Abstract
Micro abstract:
Targeted intraoperative radiotherapy (TARGIT-IORT) is delivered immediately after lumpectomy for breast cancer. We estimated its impact. At least 44,752 patients with breast cancer were treated with TARGIT-IORT in 260 centres in 35 countries, saving >20 million miles of travel and preventing ~2,000 non–breast cancer deaths. The TARGIT-IORT website (https://targit.org.uk/travel) provides maps and tools to find the nearest centre offering TARGIT-IORT and travel savings.
Background:
Targeted intraoperative radiotherapy (TARGIT-IORT) delivers radiotherapy targeted to the fresh tumour bed exposed immediately after lumpectomy for breast cancer. TARGIT-A trial found TARGIT-IORT to be as effective as whole-breast radiotherapy, with significantly fewer deaths from non–breast cancer causes. This paper documents its worldwide impact and provides interactive tools for clinicians and patients.
Method:
Centres using TARGIT-IORT provided the date of the first case and the total number of patients. We plotted these data on a customised Google Map. An interactive web-based tool provided directions to the closest centre. Using the data from the TARGIT-A trial, we estimated the total savings in travel miles, carbon footprint, and the number of non–breast cancer deaths that might be prevented.
Results:
Data from 242 (93%) of the 260 centres treating patients from 35 countries were available. From the first patient treated in 1998 to early 2020, at least 44,752 women with breast cancer have been treated with TARGIT-IORT. The TARGIT-IORT website (https://targit.org.uk/travel) displays the Google Map of centres with number of cases and an interactive tool for patients to find the nearest centre offering TARGIT-IORT and their travel savings. Scaling up to the already treated patients, >20 million miles of travel would have been saved and about 2,000 deaths prevented.
Conclusion:
One can ascertain the number of patients treated with a novel treatment. These data show how widely TARGIT-IORT has now been adopted and gives an indication of its beneficial worldwide impact on a large number of women with breast cancer.
Clinical practice points
What is already known about this subject? Targeted intraoperative radiotherapy (TARGIT-IORT) delivers radiotherapy targeted to the fresh tumour bed exposed immediately after lumpectomy for breast cancer. TARGIT-A trial found TARGIT-IORT to be as effective as whole-breast radiotherapy, with significantly fewer deaths from non–breast cancer causes. This paper documents its worldwide impact and provides interactive tools for clinicians and patients.
What are the new findings? We ascertained that by early 2020, at least 44,752 women with breast cancer have been treated with TARGIT-IORT in 260 centres in 35 countries. We provide at the TARGIT-IORT website (https://targit.org.uk/travel) the Google Map of centres with number of cases and an interactive tool for patients to find the nearest centre offering TARGIT-IORT and their travel savings. We also estimated that, by now, >20 million miles of travel would have been saved and about 2,000 deaths prevented.
How might it impact on clinical practice in the foreseeable future? In addition to hard randomised evidence proving survival and quality of life benefits clinical practice is often prompted by seeing what our peers are doing. Dissemination of these data showing widespread adoption of the technique would further increase awareness and utilisation of this patient-centred approach amongst patients, clinicians, and policymakers.
Introduction
A large proportion of patients with small breast cancers can be effectively treated by a lumpectomy and radiotherapy, rather than a mastectomy. Radiotherapy is traditionally given to the whole breast.
In the mid-1990s, targeted intraoperative radiotherapy (TARGIT-IORT) (1–3) was proposed as a radical new approach. This treatment delivers effective radiotherapy targeted to the fresh tumour bed exposed immediately after lumpectomy (4, 5) while sparing nearby tissues and nearby vital organs such as the heart and lung.
In pilot studies starting from 2 July 1998, the safety and feasibility of this novel approach combining surgery and radiotherapy were confirmed (1–3), and the TARGIT-A randomised trial was proposed in 1999 (6) comparing risk-adapted single-dose TARGIT-IORT during lumpectomy vs. conventional fractionated whole-breast external beam radiotherapy (EBRT) given daily for several weeks (6–8).
Long-term outcomes of the TARGIT-A trial found it to be as effective in terms of breast cancer outcomes and that it led to fewer deaths from other causes (9). Further pre-planned subgroup analysis found that these results are valid for all invasive ductal carcinoma tumour subtypes; there is an overall survival benefit of 4.4% at 12 years in those with grade 1 or 2 tumours (n = 1,797) and identical overall survival in grade 3 cancers (n = 443) (10). Unlike the poor prognosis faced by patients who have a local recurrence after EBRT, those who receive TARGIT-IORT maintain their excellent prognosis even after local recurrence (10). Other benefits included lower radiation related toxicity (11–18), reduced pain, and better quality of life (17, 19–23). When given a choice, TARGIT-IORT is preferred by patients over other methods of radiotherapy or “no-radiotherapy” (24–30). An online tool can guide clinicians in decisions about additional whole-breast radiotherapy after TARGIT-IORT (https://targit.org.uk/addrt) (10).
The adoption of TARGIT-IORT for standard clinical practice has grown considerably over the last 20 years. In this short paper, to assess the worldwide impact of TARGIT-IORT, we aimed to count the number of patients treated with TARGIT-IORT around the world, as well as to estimate the total benefits to the patient, in terms of the saving of travel distance, time, and reduction of transport-related carbon footprint and reduced deaths from other causes.
The TARGIT-A trial was initiated by an academic insight and collaboration with the industry was solely for the development of the device. The study was sponsored by University College London Hospitals (UCLH)/UCL Comprehensive Biomedical Research Centre. Funding was provided by UCLH Charities, National Institute for Health Research (NIHR) Health Technology Assessment programme (HTA 07/60/49), Ninewells Cancer Campaign, National Health and Medical Research Council, and German Federal Ministry of Education and Research (BMBF) FKZ 01ZP0508. The infrastructure of the trial operations office in London, UK, was supported by core funding from Cancer Research Campaign (now Cancer Research UK) when the trial was initiated. In the extended follow-up of the TARGIT-A trial (TARGIT-Ex; funded by the HTA programme of the National Institute for Health Research, Department of Health and Social Care in the UK, HTA 14/49/13), we are continuing the follow up of TARGIT-A trial patients in the UK by direct patient contact and via UK national databases.
We are also currently inviting women who would fall outside the eligibility criteria of the TARGIT-A trial to participate in the TARGIT-B(oost) trial (funded by HTA 10/104/07), already opened in 38 centres internationally, which is comparing TARGIT-IORT as a tumour bed boost with EBRT boost in younger women or women who have higher risk disease to test for superiority in terms of local control and survival.
Method
Since the first case was performed in London in 1998, an international network has been developed between centres using TARGIT-IORT. Therefore, the contact details of a large proportion of the centres were available. Using Google forms and electronic communication, we requested the date when the first patient with breast cancer was treated with TARGIT-IORT at their centre and how many such patients were treated by their centre in total. We did not restrict this to those centres that had participated in the TARGIT trials. If, after repeated attempts, there was no response from a centre, then we included the name of the centre without the number of cases. We also queried the German National Database (https://www.destatis.de/) using the codes 8.52d, 8-523.6, and 8-521. Such databases were not available for other countries. We collected data until just before the COVID-19 pandemic started. Using Google My Maps, each hospital was displayed on an interactive map, showing the date of the first case and the total number of cases performed at the centre, along with directions to a chosen hospital.
In addition to avoiding the hospital visit required to plan radiotherapy, the large majority of patients (eight out of every 10) who received TARGIT-IORT would avoid 15 to 30 daily trips to the hospital where they would have undergone conventional whole-breast radiotherapy. Therefore, we made an estimate of the total savings by the patient—in terms of travel miles, travel time, and carbon footprint, using the methodology described previously (31). Our previous work (31) had found that patients in the TARGIT-A trial, mostly from urban areas in the UK, saved an average of 305 miles of travel, whereas those in semi-urban areas saved 753 miles. This calculation was based on the total number of hospital trips the patients saved when they were randomised to the TARGIT-IORT arm compared with the EBRT arm in the randomised TARGIT-A trial. The distance travelled for each trip was individually calculated by inputting in Google Maps API, the addresses of the patient, and the treating hospital where the EBRT was given. The total miles saved were used to calculate the amount of CO2 saved using standard emissions for a medium sized car. This estimate takes into account the additional travel required in the 20% of patients who are recommended whole-breast EBRT. It has been estimated that 55% of the world population lived in urban areas in 2018 (32). In this paper, we used the UK figures for travel savings and assumed a larger proportion of patients (66% rather than 55%) will be urban dwellers. We prepared an interactive web application to make individual estimates. These tools were tested by patients, and their feedback was used for making improvements.
