- 1Department of Pathology, School of Basic Medicine, Qingdao University, Qingdao, Shandong, China
- 2Department of Hematology, Qingdao Municipal Hospital, Qingdao, Shandong, China
- 3Department of Pathology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 4Department of Pathology, People Hospital of Changzhi, Changzhi, Shanxi, China
Background: The prognostic implication of tumor-associated macrophages (TAMs) in the microenvironment of diffuse large B cell lymphoma (DLBCL) remains controversial.
Methods: A systematic and comprehensive search of relevant studies was performed in PubMed, Embase and Web of Science databases. The quality of the included studies was estimated using Newcastle-Ottawa Scale (NOS).
Results: Twenty-three studies containing a total of 2992 DLBCL patients were involved in this study. They were all high-quality studies scoring ≥ 6 points. High density of M2 TAMs in tumor microenvironment significantly associated with both advanced disease stage (OR= 1.937, 95% CI: 1.256-2.988, P = 0.003) and unfavorable overall survival (OS) (HR = 1.750, 95% CI: 1.188-2.579, P = 0.005) but not associated with poor progression free survival (PFS) (HR = 1.672, 95% CI: 0.864-3.237, P = 0.127) and international prognostic index (IPI) (OR= 1.705, 95% CI: 0.843-3.449, P = 0.138) in DLBCL patients. No significant correlation was observed between the density of CD68+ TAMs and disease stage (OR= 1.433, 95% CI: 0.656-3.130, P = 0.366), IPI (OR= 1.391, 95% CI: 0.573-3.379, P = 0.466), OS (HR=0.929, 95% CI: 0.607-1.422, P = 0.734) or PFS (HR= 0.756, 95% CI: 0.415-1.379, P = 0.362) in DLBCL patients.
Conclusion: This meta-analysis demonstrated that high density of M2 TAMs in the tumor microenvironment was a robust predictor of adverse outcome for DLBCL patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42022343045.
Background
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin lymphoma (NHL), occupying 30-40% of newly diagnosed NHL (1, 2). DLBCL was heterogenous and patients with DLBCL showed various clinical outcomes (3). Approximately 60-70% DLBCL patients can be cured by anti-CD20 based immunochemotherapy. However, relapsed and refractory patients still die from DLBCL and its complications (4–6). Further improvement of DLBCL patients’ therapeutic outcome relies on identifying high-risk patients and individualizing treatment regimens.
Recent studies by molecular profiling showed that tumor microenvironment (TME) was associated with clinical behavior of DLBCL. Lenz and colleagues demonstrated that the prognosis of DLBCL patients was influenced by differences of TME. They also demonstrated that high stromal-2 signature predicted poor outcome (7). Using gene expression and sequencing, several other studies also obtained promising results in identifying high-risk DLBCL patients (8–12). However, molecular profiling has the disadvantage of low applicability in daily practice.
Tumor-associated macrophages (TAMs) are the most abundant component of TME (13). Recent studies have demonstrated that TAMs were critical for the survival, growth, metastasis, and drug resistance of tumors (14, 15). In response to different environmental stimuli, TAMs differentiated into M1 type (classically activated phenotype) and M2 type (alternatively activated phenotype) (16). The two types of TAMs were distinguished in functions and surface markers. M1 TAMs prevented tumor growth (17, 18). whereas M2 TAMs promoted angiogenesis and was involved in the progression of tumor (17–19). CD68 is a general marker for all TAMs and CD163 is a specific marker for M2 TAMs (3).
In DLBCL, the role of TAMs in the progression of DLBCL and the prognostic value of TAMs remains inconclusive due to the contradictory results obtained by previous studies. Several studies showed that a high density of CD68+ TAMs was associated with favorable prognosis (3, 20, 21). A few other studies failed to demonstrate such association (4, 18, 22–27). By contrast, Cai et al. (28) and Carreras et al. (17) showed that high density of CD68+ TAMs correlated with inferior outcome. The correlation between M2 TAMs and survival of DLBCL patients was also unsettled. Some researchers showed that a high density of M2 TAMs was correlated with shortened survival in DLBCL (1, 3, 17, 23). However, several other studies did not demonstrate such association (20, 24, 25, 27, 29–32). Therefore, we performed this meta-analysis to explore the role of TAMs in DLBCL progression and the prognostic value of TAMs in DLBCL patients.
Methods
Literature search
Relevant articles were systemically searched in PubMed, Embase and Web of Science databases with an end date of August 5th, 2022. The searching terms were “macrophage” or “macrophages” or “TAM” or “TAMs” or “tumor-infiltrating macrophage” or “tumor-associated macrophage” or “intratumoral macrophage” and “diffuse large B-cell lymphoma” or “DLBCL”. In addition, we also searched the references of relevant studies for eligibility. The literature search was performed by two independent reviewers (Mei Lin and Shupei Ma) and disagreement was resolved by consensus.
Inclusion criteria
Our inclusion criteria were as follows (1): proven diagnosis of DLBCL; (2) CD68+ TAMs, CD163+ TAMs or CD163+/CD68+ TAMs were detected by immunohistochemical or immunofluorescence staining; (3) patients were categorized into high and low density TAMs groups; (4) Odds ratio (OR) or hazard ratio (HR) and 95% confidence interval (CI) on the density of CD68+, CD163+ or CD163+/CD68+ TAMs and disease stage, international prognostic index (IPI), overall survival (OS) or progression free survival (PFS) could be obtained.
Data extraction and quality assessment
The data extraction was performed by two reviewers (Shupei Ma and Mei Lin) independently. For each eligible study, we extracted the following data: surname of the first author, year of publication, number of patients, country, treatment, median/mean/average follow-up, method, antibody (clone), analysis. For studies that HR and its 95% CI were not reported, the data was extracted using the Tierney’s calculation method (33). The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the involved studies and any study scores ≥ 6 was considered as a high-quality literature.
Statistical analysis
Pooled OR and 95% CI were used to estimate the correlation between the density of TAMs and disease stage or IPI. Pooled HR and 95% CI were used to investigate the effect of TAMs on prognosis. To evaluate the interstudy heterogeneity, chi-squared test (Q test) and I² test were used. P > 0.10 and I² < 50% indicated no significant heterogeneity existed. In this case, fixed-effect model was used. Otherwise, random-effect model was applied. Sensitivity analysis and subgroup analysis were applied to explore the source of heterogeneity. Publication bias was evaluated by funnel plot and Egger test. All statistical analysis was performed using Stata 12.0 software. P < 0.05 was considered statistically significant.
