Abstract
Osteoclast-like giant cell tumor (OGCT) is a common bone tumor, occasionally observed in some extraosseous organs, but rarely involving the digestive system, especially the liver. Previously reported osteoclast-like giant cell carcinoma of the liver often coexists with sarcomatoid or hepatocellular carcinoma. Undifferentiated liver tumors with osteoclast-like giant cells (OGCs) are extremely rare. Due to its rarity, there is no consensus for diagnosis and treatment of undifferentiated liver tumors with OGCs. Definitive diagnosis comes from surgery, so there is often a long delay in diagnosis following the occurrence of symptoms. This case describes an extremely rare case of an undifferentiated liver tumor with OGCs in detail. It also summarizes the previously published cases based on liver tumors with OGCs from August 1980 to June 2021, providing extensive evidence to improve preoperative diagnosis and management options.
1 Introduction
Giant cell tumor of bone (GCTB) is a benign mesenchymal tumor, affecting mostly long bones. It usually develops in young adults (20–40 years old). Histologically, it is mainly composed of numerous osteoclast-like giant cells (OGCs) histologically (1). Notably this type of tumor, as reported, was also found at several extraskeletal sites (2). Among the digestive system organs, the pancreas was designated as the most vulnerable to developing this pathology (3). The occurrence of undifferentiated liver tumors manifesting as OGCs is an extremely rare event. According to the latest edition of the WHO classification of digestive system tumors, primary hepatic undifferentiated carcinoma meets the diagnostic criteria for rare liver tumors (4). A total of 18 instances associated with hepatic tumors with cell components of OGCs have been described since the first case was reported in 1980 (5). However, less than three cases are undifferentiated liver tumors, with OGCs among these instances. Due to limited information, clinical manifestations and imaging features cannot be well understood and summarized. Misdiagnosis and delayed diagnosis might easily happen, leading to a poor prognosis. Here, we present a rare case of undifferentiated liver tumor with OGCs and review previously published cases of liver tumor with OGCs from August 1980 to June 2021. We discuss the epidemiology, clinical manifestations, imaging features, pathological features, differential diagnosis, treatments, and prognosis of OGCT in detail, which is to collect more information systematically for disease decision-making.
2 Case presentation
2.1 History and examination
A 68-year-old man presented with intermittent right upper quadrant (RUQ) abdominal pain for half a year. Additionally, a temperature of up to 38.3°C occurred more than a month ago with no apparent cause. Upon arrival, he admitted having diabetes for three years, treated with metformin and glimepiride. His vital signs were stable upon initial evaluation. There is no evidence of a family history of cancer. The patient had a long history of alcohol abuse (daily consumption of >250 g alcohol over the past 30 years). Physical examination revealed no noteworthy findings other than abdominal pain. Tumor markers revealed elevated alpha-fetoprotein (AFP) levels (7.03 ng/ml) and des-gamma-carboxy prothrombin (DCP) levels (33.0 mAU/ml). A complete hemogram showed elevated RDW-CV and AMC levels, and decreased RBC, HGB, HCT, and LY% levels. Liver function tests showed elevated ALT, AST, GGT, and glucose levels and decreased TP and albumin levels. Renal function tests showed elevated serum cystatin C levels. A complete hemogram, liver function, and renal function tests of the patient are presented in Table 1. Laboratory tests also showed positive E antibody and core antibody.
Table 1
| Items | Result | Unit of measurement | Reference value | Mark |
|---|---|---|---|---|
| Red blood cell count, RBC | 2.70 | ×1012/L | 4.3–5.8 | ↓ |
| Hemoglobin, HGB | 78 | g/L | 130–175 | ↓ |
| Hematocrit in blood, HCT | 0.24 | L/L | 0.40–0.50 | ↓ |
| Red blood cell distribution width coefficient of variation, RDW-CV | 15 | % | 11.5–14.5 | ↑ |
| Lymphocyte percentage, LY% | 16.2 | % | 20–50 | ↓ |
| Absolute monocyte Count, AMC | 0.64 | ×109/L | 0.1–0.6 | ↑ |
| Alanine aminotransferase, ALT | 74 | IU/L | <50 | ↑ |
| Aspartate aminotransferase, AST | 41 | IU/L | <40 | ↑ |
| Gamma-Glutamyl transpeptidase, GGT | 72 | IU/L | <60 | ↑ |
| Total protein, TP | 63.1 | g/L | 65.0–85.0 | ↓ |
| Albumin | 34.9 | g/L | 40–55 | ↓ |
| Glucose | 6.13 | mmol/L | 3.9–5.9 | ↑ |
| Serum cystatin C | 1.13 | mg/L | 0.51–1.09 | ↑ |
Blood tests.
2.2 Abdominal imaging findings
A contrast-enhanced computed tomography (CT) scan of the upper abdomen displayed multiple slightly low-density masses and mixed-density nodules in the left lobe and the anterior segment of the right lobe. The largest mass was measured at 11.2 × 8.5 cm from an axial view. Lesions showed ring enhancement in the arterial phase and continuous enhancement in the portal phase. The sagittal part of the portal vein was infiltrated. Dilatation of the left intrahepatic bile duct combined with bile duct stones was observed. In addition, there were enlarged lymph nodes in the hepatic portal, portacaval space, and around the abdominal aorta (Figure 1).
