- 1Department of Anesthesiology and Perioperative Medicine, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Hemato-Oncology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
- 3Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 4Department of General Surgery, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
- 5Department of Food Nutrition and Health Biotechnology, College of Medical and Health Science, Asia University, Taichung, Taiwan
- 6Big Data Center, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
- 7Division of Radiation Oncology, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan
- 8Department of Healthcare Administration, College of Medical and Health Science, Asia University, Taichung, Taiwan
- 9Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, Taipei, Taiwan
- 10Centers for Regional Anesthesia and Pain Medicine, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
Purpose: We examined locoregional recurrence (LRR) in patients with breast invasive ductal carcinoma (IDC) receiving total mastectomy (TM) under propofol-based paravertebral block-regional anesthesia (PB-RA) versus sevoflurane-based inhalational general anesthesia (INHA-GA) without propofol. All-cause death and distant metastasis were secondary endpoints.
Patients and Methods: Patients with breast IDC receiving TM were recruited through propensity score matching and categorized into INHA-GA with sevoflurane and PB-RA with propofol groups. Cox regression analysis was performed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: In the multivariate Cox regression analysis, the adjusted HR (aHR; 95% CI) of LRR for the PB-RA with propofol group was 0.52 (0.28–0.96) compared with the INHA-GA with sevoflurane group. The aHRs of LRR for differentiation grade II, grade III, the American Joint Committee on Cancer clinical stage II, stage III, pathological tumor (pT) stage 2, pT stage 3–4, pathological nodal (pN) stage 1, and pN stage 2–3 were 1.16 (1.04–2.08), 1.28 (1.07–2.12), 3.71 (1.82–7.59), 4.67 (1.65–13.18), 1.09 (1.02–1.21), 1.17 (1.03–2.16), 1.10 (1.03–1.33), and 1.22 (1.06–2.41), respectively, compared with differentiation grade I, clinical stage I, pT1, and pN0. The aHR of LRR for adjuvant RT was 0.88 (0.64–0.94) compared with that for no adjuvant RT.
Conclusion: PB-RA with propofol might be beneficial for reducing LRR in women with breast IDC receiving TM compared with INHA-GA without propofol.
Introduction
Many preclinical studies including in vivo or in vitro have suggested an association between anesthetic drugs and techniques and the activity and survival of cancer cells; this association can result from changes in the immune response, modulation of the neuroendocrine stress response to surgery, or effects on cancer cell signaling (1–7). However, few studies have reported high-quality clinical outcomes. Most existing clinical studies are retrospective in nature (8–11), and most prospective trials were initially designed to study outcomes other than cancer recurrence (12–14).
Sevoflurane is one of the most widely used volatile anesthetic agents. Sevoflurane exhibited chemoresistance to cisplatin (15) and led to an increased expression of metastasis-related genes (16). By contrast, propofol is the most commonly used intravenous induction agent and is often used for maintaining anesthesia (7). In a laboratory study, propofol exhibited antitumor effects (7). However, investigating the effects of anesthetics, such as sevoflurane and propofol, on patients with cancer in a clinical trial is difficult (17, 18) because patients generally require a combination of anesthetic agents (19, 20). Patients are often managed with either inhalation agents and opioids or propofol as the anesthetic agent and regional anesthesia as the analgesic agent (19, 20). Moreover, performing surgery without providing perioperative pain relief or solely under regional anesthesia to examine the effects of specific anesthetic modalities would be unethical (19, 20). In addition, interpretation of these findings from controversial conclusions in previous studies is limited by heterogeneity resulting from the different extents of surgery, cancer types, and patient characteristics as well as other limitations associated with the retrospective nature of most studies (21). Therefore, conflicting conclusions have been reported in preclinical and clinical studies (1–7, 19, 20).
To address this crucial problem, we chose a consistent extent of surgery (total mastectomy [TM]) for patients with breast invasive ductal carcinoma (IDC), consistent anesthesia (propofol-based paravertebral block-regional anesthesia [PB-RA] vs. sevoflurane-based inhalational general anesthesia [INHA-GA]), and the primary endpoint of locoregional recurrence (LRR) to investigate LRR between INHA-GA without propofol and PB-RA with propofol in patients with breast cancer who underwent TM through propensity score matching (PSM).
Patients and Methods
Study Cohorts
This retrospective study was conducted using data from the Health and Welfare Data Center (HWDC) established by Taiwan’s Ministry of Health and Welfare. The HWDC consolidates data gathered by the Taiwanese government from various sources. These data are then deidentified and made available for research purposes based on case-by-case approval. In particular, we used the Taiwan Cancer Registry, which includes the detailed staging and treatment information of patients with cancer, the Cause of Death database, which lists all death certificates issued in Taiwan (22), and the National Health Insurance Research Database, which contains billing information on all National Health Insurance (NHI)-reimbursed examinations, medications, and treatments. We have confident are that no evidence of death is evidence of life, because all death certificates issued is the Government system-specific judgment. A death certificate is required for property inheritance, abandonment of inheritance to the court, burial or cremation. The NHI program has been implemented since 1995 and covers more than 99% of Taiwan’s population.
We established a cohort consisting of female patients with breast IDC by using data from the Taiwan Cancer Registry Database (TCRD), which is maintained by the Collaboration Center of Health Information Application. We enrolled patients who received a diagnosis of IDC between January 1, 2009, and December 31, 2018, and underwent TM. The follow-up duration was from the index date to December 31, 2019. The index date was the date of TM. The mean follow-up duration was 43.3 months (standard deviation [SD], 29.8 months) and 55.9 months (22.6 months) for patients receiving INHA-GA without propofol and those receiving PB-RA with propofol, respectively. The TCRD contains detailed cancer-related information including the clinical or pathological stage (according to the American Joint Committee on Cancer [AJCC], seventh edition), anesthesia modalities, hormone receptor (HR) status, human epidermal growth factor receptor-2 (HER2) status, and radiotherapy (RT) and chemotherapy regimens used (23–27). The study protocols were reviewed and approved by the Institutional Review Board of Tzu-Chi Medical Foundation (IRB109-015-B). Patient diagnoses were confirmed on the basis of pathological data, and patients who received a new diagnosis of breast IDC were confirmed to have no other cancers and no distant metastasis. In the PB-RA with propofol group, propofol was initially used as target-controlled infusion for conscious sedation during paravertebral block and TM (28). The optimal propofol target concentration was ≥0.8 mg/ml at least for the PB-RA with propofol group (29). In the INHA without propofol group, anesthesia was continued with sevoflurane in 100% oxygen at a flow rate of ≥5 L/min in a circle system, and the end-tidal concentration of sevoflurane was maintained at a minimum alveolar concentration of approximately ≥2 (30). Our propofol doses in our study were similar with the previous studies (20, 31). There is no association of the cost of propofol, cost of treatment, and not affected by insurance or decision to in the chose either type of anesthesia. All surgical procedures and propofol cost of treatment for breast cancer were all covered by NHI. Propofol was not used in the INHA-GA group. Other inclusion criteria were age ≥20 years and AJCC clinical stage I–III. Patients with metastasis, missing sex data, age <20 years, nonstandard adjuvant breast RT (contrast with standard adjuvant RT, consisting of irradiation to both the chest wall/whole breast and regional nodes with a minimum of 50 Gy), neoadjuvant chemotherapy, unclear differentiation of tumor grade, missing HR status, missing HER2 status, or unclear pathological tumor, node, and metastasis (TNM) staging were excluded. Adjuvant treatments such as adjuvant RT, adjuvant chemotherapy, hormone therapy, and target therapy were allowed on the basis of National Comprehensive Cancer Network (NCCN) guidelines for breast cancer in Taiwan (32). Furthermore, we excluded patients with unclear surgical procedures, ill-defined nodal surgery, unclear HR status, unclear HER2 status, unknown pathologic TNM stages, unknown American Society of Anesthesiology (ASA) physical status, unclear Charlson comorbidity index (CCI), unclear grade of differentiation, or nonrecorded hospital type (33) (academic center or community hospital) from our cohort. HR positivity was defined as ≥1% of tumor cells demonstrating positive nuclear staining through immunohistochemistry (34) and HER2 positivity was defined as an immunohistochemistry score of 3+ or a fluorescence in situ hybridization ratio of ≥2 (33, 35). Finally, we enrolled patients with breast IDC receiving TM under PB-RA with propofol or INHA-GA without propofol during perioperative anesthesia. Comorbidities were assessed using the CCI (36, 37). The CCI has prognostic significance for all-cause death in patients with breast cancer (38, 39). Only comorbidities observed 6 months before the index date were included, and new-onset comorbidities diagnosed within 6 months before the index date were excluded. On the basis of the inclusion criteria, we examined the effects of long-term comorbidities on the survival of patients. Comorbidities were identified according to primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes; diseases present at the first admission and those identified more than twice during outpatient visits were included as comorbidities.