Results
Identification of eligible studies
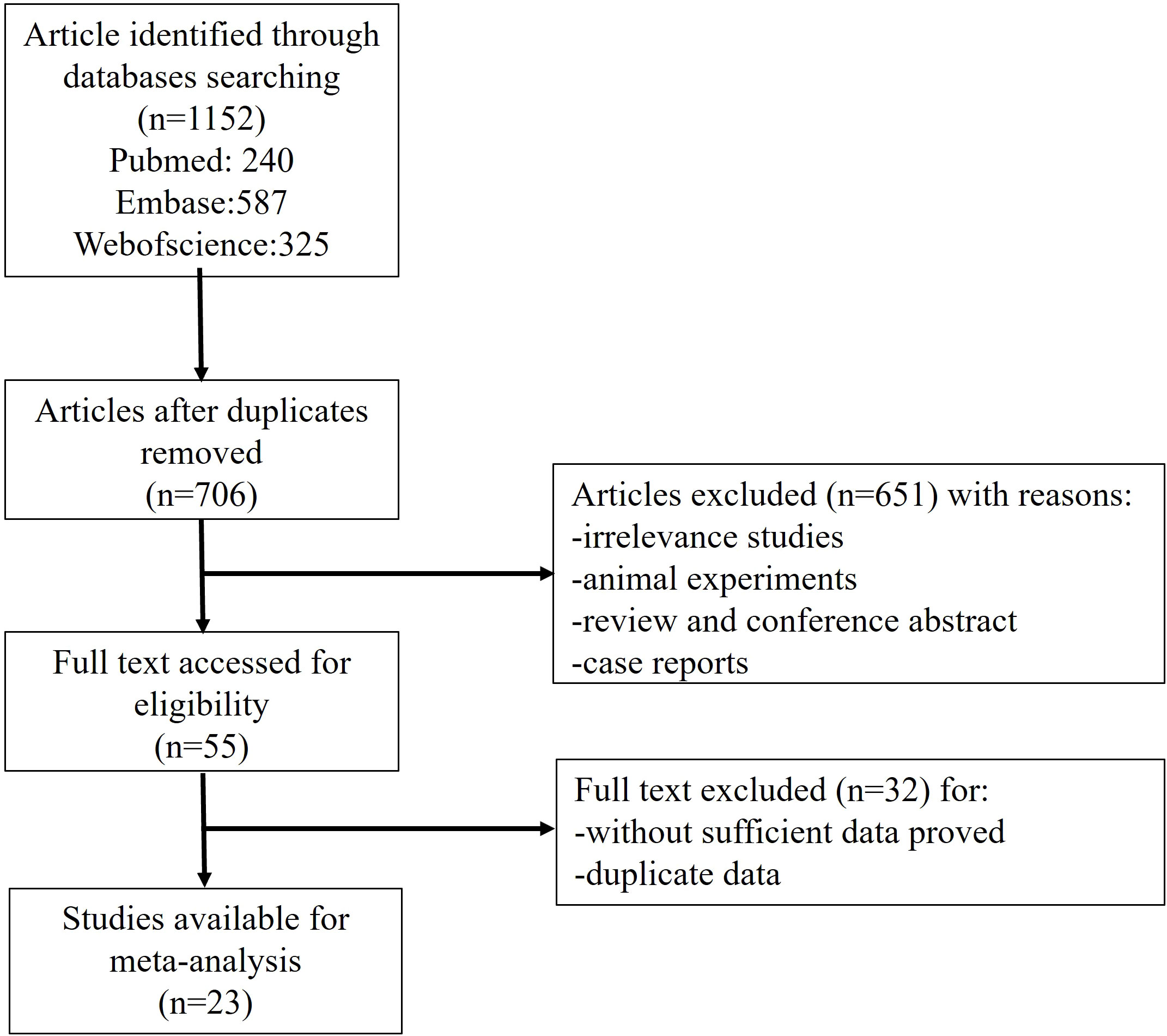
A total of 1152 literatures were retrieved according to the abovementioned searching strategy, including 240 from PubMed, 587 from Embase and 325 from Web of Science. A total of 446 duplication was excluded. By carefully reviewing the title and abstract, we excluded 651 articles which are non-original, irrelevant or laboratory studies on animals or cell lines. The remaining 55 studies were further investigated by reading the full text carefully. Thirty-two studies were then excluded due to not fulfilling the inclusion criteria. Finally, 23 studies were eligible for this meta-analysis (Figure 1).
Characteristics of included studies and quality assessment
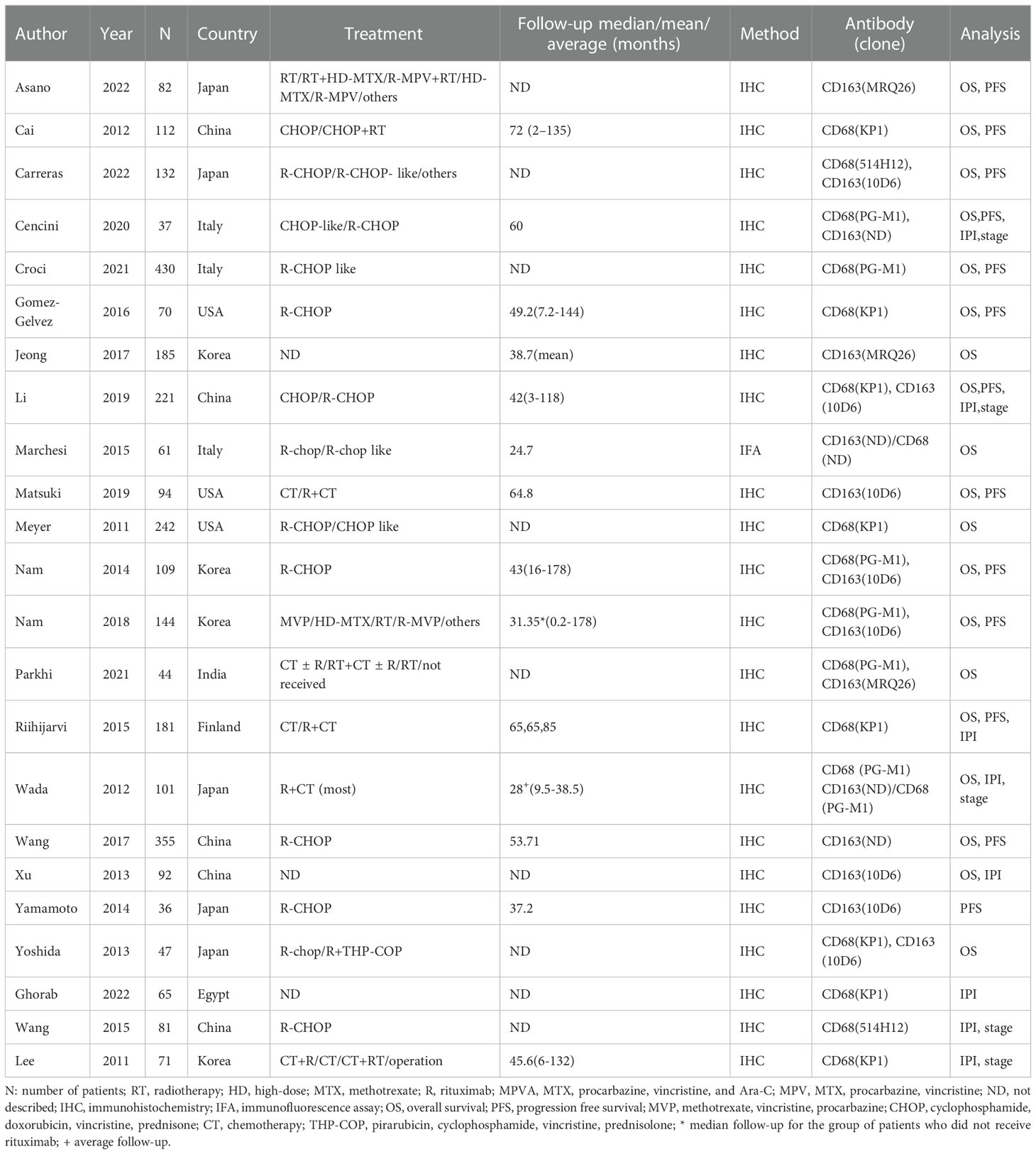
The basic characteristics of the 23 eligible studies was shown in Table 1. The included studies were published between 2011-2022 and the number of participants ranged from 36 to 430. Of the 23 included studies, 5 were from Japan (17, 26, 27, 31, 32) and China (1, 23, 28, 34, 35) respectively; 4 from Korea (3, 20, 29, 36), 3 from USA (4, 22, 30) and Italy (21, 24, 37) respectively; 1 from Finland (18), India (25) and Egypt (38) respectively. Immunohistochemistry or immunofluorescence was performed by the included studies. Antibodies against CD68 was used to detect total TAMs and anti-CD163 antibody or double staining with antibodies against CD68 and CD163 was applied to estimate M2 TAMs by the eligible studies.