Figure 1
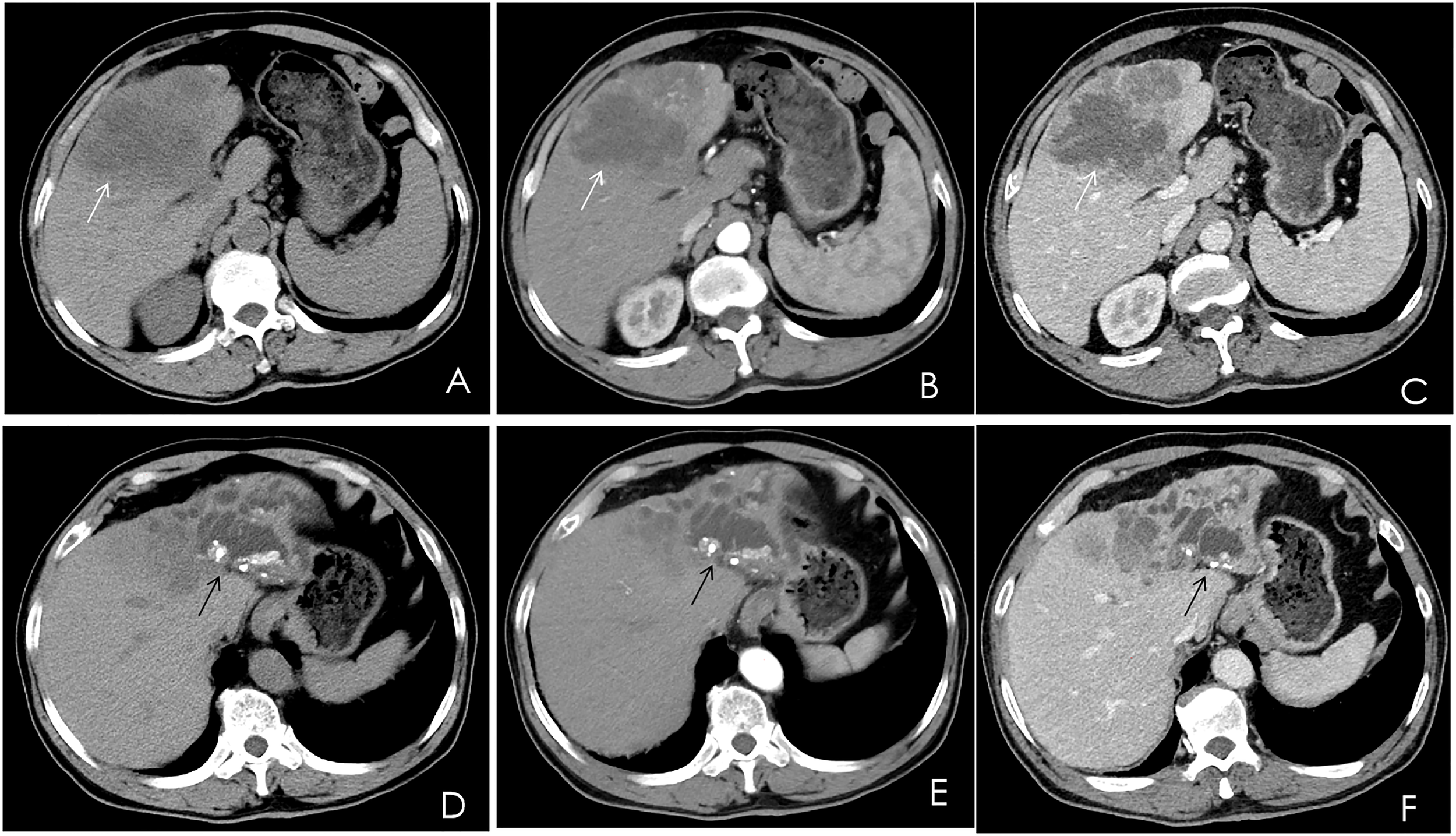
Multiphase contrast-enhanced CT of the upper abdomen, axial view. (A) A noncontrast CT showed multiple low-density masses and nodular mixed-density shadows in the left lobe and part of the anterior right lobe of the liver (white arrow). (B) The arterial phase showed heterogeneous ring enhancement (white arrow). (C) The venous phase showed continuous enhancement (white arrow). (D–F) The left branch of the intrahepatic bile duct and its branches were dilated, and high-density nodular shadows were seen in the liver (black arrow).
2.3 Surgery
A preoperative diagnosis of cholangiocarcinoma was made. Resection of the left lateral lobe, left medial lobe, right anterior lobe, and caudate lobe of the liver, hilar cholangioplasty, and cholecystectomy were performed to remove the whole mass after two days of admission, which appeared to have a complete tumor margin, a yellowish cross-section, and dilated partial bile ducts with stones inside. A lymphadenectomy was not performed. The operative time was 228 min. The blood loss was 500 cc, and a perioperative blood transfusion was not required. Surgical samples were taken and sent for pathological examination. There were no immediate postoperative complications.
2.4 Pathological findings
Histopathological analysis revealed that tumors were rich in osteoclast-like giant cells and neoplastic spindle-shaped cells (Figures 2A, B). Complete hepatic-lobule-like structures, edema of hepatocytes, intrahepatic cholestasis, lymphocyte, and plasma cell infiltration in the portal area were observed in the non-tumorous liver parenchyma of the resected specimen. Immunohistochemical staining showed that tumor cells were negative for EMA, Hepa, CK (Pan) (Figures 3A–C), desmin, CD34, S-100, SATB2, P63, P16, GS, GPC3, and CAM5.2, but positive for SMA, P53, Ki-67 (30%), ACT, CK8/18 (Figures 4A, B), and vimentin. PCR and Sanger sequencing: no H3F3A gene mutation was detected. Combined with histopathology and immunohistochemistry, the diagnosis of undifferentiated carcinoma with osteoclast-like giant cells of the liver was considered. The possibility of metastatic tumors was excluded by a comprehensive clinical examination.
