- 1Department of Internal Medicine, Clinic III – Hematology, Oncology and Palliative Care, Rostock University Medical Center, Rostock, Germany
- 2Department of Hematology, Oncology, and Cancer Immunology, Charité Campus Virchow-Klinikum, Charité, Universitätsmedizin Berlin, Berlin, Germany
- 3Krukenberg Cancer Center Halle, University Hospital Halle, Halle (Saale), Halle, Germany
- 4Department of Internal Medicine, Medical Clinic II, Carl-von-Basedow-Klinikum, Merseburg, Germany
- 5Department of Internal Medicine III, Heinrich Braun Hospital, Zwickau, Germany
- 6LeukaNET/Leukemia Online e.V., Riemerling, Germany
- 7MPN-Netzwerk e. V., Bonn, Germany
Background: Physical activity (PA) is a non-pharmacological approach to alleviate symptom burden and improve health-related quality of life (HrQoL) in cancer patients (pts). Whether pts with myeloproliferative neoplasms (MPN) PA behavior changes due to symptom burden and/or knowledge of the putative beneficial effects of PA has not yet been investigated.
Methods: We performed a large questionnaire study in MPN pts. Self-reported PA behavior and potential influencing factors of 634 MPN pts were analyzed. Questionnaires were used to assess demographics, anxiety, severity of symptoms, HrQoL, current level of everyday and sports activities, and the level of information regarding the importance/possibilities of PA. According to their PA, the pts were assigned to the three groups: “inactive”, “non-targeted active”, and “sporty active” and compared with each other.
Results: Key findings are that in 73% of the pts, the disease had an impact on PA, with 30% of pts reducing their PA. The prevalence of anxieties (e.g., occurrence of thrombosis and bleeding) regarding PA was 45%. Sporty active pts had a lower symptom burden and better HrQoL (p ≤ 0.001) compared to the other groups. Inactive pts were significantly older and had a higher body mass index than sporty active pts. Inactive and non-targeted active pts felt less informed about the importance/possibilities of PA (p = 0.002).
Conclusion: Our results suggest that especially older and non-sporty MPN pts could benefit from motivational as well as disease-specific PA information. This study was registered at the German Registry of Clinical Trials, DRKS00023698.
1. Introduction
Patients (pts) with myeloproliferative neoplasms (MPN) suffer from a variety of disease- and therapy-related symptom burden. In addition to fatigue, the most common symptoms include concentration problems, bone pain, headache, dizziness, microcirculatory symptoms, itching, night sweet, depression, and anxiety (1–4). In advanced disease, splenomegaly is common and often associated with abdominal discomfort, loss of appetite, and leads to weight loss in about one-fifth of pts (1, 5). All of these symptoms have implications on physical performance, emotional well-being, and health-related quality of life (HrQoL), and lead to work productivity impairments (1, 6, 7). Thanks to advances in diagnostics and therapy, many MPN pts have an almost normal life expectancy (8, 9). Of note, myelofibrosis (MF) -primary or secondary- is often associated with a more severe disease course and decreased overall survival (10). Pts with chronic myeloid leukemia (CML) benefit in regards to life expectancy from the effectiveness of tyrosinkinase inhibitors (TKI). Due to the predominantly chronic courses of the diseases, MPN pts suffer from symptoms throughout their lives. Thus, HrQoL is increasingly becoming a focus of MPN treatment.
Based on the evidence regarding the effects of physical activity (PA) on functionality, symptom burden, and HrQoL in pts with solid tumors, acute leukemia, lymphomas, and myelomas (11, 12), it is reasonable to assume that PA may be an effective non-pharmacological approach to reduce symptom burden and improve HrQoL in MPN pts (13). Whether MPN pts’ PA behavior changes due to symptom burden and/or knowledge of the putative beneficial effects of PA has not yet been investigated. Similarly, it is unclear whether the consequences of impaired hematopoietic system function have an impact on PA. MPN pts often have an increased risk of thrombosis and infection, an increased bleeding tendency and/or anemia, accompanied by a reduced performance capacity (3, 4, 9). Itching and skin reactions could also have an impact on PA.
To support MPN pts in maintaining or implementing a physically active lifestyle in the long term, targeted information is warranted. The present study investigated which factors show an association with PA in MPN pts. The present study investigated (I) whether and how PA behavior changes due to a MPN disease, (II) whether anxiety of certain events such as thrombosis, bleeding, and skin reactions have an influence on PA, (III) how physically inactive MPN pts differ from active pts, and (IV) whether MPN pts have knowledge of the importance and possibilities of PA.
2. Materials and methods
The study was designed as a multicenter cross-sectional study. It was approved by the Ethics Committee of the University of Rostock (A2020-0274) and registered with the German Registry of Clinical Trials: DRKS00023698. Pts ≥ 18 years with any type of MPN (14) could participate in the survey. Eligible pts of 12 institutions in the East German Study Group Hematology and Oncology (OSHO, Online Supplementary S1) were asked to participate and fill in a hard copy questionnaire (enrollment January 2021 to September 2021). From April 2021 to September 2021, the study was amended by an online version of the survey consisting of the same set of questions. Participants included pts of the LeukaNET/Leukemia-Online pts network as well as the German, Austrian, and Swiss MPN pts network.
Questionnaire
General information. The following general characteristics were collected: gender, age, education level, family status, profession, height, and weight. The Body Mass Index (BMI) was calculated.
Details of the disease. MPN subtype, year of diagnosis, disease-specific therapies (e.g. TKI, januskinase (JAK) inhibitors, cytostatics (e.g. hydroxyurea, anagrelid, busulfan, clardibrin), inteferon, and other therapies (e.g., anticoagulation, phlebotomy) were inquired.
Physical activity. Questions asked for whether and how the PA had changed in everyday life and during sports since the diagnosis of the MPN, and whether they are afraid of certain events (e.g., bleeding). Everyday activity was measured with the Godin-Shepard Leisure-Time Physical Activity Questionnaire (GSLTPAQ) and was classified into three categories: “insufficiently active”, “moderately active”, and “active” (15, 16). Additionally, the five stages of the transtheoretical model of behavioral change (SOC) were used to determine the motivation to participate regularly in sports (17, 18). In the stages of precontemplation, contemplation, and preparation, pts are not regularly active in sports. In the stages of action and maintenance, pts are active for at least 20 minutes on at least 3 days per week. The questionnaires (GSLTPAQ, SOC) are provided in the Online Supplementary S2.
HrQoL and symptoms. HrQoL was assessed by a visual analogue scale (VAS) ranging from 0 (very poor) to 100 (very good). Symptoms were assessed using single items of the MPN Symptom Assessment Form (MPN-SAF) (19), supplemented by other typical symptoms of CML, ranging from 0 (absent) to 100 (worst imaginable). Further, weight changes, potential side effects of MPN such as skin reactions, splenomegaly, as well as the number of falls in the last 12 months were inquired.
Information level. It was recorded whether the pts felt sufficiently informed about the importance and possibilities of PA and the desire for more information.
Activity groups
Pts were divided into three groups depending on their level of everyday (GSLTPAQ) and sports activities (SOC). Group 1 “inactive”: all insufficiently active pts who do no sports at all. Group 2 “non-target active”: all moderately and sufficiently active pts who do no sports at all. Group 3 “sporty active”: all moderately and sufficiently active pts who do sports regularly.
Statistical analysis
Continuous data are reported as means ± standard deviation, and categorical variables as counts and percentages. Mean differences for continuous variables were tested using Mann-Whitney U test and χ2-test for categorical variables. All data were analyzed using SPSS (version 25.0, IBM, Chicago, IL, USA). Statistical significance was assumed for p-values < 0.05.