Long-term results of the TARGIT-A trial (9) (Supplementary Figure 1) found no difference any breast cancer outcome or breast cancer–specific mortality but a significant reduction in non–breast cancer mortality (HR, 0.59; 95% CI, 0.40 to 0.86; P = 0.005) such that it was 5.41% for TARGIT-IORT and 9.85% for EBRT. The difference was 4.44% (95% CI of the difference being 2.5% to 6.4%). This estimate is consistent with that of overall survival improvement in patients with grade 1 and grade 2 cancers that formed a large subgroup of patients in the trial contributing 1,796 out of the total of 2,298. In a pre-specified subgroup analysis (with its usual caveats), overall survival was significantly better in this subgroup by 4.4% (HR, 0.72; p = 0.0361). We used this absolute difference in deaths, i.e., 4.4 fewer deaths over 12 years per 100 patients treated, to estimate the global impact of using TARGIT-IORT in terms of mortality reduction amongst patients already treated around the world.
We used STATA 16 for statistical analysis.
Results
Data from 242 (93%) of the 260 centres were available. Data from 31 of 64 centres (n = 8021) in Germany were available directly from investigators and the remaining 33 (n = 8044) from the German national database. Of these 260 centres, 33 had participated in the TARGIT-A trial.
The first patient with breast cancer was treated with TARGIT-IORT on 2 July 1998 at the Middlesex hospital (now part of University College London Hospitals), University College London. Since then, we found that TARGIT-IORT has been used in 35 countries, and at least 44,752 patients with breast cancer have been treated (Table 1). The total numbers of patients known to have been treated are approximately 30,000 in Europe, 9,000 in North America, 3,000 in Asia Pacific, 2,000 in South/Central America, 500 in the Middle East, and 200 in Africa. Table 2 has the list the collaborating centres.
Table 1
| a) Number of centres per country and region | |||
|---|---|---|---|
| Region | Country | Number of centres | Centres from where the number of patients is available |
| Africa | South Africa | 1 | 1 |
| Africa Total | 1 | 1 | |
| Asia and Pacific | Australia | 3 | 3 |
| China | 13 | 13 | |
| India | 2 | 2 | |
| Malaysia | 4 | 4 | |
| New Zealand | 1 | 1 | |
| Philippines | 1 | 1 | |
| Singapore | 1 | 1 | |
| South Korea | 1 | 1 | |
| Thailand | 1 | 1 | |
| Vietnam | 1 | 0 | |
| Asia and Pacific Total | 28 | 27 | |
| Europe | Austria | 1 | 1 |
| Belgium | 1 | 1 | |
| Bulgaria | 1 | 1 | |
| Denmark | 1 | 1 | |
| France | 12 | 12 | |
| Georgia | 1 | 1 | |
| Germany | 63 | 65 | |
| Israel | 9 | 9 | |
| Italy | 5 | 5 | |
| Norway | 1 | 1 | |
| Poland | 8 | 2 | |
| Russia | 12 | 3 | |
| Spain | 3 | 3 | |
| Switzerland | 6 | 6 | |
| Turkey | 4 | 2 | |
| United Kingdom | 11 | 11 | |
| Europe Total | 140 | 124 | |
| Middle East | Iran | 2 | 2 |
| Saudi Arabia | 3 | 3 | |
| Middle East Total | 5 | 5 | |
| North America | Canada | 2 | 2 |
| USA | 72 | 71 | |
| North America Total | 74 | 73 | |
| South/Central America | Brazil | 4 | 4 |
| Mexico | 3 | 3 | |
| Peru | 2 | 2 | |
| Venezuela | 3 | 3 | |
| South/Central America Total | 12 | 12 | |
| Grand Total | 260 | 242 | |
| b) Number of patients treated per region | |||
| Region | Number of patients treated | ||
| Africa | 179 | ||
| Asia pacific | 2,803 | ||
| Europe | 29,716 | ||
| Middle East | 1,009 | ||
| North America | 9,019 | ||
| South America | 2,026 | ||
| Total | 44,752 | ||
a) Number of centres that have treated patients with breast cancer with TARGIT-IORT around the world and b) number of patients treated in each of the world regions.
Since the initial submission, there is another country to this list: Argentina.
Table 2
| Centre | Contributors |
| University College London Hospital, London, UK | Jayant S. Vaidya, Max Bulsara, Michael Baum, Jeffrey S. Tobias, Chris Brew-Graves, Ingrid Potyka, Nick Roberts, Norman Williams |
| Sir Charles Gairdner Hospital, Perth, WA, Australia | Christobel Saunders, Tammy Corica, David Joseph |
| University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Germany | Elena Sperk, Marc Sutterlin, Frederik Wenz |
| Centro di Riferimento Oncologico, Aviano, Italy | Samuele Massarut, Lorenzo Vinante |
| Peter Mac Centre, Melbourne, Australia | Boon Chua |
| Ninewells Hospital, Dundee, Scotland, UK | Douglas Brown, Julie Lindsay |
| Klinikum der Johann-Wolfgang Goethe-Universität, Frankfurt 60596, Germany | Claus Rödel, Manfred Kaufmann |
| UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA | Michael Alvarado, Jane Wei |
| Technical University Munich and Red Cross Munich, Germany | Steffi Pigorsch, Christian Diehl |
| University of Southern California, USC, USA | Dennis Holmes |
| Department of Surgical Oncology, Medical University of Lublin, Lublin, Poland | Wojciech Polkowski |
| Ospedale San Giuseppe di Empoli, Viale Giovanni Boccaccio, 16, 50053 Empoli FI, Italy | Claudio Caponi |
| Sankt Gertrauden-Krankenhaus, and The Charité – Universitätsmedizin Berlin, Berlin, Germany | Jens Blohmer, Volker Budach, Dirk Böhmer |
| Ludwig Maximilian University of Munich, Munich, Germany | Montserrat Pazos, Claus Belka, Nadia Harbeck |
| Herlev Hospital, Copenhagen, Denmark | Henrik Flyger |
| Princess Margaret Cancer Center, Toronto, Canada | David McCready, Jaime Escallon |
| Royal Hampshire County Hospital, Winchester, UK | Siobhan Laws, Dick Rainsbury, Ajay Raj |
| Radiotherapie Hirslanden, Brust-Zentrum Seefeld, Zurich, Switzerland | Gunther Gruber, Barbara Papassotiropoulos, Christoph Tausch |
| Lafayette Surgical Clinic, 1345 Unity Pl #235, Lafayette, IN 47905, USA | Thomas Summer |
| Royal Free Hospital, Hampstead and Whittington Hospital, London, UK | Tim Davidson, Mohammed Keshtgar, Jayant S. Vaidya, Katharine Piggott |
| Sentara Surgery Specialists, Hampton, USA | Richard Hoefer, Song Kang |
| Saarland University Medical Center, Homburg, Germany | Marcus Niewald |
| University Hospital of Zurich, Switzerland | Konstantin Dedes |
| University of Science and Technology (NTNU) Trondheim, Norway | Steinar Lundgren |
| University of Nebraska Medical Center, Buffet Cancer Center, S 42nd St and Emile St, Omaha, NE 68198, USA | Deborah Spence, James Edney |
| Guy’s Hospital, London, UK (now at Oxford University Hospital) | Michael Douek, Joyce Akinwale |
| Ashikari Breast Center, St. Johns Riverside, Dobbs Ferry, NY, USA | Pond Kelemen, Andrew Ashikari |
| Vassar Brothers Medical Center, Poughkeepsie, NY, USA | Daniel Lackaye, Dan Pavord, William Rausch, Dimitrios Papadopoulous, Camilo Torres |
| Institute de Cancerologie de l’Ouest René Gauducheau, Nante, France | Magali Le-Blanc-Onfroy |
| Medical School Hannover, Germany | Michael Bremer, Park-Simon, Tjoung-Won |
| Instituto Oncologico Veneto, Padoa, Italy | Fernando Bozza, Franco Berti, Silvia Michieletto |
| Institut Bergonié, Bordeaux France | Beatrice Gonzalves, Christel Breton-callu, Adeline Petit |
| St John and St Elizabeth Hospital, London, UK | Mohammed Keshtgar |
| Whittington Hospital, London, UK | Jayant S. Vaidya, Jeffrey S. Tobias |
| CHU Morvan, Brest, France | Pierre Francoise Dupre, Pradier Olivier, Chajara Abdesslam, Sarah Quillevéré |