Figure 2
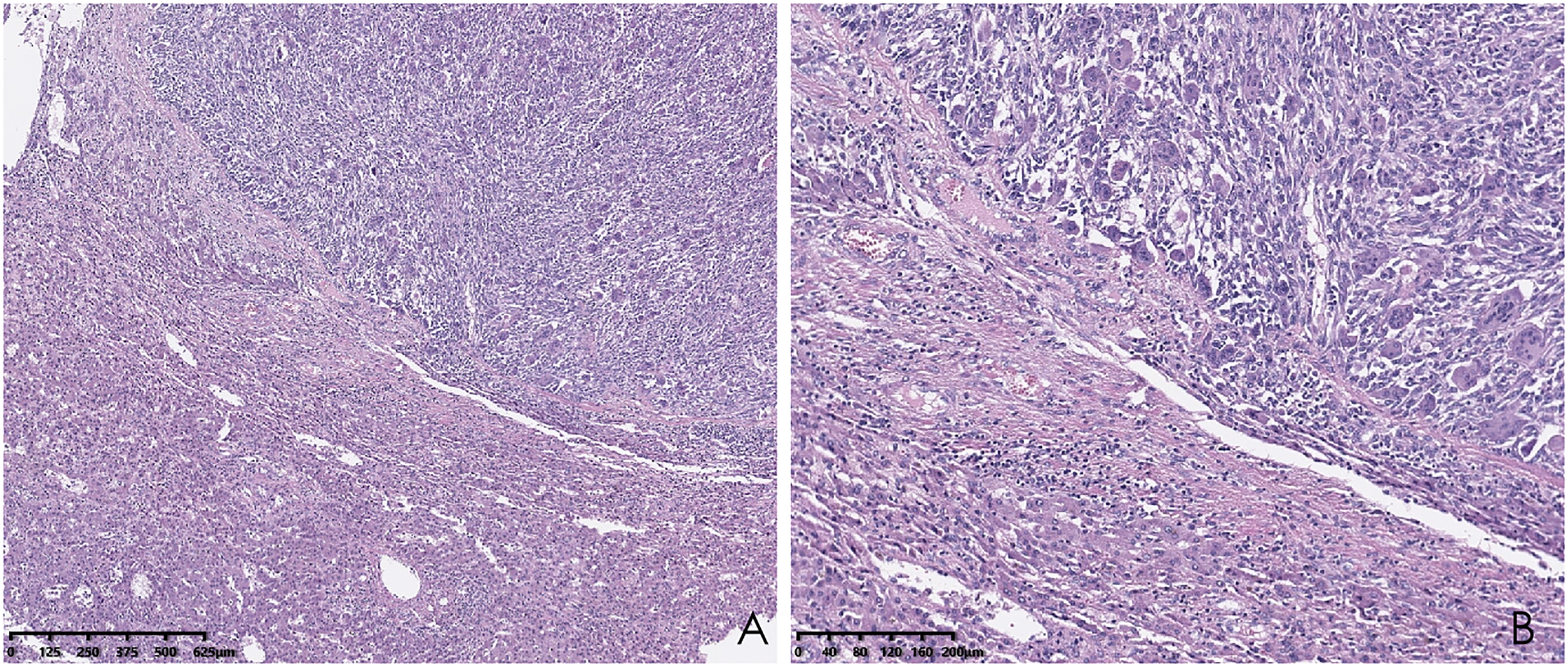
Hematoxylin and eosin (HE) staining of the liver biopsy sample shows osteoclast-like giant cells and neoplastic spindle-shaped cells. (A; hematoxylin and eosin, ×40); higher magnification of the destroyed liver structure lined by osteoclast-like giant cells and neoplastic spindle-shaped cells (B; hematoxylin and eosin, ×100).
Figure 3
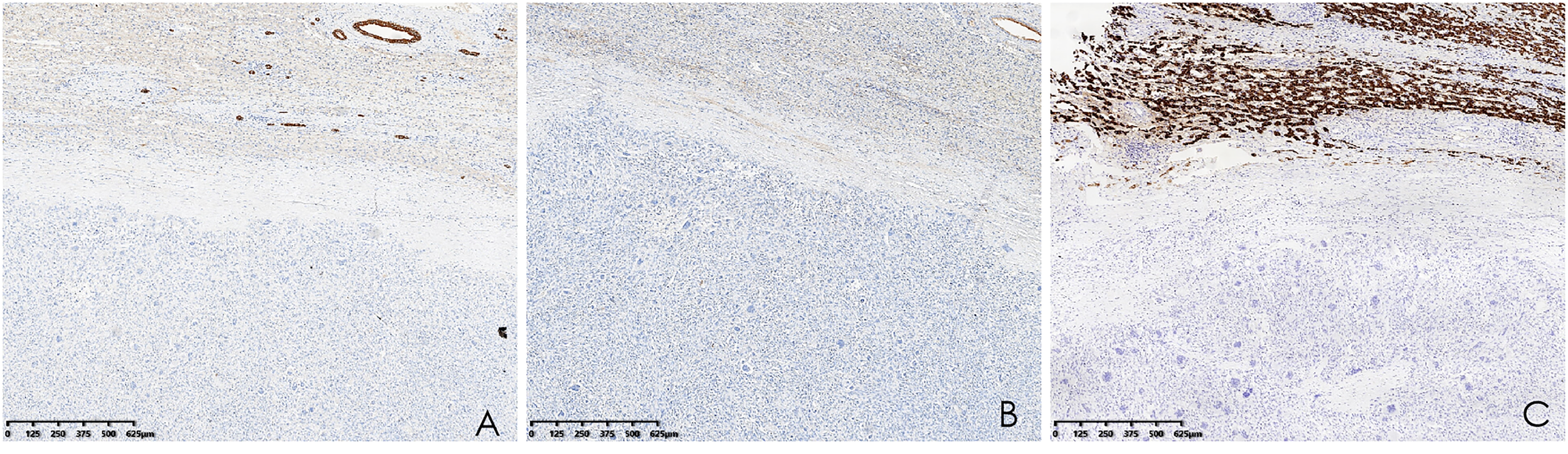
(A–C) Immunohistochemistry showed that CK (Pan), EMA, and Hepa were negative (hematoxylin and eosin, ×40).
