- 1Department of Pediatrics, Division of Oncology, The Children’s Hospital of Philadelphia, Philadelphia, PA, United States
- 2Department of Pediatrics, Division of Oncology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
- 3Department of Neurology and Neurological Sciences, Division of Child Neurology, Lucile Packard Children’s Hospital at Stanford University, Palo Alto, CA, United States
- 4Department of Radiation Oncology, Stanford University Cancer Center, Palo Alto, CA, United States
- 5Department of Pediatrics, Division of Hematology/Oncology, Children’s Healthcare of Atlanta, Atlanta, GA, United States
Purpose: Medulloblastoma is one of the most common malignant brain tumors in children. To date, the treatment of average-risk (non-metastatic, completely resected) medulloblastoma includes craniospinal radiation therapy and adjuvant chemotherapy. Modern treatment modalities and now risk stratification of subgroups have extended the survival of these patients, exposing the long-term morbidities associated with radiation therapy. Prior to advances in molecular subgrouping, we sought to reduce the late effects of radiation in patients with average-risk medulloblastoma.
Methods: We performed a single-arm, multi-institution study, reducing the dose of craniospinal irradiation by 25% to 18 Gray (Gy) with the goal of maintaining the therapeutic efficacy as described in CCG 9892 with maintenance chemotherapy.
Results: Twenty-eight (28) patients aged 3-30 years were enrolled across three institutions between April 2001 and December 2010. Median age at enrollment was 9 years with a median follow-up time of 11.7 years. The 3-year relapse-free (RFS) and overall survival (OS) were 79% (95% confidence interval [CI] 58% to 90%) and 93% (95% CI 74% to 98%), respectively. The 5-year RFS and OS were 71% (95% CI 50% to 85%) and 86% (95% CI 66% to 94%), respectively. Toxicities were similar to those seen in other studies; there were no grade 5 toxicities.
Conclusions: Given the known neurocognitive adverse effects associated with cranial radiation therapy, studies to evaluate the feasibility of dose reduction are needed. In this study, we demonstrate that select patients with average-risk medulloblastoma may benefit from a reduced craniospinal radiation dose of 18 Gy without impacting relapse-free or overall survival.
Clinical Trial Registration: ClinicalTrials.gov identifier: NCT00031590
Introduction
Medulloblastoma is the most common malignant brain tumor in children, with an annual incidence of 0.4 per 100,000 population aged 0-19 years (1). Optimal treatment of average-risk (i.e., non-metastatic, gross totally resected) medulloblastoma includes surgical resection, craniospinal irradiation (CSI) and chemotherapy. Over the past several decades, studies have aimed to mitigate the adverse neurocognitive effects of radiation by reducing the craniospinal radiation dose. In a clinical trial from the Children’s Cancer Group (CCG) and Pediatric Oncology Group (POG) for low-stage (gross totally resected tumor with no metastasis and no brainstem invasion) medulloblastoma that randomized patients to 23.4 Gy or 36 Gy CSI without adjuvant chemotherapy, early relapses and increased exoprimary failure rate were seen in the lower radiation dose arm (2). Studies that incorporated adjuvant chemotherapy in average-risk medulloblastoma demonstrated 5-year event free survival (EFS) over 80% in the late 1990s (3, 4). The increased survival of children with medulloblastoma into adulthood revealed the potentially severe late effects and resultant decreased quality of life that may occur as a consequence of radiation therapy (5–8). Thus, attempts have been made to further reduce CSI doses in lower risk patients (2, 9, 10). In the Children’s Oncology Group trial ACNS0331 children ages 3 to 7 years diagnosed with medulloblastoma were randomized to 18 Gray (Gy) or 23.4 Gy CSI with variable posterior fossa radiation boosts, followed by nine cycles of alkylator-based chemotherapy (11).
In this single-arm, multi-institution pilot study, we aimed to reduce the late neurotoxic (otologic, neuroendocrine, neurocognitive, neurovascular) effects of treatment in patients with average-risk medulloblastoma across a broader age range from 3 to 30 years at diagnosis. To do so, the craniospinal irradiation dose was decreased to 18 Gy while maintaining the standard chemotherapy as described in CCG 9892 (12) and adding cyclophosphamide and etoposide as in the contemporaneous trials CCG 9961 (13) and POG 9631 (14), respectively.