3. Results
Sample characteristics
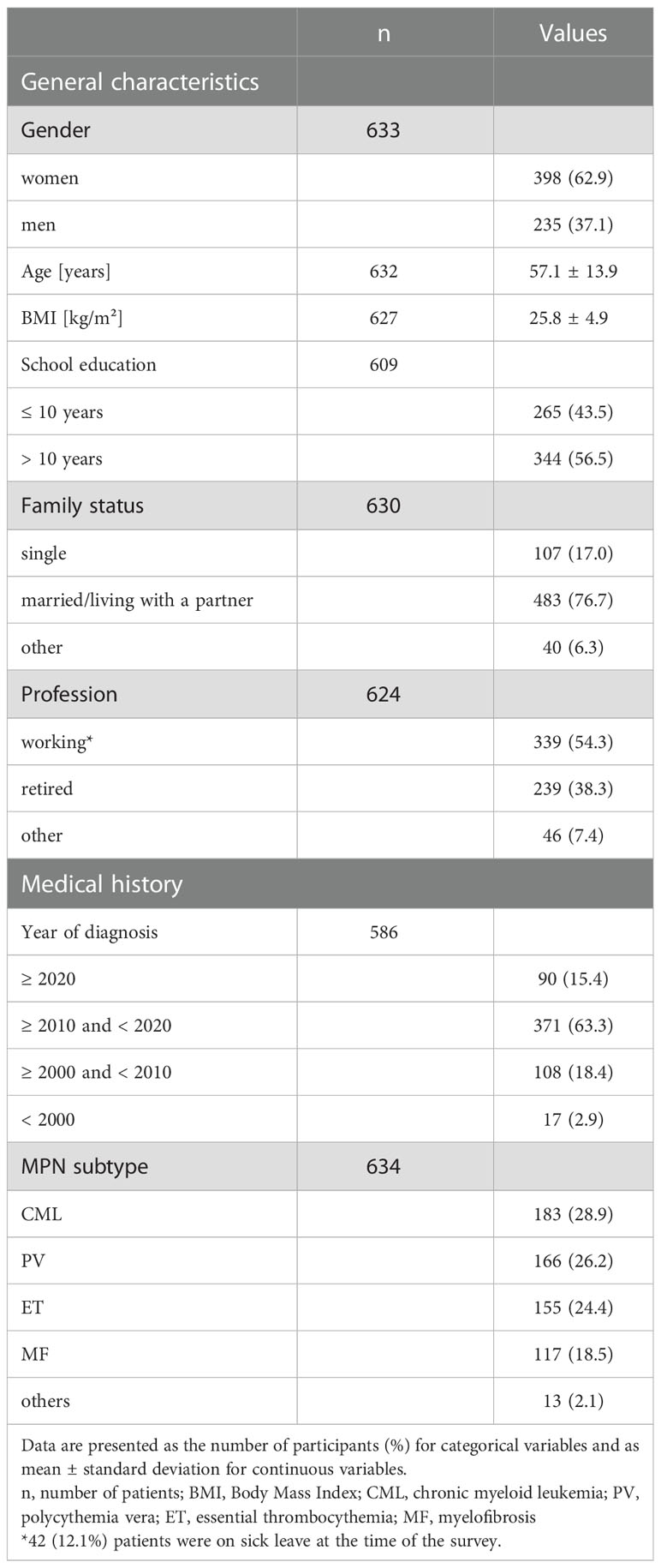
In total, 766 questionnaires were received, of which 315 (41%) were in hard copy and 451 (59%) online. The response rate (handed out/received filled in) of the hard copy survey was 78%. Reasons for exclusion of questionnaires are presented in Figure 1. The final sample cohort comprised 634 questionnaires (63% women, mean age 57 ± 14 years). General characteristics, including the medical history of this cohort, are presented in Table 1. The pts were diagnosed between 1981 and 2021. The median age of MPN onset was 50 ± 14 years.
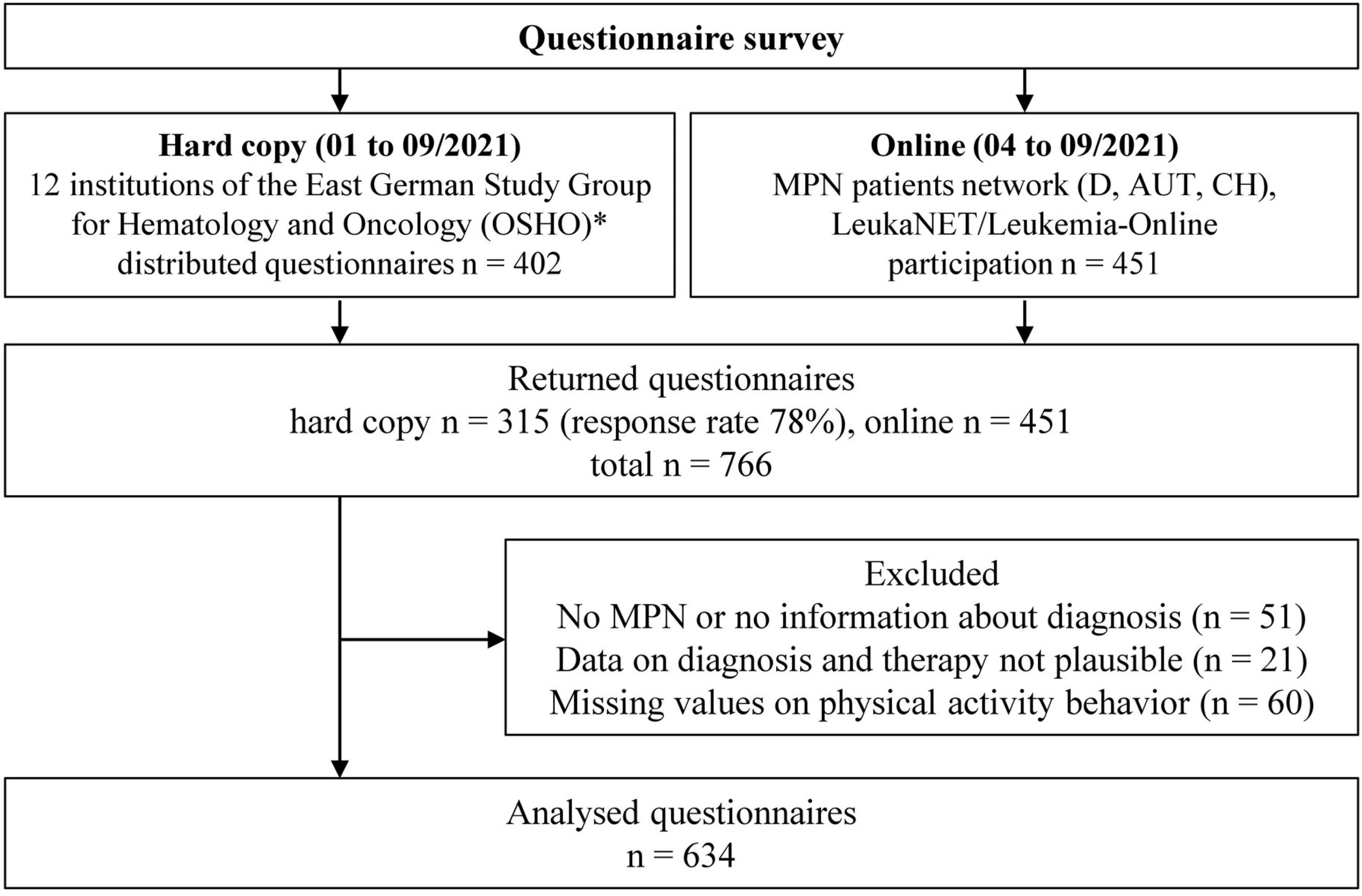
Figure 1 Flow chart of the study. D, Germany; AUT, Austria; CH, Switzerland; MPN, myeloproliferative neoplasms. *Participating institutions are presented in the supplement (Table S1).