Figure 4
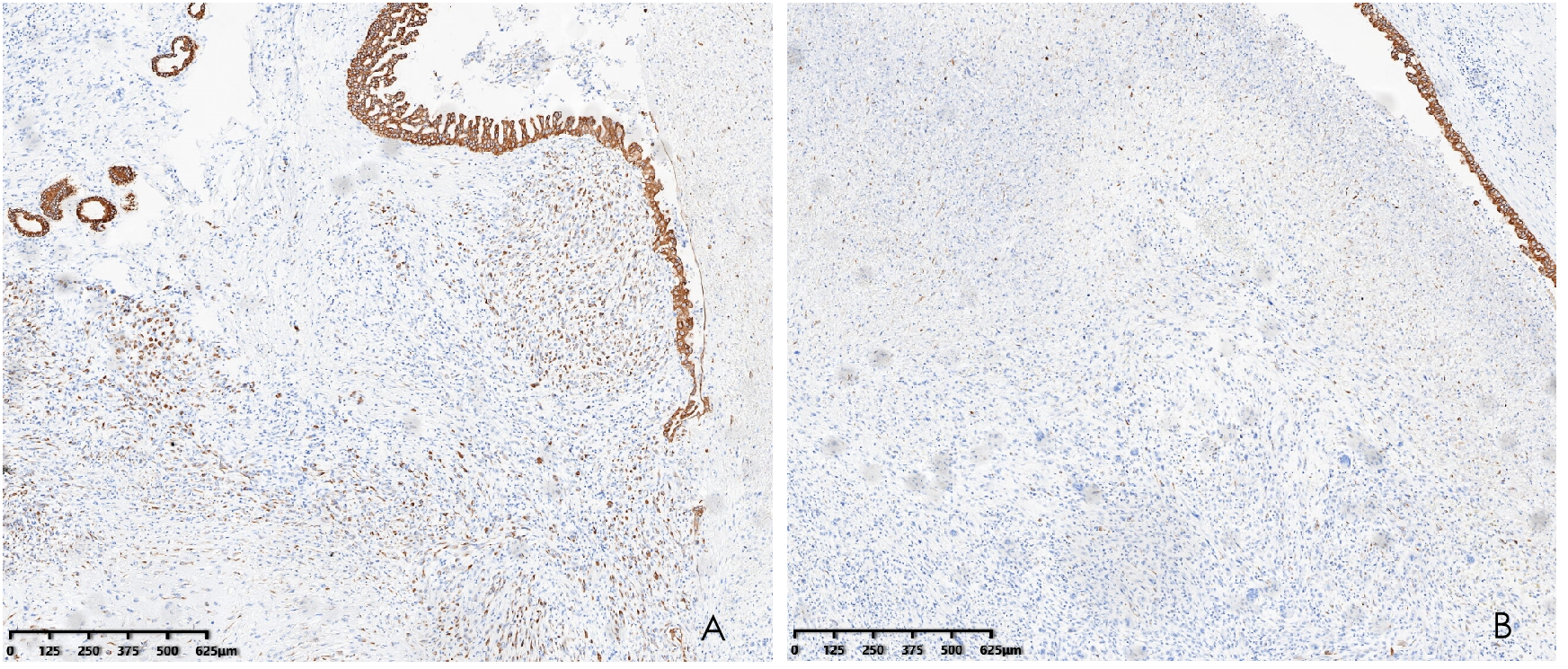
Immunohistochemistry demonstrated CK8/18 positivity in the tumor (A) and CK8/18 negativity for osteoclasts (B) (hematoxylin and eosin, ×40).
2.5 Postoperative course
The postoperative situation of the patient was stable, and he was discharged on the 12th day. At the first follow-up two months after hospital discharge, a chest CT showed that the lymph nodes in the mediastinum and right diaphragmatic corner were significantly increased. The patients and their families refused gene tests and agreed to perform GA chemotherapy was temporarily performed (gemcitabine 1.5 g d1; d8 + albumin paclitaxel 200 mg d1; d8). At the second follow-up five months after hospital discharge, metastases were found on CT scans of the head and chest. The patient and his family still refused the gene test and chose to undergo GP chemotherapy (gemcitabine 1,600 mg d1, 8 ivgtt + cisplatin 40 mg d1, 8; Q3w) and radiotherapy for the head. The current vital signs of the patient are stable. The patient is alive after 5-month follow-up.
3 Discussion
3.1 Epidemiology
Munoz et al. (5) first recorded an instance of OCGT of the liver, and some additional patients suffering from this rare tumor at the same site have been reported since then. These previously reported carcinomas were classified as tumors related to hepatocellular carcinomas, tumors related to cholangiocarcinomas or cystadenocarcinomas of the liver, tumors related to sarcomatous tumors, and undifferentiated tumors that were not related to conventional carcinomas, as presented in this case (6). Undifferentiated primary carcinoma of the liver itself is an uncommon type of cancer. The presence of osteoclast-like giant cells in undifferentiated carcinoma of the liver rarely occurs, classified as T3N0M0 and Stage III according to the third English edition of the Japanese classification of liver cancer. Only a few previous reports of liver tumors with giant cells resembling osteoclasts have been reported, so little is known about their biological behavior and clinical course.
It is well known that blood serum levels of PIVKA-II and AFP are useful indicators for the diagnosis of HCC. Suehiro et al. confirmed that a poorer prognosis is associated with a high level of serum PIVKA-II (7). In this case, the serum PIVKA-II level was elevated in this patient.
3.2 Clinical presentation
Case reports associated with hepatic tumors with OCGs, published from August 1980 to June 2021, were searched through the Biomedical Literature Database (PubMed). Clinical manifestations of hepatic tumors with OCGs are listed in Table 2. As evident from the 18 reported cases, the disease is more common in males (12, 66.7%), and its onset age ranges from 37 to 87 years old (average age: 63.7 years old), mostly affecting the left lobe of the liver. Several clinical symptoms have been reported at presentation, including abdominal pain, nausea, abdominal distension, fever, weight loss, and so on. Some cases were associated with cirrhosis (7, 38.9%) and hepatitis (6, 33.3%). AFP is a specific tumor marker for HCC, and the level of the tumor marker was elevated in four patients. As for previous history, most patients suffered from hypertension and diabetes (3, 5, 6, 8–22).