The quality of the 23 included studies was estimated by NOS. The scores were all ≥ 6 points (Supplementary Table 1). This suggested that all the eligible studies were high-quality studies.
Total TAMs and IPI, disease stage or prognosis
In this study, the density of total TAMs was not correlated with IPI (≥3/0-2: OR= 1.391, 95% CI: 0.573-3.379, P= 0.466) with significant heterogeneity (P= 0.000, I² =78.4%) (Figure 2A). No correlation was observed between the density of total TAMs and disease stage (Ann Arbor stage, III+IV/I+II: OR= 1.433, 95% CI: 0.656-3.130, P = 0.366) with evident heterogeneity (P = 0.052, I² = 61.2%) (Figure 2B).
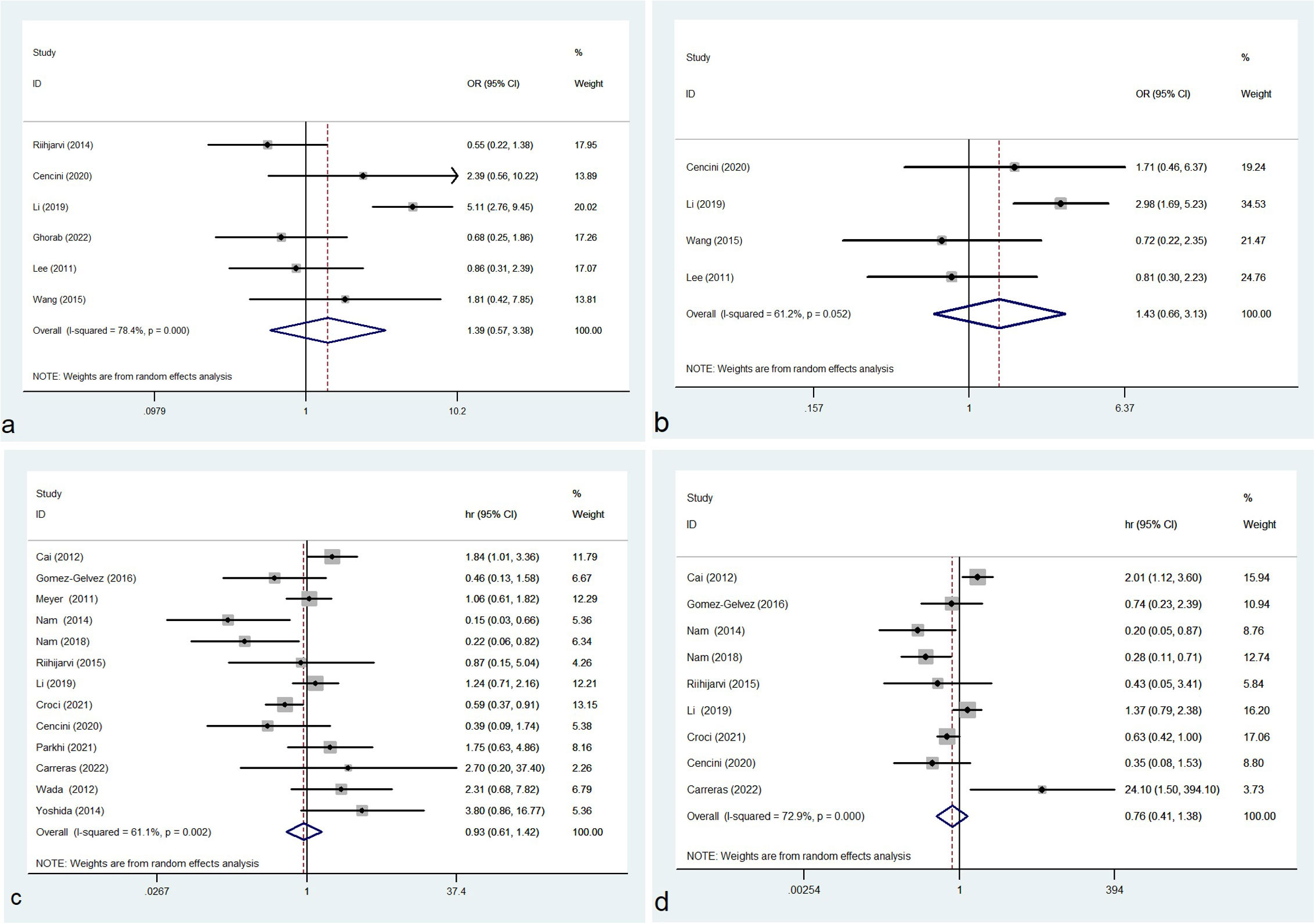
Figure 2 Forest plot of total TAMs and IPI (A), disease stage (B), OS (C) and PFS (D); OR, odds ratio; hr, hazard ratio; CI, confidence interval.
Of the 23 eligible studies, 13 or 9 studies reported the association between the density of CD68+ TAMs and OS or PFS respectively. In our meta-analysis, no significant correlation was observed between the density of total TAMs and OS (HR=0.929, 95% CI: 0.607-1.422, P = 0.734), with significant heterogeneity (P = 0.002, I² = 61.1%) (Figure 2C). No significant association was identified between the density of total TAMs and PFS (HR= 0.756, 95% CI: 0.415-1.379, P = 0.362) and the heterogeneity was significant (P = 0.000, I² = 72.9%) (Figure 2D).
M2 TAMs and IPI, disease stage or prognosis
In this study, no correlation was observed between the density of M2 TAMs and IPI (OR= 1.705, 95% CI: 0.843-3.449, P = 0.138) with evident heterogeneity (P = 0.061, I² = 59.3%) (Figure 3A). High density of M2 TAMs associated with disease stage (OR= 1.937, 95% CI: 1.256-2.988, P = 0.003) with no heterogeneity (P = 0.639, I² = 0.0%) (Figure 3B).
