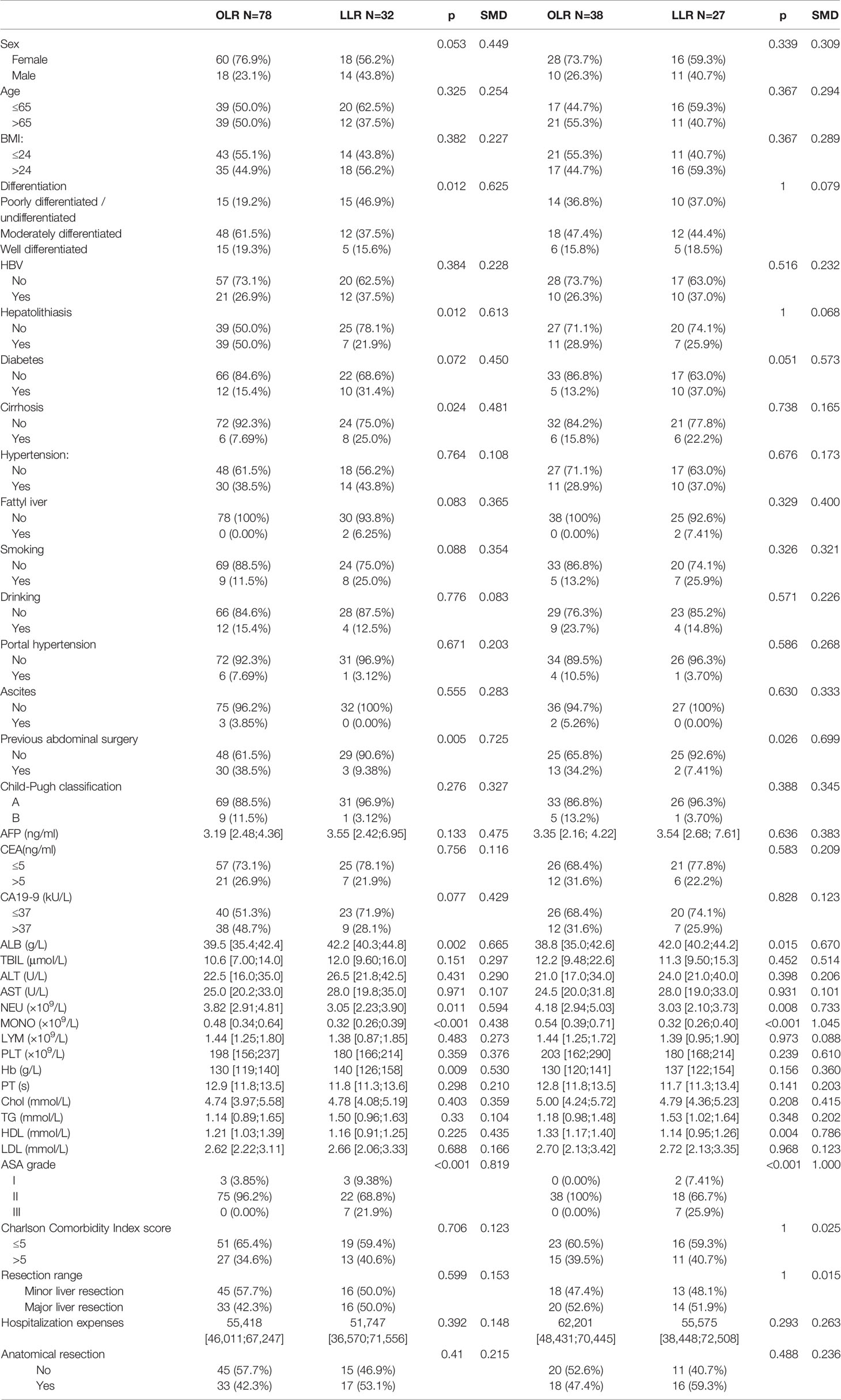
A total of 1,084 patients with ICC were selected from the three centers, and data from 150 LLR patients and 645 OLR patients were obtained according to the above exclusion criteria. After matching, a total of 244 patients (122 in the LLR group and 122 in the OLR group) were included. The median follow-up was 33.2 months in both groups. Table 1 summarizes the baseline of the variables before and after matching. Lymphatic invasion, vascular invasion, nerve invasion, differentiation, tumor size, BMI, AFP, CEA, CA19-9, previous abdominal surgery, ALB, MONO, Hb, and ASA grade showed significant differences between the groups before propensity score matching (PSM). Although the matching process does not completely eliminate all differences, these small differences are within the clinically acceptable range. Table 2 summarizes the same baseline items between the two groups of early-stage patients, and the matching results are also acceptable. The median follow-up for early-stage patients was 26.0 (for OLR) and 31.2 (for LLR) months.