| Beverley Hill Cancer Centre (Helen Rey), CA 90210, USA | Dennis Holmes |
| Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia | Maha Abdel Hadi |
| Centre Léon Bérard, 28 Prom. Léa and Napoléon Bullukian, 69008 Lyon, France | Severine Racadot, Jean-Noel Badel |
| Princess Grace Hospital, London, UK | Jayant S. Vaidya, Jeffrey S. Tobias |
| Center Georges-François Leclerc - Dijon, France | Etienne Martin, Charles Coutant, Karine Peignaux-Casasnovas, Magali Rouffiac, Gilles Truc, Fabienne Bidault, Mathieu Gonod |
| Memorial University Medical Center, Savannah, GA, USA | Aaron Pederson, William Burak |
| Universidad Fernando Pessoa Canarias. Hospital de Gran Canaria Dr Negrín, Gran Canaria, Spain | Pedro Lara, Beatriz Pinar Sedeño |
| CLEVELAND CLINIC FOUNDATION, Cleveland, OH, USA | Stephanie Valente, Sheen Cherian, Stephen Grobmyer |
| Princess Alexandra Hospital, Harlow, UK | Julian Singer, Ashraf Patel, Veronica Grassi, Bijan Ansarimohabadian |
| Gangnam Severance Hospital, Yonsei University, Seoul | Joon Jeong |
| Aurora Baycare Medical Centre, Green Bay, WI, USA | William Owens |
| Institut Universitaire du Cancer de Toulouse Oncopole, Centre Claudius Regaud, Toulouse, France | Izar Francoise |
| Institut Catalan de Oncología. Hospital de bellvitge, Hospital Duran i Reynals, Avinguda de la Gran Via de l’Hospitalet, 199-203, 08908 L’Hospitalet de Llobregat, Barcelona, Spain | Ferran Gueda, Arancha Eraso, Evelyn Martinez, Maria Laplana, Maria Jesus Pla, Pablo Saldaña, Roberto Martín Vaello |
| Great Western Hospital, Swindon, UK | Nathan Coombs, Shiroma DeSliva Minor, David Dommett |
| Morgantown, Health Sciences Centre, WV, USA | Geraldine Jacobson |
| Centre Hospitalier Universitaire (APHM CHU Nord and Hopital de la Timone), Marseille, France | Didier Cowen, Jean Baptiste Haumonte, Aubert Agostini, Corinne Aquaron, Natacha Nomikossoff |
| Beijing Cancer Hospital(2), No. 52 Fucheng Road, Haidian District, Beijing (Ding Hui Temple), China | Xinguang Wang, Chang Cheng |
| University Malaya Medical Centre (UMMC), Kuala Lumpur, Malaysia | Nur Aishah Mohd Taib, See Mee Hoong, Suniza Jamaris, Teh Mei Sze, Teoh Li Ying, Marniza Saad, Anita Zarina Bustam, Rozita Abdul Malik, Nur Fadhlina Abdul Satar |
| Centre François Bâclasse, Caen, Normandy, France | Serge S. Danhier, Julien Geffrelot, Alain Batalla, Jean Francoise Le Brun, Sandrine Martin-Francoise, Helen Planque |
| William Beaumont Hospital, Detroit, MI, USA | Nayana Dekhne, Peter Chen, Blerina Pople |
| Lakeland Health, St Joseph, MI, USA | Benjamin T. Gielda |
| Queen Sirikit Centre for Breast Cancer, King Chulalongkorn Memorial Hospital, Bangkok, Thailand | Kris Chatamara, Adhisabandh Chulakadabba, Sikrit Denariyakoon |
| Gauteng, Netcare Milpark Hospital, South Africa | Carol Benn, Yastira Ramdas |
| Rest of German centres (not all are listed) have treated a total of 7,853 patients with breast cancer | |
| New York Medical College, NY, USA | Basil Hilaris |
| Maria Skłodowska-Curie Memorial Cancer Centre and Institute of Oncology (MSCNRIO) Gliwice branch, Gliwice, Poland | Jerzy Wydmański, Żaneta Kaniszewska-Dorsz, Andrzej Tukiendorf |
| Summit Hospital (Oncologics), Baton Rouge, LA, USA | John Head, Bob Elliot |
| Carmel Medical Center, Haifa, Israel | Mariana Steiner |
| Klinikum Augsburg, University Medical Center Augsburg, Germany | Henning Kahl |
| Casa di Cura Quisisana, Rome, Italy | Stefano Drago |
| University of Regensburg Radiotherapy, Caritas - Krankenhaus St. Josef’, Germany | Oliver Kölbl |
| Klinik Hirslanden, Spital Männedorf, Männedorf, Switzerland | Gunther Gruber, Barbara Papassotiropoulos, Christoph Tausch |
| Mammazentrum, Krankenhaus Jerusalem, Moorkamp 2-6, Hamburg 20357, Germany | Florian Würschmidt (Radiologische Allianz Hamburg), Kay Friedrichs |
| Diakonie Klinikum Hamburg, Hamburg 20259, Germany | Florian Würschmidt (Radiologische Allianz Hamburg), Christoph Lindner |
| Renaissance Surgical Memorial Care Pacific Breast Care Center, Costa Mesa, CA, USA | Alice Police |
| Klinikum St. Marien Amberg, Amberg 92224, Germany | Hipp Matthias, Klaus Graaf, Tanja Eberl, Thomas Papathemelis, Tanja Hauzenberger, Anton Scharl |
| Klinikum Nürnberg Nord, Klinik für Frauenheilkunde und Geburtshilfe Universitätsklinik der Paracelsus Medizinischen Privatuniversität | Cosima Brucker |
| Indo-American Cancer Institute, Hyderabad, India | Sushila Narayan, Mohan Vamsy |
| Oregon Health Science University, Portland, OR, USA | Susha Pillai, Arpana Naik |
| University of Florida, Gainesville, FL, USA | Lisa Spiguel, Paul Okunieff, Natalie A Lockney, Jian Wu, Chihray Liu |
| Institute for Breast Diseases, Fucam Hospital, Mexico City, Mexico | Antonio Maffuz-Azis, Sergio Rodrigez-Cuevas, Judith Huerta-Bahena, Carlos Alberto Dominguez-Reyes, Jorge Anselmo Vazquez-Reyes |
| Marienhospital Bottrop, Josef-Albers-Straße 70, 46236 Bottrop, Germany | Hans-Christian Kolberg |
| University of Cologne, Faculty of Medicine and University Hospital of Cologne, Germany | Wolfram Malter, Stefan Krämer, Peter Mallmann, Karolina Jablonska, Wolfgang Baus, Simone Marnitz |
| Trinity Medical Center, Birmingham, AL, USA | William Thompson |
| California Pacific Medical Center, San Francisco, CA, USA | John Lee, Terry Pierce |
| Vorarlberger Krankenhaus- betriebsges.mbH, Carinagasse 47, A-6807 Feldkirch, Austria | Rita Alton |
| Northern Westchester Hospital, Mount Kisco, NY, USA | Stephen Iorio |
| Klinikum Westfalen, Am Knappschaftskrankenhaus 1, 44309 Dortmund, Germany | Mohammed Yossof Karim-Payab, Heidemarie Tonscheidt Head, Frank Schmolling |
| King Abdulaziz University Hospital, Jeddha, Saudi Arabia | Yasir Bahadur |
| Northwestern University Hospital, 251 E Huron St, Chicago, IL 60611, USA | Eric Donnelly, Hualin Zhang |
| Moffitt Cancer Center, Tampa, FL, USA | Christine Laronga |
| Marien Hospital and St Barbara Klinik, Hamm Heessen GmbH | Jany Ralf, Hermann Wiebringhaus, Frank Holms, Thilo Vormann, Tobias Tan-Tjen, Norbert Lang |
| Kreiskrankenhaus Gummersbach, Klinik für Strahlentherapie, Wilhelm Breckow Allee 20, 51643 Gummersbach, Germany | Peter Vacha, Golamabu Zakaria, Magdolna Bajnok, Anja Weishap |
| Raheja Hospital, Mumbai, India | Sanjay Sharma |
| Klinikum Stuttgart - Katharinen Hospital, Germany | M. Münter, U. Köppen, N. Wegner, J. Schuster, A. Golle, S. Baumbach, S. Staubus, U. Karck |
| Klinikum St. Georg GmbH, Saxony, Leipzig, Germany | André Liebemann, Marion Hindemith, Susanne Miethe, Niels-Karsten Bär, Cornelius Walter, Uwe Köhler |
| Institut Regional du Cancer de Montpellier- ICM Val d’Aurelle, Montpellier, France | Claire Lemanski, David Azria, Marian Gutowski |
| Bay Area Cancer Physicians at Summit Medical Center, Oakland, CA, USA | Valery Uhl |
| Sutter Medical Center, Sacramento, USA | Jeannine Graves |
| Städtisches Klinikum Lüneburg, Lueneburg, Germany | Stefan Dinges, Eric Boetel |
| Brustzentrum Rhein-Kreis-Neuss, Johanna-Etienne-Krankenhaus Neuss, Germany | Georg Unruh, Susanne Coslar |
| Cornell University, New York, NY, USA | Alex Swistel, Samuel Trichter, John Ng |
| Hôpitaux Universitaire de Genève, Geneva, Switzerland | Pelagia Tsoutsou, Vincent Van Hung, Odile Fargier Bochaton, Thanh Giang Lam |