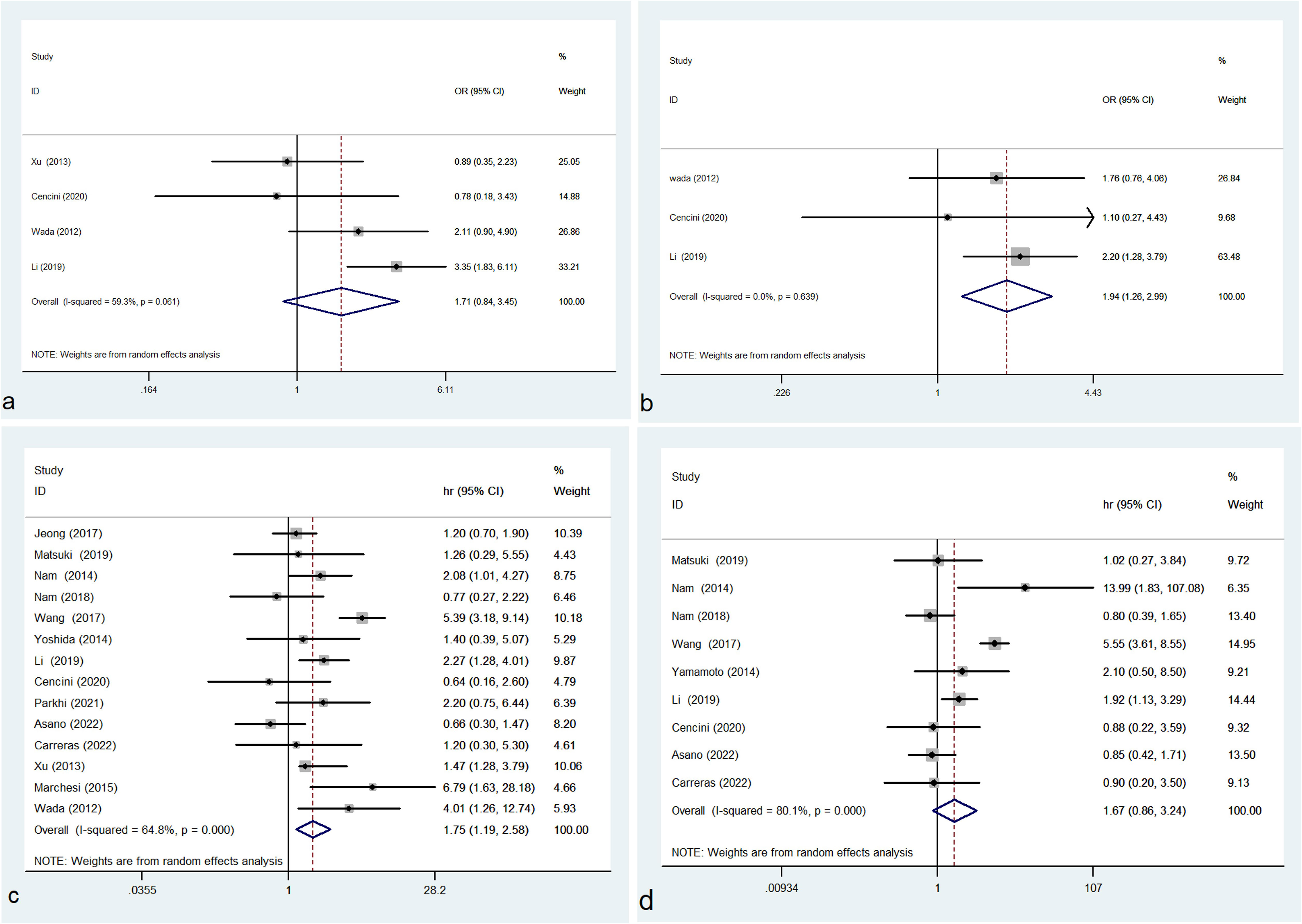
Figure 3 Forest plot of M2 TAMs and IPI (A), disease stage (B), OS (C) and PFS (D); OR, odds ratio; hr, hazard ratio; CI, confidence interval.
In this study, the pooled results of 14 studies showed that high density of M2 TAMs in the microenvironment of DLBCL patients correlated with unfavorable OS (HR = 1.750, 95% CI: 1.188-2.579, P = 0.005), with significant heterogeneity (P = 0.000, I² = 64.8%) (Figure 3C). Pooled HR for PFS in 9 studies showed that high density of M2 TAMs was not significantly associated with poor PFS (HR = 1.672, 95% CI: 0.864-3.237, P = 0.127), with evident heterogeneity (P = 0.000, I² = 80.1%) (Figure 3D).
Sensitivity analysis
Sensitivity analysis was conducted by removing one study each time and recalculating the remaining studies (39). In the analysis of M2 TAMs and OS or PFS, the heterogeneity become insignificant (M2 TAMs and OS: P = 0.108, I² = 34.3%; M2 TAMs and PFS: P = 0.115, I²= 39.6%) after removing Wang et al.’s study (1). In the study of total TAMs and IPI or disease stage, there was no heterogeneity after removing Li et al.’s study (23). In the analysis of M2 TAMs and IPI, the heterogeneity become insignificant after removing Li et al.’s study (P = 0.306, I²= 15.7%) (23) or Xu et al.’s study (P = 0.179, I²= 41.9%) (34).
After removing Xu et al.’s study (34), high density of M2 TAMs was related to high and high-intermediate IPI (OR= 2.239, 95% CI: 1.140-4.396, P = 0.019). After removing Li et al.’s study (23), the density of M2 TAMs was not correlated with disease stage (OR=1.552, 95% CI: 0.758-3.180, P = 0.229). Except the abovementioned 2 studies, no other study significantly influenced the pooled results in this meta-analysis.
Subgroup analysis
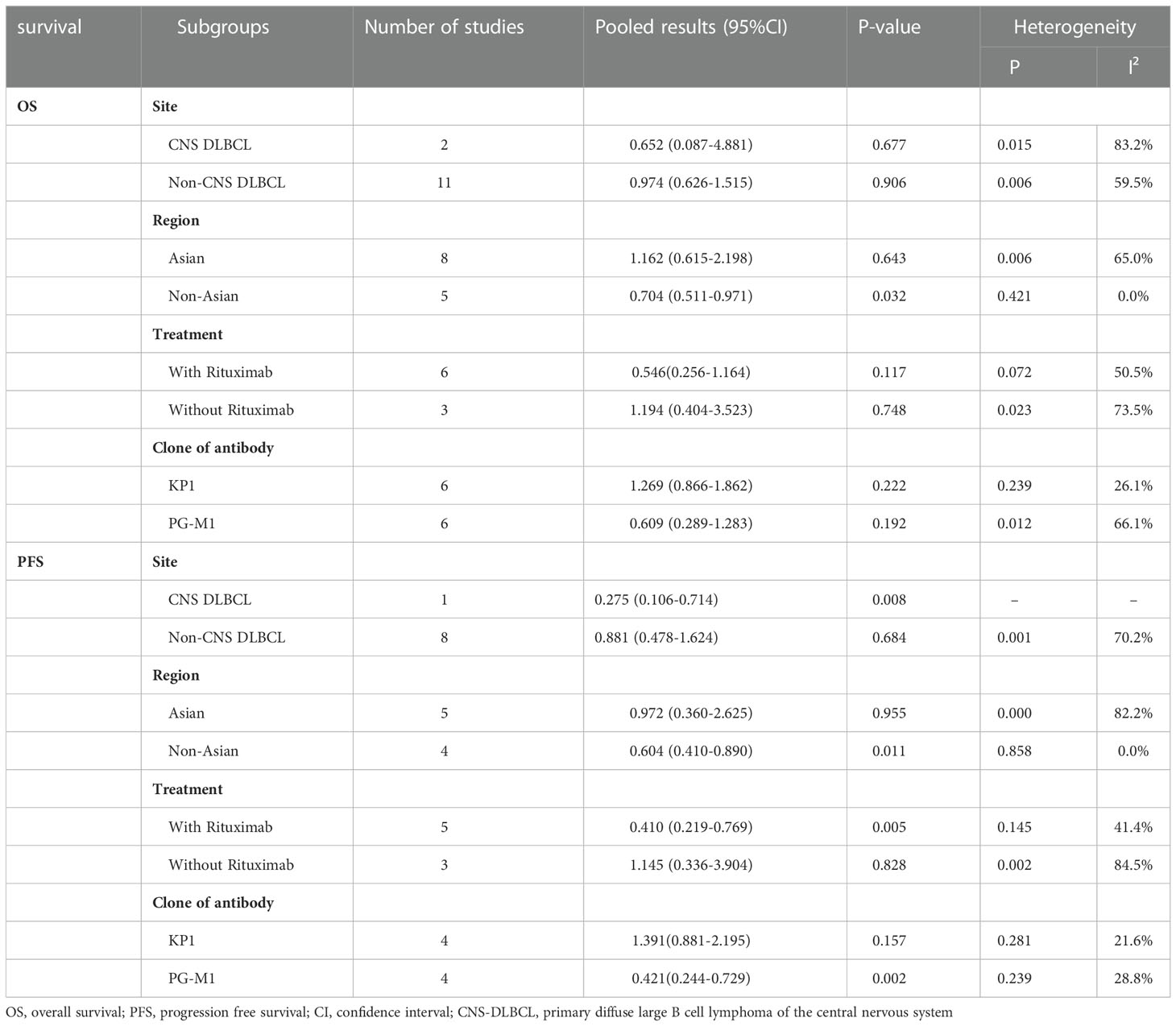
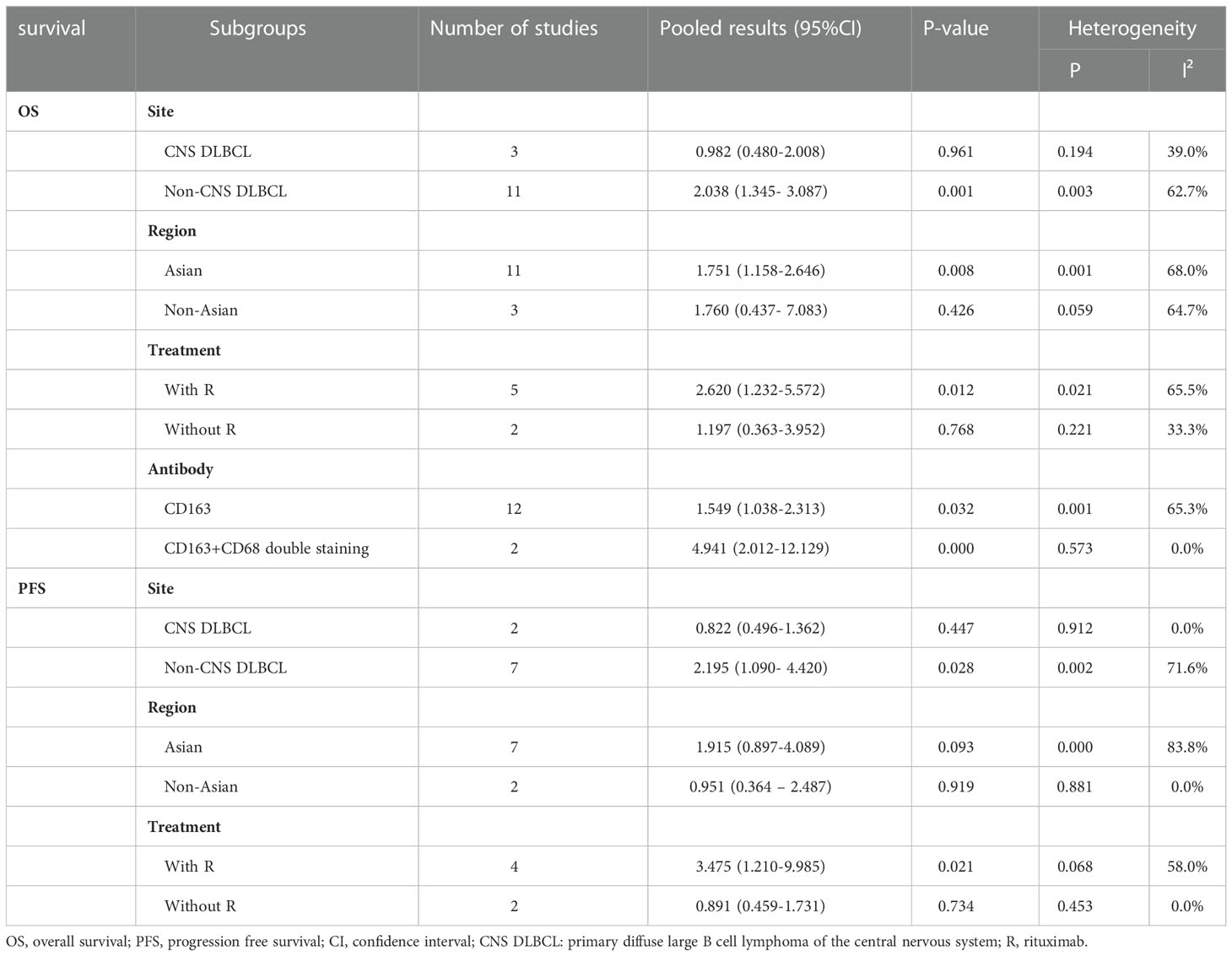
Subgroup analysis was performed in the studies of total TAMs or M2 TAMs and survival of DLBCL patients. The eligible studies were divided into two subgroups according to whether the study focused on central nervous system DLBCL (CNS DLBCL). Our results showed that high density of total TAMs was not correlated with OS in both CNS (HR=0.652, 95% CI: 0.087-4.881, P = 0.677) and non-CNS DLBCL patients (HR=0.974, 95% CI: 0.626-1.515, P = 0.906) (Table 2). A high density of total TAMs was associated with favorable PFS in CNS DLBCL patients (HR=0.275, 95% CI: 0.106-0.714, P = 0.008) but not in non-CNS patients (HR=0.881, 95% CI: 0.478-1.624, P = 0.684) (Table 2). High density of M2 TAMs correlated with poor OS (HR=2.038, 95% CI: 1.345-3.087, P = 0.001) and PFS (HR=2.195, 95% CI: 1.090-4.420, P = 0.028) in non-CNS DLBCL patients. The density of M2 TAMs was not correlated with both OS and PFS in CNS DLBCL patients (Table 3).
Based on geographic region, the included patients were classified into Asian group and non-Asian group. High density of total TAMs correlated with favorable OS (HR=0.704, 95% CI: 0.511-0.971, P = 0.032) and PFS (HR=0.604, 95% CI: 0.410-0.890, P = 0.011) in non-Asian patients with no heterogeneity (total TAMs and OS: P = 0.421, I² = 0.00%; total TAMs and PFS: P = 0.858, I² = 0.00%). However, the density of total TAMs was not correlated with both OS and PFS in Asian patients (Table 2). High density of M2 TAMs associated with poor OS (HR=1.751, 95% CI: 1.158-2.646, P = 0.008) and showed a trend of association with poor PFS (HR=1.915, 95% CI: 0.897-4.089, P = 0.093) in Asian patients. The density of M2 TAMs was not correlated with both OS and PFS in non-Asian patients (Table 3).
In the subgroup analysis according to whether rituximab was included in the treatment regimen, a high density of total TAMs was significantly correlated with favorable PFS (HR=0.410, 95%CI: 0.219-0.769, P=0.005) but not significantly correlated with OS (HR=0.546, 95% CI: 0.256-1.164, P = 0.117) in patients treated with rituximab-containing regimen (Table 2). In contrast, no correlation was observed between the density of total TAMs and OS or PFS in patients treated without rituximab (Table 2). High density of M2 TAMs significantly correlated with unfavorable OS (HR=2.620, 95% CI: 1.232-5.572, P = 0.012) and PFS (HR=3.475, 95% CI: 1.210-9.985, P = 0.021) in patients treated with rituximab-containing regimen. However, no correlation was found between the density of M2 TAMs and prognosis of patients treated without rituximab (Table 3).