Methods
Patients
Eligible patients were age 3 to 30 years at the time of diagnosis with average-risk, histologically proven medulloblastoma as determined by each participating institution. Average-risk was defined as less than 1.5 cm3 residual tumor following resection by neuroimaging, no evidence of metastases by brain and spine MRI, and no tumors cells present on cerebrospinal fluid (CSF) cytology. CSF was obtained by lumbar puncture between 10 days and 4 weeks after surgery. Postoperative MRI of the primary site with and without contrast was required within 72 hours of surgery, preferably within 48 hours; volumetrics were performed by neuroradiologists at their respective institutions.
Study Design
This single-arm study was developed by the authors and conducted at three institutions in the United States. The primary objective was to reduce the late toxicity of CSI without compromising the therapeutic efficacy compared with standard therapy [86% ± 4% standard error of the mean (SEM) three-year relapse-free survival per CCG 9892 (12)]. The secondary objectives were to assess relapse-free survival and overall survival 5 years from time of study enrollment.
Surgical resection was carried out according to institutional standards with staging as stated above. Following institutional diagnosis of medulloblastoma, histologic slides were sent to the Children’s Hospital of Philadelphia for correlative studies.
Patients began CSI within four weeks of surgical resection; concurrent weekly intravenous (IV) vincristine 1.5mg/m2 (maximum single dose 2 mg) was administered for 6 doses. Radiation therapy consisted of 18 Gy photons to the whole brain and spine in 10 fractions with a boost to the tumor bed in 21 fractions for a total dose of 55.8 Gy to the tumor bed. Four weeks after completion of radiotherapy, maintenance chemotherapy was initiated, consisting of 9 cycles of regimens A and B in an AABAABAAB pattern. Regimen A included oral lomustine 75 mg/m2 and IV cisplatin 70 mg/m2 on day 0, and IV vincristine 1.5 mg/m2 (maximum single dose 2 mg) on days 0, 7, and 14. For regimen B, patients received IV cyclophosphamide 1000 mg/m2 on days 0 and 1 and IV etoposide 150 mg/m2 on days 0 and 1, followed by oral etoposide 50 mg/m2 on days 14-34.
All patients were monitored for adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. MRI of the brain and spine with and without gadolinium was obtained prior to surgery, post-operatively, in 3-month intervals during maintenance chemotherapy and in the first year after the end of treatment. Participants then underwent imaging every 6 months for years 2 and 3, and yearly thereafter or if clinically indicated for suspected relapse. Relapse was defined as a greater than 25% increase in the product of the longest two perpendicular measurements of residual tumor on MRI, evidence of new tumor by MRI, or de novo tumor cells in the CSF. Patients were removed from study for relapse, unacceptable toxicity or other extraordinary medical circumstances, inability or refusal to comply with study treatment or follow up with required observations, or death.
Audiograms were obtained prior to the start of each chemotherapy cycle, every 6 months from one to three years after the completion of chemotherapy and yearly thereafter. Laboratory studies to evaluate liver, kidney and endocrinologic function as well as MRI were obtained serially according to study protocol. Neurocognitive testing utilizing age-appropriate Wechsler Scales was obtained within 6 months of diagnosis and at one, two and three years after the completion of chemotherapy.
The study was approved by institutional review boards at the participating institutions and conducted in accordance with the Declaration of Helsinki. All patients or their legal guardians provided written informed consent and age-appropriate assent was obtained. ClinicalTrials.gov identifier: NCT00031590.
Statistical Analysis
The target accrual of the study was 50 patients over 3 years, assuming that 15% represents an acceptable difference in relapse-free survival between the study and the standard survival rate of 86 ± 4% at 3 years in CCG 9892 (12). With a sample size of 50, 11 events would represent relapse-free survival of 78% with a 90% confidence interval (CI) of 70.8% - 85.8%. Stopping rules were designed to close accrual if local relapses, exoprimary relapses or toxicity of the protocol exceeded those reported in CCG 9892 (12). Relapse-free survival (RFS) was defined as the time to first disease progression, disease recurrence, death from any cause, or occurrence of a subsequent malignant neoplasm. Overall survival (OS) was defined as time to death from any cause. Time to first relapse was defined as the time from diagnosis to the time of tumor relapse as previously defined. RFS and OS and were calculated using the methods of Kaplan and Meier.