Further demographics and current therapies at the time of the survey are presented in Table 2. The CML pts were the youngest pts (mean 51 ± 14 years), the polycythemia vera (PV) pts were the oldest (mean 61 ± 12 years). Of the 183 CML pts, 171 received disease-specific therapy. Of these, 158 (92%) were treated with TKI. One hundred thirty-two of the 166 PV and 133 of the 155 essential thrombocythemia (ET) pts received disease-specific therapy, most frequently with cytostatics (n = 55, 42% and n = 70, 53%, respectively). Thirteen (10%) of PV and 36 (27%) of ET pts were on a watch-and-wait strategy. The most common disease-specific therapy among MF pts was treatment with a JAK-2 inhibitor, (n = 56, 52%) followed by a watch-and-wait strategy (n = 22, 20%). Regardless of disease-specific therapy, 66 (40%) PV, 87 (56%) ET, and 31 (26%) MF pts received anticoagulation. Sixty-three (38%) PV pts underwent phlebotomy. The total cohort included seven pts with splenectomy.
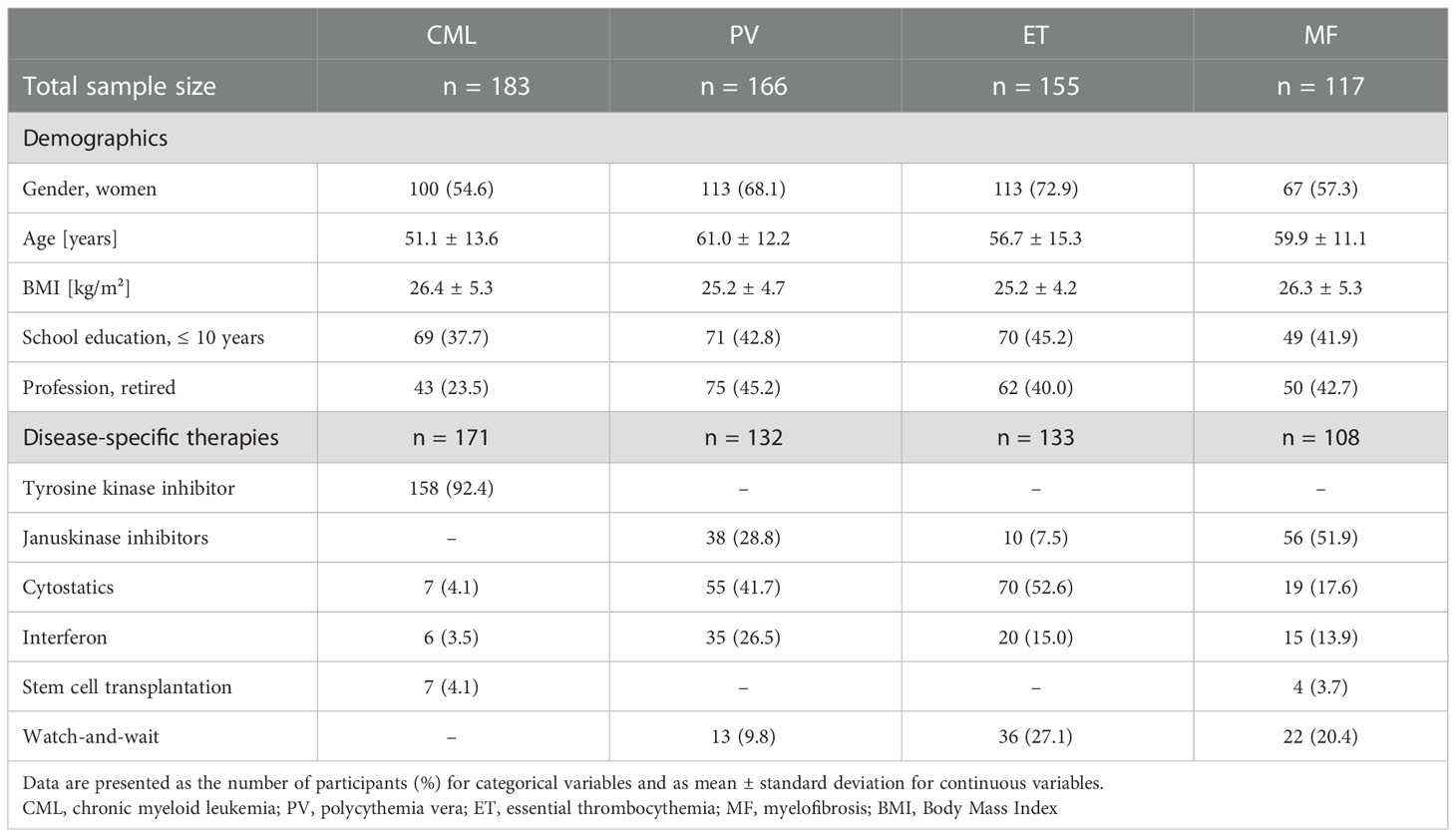
Table 2 Demographics and current therapies of patients with myeloproliferative neoplasms depending on the diagnosis.
Influence of MPN disease on physical activity and anxiety
The influence of a MPN disease on the PA of those affected is presented in Figure 2. Most participants (n = 455, 73%) changed their self-reported PA behavior in everyday life and/or sports. Both, the decade of diagnosis and the type of therapy in PV/ET/MF pts showed no significant group differences. Group differences were only found depending on the diagnosis. The percentage of those who changed their PA behavior was lowest among the CML pts (65%) and highest among the MF pts (81%). In everyday life, 177 (35%) reported being less active and 78 (15%) more active. Especially pts with MF and PV reduced their everyday activities (47% and 40% respectively). The proportion of pts who have been more active in everyday life since diagnosis is highest among ET pts (23%) and lowest among MF pts (8%). Two hundred one pts (33%) moved more consciously in everyday life and 132 (22%) moved more carefully. The latter finding was especially relevant in pts with MF and PV (29% and 25%, respectively). In sports, 191 (40%) of the 455 pts reduced their training, with the proportion being lowest among ET pts at 28% and highest among MF pts at 52%. In contrast, 76 (16%) reported exercising more since diagnosis, which was most common for ET pts (21%). One hundred and eighty-one (31%) were more conscious during sports and 123 (21%) were more careful.