Table 3 summarizes the perioperative results of the two groups of all-stage patients. There were significant differences observed in blood loss [blood loss >400 ml 27 (22.1%) for OLR vs. 6 (4.92%) for LLR, p<0.001], the duration of hospital stay (11.0 [9.00;16.0] for OLR vs. 9.00 [7.00;12.0] for LLR, p<0.001), and severe complications [18 (14.8%) for OLR vs. 7 (5.74%) for LLR, p=0.032]. Table 4 shows the perioperative results of the early-stage patients. Similar to the all-stage patients, the LLR group had less blood loss [blood loss >400 ml 12 (31.6%) for OLR vs. 2 (7.41%) for LLR, p=0.042] and a shorter duration of hospital stay [11.0 (8.50;17.8) for OLR vs. 9.00 (6.50;11.0) for LLR, p=0.011] than the OLR group. In addition, the LLR group had a shorter duration of surgery [200 (141;249) min for OLR vs. 125 (115;222) min for LLR, p=0.025].
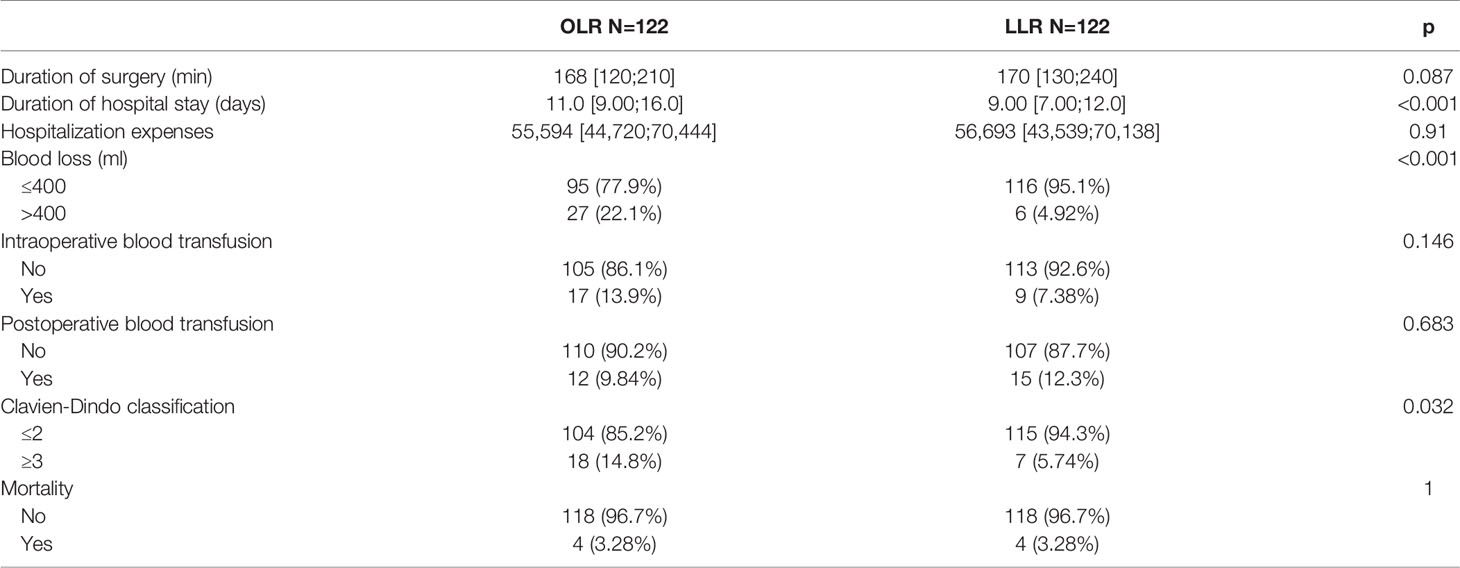
Table 3 Comparation of short-term outcomes between the two groups before and after matching in all-stage patients.
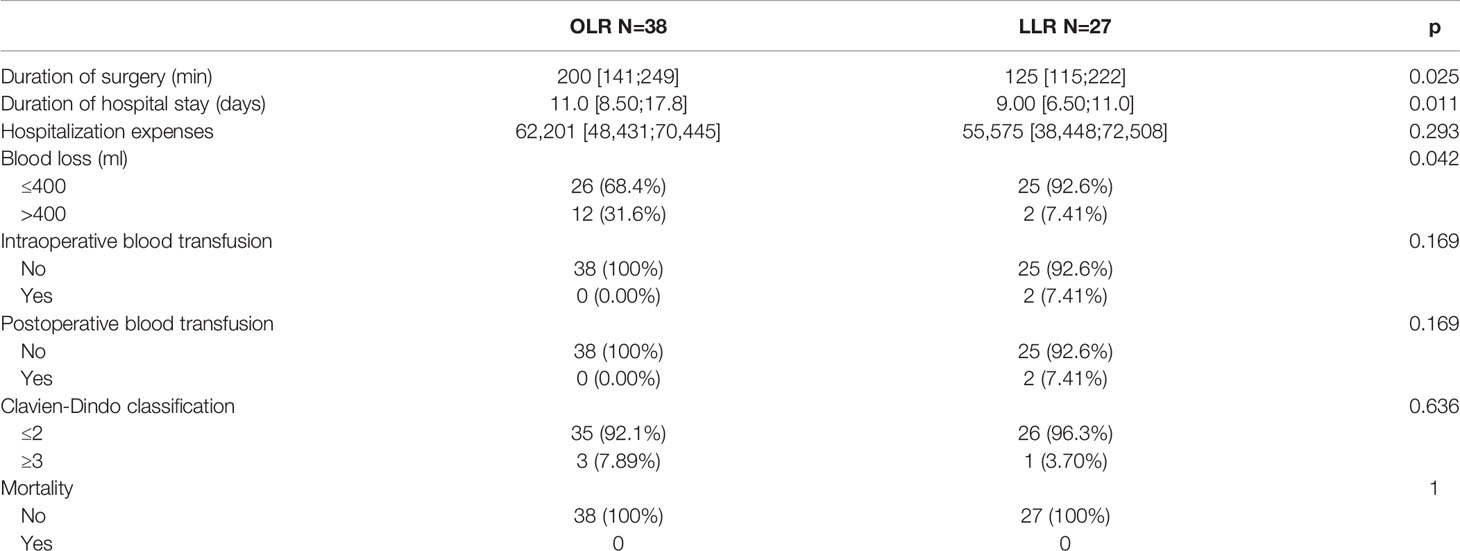
Table 4 Comparation of short-term outcomes between the two groups before and after matching in early-stage patients.