PSM and Covariates
After adjustment for confounders, we used a Cox proportional-hazards model to model time from the index date to LRR (primary endpoint) for patients with IDC receiving TM. To reduce the effects of potential confounders when LRR was compared between different anesthesia groups, PSM was performed. Matching variables used were age, menopausal status, diagnosis year, CCI score, differentiation, AJCC clinical stage, pathological tumor (pT) stage, pathological nodal (pN) stage, ASA physical status, adjuvant chemotherapy, adjuvant RT, HR status, HER2 status, nodal surgery, and hospital level. We matched the cohorts at a ratio of 1:1 by using the greedy method, with age, diagnosis year, menopausal status, CCI score, differentiation, AJCC clinical stage, pT, pN, adjuvant RT, HR status, HER2 status, and nodal surgery completely matched with a propensity score within a caliper of 0.2 (40). Matching is a common technique used for selecting controls with identical background covariates as study participants to minimize differences between individuals that the investigator believes must be controlled. A Cox model was used to regress all-cause death and distant metastasis (DM; secondary endpoints) on different anesthesia statuses, and a robust sandwich estimator was used to account for clustering within matched sets (41). Multivariate Cox regression analysis was performed to calculate hazard ratios (HRs) to determine whether factors such as different anesthesia modalities, age, menopausal status, diagnosis year, CCI score, differentiation, AJCC clinical stage, pT, pN, ASA physical status, adjuvant chemotherapy, adjuvant RT, HR status, HER2 status, nodal surgery, and hospital level are potential independent predictors of all-cause death, LRR, or DM. Potential predictors were controlled for in the analysis (Table 1), and LRR was the primary endpoint in both anesthesia groups. All-cause death and DM were the secondary endpoints in our study.
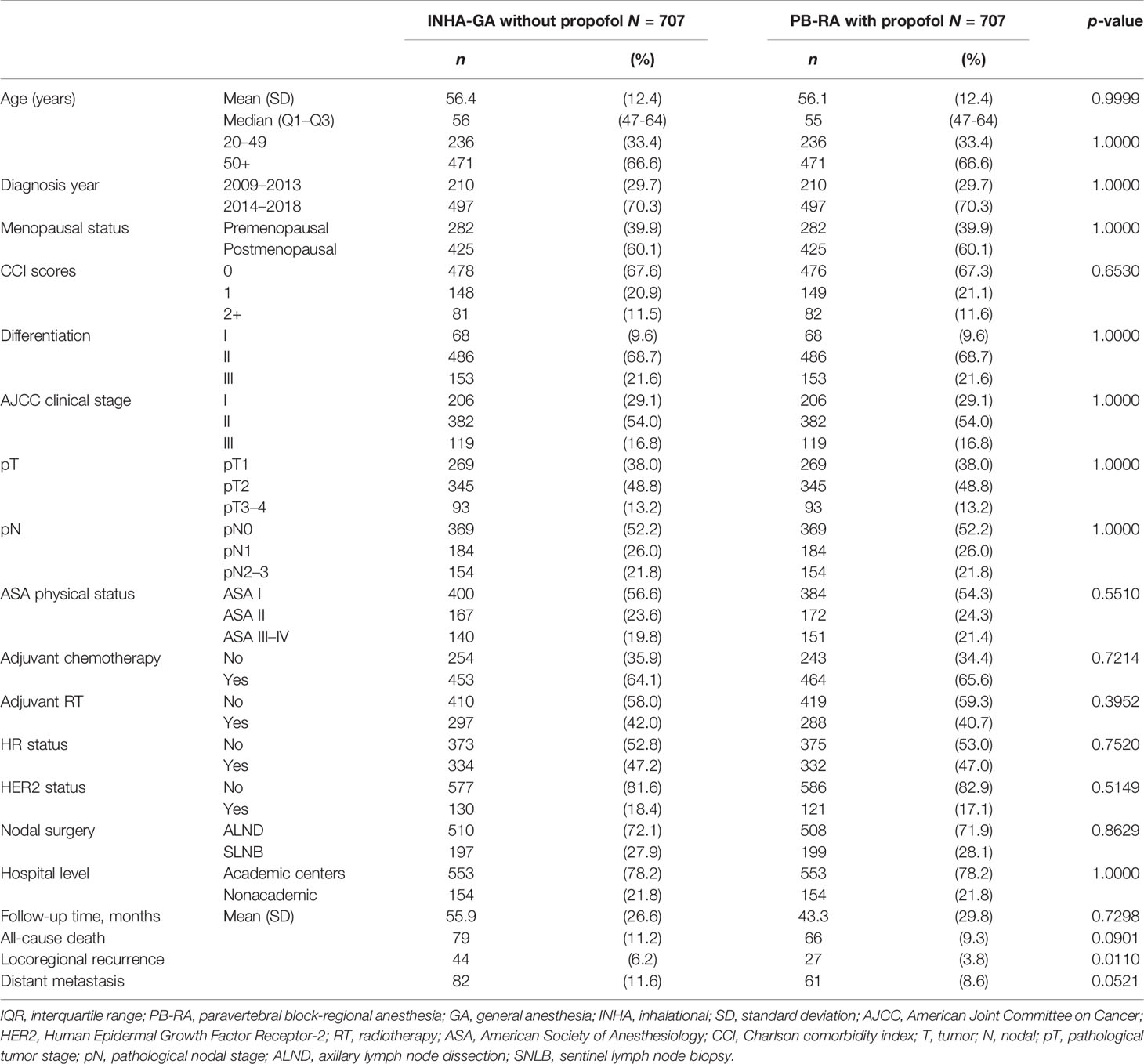
Table 1 Demographics of propensity score-matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol.
Statistics
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). In a two-tailed Wald test, p < 0.05 was considered significant. Overall survival (OS), LRR-free survival, and DM-free survival were estimated using the Kaplan–Meier method, and differences between the INHA-GA without propofol and PB-RA with propofol groups were determined using the stratified log-rank test to compare survival curves (stratified according to matched sets) (42).
Results
PSM and Study Cohort
The matching process yielded a final cohort of 1,414 patients (707 and 707 in the INHA-GA without propofol and PB-RA with propofol groups, respectively) eligible for further analysis; their characteristics are summarized in Table 1. Age distribution was balanced between the two groups (Table 1). Menopausal status, diagnosis year, CCI score, differentiation, AJCC clinical stages, pT, pN, hospital level, adjuvant RT, adjuvant chemotherapy, ASA physical status, HR status, HER2 status, and nodal surgery were similar after head-to-head PSM in the two cohorts, and no significant differences were observed in the variables between the two cohorts. The follow-up duration, LRR, DM, or all-cause death was not matched because oncological outcomes were inconsistent between the two groups (Table 1). The crude primary endpoint of LRR in women with breast IDC receiving TM under INHA-GA without propofol and PB-RA with propofol varied significantly (p = 0.0110; Table 1).
