- 1Department of Thyroid and Parathyroid Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Laboratory of Thyroid and Parathyroid Diseases, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3West China School of Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 4State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center, Chengdu, Sichuan, China
- 5Respiratory Health Institute, Frontiers Science Center for Disease Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Lymph node metastasis is widespread in papillary thyroid cancer (PTC). Patients are more vulnerable than those with central lymph node metastasis if they have lateral neck lymph node metastasis (LLNM). There are few researches focus on the correlation between clinical characteristics and genetic profile of PTC with LLNM. In this study, we aimed to analyze the clinical and genetic features of PTC with LLNM.
Methods: A total of 160 primary tumor samples derived from PTC patients with LLNM were involved. Targeted next-generation sequencing was carried out on all samples with 57 known thyroid-cancer-related genes. The associations between genomic alternations and clinical characteristics of PTC with LLNM were statistically evaluated.
Results: The median age of patients was 37 years, ranging from 5 to 77 years and the female/male ratio was 1.86. The most frequently altered genes in our series were BRAF mutation (68%), followed by RET fusion (17%), TERT promoter mutation (5%) and PIK3CA mutation (2%). To be noted, all PTC patients with LLNM of TERT promoter mutations appeared along with BRAF mutations (8/8) and half of them experienced a relapse. Intriguingly, we found more metastatic lymph nodes in patients with RET fusion, but there was no statistically significant difference in metastatic lymph node ratio than those with BRAF mutation or without mutation. A high rate of gene fusion (70%) was found in the pediatric population, with aggressive late-onset disease.
Conclusions: PTC patients with LLNM is characterized by a high rate of BRAF mutation. Due to the observed clinicopathological differences in those patients among different alterations, further prospective studies are needed to verify our results and to evaluate the most suitable treatment strategies.
Introduction
The incidence rate of thyroid cancer has steadily climbed in recent years due to the rapid development of ultrasound imaging (1), making thyroid cancer one of the most prevalent endocrine tumors (2). Papillary thyroid carcinoma (PTC), so named because of its histological architecture, accounts for more than 80 percent of thyroid cancers. PTC typically has a five-year survival of over 95%, making it a docile disease (3). However, there are approximately 30-80% of patients undergoing thyroidectomy for papillary thyroid cancers with clinically meaningful lateral neck lymph node metastases (LLNM). Additionally, studies demonstrate that these patients are more vulnerable (4). Therefore, it is crucial for future treatments to comprehend and depict the clinical characteristics and genetic features (5).
Although newly diagnosed PTCs have risen dramatically, the mortality rate has stayed constant which prompted us to explore which gene alteration may closely correlated with disease progression and patients survival (6). Thyroid cancer is brought on by point mutations or gene rearrangements/fusions of critical genes in thyroid cells that activate the MAPK pathway and PI3K/AKT pathway (7). Therefore, with the in-depth research on the molecular genetics of thyroid cancer, genetic detection has begun to assist the clinical treatment of patients (8). Genetic detection may increase the precision of distinguishing benign from malignant thyroid nodules, forecast cancer risk stratification, and choose targeted drug therapy for advanced thyroid cancer. A comprehensive characterization of the genomic landscape of PTC has been reported by The Cancer Genome Atlas (TCGA) as a homogeneous cohort (9). It demonstrated that PTC had a low frequency of somatic alternations. BRAF (60%) gene mutation is the most common and specific genetic alteration, determining papillary carcinoma’s clinical and pathological manifestations (10). For 20-30% of thyroid nodules that cannot be definitively diagnosed by fine needle aspiration (FNA), thyroid cancer is highly suspected if a BRAF gene mutation is detected. This study dramatically improved our understanding of genotype-phenotype relationships in thyroid cancer. Miguel et al. (11) evaluated the frequency of TERT promoter, BRAF, and NRAS mutations in the primary tumor, lymph node metastases, and distant metastases which showed a high concordance in primary tumor and metastatic sites. However, limited data exist regarding the genetic profile in PTC with LLNM. Dilmi et al. (12) reported the most extensive series of gene-targeted sequencing of PTC with LLNM to date, which focused on papillary microcarcinomas. However, there are few studies on the correlation between clinical characteristics and the genetic profile of PTC with LLNM so far.
Aiming to understand the clinical-molecular correlation and the prognostic factors of PTC with LLNM, we present a sizeable comprehensive retrospective cohort of 160 PTC patients with LLNM underwent surgical resection and targeted NGS at the molecular level in order to provide guidance for clinical treatment and policy makers.
Materials and methods
Patients and tissue samples collection
Tumor samples were collected from 160 PTC patients with LLNM between 2019 and 2022 at West China Hospital, Chengdu, China. We obtained informed consent from each patient. West China Hospital’s institutional review board approval was obtained for retrospective data collection and subsequent analyses.