Table 2
| Study | Year | Sex | Age | Clinical manifestation | Tumor location | Tumor size | Hepatic disease | Cirrhosis | Previous history | AFP |
|---|---|---|---|---|---|---|---|---|---|---|
| Phillip a. Munoz (5) | 1980 | man | 87 | lethargy, anorexia, and fluid retention | right lobe of the liver | 11 cm in diameter | portal hypertension and chronic hepatic failure | macronodular cirrhosis of the liver | insulin-dependent diabetes; resection of Grade I1 papillary transitional cell carcinoma of the urinary bladder | normal |
| Hlroyukl Kuwano (8) | 1984 | man | 54 | general fatigue with slight fever, night sweating, weight loss | right lobe of the liver | 6x5x12 cm | type b hepatilis | hepatic cirrhosis | negative | 206.3 ng/ml |
| Salvatore Andreola (9) | 1985 | man | 71 | eneral fatigue and upper abdominal discomfort | right lobe of the liver | 12 cm in diameter | hepatitisB | hepatic cirrhosis | chronic alcoholism | normal |
| Yutaka Hori (10) | 1987 | male | 66 | hematemesis, nausea, epigastric dullness, abdominal pain | inferior surface of the liver | unknown | negative | negative | negative | normal |
| Daniel L. Hood (11) | 1989 | woman | 37 | right upper quadrant abdominal pain and right shoulder pain | left hepatic lobe | 1,761.25 ml | negative | negative | ovarian mucinous cystadenocarcinoma, total abdominal hysterectomy and bilateral salpingo-oophorectomy | normal |
| Atsushi Sasaki (12) | 1997 | Male | 42 | right abdominal pain and high-grade fever | posterior and medial segment | 6.0 cm in diameter; 2.5 cm in diameter | hepatitis B | hepatic cirrhosis | negative | normal |
| Tohru Ikeda (13) | 2003 | Man | 76 | unremarkable | S4 and S7–8 regions of the liver | unknown | type C hepatitis | liver cirrhosis | negative | unknown |
| M. Ahaouche (14) | 2005 | man | 57 | abdominal pain | IVth hepatic segment | 6 × 5.5 cm | negative | alcoholic cirrhosis | obesity, diabetes mellitus | 12 ng/ml |
| Udo Rudloff (3) | 2005 | woman | 61 | abdominal pain | right lobe of the liver | 7 × 7 × 10 cm | fatty liver; no hepatitis | negative | laparoscopic cholecystectomy; coronary artery disease; hypertension | normal |
| Juergen Bauditz (15) | 2006 | man | 54 | no clinical symptoms | segments II and III | 7 cm | negative | negative | peripheral arterial disease, hypertension, esophageal reflux, hiatal hernia, and sleep apnea | normal |
| Chisato Tanahashi (16) | 2009 | woman | 74 | unremarkable | left lobe of the liver | 10 × 5 cm | negative | negative | negative | unknown |
| Ryusuke Matsumoto (17) | 2012 | man | 57 | jaundice | left hepatic lobe | 10 cm | negative | negative | negative | normal |
| Kyoung-Bun Lee (18) | 2014 | man | 64 | resection of a hepatic mass | segment 6 | 6.0 × 4.0 × 2.2 cm | hepatitis B | hepatic cirrhosis | liver cancer; transarterial embolization (TAE) three times for a 1.4-cm multinodular mass in segment 6 first and percutaneous ethanol injection (PEI) and TAE of a new lesion in segment 6 after 4 years. | 36.1ng/mL |
| Lorenzo Dioscorid (19) | 2015 | woman | 74 | dull pain in the right upper quadrant associated with mild anemia | V–VI segments | 10 × 7cm | negative | negative | negative | normal |
| Hans Helmut Dahm (6) | 2015 | man | 68 | a tumor of the right lobe of the liver based on ultrasonography that was performed during a routine examination |
in segments 7 and 8 of the right liver lobe that adhered to the retroperitoneum. | 6 cm in diameter | chronic periportal hepatitis | negative | Insulin dependent type 2 diabetes mellitus and hypertension | unknown |
| Bita Geramizadeh (20) | 2017 | woman | 64 | abdominal pain | right lobe of the liver | 20 cm in the greatest diameter | negative | negative | negative | unknown |
| Meera Balakrishnn (21) | 2021 | woman | 64 | abdominal pain associated with nausea | fourth and fifth segments of the liver | 11 × 7 cm2 | negative | negative | hypertension dyslipidemia | normal |
| Anke H. C. Gielen (22)] | 2021 | male | 77 | icterus, general fatigue | segment 8, parts of segments 4 and 5 | 8cm | negative | negative | hypertension, type 2 diabetes, and prostate cancer | 215.5ng/ml |
Clinical manifestation of hepatic tumor with OCGTs.
3.3 Radiologic characteristics
Hepatocellular carcinoma (HCC) accounts for most liver cancer, and classical HCC appears as arterial phase enhancement on CT followed by washout in the portal phase (23). Cholangiocarcinoma is prevalent in Asia and is divided into intrahepatic, hilar, and distal types. Intrahepatic cholangiocarcinomas show edge enhancement in the arterial phase and a progressive centripetal filling of the fibrous stroma in the delayed phase, with relative low density surrounding it. The imaging features of hilar cholangiocarcinoma are irregular thickening of the hilar bile duct wall, an eccentrically narrowed lumen, and dilation of the upper bile duct. Angiographic findings of distal cholangiocarcinoma included dilatation of the proximal bile duct, confluence of the bile ducts, and enlargement of the gallbladder with normal bile ducts below the stenotic segment. In our case, CT findings of undifferentiated carcinoma with osteoclast-like giant cells of the liver resembled cholangiocarcinoma. We suppose that large intrahepatic masses might contribute to obstruction of bile flow. These were risk factors for the development of cholestasis, bile duct dilatation, and bile duct stones. Lesions showed ring enhancement in the arterial phase and continuous enhancement in the portal phase. These enhancement characteristics suggested that tumors might be abundant in mesenchymal tissue. We summarized the imaging findings of 18 cases of HCC with OCGs reported so far (Table 3) (3, 5, 6, 8–22).