Prognostic Factors for All-Cause Death After Multivariate Cox Regression Analysis
No significant differences in OS were observed in explanatory variables except for age ≥ 50 years, differentiation grade II (moderate differentiation), grade III (poor differentiation), AJCC clinical stage II–III, pT2, pT3–4, pN1, and pN2–3 (Table 2). In the multivariate Cox regression analysis, the adjusted HR (aHR; 95% CI) of all-cause death for PB-RA with propofol compared with INHA-GA without propofol was 1.01 (0.68–1.51). The aHRs (95% CIs) of all-cause death for age ≥ 50 years, differentiation grade II, grade III, AJCC clinical stage II, clinical stage III, pT2, pT3–4, pN1, and pN2–3 were 1.64 (1.03–2.62), 2.85 (1.13–7.15), 3.83 (1.48–9.93), 1.42 (1.12–2.45), 1.56 (1.28–3.13), 1.70 (1.07–2.72), 3.06 (1.72–5.43), 1.74 (1.07–2.83), and 3.55 (2.10–6.01), respectively, compared with age < 50 years, differentiation grade 1, AJCC clinical stage I, pT1, and pN0. The aHR of all-cause death for adjuvant chemotherapy was 0.40 (0.27–0.60) compared with no adjuvant chemotherapy.
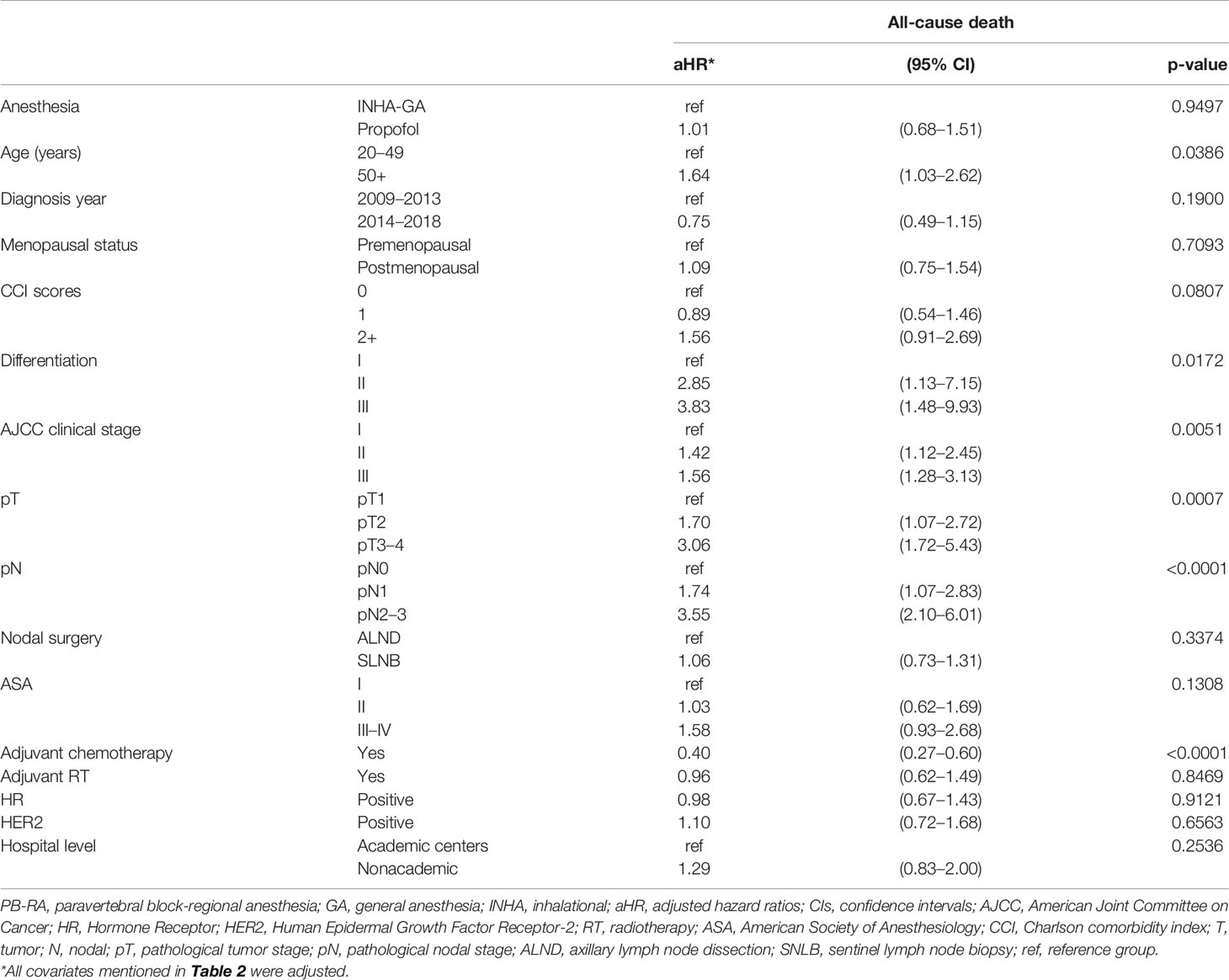
Table 2 Multivariate analysis of all-cause death for propensity score-matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol.
Prognostic Factors for LRR After Multivariate Cox Regression Analysis
The aHR (95% CI) of LRR for the PB-RA with propofol group was 0.52 (0.28–0.96) compared with the INHA-GA without propofol group (Table 3). The aHRs of LRR for differentiation grade II, grade III, clinical stage II, stage III, pT2, pT3–4, and pN2–3 were 1.16 (1.04–2.08), 1.28 (1.07–2.12), 3.71 (1.82–7.59), 4.67 (1.65–13.18), 1.09 (1.02–1.21), 1.17 (1.03–2.16), 1.10 (1.03–1.33), and 1.22 (1.06–2.41), respectively, compared with differentiation grade I, clinical stage I, pT1, and pN0. The aHR of LRR for adjuvant RT was 0.88 (0.64–0.94) compared with that for no adjuvant RT.
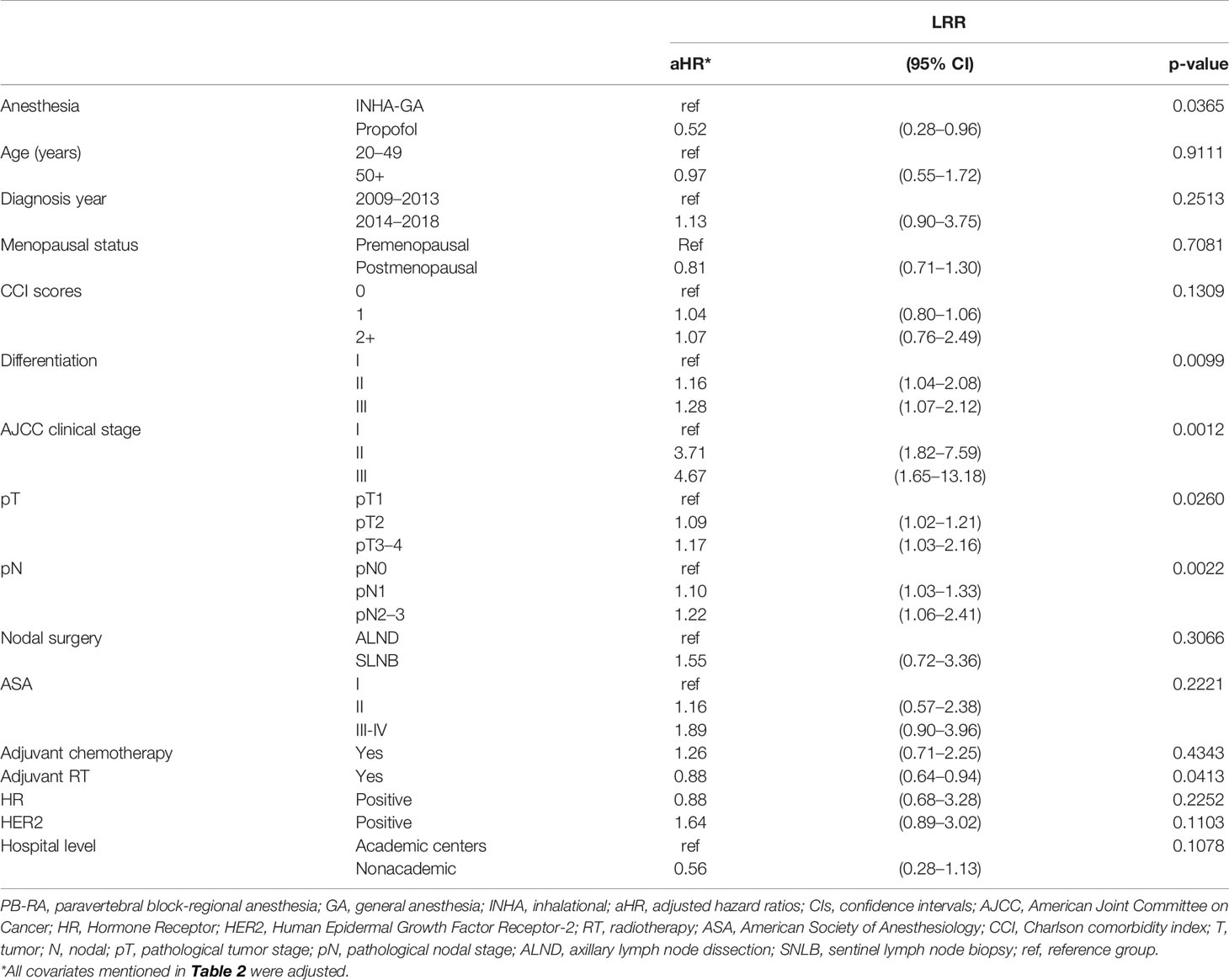
Table 3 Multivariate analysis of locoregional recurrence for propensity score-matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol.