Subgroup analysis according to different clones of anti-CD68 antibody was also performed. In the subgroup detected CD68 with clone PG-M1, total TAMs correlated with PFS (HR=0.421, 95% CI: 0.244-0.729, P = 0.002) but not correlated with OS (HR=0.609, 95% CI: 0.289-1.283, P = 0.192) (Table 2). In KP1 subgroup, no correlation was observed between total TAMs and OS (HR=1.269, 95% CI: 0.866-1.862, P = 0.222) or PFS (HR=1.391, 95% CI: 0.881-2.195, P = 0.157) (Table 2).
Among the 14 studies reported the correlation between M2 TAMs and OS, 12 studies used anti-CD163 antibody and 2 studies applied double staining with antibodies against CD163 and CD68 to estimate M2 TAMs. The density of CD163+ TAMs and CD163+/CD68+ TAMs was both correlated with OS (CD163+ TAMs: HR=1.549, 95% CI: 1.038-2.313, P = 0.032; CD163+/CD68+ TAMs: HR=4.941, 95% CI: 2.012-12.129, P = 0.000) (Table 3).
Publication bias
Funnel plots and Egger tests were used to assess the publication bias. The funnel plots of this meta-analysis were shown in Supplementary Figure 1 and Supplementary Figure 2. The yielded P values of Egger test were: 0.180 for total TAMs and disease stage, 0.302 for total TAMs and IPI, 0.860 for total TAMs and OS, 0.707 for total TAMs and PFS, 0.018 for M2 TAMs and disease stage, 0.150 for M2 TAMs and IPI, 0.598 for M2 TAMs and OS, 0.321for M2 TAMs and PFS. The results of Egger test showed that there was publication bias in the analysis of M2 TAM and disease stage. Except this, no publication bias existed in the other analysis of this meta-analysis.
Discussion
Previous studies showed that TME is critical for the progression of tumors (14). TAMs are important component of TME (28). The prognostic significance of total TAMs and M2 TAMs have been investigated in a variety of cancers by meta-analysis (40–44). In lymphoma, meta-analysis investigated the association of total TAMs or M2 TAMs and outcome of patients have been reported in non-Hodgkin’s lymphoma (NHL) (45) and Hodgkin’s lymphoma (HL) (46). DLBCL is the most common type of NHL. However, meta-analysis investigating the prognostic value of TAMs in DLBCL is still unavailable.
Consistent with the results obtained by meta-analysis in HL (46), gastric cancer (40) and NHL (45), we demonstrated that high density of M2 TAMs correlated with unfavorable prognosis in DLBCL. This suggested that high density of M2 TAMs can be used as an indicator of poor prognosis in DLBCL patients. Xu et al.’s study reported that high density of CD68+ TAMs correlated with poor OS and PFS in NHL (45) and several previous studies suggested that TAMs’ infiltration was significantly correlated with favorable (3, 20, 21) or poor outcome (17, 28) in DLBCL. However, no correlation between the density of CD68+ TAMs and prognosis in DLBCL patients was found in this meta-analysis. This was in accordance with previous studies in bladder (41) and ovarian cancers (42). Taken together, these results suggested that M2 TAMs rather than total TAMs might contribute to the progression of DLBCL and lead to unfavorable outcome in DLBCL patients.
In this study, high density of M2 TAMs was correlated with unfavorable prognosis in Asian subgroup but not in non-Asian subgroup. These suggested that M2 TAMs may play an important role in the disease progression and acted as an indicator of poor prognosis in Asian patients. In this meta-analysis, high density of CD68+ TAMs associated with favorable outcome in non-Asian DLBCL patients but not in Asian patients. This suggested that high density of CD68+ TAMs predicted favorable survival in non-Asian patients but not in Asian patients.
Rituximab, a human/murine chimeric antibody, shows high affinity and specificity for CD20 which is a transmembrane protein of B-lymphocyte. Rituximab has become a standard component of treatment modality for a number of B-cell malignancies including DLBCL (47). Taskinen and colleagues reported that addition of rituximab to the same group of patients at relapse reversed the negative prognostic effect of high density CD68+ TAMs in tumor environment to favorite (48). In this meta-analysis, high density of CD68+ TAMs correlated with favorable outcome in the subgroup of patients treated with rituximab-containing regimen. In contrast, no correlation was found between high density of CD68+ TAMs and prognosis in patients treated without rituximab. These results were in accordance with previous study and suggested that TAMs might obtain tumor-inhibiting function in response to rituximab or TAMs modulated the therapeutic efficiency of rituximab.
The results of our meta-analysis showed that the association of M2 TAMs and outcome of DLBCL patients was also influenced by whether rituximab was included in the treatment regimen. Pooled results of this meta-analysis showed that high density of M2 TAMs in tumor microenvironment associated with unfavorable outcome in patients treated with rituximab. In contrast, no correlation was observed between the density of M2 TAMs and prognosis in patients treated without rituximab. This emphasized the importance of targeting M2 macrophage in rituximab era.
TAMs centered therapeutic strategies includes suppressing the recruitment of TAMs, depletion of TAMs and reprogramming M2 TAMs to M1 type (49). Administration of antibody against chemokine (C-C motif) ligand-2 (CCL2) led to decreased infiltration of TAMs and impacted tumor growth in animal models of human cancers (50, 51). Colony stimulating factor 1 (CSF-1) is a major factor for the survival of TAMs. Targeting CSF-1 receptor with a humanized antibody RG7155 led to obvious reduction of TAMs in various tumor tissues (52). Maeda et al. showed that toll-like receptor 3 (TLR3) agonist Poly (I:C) was effective in reprogramming macrophage to anti-tumor type by an in vitro study (53). Repolarization of TAMs can also be achieved through manipulation of CD40 (54) and CD47 pathways (55). Currently, a variety of antibodies against CD40 (56) or CD47 (57) are being evaluated in clinical trials. A phase IIb clinical trial was performed to investigate the therapeutic efficacy of anti-CD40 antibody dacetuzumab plus rituximab, ifosfamide, carboplatin, and etoposide in 151 patients with relapsed and refractory DLBCL. The complete remission (CR) rate of the dacetuzumab group was not superior compared to the group using placebo in place of dacetuzumab (58). A phase Ib/II clinical trials of anti-CD47 antibody Hu5F9-G4 combined with rituximab in 75 patients with relapsed and refractory lymphoma showed promising results (57). In DLBCL patients treated with rituximab-containing regimen, the pooled results of this meta-analysis showed that high density of total TAMs significantly correlated with favorable outcome while high density of M2 TAMs significantly associated with poor prognosis. This suggested that repolarization of TAMs from M2 to M1 might have more clinical benefit than the methods of merely reducing the number of M2 TAMs in the treatment of DLBCL patients who received rituximab-containing regimen.
In the subgroup analysis according to different clones of anti-CD68 antibody, high density of total TAMs correlated with favorable PFS in the subgroup using clone PG-M1. While no association was identified between total TAMs and OS or PFS in KP1 subgroup. These suggested that high density of total TAMs detected by PG-M1 rather than KP1 was an indicator of favorable prognosis in DLBCL patients.
The current study is the first systemic meta-analysis investigating the association between the density of total TAMs or M2 TAMs and prognosis in DLBCL patients. However, several limitations in this study need to be addressed. First, some of the involved studies did not report HR. We extracted data from the Kaplan-Meier curves of these studies. In this case, deviation from the real value of HR may be caused. Second, the treatment of DLBCL patients in the included studies are variable. This may influence the survival of patients and contribute to heterogeneity. Third, significant heterogeneity exists in this meta-analysis. The interstudy heterogeneity might be derived from the differences in origin of patients, sample size, location of locus, tumor stages, inconsistency of cut-off value and the antibody used to estimate TAMs. Forth, significant publication bias was observed in the study of M2 TAMs and disease stage in this meta-analysis. This may be due to that studies with positive results are more likely to be published than those reporting negative results. In addition, only three studies were eligible for this analysis. Therefore, more studies are needed to verify our results.