Figures 2A1, A2 show the overall survival (OS) and recurrence-free survival (RFS) of the two groups of all-stage patients after PSM, and Supplementary 1 shows the same items before PSM. Different from the trend before matching (p=0.0013 for OS, p=0.0019 for RFS), no significant differences were found for all-stage patients (p=0.28 for OS, p=0.41 for RFS) after PSM. For early-stage patients, there were significant differences before (p=0.0014 for OS, p=0.0028 for RFS) (Supplementary 1) and after matching (p=0.013 for OS, p=0.014 for RFS) (Figures 2B1, B2). After matching, the 1-, 3-, and 5-year OS rates of the OLR group of all-stage patients were 74.4, 39.8, and 27.6%, respectively, and those of the LLR group were 77.3, 51.4, and 25.7%, respectively. The RFS rates of the OLR group were 60.6, 36.9, and 23.4%, respectively, and those of the LLR group were and 63.7, 53.5, and 26.7% (RFS), respectively. Correspondingly, the 1-, 3-, and 5-year OS rates of the OLR group of early-stage patients were 84.2, 65.8, and 41.1%, and the RFS rates were 84.2, 66.7, and 41.7%, respectively. The OS and RFS rates of the LLR group were 100%, 90.9%, and 90.9% and 92.3, 92.3, and 92.3%, respectively (Supplementary 2).
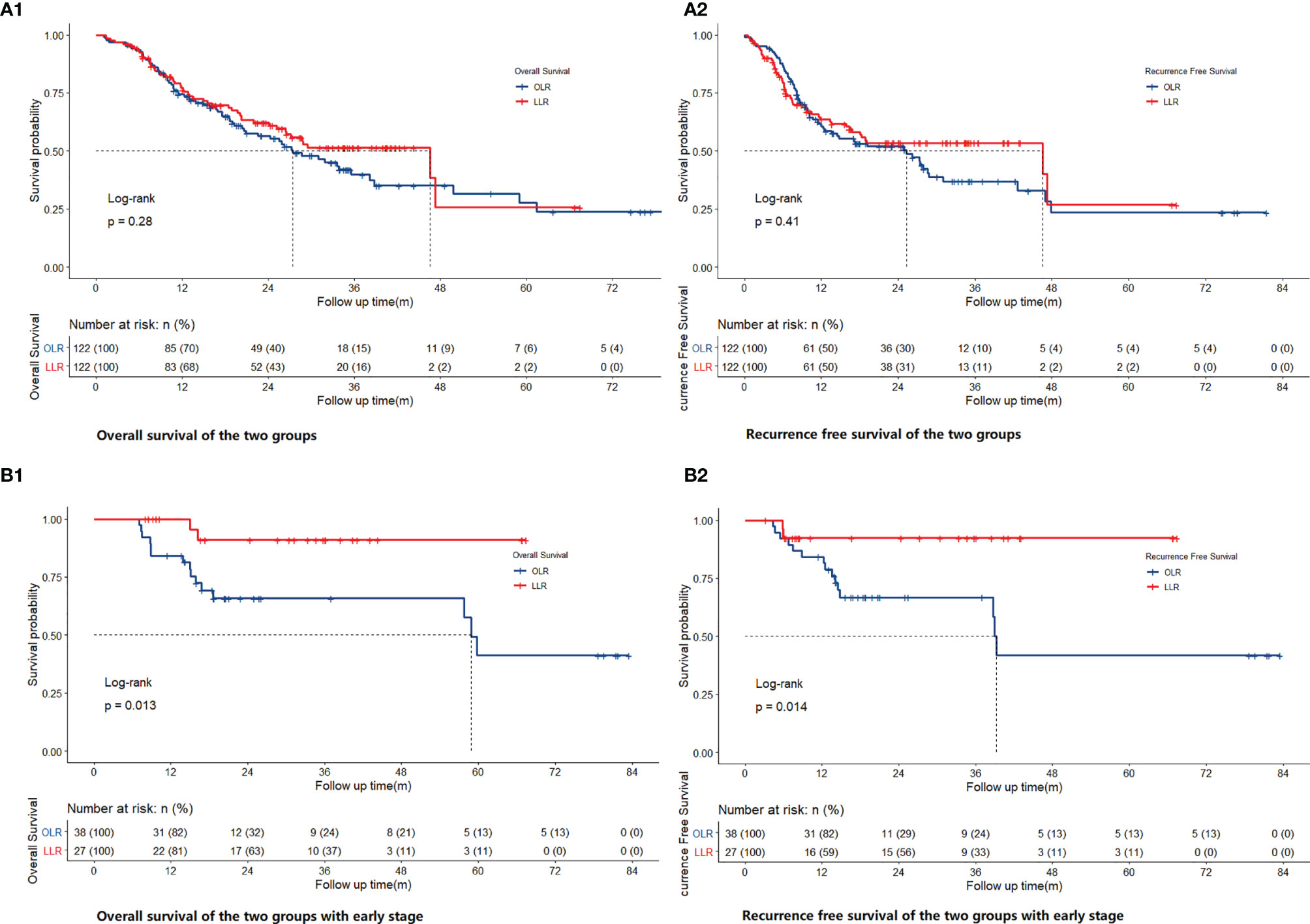
Figure 2 Comparison of OS [(A1) for all stage, (B1) for early stage] and RFS [(A2) for all stage, (B2) for early stage] of OLR and LLR after PSM.
For all-stage patients, TNM stage, differentiation, tumor size, HBV infection, hepatolithiasis, postoperative blood transfusion, resection range, and comorbidity were independent prognostic factors for OS. TNM stage, HBV infection, hepatolithiasis, resection range, and postoperative blood transfusion were independent prognostic factors for RFS. For early-stage patients, CEA >5 ng/ml and blood loss >400 ml were independent prognostic factors for OS. Hepatolithiasis, CEA >5 ng/ml, blood loss >400 ml, and Child–Pugh classification were independent prognostic factors for RFS (Supplementary 3).
Discussion
The widespread use of laparoscopic hepatectomy stands in stark contrast to its small-scale application in ICC. This may be related to the following factors. On the one hand, ICC has a very low incidence rate, comprising approximately 1.5~3% of all primary liver tumors (20). Even the increasing incidence rate in recent years (21) cannot compensate for the small total number. On the other hand, most ICC patients lose the opportunity for surgery at the time of the initial diagnosis because of its asymptomatic nature. An analysis of the data of ICC patients in the SEER database from 1983 to 2010 revealed that only approximately 12.5% of the patients underwent surgical treatment (22). Third, patients often need major liver resection because of the unique oncological characteristics of ICC. This means the reconstruction of large vessels and bile ducts, as well as adequate lymph node dissection, increases the difficulty of laparoscopic resection. Studies have shown that 50–70% of resectable ICC patients undergo hemihepatectomy or extended hepatectomy (23–25).