Prognostic Factors for DM After Multivariate Cox Regression Analysis
The aHR (95% CI) of DM for the PB-RA with propofol group was 0.74 (0.49–1.10) compared with the INHA-GA without propofol group (Table 4). The aHRs of DM for clinical stage II, stage III, pT2, pT3–4, pN1, pN2–3, and HER2 positivity were 1.15 (1.06–2.46), 1.35 (1.12–2.92), 1.12 (1.02–2.21), 2.01 (1.12–3.59), 1.24 (1.11–2.29), 2.11 (1.22–3.64), and 2.06 (1.07–3.52), respectively, compared with clinical stage I, pT1, pN0, and HER2 negativity. The aHR of DM for adjuvant chemotherapy was 0.70 (0.46–0.96) compared with that for no adjuvant chemotherapy.
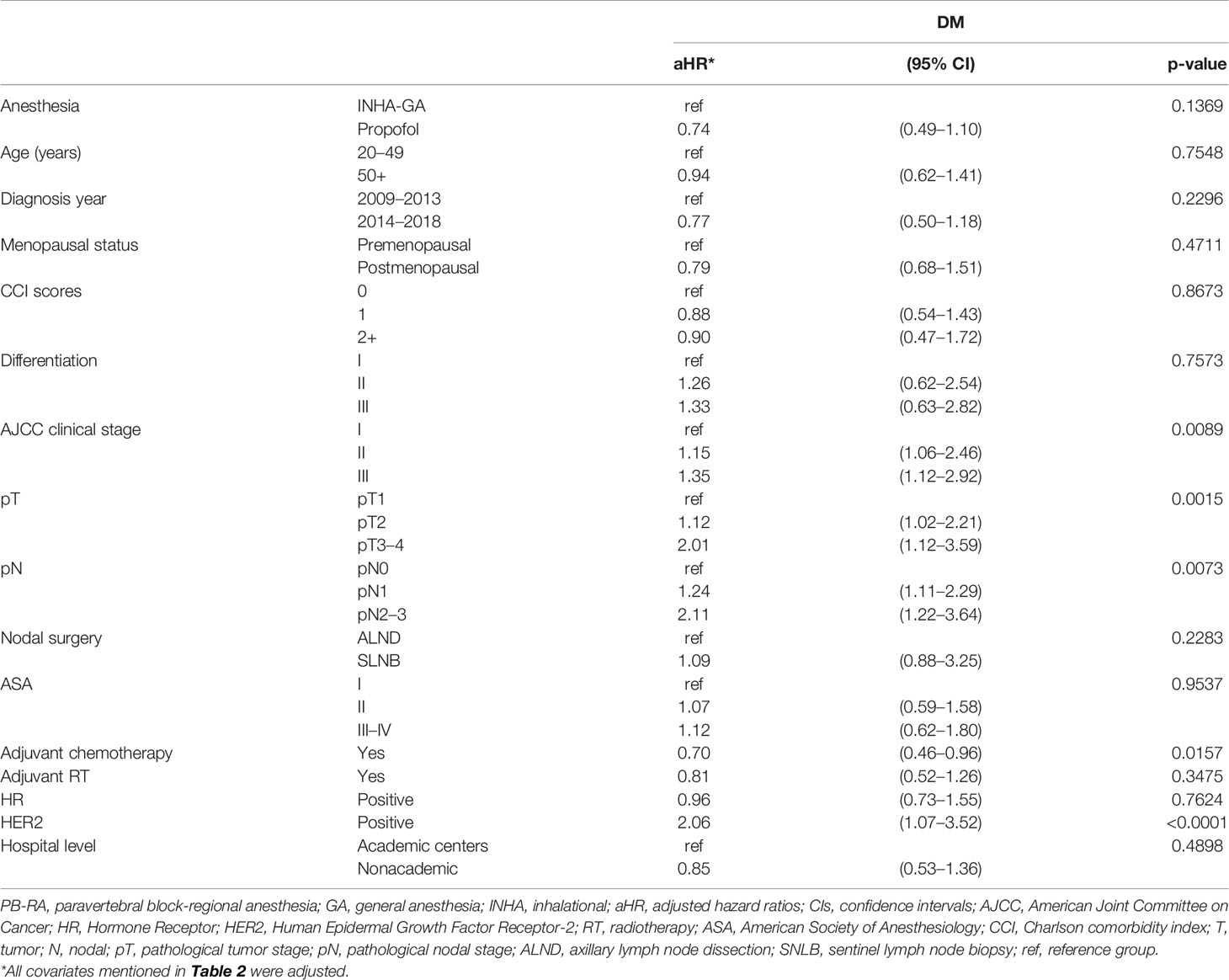
Table 4 Multivariate analysis of distant metastasis for propensity score-matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol.
Discussion
Most existing clinical studies were retrospective in nature or included a small sample, and meta-analyses included heterogeneous cancers, surgical techniques, patient populations, and follow-up (8, 9, 11, 16). Multiple factors can be responsible for differences in study findings; for instance, the characteristics and treatments varied among patients with breast IDC in clinical studies, whereas fixed conditions were examined in preclinical studies (22–26, 43–45). Factors affecting breast cancer prognosis are diverse and complex (43–45). For example, adjuvant chemotherapy is indicated for women with advanced pathological stages of breast IDC receiving breast surgery (46, 47); however, no adjuvant chemotherapy was administered in preclinical studies (1–7). Clinical covariates including molecular status (HR or HER2 status) and adjuvant treatment (adjuvant RT or chemotherapy) might result in inconsistent findings in preclinical and clinical studies (1–7, 22–26, 43–45). The only published randomized controlled trial (RCT) including breast-conserving surgery (BCS) or TM for breast cancer showed that the administration of INHA-GA without propofol or PB-RA with propofol exerted no effect on the primary endpoint of cancer recurrence including LRR and DM in patients with breast cancer (20). Moreover, the findings of this RCT are different from those of preclinical studies (1–7). Thus, to address these problems, we included LRR as the primary endpoint and performed PSM to control for all potential covariates in this study with the consistent surgical procedure.