DNA extraction and targeted NGS
All the samples in this study were fresh tissue samples without any process. We performed simultaneous extraction of tissue DNA using the QIAamp Genomic DNA kit (Qiagen) for downstream genomic analysis according to the manufacturer’s instructions.
Targeted NGS was performed for 57 known thyroid-cancer-related genes shown in Supplementary Table 1. These 57 genes were considered to be used for auxiliary diagnosis, prognostic risk assessment, targeted drug assessment, and genetic screening, involving single-nucleotide variation, small fragment insertion or deletion, gene copy number variation, and gene fusion. We used a HiSeqTM 2500 platform (Illumina) for sequencing.
Definition of metastatic lymph node ratio
Conventionally, the lymph node ratio (LNR) was calculated as the number of metastatic lymph nodes or lymph node metastases (LNM) divided by the number of retrieved lymph nodes or lymph node yield (LNY) (13).
Statistical analysis
Statistical analyses were performed using R Programming, Graphpad Prism 9 (GraphPad Software, California, CA), and SPSS software 27.0 (IBM Corporation, New York, NY). Categorical relationships were detected using the Chi-square test or Fisher’s exact test. The difference in continuous and discrete variables with normal distribution between groups was analyzed by student’s t-test or two-way ANOVA test. Risk factors of the number of metastatic lymph nodes were calculated from binary logistic regression for univariate and multivariate analysis. A two-sided P. value<0.05 was considered statistically significant.
Results
Clinical characteristics of patients and tumor samples
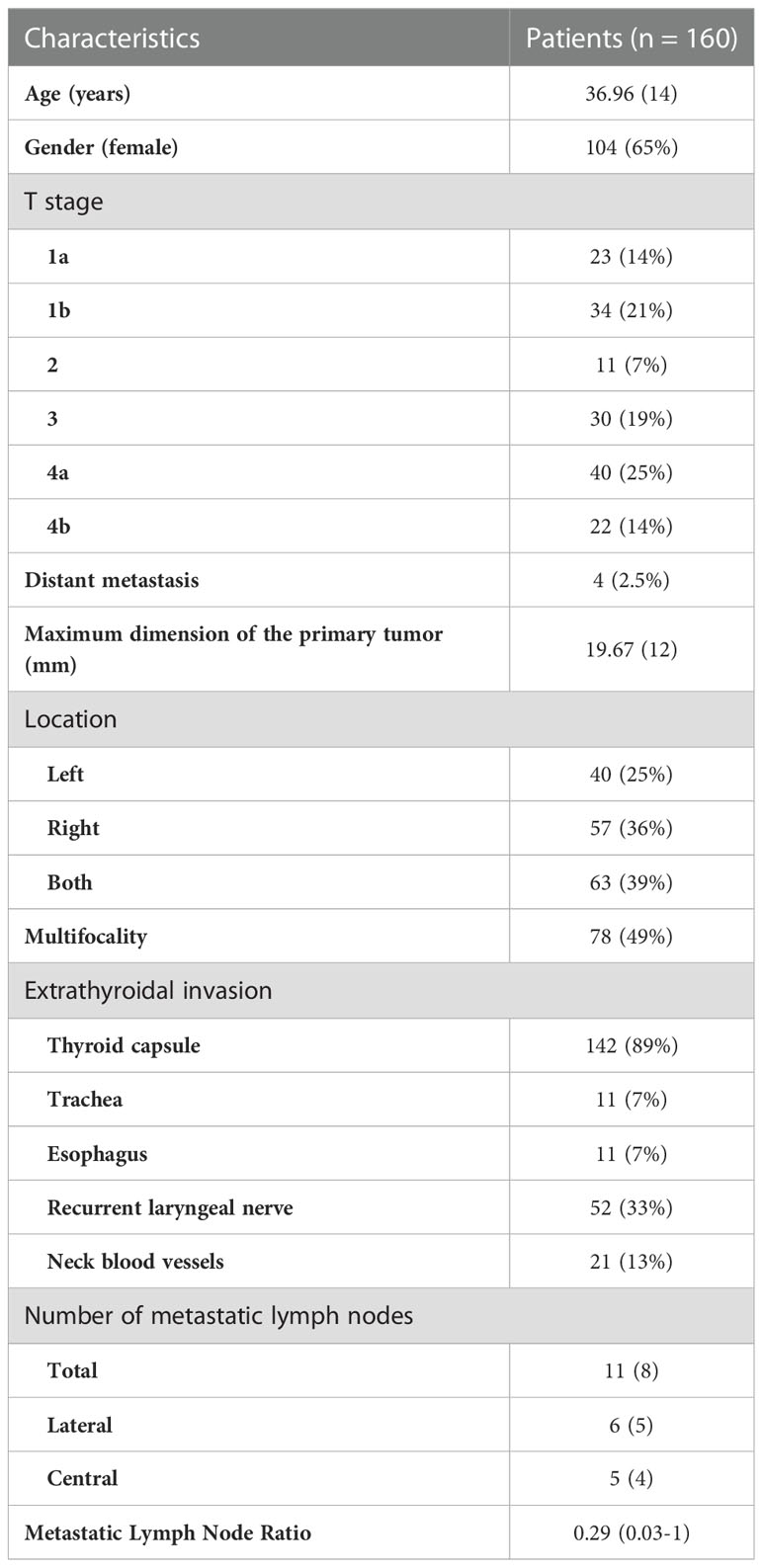