Despite the above limitations, based on the reported ICC cases, it has been concluded that laparoscopic treatment is no less effective than open hepatectomy (14–16). However, comparative studies of LLR and OLR in early-stage ICC are rare, so the benefits of minimally invasive surgery for early-stage patients remain unclear. Propensity score matching (PSM) has been favored by researchers in recent years. Despite the controversies regarding the selection of variables for generating propensity score models, some scholars have suggested that potential confounding variables not related to exposure but related to the results should be included in the propensity score model (26).
After matching, based on the short-term results, the LLR group had less blood loss (p=0.001 in all-stage, p=0.042 in early-stage) and a shorter hospital stay (p<0.001 in all-stage, p=0.011 in early-stage) than the OLR group, regardless of whether all-stage or early-stage patients were analyzed. The lower blood loss in the LLR group indicates that skilled vascular management may be key to the successful implementation of LLR. The three aspects of vascular management can be summarized as follows: The first point is to control hepatic blood inflow. The main methods at present are the Pringle maneuver and regional blood flow occlusion of tumor-bearing liver segments. The second aspect is the control of blood outflow, especially for patients with tumors located in I, VII, VIII, IVa, or right hemihepatectomy. Dissecting and exposing the hepatic veins of the second hepatic portal (Figure 3B) and the short hepatic vessels of the third hepatic portal (Figure 3A) are very important for safe resection and to reduce bleeding. High-definition magnified laparoscopic images provide a more precise perspective for managing “difficult” hepatic vessels, while the application of some tools (such as “golden finger,” right angle forceps, etc.) (Figure 3A) makes the process of vascular disconnection safer and simpler. The third point is the management of accidental bleeding. Skill in the laparoscopic suture technique is key to avoiding unnecessary conversion (Figure 3D). In many cases, large blood vessels are regarded as a “forbidden zone,” but the actual situation is not “the farther away, the better.” If the structures around the large vessels are not dissected clearly, it can be difficult to suture under laparoscopy or after conversion to open surgery because the large vessels are ruptured. Thus, we suggest that dissociating the perivascular structure and clamping it safely should be done first to prevent possible accidental bleeding. The incidence of complications is also an important indicator of surgical quality and is also related to the duration of hospital stay. For all-stage patients, the incidence of severe complications (grade III and above) in the LLR group was lower (p=0.032) than that in the OLR group, which is also consistent with the results of previous studies on laparoscopic hepatectomy (1). This may be one of the reasons for the shorter hospital stay, but no similar difference was found in the comparison of patients with early-stage ICC (p=0.636). Therefore, the shorter hospital stay may be attributed to the lighter trauma burden of minimally invasive technology, not just complications. However, no difference in hospitalization costs was seen despite the difference in hospital stay duration (p=0.91 for all stage, p=0. 0.293 for early stage).