The novelty of our study is the inclusion of LRR as the primary endpoint. No study has included LRR as a study endpoint. We controlled for all the potential covariates of LRR (Table 1) and observed no bias between the INHA-GA without propofol and PB-RA with propofol groups through PSM. Additionally, the various extent of surgery might be associated with different hypoxia time related with local recurrence (48, 49). Thus, in our study we maintain a consistent surgical procedure (all patients receiving TM) for breast IDC patients. Our results revealed that patients with breast IDC receiving TM under PB-RA with propofol had a significantly decreased risk of LRR compared with those receiving TM under INHA-GA (sevoflurane) without propofol (Table 3). A similar benefit was not observed for OS, possibly because adjuvant treatments might have masked the benefits of PB-RA with propofol; studies with longer follow-up duration should be conducted to examine the effect on OS. In addition, the proportion of patients who developed LRR in our study was small (3.8% and 6.2% for non-propofol and propofol groups, respectively); a larger sample size would be necessary to examine OS. However, our study is the first to investigate the effect of the administration of INHA-GA without propofol or PB-RA with propofol on LRR in patients with breast IDC receiving TM. Our findings for LRR are different from those reported by Sessler et al. who included DM and LRR together to examine cancer recurrence (20). Moreover, to maintain a consistent extent of surgery, we enrolled patients who received TM only and matched them at a ratio of 1:1 by using the greedy method (Table 1). In theory, the consistent time and the same extent of surgery related with similar levels of hypoxia (49) could be more consistent between the two anesthesia techniques in our study than Sessler et al.’s study (20). Tissue hypoxia causes an upregulated expression of the transcription factor hypoxia-inducible factor 1-alpha, which is crucial for the promotion of cellular pathways for angiogenesis, cell proliferation, and metastasis (48). Moreover, preclinical studies have reported that propofol exhibits the anticancer property by exerting an immune effect (4, 50, 51). Patients receiving PB-RA with propofol demonstrated an increased level of immune cell infiltration into the breast cancer tissue, an increased level of cancer cell apoptosis, and preserved cytotoxicity of natural killer cells (4, 50, 51). The advantages of PB-RA with propofol observed in preclinical studies were reproduced in our clinical study through head-to-head PSM. Our clinical study indicated differentiation grade II–III, clinical stage II–III, pT2, pT3–4, and pN2–3 as independent poor prognostic factors of LRR; this finding is compatible with those of previous clinical studies (22–26) Adjuvant RT reduced the risk of LRR in patients with breast IDC receiving TM (Table 3); this result is also in agreement with that of a previous clinical study (52).
In our study, we examined OS (the secondary endpoint) in patients with breast IDC receiving TM under INHA-GA without propofol and PB-RA with propofol (Table 2). We observed that the administration of INHA-GA without propofol or PB-RA with propofol did not exert any effect on the OS of these patients; this finding is compatible with those of previous clinical studies (Table 2) (8, 11, 53). All existing studies examining the endpoint of OS were retrospective in nature and included a small sample size, heterogeneous cancers, various surgical techniques, different patient populations, and short follow-up durations (8, 9, 11, 16). A meta-analysis conducted in 2014 found no difference in OS among patients with breast, prostate, colon, and gastroesophageal cancers who received general epidural anesthesia versus GA alone (8). Similarly, in 2017, a meta-analysis of 28 studies (retrospective, observational, and randomized) reported that OS was similar in patients with various cancers who underwent surgery under RA with or without GA and those who underwent surgery under GA alone (11). A meta-analysis of 10 retrospective studies including approximately 13,760 patients who underwent radical prostatectomy for cancer found that RA with or without GA was associated with improved OS but similar cancer recurrence compared with GA alone (9). Furthermore, a meta-analysis suggested that RA was associated with improved OS, particularly in patients with colorectal cancer, as well as a reduced risk of cancer recurrence (10). Therefore, conflicting results have been reported in clinical studies including different cancer types, extents of surgery, and adjuvant treatments (8–11, 16). The inconsistency in the results of clinical and preclinical studies might be attributed to the use of different therapeutic modalities, such as adjuvant chemotherapy, hormone therapy, and tyrosine kinase inhibitors, and different surgical procedures, which might have masked the effects of different anesthesia techniques (RA with propofol or sevoflurane-based INHA-GA) on patients’ OS (22–26, 43–45). By contrast, the findings of multivariate analysis performed in our study indicated that old age, moderate-poor differentiation (grade II–III) (54), clinical stage II–III, pT2, pT3–4, pN1, and pN2–3 were independent poor prognostic factors for all-cause death; this finding is compatible with those of previous clinical studies (20, 22–26). Adjuvant chemotherapy was associated with better OS in patients with breast IDC receiving TM (Table 2); this finding is also in accordance with those of previous clinical studies (46, 47, 52). Because the trend of oncological outcomes and prognostic factors for OS in our study was similar to that reported in other studies (20, 22–26, 46, 47, 52, 54–56), the effect of the administration of INHA-GA without propofol or PB-RA with propofol on oncological outcomes (OS, LRR, and DM) in patients with IDC receiving TM might truly exist in real-world clinical practice, although clinical outcomes might vary for different molecular breast types, adjuvant treatments, or extents of surgery. In the current study, most confounding factors like molecular breast types, adjuvant treatments, or extents of surgery (BCS or TM) were consistent or adjusted in our analysis.
As shown in Table 4, we observed that the risk of DM was not associated with the administration of INHA-GA without propofol or PB-RA with propofol in patients with IDC receiving TM; this finding differs from those of previous preclinical studies (1–7). Although many preclinical studies have reported that volatile anesthetics can enhance metastasis, such as by exerting direct survival-enhancing effects on cancer cells, suppressing immune cell functions, and killing tumor cells (2–4, 51), no association of DM with the administration of INHA-GA without propofol or PB-RA with propofol in patients with breast IDC receiving TM was observed in our clinical study. In laboratory studies, propofol exhibited antitumor effects by directly regulating key ribonucleic acid pathways and signaling in cancer cells (7). In addition, propofol exerts anti-inflammatory and antioxidative effects (1, 6, 50), which may protect against perioperative immune suppression. Although many preclinical studies have shown that propofol might inhibit cancer metastasis and INHA-GA can enhance cancer metastasis (1–4, 6, 50, 51), these phenomena were not observed in our study (Table 4). This difference might be attributed to the use of different adjuvant treatments and the inclusion of various breast cancer molecular types that might have obscured the effects of propofol and sevoflurane (43–45). However, other independent poor or better prognostic factors such as clinical stage II–III, pT2, pT3–4, pN2–3, HER2 positivity, and adjuvant chemotherapy determined in this study are compatible with those observed in previous clinical studies (22–26). Supplementary Figures 1A–C present survival curves for OS and LRR-free and DM-free survival obtained using the Kaplan–Meier method for the propensity score-matched cohort of patients with breast IDC receiving TM under PB-RA with propofol or INHA-GA without propofol. The crude LRR-free survival without adjustment for PB-RA with propofol was not significantly longer than that for INHA-GA without propofol for all patients with breast IDC receiving TM (p = 0.1430).
The strength of our study is that this is the first and largest cohort study to estimate the primary endpoint of LRR for patients with breast IDC receiving TM under INHA-GA without propofol and PB-RA with propofol. The covariates between the two anesthesia techniques were homogenous for women with breast IDC receiving TM; no selection bias was observed for the two anesthesia techniques through PSM (Table 1). No study has examined the effect of PB-RA with propofol on LRR in patients with breast cancer receiving TM, and all prognostic factors including clinical and pathologic stages and molecular types were evaluated. Poor prognostic factors for OS, LRR, or DM determined in patients with breast cancer receiving TM in the present study, namely, moderate-poor differentiation, advanced clinical stages, advanced pathologic TN stages, HER2 positivity, adjuvant RT, and adjuvant chemotherapy (Tables 2–4), are similar to those reported in previous studies (57–61). In patients with breast IDC receiving TM, adjuvant RT reduced the risk of LRR and adjuvant chemotherapy reduced the risk of DM. However, PB-RA with propofol in patients with breast IDC receiving TM was beneficial only for LRR instead of all-cause death and DM. This is the first study to show that PB-RA with propofol reduced the risk of LRR. Previous studies did not focus on recurrence; thus, LRR and DM could not be distinguished (20, 57–65). Our study is the first to examine the effects of INHA-GA without propofol or PB-RA with propofol on LRR and DM individually instead of breast cancer recurrence including LRR and DM. Our findings should be considered in future clinical practice and prospective clinical trials.