This study collected one hundred and sixty samples from PTC patients with LLNM. Table 1 summarizes the clinical characteristics of those patients. The age was 37 ± 13.75 years (Mean ± s.d.), ranging from 5 to 77 years. There was a 1.86 female to male ratio (104/56), which indicates a modest female predominance. The tumor (T) stage of patients was mainly T4a (25.00%), followed by T1b (21.25%), T3 (18.75%), T1a (14.38%), T4b (13.75%), and T2 (6.87%). Four patients had distant metastases, and the lung has been the site of all metastatic growths. Eleven patients with lateral neck lymph node metastases presented with disease recurrence. An abnormal index of human thyroglobulin (HTG) was found in 36.25% of the patients due to dysfunction of PTC cells. 25-Hydroxy-Vitamin D (25-OH-VD) in 55.00% of patients was lower than the bottom line of the normal range for several reasons, such as region, sun exposure, dietary habits (Supplementary Table 2). The mean maximum dimension of the primary tumor was 19.67mm (3 to 59 mm), with 48.75% demonstrating multifocality lesion within the thyroid gland. Invasion of the thyroid capsule was the most common type of extrathyroidal invasion (88.75%), followed by the invasion to laryngeal nerve (32.50%), neck blood vessels (13.13%), esophagus (6.88%) and trachea (6.88%). In this study, patients had an average of 42 resected lymph nodes (1 to 109 lymph nodes), and 11 metastatic lymph nodes (1 to 41 lymph nodes). 92.50% of patients (148 in 160) also had lymph node metastases in the central region. The mean LNR was 0.29 (0.03-1).
Genomic alterations from DNA profiling
Among the patients enrolled in this study, targeted NGS of 160 primary thyroid carcinomas detected a median of one (0 to 3) mutation. The frequency of mutations across all cases is shown in Figure 1. The most common alternations in our series of PTC with LLNM were BRAF mutation (68%), followed by RET fusion (17%), which both are genes encoding effectors in the mitogen-activated protein kinase (MAPK) signaling pathway (14). BRAF gene mutation plays a vital role in developing PTC, the most common being the V600E subtype (15). Interestingly, none of the patients had co-alterations of BRAF and RET. Mutations in the promoter of TERT, which always co-occurred with BRAF, known to upregulate telomerase and promote tumor progression, were detected in 5% of tumors. 