Results
Patient Characteristics
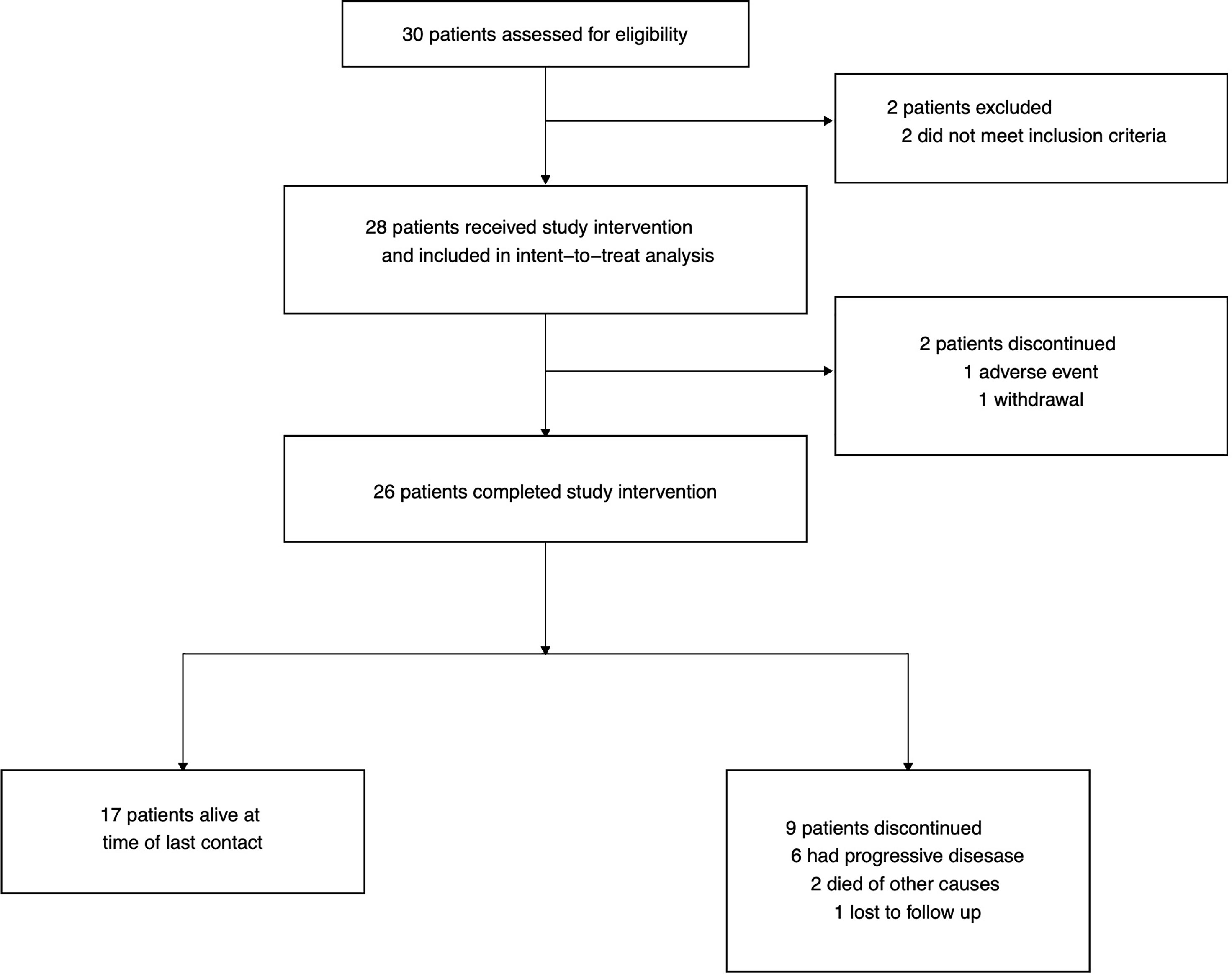
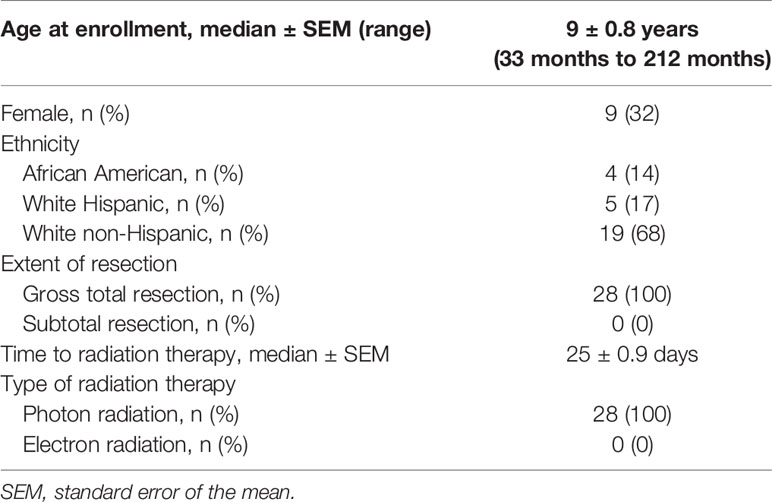
Thirty patients were assessed for eligibility; 2 were excluded as they did not meet inclusion criteria: one patient due to presence of tumor cells in CSF by cytology at diagnosis, and the other due to a delay in starting radiation therapy (Figure 1). A total of 28 patients were enrolled on study between April 2001 and December 2010 across three institutions (Children’s Hospital of Philadelphia, Children’s Hospital of Atlanta, and Lucile Packard Children’s Hospital at Stanford University). Median age at enrollment was 9 ± 0.8 years (range: 2.8 to 17.7 years). Collected patient characteristics are listed in Table 1. The study was terminated early due to slow accrual in light of evolving knowledge of molecular subgrouping and risk stratification in medulloblastoma.
Safety and Tolerability
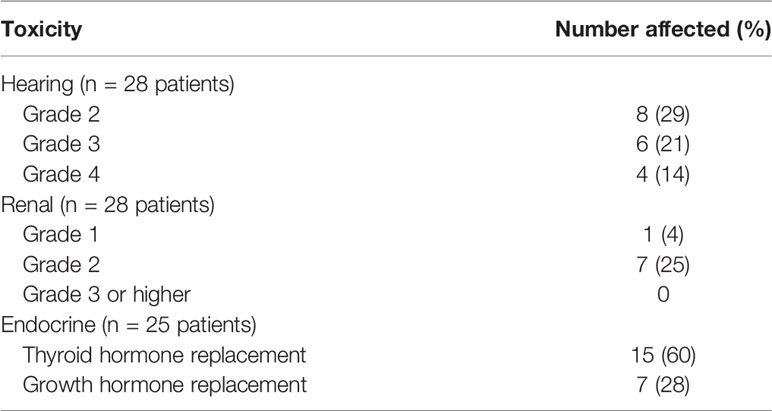
Two patients (7%) withdrew from study; one due to toxicity from chemotherapy and the other due to withdrawal of consent. Both patients were included in the intent-to-treat analysis. Eight patients (29%) received all 6 cycles of cisplatin-containing chemotherapy whereas the remainder (20, 71%) had dose reductions, omissions or were changed to carboplatin for renal toxicity, ototoxicity, or parental preference, with approval of the study chair. No dose reductions in regimen B (IV cyclophosphamide, IV and oral etoposide) were required. Of the 25 patients with endocrinologic monitoring data, 15 (60%) required thyroid hormone replacement and 7 (28%) required growth hormone replacement at some point during or after their treatment course. Collected toxicity data are summarized in Table 2. There were no grade 5 toxicities.
Survival
The median follow-up time was 140 months (11.7 years; interquartile range 111 to 158 months) by reverse Kaplan-Meier estimator (15). The 3-year RFS and OS were 79% (95% CI 58% to 90%) and 93% (95% CI 74% to 98%), respectively. The 5-year RFS and OS were 71% (95% CI 50% to 85%) and 86% (95% CI 66% to 94%), respectively (Figures 2A, B). Nineteen patients (68%) were alive at the time of last contact with no evidence of disease. Seven patients (25%) died of disease, whereas one patient died of secondary glioblastoma and one (who was later diagnosed with a germline TP53 mutation) of secondary skull base sarcoma.