This study has some limitations. First, because all patients with breast IDC were enrolled from an Asian population, the corresponding ethnic susceptibility compared with the non-Asian population remains unclear; hence, our results should be cautiously extrapolated to non-Asian populations. However, no evidence has demonstrated differences in oncological outcomes between Asian and non-Asian patients with breast IDC receiving TM. Second, recently, the propensity score could be currently recommended as a standard tool for investigators trying to estimate the effects of intervention in studies where any potential bias may exist. Although the main advantage of the propensity score methodology is in its contribution to the more precise estimation of intervention response, PSM cannot control for factors not accounted for in the model and is predicated on an [explicit selection bias] of those whom could be a match (i.e., those who could not be matched are not part of the scope of inference). Third, the diagnoses of all comorbid conditions were based on ICD-9-CM codes. Nevertheless, the Taiwan Cancer Registry Administration randomly reviews charts and interviews patients to verify the accuracy of diagnoses, and hospitals with outlier chargers or practices may be audited and subsequently be heavily penalized if malpractice or discrepancies are identified. Accordingly, to obtain crucial information regarding population specificity and disease occurrence, a large-scale randomized trial comparing carefully selected patients undergoing suitable extent of surgery, consistent molecular types, and treatments is essential. Finally, the Taiwan Cancer Registry database does not contain information regarding dietary habits, lifestyle factors, socioeconomic status, or body mass index, all of which may be risk factors for LRR or mortality. However, considering the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to affect the conclusions.
Conclusions
PB-RA with propofol might be beneficial in reducing LRR in women with breast IDC receiving TM compared with INHA-GA without non-propofol. INHA-GA without propofol or PB-RA with propofol was not associated with the risk of OS or DM in patients with breast IDC receiving TM.
Data Availability Statement
The datasets presented in this article are not readily available because we used data from the National Health Insurance Research Database (NHIRD) and Taiwan Cancer Registry database. The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data utilized in this study cannot be made available in the manuscript, the Supplementary Material, or in a public repository due to the “Personal Information Protection Act” executed by Taiwan’s government, starting from 2012. Requests for data can be sent as a formal proposal to obtain approval from the ethics review committee of the appropriate governmental department in Taiwan. Specifically, links regarding contact info for which data requests may be sent to are as follows: http://nhird.nhri.org.tw/en/Data_Subsets.html#S3 and http://nhis.nhri.org.tw/point.html. Requests to access the datasets should be directed to http://nhird.nhri.org.tw/en/Data_Subsets.html#S3 and http://nhis.nhri.org.tw/point.html.
Ethics Statement
Our study protocols were reviewed and approved by the Institutional Review Board of Tzu-Chi Medical Foundation (IRB109-015-B). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception and Design: JZ, C-LC, C-YL, H-MC, and S-YW. Collection and Assembly of Data: H-MC and S-YW. Data Analysis and Interpretation: JZ, C-LC, and S-YW. Administrative Support: S-YW. Manuscript Writing: JZ, C-LC, and S-YW. Final Approval of Manuscript: All authors. All authors contributed to the article and approved the submitted version.
Funding
Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908, 10909, 11001, 11002, 11003, 11006, and 11013).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.708632/full#supplementary-material
Supplementary Figure 1 | (A) Kaplan–Meier overall survival curves of propensity score–matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol. (B) Kaplan–Meier locoregional recurrence-free survival curves of propensity score–matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol. (C) Kaplan–Meier distant metastasis-free survival curves of propensity score–matched patients with breast cancer receiving total mastectomy under PB-RA with propofol or INHA-GA without propofol.
Abbreviations
OS, overall survival; LRR, locoregional recurrence; DM, distant metastasis; IDC, invasive ductal carcinoma; TM, total mastectomy; PB-RA, paravertebral block-regional anesthesia; GA, general anesthesia; INHA, inhalational; HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; RCT, randomized controlled trial; PSM, propensity score matching; TCRD, Taiwan Cancer Registry database; SD, standard deviation; AJCC, American Joint Committee on Cancer; HR, Hormone Receptor; HER2, Human Epidermal Growth Factor Receptor-2; RT, radiotherapy; ASA, American Society of Anesthesiology; CCI, Charlson comorbidity index; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; TNM, Tumor, Node, and Metastasis; T, tumor; N, nodal; pT, pathological tumor stage; pN, pathological nodal stage; NCCN, National Comprehensive Cancer Network; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
References
1. Baki ED, Aldemir M, Kokulu S, Koca HB, Ela Y, Sivaci RG, et al. Comparison of the Effects of Desflurane and Propofol Anesthesia on the Inflammatory Response and S100beta Protein During Coronary Artery Bypass Grafting. Inflammation (2013) 36:1327–33. doi: 10.1007/s10753-013-9671-6
2. Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, et al. Isoflurane, a Commonly Used Volatile Anesthetic, Enhances Renal Cancer Growth and Malignant Potential via the Hypoxia-Inducible Factor Cellular Signaling Pathway In Vitro. Anesthesiology (2013) 119:593–605. doi: 10.1097/ALN.0b013e31829e47fd
3. Ecimovic P, Mchugh B, Murray D, Doran P, Buggy DJ. Effects of Sevoflurane on Breast Cancer Cell Function In Vitro. Anticancer Res (2013) 33:4255–60.
4. Buckley A, Mcquaid S, Johnson P, Buggy DJ. Effect of Anaesthetic Technique on the Natural Killer Cell Anti-Tumour Activity of Serum From Women Undergoing Breast Cancer Surgery: A Pilot Study. Br J Anaesth (2014) 113 Suppl 1:i56–62. doi: 10.1093/bja/aeu200
5. Chen Y, Liang M, Zhu Y, Zhou D. The Effect of Propofol and Sevoflurane on the Perioperative Immunity in Patients Under Laparoscopic Radical Resection of Colorectal Cancer. Zhonghua Yi Xue Za Zhi (2015) 95:3440–4.
6. Kim R. Anesthetic Technique and Cancer Recurrence in Oncologic Surgery: Unraveling the Puzzle. Cancer Metastasis Rev (2017) 36:159–77. doi: 10.1007/s10555-016-9647-8
7. Jiang S, Liu Y, Huang L, Zhang F, Kang R. Effects of Propofol on Cancer Development and Chemotherapy: Potential Mechanisms. Eur J Pharmacol (2018) 831:46–51. doi: 10.1016/j.ejphar.2018.04.009
8. Pei L, Tan G, Wang L, Guo W, Xiao B, Gao X, et al. Comparison of Combined General-Epidural Anesthesia With General Anesthesia Effects on Survival and Cancer Recurrence: A Meta-Analysis of Retrospective and Prospective Studies. PloS One (2014) 9:e114667. doi: 10.1371/journal.pone.0114667
9. Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional Anesthesia/Analgesia and the Risk of Cancer Recurrence and Mortality After Prostatectomy: A Meta-Analysis. Pain Manag (2015) 5:387–95. doi: 10.2217/pmt.15.30
10. Weng M, Chen W, Hou W, Li L, Ding M, Miao C. The Effect of Neuraxial Anesthesia on Cancer Recurrence and Survival After Cancer Surgery: An Updated Meta-Analysis. Oncotarget (2016) 7:15262–73. doi: 10.18632/oncotarget.7683
11. Grandhi RK, Lee S, Abd-Elsayed A. The Relationship Between Regional Anesthesia and Cancer: A Metaanalysis. Ochsner J (2017) 17:345–61.