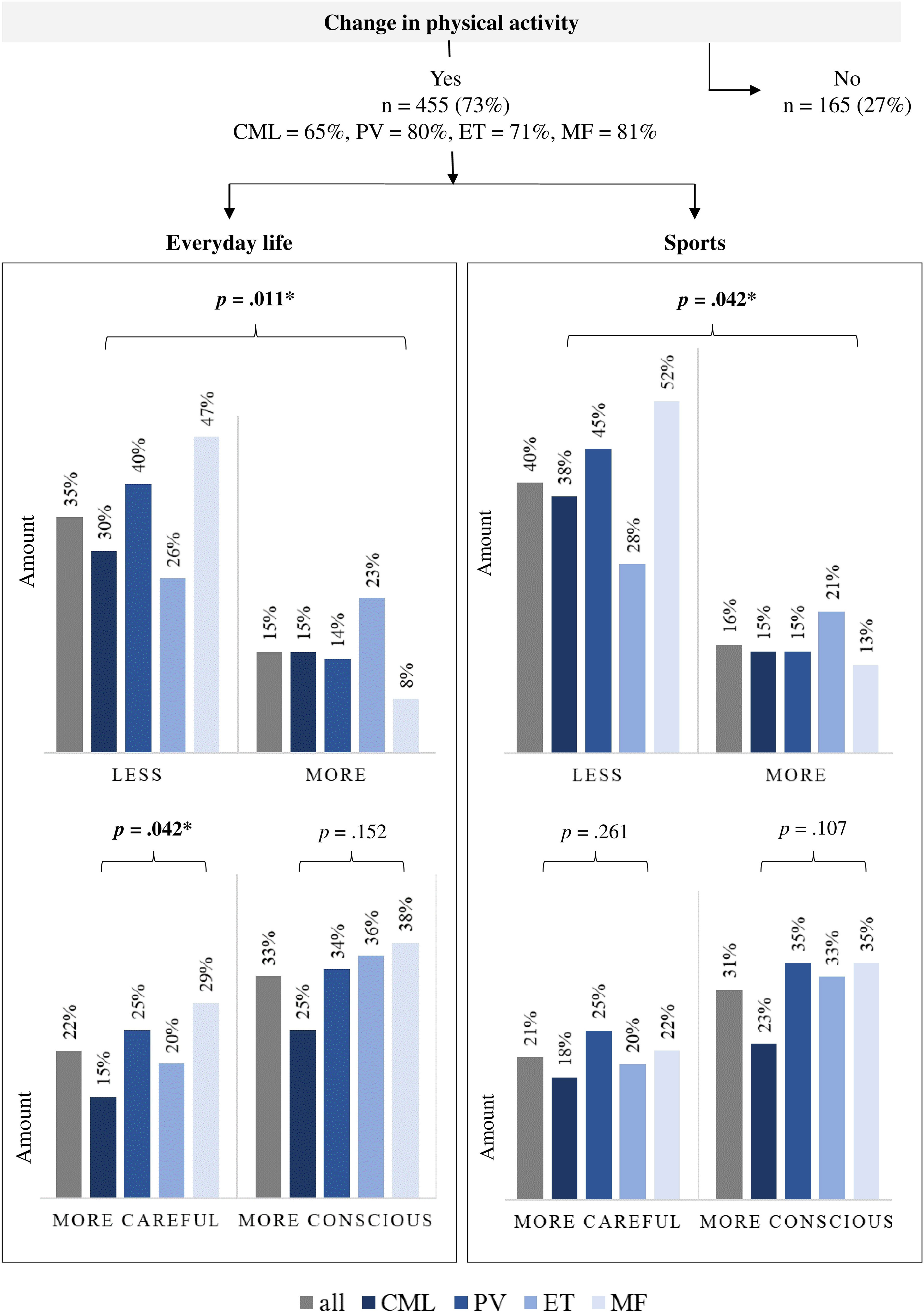
Figure 2 Influence of a myeloproliferative neoplasia disease on self-reported physical activity (n = 620). n, number of patients; CML, chronic myeloid leukemia (n = 169); PV, polycythemia vera (n = 166); ET, essential thrombocythemia (n = 155); MF, myelofibrosis (n = 117). Bold: statistically significance, *p ≤.05.
Thirty percent of those who moved/exercised more consciously were more physically active. In contrast, 68% of those who were more careful were less physically active. Pts who reported moving less after diagnosis tended to have more anxiety about certain events than pts who reported moving as much or more (54% vs. 31% and 33%, respectively, p ≤ 0.001). Which events MPN pts are most afraid of during PA are presented in Figure 3. Overall, 278 (45%) participants reported that they were afraid of at least one event. There were no significant group differences in the prevalence of anxiety according to diagnosis. Anxiety about infections (52%) and thrombosis (51%) were mentioned most frequently, followed by bleeding (32%) and skin reactions (31%). Group differences were found in anxiety about infections (p = 0.038) and thrombosis (p ≤ 0.001). While CML pts had more anxiety about infections, ET and PV pts had more anxiety about thrombotic events compared to the other MPN subtypes. MF pts tended to have more anxiety about splenic rupture compared to the other MPN subtypes (22% vs. 8-11%, respectively, p = 0.091).
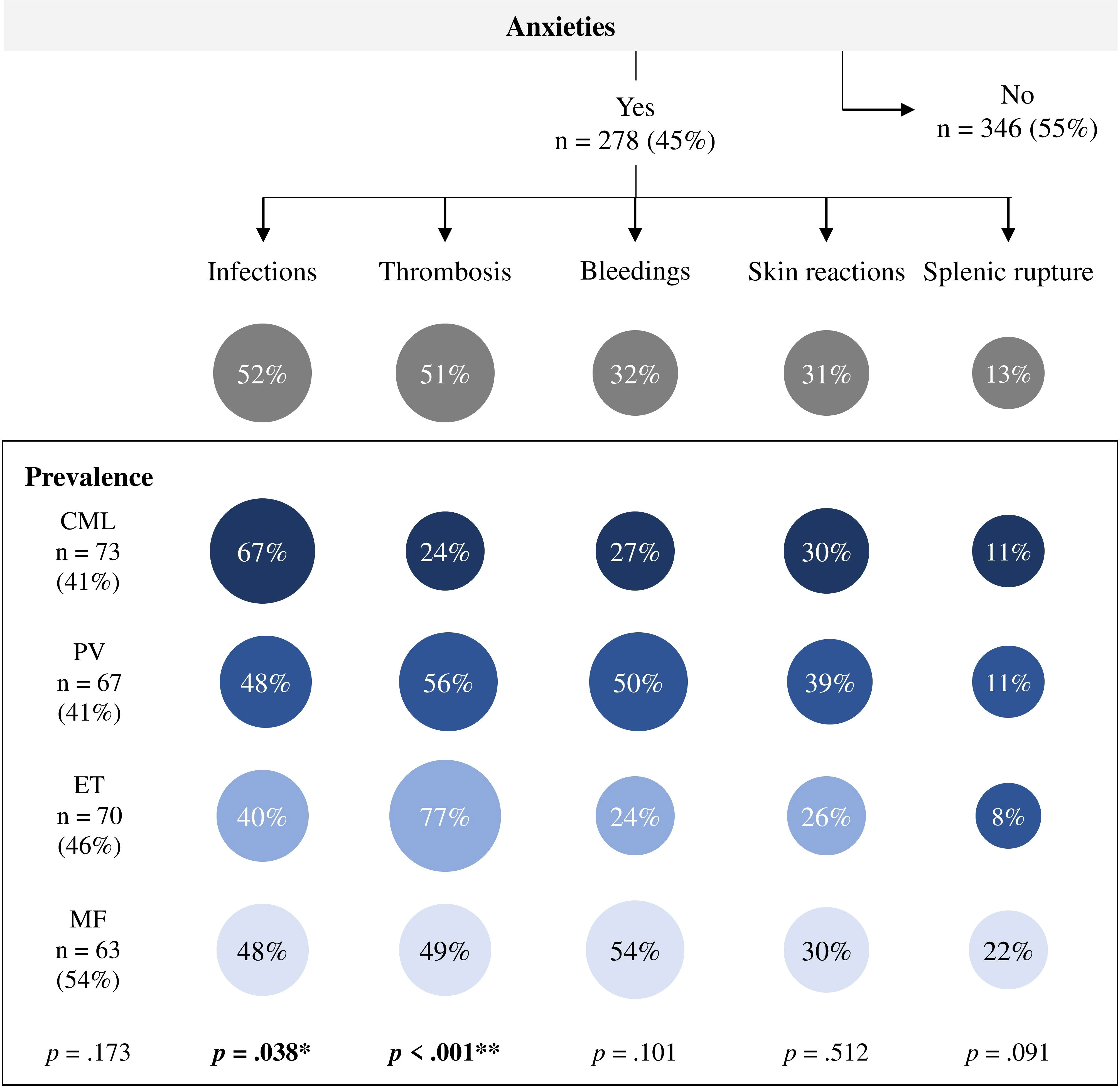
Figure 3 Anxieties during physical activity in patients with myeloproliferative neoplasms (n = 624). n, number of patients; CML, chronic myeloid leukemia (n = 178); PV, polycythemia vera (n = 164); ET, essential thrombocythemia (n = 155); MF, myelofibrosis (n = 117). bold: statistically significance, *p ≤ 05, **p ≤ 001.