Conclusion
This meta-analysis demonstrated that a high density of M2 TAMs was a robust predictor of unfavorable outcome for DLBCL patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ML designed the study and revised the manuscript, ML, SM, LS, and ZQ selected the study, extracted and analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1094400/full#supplementary-material
Supplementary Figure 1 | Funnel plot for publication bias of total TAMs and IPI (A), disease stage (B), OS (C) and PFS (D).
Supplementary Figure 2 | Funnel plot for publication bias of M2 TAMs and IPI (A), disease stage (B), OS (C) and PFS (D).
Supplementary Table 1 | Newcastle-Ottawa Quality Assessment Scale. * A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
References
1. Wang J, Gao K, Lei W, Dong L, Xuan Q, Feng M, et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: Correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget (2017) 8(3):5414–25. doi: 10.18632/oncotarget.14289
2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
3. Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large b-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. (2014) 55(11):2466–76. doi: 10.3109/10428194.2013.879713
4. Gomez-Gelvez JC, Salama ME, Perkins SL, Leavitt M, Inamdar KV. Prognostic impact of tumor microenvironment in diffuse large B-cell lymphoma uniformly treated with r-CHOP chemotherapy. Am J Clin Pathol (2016) 145(4):514–23. doi: 10.1093/ajcp/aqw034
5. Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26 Suppl 5:v116–25. doi: 10.1093/annonc/mdv304
6. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the groupe d’Etudes des lymphomes de l’Adulte. Blood (2010) 116(12):2040–5. doi: 10.1182/blood-2010-03-276246
7. Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med (2008) 359(22):2313–23. doi: 10.1056/NEJMoa0802885
8. Staiger AM, Altenbuchinger M, Ziepert M, Kohler C, Horn H, Huttner M, et al. A novel lymphoma-associated macrophage interaction signature (LAMIS) provides robust risk prognostication in diffuse large B-cell lymphoma clinical trial cohorts of the DSHNHL. Leukemia (2020) 34(2):543–52. doi: 10.1158/2159-8290.CD-20-0839
9. Vegliante MC, Mazzara S, Zaccaria GM, De Summa S, Esposito F, Melle F, et al. NR1H3 (LXRα) is associated with pro-inflammatory macrophages, predicts survival and suggests potential therapeutic rationales in diffuse large B-cell lymphoma. Hematol Oncol (2022) 40(5):864–75. doi: 10.1038/nrclinonc.2016.217
10. Autio M, Leivonen SK, Brück O, Mustjoki S, Mészáros Jørgensen J, Karjalainen-Lindsberg ML, et al. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica (2021) 106(3):718–29. doi: 10.1016/j.cub.2020.06.081
11. Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell (2020) 37(4):551–568.e14. doi: 10.3390/cancers14061469
12. Kotlov N, Bagaev A, Revuelta MV, Phillip JM, Cacciapuoti MT, Antysheva Z, et al. Clinical and biological subtypes of B-cell lymphoma revealed by microenvironmental signatures. Cancer Discovery (2021) 11(6):1468–89. doi: 10.1155/2016/9720912
13. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol (2017) 14(7):399–416. doi: 10.1111/cas.15179
14. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol (2020) 30(16):R921–5. doi: 10.3324/haematol.2014.113472
15. García-Domínguez DJ, Hontecillas-Prieto L, Palazón-Carrión N, Jiménez-Cortegana C, Sánchez-Margalet V, de la Cruz-Merino L. Tumor immune microenvironment in lymphoma: Focus on epigenetics. Cancers (Basel) (2022) 14(6):1469. doi: 10.3390/jcm9082418
16. Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H, et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: Recent research advances and potential targets for tumor immunotherapy. J Immunol Res (2016) 2016:9720912. doi: 10.1080/2162402X.2018.1442164
17. Carreras J, Kikuti YY, Hiraiwa S, Miyaoka M, Tomita S, Ikoma H, et al. High PTX3 expression is associated with a poor prognosis in diffuse large B-cell lymphoma. Cancer Sci (2022) 113(1):334–48. doi: 10.1016/j.annonc.2021.08.1991
18. Riihijärvi S, Fiskvik I, Taskinen M, Vajavaara H, Tikkala M, Yri O, et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: A correlative study from a Nordic phase II trial. Haematologica (2015) 100(2):238–45. doi: 10.1309/AJCPJX4BJV9NLQHY
19. Tamma R, Ranieri G, Ingravallo G, Annese T, Oranger A, Gaudio F, et al. Inflammatory cells in diffuse large B cell lymphoma. J Clin Med (2020) 9(8):2418. doi: 10.1186/s12885-019-6208-x
20. Nam SJ, Kim S, Kwon D, Kim H, Kim S, Lee E, et al. Prognostic implications of tumor-infiltrating macrophages, M2 macrophages, regulatory T-cells, and indoleamine 2,3-dioxygenase-positive cells in primary diffuse large B-cell lymphoma of the central nervous system. Oncoimmunology (2018) 7(7):e1442164. doi: 10.1080/2162402X.2018.1442164
21. Croci GA, Au-Yeung RKH, Reinke S, Staiger AM, Koch K, Oschlies IR, et al. SPARC-Positive macrophages are the superior prognostic factor in the microenvironment of diffuse large B-cell lymphoma and independent of MYC rearrangement and double-/triple-hit status. Ann Oncol (2021) 32(11):1400–9. doi: 10.1111/apm.13195
22. Meyer PN, Fu K, Greiner T, Smith L, Delabie J, Gascoyne R. The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol (2011) 135(1):54–61. doi: 10.1309/AJCPJX4BJV9NLQHY
23. Li YL, Shi ZH, Wang X, Gu KS, Zhai ZM. Tumor-associated macrophages predict prognosis in diffuse large B-cell lymphoma and correlation with peripheral absolute monocyte count. BMC Cancer. (2019) 19(1):1049. doi: 10.1371/journal.pone.0078730
24. Cencini E, Fabbri A, Schiattone L, Sicuranza A, Mecacci B, Granai M. Prognostic impact of tumor-associated macrophages, lymphocyte-to-monocyte and neutrophil-to-lymphocyte ratio in diffuse large B-cell lymphoma. Am J Blood Res (2020) 10(4):97–108. doi: 10.1007/s12032-011-0123-6
25. Parkhi M, Chatterjee D, Bal A, Vias P, Yadav BS, Prakash G. Prognostic implications of the tumor immune microenvironment and immune checkpoint pathway in primary central nervous system diffuse large B-cell lymphoma in the north Indian population. APMIS (2022) 130(2):82–94. doi: 10.1016/j.humpath.2017.04.012
26. Wada N, Zaki MA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y, et al. Osaka Lymphoma study group. Tumour-associated macrophages diffuse large B-cell lymphoma: Study Osaka Lymphoma Study Group Histopathology. (2012) 60(2):313–9. doi: 10.1097/PAI.0000000000000645
27. Yoshida N, Oda M, Kuroda Y, Katayama Y, Okikawa Y, Masunari T, et al. Clinical significance of sIL-2R levels in B-cell lymphomas. PloS One (2013) 8(11):e78730. doi: 10.1007/s10014-022-00427-4
28. Cai QC, Liao H, Lin SX, Xia Y, Wang XX, Gao Y, et al. High expression of tumor-infiltrating macrophages correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med Oncol (2012) 29(4):2317–22. doi: 10.3109/10428194.2014.893311
29. Jeong J, Oh EJ, Yang WI, Kim SJ, Yoon SO. Implications of infiltrating immune cells within bone marrow of patients with diffuse large B-cell lymphoma. Hum Pathol (2017) 64:222–31. doi: 10.1186/1745-6215-8-16
30. Matsuki E, Bohn OL, El Jamal S, Pichardo JD, Zelenetz AD, Younes A, et al. Lymphocyte-to-monocyte ratio may serve as a better prognostic indicator than tumor-associated macrophages in DLBCL treated with rituximab. Appl Immunohistochem Mol Morphol. (2019) 27(8):572–80. doi: 10.1097/PAI.0000000000000645
31. Asano K, Yamashita Y, Ono T, Natsumeda M, Beppu T, Matsuda K, et al. Clinicopathological risk factors for a poor prognosis of primary central nervous system lymphoma in elderly patients in the tohoku and niigata area: A multicenter, retrospective, cohort study of the tohoku brain tumor study group. Brain Tumor Pathol (2022) 39(3):139–50. doi: 10.1016/j.anndiagpath.2015.04.008
32. Yamamoto W, Nakamura N, Tomita N, Takeuchi K, Ishii Y, Takahashi H, et al. Human leukocyte antigen-DR expression on flow cytometry and tumor-associated macrophages in diffuse large B-cell lymphoma treated by rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone therapy: retrospective cohort study. Leuk Lymphoma. (2014) 55(12):2721–7. doi: 10.4132/KoreanJPathol.2011.45.4.361
33. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1002/hon.2142
34. Xu YL, Wang HQ, Qian ZZ, Song Z, Zhou SY, Zhang HL, et al. Expression and prognostic value of regulatory T cells and M2 macrophages in diffuse large B-cell lymphoma tissues. Zhonghua Zhong Liu Za Zhi. (2013) 35(6):450–5.