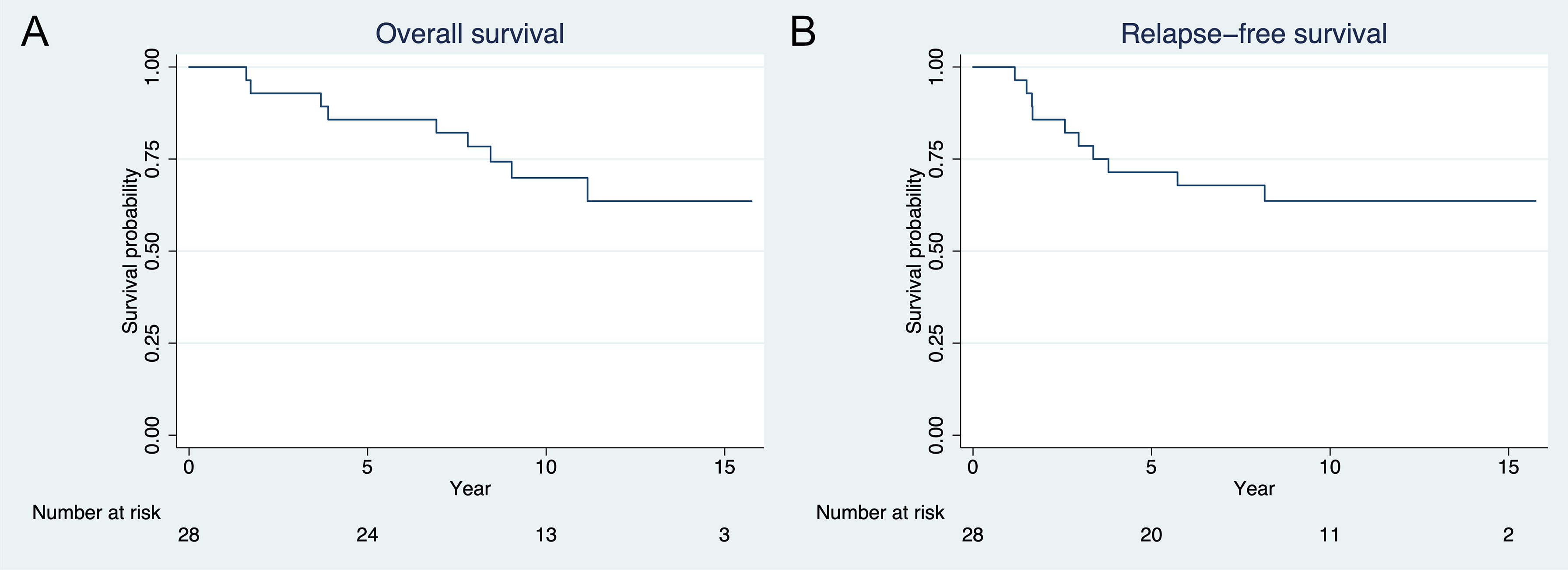
Figure 2 Kaplan-Meier plot of (A) overall survival and (B) recurrence free survival. Three- and 5-year overall survival are 93% and 86%, respectively. Three- and 5-year relapse-free survival are 79% and 71%, respectively.
Eighteen patients (64%) were greater than 7 years of age at enrollment. For this subgroup, the 3- and 5-year OS were 83% (95% CI 68% to 100%) and 78% (95% CI 61% to 100%). Of these 18 patients, 11 (61%) were alive at the time of last contact with no evidence of disease at a median follow up time of 11.7 ± 1.0 years. There was no difference in 3- and 5-year OS when compared to patients less than or equal to 7 years of age or in all patients when stratified by sex.
Patterns of Relapse
Seven patients (25%) developed relapsed disease: two patients with diffuse or leptomeningeal relapse including the primary site; three with isolated intraspinal tumor; one with exoprimary brain relapse solely in the pituitary; and one patient who initially developed primary site relapse and subsequently disseminated disease.
Neurocognitive Outcomes
Partial neurocognitive data was available on 15 patients at multiple different time points, limiting complete analysis. Collected data demonstrated mixed changes over time (Supplementary Figure 1). One year from the initiation of therapy, one patient showed an increase of greater than 10 IQ points, one patient demonstrated a decrease of greater than 10 IQ points. The remaining six patients from whom data was collected remained within 10 IQ points of their baseline. At 3 years, two patients experienced a decline in greater than 10 IQ points, one remained stable and another had an increase of greater than 10 IQ points from baseline.
