- 1Department of Thoracic Oncology, Thoraxklinik and National Center for Tumor Diseases (NCT) at Heidelberg University Hospital, Heidelberg, Germany
- 2Translational Lung Research Center Heidelberg (TLRC-H), member of the German Center of Lung Research (DZL), Heidelberg, Germany
- 3Department of Internal Medicine V, Hematology, Oncology and Rheumatology, Heidelberg University Hospital, Heidelberg, Germany
- 4Division of Cancer Genome Research (B063), German Cancer Research Center (DKFZ) and German Cancer Consortium (DKTK), Heidelberg, Germany
- 5Department of Thoracic Surgery, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany
- 6Department of Molecular Pathology Institute of Pathology Heidelberg, Heidelberg, Germany
- 7Translational Research Unit, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany
- 8Department of Pharmacy, Thoraxklinik and National Center for Tumor Diseases (NCT) at Heidelberg University Hospital, Heidelberg, Germany
- 9Center for Infectious Diseases, Virology, University Hospital Heidelberg, Heidelberg, Germany
Introduction: PD-(L)1 inhibitors (IO) have improved the prognosis of non-small-cell lung cancer (NSCLC), but more reliable predictors of efficacy and immune-related adverse events (irAE) are urgently needed. Cytokines are important effector molecules of the immune system, whose potential clinical utility as biomarkers remains unclear.
Methods: Serum samples from patients with advanced NSCLC receiving IO either alone in the first (1L, n=46) and subsequent lines (n=50), or combined with chemotherapy (ICT, n=108) were analyzed along with age-matched healthy controls (n=15) at baseline, after 1 and 4 therapy cycles, and at disease progression (PD). Patients were stratified in rapid progressors (RP, progression-free survival [PFS] <120 days), and long-term responders (LR, PFS >200 days). Cytometric bead arrays were used for high-throughput quantification of 20 cytokines and other promising serum markers based on extensive search of the current literature.
Results: Untreated NSCLC patients had increased levels of various cytokines and chemokines, like IL-6, IL-8, IL-10, CCL5, G-CSF, ICAM-1, TNF-RI and VEGF (fold change [FC]=1.4-261, p=0.026-9x10-7) compared to age-matched controls, many of which fell under ICT (FC=0.2-0.6, p=0.014-0.002), but not under IO monotherapy. Lower baseline levels of TNF-RI were associated with longer PFS (hazard ratio [HR]= 0.42-0.54; p=0.014-0.009) and overall survival (HR=0.28-0.34, p=0.004-0.001) after both ICT and IO monotherapy. Development of irAE was associated with higher baseline levels of several cytokines, in particular of IL-1β and angiogenin (FC=7-9, p=0.009-0.0002). In contrast, changes under treatment were very subtle, there were no serum correlates of radiologic PD, and no association between dynamic changes in cytokine concentrations and clinical outcome. No relationship was noted between the patients’ serologic CMV status and serum cytokine levels.
Conclusions: Untreated NSCLC is characterized by increased blood levels of several pro-inflammatory and angiogenic effectors, which decrease under ICT. Baseline serum cytokine levels could be exploited for improved prediction of subsequent IO benefit (in particular TNF-RI) and development of irAE (e.g. IL-1β or angiogenin), but they are not suitable for longitudinal disease monitoring. The potential utility of IL-1/IL-1β inhibitors in the management and/or prevention of irAE in NSCLC warrants investigation.
Introduction
Non-small-cell lung cancer (NSCLC) is the deadliest malignancy with an estimated 1.8 million deaths worldwide in 2021 (1). Most patients are diagnosed with advanced, incurable disease and a median life expectancy below two years (2). Immunotherapy (IO) with programmed death-(ligand) 1 [PD-(L)1] inhibitors, like pembrolizumab, atezolizumab and nivolumab, was a major step forward in the management of stage IV disease, facilitating long-term disease control and 5-year overall survival (OS) rates of 20-30% (3, 4). However up to 1/3 of patients do not respond, while potentially life-threatening grade III-IV immune-related adverse events (irAE) occur in approximately 10% of cases (5–7). One major unmet need is finding more reliable predictors of efficacy and toxicity to improve guidance of patient management. Tumor PD-L1 is the only currently approved biomarker (8, 9), and has also demonstrated association with the development of oligoprogression under IO (10), as well as irAE (5), but these associations are weak and complicated by considerable spatial heterogeneity of PD-L1 expression (11). Besides, the tumor mutational burden (TMB) is a pure genetic biomarker less prone to sampling errors, whose implementation has nevertheless been hampered by insufficient predictive potential and considerable technical variability (12–14). Immunologic parameters of the tumor tissue, like the emerging association of B cells and tertiary lymphoid structures with long-term IO benefit (15, 16), are an attractive alternative that directly reflects immunobiologic processes (17), but their use is limited by the scant material obtainable through small biopsies and the high procedural risk of repeated assessments. Therefore, there is increasing interest in soluble blood biomarkers that could be used to stratify patients and monitor treatment in a non-invasive manner (18). While rudimentary parameters based on routine laboratory tests, like the neutrophil-to-lymphocyte ratio (NLR, from the differential hemogram), and the advanced lung inflammation index (ALI, also incorporating the body-mass-index and serum albumin), have demonstrated predictive and prognostic utility for IO-treated NSCLC (19), a more detailed analysis of immunologic effector molecules in the blood would be expected to provide refined information and thus improve accuracy (17). Aim of this study was to systematically investigate the potential clinical utility of serum cytokines for the management of NSCLC patients treated with PD-(L)1 inhibitors.
Materials and methods
Patients and samples
This study included all patients with metastatic NSCLC and available serum samples, who received immunotherapy in the Thoraxklinik Heidelberg between 2012-2020, with a data cut-off on October 12th 2021. PD-(L)1 inhibitors were administered either alone in the first (1L-IO) or subsequent lines (2+L-IO, after preceding chemotherapy), or in the 1L combined with chemotherapy (ICT). Serum samples were collected prospectively at baseline before treatment start, and longitudinally after 1 (1C) and 4 cycles (4C) of therapy, as well as at the time of disease progression (PD) (20). In order to capture more clear signals of efficacy, the focus was placed on cases with either rapid progression (RP), i.e. progression-free survival (PFS) < 120 days, or long-time response (LR), i.e. PFS > 200 days, while patients with intermediate PFS (120-200 days) were excluded from analysis. A group of age-matched healthy subjects without NSCLC was analyzed as controls.
Histological diagnosis and molecular profiling of NSCLC using combined DNA/RNA next-generation sequencing (NGS) were performed in the Institute of Pathology Heidelberg, as published (21). Patients with routinely treatable genetic alterations, like EGFR and BRAF-V600 mutations, or ALK/ROS1/RET/NTRK fusions, received tyrosine kinase inhibitors and were excluded from this study. The only two cases with mutations of these genes were one patient with BRAF p.G466E, for which no targeted therapy has been approved yet by the EMA or FDA, and one case with MET exon 14 skipping, for which MET inhibitors had not been approved yet at the time of the patient’s treatment, both of which received first-line chemoimmunotherapy. Clinicopathological parameters were collected from the patients’ records. The following parameters were extracted: demographics, baseline clinical and tumor characteristics including the Eastern Cooperative Oncology Group performance status (ECOG PS) and smoking status, PD-L1 tumor proportion score (TPS), results of differential blood counts, irAE characteristics, systemic anticancer treatments, date of progression, date of the last follow-up, and date of death. PD-L1 TPS was assessed using the clone SP263 (Ventana/Roche, Mannheim, Germany) and trichotomized for analysis as <1, 1–49, and ≥50%. IgG and IgM against human cytomegalovirus (CMV) was quantified using ELISA according to the manufacturer’s instructions (Euroimmun, Lübeck, Germany), and presence of either antibody class was considered to reflect positive serologic status.
PFS was defined as the time from immunotherapy start to death or progression. Overall survival (OS) was defined as the time from immunotherapy start to death or last follow-up. The progression date under immunotherapy was verified by the investigators with review of radiologic images, i.e. chest/abdomen CT and brain MRI-based restaging every 6–12 weeks, without formal RECIST reevaluation, as several studies have demonstrated very good agreement between real-world and RECIST-based assessments (22, 23). Diagnosis of irAE was based on standard clinicolaboratory criteria (24). This study was approved by the ethics committee of Heidelberg University (S-579/2019), and all participants gave informed consent.
Selection of target cytokines
The cytokines in the panel were selected based on a search for original articles in PubMed on serum markers potentially associated with PD-(L)1 inhibitor efficacy in NSCLC or the development of irAE. Search terms were ((predictive biomarker[Title/Abstract]) AND ((NSCLC[Title/Abstract]) OR (lung cancer[Title/Abstract]))) with 624 results. Review articles, publications about treatments other than immunotherapy, about other tumor entities (e.g. SCLC), or about non-soluble biomarkers were excluded. After compilation of the first database, 10 additional papers were found through manual search focused on already identified potential markers. According to the published evidence and technical feasibility of multiplex measurements, the following 20 markers were selected for the current analysis: interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17F, interferon gamma (IFN-γ), tumor necrosis factor (TNF), intercellular adhesion molecule 1 (ICAM-1), interferon-gamma induced protein 10 (IP-10), vascular endothelial growth factor (VEGF), angiogenin, soluble CD40 ligand (sCD40L), granulocyte-colony stimulating factor (G-CSF), CC motif chemokine ligand 5 (CCL5), granzyme a, and soluble TNF-receptor I (TNF-RI). The results of the literature search and the rationale for selection of analyzed markers are shown in Supplementary Table 1.