13% of patients had not detected any gene alternation in this panel. Mutations of PIK3CA in members of the phosphoinositide 3-kinase (PI3K) pathway had also been detected at a low frequency (2%).
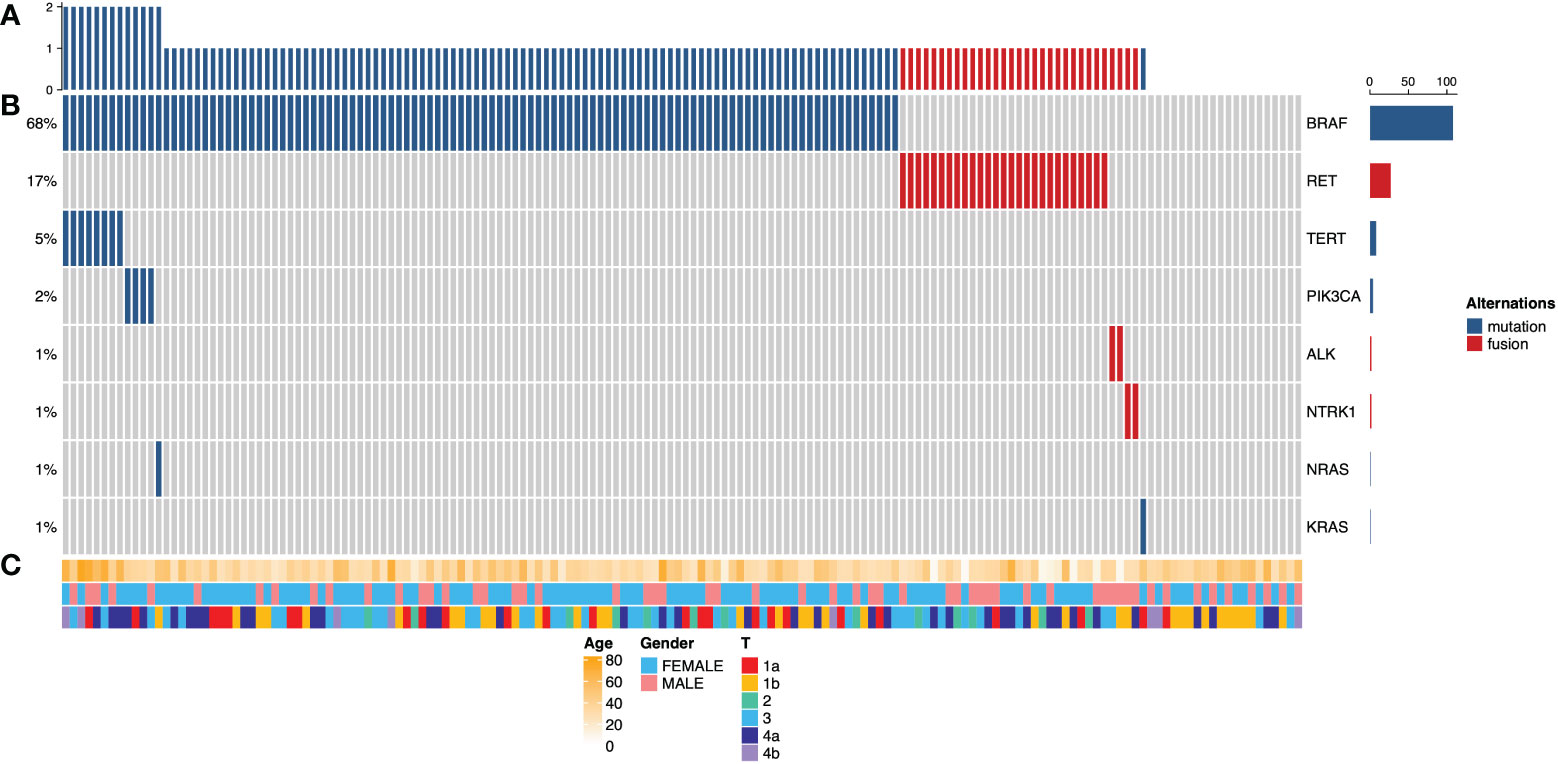
Figure 1 Cancer genome alterations in 160 papillary thyroid carcinomas with lateral lymph node metastasis. (A) Mutation density across the PTC with lateral lymph node metastasis cohorts (n = 160), expressed as a number of genetic alterations found in 57 known thyroid-cancer-related genes. (B) Oncoprints of PTC with LLNM. The left side shows the percentage of tumors altered for each event; the Color key for genetic alterations is shown in the right panel. (C) Clinicopathological features, including patients’ age, gender, and tumor stage. Color keys are shown in the bottom panel.
Differences in clinical characters among BRAF, RET, and non-mutation
A clinical comparison of patients with alternation of BRAF (n=95), RET fusion (n=27), and no mutation (n=20) is summarized in Supplementary Table 3. The difference in age of groups is statistically significant (ANOVA test, p<0.01). Patients with RET fusion were much younger than the other two groups (Figure 2A). The group with RET fusion had a slightly increased average number of LNM (15, 2 to 27 lymph nodes) when compared to groups with BRAF mutation (10, 1 to 41 lymph nodes) or without gene alternation (10, 3 to 24 lymph nodes) (Figure 2B). However, there was no statistical difference in the LNR of the RET group compared to the other 2 groups after the PSM matching (t-test, p=0.40 and 0.98). The proportion of T4 was higher in the RET group than other two groups (Chi-square test, p=0.02) (Figure 2C). There is no significant difference in the extrathyroidal invasion of the three groups (Figure 2D).