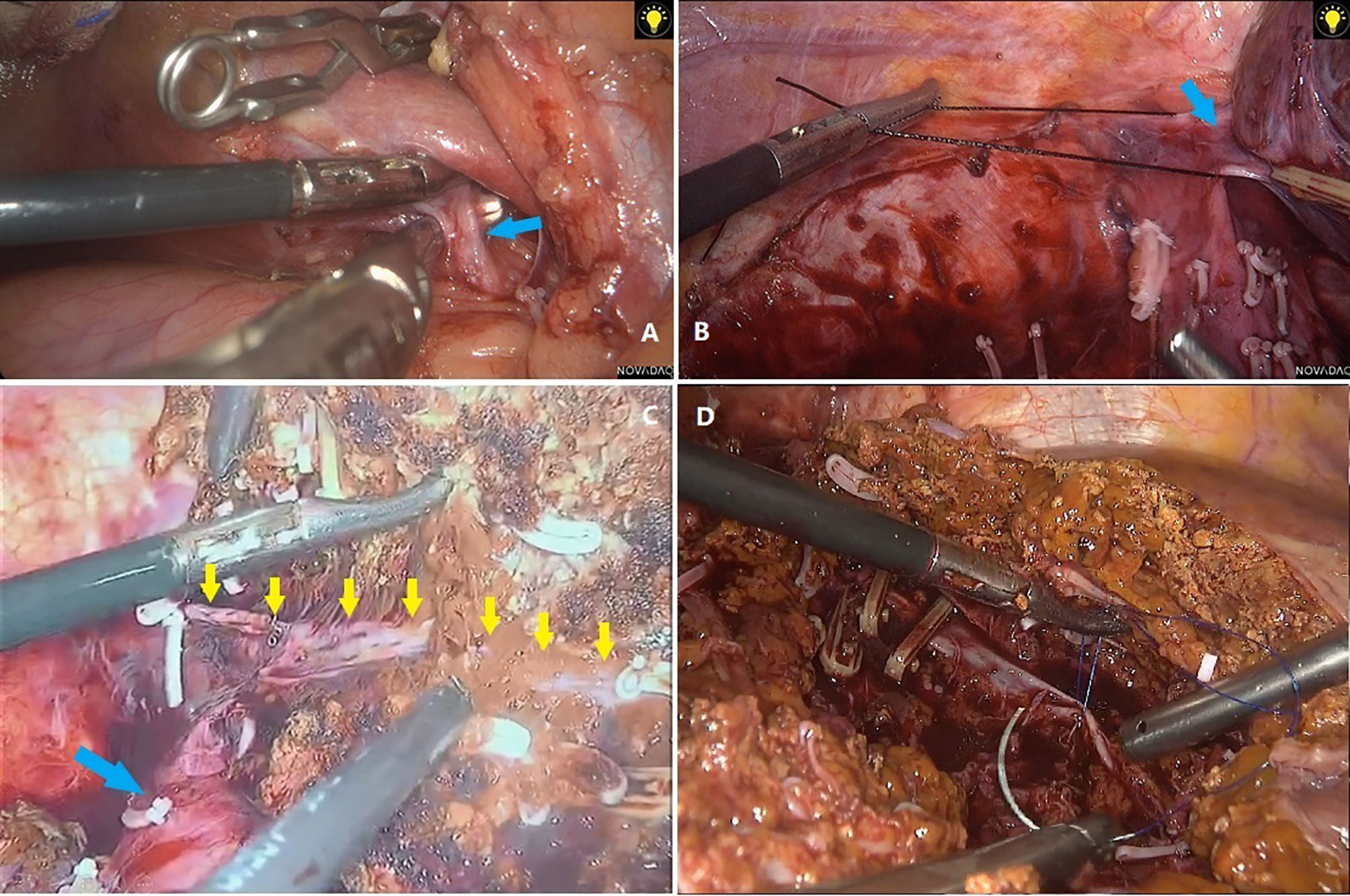
Figure 3 (A) Separation of short hepatic veins with right angle forceps. (B) Dissociation of right hepatic vein. (C) Anatomical resection showed right hepatic vein (yellow arrows) and inferior vena cava (blue arrow). (D) Suture of bleeding hepatic veins.