Sample processing and cytokine testing
Blood was collected in lithium-heparin tubes, centrifuged at 2000 g for 10 minutes within 1 h from venipuncture, followed by removal, aliquoting and storage of serum at -80°C. For the quantification of cytokines, aliquots were thawed on ice and measured using cytometric bead arrays (CBA) according to the manufacturer’s instructions (Beckton Dickinson, Heidelberg, Germany) with standard (S) or enhanced (E) sensitivity kits, as appropriate. The limit of detection and range of assays used in this study is shown in Supplementary Table 2. In brief, each sample was centrifuged at 12000 g for 2 min at 4°C, and the supernatant transferred into a new tube and diluted 1:3 (S) or 1:4 (E) to a total volume of 50 µl. Capture beads were diluted 1:20 (for E kits, followed by a single wash step using 1 ml of wash buffer at 200g for 5 minutes) or 1:50 (for S kits), added to the samples (E: 20 µl; S: 50 µl), and incubated at room temperature (E: 2 h; S: 1 h). Next, the phycoerythrin (PE) detection reagent was added (E: 20 µl diluted 1:20; E: 50 µl diluted 1:50), samples were incubated for 2 h at room temperature in the dark, for E kits the second component of the detection diluent 1:10 diluted was added, and all samples underwent a final washing step with resuspension in 200 μl of wash buffer for measurement. Standard curves were generated by processing the lyophilized standards provided with the kits in a similar way as the patients’ samples. For sample acquisition, an LSR-Fortessa Flow Cytometer (Beckton Dickinson, Heidelberg, Germany) was used. Cytokine concentrations were calculated from the raw CBA data using the Fcap Array™ version 3.0 software (Soft Flow, Pecs, Hungary).
Statistical analysis
Statistical comparisons between patient groups (e.g. RP vs. LR) were performed using Wilcoxon tests, while paired Wilcoxon tests were used to analyze different time-points of the same patients (e.g. baseline vs. 4C). Fold change (FC) was calculated trough division of mean values. Survival was analyzed according to Kaplan-Meier and compared between groups using log-rank tests, after determining the optimal cut-off based on ROC and Youden-index analysis. The association with various parameters with survival was explored using Cox regression. The correlation between clinicopathological variables and serum markers was analyzed according to Spearman, while correlations classified as very weak (|r|<0.2), weak (|r|=0.2-0.3), moderate (|r|=0.3-0.5), and strong (|r|=0.5-0.7). Multiple testing correction was performed according to Benjamini- Hochberg. Statistical calculations were performed with SPSS version 28 (IBM Corp., Armonk, NY, USA) and R version 4.2.1 (www.R-project.org). Two-tailed p-values lower than 0.05 and with false discovery rate (FDR) lower 0.1 were considered significant.
Results
Patient characteristics
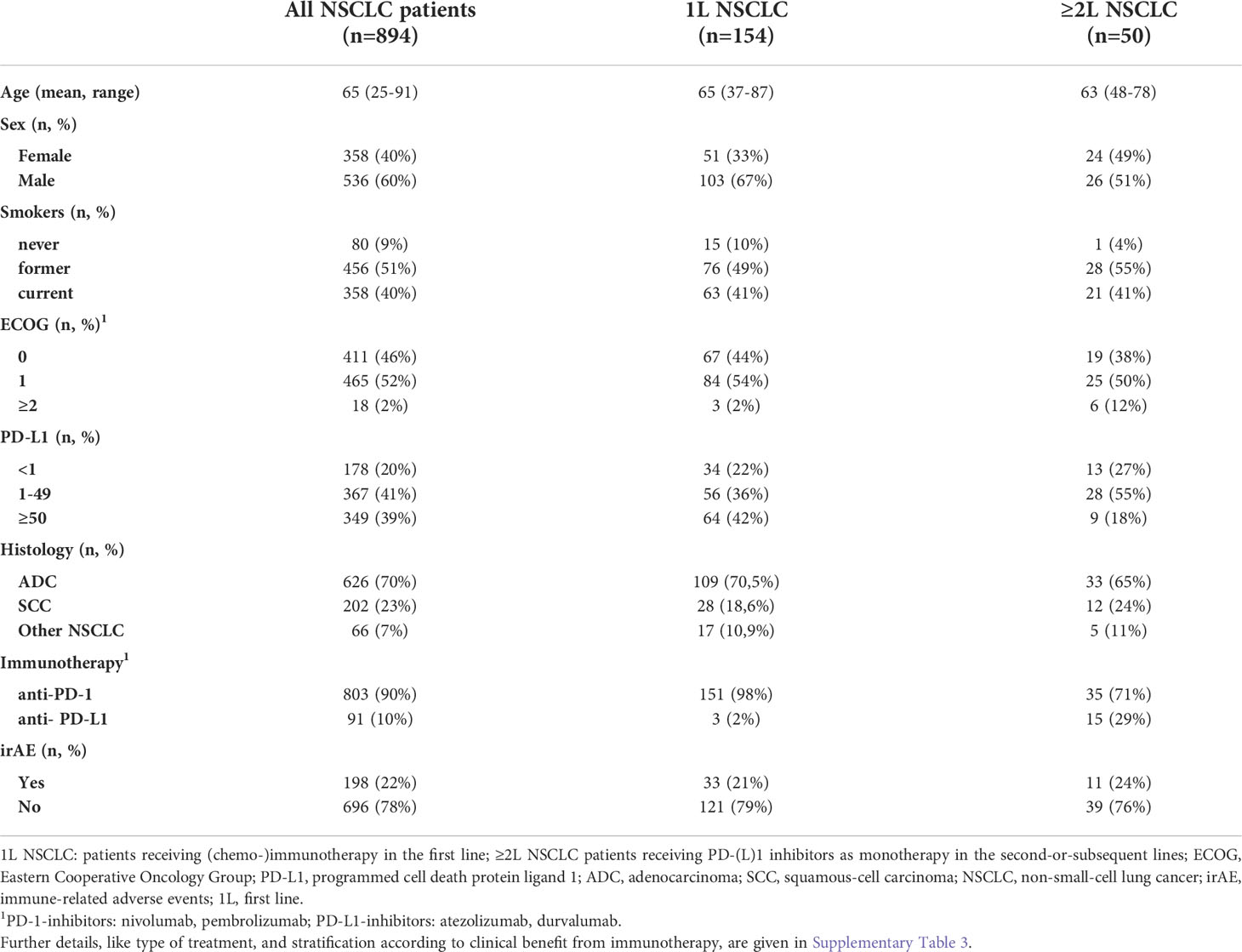
A total of 204 patients with metastatic NSCLC who received PD-(L)1 inhibitors in the first or subsequent lines could be included in the study (Figure 1). An overview of characteristics for study patients is given in Table 1, while more details about treatment type and stratification based on IO efficacy are provided in Supplementary Table 3. Mean age was 65 years (range 37-87) for first-line patients (n=154), 63 years (range 48-78) for patients in subsequent lines (n=50, Table 1), and 66 years (range 58-81) for age-matched healthy donors (n=15).
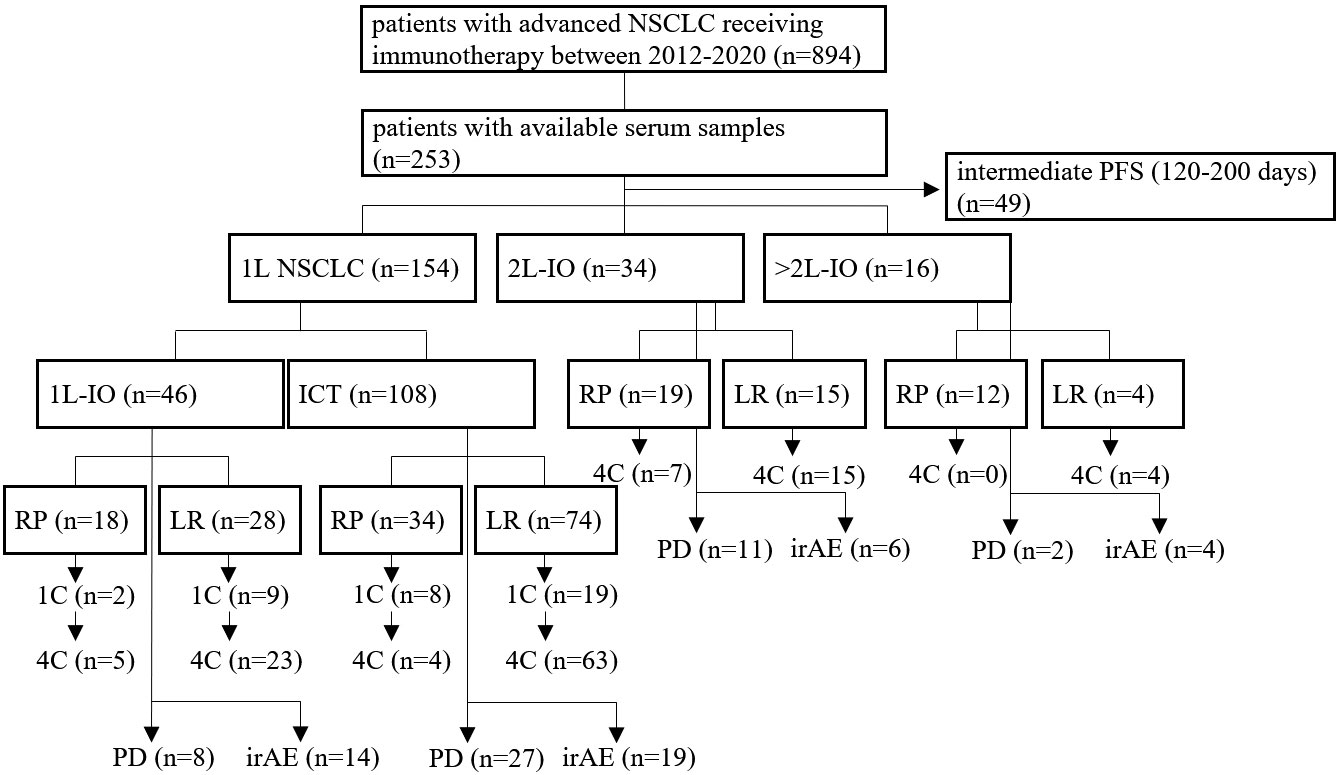
Figure 1 Flowchart of study patients. A total of 894 patients with metastatic NSCLC from 2012-2020 was treated with immunotherapy (IO), i.e. either PD-(L)1 inhibitors alone or in combination with chemotherapy (ICT), of which serum samples were available for 253. This study focused on patients with either rapid progression (RP, i.e. within 120 days from immunotherapy start), or long-term benefit (LR, i.e. progression-free survival > 200 days), as explained in the Materials and Methods. Treatment was either in the first (1L) or in the second and subsequent lines (2+L IO). Samples were collected at baseline, after 1 cycle of treatment (1C), after 4 cycles of treatment (4C), or at the time of disease progression (PD), as possible. Some patients developed immune-related adverse-events (irAE), which were analyzed separately.