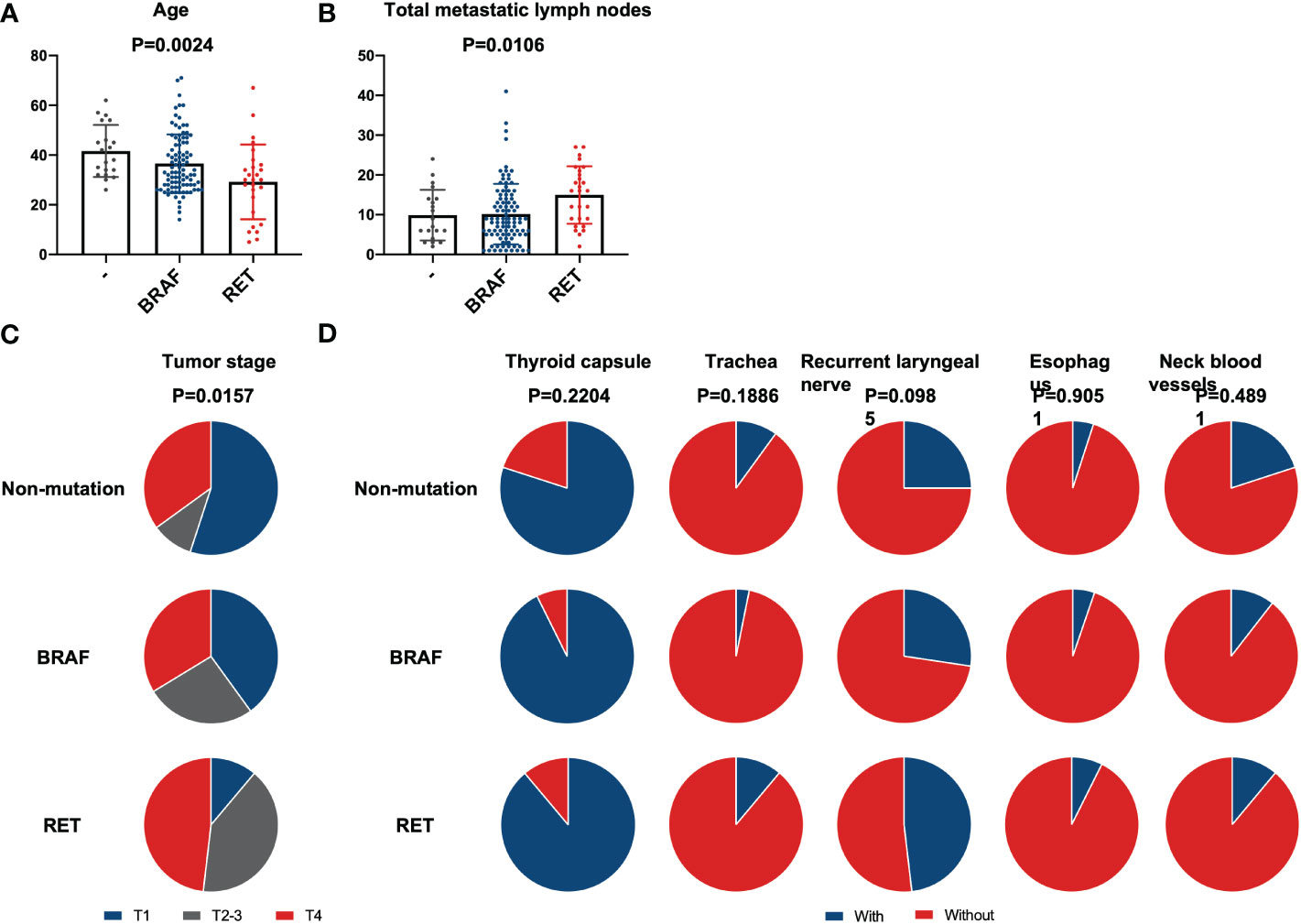
Figure 2 Comparison between BRAF, RET, and non-mutation. Differences in clinical characteristics between patients with BRAF mutation, RET fusion, and without mutation. (A) Patients’ age; (B) Number of lymph node metastases; (C) Tumor stage; (D) Extrathyroidal invasion. No statistical differences in any extrathyroidal invasion exist between these three groups.
Clinical characters of patients with TERT promoter mutation
We identified 8 TERT promoter mutations from informative primary tumors. Interestingly, all TERT promoter mutations were accompanied by BRAF mutations. The age of these patients was 61 ± 12.45 (Mean ± s.d.) and majority of them (7/8) with an advanced tumor stage of T4. Additionally, the largest tumor size in 5 individuals exceeded 40mm, compared to 19.70mm in the entire PTCs with LLNM group. Thus, we supposed that TERT promoter mutation aided in the growth of tumor. Besides, four patients with relapse in the TERT promoter mutation group showed a high recurrence rate. TERT promoter mutations were rare in PTCs but high-risk and highly prevalent in advanced cancers (16).
Characters of the pediatric population
This study collected ten samples of primary thyroid tumors from pediatric PTC patients (age < 18 years) with LLNM. The mean age was 10 years, ranging from 5 to 17 years. The most common alternation in pediatric PTC with LLNM was RET fusion (70%), an inherited genetic mutation. The primary tumor stage of patients was mainly T4 (50%), and distant metastases were found in two patients. The mean maximum dimension of the primary tumor was 27.30mm (range, 13 to 45 mm). Invasion of the thyroid capsule was the most common type of extrathyroidal invasion (100%), found in all pediatric patients. Five patients’ tumors had invaded the recurrent laryngeal nerve. Pediatric patients demonstrated a mean of 5 lateral neck metastatic lymph nodes (range, 3 to 16 lymph nodes).