Table 3
| Study | Imaging findings |
|---|---|
| Phillip A. Munoz (5) | A liver scan with technetium sulfur colloid revealed diffuse hepatic parenchymal disease with a large focal lesion in the right lobe. A 67-gallium citrate scan suggested a primary liver tumor in addition to cirrhosis. |
| Hiroyuki Kuwano (8) | Celiac angiography showed a hypervascular tumor shadow, 7.5 × 6.5 cm, in the posterior area of the right lobe of the liver |
| Salvatore Andreola (9) | A liver echography showed a hypoechoic mass in the right lobe of the liver. |
| Yutaka Hori (10) | Serial CT scans showed a clustering tumor mass arising from the inferior surface of the liver with low attenuated areas in the mass |
| Daniel L. Hood (11) | n abdominal computed tomography (CT) scan showed a large hepatic mass |
| Atsushi Sasaki (12) | Two hepatic tumors were detected in the posterior segment (6.0 cm in diameter) and the medial segment (2.5 cm in diameter), respectively, by abdominal ultrasonography, computed tomography and abdominal angiography. |
| Tohru Ikeda (13) | unknown |
| M. Ahaouche (14) | ultrasound and CT scan identified a mass measuring 60 mm in maximum dimension located in the IVth hepatic segment. This nodule had extrahepatic extension and invaded the diaphragm. It was hypervascular and homogeneous. |
| Udo Rudloff (3) | Magnetic resonance imaging (MRI) of the abdomen confirmed an 8 × 8 × 10 cm mass within the right lobe of the liver involving segments V, VI, and VII. Endoscopic retrograde cholangiopancreatography (ERCP) showed common bile duct (CBD) dilation to 1.3 cm. |
| Juergen Bauditz (15) | CT demonstrated a 7 cm large inhomogeneous solid liver tumor involving segments II and III.B-mode sonography (HDI 5000, Philips) demonstrated a well-defined, inhomogeneous, cauliflower-like tumor with multiple small calcifications, causing retraction of the liver contour. Within the center of the tumor, a focal nodular hyperplasia (FNH)-like stellar scar was present. Contrast-enhanced sonography demonstrated an inhomogeneous perfused tumor with a large feeding artery heading towards the center of the tumor, radially branching to the periphery. |
| Chisato Tanahashi (16) | unknown |
| Ryusuke Matsumoto (17) | Abdominal computed tomography showed a 10-cm left hepatic lobe heterogeneous solid mass with low attenuated areas in the mass, multiple liver metastases and lung metastasis. |
| Kyoung-Bun Lee (18) | Magnetic resonance imaging showed a 5-cm lobulating soft tissue mass with an internal hemorrhagic component |
| Lorenzo Dioscorid (19) | the hepatic neoplasm was confirmed and shown to grow from a Riedel’s segment towards the right iliac fossa with a close contiguity with ascending colon and caecum |
| Hans Helmut Dahm (6) | A computed tomography (CT) scan showed evidence of a malignant liver neoplasm. |
| Bita Geramizadeh (20) | CT scan showed an enlarged liver with a large mass in the right lobe of the liver with irregular borders and central necrosis, measuring 20 cm in the greatest diameter. A few smaller lesions were also present. Portal vein thrombosis was also identified. |
| Meera Balakrishnn (21) | Computed tomography (CT) scan showed a heterogeneous focal mass measuring 11x7cm2 occupying the fourth and fifth segments of the liver with areas of necrosis and prominent vessels passing through it. The mass infiltrated the gallbladder wall |
| Anke H. C. Gielen (22) | A computed tomography (CT) scan showed a liver neoplasm of 6.7 cm in segment 8 |
Imaging findings of hepatic tumors with OCGTs.
3.4 Pathological features
There are many controversies surrounding the origin and histogenesis of OCGT. A few studies have suggested that osteoclast-like giant cells were probably neoplastic and epithelial (24). Other studies held the opposite view, that tumors originated from mesenchymal tissue (25, 26). Munoz et al. first reported the case of an osteoclastoma-like giant cell tumor of the liver in 1980. They hypothesized that reticuloendothelial cells were involved in the origin of the tumor (5). Rosai proposed that multinucleated giant cells are always derived from non-epithelial cells with an osteoclastic phenotype and are essentially non-neoplastic, rather than determined by the location of the tumor and the presence of identifiable cancer components in the tumor (2). Based on immunohistochemistry, Sasaki et al. confirmed that the OGC expressed only histiocytic and mesenchymal markers (ACT, AAT, MUR, VIM, and CD68), and they remained negative for epithelial markers (EMA, CK 7, CK 8, and CK 19). Their findings support the view that OCG is non-tumor and non-epithelial (12). In this case, tumors were more likely to be of non-epithelial and mesenchymal origin based on the negative results of EMA and CK (Pan) and the positivity of Vim. The diagnosis of giant cell tumors of bone was unconsidered due to no H3F3A mutation. P53 expression tends to be associated with the differentiation degree of liver tumor cells, especially in poorly differentiated liver tumors, indicating a worse prognosis (27). Hepatic undifferentiated carcinoma is poorly defined from a clinicopathological and molecular perspective. After a full study of 14 cases of primary hepatic undifferentiated carcinoma, high mutation rates of the TERT and TP53 genes and high expression rates of PD-L1 were found, which may be useful biomarkers for potential immunotherapy strategies (28). However, despite a thorough explanation, the patient declined further genetic testing in our case.
3.5 Differential diagnosis
There are several subtypes of liver tumors that may be related to multinuclear giant cells and therefore require a differential diagnosis. Hepatocellular carcinoma with syncytial giant cells is a special variety of liver tumor. As the tumor exhibits positive cytokeratin 8 and Hep markers, hepatocellular carcinoma with syncytial giant cells is epithelial and probably arises from hepatocytes (29). Differently, the immunohistological results suggest a different origin of the giant cells, which is more likely to be mesenchymal for our type. As another type of liver tumor containing multinuclear giant cells, sarcomatoid HCC is featured by reactivity for CK 8, ALB, and fibrinogen, as well as for VIM (30). In addition, OCG-associated hepatocellular carcinoma contains two components, including a well-differentiated HCC characterized by partial steatohepatitic morphology and abundant OCG forms mixed with hepatocellular cancer cells (22). The tumor in our case report showed coexistence of undifferentiated carcinoma of the liver and osteoclast-like giant cells, exhibiting negativity for hepatocellular and epithelial markers and positivity for cytokeratin 8/18 (CK8/18).
3.6 Treatment
Due to the rarity of the condition, there is not yet a standard therapy for it. It seems that surgery is still the main treatment for this ailment. Masatsugu et al. described radical surgery without any chemotherapy for an undifferentiated liver tumor that achieved good results (31). According to Hood et al., a woman with recurrent OCGT of the liver was treated with 5-fluorouracil and adriamycin, external beam radiation, and radioimmune therapy (IgG, labeled with I-131) (11). The case reported by Kamitani et al. was treated with neoadjuvant chemotherapy before radical hepatectomy. After metastasis occurred two months after surgery, targeted treatment was adopted; however, the patient died five months later (32). In this case, the patient underwent surgery and postoperative adjuvant chemotherapy. The patient is alive after 5-month follow-up.
3.7 Prognosis
OCGTs with liver involvement tend to proliferate aggressively. It is apparent that nodal metastasis is the predominant mode of spreading, and the prognosis, even after resection, is usually dismal. Patients survive for weeks to months after surgery for OCGT of the liver. We summarized published cases associated with the prognosis of hepatic tumors with OCGTs from August 1980 to June 2021 on PubMed (Table 4) (3, 5, 6, 8–22). As evident from the 18 published cases, most patients died within 3 months of surgery, and only one patient was alive and in good condition.