| Institut Paoli Calmettes, Marseille, France | Agnes Tallet, Gilles Houvenaeghel, Monique Cohen, Leonel Varela-Cagetti, Laurence Gonzague, Véronique Favrel, Marguerite Tyran, Pierre Annède, Eric Lambaudie, Sandrine Rua, Max Buttarrelli |
| Advocate Good Shepherd Hosp, Barrington, 1301 S Barrington Rd, Barrington, IL, USA | Barry Rosen, Brian Tom |
| Community Surgery Center North, 1550 East County Line Road, Indianapolis, IN 46227, USA | Susan Chace Lottich, Darrel Ross |
| University of Iowa Hospitals and Clinics, Iowa City, IA, USA | Timothy Waldron, Wenqung Sun, Allison W Lorenzen |
| Ammerlandklinik Westerstede, Germany | Robert M. Hermann |
| National Cancer Centre, 11 Hospital Drive, Chow, Singapore | Kong Wee Ong, Veronique K.M. Tan, Fuh Yong Wong, Eu Tiong Chua, Richard M.C. Yeo, Sue Zann Lim |
| Riyadh Military Hospital, Riyadh, Saudi Arabien | Esam Murshid, Marouf Adili |
| St. Louis Hospital, APHP, Paris, France | Christophe Hennequin |
| Specialist Center for Radiation Therapy and Laboratory Medicine, Steinbacher Hohl 2-26, 60488 Frankfurt am Main, Germany | Uta Kraus-Tiefenbacher, Volker Möbus |
| Littleton Adventist Hospital, Littleton, CO, USA | Darlene Bugoci, Ellen Buchannan, Jodi Widner, Justin Keener |
| The Hoffberger Breast Center at Mercy, 227 St Paul Pl, Baltimore, MD 21202, USA | Neil B. Friedman |
| Holy Cross Hospital, Ford Lauderdale, FL, USA | Omar Rashid, Joseph J Casey, Marnie Kaplan, Lav Goyal, Irina Frosman |
| OLV Hospital Aalst, Moorselbaan 164, 9300 Aalst, Belgium | Adelheid Roelstraete, Koen Traen |
| Washington Hospital Center, Washington, D.C., USA | Eleni A Tousimis, Marc Boisvoir |
| Kantonsspital Münsterlingen und Frauenfeld, Spital Thurgau AG, Switzerland | Hans Reichardt, Christiane Reuter |
| Military Region General Hospital of Lanzhou, No. 333, South Binhe Road, Qilihe District, Lanzhou City, China | Zhao Qingli |
| Lindenhofgruppe Engeriedspital, Bern, Switzerland | Armin Thoeni, Gilles Berclaz, Jacqueline Vock, Karin Muench |
| St. Thomas Ascension Midtown Hospital, (previously Baptist Hospital), Nashville, TN, USA | Pat Whitworth, Kenneth Lloyd, Julian Heitz |
| Academician F. Todua Medical Center- Research Institute of Clinical Medicine, Tbilisi, Georgia | Mikheil Janjalia, Irakli Sixarulidze, Natalia Jankarashvili, Maia Topeshashvili, Mikheil Kavtaradze |
| The First Affiliated Hospital of Guangzhou Medical University, No. 151, Yanjiang West Road, Yuexiu district, Guangzhou, China | Wenbo Zheng |
| Instituto Nacional De Cancerologia (INCAN), Mexico City, Mexico | Enrique Bargallo, Christian Flores, Gabriel Santiago |
| MedStar Georgetown University Hospital, 3800 Reservoir Rd NW, Washington, DC 20007, USA | Eleni Tousimis |
| Guangdong Provincial People’s Hospital, No. 106 Zhongshan 2nd Road, Guangzhou City, Guangdong Province, China | Yi Pan, Wei Huang |
| Hudson Valley Hospital Center, Cortland Manor, NY, USA | Pond Keleman |
| Franziskushospital Harderber, Radiologische Klinik Alte Rothenfelder Landstrasse 23 D-49124 Georgsmarienhütte, Germany | Otfried Sauer, Albert von der Assen |
| St.Luke’s Hospital Anderson Campus, Easton, PA, USA | Lee Riley |
| Cancer Treatment Centers of America at Southeastern, Newnan, GA, USA | Anita Johnson, John Swanson, Christian Hyde, Joseph Dick, Patricia Young |
| Cancer Treatment Centers of America @ Western Regional Medical Center, Goodyear, AZ, USA | Simon Lam, Matt West |
| The First Pavlov State Medical University of St. Petersburg, Academition Pavlov Str.9, St. Petersburg, Russia | Alexey G. Manihas, Babeshkin Roman Nikolaevich |
| American British Cowdray (ABC) Medical Center, Mexico City, Mexico | Jorge Omar Hernandez Oviedo, Dolores De La Mata, Jose Hinojoso, Fabiola Flores, Carlos Robles, Bargallo Enrique, Antonio Maffuz-Azis |
| Marietta Memorial Hospital, Marietta, OH, USA | Teressa Valentine, Rajendra Bhati, Srini Vasan |
| Focus Radiotherapy, 209 Shakespeare Rd, Milford, Auckland, New Zealand | Erica Whineray Kelly |
| Columbia University Medical Center, New York, NY, USA | Eileen Connolly, Sheldon Feldman, Bret Taback |
| Clinica Leopoldo Aguerrevere, Caracas 1080, Miranda, Venezuela | Alecia Cosson, Ricardo Paredes, Gerardo Hernandez, Juan Rasquin, Adriana Pesci, Francisco Dona, Elizabeth González |
| John Muir Health Care, Walnut Creek, CA, USA | William Bice, Marjaneh Moini, Suzanne Clements |
| Moscow City Hospital №. 57, Moscow, Russia | Dmitry Bondar |
| McGill University Health Center, 1001 Decarie Blvd, Montreal, Quebec H4A 3J1, Canada | Marija Popovic, Bassam Abdulkarim, Peter Watson, Jan Seuntjens |
| Loyola University Medical Center, Maywood, IL, USA | William Small Jr., T. Refaat, T. Thomas, C. Hentz, S. Gros |
| North Shore Long Island Jewish, Health System Center for Advanced Medicine, 450 Lakeville Road, Lake Success NY 11042, USA | Lin Wang |
| Lenox Hill Hospital, New York, NY, USA | Alice Police |
| Diagnosticos C.A., Barcelona, Estado Anzoategui, Venezuela | Eduardo Benavides, Ivan Gonzalez |
| Instituto Imor, Instituto Médico de Onco-Radioterapia. Carrer de les Escoles Pies, 81, 08017 Barcelona, Spain | Benjamin Guix, Iván García, Manel Algara, Miquel Puig |
| Lahey Hospital and Medical Center, 41 Burlington Mall Road, Burlington, MA 01805, USA | Per Halvorsen, Andrea McKee |
| Meir Medical Center, Israel | Bella Nisenbaum |
| Medipol University, Istambul, Turkey | Hale Basak Caglar, Dilek Unal |
| Kaplan Medical Center, Rehovot, Israel | Tanir M Allweis |
| Hospital Sao Rafael, Salvador, Brazil | Arthur Rosa, Ezio Novais Dias |
| Kaiser Oakland Medical Center, Oakland, CA, USA | Veronica Shim |
| Cancer Research Center, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran | Mohammad Esmail Akbari |
| Instituto Nacional de Enfermedades Neoplásicas, Suquilo, Lima (INEN), Peru | Gustavo Sarria, Jose Antonio Galarreta, Julio Abugattas |
| Ha’emek Medical Center, Afula, Israel | Hershko Da |
| Lee Health Regional Cancer Centre, Fort Myers, FL, USA | David Rock, Alan Brown Jr. |
| Krankenhaus Weinheim, Gesundheitszentren Rhein-Neckar GmbH, Germany | Lelia Bauer, Bettina Müller |
| Universitätsklinikum Bonn, Germany | Frank Giordano, Stephan Garbe, Christopher Schmeel |
| University of California Irvine Medical Center, Orange, USA | Alice Police, Erin Lin, Jeffery Kuo |
| Assuta Medical Centers, HaBarzel St 20, Tel Aviv-Yafo, Israel | Daphne Levin, Yonina Tova, Vladislav Greenberg |
| Beilinson/Rabin Medical Center, Petah Tikva, Israel | Eran Sharon |
| The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe Dong Road, Zhongyuan District, Zhengzhou City, Henan Province, China | Li Guowen |
| University of California Los Angeles (UCLA), Medical Center Harbor, Torrance, USA | Christine Dauphine, Junko Ozao-Choy, Chad Sila, Eric Frank, Katherine Magat |
| Soroka Medical Center, Beer Sheba, Israel | Ravit Agassi |
| Bethesda North Hospital, OH, USA | Jessica Guarnaschelli, Ching Ho, Peter Sandwall |
| Helios Klinikum Bad Saarow, Germany | Stephan Koswig, Gerlinda Kho, Marén Sawatzki, Justyna Polowy |