Discussion
Currently, next-generation sequencing technology simultaneously analyzes hundreds of genes of interest using targeted sequencing panels (17). Understanding the molecular mechanisms of tumor formation is mandatory for accurate diagnoses and personalized treatments (18). Thus, NGS-based molecular tests for oncology research and clinical practice appear rapidly evolving. In PTC, NGS using multigene panels is discussed as an adjunct to ultrasound-guided fine-needle aspiration biopsy to better triage the risk of malignancy in cytologically indeterminate thyroid nodules, potentially reducing the risk of the need for diagnostic surgery with its attendant morbidity (19, 20). It may also contribute to the development of systemic molecular therapies for papillary thyroid cancers that are refractory to standard treatments (21). The discovery of specific genetic alterations and mechanisms of thyroid cancer development is expected to lead to more personalized therapies for patients and achieve precision medicine (22). In addition, the linkage between genetic alterations and clinical features of tumors can be of greater clinical value.
This study evaluated papillary thyroid carcinoma’s mutational status and clinical data of PTC with LLNM. Our result was in accordance with the previous PTC TCGA study (9) that the PTC population had female prominence at 73%. Applying targeted NGS of 57 thyroid-cancer-related genes demonstrates the expected mutations in BRAF and RET. In our cohort, BRAF mutation is the most prevalent alternation, aligned with many landmark researches (9, 23, 24). Tumors driven by BRAF do not respond to the negative feedback from ERK to RAF, resulting in high MAPK-signaling (25). There are 27 patients with RET fusion common in this study. However, none of the patients had a co-alternation of BRAF and RET, suggesting that the BRAF mutation appears to be an alternative to RET in PTC. BRAF mutation and RET fusion may be two mutually exclusive drivers with distinct signaling consequences, like exclusive status between RAS and BRAF mutation (9, 26).
According to our result, patients with RET fusion tend to have more lymph node metastases. This result is consistent with that found in the TCGA database (9), with RET fusion patients seeing a marginally higher total number of metastatic lymph nodes than BRAF mutant patients. To be noted, this discrepancy was more pronounced when compared to the pediatric population (27). Nevertheless, no statistical difference was found in LNR between mutation types after PSM matching. LNR, a way to mitigate the effects of surgery, is computed by dividing the number of metastatic lymph nodes by the total number of lymph nodes evaluated (13). The discrepancy in LNR and absolute values may be related to the insufficient sample size after matching and the limitation of retrospective studies. Only 18 patients in each of the BRAF versus RET groups and 9 patients in each of the non-mutation against RET groups remained after PSM matching. In order to further investigate this data, a bigger sample size is required.
The genetic duet of BRAF and TERT promoter is rare in the entire population of PTCs (9, 28, 29). A genetic-clinical correlation study demonstrated PTCs with a disease-specific mortality risk order of the genetic duet>>>>BRAF V600E alone = TERT promoter mutation alone > wild-type for both genes (29). It presents an essential indication in papillary thyroid cancers with clinically aggressive features and advanced disease (30, 31). Particularly, BRAF and TERT promoter mutational statuses have already been included as a criterion for the risk stratification system in the American Thyroid Association guidelines (32). Our study is consistent with the proposed mechanism, whereby the TERT promoter mutations generate de novo binding elements for ETS-family transcription factors activated by MAPK signaling (33).
We included 10 pediatric patients in this study. It is well known that pediatric thyroid cancer has potential molecular and biological differences compared to thyroid cancer in adults. Specifically, our study found a high rate of gene fusion in the pediatric population, up to 70% in line with other studies (27, 34), compared to 15% in the adult population. The aggressive late-onset disease is more frequent in children than in adults (35). It often spreads to the lymph nodes in the neck and may also spread to the lung (36). Lung metastases were also found in 2 patients in our study (2/10), which is more common than adult thyroid cancer. However, additional efforts with broader groups and larger cohorts are needed if we are to better define the genomic landscape of pediatric thyroid cancer and whether there is an association between histology and/or outcome.