Serum cytokine profile of advanced NSCLC at baseline
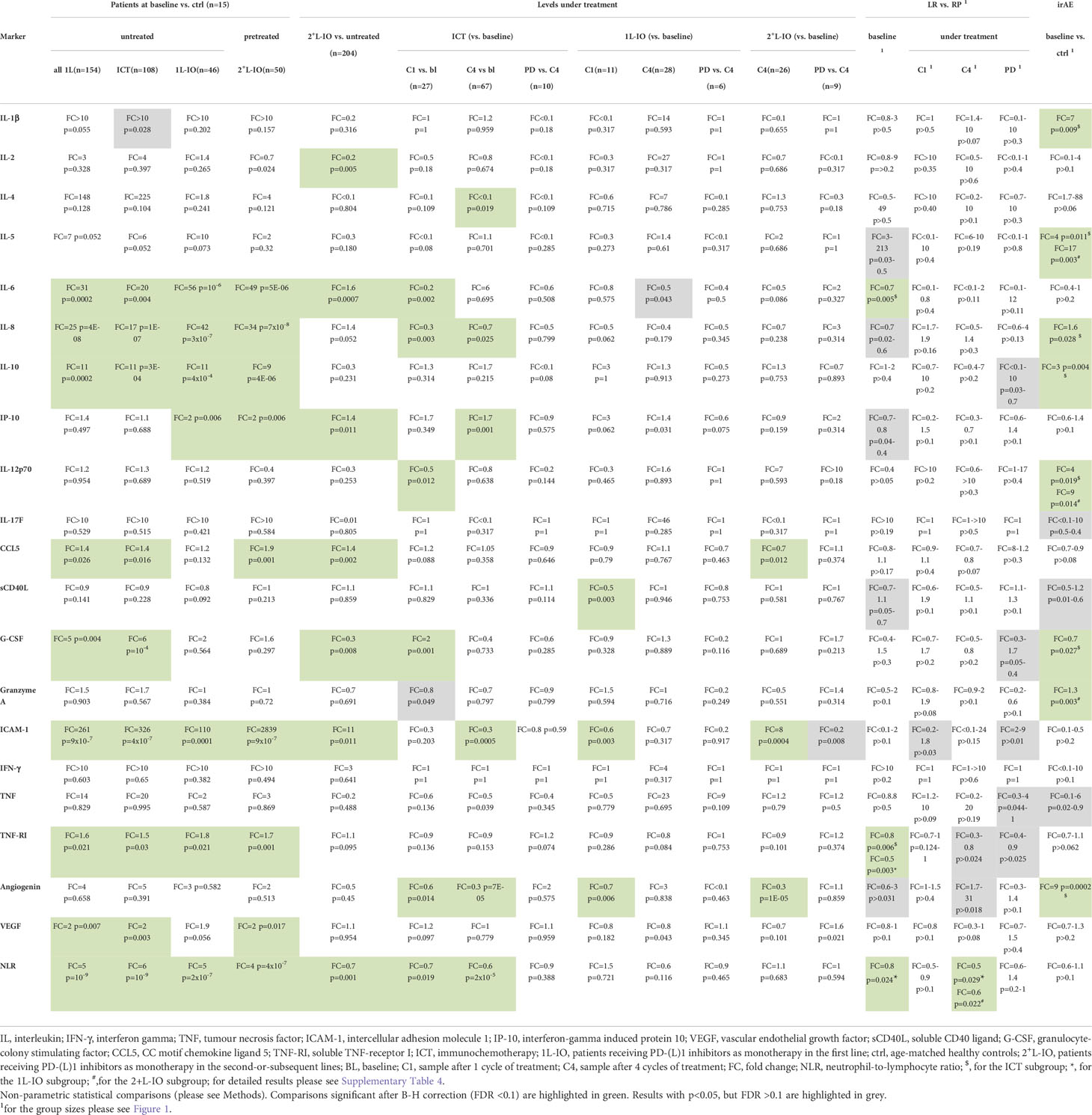
Several cytokines were significantly increased in the serum of untreated patients compared to age-matched healthy controls, i.e. IL-6, IL-8, IL-10, CCL5, G-CSF, ICAM-1, TNF-RI and VEGF (fold-change [FC]=1.4-261, p=0.026-9x10-7, Table 2 and Figure 2). Besides, chemotherapy-pretreated 2+L patients before start of immunotherapy in later lines showed similar changes, with significantly increased serum concentrations of IL-6, IL-8, IL-10, IP-10, CCL5, ICAM-1, TNF-RI and VEGF compared to the controls (FC= 1.7-2839; p=0.017–7x10-8), but mixed changes compared to untreated, newly diagnosed patients: IL-6, IP-10, CCL5 and ICAM-1 were significantly increased (FC= 1.4-11; p=0.011–0.0007), while IL-2 and G-CSF were decreased (FC= 0.2-0.3; p=0.005–0.008, Table 2 and Figure 2). Of note, the baseline NLR was increased in all patient subgroups compared to controls, but decreased in 2+L compared to untreated patients (Table 2).
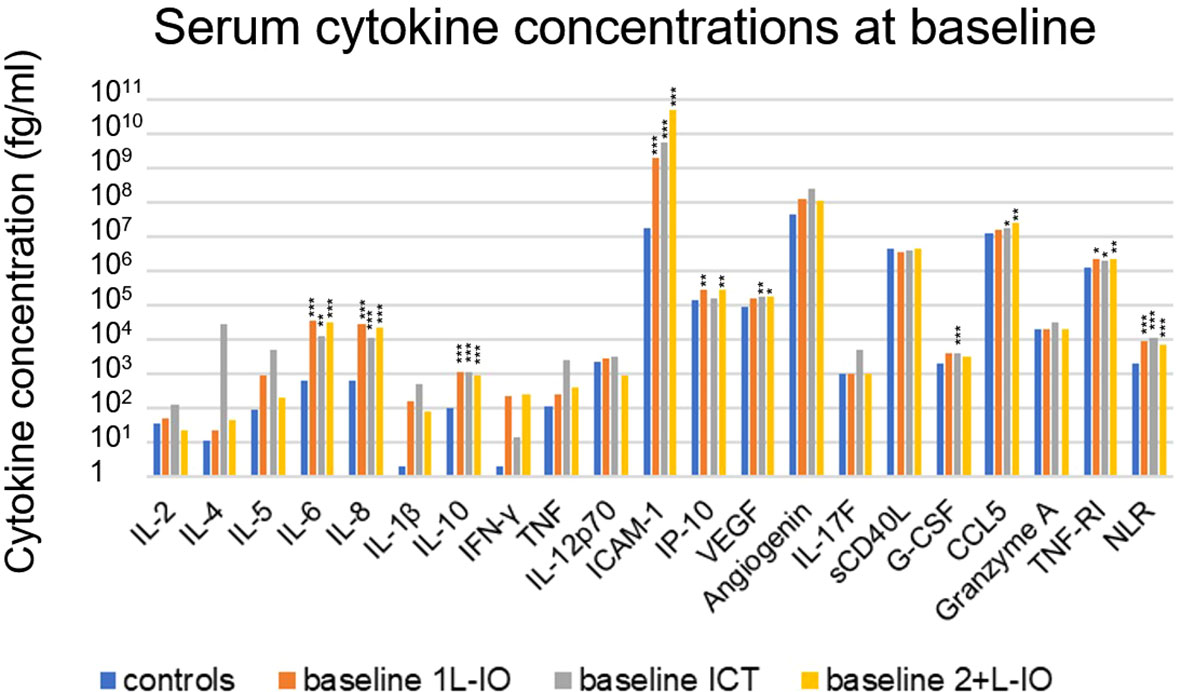
Figure 2 Cytokine levels before immunotherapy start in newly diagnosed and chemotherapy-pretreated NSCLC compared to age-matched healthy control donors. Shown is the mean concentration of each cytokine in first-line patients at baseline, as well as in age-matched healthy controls. An overview of significant results and the explanation of abbreviations is shown in Table 2. In newly diagnosed patients IL-6 (FC=31, p=0.0002), IL-8 (FC=25, p=4x10-8), IL-10 (FC=11, p=0.0002), CCL5 (FC=1.4, p=0.026), G-CSF (FC=5, p=0.004), ICAM-1 (FC=261, p=9x10-7), TNF-RI (FC=1.6, p=0.021), VEGF (FC=2, p=0.007) and NLR (FC=5, p=10-9) were elevated. In pretreated patients IL-6 (FC=49, p=5x10-6), IL-8 (FC=34, p=7x10-8), IL-10 (FC=9 p=4x10-6), IP-10 (FC=2, p=0.006), CCL5 (FC=1.9, p=0.001), ICAM-1 (FC=2839, p=9x10-7), TNF-RI (FC=1.7, p=0.001), VEGF (FC=2, p=0.017) and NLR (FC=4, p=4x10-7) were elevated as well; *=p<0.05; **=p<0.01; ***=p<0.001.