Physical activity level and motivation for regular sports
Regarding the GSLTPAQ score, 110 (19%) of the total cohort were categorized as insufficiently active, 109 (19%) as moderately active, and 361 (62%) as active. The analysis of SOC revealed that 203 (34%) pts were not action-oriented (stage of precontemplation), 98 (16%) and 41 (7%) were in the stages of contemplation and preparation, respectively. In total, 257 (43%) pts reported regular sports (stages of action and maintenance). The results for the different diagnoses are available in the Online Supplementary S3. There are no significant group differences in everyday activity (GSLTPAQ) or motivation to do regular sports (SOC) depending on diagnosis or therapy in PV/ET/MF pts.
Activity groups
All MPN pts who reported both GSLTPAQ and SOC (n = 559) were assigned to an activity group according to the information provided. For 18 (3%) pts, the information provided was not plausible. These were excluded from the subgroup analysis. The SOC as a relation to the GSLTPAQ scores are presented in Figure 4. Eighty-six (15%) pts were assigned to Group 1 “inactive”, 229 (41%) to Group 2 “non-targeted active”, and 226 (40%) to Group 3 “sporty active”.
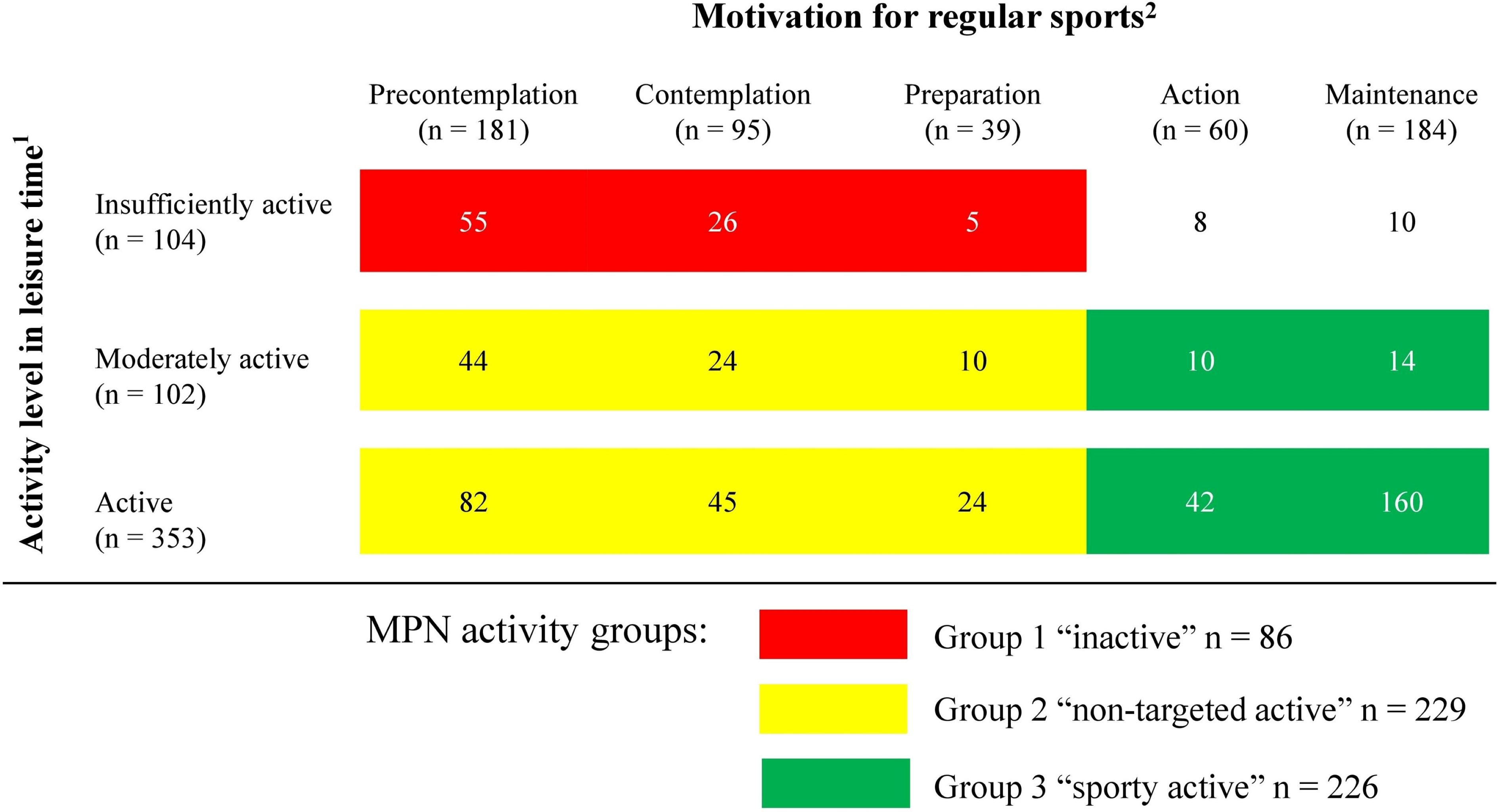
Figure 4 Physical activity in patients with myeloproliferative neoplasms (n = 559). Data are presented as the number of participants. 1Activity levels in leisure time were grouped according to the Godin-Shepard Leisure-Time Physical Activity Questionnaire (GSLTPAQ) into three categories: “insufficiently active”, “moderately active”, and “active” (15, 16). 2Five stages of the transtheoretical model of behavioral change (SOC) were used to determine the motivation to participate in sports. In the stages of precontemplation, contemplation, or preparation, patients are not regularly active in sports. In the stages of action and maintenance, patients are active for at least 20 minutes on at least 3 days per week (17, 18).
Demographics, HrQoL, and symptom burden depending on the activity group
The demographics, HrQoL, symptoms, and side effects of the MPN pts, depending on the activity group, are presented in Table 3. The inactive pts were significantly older than those in the two active groups (Group 2: 60 ± 16 years vs. 56 ± 13 years; p = 0.018; Group 3: 55 ± 13 years; p = 0.004), and had a higher BMI than the sporty active group (27 ± 5 vs. 25 ± 5, p = 0.013). The sporty active group rated their HrQoL significantly higher than the other two groups (Group 1: 73 ± 20 vs. 60 ± 23, p ≤ 0.001 vs. Group 2: 63 ± 21, p ≤ 0.001). In addition, the sporty active group reported significantly less fatigue, bone and muscle pain, and concentration problems (all: p ≤ 0.05), compared to both groups. There were no differences in HrQoL and symptom burden between the inactive and non-targeted active pts.