We conduct a contemporary large-scale clinical and molecular cohort with a clinically meaningful group of PTCs with LLNM and analyze the relationship between clinical data and genetic profile. LLNM is always known as a higher risk. Lee et al. (4) evaluated the effect of lymph node-related factors on the risk and location of recurrence in patients with PTCs with LLNM. There was no analysis of genetic detection talked about in Lee’s study. Zheng et al. (37) analyzed factors including clinical results, pathology records, ultrasound results, and BRAFV600E status of PTC and found risk factors for cervical lymph node metastasis. However, it explained the relationship between clinical data and BRAF mutation rather than a genetic profile. Thus, the present investigation suffered from limitations inherent to the lack of comprehensive clinical data and polygenic detection results. However, our study spends a lot of time and effort to collect exhaustive patient information and perform targeted NGS on large primary tumor samples from 2019 to 2022. And advanced patients and patients with distant metastasis are included in this study, which is close to the constitution of PTC with lateral neck lymph node metastasis group. Thus, this study cohort comprised a large-size group of patients with several advanced samples and comprehensive clinical and genetic information, which may help to make a more sensible clinical decision.
This study analyzes the most frequently thyroid-cancer-related mutated gene. Further studies will be required to apply highly sensitive sequencing techniques to evaluate more oncogenic mutations and whether these results can be verified in a larger sequencing panel. Furthermore, we do not explore the basic mechanism of the clinical difference shown in different genotypes. Thus, a lot of room for improvement is given to this research.
Conclusions
In conclusion, we analyzed the clinical and genetic data from a cohort of PTCs with LLNM in a comprehensive manner. We identified the relationship between certain genotype and clinical feature. RET fusion is associated with more metastatic lymph nodes but no significant increase on metastatic lymph node ratio. And the genetic duet of BRAF and TERT was related to advanced cancers. Such an effort may help to make a more sensible clinical decision in the treatment of PTC with LLNM.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Concept and design: YF, XZ. Acquisition of data: ZY, XHZ, JLe. Analysis and interpretations of data: YF, ZL. Drafting of the manuscript: YF. Critical revision of the paper for important intellectual content: JLi, XZ, XHZ. Supervision: ZL, JLi. All authors contributed to the article and approved the submitted version.
Funding
This research is supported by the fellowship of China Postdoctoral Science Foundation (2021M702340), Sichuan Science and Technology Program (2020YFS0573, 2022NSFSC 1441), National Natural Science, Foundation of China (22004088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1057532/full#supplementary-material
References
1. Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol (2021) 17(3):176–88. doi: 10.1038/s41574-020-00448-z
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Kim SH, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Differences in the recurrence and survival of patients with symptomatic and asymptomatic papillary thyroid carcinoma: An observational study of 11,265 person-years of follow-up. Thyroid (2016) 26(10):1472–9. doi: 10.1089/thy.2016.0238
4. Lee YM, Sung TY, Kim WB, Chung KW, Yoon JH, Hong SJ. Risk factors for recurrence in patients with papillary thyroid carcinoma undergoing modified radical neck dissection. Br J Surg (2016) 103(8):1020–5. doi: 10.1002/bjs.10144
5. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the united states, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
6. Hoang JK, Nguyen XV, Davies L. Overdiagnosis of thyroid cancer: Answers to five key questions. Acad Radiol (2015) 22(8):1024–9. doi: 10.1016/j.acra.2015.01.019
7. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol (2011) 7(10):569–80. doi: 10.1038/nrendo.2011.142
8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
9. Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159(3):676–90. doi: 10.1016/j.cell.2014.09.050
10. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA (2013) 309(14):1493–501. doi: 10.1001/jama.2013.3190
11. Melo M, Gaspar da Rocha A, Batista R, Vinagre J, Martins MJ, Costa G, et al. TERT, BRAF, and NRAS in primary thyroid cancer and metastatic disease. J Clin Endocrinol Metab (2017) 102(6):1898–907. doi: 10.1210/jc.2016-2785
12. Perera D, Ghossein R, Camacho N, Senbabaoglu Y, Seshan V, Li J, et al. Genomic and transcriptomic characterization of papillary microcarcinomas with lateral neck lymph node metastases. J Clin Endocrinol Metab (2019) 104(10):4889–99. doi: 10.1210/jc.2019-00431
13. Kim J, Park J, Park H, Choi MS, Jang HW, Kim TH, et al. Metastatic lymph node ratio for predicting recurrence in medullary thyroid cancer. Cancers (Basel) (2021) 13(22):5842. doi: 10.3390/cancers13225842