12. Rodgers A, Walker N, Schug S, Mckee A, Kehlet H, Van Zundert A, et al. Reduction of Postoperative Mortality and Morbidity With Epidural or Spinal Anaesthesia: Results From Overview of Randomised Trials. BMJ (2000) 321:1493. doi: 10.1136/bmj.321.7275.1493
13. Urwin SC, Parker MJ, Griffiths R. General Versus Regional Anaesthesia for Hip Fracture Surgery: A Meta-Analysis of Randomized Trials. Br J Anaesth (2000) 84:450–5. doi: 10.1093/oxfordjournals.bja.a013468
14. Landoni G, Pisano A, Lomivorotov V, Alvaro G, Hajjar L, Paternoster G, et al. Randomized Evidence for Reduction of Perioperative Mortality: An Updated Consensus Process. J Cardiothorac Vasc Anesth (2017) 31:719–30. doi: 10.1053/j.jvca.2016.07.017
15. Ciechanowicz S, Zhao H, Chen Q, Cui J, Mi E, Mi E, et al. Differential Effects of Sevoflurane on the Metastatic Potential and Chemosensitivity of Non-Small-Cell Lung Adenocarcinoma and Renal Cell Carcinoma In Vitro. Br J Anaesth (2018) 120:368–75. doi: 10.1016/j.bja.2017.11.066
16. Iwasaki M, Zhao H, Jaffer T, Unwith S, Benzonana L, Lian Q, et al. Volatile Anaesthetics Enhance the Metastasis Related Cellular Signalling Including CXCR2 of Ovarian Cancer Cells. Oncotarget (2016) 7:26042–56. doi: 10.18632/oncotarget.8304
17. Hong B, Lee S, Kim Y, Lee M, Youn AM, Rhim H, et al. Anesthetics and Long-Term Survival After Cancer Surgery-Total Intravenous Versus Volatile Anesthesia: A Retrospective Study. BMC Anesthesiol (2019) 19:233. doi: 10.1186/s12871-019-0914-4
18. Li R, Huang Y, Lin J. Distinct Effects of General Anesthetics on Lung Metastasis Mediated by IL-6/JAK/STAT3 Pathway in Mouse Models. Nat Commun (2020) 11:642. doi: 10.1038/s41467-019-14065-6
19. Buggy DJ, Wall T. Can Anaesthetic-Analgesic Technique During Cancer Surgery of Curative Intent Influence Recurrence or Metastasis? An Update. Br J Anaesth (2019) 123:e525–6. doi: 10.1016/j.bja.2019.07.023
20. Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, et al. Recurrence of Breast Cancer After Regional or General Anaesthesia: A Randomised Controlled Trial. Lancet (2019) 394:1807–15. doi: 10.1016/S0140-6736(19)32313-X
21. Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B, Global Onco-Anesthesia Research Collaboration, G. Anesthetic Technique and Cancer Outcomes: A Meta-Analysis of Total Intravenous Versus Volatile Anesthesia. Can J Anaesth (2019) 66:546–61. doi: 10.1007/s12630-019-01330-x
22. Zhang J, Lu CY, Chen HM, Wu SY. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast With High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw Open (2021) 4:e211785. doi: 10.1001/jamanetworkopen.2021.1785
23. Zhang J, Lu CY, Chen CH, Chen HM, Wu SY. Effect of Pathologic Stages on Postmastectomy Radiation Therapy in Breast Cancer Receiving Neoadjuvant Chemotherapy and Total Mastectomy: A Cancer Database Analysis. Breast (2020) 54:70–8. doi: 10.1016/j.breast.2020.08.017
24. Zhang J, Lu CY, Chen HM, Wu SY. Pathologic Response Rates for Breast Cancer Stages as a Predictor of Outcomes in Patients Receiving Neoadjuvant Chemotherapy Followed by Breast-Conserving Surgery. Surg Oncol (2020) 36:91–8. doi: 10.1016/j.suronc.2020.11.015
25. Zhang J, Lu CY, Qin L, Chen HM, Wu SY. Breast-Conserving Surgery With or Without Irradiation in Women With Invasive Ductal Carcinoma of the Breast Receiving Preoperative Systemic Therapy: A Cohort Study. Breast (2020) 54:139–47. doi: 10.1016/j.breast.2020.09.010
26. Zhang J, Sun M, Chang E, Lu CY, Chen HM, Wu SY. Pathologic Response as Predictor of Recurrence, Metastasis, and Survival in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy and Total Mastectomy. Am J Cancer Res (2020) 10:3415–27. doi: 10.2139/ssrn.3680079
27. Zhang JQ, Lu CY, Qin L, Chen HM, Wu SY. Outcome of Post-Mastectomy Radiotherapy After Primary Systemic Treatment in Patients With Different Clinical Tumor and Nodal Stages of Breast Cancer: A Cohort Study. Am J Cancer Res (2020) 10:2185–98.
28. Pawa A, Wight J, Onwochei DN, Vargulescu R, Reed I, Chrisman L, et al. Combined Thoracic Paravertebral and Pectoral Nerve Blocks for Breast Surgery Under Sedation: A Prospective Observational Case Series. Anaesthesia (2018) 73:438–43. doi: 10.1111/anae.14213
29. Souron V, Delaunay L, Bonner F. Sedation With Target-Controlled Propofol Infusion During Shoulder Surgery Under Interscalene Brachial Plexus Block in the Sitting Position: Report of a Series of 140 Patients. Eur J Anaesthesiol (2005) 22:853–7. doi: 10.1017/S0265021505001444
30. Fragen RJ, Dunn KL. The Minimum Alveolar Concentration (MAC) of Sevoflurane With and Without Nitrous Oxide in Elderly Versus Young Adults. J Clin Anesth (1996) 8:352–6. doi: 10.1016/0952-8180(96)00082-7
31. Pei L, Zhou Y, Tan G, Mao F, Yang D, Guan J, et al. Ultrasound-Assisted Thoracic Paravertebral Block Reduces Intraoperative Opioid Requirement and Improves Analgesia After Breast Cancer Surgery: A Randomized, Controlled, Single-Center Trial. PloS One (2015) 10:e0142249. doi: 10.1371/journal.pone.0142249
32. Oncology, N C P G I. NCCN Clinical Practice Guidelines in Oncology [Online] (2020). 94 N Woodhull Rd, Huntington, NY 11743: Harborside Press, LLC. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (Accessed 2020).