Serum cytokine changes under treatment with PD-(L)1 inhibitors
In contrast to prominent aberrations at the time of immunotherapy start, serum cytokine changes under treatment with PD-(L)1 inhibitors were subtle (Figures 3A, B). Most consistent was a decrease in angiogenin after 1 cycle in the first line (FC=0.6-0.7, p=0.014-0.006) or after 4 cycles in later lines (FC=0.3, p=10-5). Other changes were either inconsistent, i.e. ICAM-1 dropped under IO-monotherapy in the first line, but increased under treatment in later lines; sCD40L was lower under treatment in the first, but not in subsequent lines; CCL5 was decreased under treatment in subsequent, but not in the first line; or concerned chemoimmunotherapy only, but not PD-(L)1 monotherapy, i.e. decreases of IL-6, IL-8, IL-12p70, and increases in IP-10 and G-CSF (Figures 3A, B). The NLR also decreased under treatment, but only in patients receiving ICT (Figures 3A, B). No significant changes were observed at the time of disease progression compared to the levels after 4 treatment cycles (Figures 3A, B).
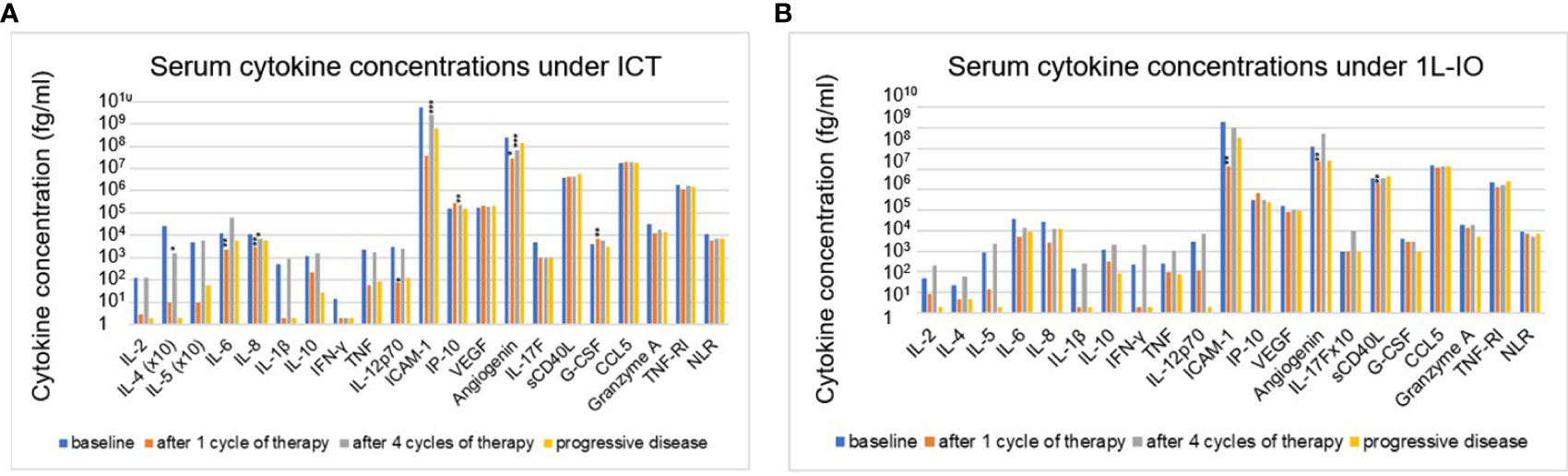
Figure 3 Changes of serum cytokines levels under immunotherapy in NSCLC patients compared to baseline values. (A) for patients receiving first-line immunochemotherapy (ICT): baseline (BL), after 1 cycle of treatment (C1), after 4 cycles of treatment (C4), at the time of disease progression (PD). (B) for patients receiving first-line PD-(L)1 monotherapy (1L-IO): baseline (BL), after 1 cycle of treatment (C1), after 4 cycles of treatment (C4), at the time of disease progression (PD). Shown is the mean concentration of each cytokine in the respective patients. An overview of significant results and the explanation of abbreviations is shown in Table 2; *=p<0.05; **=p<0.01; ***=p<0.001.
Serum cytokine changes associated with survival
For both first-line PD-(L)1 inhibitor monotherapy and ICT cohorts, LR patients had significantly lower TNF-RI levels at baseline compared to RP patients (FC=0.5-0.8, p=0.006-0.003, Table 2). The respective TNF-RI cut-off was 2139.7 pg/ml, as determined by receiver operating characteristic (ROC) curve and Youden index analysis (Supplementary Figure 1). Patients with low TNF-RI baseline levels receiving PD-(L)1 monotherapy showed longer PFS (442 vs. 80 days in median, hazard ratio [HR] = 0.42, p=0.014) and OS (not reached vs. 229 days, HR = 0.28, p=0.004) compared to patients with high TNF-RI levels, Figures 4A, B). Besides, patients with low TNF-RI baseline levels receiving ICT showed longer PFS (409 vs. 212 days in median, hazard ratio [HR] = 0.53, p=0.009) and OS (not reached vs. 493 days, HR = 0.34, p=0.001) compared to patients with high TNF-RI levels, Figures 4C, D). Additionally, lower IL-6 levels were also significantly linked with IO efficacy for patients receiving ICT (Table 2), but other associations did not exceed the FDR<0.1 threshold (Supplementary Table 4). Patients with lower IL-6 levels at baseline receiving ICT showed longer PFS (436 vs. 212 days in median, HR = 0.50, p=0.003) and OS (not reached vs. 514 days in median, HR = 0.29, p=0.0003) than patients with higher IL-6 levels. No differences in cytokine levels under treatment and during disease progression were observed between LR and RP after correction for multiple testing (Table 2 and Supplementary Table 4). The NLR at baseline and after 4 cycles of treatment was associated with LR in patients receiving PD-(L)1 monotherapy, but not in patients receiving ICT (Table 2).
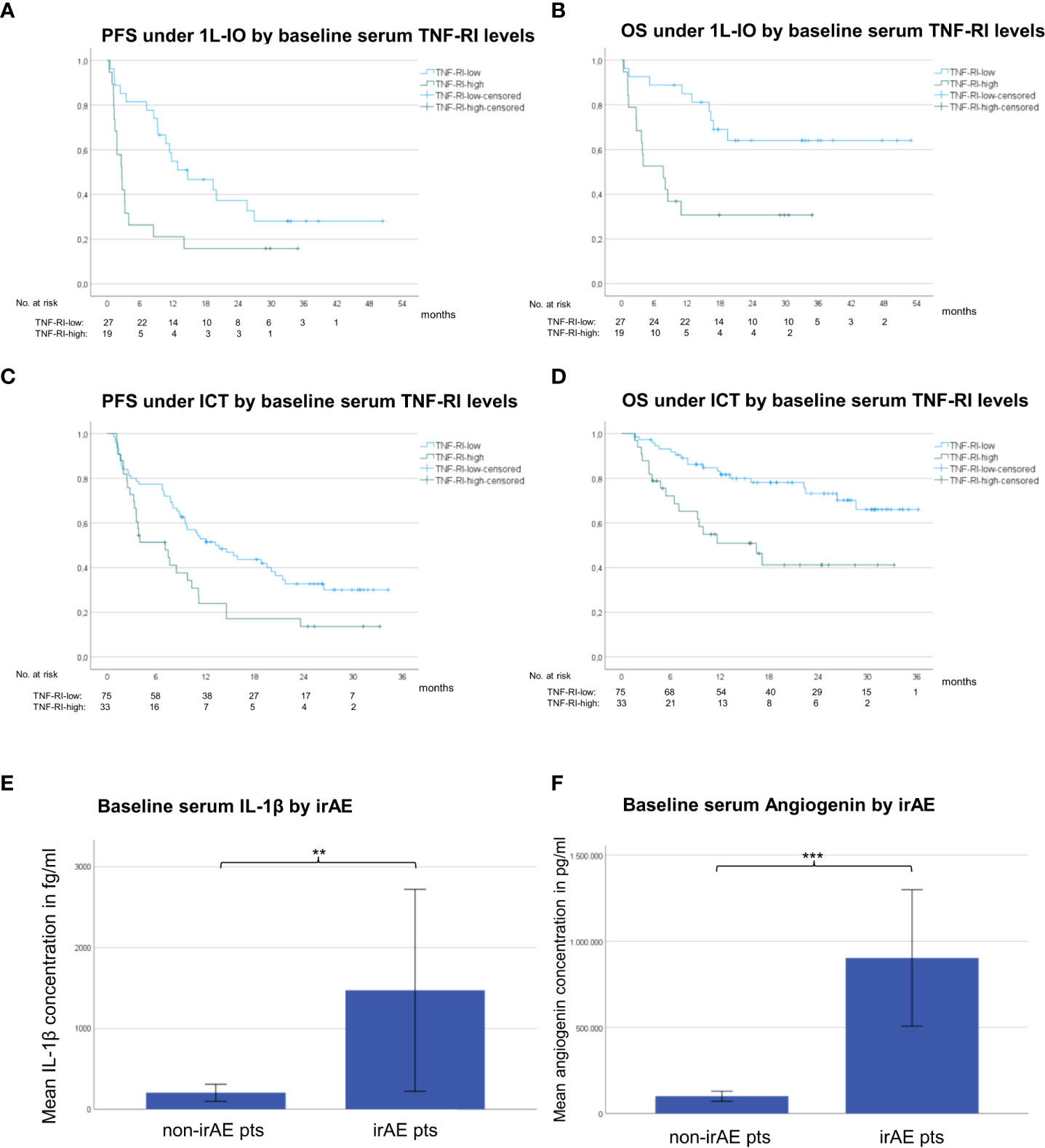
Figure 4 Serum cytokines associated with immunotherapy efficacy and toxicity in NSCLC. (A) The median progression-free survival (PFS) under PD-(L)1 monotherapy for patients with low TNF-RI at diagnosis (<2139.7 pg/ml, please see Supplementary Figure 1) was 442 days vs. 80 days for patients with high TNF-RI. (B) The median overall survival (OS) under PD-(L)1 monotherapy for patients with low TNF-RI at diagnosis (<2139.7 pg/ml) was not reached vs. 229 days for patients with high TNF-RI. (C) The median PFS under immunochemotherapy (ICT) for patients with low TNF-RI at diagnosis (<2139.73 pg/ml, please see Supplementary Figure 1) was 409 days vs. 212 days for patients with high TNF-RI. (D) The median OS under ICT for patients with low TNF-RI at diagnosis (<2139.73 pg/ml) was not reached vs. 493 days for patients with high TNF-RI. (E) The mean baseline serum IL-1β concentration for patients with metastatic NSCLC receiving first-line ICT who subsequently developed irAE was 1472 fg/ml vs. 206 fg/ml for patients without irAE; **=p<0.01. Error bars indicated standard error of the mean. (F) The mean baseline serum angiogenin concentration for patients with metastatic NSCLC receiving first-line ICT who subsequently developed irAE was 903,463 pg/ml vs. 100,254 pg/ml for patients without irAE; ***=p<0.001. Error bars indicated standard error of the mean.