| Inova Fairfax Hospital, Falls Church, VA, USA | Stella Hetelekidis, Lonika Majithia, Ashish Chawla, Michael Eblan, Sara Bruce, David Weintritt, Constanza Cocilovo, Robert Cohen, Kirsten Edmiston |
| Hospital Alemão Oswaldo Cruz, São Paulo, Brazil | Rodrigo Hanriot, Patricia B. Aguilar, Douglas G. Castro, Guilherme RM Gondim |
| The First Affiliated Hospital, Sun Yat-sen University, No. 58, Zhongshan Second Road, Yuexiu District, Guangzhou, China | Ying Lin |
| Emory University Midtown Hospital, Atlanta, GA, USA | Rogsbert Phillips, Karen Godette |
| Ospedale dell’Angelo - Mestre VENEZIA, Via Paccagnella, 11, 30174 Venice VE, Italy | Sonia Reccanello |
| Medicana International Ankara Hospital, Cankaya/Ankara, Turkey | Kaan Oysul |
| The Second Affiliated Hospital, Sun Yat-sen University(2), No. 107 West Yanjiang road, Guangzhou, Guangdong, China | Lin, Huang, Shi Juntian |
| The London Clinic, 20 Devonshire Avenue, London, UK | Gerald Gui, Jeffrey S. Tobias, Jayant S. Vaidya, Tim Davidson, Susan Cleator, Simon Stevens |
| RF Magadan Regional Oncology Centre | Roman Shumel |
| Newport Beach Surgery Center, CA, USA | Alice Police |
| Haerbin Medical University Cancer Hospital, No. 150 Haping Road, Nangang District, Harbin City, Heilongjiang Province, China | Zhao Chunbo |
| Greenwich Hospital, Greenwich, USA | Barbara Ward, Sana Quirk |
| University Hospital “Tzaritza Joanna – ISUL”, Medical University of Sofia, Bulgaria | Theophil Sedloev, Slavyana Usheva, Iliya Gabrovski, Ivan Terziev |
| Clinica AUNA Oncosalud, Lima, Peru | Gustavo Sarria, David Martinez |
| Inova Alexandria Hospital, Alexandria, VA, USA | David Weintritt, Sara Bruce, Tobias Chapman, Lonika Majithia |
| Fundação Antonio Prudente - Hospital AC Camargo Cancer Center, Sao Paolo, Brazil | Antonio Cassio De Assis Pellizzon, Fabiana Makdissi, Ricardo Fogarolli, Juan Bautista Donoso Collins, Guilherme Rocha Gondim |
| University of Würzburg, Würzburg, Germany | Bülent Polat, Achim Wöckel, Marcus Zimmermann |
| California Hospital Medical Center, Los Angeles, CA, USA | Dennis Holmes |
| Mount Carmel Hospital, Columbus, OH, USA | Shilpa Padia, Malouan Rajagopolan |
| Sha’arei Zedek Medical Center | Carmon Moshe |
| Pastornow Cancer Research Center, and Medical Physics Research Center, Mashhad University of Medical Sciences, Mashhad, Iran | Hamid Gholamhosseinian, Roham Salek, Mohammad Naser Forghani, Mahboobeh Sadeghi ivari, Fatemeh Homaei, Kazem Anvari, Gholamhossein Noferesti, Amir Aledavood, |
| Clinique du Sein, Centre Republique, 99 avenue de la République, 63100 Clermont-Ferrand, France | Christophe Scherer, Doridot Virgenie |
| The Second Affiliated Hospital of Guangzhou Medical University, 250 Changgang Middle Rd, Haizhu, Guangzhou, Guangdong, China | Hu Xiaowu, Yong He |
| HELIOS Medical Center Krefeld, Germany | Stefan Krämer, Michael Friedrich, Michael Daum-Marzian, Dilek Saylan, Maike Sellinger |
| Helios University Hospital Wuppertal, University Witten/Herdecke, Germany | Marc D Piroth, Vesna Bjelic-Radisic, Markus Fleisch, Steffi Marzotko, Bianca Böning, Arnd Röser |
| The First Hospital Affiliated To AMU(Southwest Hospital), Lihui road, Beibei district, Chongqing, China | Yi. Zhang |
| Hospital Dr Domingos Luciani, Caracas 1073, Miranda, Venezuela | Carlos Nunez, Berta Prato |
| Wellington Regional Medical Center, Wellington, FL, USA | Kathleen Minnick, Kishore Dass, Andrew J. Shapiro |
| Sunway Medical Centre, 5, Jalan Lagoon Selatan, Bandar Sunway, 47500 Petaling Jaya, Selangor, Malaysia | Char Hong Ng |
| Inova Fair Oaks, 3600 Joseph Siewick Dr, Fairfax, VA 22033, USA | Stella Hetelekidis, Ashish Chawla, Michael Taylor, H Vargas, Moonseong Oh, Kirsten Edmiston |
| Halifax Hospital, Daytona Beach, FL USA | Domenico Dellicarpini |
| Advocate Masonic Hospital, Chicago, IL, USA | Barry Rosen |
| New Mexico Cancer Care Alliance, Albuquerque, New Mexico | Calvin Ridgway |
| Sun Yat-sen University Cancer Center, No. 651 East Dongfeng road, Yuexiu District, Guangzhou, Guangdong, China | A Long Chen |
| Subang Jaya Medical Centre, No. 1, Jalan SS12/1A, Ss 12, 47500 Subang Jaya, Selangor, Malaysia | Yip Cheng-Har |
| Assuta Medical Centre, Haifa, Israel | Abdah-Bortnyak Roxolyana, Rafi Klein |
| Phelps Hospital, Sleepy Hollow, NY, USA | Alice Police |
| University of Miami/Jackson Memorial Hospital, Miami, FL, USA | Eli Avisar, Cristiane Takita |
| Montifiore Hospital, New York, NY, USA | Sheldon Feldman |
| Rochester Regional Health, 100 Kings Highway South Rochester, NY 14617, USA | Lori Medeiros, Deore Shivaji, Michelle Beaty, Xunyi Xu, Mubin Shaikh, Adi Robinson, Joel Yellin, Meri Atanas |
| Mount Sinai Hospital, 1468 Madison Ave, New York, NY 10029, USA | Sheryl Green |
| Hainan Cancer Hospital, No 6, Changbin West 4th St, Xiuying district, Haikou City, Hainan Province, China | Haonan Ran |
| No. 12 Jiankang Rd, Changan District, Shijiazhuang City, Hebei Province, China | Zhang Ruohui |
| IMO- Instituto de Mastologia e Oncologia - Goiania - GO - Brazil | Nilceana Maya Aires Freitas, Ruffo Freitas Junior, Alexandre Marchiori, Jean Teixeira Paiva, Lais Tomaz Maya |
| Legacy Health, Portland, OR, USA | Mark Schray, Nathalie Johnson, Cynthia Aks |
| Prince Court Medical Centre, 39, Jalan Kia Peng, Kuala Lumpur, 50450 Kuala Lumpur, Wilayah Persekutuan Kuala Lumpur, Malaysia | Harjit Kaur Perdamen |
| The Medical City, Ortigas Ave, Pasig, Metro Manila, Philippines | Aldine Astrid Arive Basa |
| Inova Loudoun Hospital, Leesburg, VA, USA | Virginia Chiantella, Lonika Majithia |
| St John of God Hospital, Subiaco, Perth, Australia | Christobel Saunders |
| University of Kansas Medical Center (KUMC), Overland Park, Kansas, KS, USA (centre started after the initial submission so numbers not included in the total) | Kelsey Larson, James Coster |
| Hospital Italiano de Buenos Aires, MEVATERAPIA, Argentina (centre started after the initial submission so numbers not included in the total) | Carola Allemand, Federico Diaz |
| Fortis Hospital, Bangalore, India (centre started after the initial submission so numbers not included in the total) | Sandeep P. Nayak, Nisha Vishnu |
TARGIT-IORT global collaborators.
Jayant S. Vaidya, Uma J. Vaidya, Michael Baum, Max Bulsara, David Joseph, and Jeffrey S. Tobias, on behalf of the TARGIT-IORT Global Collaborators. The centres are listed in order when the first case was treated firstly within TARGIT-A trial, then TARGIT-B trial, and then those outside these two trials. This table is not an exhaustive list and includes only those given by contributors who have responded to our emails for collaboration. We apologise for the omission of any names. Up to date list at https://targit.org.uk/travel.
Figure 1 is the screenshot of an interactive Google Map that shows the centres that have offered TARGIT-IORT for breast cancer, the year of their first case, and the number of cases performed as of August 2020. Once the reader clicks on a particular centre, they can get directions to the centre by clicking on the direction arrow on top left corner, next to the name of the centre. The interactive map in Supplementary Figure 2 shows the number of centres in each country. Supplementary Figure 3 shows how they have increased since 1998.