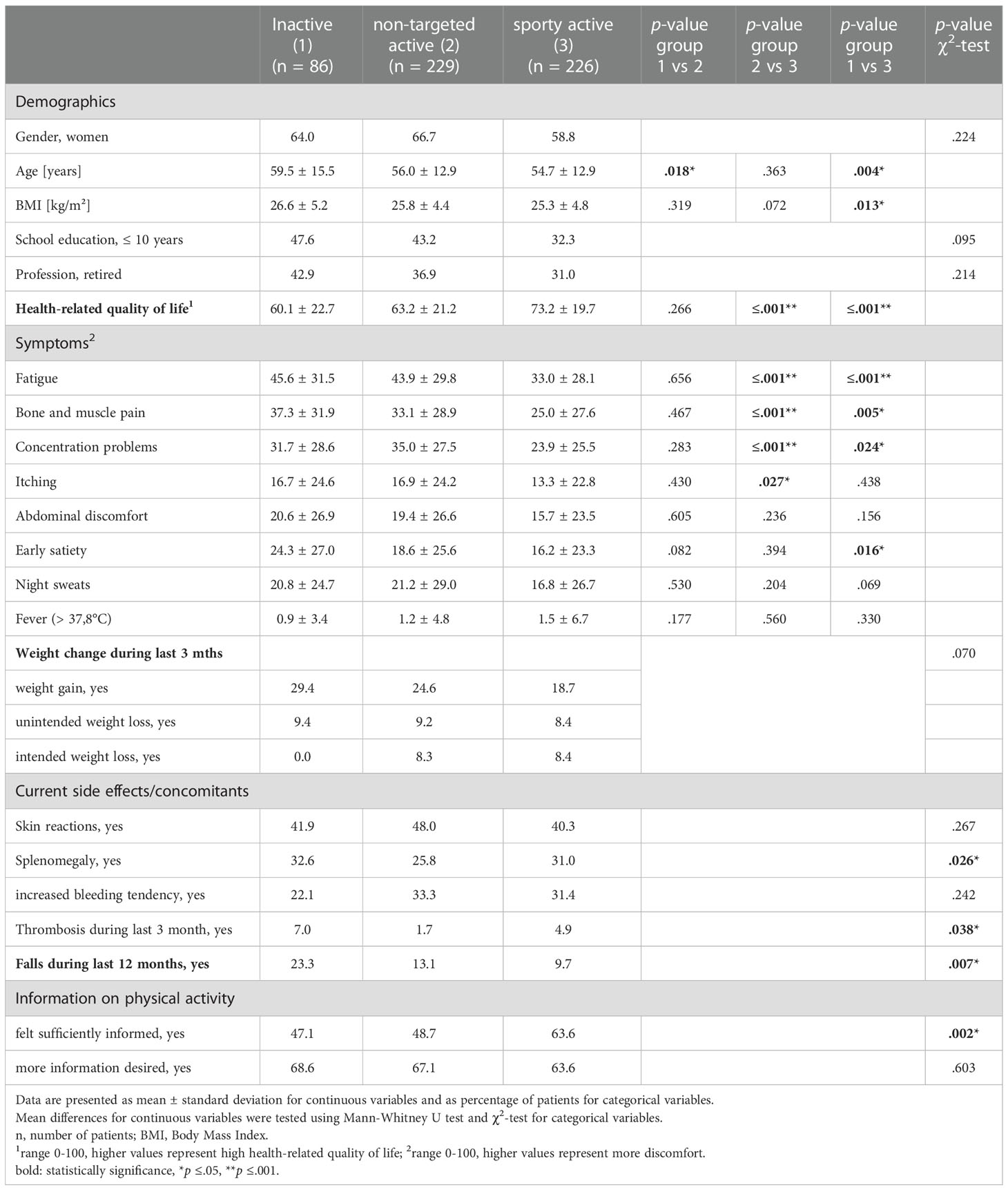
Table 3 Characteristics of patients with myeloproliferative neoplasms depending on the level of physical activity.
Inactive MPN pts tended to gain weight more often than the active ones (29% vs. 25% and 19%, respectively, p = 0.070). Eight percent of the active pts intentionally lost weight.
The inactive pts reported thromboses more often than the active pts (7% vs. 2% and 5%, respectively, p = 0.038). Falls during the last 12 months were reported significantly more often in this group, as well (23% vs. 13% and 10%, respectively, p = 0.007). Thirty-one percent of the sporty active group reported a splenomegaly, which was in the range to the inactive (33%, p = ns), but of note: in higher proportion compared to the non-targeted active (26%, p = 0.026).
Association between information level and physical activity
Two hundred and seventy-two (43%) of all participants stated that they did not feel sufficiently informed about the importance and possibilities of PA for their disease. The differences in the level of information depending on the activity group are presented in Table 3. Uninformed pts belonged significantly more often to the group of inactive and non-targeted active pts (p = 0.002). All pts, regardless of their activity level, expressed their wish to receive more information about PA.
4. Discussion
This is the first study to investigate the PA behavior of MPN pts and provide an overview of which factors show an association with PA in this population. The most important results are discussed below and information is derived for which pts may need to lead an active lifestyle for as long as possible.
According to the presented data, the MPN disease and associated therapies had an impact on PA in 65-81% of the pts, depending on the MPN subtype. Approximately one in three pts reported a reduction in PA because of the disease, with the proportion highest in MF pts and lowest in ET pts. This is about as expected, as symptom burden varies according to diagnosis and consequently has different effects on HrQoL. Furthermore, the prevalence of moderate to severe fatigue, which is associated with a reduction in PA, is about 50% in MPN pts (1, 11).
Of importance is the result that in many MPN pts, depending on the MPN subtype, fear of certain events - especially infections, thromboses, bleeding, and skin reactions - had a negative influence on PA behavior. The reduction was not only limited to sports activities, but also affected everyday activities. Of particular interest is our finding that PV and ET pts more frequently reported anxieties compared to CML and MF pts in regards to the occurrence of thromboses. This might reflect the fact, that in PV and ET thromboembolism is often the initial disease complication that leads to the diagnosis. The rare occurrence of thrombosis in the present cohort (3%) suggests that the fear especially of PV and ET pts has to be addressed in order to avoid negative effects on PA. The high proportion of pts in the present study who regularly participate in sports despite anticoagulation and/or skin reaction suggests that these symptoms do not represent a limitation to sports activities. The numerous exercise interventions available for pts following high-dose chemotherapy and hematopoietic stem cell transplantation also suggests that exercise is safe for pts at increased risk of bleeding and infection, and that pts may benefit from numerous positive effects (20). It cannot be excluded that the COVID-19 pandemic prevailing at the time of the survey increased the fear of infection, which was not further specified. The present results suggest that MPN pts should be informed about the real risk of thrombosis or serious bleeding during PA. To support patients’ active lifestyles, ways to reduce the risk of infection, bleeding, and/or skin reactions during daily activities and sports should be emphasized (e.g., hand disinfection, face mask during group exercise, low-injury sports/forms of exercise, and if necessary, refrain from water sports, add sun protection, etc.).
According to the presented data, 62% of MPN pts were sufficiently physically active in their daily lives at the time of the survey (self-reported), and 43% stated that they regularly played sports. These are unexpectedly high percentages and might be due to the survey design. Due to the voluntariness, it cannot be ruled out that more pts with an affinity for sports took part in the survey. In addition, 10% of the participants did not answer the questions on PA behavior or answered them inadequately and were excluded from the analysis. Furthermore, the cohort is quite young with an average age of 57 years and it is known that PA tends to decrease with age (21). Likewise, socially desirable responses cannot be excluded. Based on the results of large American and British cohort studies of cancer survivors, it must be assumed that the proportion of insufficiently physically active MPN pts is higher than the results of this study show (22, 23). Regardless, the participants could be divided into three groups (inactive, non-targeted active, and sporty active) according to their PA statements, which were sufficiently large for the statistical analyses.