Discussion
In this study, CSI dose reduction to 18 Gy in select patients with average-risk medulloblastoma was associated with similar 3- and 5-year relapse-free and overall survival as well as toxicity profiles compared with historical clinical trials. Considering the burgeoning literature on the long-term effects of chemoradiotherapy in the treatment of pediatric cancers, many studies have sought to risk stratify patients while maintaining efficacy. In this study, there was no significant difference in 3-year RFS in comparison to CCG 9892 (3-year EFS 86% ± SEM 4%); however, there were 4 out of 28 isolated exoprimary relapses (compared to 3 out of 14 in CCG 9892). The Children’s Oncology Group ACNS0331 study has also evaluated CSI dose reduction in patients 3 to 7 years of age with newly diagnosed average-risk medulloblastoma. The data demonstrated inferiority of low dose compared to standard dose craniospinal irradiation in both EFS and OS. However, when post-hoc molecular subtyping was performed, the inferior outcomes in the low-dose CSI arm were largely driven by group 4 tumors (11). In review of the published literature, this is the only clinical study that addresses the utilization of 18 Gy CSI in a patient greater than 7 years of age. These data suggest that reduced dose CSI in select patients with average-risk medulloblastomas should be studied.
Although chemotherapy was relatively well tolerated, 71% of patients on this study had dose reductions of cisplatin or substitutions for carboplatin due to ototoxicity and/or nephrotoxicity, both well-recognized adverse events associated with cisplatin (16–18). Indeed, newer medulloblastoma protocols seeking to limit cisplatin-associated toxicity have reduced the total cumulative dosage in patients with average-risk medulloblastoma without decreases in overall survival (19, 20). In this study, 2 out of 28 patients (7%) developed secondary malignancies. Although one was later diagnosed with a germline TP53 mutation, this rate is higher than the 3.4% seen for all patients in ACNS0331.
This study is not without limitations. As a single-arm trial with a small sample size, the results are difficult to generalize, and subgroup analyses were not performed. This study took place prior to the widespread implementation of molecular subtyping, which is now essential in medulloblastoma clinical trial risk stratification (21), and ultimately led to early closure prior to meeting accrual goals. Residual tissue for post-hoc testing was limited due to use in correlative studies; in addition, retrospective molecular analysis was not provided for in the protocol, nor is the number of subjects large enough for meaningful comparison, significantly limiting conclusions in this era of integrated molecular diagnostics. Of note, wingless (WNT) pathway activation has been associated with an excellent prognosis in patients with medulloblastoma (22); the concept of dose reduction in this subgroup is currently being studied in clinical trials. While the sample size is small, it is statistically improbable based on age and sex distributions that all patients recruited in this study had WNT-activated tumors (22–24). Thus, the similarity in OS and EFS of this study to others is likely not spurious. The exclusion of anaplastic histologies from average risk medulloblastoma trials was implemented in 2008. Finally, the interpretation of neurocognitive data is significantly impacted by incomplete collection and indeed precludes the analysis of the study’s primary objective. The limited psychometrics implemented in this study were typical of the era and have since been supplanted by more precise and standardized batteries of tests, e.g., the Children’s Oncology Group study ALTE07C1 (25), which was added to ACNS0331 as an amendment in February 2011.
Overall, though the results of the study were somewhat disappointing compared to prior studies, many patients achieved long-term survival without relapse with 18 Gy CSI, potentially limiting late neurocognitive deficits and neurovascular disease and improving quality of life. With the emerging knowledge of differing clinical behaviors by subgroup of medulloblastoma, it is possible that the small differences in outcomes in this study are explained by molecular subtype. Larger, randomized controlled trials with molecular classification and integrated risk adapted staging are needed.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by participating sites’ respective institutional review boards. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Study conception and design: JM, JB, IG, BL, PP, PF, MF, and AJ. Acquisition, analysis, and interpretation of data: JM, AM, SP, YL, PF, MF, and AJ. Drafting of manuscript: AM and SP. Critical revision: all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge Hui-Kuo Shu and Avital Cnaan for their contributions to this project. The content of this manuscript has been presented at the International Society of Pediatric Neuro-Oncology 2020 meeting: AM, AJ, SP, PF, YL, MF, and JM. A study of low-dose craniospinal radiation therapy in patients with newly diagnosed average-risk medulloblastoma. (2020). Neuro Oncol. 22 (suppl 3), iii390-iii391.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.744739/full#supplementary-material
Supplementary Figure 1 | Collected raw full scale intelligence quotients (FSIQ) for patients on study over time.