Table 4
| Study | Diagnosis | Treatment | Postoperative treatment | Outcome |
|---|---|---|---|---|
| Phillip A. Munoz (5) | hepatic tumor with OCGTs | no | no | died 32 days later |
| Hiroyukl Kuwano (8) | HCC with OCGTs | A posterior segmentectomy of the right hepatic lobe with resection of the right diaphragm was performed. | no | metastasis; died four 42 days later |
| Salvatore Andreola (9) | hepatic tumor with OCGTs | cholecystectomy | no | metastasis; died 20 days later |
| Yutaka Hori (10) | hepatic tumor with OCGTs | TACE | TACE | died 42 days later |
| Daniel L Hood (11) | hepatic tumor with OCGTs | resection of the left hepatic lobe and part of the anterior abdominal wall | chemoradiotherapy | metastasis; died three months later |
| Atsushi Sasaki (12) | Sarcomatoid hepatocellular carcinoma with OCGT | atypical segmentectomy | transcatheteral arterial chemoembolization therapy (TACE) | metastasis, died 28 days later |
| Tohru Ikeda (13) | sarcomatoid tumor cells with OCGTs | trans-arterial embolization (TAE); Partial hepatectomy | Radiation therapy | metastasis; died one months later |
| M. Ahaouche (14) | hepatic tumor with osteoclast-like giant cells | lobectomy | no | died 3 months later |
| Udo Rudloff (3) | hepatic tumor with OCGTs | right hepatic lobectomy | no | metastasis; died three months later |
| Juergen Bauditz (15) | hepatic tumor with OCGTs | surgical resection of liver segments II and III | chemotherapy | metastasis; alive |
| Chisato Tanahashi (16) | HCC with OCGTs | left lobectomy | no | died of the disease at 110 days after operation. |
| Ryusuke Matsumoto (17) | hepatic tumor with OCGTs | no | no | died a few weeks later |
| Kyoung-Bun Lee (18) | Sarcomatoid hepatocellular carcinoma with OCGT | peripheral segmentectomy of segment 6 | no | metastasis |
| Lorenzo Dioscorid (19) | HCC with OCGTs | hepatic resection of the V-VI segments | no | metastasis; died four months later |
| Hans Helmut Dahm (6) | HCC, sarcoma with OCGT | atypical segmentectomy | no | metastasis; tumor recurrence |
| Bita Geramizadeh (20) | hepatic tumor with OCGTs | atypical segmentectomy | no | died two months later |
| Meera Balakrishnn (21) | hepatic tumor with OCGTs | segmentectomy | chemotherapy | metastasis; died after 108 days |
| Anke H. C. Gielen (22) | HCC with OCGTs | resection of segment 8 and parts of segment 4 and 5 was performed. | no | alive in a good condition |
Prognosis of hepatic tumor with OCGTs.
4 Conclusion
Our study provided an in-depth look at imaging observations, treatment modalities, patterns of spread, and clinical outcomes to gain a more comprehensive understanding of this disease. Further studies on undifferentiated hepatic tumors with OGCs are recommended to analyze a suitable therapeutic strategy for this rare condition in the future.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YD, YW, YZ, and NY conducted the radiological analysis of CT images, XJ conducted the pathological analysis, and YD prepared the manuscript. BW revised the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was partially supported by the Sichuan Province Science and Technology Support Program (Grant Number: 2021YJ0241).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Raskin KA Schwab JH Mankin HJ Springfield DS Hornicek FJ . Giant cell tumor of bone. J Am Acad Orthop Surg (2013) 21(2):118–26. doi: 10.5435/JAAOS-21-02-118
2
Rosai J . Liver cell carcinoma with osteoclast-like giant cells: nonepitheliogenic giant cells in diverse malignancies. Hepatology (1990) 12(4 Pt 1):782–3. doi: 10.1002/hep.1840120425
3
Rudloff U Gao ZQ Fields S Gecelter GR . Osteoclast-like giant cell tumor of the liver: A rare neoplasm with an aggressive clinical course. J Gastroi Surg (2005) 9(2):207–14. doi: 10.1016/j.gassur.2004.07.007
4
WHO Classification of Tumours Editorial Board . Digestive system tumours. In: Lyon(France): International agency for research on cancer. (IARC publications; France), 5th ed, vol. 1. (2019). p. 262. Available at: https://publications.iarc.fr/579.
5
Munoz PA Rao MS Reddy JK . Osteoclastoma-like giant cell tumor of the liver. Cancer (1980) 46:771–9. doi: 10.1002/1097-0142(19800815)46
6
Dahm HH . Immunohistochemical evaluation of a sarcomatoid hepatocellular carcinoma with osteoclastlike giant cells. Diagn Pathol (2015) 10:40. doi: 10.1186/s13000-015-0274-4
7
Suehiro T Sugimachi K Matsumata T ltasaka H Taketomi A Maeda T . Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatoceflular carcinoma: Comparison with alpha-fetoprotein. Cancer (1994) 73:2464–71. doi: 10.1002/1097-0142(19940515)73
8
Kuwano H Sonoda T Hashimoto H Enjoji. M . Hepatocellular carcinoma with osteoclast-like giant cells. Cancer (1984) 54(5):837–42. doi: 10.1002/1097-0142(19840901)54
9
Andreola S Lombardi L Scurelli A Bersiga. A . Osteoclastoma-like giant-cell tumor of the liver. case report. Tumori (1985) 71(6):615–20. doi: 10.1177/030089168507100616
10
Horie Y Hori T Hirayama C Hashimoto K Yumoto T Tanikawa. K . OSTEOCLAST-LIKE GIANT CELL TUMOR OF THE LIVER. Acta Pathol Jpn (1987) 37(8):1327–35. doi: 10.1111/j.1440-1827.1987.tb00465.x
11
Hood DL Bauer TW Leibel SA McMahon JT . Hepatic giant cell carcinoma. an ultrastructural and immunohisto-chemical study. Am J Clin Pathol (1990) 93:111–6. doi: 10.1093/ajcp
12