Figure 1
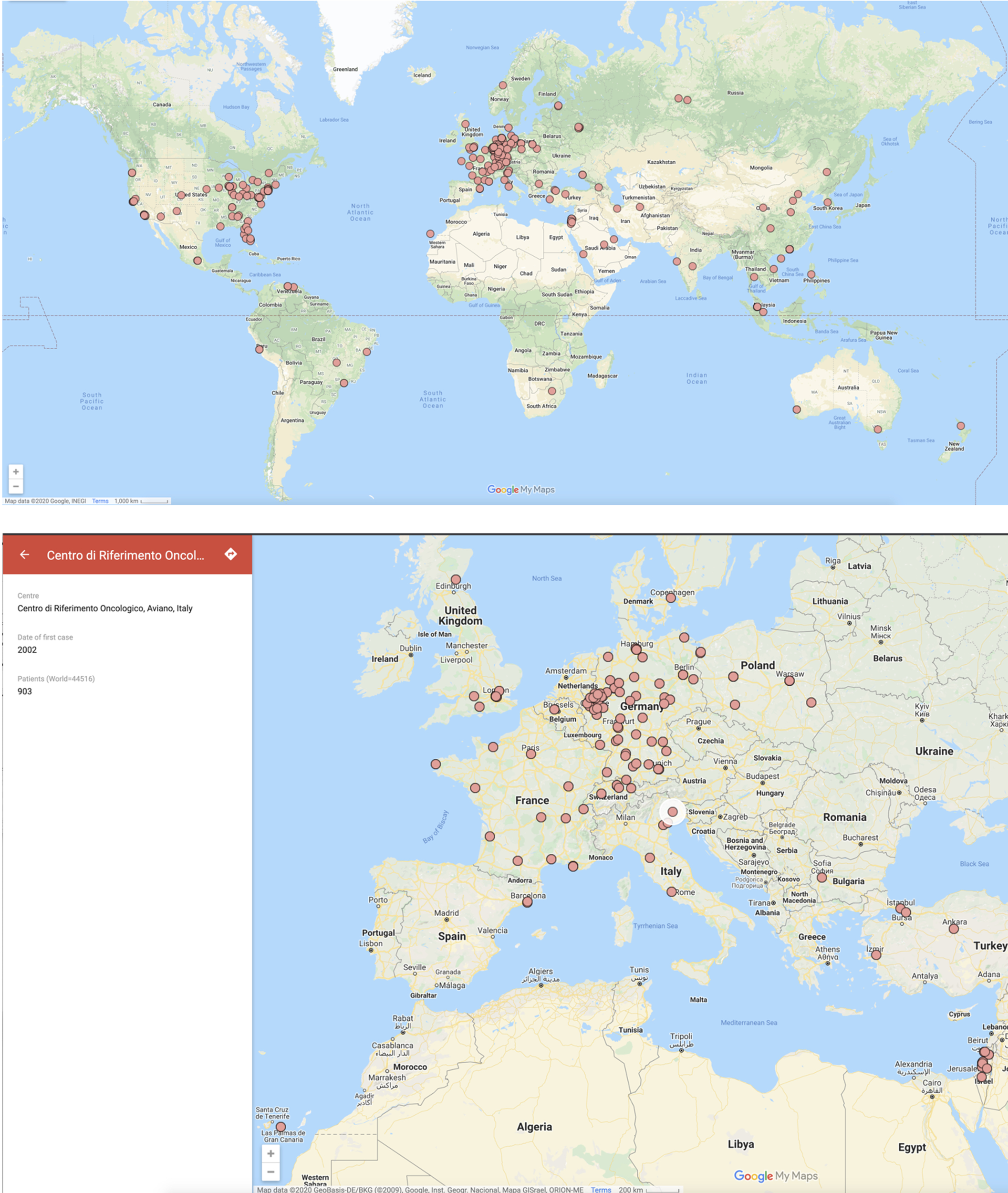
Screenshots of the map of the world with each dot representing a centre that has treated breast cancer with TARGIT-IORT. The name of the centre and the number of cases treated by the centre (if available) are seen in the left-hand pane when you click on the centre in 1b below (the map can be zoomed in). This map is interactive and available at https://targit.org.uk/travel.
Scaling up the journeys saved by avoiding EBRT, because of the use of TARGIT-IORT, to the 44,752 patients, we estimate that over 20 million (20,134,909) miles of travel have already been saved, representing a carbon footprint reduction of 5.6 million kg of CO2 emissions.
Figure 2 is the screenshot of the interactive tool with which one can find the centre offering TARGIT-IORT closest to one’s home. It will also estimate how much an individual patient would save by using TARGIT-IORT in terms of travel distance, time, and carbon footprint.
Figure 2
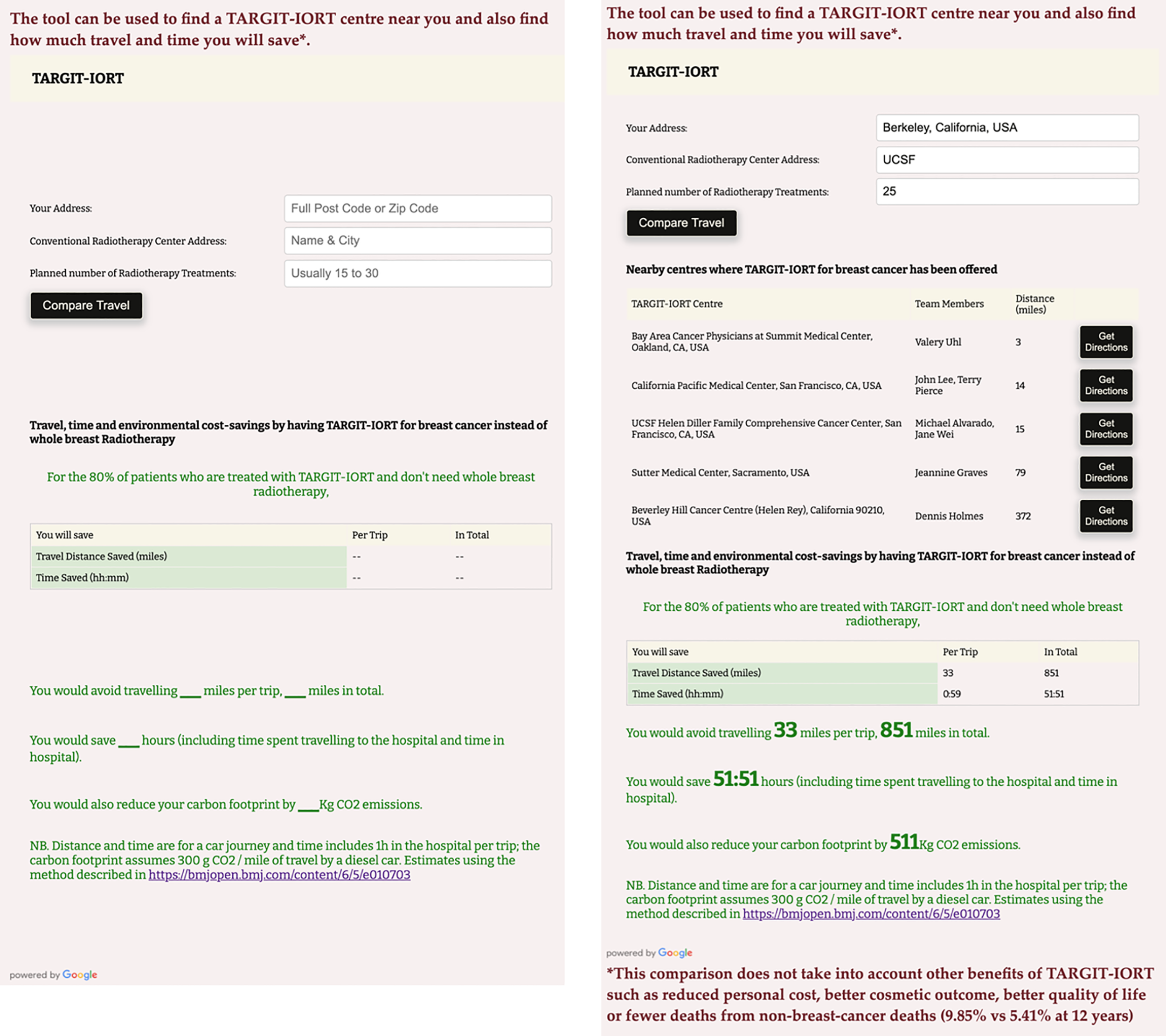
A screenshot of the interactive tool to assess how much an individual patient would save by using TARGIT-IORT in terms of travel distance, time, and carbon footprint. This example is for someone living in Berkeley, California, USA, for example, and going for radiotherapy at the University of Califoria San Francisco UCSF hospital, the closest radiotherapy centre from this house. This interative tool can be accessed at https://targit.org.uk/travel.
These interactive maps and tools can be accessed at https://targit.org.uk/travel.