Baseline serum cytokines levels associated with the development of irAE
Several cytokine abnormalities were evident in baseline samples of patients receiving ICT, who subsequently developed irAE (Figures 4E, F), i.e. in order of decreasing degree of association: elevated baseline levels of angiogenin (FC=9, p=0.0002), IL-1β (FC=7, p=0.009), IL-5 (FC=4, p=0.011), IL-12p70 (FC=4, p=0.019), IL-10 (FC=3, p=0.004), and IL-8 (FC=1.6, p=0.0285), as well as reduced baseline levels of G-CSF (FC=0.7, p=0.007). In addition, patients developing irAE under PD-(L)1 monotherapy in later lines showed higher baseline levels of IL-5 (FC=17, p=0.003), IL-12p70 (FC=9, p=0.014), and granzyme A (FC=1.3, p=0.003, Table 2). The characteristics of irAE are shown in Supplementary Table 5. No significant changes of the aforementioned cytokines were noted according to the severity or irAE (grade 1/2 vs. 3/4) or the use of steroids or not (data not shown).
Serum cytokine levels associated with clinical characteristics
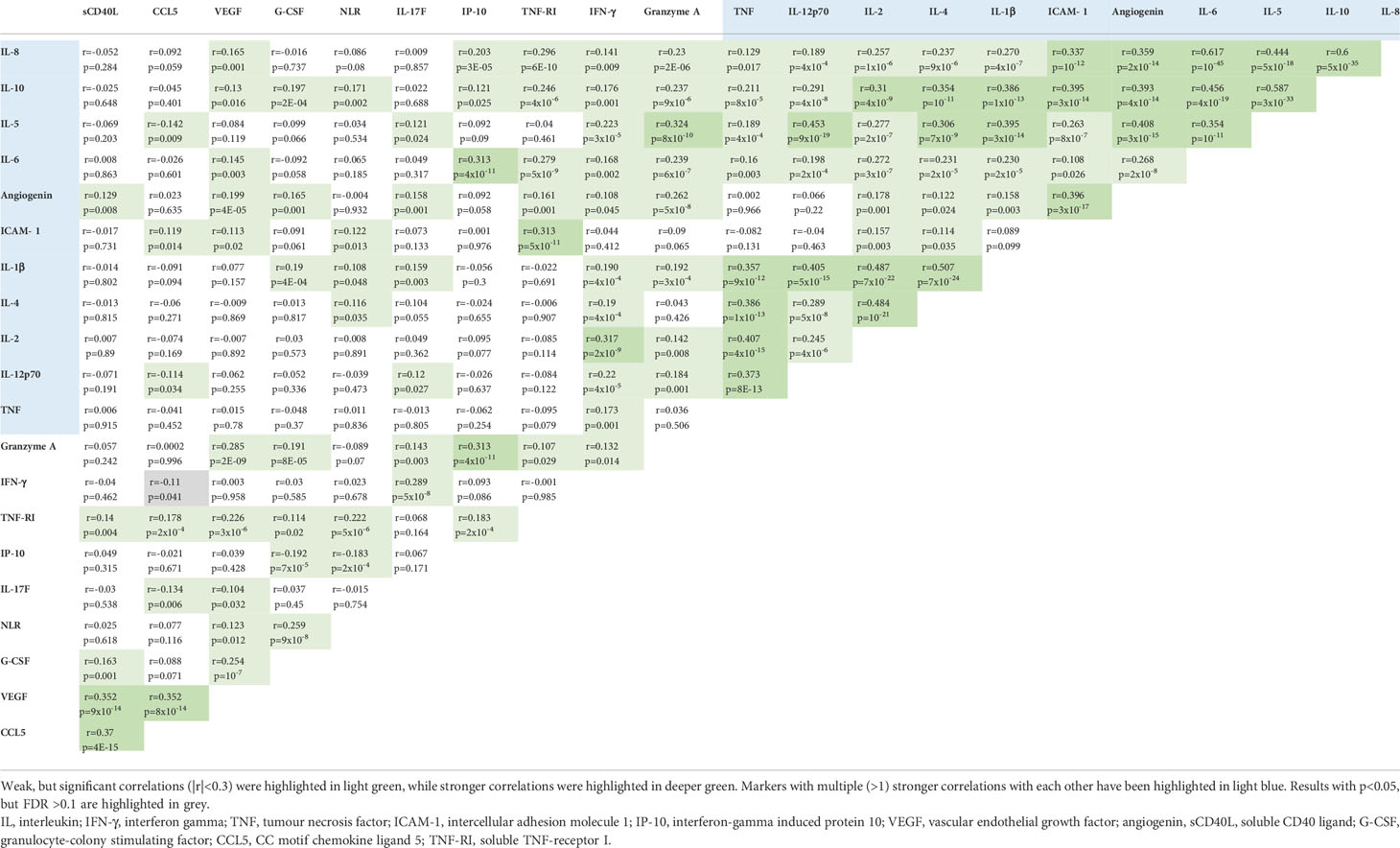
Several cytokines were associated with each other, most notably IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, ICAM-1, TNF and angiogenin formed a cluster with multiple (>1) moderate (r>0.3) significant correlations with each other (Table 3). Notable was also a weak, but significant (r=0.28, p=5x10-9) correlation between TNF-RI and IL-6, the two cytokines more prominently linked to immunotherapy efficacy, as described in the previous section Serum cytokine changes associated with survival.
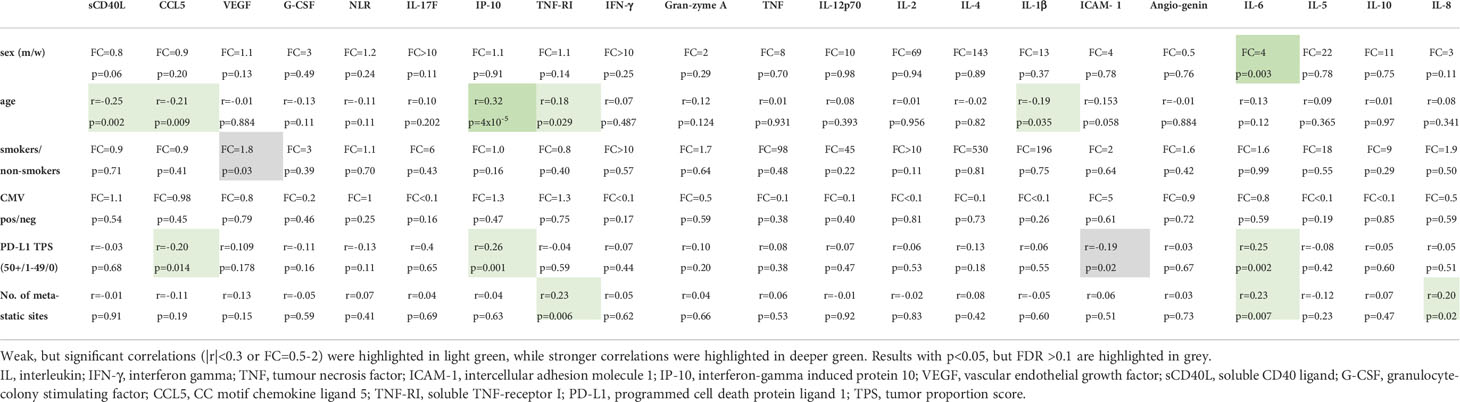
Furthermore, the correlations between baseline serum cytokine levels and clinical characteristics are summarized in Table 4. IL-6 levels were higher in men (FC=4, p=0.003), while IP-10 showed a moderate positive correlation with age (r=0.323, p=4x10-5). All other associations noted were either non-significant or weak (|r|<0.3). In particular, no association was found between the serologic CMV status of patients and serum cytokine levels.