35. Wang X, Li X, Zhang X, Zang L, Yang H, Zhao W, et al. Toll-like receptor 4-induced inflammatory responses contribute to the tumor-associated macrophages formation and infiltration in patients with diffuse large B-cell lymphoma. Ann Diagn Pathol (2015) 19(4):232–8. doi: 10.1016/j.anndiagpath.2015.04.008
36. Lee J, K wak Y, Kim C, Kim I. Association of CD57+ natural killer cells with better overall survival in DLBCL patients. Korean J Path. (2011) 45:361–70. doi: 10.1371/journal.pone.0170042
37. Marchesi F, Cirillo M, Bianchi A, Gately M, Olimpieri OM, Cerchiara E, et al. High density of CD68+/CD163+ tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematol Oncol (2015) 33(2):110–2. doi: 10.18632/oncotarget.25334
38. Ghorab DS, Helaly AM, El Mahdi HS, Khatatbeh M, Ibrahiem AT. Prognostic role of tumor microenvironment in DLBCL and relation to patients’ clinical outcome: A clinical and immunohistochemical study. Anal Cell Pathol (Amst). (2022) 2022:9993496. doi: 10.1016/j.ygyno.2017.07.007
39. Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions Version5.2.0. Cochrane (2022). Available at: www.training.cochrane.org/handbook.
40. Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, et al. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PloS One (2017) 12(1):e0170042. doi: 10.7150/jca.46282
41. Wu SQ, Xu R, Li XF, Zhao XK, Qian BZ. Prognostic roles of tumor associated macrophages in bladder cancer: A system review and meta-analysis. Oncotarget (2018) 9(38):25294–303. doi: 10.18632/oncotarget.25334
42. Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol (2017) 147(1):181–7. doi: 10.1186/s12916-016-0711-6
43. Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, et al. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget (2017) 8(18):30576–86. doi: 10.1007/s12325-017-0612-x
44. Xie W, Yan O, Liu F, Han Y, Wang H. Prognostic value of survivin in nasopharyngeal carcinoma: A systematic review and meta-analysis. J Cancer. (2021) 12(14):4399–407. doi: 10.1158/1078-0432.CCR-07-0778
45. Xu X, Li Z, Liu J, Zhu F, Wang Z, Wang J, et al. The prognostic value of tumour-associated macrophages in non-hodgkin’s lymphoma: A systematic review and meta-analysis. Scand J Immunol (2020) 91(1):e12814. doi: 10.3390/ijms22136995
46. Guo B, Cen H, Tan X, Ke Q. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med (2016) 14(1):159. doi: 10.1158/1078-0432.CCR-16-0870
47. Salles G, Barrett M, Foà R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-cell hematologic malignancies: A review of 20 years of clinical experience. Adv Ther (2017) 34(10):2232–73. doi: 10.1002/cam4.886
48. Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppä S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res (2007) 13(19):5784–9. doi: 10.1016/j.ccr.2014.05.016
49. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci (2021) 22(13):6995. doi: 10.1002/eji.201847888
50. Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, et al. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res (2017) 23(1):137–48. doi: 10.1158/1078-0432.CCR-16-0870
51. Arakaki R, Yamasaki T, Kanno T, Shibasaki N, Sakamoto H, Utsunomiya N. CCL2 as a potential therapeutic target for clear cell renal cell carcinoma. Cancer Med (2016) 5(10):2920–33. doi: 10.1371/journal.pone.0153550
52. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell (2014) 25(6):846–59. doi: 10.3892/ol.2020.12037
53. Maeda A, Digifico E, Andon FT, Mantovani A, Allavena P. Poly(I:C) stimulation is superior than imiquimod to induce the antitumoral functional profile of tumor-conditioned macrophages. Eur J Immunol (2019) 49(5):801–11. doi: 10.1186/s13045-021-01197-w
54. Jensen JL, Hope C, Asimakopoulos F. Deploying myeloid cells against myeloma. Oncoimmunology (2015) 5(3):e1090076. doi: 10.3109/10428194.2015.1007504
55. Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages In vivo. PloS One (2016) 11(4):e0153550. doi: 10.1038/s41375-019-0573-y
56. Li DK, Wang W. Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies. Oncol Lett (2020) 20(5):176. doi: 10.1002/hon.3050
57. Jiang Z, Sun H, Yu J, Tian W, Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol (2021) 14(1):180. doi: 10.1186/s13045-021-01197-w
58. Fayad L, Ansell SM, Advani R, Coiffier B, Stuart R, Bartlett NL, et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: A randomized, double-blind, placebo-controlled phase 2b trial. Leuk Lymphoma. (2015) 56(9):2569–78. doi: 10.1016/j.ccell.2020.03.015
Keywords: diffuse large B cell lymphoma (DLBCL), prognosis, meta-analysis, tumor-associated macrophages (TAMs), M2 TAMs
Citation: Lin M, Ma S, Sun L and Qin Z (2023) The prognostic value of tumor-associated macrophages detected by immunostaining in diffuse large B cell lymphoma: A meta-analysis. Front. Oncol. 12:1094400. doi: 10.3389/fonc.2022.1094400
Received: 10 November 2022; Accepted: 29 December 2022;
Published: 20 January 2023.
Edited by:
Alberto Fabbri, Siena University Hospital, ItalyReviewed by:
Emanuele Cencini, Siena University Hospital, ItalySabino Ciavarella, National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 Lin, Ma, Sun and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Lin, bGlubWVpNzBAaG90bWFpbC5jb20=
 Mei Lin
Mei Lin Shupei Ma2
Shupei Ma2