Relatively speaking, major resections are more often performed in ICC patients and can cause excessive blood loss, the need for blood transfusion, severe complications, and a longer surgery compared with minor resection (5, 7). In this study, major LLR included right hemihepatectomy, right posterior lobectomy, left half + left caudate lobe, right half + partial caudate lobe, middle hepatectomy, and other resections involving segments IVA, VII, VIII, and I. This process requires anatomical or non-anatomical resection, which depends on the volume of the remnant liver or lesion location (Figure 3C).
Surgical experience is also an important factor affecting short-term and long-term outcomes. A lack of experience with certain techniques may have disastrous oncological consequences for ICC patients with a high metastatic burden. The recent European consensus suggested that the learning curve for minor resections is 60 cases, while that for major resections is 55 cases on the basis of minor resections (10). Therefore, the years of experience (≥6 years) and the cumulative number of LLR cases (≥60 cases) should be considered, and patients who did not meet these criteria were excluded (Figure 1).
For the long-term outcomes, no significant difference was found in the all-stage groups after PSM (p=0.28 for OS, p=0.41 for RFS). This conclusion is consistent with that of recent studies on the same subject (15, 17, 27). In fact, most of the current studies on HCC have not found significant differences in long-term oncological outcomes between laparoscopic and open approaches. However, based on the long-term outcomes of the early-stage groups, significant differences were found between the two groups before (p=0.0014 for OS, p=0.0028 for RFS) and after matching (p=0.013 for OS, p=0.014 for RFS). This may be due to the oncological benefit of laparoscopy. As mentioned above, high-definition surgical images, the convenience and safety of laparoscopic lenses and instruments when dealing with “difficult” posterosuperior segments are all possible reasons. Another possible reason is that surgery itself profoundly suppresses cell-mediated immunity (CMI) (28). The exaggerated and prolonged inflammatory, metabolic, and catabolic responses caused by major surgery induce clinical complications, delay recovery, increase mortality (29), and promote cancer metastasis (30–32). Some studies have also confirmed that surgical stress has a negative impact on long-term survival outcomes in patients with colorectal cancer (33). Under the same conditions, we consider that the trauma incurred from laparoscopy is milder than that incurred from open surgery, which also provides a possible explanation to promote this approach for patients with cholangiocarcinoma. In addition, it is not easy to assess whether there are differences in long-term survival outcomes due to anthropic factors. For example, we speculate that if the preoperative assessment indicates that the tumor is adjacent to larger vessels, some surgeons may prefer open surgery; otherwise, laparoscopic surgery may be considered. Therefore, as ICC is more invasive than HCC in terms of its oncological characteristics, it is necessary to be cautious when making conclusions regarding tumor differences or the equivalence of laparoscopic treatment for ICC. Preoperative imaging may not be able to accurately distinguish some ICCs from HCC in clinical practice, but in view of the benefits of laparoscopic treatment compared with open surgery (34, 35) and the similar results of this retrospective study for patients with early HCC, the clinical significance of this study is that laparoscopic surgery can be recommended for patients with a tumor diameter of ≤3 cm and no vascular invasion—even if it is difficult to distinguish ICC from HCC on imaging. Higher-quality studies should be carried out to further verify the role of laparoscopy in early ICC patients, which may have a certain impact on the choice of surgical methods.
Conclusion
Patients treated with laparoscopy seem to have better short-term outcomes, such as less blood loss, shorter operation duration, and shorter hospital stay, than patients undergoing open surgery. Based on the long-term results, no significant difference was found between OS and RFS in all-stage patients, but the differences were more obvious in early-stage patients. Future research needs to examine the outcomes of early-stage ICC patients from different aspects, such as long-term complications, and focus on the improving the quality of these patients’ lives.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University. The data used in this article are all items that must be checked according to medical standards during the hospitalization, and collected retrospectively when designing the study, without adding any additional medical examination or test outside the normal diagnosis and treatment procedures.