Discussion
Main objective of this study was to characterize the potential clinical utility of serum cytokine concentrations in IO-treated NSCLC. One prominent finding was the profoundly altered cytokine profile of newly diagnosed NSCLC patients compared to age-matched healthy controls (Table 2 and Figure 2). Several interrelated (Table 4) mediators were significantly elevated, including the proinflammatory cytokines IL-6, IL-8, G-CSF and the proinflammatory chemokine CCL5 (25), the proinflammatory adhesion glycoprotein ICAM-1, soluble TNF-RI, which is increased in inflammatory states to curb the bioactivity of TNF (26, 27), the anti-inflammatory IL-10 (28), as well as the proangiogenic VEGF (29). These results illustrate the systemic inflammation and immune dysregulation present in metastatic NSCLC, which explains several disease manifestations and offers specific therapeutic vulnerabilities. For example, the increased NLR in the blood of these patients is facilitated by elevated levels of various interleukins and G-CSF, which stimulate granulopoiesis (30), ICAM-1 expression is induced by inflammation and associated with worse prognosis in NSCLC and other cancers likely by facilitating the metastatic cascade (31), while the therapeutic relevance of elevated VEGF in the circulation is reflected by the success of bevacizumab and other angiogenesis inhibitors in the treatment of metastatic NSCLC (32). Of note, association of blood cytokine levels with the tumor PD-L1 expression were generally absent or very weak (Table 4), so that these parameters capture different aspects of NSCLC immunobiology. For example, alterations of the lung and gut microbiome were associated with increased levels of several inflammatory serum cytokines, including IL-6 and TNF-α, in preclinical models of lung cancer, which could in part be remedied by the administration of probiotics (33, 34). At the same time, the association of IP-10 and CCL-5 with PD-L1 TPS (Table 3) probably explain why these were elevated only in 1L-IO (with higher PD-L1 expression ≥ 50%) or ICT patients (with lower average PD-L1 expression), respectively (Table 2).
In contrast, serum cytokine changes under treatment were subtle. Consistent was only a decrease in angiogenin, also known as ribonuclease 5, a small, 123 amino acid protein that stimulates angiogenesis alongside several other pleotropic effects (35). Recently it has been reported, that increased serum angiogenin correlated with dynamic contrast-enhanced MR-PET parameters in NSCLC patients, which were improved under anti-angiogenic therapy and linked with OS (36). However, in our study the change in angiogenin under immunotherapy was neither accompanied by a decrease in circulating VEGF nor associated with OS (Table 2), therefore its significance remains unclear. From a clinical perspective, an important conclusion from the results of this study is that serial serum cytokine measurements are not suitable for disease monitoring, since there were minimal changes under treatment in our cohort (Figures 3A, B). This is in contrast to longitudinal ctDNA assays, which have demonstrated potential clinical utility in the context of both immunotherapy-treated (37) and oncogene-driven disease (38), based on the strong association of higher tumor mutation levels in the blood under therapy with refractory disease and shorter PFS (39). In particular, serum cytokines are obviously not suitable for early detection of treatment failure, since no consistent changes accompanied PD in our patients, contrary to ctDNA-based liquid biopsies, which can reveal emergence of novel mutations of increases in allelic frequencies of preexisting variants as a sensitive marker of PD several months earlier than radiologic tumor growth according to recent pivotal studies (40). Moreover, dynamic changes of cytokine levels after 1 and 4 treatment cycles were not associated with immunotherapeutic efficacy (LR vs. RP, Table 2), while dynamic changes of the NLR after 4 cycles (12 weeks) correlated with clinical outcome, as has also been observed by other investigators (41). Besides low sensitivity, as demonstrated by the current study, another problem of disease monitoring using serum cytokines would be susceptibility to external influences by factors unrelated to the tumor remission status, like use of steroids and concomitant infection (42, 43). The lack of association between serum cytokine concentrations in NSCLC and the serologic CMV status (Table 4), itself linked to mild chronic immune activation and immunosenescence (44), also reflects the inability of cytokines to capture subtle systemic changes of the adaptive immunity, as those expected to occur longitudinally under PD-(L)1 blockade.
Another important question is whether baseline cytokine levels could be used for improved prediction of immunotherapy benefit. In general, few differences in the blood levels of analyzed cytokines between patients with LR vs. RP were noted, collectively suggesting an association between lower levels of inflammatory markers, such as IL-6, IL-8, IP-10, TNF-RI and the NRL, with better immunotherapy outcome (Table 2). Similar observations have recently been reported by other investigators, as well, for example lower IL-6 and IL-8 levels at baseline as well as after 1 cycle of treatment were strongly linked to longer survival under immunotherapy in patients with lung cancer and melanoma from a prospective multicenter study in Italy (45). Based on the results of the current study, low levels of TNF-RI appear to be a particularly promising marker for several reasons: first, for patients receiving PD-(L)1 inhibitor monotherapy, the TNF-RI differences between LR vs. RP were more pronounced than those observed for the established marker NLR (FC=0.5 with p=0.003 vs. FC=0.8 with p=0.024, Table 2) (46); second, TNF-RI retained prognostic utility also for patients treated with ICT (FC=0.8 with p=0.006, Table 2 and Figure 4), which is a major unmet need, because the NLR, ALI, PD-L1 and other biomarkers of PD-(L)1 monotherapy become useless, when additional chemotherapy is administered (19); third, blood TNF-RI levels showed no correlation with tissue PD-L1 expression (Table 4), which means that it represents an independent biomarker; finally, the signal of TNF-RI observed in this study appears to be stronger than that of several other inflammatory biomarkers described in the literature, like the blood levels of IL-6 (47), IL-8 (48), IP-10 (49), ICAM-1 (50) and VEGF (51). Actually, all these molecules showed significantly increased levels at baseline in our patients, similar to TNF-RI, but lower levels of TNF-RI could much better discriminate LR vs. RP patients (Table 2 and Supplementary Table 4).
Remarkable was also the association of several serum cytokines, in particular increased angiogenin and IL-1β, but also IL-5, IL-8, IL-10, IL-12p70, and granzyme A, or decreased G-CSF at baseline, with the subsequent development of irAE (Figures 4E, F). Higher baseline IL-1β, IL-10 and IL-12p70 serum concentrations in NSCLC patients who later developed rheumatic irAE have been independently confirmed in a different patient cohort (personal communication with KB and MMSC). Such an association between preexisting systemic inflammation and autoimmunity has also been observed in other tumor types, like indolent B-cell lymphomas (52) and malignant thymoma (53). These results corroborate previous reports of a higher propensity for the development of irAE in IO-treated cancer patients with higher blood IL-10 (54) and other inflammatory mediators (55). Of note, irAE-related cytokines did not include TNF-RI and IL-6, whose lower levels were associated with longer survival in this study (Table 2), so that a complex protein panel may be able to independently predict both IO efficacy and IO toxicity. The association of baseline IL-1β levels with irAE is particularly interesting, because it had not been reported in NSCLC patients before, and because the IL-1β inhibitor canakinumab and other IL-1 drugs are widely, in part off-label, used to treat a variety of mainly autoinflammatory disorders in rheumatology (56). There is also evidence suggesting anticancer activity of canakinumab, for example its use was associated with a reduced incidence of lung cancer in the phase 3 CANTOS study (57), so that phase 3 trials of this drug in combination with chemotherapy for the treatment of NSCLC in various stages are ongoing (58). The findings of this study suggest that these drugs could potentially be useful in the treatment or even prevention of irAE, as well.
Main advantages of this work are the relatively large number of cytokines based on a preceding systematic literature review, the relatively large number of patients in several dedicated cohorts, i.e. first-line PD-(L)1 monotherapy, ICT, or PD-(L)1 monotherapy in subsequent lines, the prospective longitudinal sample collection at defined uniform time-points, the simultaneous consideration of IO efficacy and toxicity, and the rigorous statistical testing including correction for multiple comparisons and multivariable testing. Main limitations are the smaller number of available samples under treatment and at disease progression, as well as the inability to exclude potential confounders. Therefore, the results will need to be validated in future studies, which might pave the way for building a complex score based on several cytokines and data mining analysis (59). Other emerging approaches to refine patient stratification are measurement of circulating tumor cells, ctDNA, miRNA, blood exosomes, gene expression profiling, or analysis of the T-cell receptor (TCR) repertoire (18, 37, 60–64).
Conclusion
In conclusion, this study could demonstrate that several altered serum cytokines in patients with advanced NSCLC could be exploited in order to predict efficacy and toxicity of PD-(L)1 monotherapy or ICT more accurately, but they are not suitable for longitudinal disease monitoring and early detection of tumor escape.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of Heidelberg University (S-579/2019). All patients and healthy controls provided written informed consent to participate in this study.