Among physically active pts, the proportion of pts with increased bleeding tendency or skin reactions is as high as among inactive pts. Consequently, these side effects/concomitants are not, or only to a limited extent, barriers to PA or sports. Mean comparisons showed comparable symptom burden and HrQoL for the inactive and non-targeted active pts. This suggests that a lack of motivation is the reason for inactivity rather than symptom burden. As behaviors tend to become entrenched over time, they are often difficult to change. To motivate previously inactive MPN pts to adopt an active lifestyle, a psychologist’s involvement may be beneficial. The most effective strategies to motivate cancer pts to be more physically active in the long term include motivational interviewing, coaching, and Bandura’s socio-cognitive learning (model learning) approach (24).
In all groups, fatigue, bone and muscle pain, and concentration problems represented the most common severe symptoms. The reported prevalence and severity of these symptoms is comparable to results of other studies (1, 11). Inactive and non-targeted active pts showed no differences in symptom burden and HrQoL. Based on the findings that PA and fatigue correlate negatively, and PA and HrQoL correlate positively (5, 11), this is an unexpected result. However, the result could be an indication that it is not the amount of PA that is decisive for the symptom burden, but the quality/targeting of the PA. This is also in line with the general recommendations for reducing fatigue. Moderate-intensity exercise is recommended here, as the effect is unlikely at low intensities. Moreover, there is no evidence for a dose-response relationship (25).
The lower symptom burden and higher HrQoL of the sporty active pts in our study is consistent with the assumption of Eckert et al. (13) that targeted PA could also have positive effects in MPN pts. However, as there is a bidirectional relationship between symptom burden or HrQoL and PA, no statement on causality can be made on the basis of the available cross-sectional data. This should be investigated in subsequent studies. Due to the small differences in symptom burden and HrQoL between the groups (about 10%), it is assumed that the effects of targeted PA on symptoms and HrQoL in MPN pts are modest. This is also confirmed by the results of Huberty et al. (26, 27). Thus, small to moderate effect size for sleep disturbance, pain intensity, anxiety, and depression were generated by approximately one hour of yoga training per week over a period of 12 weeks. Although Pedersen et al. (28) demonstrated that a 12-week self-exercising program, after a 5-day interdisciplinary exercise-based rehabilitation intervention, significantly increased physical performance of MPN pts, but no improvements were seen with respect to HrQoL and fatigue. However, since PA has a multitude of health potentials, MPN pts should be motivated to be physically active regularly and for as long as possible. The available data suggest that both daily activities and sports can reduce the risk of falls and regulate body weight. The fact that 12% of pts surveyed reported being more conscious and/or increasing their PA since diagnosis suggests that adequate patient education can alleviate potential fears and possibly increase motivation to engage in PA or sports. The focus should be specifically on older and physically inactive pts (29). Similarly, overweight MPN pts should be addressed, appropriately educated, and motivated to be physically active. Reducing obesity in MPN pts might have several positive effects on outcome. Reducing obesity associated diseases such as atherosclerosis and risk factors is a general goal. In particular as some MPN treatment approaches such as TKI-treatment or stem cell transplantation might increase the risk for e.g. atherosclerosis themselves. Furthermore, obesity might influence pharmacokinetics of drugs used in MPN treatment, although data is limited (30–33).
The presented data is based on a large cohort, but due to the survey design, there are inevitable limitations that should be taken into consideration when interpreting the data. First, due to the online format, no information is available on how many potential participants were informed about the study and declined to participate. Due to the high proportion of online questionnaires, a bias towards more women and “younger” respondents is suspected. Even though it is known that women often report a higher symptom burden, we suspect that the bias of the results due to the unbalanced participation of the genders is small (34). This assumption is strengthened by the fact that the proportion of women in the three activity groups did not differ significantly. Second, all data were assessed retrospectively. Third, due to the cross-sectional design of the study, it is not possible to distinguish between cause and effect in terms of symptom burden and PA. Regardless of whether and to what extent individual symptoms can be reduced by PA, our results highlight the importance of PA because of its multitude of other potentials, such as reducing the risk of falls and weight control. Forth, in order to reduce the length of the questionnaire, no validated questionnaire was used for the assessment of HrQoL, but a VAS scale from 0 to 100. Since the VAS allows a more differentiated assessment of HrQoL compared to a Likert scale, it can be assumed that the HrQoL of cancer pts can be measured just as adequately (35). A major advantage of our study is the relatively large sample size. This representative sample of the population-based study thus enables the transfer of the results to clinical practice.
5. Conclusion
In conclusion, it could be shown that the majority of MPN pts change their self-reported PA behavior due to the MPN disease or therapy. About one third of all MPN pts reduce the amount of PA, especially pts with PV and MF. In addition to fears, especially of infection, thrombosis and bleedings depending on the MPN subtype, higher age and motivation level also seem to influence PA. Sporty pts have a lower symptom burden and higher HrQoL than non-sporty pts. Physically inactive pts have a significantly higher prevalence of falls and higher BMI compared to physically active pts. Inactive and non-targeted active pts were significantly less likely to be informed about the importance and possibilities of PA. Our data clearly suggest that PA information and education, as well as sports programs, should be integrated into the treatment of MPN pts. Further studies, especially longitudinal studies are needed to verify the results of the survey study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University of Rostock. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception and design: SF, CJ. Statistical analysis and interpretation: SF, JR, CJ. Data collection: PC, HA-A, SS, L-OM, JuG, JaG, VK-K. Writing the article: SF, JR, CJ. Critical revision of the article: SF, JR, PC, HA-A, SS, L-OM, JuG, JaG, VK-K, CJ. Obtained funding: SF. Overall responsibility: SF, CJ. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the East German Study Group Hematology and Oncology (OSHO), file number OSHO #97.