References
1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol (2020) 22:iv1–96. doi: 10.1093/neuonc/noaa200
2. Thomas PR, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, et al. Low-Stage Medulloblastoma: Final Analysis of Trial Comparing Standard-Dose With Reduced-Dose Neuraxis Irradiation. J Clin Oncol (2000) 18:3004–11. doi: 10.1200/JCO.2000.18.16.3004
3. Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a Randomized Study of Preradiation Chemotherapy Versus Radiotherapy Alone for Nonmetastatic Medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol (2003) 21:1581–91. doi: 10.1200/JCO.2003.05.116
4. Kortmann R-D, Kühl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative Neoadjuvant Chemotherapy Before Radiotherapy as Compared to Immediate Radiotherapy Followed by Maintenance Chemotherapy in the Treatment of Medulloblastoma in Childhood: Results of the German Prospective Randomized Trial Hit ‘91. Int J Radiat OncologyBiologyPhysics. (2000) 46:269–79. doi: 10.1016/S0360-3016(99)00369-7
5. Olshan JS, Gubernick J, Packer RJ, D’Angio GJ, Goldwein JW, Willi SM, et al. The Effects of Adjuvant Chemotherapy on Growth in Children With Medulloblastoma. Cancer (1992) 70:2013–7. doi: 10.1002/1097-0142(19921001)70:7<2013::AID-CNCR2820700734>3.0.CO;2-J
6. Radcliffe J, Packer RJ, Atkins TE, Bunin GR, Schut L, Goldwein JW, et al. Three- and Four-Year Cognitive Outcome in Children With Noncortical Brain Tumors Treated With Whole-Brain Radiotherapy. Ann Neurol (1992) 32:551–4. doi: 10.1002/ana.410320411
7. Silber JH, Radcliffe J, Peckham V, Perilongo G, Kishnani P, Fridman M, et al. Whole-Brain Irradiation and Decline in Intelligence: The Influence of Dose and Age on IQ Score. J Clin Oncol (1992) 10:1390–6. doi: 10.1200/JCO.1992.10.9.1390
8. Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML. Neuropsychological Sequelae of the Treatment of Children With Medulloblastoma. J Neurooncol. (1996) 29:91–101. doi: 10.1007/BF00165522
9. Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III Study of Craniospinal Radiation Therapy Followed by Adjuvant Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J Clin Oncol (2006) 24:4202–8. doi: 10.1200/JCO.2006.06.4980
10. Dietzsch S, Placzek F, Pietschmann K, von Bueren AO, Matuschek C, Gluck A, et al. Evaluation of Prognostic Factors and Role of Participation in a Randomized Trial or a Prospective Registry in Pediatric and Adolescent Nonmetastatic Medulloblastoma - A Report From the HIT 2000 Trial. Adv Radiat Oncol (2020) 5:1158–69. doi: 10.1016/j.adro.2020.09.018
11. Michalski JM, Janss AJ, Vezina LG, Smith KS, Billups CA, Burger PC, et al. Children’s Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J Clin Oncol (2021) 39:2685–97. doi: 10.1200/JCO.20.02730
12. Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of Children With Medulloblastomas With Reduced-Dose Craniospinal Radiation Therapy and Adjuvant Chemotherapy: A Children’s Cancer Group Study. J Clin Oncol (1999) 17:2127–36. doi: 10.1200/JCO.1999.17.7.2127
13. Donahue B, Marymont MA, Kessel S, Iandoli MK, Fitzgerald T, Holmes E, et al. Radiation Therapy Quality in CCG/POG Intergroup 9961: Implications for Craniospinal Irradiation and the Posterior Fossa Boost in Future Medulloblastoma Trials. Front Oncol (2012) 2:185. doi: 10.3389/fonc.2012.00185
14. Esbenshade AJ, Kocak M, Hershon L, Rousseau P, Decarie JC, Shaw S, et al. A Phase II Feasibility Study of Oral Etoposide Given Concurrently With Radiotherapy Followed by Dose Intensive Adjuvant Chemotherapy for Children With Newly Diagnosed High-Risk Medulloblastoma (Protocol POG 9631): A Report From the Children’s Oncology Group. Pediatr Blood Cancer (2017) 64:e26373. doi: 10.1002/pbc.26373