Scaling up the 4.44% (95% CI, 2.5% to 6.4%) reduction in non–breast cancer mortality to the 44,752 patients treated to date (mid-2020), we estimate that 1,987 (95% CI, 1,129 to 2,845) non–breast cancer deaths from causes other than breast cancer such as cardiovascular and lung problems and other cancers would be prevented.
Discussion
This paper describes the worldwide adoption of TARGIT-IORT for treatment of early breast cancer over the past two decades. We could confirm that TARGIT-IORT has been used in 260 centres in 35 countries and about 45,000 patients in six continents have been treated. In the process, an estimated 20 million miles of journeys were avoided. Applying the reduction in non–breast cancer mortality, found in the TARGIT-A trial, to the patients already treated around the world suggests that the use of TARGIT-IORT would lead to 2,000 fewer deaths from causes other than breast cancer.
Over the last decade, there has been growing support for the use of partial breast irradiation (PBI) instead of whole-breast radiation therapy, and it is arguable that TARGIT-IORT is much better for patients than other methods of PBI (30, 33–35). The TARGIT-A trial cohort comprised a medium-risk population, with a substantial number of patients at a higher risk of relapse: 1,898 (83%) were younger than 70 years, 366 (16%) had tumours >2 cm in size, 443 (20%) patients had grade 3 cancers, 488 (22%) patients had involved nodes, and 426 (19%) had ER- or PgR-negative tumours. Therefore, its results would also be applicable to patients with breast cancer suitable for breast conserving surgery more widely than other methods of PBI (9, 30).
In many countries, patients live a considerable distance from the radiotherapy centre (31, 36, 37) and are more likely to receive a mastectomy than breast conservation (38). Even in the USA as recently as 2015, patients who lived farther away from the radiation facility (> 9.2 miles/19 min away by road) were 36%–44% more likely to receive a mastectomy than breast conservation (38). TARGIT-IORT is a much more convenient option (28, 39). We pointed out that wider availability and applicability of TARGIT-IORT should enable many more women to have the choice of having breast conservation when they would otherwise have a mastectomy because they are not able to have conventional radiotherapy (40–49). TARGIT-IORT also reduces the cost of providing treatment (50–55).
Importantly, TARGIT-IORT lowers the toxicity and reduces deaths from cardiovascular causes and other cancers by a substantial amount (4.4% by 12 years) (30), which has become increasingly important with the rising rates of survival with modern breast cancer treatment. This effect of improving survival appears to be a combination of avoiding the risks due to inadvertent scattered radiation from whole-breast radiotherapy and from a potential abscopal effect of delivery of intraoperative radiotherapy during the surgical excision of the cancer (10).
The strengths of this study are that the data were provided directly by the physicians and staff from each centre and that the response rate of 93% was excellent. In addition, we provide user-friendly interactive links (https://targit.org.uk/travel) for use by clinicians and patients. The obvious weakness is that this paper does not describe data about outcomes, but this is not the intention of this manuscript. Outcome data are best gained from comparative analysis within the prospective TARGIT-A randomised trial (9, 10), as well as data from several centres that have published their own experience of TARGIT-IORT and from prospective registry studies (https://targit.org.uk/publications) (18, 28, 39, 55–65). In addition, as the list of centres using TARGIT-IORT was compiled using personal contacts, we may have missed some centres, underestimating the number of cases. The network of centres using this approach is now been greatly strengthened and will in due course provide the foundation for a unified collection of outcome data.
TARGIT-IORT is now included in several national and international guidelines (66–79) (https://www.targit.org.uk/targit-iort-in-guidelines) for breast cancer treatment. Several of these guidelines specifically recommend using TARGIT-IORT during the COVID-19 pandemic caused by the SARS-CoV-2 virus to give the added advantage of reducing patient exposure to hospital environments and public places.
In this paper, we described the impact of a new treatment proven in a randomised clinical trial over the worldwide breast cancer community. It demonstrates how widely this evidence-based approach has now been adopted and how it has benefitted women with breast cancer around the world.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author’s note
A pre-print of this paper is available from UCL Discovery http://doi.org/10.14324/000.wp.10121050
Data availability statement
A pre-print of this paper is available from UCL Discovery http://doi.org/10.14324/000.wp.10121050
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JV conceived the project and discussed it with UV, MBa, JST, and MBu and wrote the first draft; UV helped in making contacts, collecting data from centres and collating data, and programming for creating the figures and tables; JV, MBa, MBu, JT, DJ, and UV contributed to finalizing the draft. All other authors and contributors/collaborators contributed by treating patients and returning their own data for the compilation and approving the manuscript for submission.
Conflict of interest
JV has received a research grant from Photoelectron Corp (1996–99) and from Carl Zeiss for supporting data management at the University of Dundee (Dundee, UK, 2004–2008) and has received honorariums. JV and JT received funding from HTA, NIHR, Department of Health and Social Care for some activities related to the TARGIT trials. MBa was briefly on the scientific advisory board of Carl Zeiss and was paid consultancy fees before 2010. Carl Zeiss sponsors some of the travel and accommodation for meetings of the international steering committee and data monitoring committee and when necessary for conferences where a presentation about targeted intraoperative radiotherapy is being made for all authors apart from UV, who has declared no conflict of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.786515/full#supplementary-material
Supplementary Figure 1Kaplan-Meier curves showing breast cancer mortality (top left) and non–breast cancer mortality (top right), overall mortality for grade 1 or 2 cancers (bottom left), and grade 3 cancers (bottom left) for TARGIT-IORT v EBRT in the TARGIT-A trial. Figures under titles are hazard ratios (95% confidence intervals) and log rank test P values. EBRT=external beam radiotherapy; TARGIT = targeted intraoperative radiotherapy = TARGIT-IORT (taken from BMJ 2020;370:m2836 https://www.bmj.com/content/370/bmj.m2836.full.pdf and BJC 2021 125, pages380–389 (2021) https://www.nature.com/articles/s41416-021-01440-8.pdf.
Supplementary Figure 2World map showing countries in which TARGIT-IORT is offered for breast cancer. The shading correlates with the number of centres in each country. For an interactive map see https://targit.org.uk/travel.
Supplementary Figure 3The number of centres offering TARGIT-IORT increased worldwide from 1998 onward. The graph below includes only those centres from which the date of first case was returned to us.
References
1
VaidyaJSBaumMTobiasJSet al. Targeted intra-operative radiotherapy (TARGIT): an innovative method of treatment for early breast cancer. Ann Oncol Off J Eur Soc Med Oncol / ESMO (2001) 12(8):1075–80.
2
VaidyaJSBaumMTobiasJSMorgan SSD'SouzaD. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2002) 28(4):447–54. doi: S0748798302912758
3
VaidyaJS. A novel approach for local treatment of early breast cancer. PhD thesis, university college London. University of London (2002). Available at: http://www.ucl.ac.uk/~rmhkjsv/papers/thesis.htm.
4
VaidyaJSVyasJJChinoyRFMerchantNSharmaOPMittraIet al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer (1996) 74(5):820–4.
5
BaumMVaidyaJSMittraI. Multicentricity and recurrence of breast cancer [letter; comment]. Lancet (1997) 349(9046):208–08.
6
VaidyaJSBaumMTobiasJSHoughtonJ. Targeted intraoperative radiothearpy (TARGIT)- trial protocol. Lancet (1999).
7
VaidyaJSJosephDJTobiasJSBulsaraMWenzFSaundersCet al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-a trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet (2010) 376(9735):91–102. doi: 10.1016/S0140-6736(10)60837-9
8
VaidyaJSWenzFBulsaraMTobiasJSJosephDJKeshtgarMet al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-a randomised trial. Lancet (2014) 383(9917):603–13. doi: 10.1016/S0140-6736(13)61950-9
9
VaidyaJSBulsaraMBaumMWenzFMassarutSPigorschSet al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-a randomised clinical trial. BMJ (2020) 370:m2836. doi: 10.1136/bmj.m2836
10
VaidyaJSBulsaraMBaumMWenzFMassarutSPigorschSet al. New clinical and biological insights from the international TARGIT-a randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer. Br J Cancer (2021) 125(3):380–89. doi: 10.1038/s41416-021-01440-8
11
Kraus-TiefenbacherUBauerLKehrerTHermannBMelchertFWenzFet al. Intraoperative radiotherapy (IORT) as a boost in patients with early-stage breast cancer – acute toxicity. Onkologie (2006) 29(3):77–82.
12
Kraus-TiefenbacherUBauerLSchedaAFleckensteinKKellerAHerskindCet al. Long-term toxicity of an intraoperative radiotherapy boost using low energy X-rays during breast-conserving surgery. Int J Radiat Oncol Biol Phys (2006) 66(2):377–81.
13
WenzFWelzelGKellerABlankEVorodiFHerskindCet al. Early initiation of external beam radiotherapy (EBRT) may increase the risk of long-term toxicity in patients undergoing intraoperative radiotherapy (IORT) as a boost for breast cancer. Breast (2008) 17(6):617–22. doi: 10.1016/j.breast.2008.05.009. doi: S0960-9776(08)00148-3.