14. Mitsutake N, Miyagishi M, Mitsutake S, Akeno N, Mesa C Jr., Knauf JA, et al. BRAF mediates RET/PTC-induced mitogen-activated protein kinase activation in thyroid cells: functional support for requirement of the RET/PTC-RAS-BRAF pathway in papillary thyroid carcinogenesis. Endocrinology (2006) 147(2):1014–9. doi: 10.1210/en.2005-0280
15. Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simoes M. ENDOCRINE TUMOURS: Genetic predictors of thyroid cancer outcome. Eur J Endocrinol (2016) 174(4):R117–26. doi: 10.1530/EJE-15-0605
16. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest (2016) 126(3):1052–66. doi: 10.1172/JCI85271
17. LeBlanc VG, Marra MA. Next-generation sequencing approaches in cancer: Where have they brought us and where will they take us? Cancers (Basel) (2015) 7(3):1925–58. doi: 10.3390/cancers7030869
18. Cha YJ, Koo JS. Next-generation sequencing in thyroid cancer. J Transl Med (2016) 14(1):322. doi: 10.1186/s12967-016-1074-7
19. Song Y, Zhang B, Ma T. Highly accurate NGS-based multi-gene testing in the diagnosis of thyroid nodules with indeterminate cytology. J Clin Oncol (2020) 38(15_suppl):e13579–e. doi: 10.1200/JCO.2020.38.15_suppl.e13579
20. Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ 3rd, Ganly I, et al. American Thyroid association statement on surgical application of molecular profiling for thyroid nodules: Current impact on perioperative decision making. Thyroid (2015) 25(7):760–8. doi: 10.1089/thy.2014.0502
21. Pstrag N, Ziemnicka K, Bluyssen H, Wesoly J. Thyroid cancers of follicular origin in a genomic light: in-depth overview of common and unique molecular marker candidates. Mol Cancer (2018) 17(1):116. doi: 10.1186/s12943-018-0866-1
22. Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat Rev Endocrinol (2021) 17(5):296–306. doi: 10.1038/s41574-021-00470-9
23. Morton LM, Karyadi DM, Stewart C, Bogdanova TI, Dawson ET, Steinberg MK, et al. Radiation-related genomic profile of papillary thyroid carcinoma after the Chernobyl accident. Science (2021) 372(6543):725–9. doi: 10.1126/science.abg2538
24. Liang J, Cai W, Feng D, Teng H, Mao F, Jiang Y, et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. J Pathol (2018) 244(2):215–26. doi: 10.1002/path.5005
25. Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A (2009) 106(11):4519–24. doi: 10.1073/pnas.0900780106
26. Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene (2003) 22(29):4578–80. doi: 10.1038/sj.onc.1206706
27. Franco AT, Ricarte-Filho JC, Isaza A, Jones Z, Jain N, Mostoufi-Moab S, et al. Fusion oncogenes are associated with increased metastatic capacity and persistent disease in pediatric thyroid cancers. J Clin Oncol (2022) 40(10):1081–90. doi: 10.1200/JCO.21.01861
28. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocrine-related Cancer (2013) 20(4):603–10. doi: 10.1530/ERC-13-0210
29. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: Genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol (2017) 3(2):202–8. doi: 10.1001/jamaoncol.2016.3288
30. Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, et al. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab (2013) 98(9):E1562–6. doi: 10.1210/jc.2013-2383
31. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol (2014) 32(25):2718–26. doi: 10.1200/JCO.2014.55.5094
32. Balmelli C, Railic N, Siano M, Feuerlein K, Cathomas R, Cristina V, et al. Lenvatinib in advanced radioiodine-refractory thyroid cancer - a retrospective analysis of the Swiss lenvatinib named patient program. J Cancer (2018) 9(2):250–5. doi: 10.7150/jca.22318
33. Bell RJA, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, et al. Cancer. the transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science (2015) 348(6238):1036–9. doi: 10.1126/science.aab0015
34. Bauer AJ. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metab Clin North Am (2017) 46(2):389–403. doi: 10.1016/j.ecl.2017.01.014
35. Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes (Basel) (2019) 10(9):723. doi: 10.3390/genes10090723
36. Pekova B, Dvorakova S, Sykorova V, Vacinova G, Vaclavikova E, Moravcova J, et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr Connect (2019) 8(6):796–805. doi: 10.1530/EC-19-0069
Keywords: papillary thyroid carcinoma, genetic alternation, lateral lymph node metastasis, BRAF, RET
Citation: Fang Y, Zheng X, Zou X, Ye Z, Liu J, Lei J and Li Z (2023) Genetic and clinical profiles of 160 papillary thyroid cancers with lateral neck lymph node metastasis. Front. Oncol. 12:1057532. doi: 10.3389/fonc.2022.1057532
Received: 29 September 2022; Accepted: 19 December 2022;
Published: 12 January 2023.
Edited by:
Yin Detao, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Mingzhao Xing, Johns Hopkins Medicine, United StatesYan-Song Lin, Peking Union Medical College Hospital, China
Copyright © 2023 Fang, Zheng, Zou, Ye, Liu, Lei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Li, cm9ja29saXZlckB2aXAuc2luYS5jb20=
†These authors have contributed equally to this work
 Yiqiao Fang
Yiqiao Fang Xun Zheng
Xun Zheng Xiuhe Zou1,2
Xiuhe Zou1,2 Jianyong Lei
Jianyong Lei Zhihui Li
Zhihui Li