15. Schemper M, Smith TL. A Note on Quantifying Follow-Up in Studies of Failure Time. Controlled Clin Trials. (1996) 17:343–6. doi: 10.1016/0197-2456(96)00075-X
16. Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum Compound-Related Ototoxicity in Children: Long-Term Follow-Up Reveals Continuous Worsening of Hearing Loss. J Pediatr Hematol Oncol (2004) 26:649–55. doi: 10.1097/01.mph.0000141348.62532.73
17. Li Y, Womer RB, Silber JH. Predicting Cisplatin Ototoxicity in Children: The Influence of Age and the Cumulative Dose. Eur J Cancer. (2004) 40:2445–51. doi: 10.1016/j.ejca.2003.08.009
18. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin Nephrotoxicity. Toxins (Basel). (2010) 2:2490–518. doi: 10.3390/toxins2112490
19. Nageswara Rao AA, Wallace DJ, Billups C, Boyett JM, Gajjar A, Packer RJ. Cumulative Cisplatin Dose is Not Associated With Event-Free or Overall Survival in Children With Newly Diagnosed Average-Risk Medulloblastoma Treated With Cisplatin Based Adjuvant Chemotherapy: Report From the Children’s Oncology Group. Pediatr Blood Cancer. (2014) 61:102–6. doi: 10.1002/pbc.24670
20. Fouladi M, Chintagumpala M, Ashley D, Kellie S, Gururangan S, Hassall T, et al. Amifostine Protects Against Cisplatin-Induced Ototoxicity in Children With Average-Risk Medulloblastoma. J Clin Oncol (2008) 26:3749–55. doi: 10.1200/JCO.2007.14.3974
21. Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, et al. Risk Stratification of Childhood Medulloblastoma in the Molecular Era: The Current Consensus. Acta Neuropathol. (2016) 131:821–31. doi: 10.1007/s00401-016-1569-6
22. Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, et al. Wnt/Wingless Pathway Activation and Chromosome 6 Loss Characterize a Distinct Molecular Sub-Group of Medulloblastomas Associated With a Favorable Prognosis. Cell Cycle (2006) 5:2666–70. doi: 10.4161/cc.5.22.3446
23. Cambruzzi E. Medulloblastoma, WNT-Activated/SHH-Activated: Clinical Impact of Molecular Analysis and Histogenetic Evaluation. Childs Nerv Syst (2018) 34:809–15. doi: 10.1007/s00381-018-3765-2
24. Goschzik T, Zur Muhlen A, Kristiansen G, Haberler C, Stefanits H, Friedrich C, et al. Molecular Stratification of Medulloblastoma: Comparison of Histological and Genetic Methods to Detect Wnt Activated Tumours. Neuropathol Appl Neurobiol (2015) 41:135–44. doi: 10.1111/nan.12161
Keywords: medulloblastoma, craniospinal irradiation (CSI), neurotoxicity, late effects after cancer therapy, survivorship
Citation: Minturn JE, Mochizuki AY, Partap S, Belasco JB, Lange BJ, Li Y, Phillips PC, Gibbs IC, Fisher PG, Fisher MJ and Janss AJ (2021) A Pilot Study of Low-Dose Craniospinal Irradiation in Patients With Newly Diagnosed Average-Risk Medulloblastoma. Front. Oncol. 11:744739. doi: 10.3389/fonc.2021.744739
Received: 20 July 2021; Accepted: 17 August 2021;
Published: 02 September 2021.
Edited by:
Jose R. Pineda, University of the Basque Country, SpainReviewed by:
Yonehiro Kanemura, Osaka National Hospital (NHO), JapanAndré O. von Bueren, Hôpitaux Universitaires de Genève, Switzerland
Copyright © 2021 Minturn, Mochizuki, Partap, Belasco, Lange, Li, Phillips, Gibbs, Fisher, Fisher and Janss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna J. Janss, YW5uYS5qYW5zc0BjaG9hLm9yZw==
 Jane E. Minturn
Jane E. Minturn Aaron Y. Mochizuki
Aaron Y. Mochizuki Sonia Partap
Sonia Partap Jean B. Belasco1
Jean B. Belasco1 Yimei Li
Yimei Li Paul G. Fisher
Paul G. Fisher