Acknowledgments
The authors would like to thank the LeukaNET/Leukemia-Online and the German, Austrian and Swiss MPN Network for her support for assistance in recruiting MPN patients for conducting online survey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1056786/full#supplementary-material
References
1. Mesa R, Boccia RV, Grunwald MR, Oh ST, Colucci P, Paranagama D, et al. Patient-reported outcomes data from REVEAL at the time of enrollment (Baseline): A prospective observational study of patients with polycythemia Vera in the united states. Clin Lymphoma Myeloma Leuk (2018) 18(9):590–6. doi: 10.1016/j.clml.2018.05.020
2. Brochmann N, Flachs EM, Christensen AI, Bak M, Andersen CL, Juel K, et al. Anxiety and depression in patients with Philadelphia-negative myeloproliferative neoplasms: a nationwide population-based survey in Denmark. Clin Epidemiol (2019) 11:23–33. doi: 10.2147/CLEP.S162688
3. Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol (2016) 91(12):1262–71. doi: 10.1002/ajh.24592
4. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol (2017) 92(1):94–108. doi: 10.1002/ajh.24607
5. Tolstrup Larsen R, Tang LH, Brochmann N, Meulengracht Flachs E, Illemann Christensen A, Hasselbalch HC, et al. Associations between fatigue, physical activity, and QoL in patients with myeloproliferative neoplasms. Eur J Haematol (2018) 100(6):550–9. doi: 10.1111/ejh.13048
6. Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer (2007) 109(1):68–76. doi: 10.1002/cncr.22365
7. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Quality-of-life issues and symptoms reported by patients living with haematological malignancy: a qualitative study. Ther Adv Hematol (2020) 11):1–14. doi: 10.1177/2040620720955002
8. Maas CCHM, van Klaveren D, Ector GICG, Posthuma EFM, Visser O, Westerweel PE, et al. The evolution of the loss of life expectancy in patients with chronic myeloid leukaemia: a population-based study in the Netherlands, 1989-2018. Br J Haematol (2022) 196(5):1219–24. doi: 10.1111/bjh.17989
9. Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med (2004) 117(10):755–61. doi: 10.1016/j.amjmed.2004.06.032
10. Verstovsek S, Mesa RA, Gotlib J, Gupta V, DiPersio JF, Catalano JV, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol (2017) 10(1):55. doi: 10.1186/s13045-017-0417-z
11. Janssen L, Blijlevens NMA, Drissen MMCM, Bakker EA, Nuijten MAH, Janssen JJWM, et al. Fatigue in chronic myeloid leukemia patients on tyrosine kinase inhibitor therapy: predictors and the relationship with physical activity. Haematologica (2021) 106(7):1876–82. doi: 10.3324/haematol.2020.247767
12. Sweegers MG, Altenburg TM, Chinapaw MJ, Kalter J, Verdonck-de Leeuw IM, Courneya KS, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med (2018) 52(8):505–13. doi: 10.1136/bjsports-2017-097891
13. Eckert R, Huberty J, Gowin K, Mesa R, Marks L. Physical activity as a nonpharmacological symptom management approach in myeloproliferative neoplasms: Recommendations for future research. Integr Cancer Ther (2017) 16(4):439–50. doi: 10.1177/1534735416661417
14. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood (2016) 127(20):2391–405. doi: 10.1182/blood-2016-03-643544
15. Amireault S, Godin G. The godin-shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills (2015) 120(2):604–22. doi: 10.2466/03.27.PMS.120v19x7
16. Godin G. The godin-shephard leisure-time physical activity questionnaire. Health Fitness J Canada (2011) 4(1):18–22. doi: 10.14288/hfjc.v4i1.82
17. Prochaska JO, Marcus BH. The transtheoretical model: Applications to exercise. In: Dishman RK, editor. Advances in exercise adherence. Champaign, IL, England: Human Kinetics Publishers (1994). p. 161–80.
18. Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: Theory, research and practice. Hoboken, NJ: Jossey-Bass (2008). 97–122.
19. Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood (2011) 118(2):401–8. doi: 10.1182/blood-2011-01-328955
20. Prins MC, van Hinte G, Koenders N, Rondel AL, Blijlevens NMA, van den Berg MGA. The effect of exercise and nutrition interventions on physical functioning in patients undergoing haematopoietic stem cell transplantation: a systematic review and meta-analysis. Support Care Cancer (2021) 29(11):7111–26. doi: 10.1007/s00520-021-06334-2
21. Dorner TE, Wilfinger J, Hoffman K, Lackinger C. Association between physical activity and the utilization of general practitioners in different age groups. Wien Klin Wochenschr (2019) 131(11-12):278–87. doi: 10.1007/s00508-019-1503-8
22. Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American cancer society's SCS-II. JCO (2008) 26(13):2198–204. doi: 10.1200/JCO.2007.14.6217
23. Mayer DK, Terrin NC, Menon U, Kreps GL, McCance K, Parsons SK, et al. Health behaviors in cancer survivors. Oncol Nurs Forum (2007) 34(3):643–51. doi: 10.1188/07.ONF.643-651
24. Berkman AM, Gilchrist SC. Behavioral change strategies to improve physical activity after cancer treatment. Rehabil Oncol (2018) 36(3):152–60. doi: 10.1097/01.REO.0000000000000112
25. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc (2019) 51(11):2375–90. doi: 10.1249/MSS.0000000000002116
26. Huberty J, Eckert R, Dueck A, Kosiorek H, Larkey L, Gowin K, et al. Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research. BMC Complement Altern Med (2019) 19(1):121. doi: 10.1186/s12906-019-2530-8
27. Huberty J, Eckert R, Gowin K, Mitchell J, Dueck AC, Ginos BF, et al. Feasibility study of online yoga for symptom management in patients with myeloproliferative neoplasms. Haematologica (2017) 102(10):e384–8. doi: 10.3324/haematol.2017.168583
28. Pedersen KM, Zangger G, Brochmann N, Grønfeldt BM, Zwisler A-D, Hasselbalch HC, et al. The effectiveness of exercise-based rehabilitation to patients with myeloproliferative neoplasms-an explorative study. Eur J Cancer Care (Engl) (2018) 27(5):e12865. doi: 10.1111/ecc.12865
29. Felser S, Behrens M, Lampe H, Henze L, Grosse-Thie C, Murua Escobar H, et al. Motivation and preferences of cancer patients to perform physical training. Eur J Cancer Care (Engl) (2020) 29(4):e13246. doi: 10.1111/ecc.13246
30. Abdulla MAJ, Chandra P, Akiki SE, Aldapt MB, Sardar S, Chapra A, et al. Clinicopathological variables and outcome in chronic myeloid leukemia associated with BCR-ABL1 transcript type and body weight: An outcome of European LeukemiaNet project. Cancer Control (2021) 28:10732748211038429. doi: 10.1177/10732748211038429
31. Chen X, Williams WV, Sandor V, Yeleswaram S. Population pharmacokinetic analysis of orally-administered ruxolitinib (INCB018424 phosphate) in patients with primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF) or post-essential thrombocythemia myelofibrosis (PET MF). J Clin Pharmacol (2013) 53(7):721–30. doi: 10.1002/jcph.102
32. Molica M, Canichella M, Colafigli G, Latagliata R, Diverio D, Alimena G, et al. Body mass index does not impact on molecular response rate of chronic myeloid leukaemia patients treated frontline with second generation tyrosine kinase inhibitors. Br J Haematol (2018) 182(3):427–9. doi: 10.1111/bjh.14783
33. Yassin MA, Kassem N, Ghassoub R. How I treat obesity and obesity related surgery in patients with chronic myeloid leukemia: An outcome of an ELN project. Clin Case Rep (2021) 9(3):1228–34. doi: 10.1002/ccr3.3738
34. Langlais B, Mazza GL, Scherber RM, Geyer H, Gowin KL, Palmer J, et al. Impact of imbalanced gender participation in online myeloproliferative neoplasm symptom surveys. JCO (2022) 40(16_suppl):e19078–8. doi: 10.1200/JCO.2022.40.16_suppl.e19078
Keywords: anxieties, education, fatigue, fears, health-related quality of life (HrQoL), myeloproliferative neoplasms (MPN), physical activity, sports
Citation: Felser S, Rogahn J, le Coutre P, Al-Ali HK, Schulze S, Muegge L-O, Gruen J, Geissler J, Kraze-Kliebhahn V and Junghanss C (2023) Anxieties, age and motivation influence physical activity in patients with myeloproliferative neoplasms - a multicenter survey from the East German study group for hematology and oncology (OSHO #97). Front. Oncol. 12:1056786. doi: 10.3389/fonc.2022.1056786
Received: 29 September 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Massimo Breccia, Sapienza University of Rome, ItalyReviewed by:
Silvia Riva, St Mary’s University, United KingdomZefeng Xu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Felser, Rogahn, le Coutre, Al-Ali, Schulze, Muegge, Gruen, Geissler, Kraze-Kliebhahn and Junghanss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabine Felser, c2FiaW5lLmZlbHNlckBtZWQudW5pLXJvc3RvY2suZGU=
 Sabine Felser
Sabine Felser Julia Rogahn1
Julia Rogahn1 Philipp le Coutre
Philipp le Coutre Haifa Kathrin Al-Ali
Haifa Kathrin Al-Ali Christian Junghanss
Christian Junghanss