Author Contributions
YJ is responsible for the conception, design, and writing of the article. WYi and ZY are responsible for the data processing and analysis. MD, CX, WY, DL, YH, WL, DT, CK, HJ, ZC, and WD are responsible for collecting the original data. JB and CG are responsible for reviewing and guiding the revision of the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81772628, 81703310, 82072685), the Research Foundation of National Health Commission of China–Major Medical and Health Technology Project for Zhejiang Province (WKJ-ZJ-1706).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.742544/full#supplementary-material
References
1. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Short-Term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg (2016) 263(4):761–77. doi: 10.1097/SLA.0000000000001413
2. Ibuki S, Hibi T, Tanabe M, Geller DA, Cherqui D, Wakabayashi G. Short-Term Outcomes of "Difficult" Laparoscopic Liver Resection at Specialized Centers: Report From INSTALL (International Survey on Technical Aspects of Laparoscopic Liver Resection)-2 on 4478 Patients. Ann Surg (2020). doi: 10.1097/SLA.0000000000004434
3. Ban D, Tanabe M, Kumamaru H, Nitta H, Otsuka Y, Miyata H, et al. Safe Dissemination of Laparoscopic Liver Resection in 27,146 Cases Between 2011 and 2017 From the National Clinical Database of Japan. Ann Surg (2020) 274(6):1043–50. doi: 10.1097/SLA.0000000000003799
4. Franken C, Lau B, Putchakayala K, DiFronzo LA. Comparison of Short-Term Outcomes in Laparoscopic vs Open Hepatectomy. JAMA Surg (2014) 149(9):941–6. doi: 10.1001/jamasurg.2014.1023
5. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for Laparoscopic Liver Resection: A Report From the Second International Consensus Conference Held in Morioka. Ann Surg (2015) 261(4):619–29. doi: 10.1097/SLA.0000000000001184
6. Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, et al. Long-Term Survival Analysis of Pure Laparoscopic Versus Open Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Single-Center Experience. Ann Surg (2013) 257(3):506–11. doi: 10.1097/SLA.0b013e31827b947a
7. Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg (2018) 267(1):13–7. doi: 10.1097/SLA.0000000000002176
8. Dagher I, O'Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, et al. Laparoscopic Major Hepatectomy: An Evolution in Standard of Care. Ann Surg (2009) 250(5):856–60. doi: 10.1097/SLA.0b013e3181bcaf46
9. Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The International Position on Laparoscopic Liver Surgery: The Louisville Statement, 2008. Ann Surg (2009) 250(5):825–30. doi: 10.1097/SLA.0b013e3181b3b2d8
10. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg (2018) 268(1):11–8. doi: 10.1097/SLA.0000000000002524
11. Syn NL, Kabir T, Koh YX, Tan HL, Wang LZ, Chin BZ, et al. Survival Advantage of Laparoscopic Versus Open Resection For Colorectal Liver Metastases: A Meta-Analysis of Individual Patient Data From Randomized Trials and Propensity-Score Matched Studies. Ann Surg (2020) 272(2):253–65. doi: 10.1097/SLA.0000000000003672
12. Black N. Why We Need Observational Studies to Evaluate the Effectiveness of Health Care. BMJ (1996) 312(7040):1215–8. doi: 10.1136/bmj.312.7040.1215
13. Silverman SL. From Randomized Controlled Trials to Observational Studies. Am J Med (2009) 122(2):114–20. doi: 10.1016/j.amjmed.2008.09.030
14. Lee W, Park JH, Kim JY, Kwag SJ, Park T, Jeong SH, et al. Comparison of Perioperative and Oncologic Outcomes Between Open and Laparoscopic Liver Resection for Intrahepatic Cholangiocarcinoma. Surg Endosc (2016) 30(11):4835–40. doi: 10.1007/s00464-016-4817-x
15. Kang SH, Choi Y, Lee W, Ahn S, Cho JY, Yoon YS, et al. Laparoscopic Liver Resection Versus Open Liver Resection for Intrahepatic Cholangiocarcinoma: 3-Year Outcomes of a Cohort Study With Propensity Score Matching. Surg Oncol (2020) 33:63–9. doi: 10.1016/j.suronc.2020.01.001
16. Uy BJ, Han HS, Yoon YS, Cho JY. Laparoscopic Liver Resection for Intrahepatic Cholangiocarcinoma. J Laparoendosc Adv Surg Tech A (2015) 25(4):272–7. doi: 10.1089/lap.2014.0233
17. Ratti F, Cipriani F, Ariotti R, Gagliano A, Paganelli M, Catena M, et al. Safety and Feasibility of Laparoscopic Liver Resection With Associated Lymphadenectomy for Intrahepatic Cholangiocarcinoma: A Propensity Score-Based Case-Matched Analysis From a Single Institution. Surg Endosc (2016) 30(5):1999–2010. doi: 10.1007/s00464-015-4430-4
18. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile Leakage After Hepatobiliary and Pancreatic Surgery: A Definition and Grading of Severity by the International Study Group of Liver Surgery. Surgery (2011) 149(5):680–8. doi: 10.1016/j.surg.2010.12.002
19. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy Liver Failure: A Definition and Grading by the International Study Group of Liver Surgery (ISGLS). Surgery (2011) 149(5):713–24. doi: 10.1016/j.surg.2010.10.001
20. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: The Next Horizon in Mechanisms and Management. Nat Rev Gastroenterol Hepatol (2020) 17(9):557–88. doi: 10.1038/s41575-020-0310-z
21. Wu L, Tsilimigras DI, Paredes AZ, Mehta R, Hyer JM, Merath K, et al. Trends in the Incidence, Treatment and Outcomes of Patients With Intrahepatic Cholangiocarcinoma in the USA: Facility Type is Associated With Margin Status, Use of Lymphadenectomy and Overall Survival. World J Surg (2019) 43(7):1777–87. doi: 10.1007/s00268-019-04966-4
22. Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal Trends in Liver-Directed Therapy of Patients With Intrahepatic Cholangiocarcinoma in the United States: A Population-Based Analysis. J Surg Oncol (2014) 110(2):163–70. doi: 10.1002/jso.23605
23. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis of Prognostic Factors and Lymph Node Assessment. J Clin Oncol (2011) 29(23):3140–5. doi: 10.1200/JCO.2011.35.6519
24. Sotiropoulos GC, Bockhorn M, Sgourakis G, Brokalaki EI, Molmenti EP, Neuhäuser M, et al. R0 Liver Resections for Primary Malignant Liver Tumors in the Noncirrhotic Liver: A Diagnosis-Related Analysis. Dig Dis Sci (2009) 54(4):887–94. doi: 10.1007/s10620-008-0408-6
25. Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic Cholangiocarcinoma: Rising Frequency, Improved Survival, and Determinants of Outcome After Resection. Ann Surg (2008) 248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3
26. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable Selection for Propensity Score Models. Am J Epidemiol (2006) 163(12):1149–56. doi: 10.1093/aje/kwj149
27. Ratti F, Rawashdeh A, Cipriani F, Primrose J, Fiorentini G, Abu Hilal M, et al. Intrahepatic Cholangiocarcinoma as the New Field of Implementation of Laparoscopic Liver Resection Programs. A Comparative Propensity Score-Based Analysis of Open and Laparoscopic Liver Resections. Surg Endosc (2021) 35(4):1851–62. doi: 10.1016/j.hpb.2020.04.458
28. Shakhar G, Ben-Eliyahu S. Potential Prophylactic Measures Against Postoperative Immunosuppression: Could They Reduce Recurrence Rates in Oncological Patients. Ann Surg Oncol (2003) 10(8):972–92. doi: 10.1245/ASO.2003.02.007
29. Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The Surgically Induced Stress Response. JPEN J Parenter Enteral Nutr (2013) 37(5 Suppl):21S–9S. doi: 10.1177/0148607113496117
30. Neeman E, Ben-Eliyahu S. Surgery and Stress Promote Cancer Metastasis: New Outlooks on Perioperative Mediating Mechanisms and Immune Involvement. Brain Behav Immun (2013) 30 Suppl(Suppl):S32–40. doi: 10.1016/j.bbi.2012.03.006
31. Kumagai Y, Ohzawa H, Miyato H, Horie H, Hosoya Y, Lefor AK, et al. Surgical Stress Increases Circulating Low-Density Neutrophils Which May Promote Tumor Recurrence. J Surg Res (2020) 246:52–61. doi: 10.1016/j.jss.2019.08.022
32. Chen Z, Zhang P, Xu Y, Yan J, Liu Z, Lau WB, et al. Surgical Stress and Cancer Progression: The Twisted Tango. Mol Cancer (2019) 18(1):132. doi: 10.1186/s12943-019-0943-0
33. Shibutani M, Nakao S, Maeda K, Nagahara H, Fukuoka T, Iseki Y, et al. Inflammation Caused by Surgical Stress Has a Negative Impact on the Long-Term Survival Outcomes in Patients With Colorectal Cancer. Anticancer Res (2020) 40(6):3535–42. doi: 10.21873/anticanres.14342
34. Tsai KY, Chen HA, Wang WY, Huang MT. Long-Term and Short-Term Surgical Outcomes of Laparoscopic Versus Open Liver Resection for Hepatocellular Carcinoma: Might Laparoscopic Approach be Better in Early HCC. Surg Endosc (2019) 33(4):1131–9. doi: 10.1007/s00464-018-6372-0
Keywords: intrahepatic cholangiocarcinoma, laparoscopic hepatectomy, liver resection, overall survival, recurrence-free survival
Citation: Jinhuan Y, Yi W, Yuanwen Z, Delin M, Xiaotian C, Yan W, Liming D, Haitao Y, Lijun W, Tuo D, Kaiyu C, Jiawei H, Chongming Z, Daojie W, Bin J and Gang C (2022) Laparoscopic Versus Open Surgery for Early-Stage Intrahepatic Cholangiocarcinoma After Mastering the Learning Curve: A Multicenter Data-Based Matched Study. Front. Oncol. 11:742544. doi: 10.3389/fonc.2021.742544
Received: 16 July 2021; Accepted: 08 December 2021;
Published: 07 January 2022.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Federica Cipriani, San Raffaele Scientific Institute (IRCCS), ItalyEphraim Tang, Western University, Canada
Copyright © 2022 Jinhuan, Yi, Yuanwen, Delin, Xiaotian, Yan, Liming, Haitao, Lijun, Tuo, Kaiyu, Jiawei, Chongming, Daojie, Bin and Gang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Gang, Y2hlbi5nYW5nQHdtdS5lZHUuY24=; Jin Bin, amluYmluOTQ0OUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Yang Jinhuan
Yang Jinhuan Wang Yi
Wang Yi Zheng Yuanwen3†
Zheng Yuanwen3† Ma Delin
Ma Delin Chen Xiaotian
Chen Xiaotian Deng Liming
Deng Liming Yu Haitao
Yu Haitao Chen Kaiyu
Chen Kaiyu Hu Jiawei
Hu Jiawei Chen Gang
Chen Gang