33. Bahreini F, Soltanian AR, Mehdipour P. A Meta-Analysis on Concordance Between Immunohistochemistry (IHC) and Fluorescence in Situ Hybridization (FISH) to Detect HER2 Gene Overexpression in Breast Cancer. Breast Cancer (2015) 22:615–25. doi: 10.1007/s12282-014-0528-0
34. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol (2010) 28:2784–95. doi: 10.1200/JCO.2009.25.6529
35. Fehrenbacher L, Cecchini RS, Geyer CE Jr., Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J Clin Oncol (2020) 38:444–53. doi: 10.1200/JCO.19.01455
36. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a Combined Comorbidity Index. J Clin Epidemiol (1994) 47:1245–51. doi: 10.1016/0895-4356(94)90129-5
37. Chen JH, Yen YC, Yang HC, Liu SH, Yuan SP, Wu LL, et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Med (Baltimore) (2016) 95:e3268. doi: 10.1097/MD.0000000000003268
38. West DW, Satariano WA, Ragland DR, Hiatt RA. Comorbidity and Breast Cancer Survival: A Comparison Between Black and White Women. Ann Epidemiol (1996) 6:413–9. doi: 10.1016/S1047-2797(96)00096-8
39. Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An Electronic Application for Rapidly Calculating Charlson Comorbidity Score. BMC Cancer (2004) 4:94. doi: 10.1186/1471-2407-4-94
40. Austin PC. Optimal Caliper Widths for Propensity-Score Matching When Estimating Differences in Means and Differences in Proportions in Observational Studies. Pharm Stat (2011) 10:150–61. doi: 10.1002/pst.433
41. Austin PC. The Performance of Different Propensity Score Methods for Estimating Marginal Hazard Ratios. Stat Med (2013) 32:2837–49. doi: 10.1002/sim.5705
42. Austin PC. The Use of Propensity Score Methods With Survival or Time-to-Event Outcomes: Reporting Measures of Effect Similar to Those Used in Randomized Experiments. Stat Med (2014) 33:1242–58. doi: 10.1002/sim.5984
43. Yersal O, Barutca S. Biological Subtypes of Breast Cancer: Prognostic and Therapeutic Implications. World J Clin Oncol (2014) 5:412–24. doi: 10.5306/wjco.v5.i3.412
44. Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis (2018) 5:77–106. doi: 10.1016/j.gendis.2018.05.001
45. Wu S, Zhu W, Thompson P, Hannun YA. Evaluating Intrinsic and non-Intrinsic Cancer Risk Factors. Nat Commun (2018) 9:3490. doi: 10.1038/s41467-018-05467-z
46. Bonadonna G, Zambetti M, Valagussa P. Sequential or Alternating Doxorubicin and CMF Regimens in Breast Cancer With More Than Three Positive Nodes. Ten-Year Results. JAMA (1995) 273:542–7. doi: 10.1001/jama.1995.03520310040027
47. Recht A, Come SE, Henderson IC, Gelman RS, Silver B, Hayes DF, et al. The Sequencing of Chemotherapy and Radiation Therapy After Conservative Surgery for Early-Stage Breast Cancer. N Engl J Med (1996) 334:1356–61. doi: 10.1056/NEJM199605233342102
48. Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, et al. Prostate Cancer Cell Malignancy via Modulation of HIF-1alpha Pathway With Isoflurane and Propofol Alone and in Combination. Br J Cancer (2014) 111:1338–49. doi: 10.1038/bjc.2014.426
49. Manella G, Aviram R, Bolshette N, Muvkadi S, Golik M, Smith DF, et al. Hypoxia Induces a Time- and Tissue-Specific Response That Elicits Intertissue Circadian Clock Misalignment. Proc Natl Acad Sci USA (2020) 117:779–86.
50. Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential Effects of Serum From Patients Administered Distinct Anaesthetic Techniques on Apoptosis in Breast Cancer Cells In Vitro: A Pilot Study. Br J Anaesth (2014) 113 Suppl 1:i63–67. doi: 10.1093/bja/aet581
51. Desmond F, Mccormack J, Mulligan N, Stokes M, Buggy DJ. Effect of Anaesthetic Technique on Immune Cell Infiltration in Breast Cancer: A Follow-Up Pilot Analysis of a Prospective, Randomised, Investigator-Masked Study. Anticancer Res (2015) 35:1311–9.
52. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
53. Hasselager RP, Hallas J, Gogenur I. Inhalation or Total Intravenous Anaesthesia and Recurrence After Colorectal Cancer Surgery: A Propensity Score Matched Danish Registry-Based Study. Br J Anaesth (2021) 126:921–30. doi: 10.1016/j.bja.2020.11.019
54. Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical Nomogram for Predicting Survival Outcomes in Early Mucinous Breast Cancer. PloS One (2016) 11:e0164921. doi: 10.1371/journal.pone.0164921
55. Land LH, Dalton SO, Jorgensen TL, Ewertz M. Comorbidity and Survival After Early Breast Cancer. A Review. Crit Rev Oncol Hematol (2012) 81:196–205. doi: 10.1016/j.critrevonc.2011.03.001
56. Pares-Badell O, Banque M, Macia F, Castells X, Sala M. Impact of Comorbidity on Survival by Tumour Location: Breast, Colorectal and Lung Cance-2014). Cancer Epidemiol (2017) 49:66–74. doi: 10.1016/j.canep.2017.05.010
57. Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of Propofol-Based Total Intravenous Anesthesia on Recurrence and Overall Survival in Patients After Modified Radical Mastectomy: A Retrospective Study. Korean J Anesthesiol (2016) 69:126–32. doi: 10.4097/kjae.2016.69.2.126
58. Oh TK, Kim HH, Jeon YT. Retrospective Analysis of 1-Year Mortality After Gastric Cancer Surgery: Total Intravenous Anesthesia Versus Volatile Anesthesia. Acta Anaesthesiol Scand (2019) 63:1169–77. doi: 10.1111/aas.13414
59. Yoo S, Lee HB, Han W, Noh DY, Park SK, Kim WH, et al. Total Intravenous Anesthesia Versus Inhalation Anesthesia for Breast Cancer Surgery: A Retrospective Cohort Study. Anesthesiology (2019) 130:31–40. doi: 10.1097/ALN.0000000000002491
60. Enlund M, Berglund A, Ahlstrand R, Wallden J, Lundberg J, Warnberg F, et al. Survival After Primary Breast Cancer Surgery Following Propofol or Sevoflurane General Anesthesia-A Retrospective, Multicenter, Database Analysis of 6305 Swedish Patients. Acta Anaesthesiol Scand (2020) 64:1048–54. doi: 10.1111/aas.13644
61. Makito K, Matsui H, Fushimi K, Yasunaga H. Volatile Versus Total Intravenous Anesthesia for Cancer Prognosis in Patients Having Digestive Cancer Surgery. Anesthesiology (2020) 133:764–73. doi: 10.1097/ALN.0000000000003440
62. Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The Choice of Anaesthetic–Sevoflurane or Propofol–and Outcome From Cancer Surgery: A Retrospective Analysis. Ups J Med Sci (2014) 119:251–61. doi: 10.3109/03009734.2014.922649
63. Wigmore TJ, Mohammed K, Jhanji S. Long-Term Survival for Patients Undergoing Volatile Versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology (2016) 124:69–79. doi: 10.1097/ALN.0000000000000936
64. Oh TK, Kim K, Jheon S, Lee J, Do SH, Hwang JW, et al. Long-Term Oncologic Outcomes for Patients Undergoing Volatile Versus Intravenous Anesthesia for Non-Small Cell Lung Cancer Surgery: A Retrospective Propensity Matching Analysis. Cancer Control (2018) 25:1073274818775360. doi: 10.1177/1073274818775360
65. Lai HC, Lee MS, Lin C, Lin KT, Huang YH, Wong CS, et al. Propofol-Based Total Intravenous Anaesthesia Is Associated With Better Survival Than Desflurane Anaesthesia in Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study. Br J Anaesth (2019) 123:151–60. doi: 10.1016/j.bja.2019.04.057
Keywords: propofol, general anesthesia, survival, invasive ductal carcinoma, total mastectomy
Citation: Zhang J, Chang C-L, Lu C-Y, Chen H-M and Wu S-Y (2022) Anesthesia With Propofol Sedation Reduces Locoregional Recurrence in Patients With Breast Cancer Receiving Total Mastectomy Compared With Non-Propofol Anesthesia. Front. Oncol. 12:708632. doi: 10.3389/fonc.2022.708632
Received: 12 May 2021; Accepted: 07 February 2022;
Published: 03 March 2022.
Edited by:
Ryungsa Kim, Hiroshima Mark Clinic, JapanReviewed by:
Mohammad A. Y. Alqudah, Jordan University of Science and Technology, JordanSreejoyee Ghosh, University of Texas MD Anderson Cancer Center, United States
Copyright © 2022 Zhang, Chang, Lu, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Szu-Yuan Wu, c3p1eXVhbnd1NTM5OUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Jiaqiang Zhang
Jiaqiang Zhang Chia-Lun Chang2,3†
Chia-Lun Chang2,3† Szu-Yuan Wu
Szu-Yuan Wu