Sasaki A Yokoyama S Nakayama I Nakashima K Kim YI Kitano. S . Sarcomatoid hepatocellular carcinoma with osteoclast-like giant celIs: Case report and i mmunohistochemical obsewations. Pathol Int (1997) 47(5):318–24. doi: 10.1111/j.1440-1827.1997.tb04500.x
13
Ikeda T Seki S Maki M Noguchi N Kawamura T Arii S et al . Hepatocellular carcinoma with osteoclast-like giant cells: Possibility of osteoclastogenesis by hepatocyte-derived cells. Pathol Int (2003) 53(7):450–6. doi: 10.1046/j.1440-1827.2003.01503.x
14
Ahaouche M Cazals-Hatem D Sommacale D Cadranel J-F Belghiti J Degott. C . A malignant hepatic tumour with osteoclast-like giant cells. Histopathology (2005) 46(5):590–2. doi: 10.1111/j.1365-2559.2005.02018.x
15
Bauditz J Rudolph B Wermke W . Osteoclast-like giant cell tumors of the pancreas and liver. World J Gastroenterol (2006) 12(48):7878–83. doi: 10.3748/wjg.v12.i48.7878
16
Tanahashi C Nagae H Nukaya T Hasegawa M Yatabe Y . Combined hepatocellular carcinoma and osteoclast-like giant cell tumor of the liver: Possible clue to histogenesis. Pathol Int (2009) 59(11):813–6. doi: 10.1111/j.1440-1827.2009.02450.x
17
Matsumoto R Yoshida K Nakajima J Atarashi T Takeda T Yanagisawa H et al . Osteoclast-like giant cell tumor (OGCT) of the liver: Report of a case. Nihon Shokakibyo Gakkai Zasshi (2012) 109(2):255–62. doi: 10.11405/nisshoshi.109.255
18
Lee K-B . Sarcomatoid hepatocellular carcinoma with mixed osteoclast-like giant cells and chondroid differentiation. Clin Mol Hepatol (2014) 20(3):313–6. doi: 10.3350/cmh.2014.20.3.313
19
Dioscoridi L Bisogni D Freschi G . Hepatocellular carcinoma with osteoclast-like giant cells: report of the seventh case in the literature. Case Rep Surg (2015) 2015:836105. doi: 10.1155/2015/836105
20
Geramizadeh B Kazemi. K . Osteoclastoma-like giant cell tumor of the liver, an extremely rare tumor. Hepatitis Monthly (2017) 17(9):e56097. doi: 10.5812/hepatmon.56097
21
Balakrishnan M Pathan SK Mallik MK Hussein SAB Shatti RAl Kapila. K . Fine-needle aspiration cytology of osteoclast-like giant cell tumor of liver–a case report with review of literature. Diagn Cytopathol. (2022) 50(1):E18–22. doi: 10.1002/dc.24869
22
Gielen AHC Samarska I Dulk MD Beckervordersandforth J Dejong KHC Bouwense SAW et al . Osteoclast-like giant cells in hepatocellular carcinoma case description and review of the literature. Acta Chir Belg. (2021) 21:1–7. doi: 10.1080/00015458.2021.1940443
23
Hennedige T Venkatesh SK . Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging (2013) 12:530–47. doi: 10.1102/1470-7330.2012.0044
24
Silverberg SG DiGiorgi LS . Osteoclastoma-like giant cell tumor of the thyroid report of a case of prolonged survival following partial excision and radiotherapy. Cancer (1973) 31:62 1–625. doi: 10.1002/1097-0142(197303)31:3<621::aid-cncr2820310319>3.0.co;2-n
25
Oyasu R Battifora HA Buckingham WF Hidvegi D . Metaplastic squamous cell carcinoma of bronchus simulating giant cell tumor of bone. Cancer (1977) 39:1119–28. doi: 10.1002/1097-0142(197703)39:3<1119::aid-cncr2820390317>3.0.co;2-7
26
Udea G Furth J . Sarcomatoid transformation of transplanted thyroid carcinoma. Arch Puthol. (1967) 83:3–12.
27
Nakano A Watanabe N Nishizaki Y Takashimizu S Matsuzaki S . Immunohistochemical studies on the expression of p-glycoprotein and p53 in relation to histological differentiation and cell proliferation in hepatocellular carcinoma. Hepatol Res (2003) 25:158–65. doi: 10.1016/s1386-6346(02)00207-3
28
Tsai JH Jeng YM Lee CH Liau JY . Molecular features of primary hepatic undifferentiated carcinoma. Mod Pathol (2022) 35(5):680–7. doi: 10.1038/s41379-021-00970-z
29
Atra A Al-Asiri R Wali S Al-Husseini H Al-Bassas A Zimmermann A . Hepatocellular carcinoma, syncytial giant cell: a novel variant in children: a case report. Ann Diagn Pathol (2007) 11(1):61–3. doi: 10.1016/j.anndiagpath.2005.12.005
30
Haratake J Hone A . An immunohistochemical study of sarmmatoid liver carcinomas. Cancer (1991) 68:93–7. doi: 10.1002/1097-0142(19910701)68
31
Hiraki M Kitahara K Miyoshi A Koga H Nakamura H Kubo H et al . A long-term survivor of undifferentiated carcinoma of the liver successfully treated with surgical treatments: A case report and literature review. Int J Surg Case Rep (2018) 51:45–9. doi: 10.1016/j.ijscr.2018.07.047
32
Kamitani N Nomi T Hokuto D Yoshikawa T Matsuo Y Sho M . Primary undifferentiated carcinoma with osteoclast−like giant cells in liver and rapidly developing multiple metastases after curative hepatectomy: a case report. Int Cancer Conf J (2020) 9(4):244–8. doi: 10.1007/s13691-020-00436-0
Summary
Keywords
osteoclast-like giant cell, undifferentiated hepatic carcinoma, diagnosis, treatment, prognosis
Citation
Deng Y, Wang Y, Zhang Y, Yang N, Ji X and Wu B (2023) Undifferentiated hepatic carcinoma with osteoclast-like giant cells: A case report and literature review. Front. Oncol. 12:1018617. doi: 10.3389/fonc.2022.1018617
Received
13 August 2022
Accepted
12 December 2022
Published
09 January 2023
Volume
12 - 2022
Edited by
Riccardo Inchingolo, Ospedale Generale Regionale F. Miulli, Italy
Reviewed by
Rahul Gupta, Synergy Institute of Medical Sciences, India; Zongming (Eric) Chen, Mayo Clinic, United States
Updates
Copyright
© 2023 Deng, Wang, Zhang, Yang, Ji and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Wu, 2244096945@qq.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers, a section of the journal Frontiers in Oncology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.