Author contributions
HaS: Conceptualization, Methodology, Investigation; Data curation, Formal analysis, Visualization, Writing - Original Draft, Writing - Review and Editing; FL: Methodology, Investigation; Data curation, Formal analysis, Visualization, Writing - Review and Editing; ME: Investigation, Data curation, Validation, Writing - Review and Editing; LD: Investigation, Data curation, Validation, Writing - Review and Editing; LG: Investigation, Data curation, Validation, Writing - Review and Editing; KB: Data curation, Validation, Writing – Review and Editing; MS-C: Data curation, Validation, Writing – Review and Editing; AA: Validation, Writing – Review and Editing; FJ: Validation, Writing - Review and Editing; FE: Validation, Writing - Review and Editing; DK: Data curation, Validation, Writing – Review and Editing; MS: Investigation, Writing - Review and Editing; SL: Data curation, Validation, Writing - Review and Editing; SK: Investigation; Data curation, Formal analysis, Writing - Review and Editing; PS: Investigation, Data curation, Validation, Writing - Review and Editing; AS: Validation, Supervision, Writing - Review and Editing; HoS: Investigation, Data curation, Validation, Writing - Review and Editing; MT: Conceptualization, Methodology, Validation, Supervision, Funding acquisition, Writing - Review and Editing; PC: Conceptualization, Methodology, Investigation; Data curation, Formal analysis, Visualization, Supervision, Project administration; Writing - Original Draft, Writing - Review and Editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the German Center for Lung Research (DZL 3.0).
Acknowledgments
We would like to thank Simone Kuder for help with the data collection, Ingrid Heinzmann-Groth for help with the collection of patient samples, as well as Tamara Hedinger, Simone Karcher-Bausch and Simone Butz for expert technical assistance.
Conflict of interest
Author KB received consultancy and/or speaker fees and/or travel reimbursements from Abbvie, Bristol Myers Squibb BMS, Gilead/Galapagos, Janssen, Merck Sharp & Dohme MSD, Mundipharma, Novartis, Pfizer, Roche, Viatris, UCB, as well as scientific support from the Medical Faculty of University of Heidelberg, Rheumaliga Baden-Württemberg e.V., AbbVie, and Novartis. Author FE received personal fees from Roche and BMS; DK: advisory board and speaker’s honoraria from AstraZeneca, BMS, Pfizer. Author AS received advisory board honoraria from BMS, AstraZeneca, ThermoFisher, Novartis, speaker’s honoraria from BMS, Illumina, AstraZeneca, Novartis, ThermoFisher, MSD, Roche, and research funding from Chugai. Author HoS received research grants and personal fees from Roche Sequencing Solutions, outside the submitted work. Author MT received advisory board honoraria from Novartis, Lilly, BMS, MSD, Roche, Celgene, Takeda, AbbVie, Boehringer, speaker’s honoraria from Lilly, MSD, Takeda, research funding from AstraZeneca, BMS, Celgene, Novartis, Roche and travel grants from BMS, MSD, Novartis, Boehringer. Author PC received research funding from Amgen, AstraZeneca, Merck, Novartis, Roche, Takeda, and advisory board/lecture fees from AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Gilead, Novartis, Pfizer, Roche, Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1010660/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
3. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-Small-Cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol (2021) 39:2339–49. doi: 10.1200/JCO.21.00174
4. Gray J, Rodriguez-Abreu D, Powell S, Hochmair M, Gadgeel S, Esteban E, et al. FP13.02 pembrolizumab + pemetrexed-platinum vs pemetrexed-platinum for metastatic NSCLC: 4-year follow-up from KEYNOTE-189. J Thorac Oncol (2021) 16:S224. doi: 10.1016/j.jtho.2021.01.141
5. Daniello L, Elshiaty M, Bozorgmehr F, Kuon J, Kazdal D, Schindler H, et al. Therapeutic and prognostic implications of immune-related adverse events in advanced non-Small-Cell lung cancer. Front Oncol (2021) 11:703893. doi: 10.3389/fonc.2021.703893
6. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
7. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res (2018) 6:1093–9. doi: 10.1158/2326-6066.CIR-17-0755
8. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29:iv192–237. doi: 10.1093/annonc/mdy275
9. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. ESMO clinical practice living guidelines - metastatic non-Small-Cell lung cancer (2021). Available at: https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer.
10. Rheinheimer S, Heussel C-P, Mayer P, Gaissmaier L, Bozorgmehr F, Winter H, et al. Oligoprogressive non-Small-Cell lung cancer under treatment with PD-(L)1 inhibitors. Cancers (Basel) (2020) 12:1046. doi: 10.3390/cancers12041046
11. Schoenfeld AJ, Rizvi H, Bandlamudi C, Sauter JL, Travis WD, Rekhtman N, et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann Oncol (2020) 31:599–608. doi: 10.1016/j.annonc.2020.01.065
12. Budczies J, Kazdal D, Allgäuer M, Christopoulos P, Rempel E, Pfarr N, et al. Quantifying potential confounders of panel-based tumor mutational burden (TMB) measurement. Lung Cancer (2020) 142:114–9. doi: 10.1016/j.lungcan.2020.01.019
13. Budczies J, Allgäuer M, Litchfield K, Rempel E, Christopoulos P, Kazdal D, et al. Optimizing panel-based tumor mutational burden (TMB) measurement. Ann Oncol (2019) 30:1496–506. doi: 10.1093/annonc/mdz205
14. Kazdal D, Endris V, Allgäuer M, Kriegsmann M, Leichsenring J, Volckmar A-L, et al. Spatial and temporal heterogeneity of panel-based tumor mutational burden in pulmonary adenocarcinoma: Separating biology from technical artifacts. J Thor Oncol (2019) 14:1935–47. doi: 10.1016/j.jtho.2019.07.006
15. Budczies J, Kirchner M, Kluck K, Kazdal D, Glade J, Allgäuer M, et al. A gene expression signature associated with b cells predicts benefit from immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology (2021) 10:1860586. doi: 10.1080/2162402X.2020.1860586
16. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
17. Gaissmaier L, Christopoulos P. Immune modulation in lung cancer: Current concepts and future strategies. Respiration (2020) 99:1–27. doi: 10.1159/000510385
18. Honrubia-Peris B, Garde-Noguera J, García-Sánchez J, Piera-Molons N, Llombart-Cussac A, Fernández-Murga ML. Soluble biomarkers with prognostic and predictive value in advanced non-small cell lung cancer treated with immunotherapy. Cancers (Basel) (2021) 13:4280. doi: 10.3390/cancers13174280
19. Mountzios G, Samantas E, Senghas K, Zervas E, Krisam J, Samitas K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open (2021) 6:100254. doi: 10.1016/j.esmoop.2021.100254
20. Wessels S, Muley T, Christopoulos P, Meister M, Heinzmann-Groth I, Warth A, et al. Comprehensive serial biobanking in advanced NSCLC: feasibility, challenges and perspectives. Transl Lung Cancer Res (2020) 9:1000–14. doi: 10.21037/tlcr-20-137
21. Volckmar A-L, Leichsenring J, Kirchner M, Christopoulos P, Neumann O, Budczies J, et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: Analysis of the first 3,000 Heidelberg cases. Int J Cancer (2019) 145:649–61. doi: 10.1002/ijc.32133
22. Bartlett CH, Mardekian J, Cotter M, Huang X, Zhang Z, Parrinello CM, et al. Abstract P3-17-03: Concordance of real world progression free survival (PFS) on endocrine therapy as first line treatment for metastatic breast cancer using electronic health record with proper quality control versus conventional PFS from a phase 3 trial. Cancer Res (2018) 78:P3–17-03-P3-17-03. doi: 10.1158/1538-7445.SABCS17-P3-17-03
23. Ma X, Nussbaum NC, Magee K, Bourla AB, Tucker M, Bellomo L, et al. Comparison of real-world response rate (rwRR) to RECIST-based response rate in patients with advanced non-small cell lung cancer (aNSCLC). Ann Oncol (2019) 30:v651. doi: 10.1093/annonc/mdz260.103
24. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) | protocol development | CTEP (Maryland, USA: National Institutes of Health. National Cancer Institute) (2022).
25. Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin (2007) 45:27–37. doi: 10.1097/AIA.0b013e318034194e
26. Aderka D, Englemann H, Hornik V, Skornick Y, Levo Y, Wallach D, et al. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res (1991) 51:5602–7. Available at: https://aacrjournals.org/cancerres/article/51/20/5602/497130/Increased-Serum-Levels-of-Soluble-Receptors-for.
27. Aderka D, Wysenbeek A, Engelmann H, Cope AP, Brennan F, Molad Y, et al. Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthritis Rheum (1993) 36:1111–20. doi: 10.1002/art.1780360812
28. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol (2008) 180:5771–7. doi: 10.4049/jimmunol.180.9.5771
29. Frezzetti D, Gallo M, Maiello MR, D’Alessio A, Esposito C, Chicchinelli N, et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets (2017) 21:959–66. doi: 10.1080/14728222.2017.1371137
30. Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med (2014) 3:737–46. doi: 10.1002/cam4.239
31. Qian Q, Zhan P, Yu L, Shi Y, Cheng J, Wei S, et al. Baseline levels and decrease in serum soluble intercellular adhesion molecule-1 during chemotherapy predict objective response and survival in patients who have advanced non-small-cell lung cancer. Clin Lung Cancer (2011) 12:131–7. doi: 10.1016/j.cllc.2011.03.009
32. Crinò L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev (2014) 23:79–91. doi: 10.1183/09059180.00008913
33. Perrone F, Belluomini L, Mazzotta M, Bianconi M, Di Noia V, Meacci F, et al. Exploring the role of respiratory microbiome in lung cancer: A systematic review. Crit Rev Oncol Hematol (2021) 164:103404. doi: 10.1016/j.critrevonc.2021.103404
34. Deng Z, Li Z, Sun C, Xie H, Chen Z, Liu J, et al. The association between inflammation, the microbiome and urethane-induced pulmonary adenocarcinoma. Oncol Lett (2018) 15:6352–60. doi: 10.3892/ol.2018.8167
35. Sheng J, Xu Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim Bbiophys Sin (2016) 48:399–410. doi: 10.1093/abbs/gmv131
36. Huang Y-S, Chen JL-Y, Chen H-M, Yeh L-H, Shih J-Y, Yen R-F, et al. Assessing tumor angiogenesis using dynamic contrast-enhanced integrated magnetic resonance-positron emission tomography in patients with non-small-cell lung cancer. BMC Cancer (2021) 21:348. doi: 10.1186/s12885-021-08064-4
37. Bratman SV, Yang SY, Iafolla MA, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer (2020) 1:873–81. doi: 10.1038/s43018-020-0096-5
38. Dietz S, Christopoulos P, Yuan Z, Angeles AK, Gu L, Volckmar A-L, et al. Longitudinal therapy monitoring of ALK-positive lung cancer by combined copy number and targeted mutation profiling of cell-free DNA. EBioMedicine (2020) 62:103103. doi: 10.1016/j.ebiom.2020.103103
39. Zulato E, Attili I, Pavan A, Nardo G, Del Bianco P, Boscolo Bragadin A, et al. Early assessment of KRAS mutation in cfDNA correlates with risk of progression and death in advanced non-small-cell lung cancer. Br J Cancer (2020) 123:81–91. doi: 10.1038/s41416-020-0833-7
40. Angeles AK, Christopoulos P, Yuan Z, Bauer S, Janke F, Ogrodnik SJ, et al. Early identification of disease progression in ALK-rearranged lung cancer using circulating tumor DNA analysis. NPJ Prec Oncol (2021) 5:100. doi: 10.1038/s41698-021-00239-3
41. Russo A, Franchina T, Ricciardi G, Battaglia A, Schifano S, Schifilliti M, et al. Dynamic changes of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lactate dehydrogenase (LDH) during treatment with immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC). J Clin Oncol (2019) 37:2596. doi: 10.1200/JCO.2019.37.15_suppl.2596
42. Iwase S, Nakada T-A, Hattori N, Takahashi W, Takahashi N, Aizimu T, et al. Interleukin-6 as a diagnostic marker for infection in critically ill patients: A systematic review and meta-analysis. Am J Emerg Med (2019) 37:260–5. doi: 10.1016/j.ajem.2018.05.040
43. Frieri M. Corticosteroid effects on cytokines and chemokines. Allergy Asthma Proc (1999) 20:147–59. doi: 10.2500/108854199778553082
44. Solana R, Tarazona R, Aiello AE, Akbar AN, Appay V, Beswick M, et al. CMV and immunosenescence: from basics to clinics. Immun Ageing (2012) 9:23. doi: 10.1186/1742-4933-9-23
45. Pasello G, Fabricio AS, Del Bianco P, Favaretto A, Salizzato V, Piccin L, et al. Circulating cytokines as predictors of response to immune checkpoint inhibitors (ICIs) in patients (pts) with melanoma (Mel) and non–small cell lung cancer (NSCLC). J Clin Oncol (2022) 40:2549. doi: 10.1200/JCO.2022.40.16_suppl.2549
46. Wang Z, Zhan P, Lv Y, Shen K, Wei Y, Liu H, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res (2019) 8:214–26. doi: 10.21037/tlcr.2019.06.10
47. Da Kang H, Park C-K, Chung C, Oh I-J, Kim Y-C, Park D, et al. Baseline serum interleukin-6 levels predict the response of patients with advanced non-small cell lung cancer to PD-1/PD-L1 inhibitors. Immune Netw (2020) 20:e27. doi: 10.4110/in.2020.20.e27
48. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med (2020) 26:688–92. doi: 10.1038/s41591-020-0856-x
49. Wu L, Xie S, Wang L, Li J, Han L, Qin B, et al. The ratio of IP10 to IL-8 in plasma reflects and predicts the response of patients with lung cancer to anti-PD-1 immunotherapy combined with chemotherapy. Front Immunol (2021) 12:665147. doi: 10.3389/fimmu.2021.665147
50. Kotteas EA, Boulas P, Gkiozos I, Tsagkouli S, Tsoukalas G, Syrigos KN. The intercellular cell adhesion molecule-1 (icam-1) in lung cancer: implications for disease progression and prognosis. Anticancer Res (2014) 34:4665–72. Available at: https://ar.iiarjournals.org/content/34/9/4665.
51. Shibaki R, Murakami S, Shinno Y, Matsumoto Y, Yoshida T, Goto Y, et al. Predictive value of serum VEGF levels for elderly patients or for patients with poor performance status receiving anti-PD-1 antibody therapy for advanced non-small-cell lung cancer. Cancer Immunol Immunother (2020) 69:1229–36. doi: 10.1007/s00262-020-02539-2
52. Christopoulos P, Pfeifer D, Bartholomé K, Follo M, Timmer J, Fisch P, et al. Definition and characterization of the systemic T-cell dysregulation in untreated indolent b-cell lymphoma and very early CLL. Blood (2011) 117:3836–46. doi: 10.1182/blood-2010-07-299321
53. Morra R, de PP, Pietroluongo E, Ottaviano M, Tortora M, Mucci B, et al. Immunological signature of patients with thymic epithelial tumors. J Clin Oncol (2022) 40:8589. doi: 10.1200/JCO.2022.40.16_suppl.8589
54. Wang H, Zhou F, Zhao C, Cheng L, Zhou C, Qiao M, et al. Interleukin-10 is a promising marker for immune-related adverse events in patients with non-small cell lung cancer receiving immunotherapy. Front Immunol (2022) 13:840313. doi: 10.3389/fimmu.2022.840313
55. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-Based immunotherapy. Clin Cancer Res (2019) 25:1557–63. doi: 10.1158/1078-0432.CCR-18-2795
56. Stefania S, Colia R, Cinzia R, Corrado A, Cantatore FP. Off-label use of anti-IL-1 drugs in rheumatic diseases. Int J Immunopathol Pharmacol (2021) 35:20587384211006584. doi: 10.1177/20587384211006584
57. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (2017) 390:1833–42. doi: 10.1016/S0140-6736(17)32247-X
58. Lythgoe MP, Prasad V. Repositioning canakinumab for non-small cell lung cancer-important lessons for drug repurposing in oncology. Br J Cancer (2022) 127:785–7. doi: 10.1038/s41416-022-01893-5
59. Barrera L, Montes-Servín E, Barrera A, Ramírez-Tirado LA, Salinas-Parra F, Bañales-Méndez JL, et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann Oncol (2015) 26:428–35. doi: 10.1093/annonc/mdu549
60. Angeles AK, Janke F, Bauer S, Christopoulos P, Riediger AL, Sültmann H. Liquid biopsies beyond mutation calling: Genomic and epigenomic features of cell-free DNA in cancer. Cancers (Basel) (2021) 13:5615. doi: 10.3390/cancers13225615
61. Christopoulos P, Schneider MA, Bozorgmehr F, Kuon J, Engel-Riedel W, Kollmeier J, et al. Large Cell neuroendocrine lung carcinoma induces peripheral T-cell repertoire alterations with predictive and prognostic significance. Lung Cancer (2018) 119:48–55. doi: 10.1016/j.lungcan.2018.03.002
62. Mathew M, Zade M, Mezghani N, Patel R, Wang Y, Momen-Heravi F. Extracellular vesicles as biomarkers in cancer immunotherapy. Cancers (Basel) (2020) 12:2825. doi: 10.3390/cancers12102825
63. Rajakumar T, Horos R, Jehn J, Schenz J, Muley T, Pelea O, et al. A blood-based miRNA signature with prognostic value for overall survival in advanced stage non-small cell lung cancer treated with immunotherapy. NPJ Prec Oncol (2022) 6:19. doi: 10.1038/s41698-022-00262-y
Keywords: immune-checkpoint inhibitors, immunotherapy, immune-related adverse events, lung cancer, biomarker, cytokines
Citation: Schindler H, Lusky F, Daniello L, Elshiaty M, Gaissmaier L, Benesova K, Souto-Carneiro M, Angeles AK, Janke F, Eichhorn F, Kazdal D, Schneider M, Liersch S, Klemm S, Schnitzler P, Stenzinger A, Sültmann H, Thomas M and Christopoulos P (2022) Serum cytokines predict efficacy and toxicity, but are not useful for disease monitoring in lung cancer treated with PD-(L)1 inhibitors. Front. Oncol. 12:1010660. doi: 10.3389/fonc.2022.1010660
Received: 03 August 2022; Accepted: 14 October 2022;
Published: 31 October 2022.
Edited by:
Benjamin Frey, University Hospital Erlangen, GermanyReviewed by:
Lorenzo Belluomini, University of Verona, ItalyAlberto Pavan, Azienda ULSS 3 Serenissima, Italy
Copyright © 2022 Schindler, Lusky, Daniello, Elshiaty, Gaissmaier, Benesova, Souto-Carneiro, Angeles, Janke, Eichhorn, Kazdal, Schneider, Liersch, Klemm, Schnitzler, Stenzinger, Sültmann, Thomas and Christopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petros Christopoulos, cGV0cm9zLmNocmlzdG9wb3Vsb3NAbWVkLnVuaS1oZWlkZWxiZXJnLmRl
†ORCID: Petros Christopoulos, orcid.org/0000-0002-7966-8980
 Hannah Schindler
Hannah Schindler Fabienne Lusky1,2
Fabienne Lusky1,2 Lea Daniello
Lea Daniello Margarida Souto-Carneiro
Margarida Souto-Carneiro Arlou Kristina Angeles
Arlou Kristina Angeles Daniel Kazdal
Daniel Kazdal Marc Schneider
Marc Schneider Michael Thomas
Michael Thomas Petros Christopoulos
Petros Christopoulos