14
Kraus-TiefenbacherUWelzelGBradeJHermannBSiebenlistKWasserKSet al. Postoperative seroma formation after intraoperative radiotherapy using low-kilovoltage X-rays given during breast-conserving surgery. Int J Radiat Oncol Biol Phys (2010) 77(4):1140–5. doi: 10.1016/j.ijrobp.2009.06.008
15
AzizMHSchneiderFClausenSBlankEHerskindCAfzalMet al. Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x-rays? Radiat Oncol (2011) 6:174. doi: 10.1186/1748-717X-6-174
16
SperkEWelzelGKellerAKraus-TiefenbacherUGerhardtASutterlinMet al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT a. Breast Cancer Res Treat (2012) 135(1):253–60. doi: 10.1007/s10549-012-2168-4
17
WelzelGBochASperkEHofmannFKraus-TiefenbacherUGerhardtAet al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-a. Radiat Oncol (2013) 8(1):9. doi: 10.1186/1748-717X-8-9
18
CelejewsakAWydmanskyJMajewskiWMajewskiWWozniakGKaniewska-DorszZet al. The evaluation of tolerance and efficacy of intraoperative radiation therapy (IORT) combined with external beam radiation therapy (EBRT) in patients with breast cancer, after breast-conserving surgery (BCT). Int J Radiat Oncol Biol Phys (2016) 96(2 Suppl):D.
19
AndersenKGGartnerRKromanNFlygerHKehletH. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: A randomized trial. Breast (2012) 21(1):46–9. doi: 10.1016/j.breast.2011.07.011
20
KeshtgarMRWilliamsNRBulsaraMSaundersCFlygerHCardosoJSet al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat (2013) 140(3):519–25. doi: 10.1007/s10549-013-2641-8
21
CoricaTNowakAKSaundersCMBulsaraMTaylorMVaidyaJSet al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: A substudy of the TARGIT-a trial. Int J Radiat Oncol Biol Phys (2016) 96(1):55–64. doi: 10.1016/j.ijrobp.2016.04.024
22
CoricaTNowakAKSaundersCMBulsaraMKTaylorMWilliamsNRet al. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-a trial. Radiat Oncol (2018) 13(1):68. doi: 10.1186/s13014-018-0998-x
23
SosinMGuptaSSWangJSCostellicCDGullaABartholomewAJet al. A prospective analysis of quality of life and toxicity outcomes in treating early breast cancer with breast conservation therapy and intraoperative radiation therapy. Front Oncol (2018) 8:545. doi: 10.3389/fonc.2018.00545
24
CoricaTNowakASaundersCBulsaraMJosephDet al. Patient preferences for adjuvant radiotherapy in early breast cancer – an Australian Sub-study of the international TARGIT trial. Eur J Cancer (2012) 48(Suppl 1):S187: Abstract 482.
25
AlvaradoMDConollyJParkCSakataTMohanAJHarrisonBLet al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat (2014) 143(1):135–40. doi: 10.1007/s10549-013-2782-9
26
CoricaTJosephDSaundersCSaundersCBulsaraMNowakAKet al. Intraoperative radiotherapy for early breast cancer: do health professionals choose convenience or risk? Radiat Oncol (2014) 9:33. doi: 10.1186/1748-717X-9-33
27
SpaichSKrickebergSHetjensSWenzFGerhardtASutterlinMet al. Patient preferences regarding intraoperative versus external beam radiotherapy for early breast cancer and the impact of socio-demographic factors. Arch Gynecol Obstet (2019) 299(4):1121–30. doi: 10.1007/s00404-018-5025-9
28
RamdasYBennC-AHeerdenMV. First intraoperative radiation therapy center in Africa: First 2 years in operation, including COVID-19 experiences. JCO Global Oncol2020(6):1696–703. doi: 10.1200/go.20.00258
29
TangACohanCMBeattieGCuretonELSvahnJDLyonLLet al. Patients older 65 years with early breast cancer prefer intraoperative radiation as a locoregional treatment choice. Ann Surg Oncol (2021). doi: 10.1245/s10434-021-09618-3
30
VaidyaJSBulsaraMBaumMTobiasJS. Single-dose intraoperative radiotherapy during lumpectomy for breast cancer: an innovative patient-centred treatment. Br J Cancer (2021) 124(9):1469–74. doi: 10.1038/s41416-020-01233-5
31
CoombsNJCoombsJMVaidyaUJSingerJBulsaraMTobiasJSet al. Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-a trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ Open (2016) 6(5):e010703. doi: 10.1136/bmjopen-2015-010703
32
RitchieH. How urban is the world? (2018). University of Oxford. Available at: https://ourworldindata.org/how-urban-is-the-world#un-estimates-55-of-people-live-in-urban-areas (Accessed 1 Feb 2021 2018).
33
DouekMDe Silva-MinorSDaviesLJonesB. Breast cancer radiation therapy. Lancet (2020) 396(10262):1558–59. doi: 10.1016/S0140-6736(20)32323-0
34
VaidyaJSBulsaraMSperkEMassarutSDouekMAlvaradoMet al. TARGIT-IORT during lumpectomy for breast cancer - better for patients than other PBI approaches. Int J Radiat Oncol Biol Phys (2021). doi: 10.1016/j.ijrobp.2021.01.059
35
VaidyaJSBulsaraMBaumMAlvaradoMBernsteinMMassarutSet al. Intraoperative radiotherapy for breast cancer: powerful evidence to change practice. Nat Rev Clin Oncol (2021) 18(3):187–88. doi: 10.1038/s41571-021-00471-7
36
Bargallo-RochaJESoto-Perez-de-CelisEPico-GuzmanFJQuintero-RodriguezCEAlmogDSantiago-ConchaGet al. The impact of the use of intraoperative radiotherapy on costs, travel time and distance for women with breast cancer in the Mexico city metropolitan area. J Surg Oncol (2017) 116(6):683–89. doi: 10.1002/jso.24712
37
LarsonKEValenteSAShahCTendulkarRDCherianSYandaCet al. Are patients traveling for intraoperative radiation therapy? Int J Breast Cancer (2017) 2017:6395712. doi: 10.1155/2017/6395712
38
GoyalSChandwaniSHafftyBGDemissieK. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol (2015) 22(4):1095–101. doi: 10.1245/s10434-014-4093-8
39
LorenzenAWKiriazovBDe AndradeJPLizarragaIMScott-ConnerCESuggSLet al. Intraoperative radiotherapy for breast cancer treatment in a rural community. Ann Surg Oncol (2018) 25(10):3004–10. doi: 10.1245/s10434-018-6574-7
40
AthasWFAdams-CameronMHuntWCHuntWCAmir-FazliAKeyC. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. JNCI J Natl Cancer Inst (2000) 92(3):269–71.
41
MalterWKirnVRichtersLFridrichCMarkiefkaBBongartzRet al. Intraoperative boost radiotherapy during targeted oncoplastic breast surgery: Overview and single center experiences. Int J Breast Cancer (2014) 2014:637898. doi: 10.1155/2014/637898
42
BanksACoronadoGZimmermanRIyengarGHolmesDR. Breast conserving surgery with targeted intraoperative radiotherapy for the management of ductal carcinoma in situ. J Surg Oncol (2019) 119(4):409–20. doi: 10.1002/jso.25347 [published Online First: 2018/12/28
43
ChinCHirjiSOnishiMHaRTabackBHorowitzDPet al. A single-institution experience in the preoperative selection of DCIS patients for IORT using the ASTRO consensus guidelines. Adv Radiat Oncol (2019) 4(2):253–60. doi: 10.1016/j.adro.2018.11.004
44
Kraus-TiefenbacherUBauerLSchedaASchoeberCSchaeferJSteilVet al. Intraoperative radiotherapy (IORT) is an option for patients with localized breast recurrences after previous external-beam radiotherapy. BMC Cancer (2007) 7:178. doi: 10.1186/1471-2407-7-178
45
KeshtgarMRVaidyaJSTobiasJSWenzFJosephDStaceyCet al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Radiat Oncol Biol Phys (2011) 80(1):31–8. doi: 10.1016/j.ijrobp.2010.01.045
46
Kraus-TiefenbacherUBlankEWenzF. Intraoperative radiotherapy during a second breast-conserving procedure for relapsed breast cancer after previous external beam radiotherapy. Int J Radiat Oncol Biol Phys (2011) 80(4):1279–80. doi: 10.1016/j.ijrobp.2011.02.038
47
ThangarajahFHeilmannJMalterWKunzeSMarnitzSMallmannPet al. Breast conserving surgery in combination with intraoperative radiotherapy after previous external beam therapy: an option to avoid mastectomy. Breast Cancer Res Treat (2018) 168(3):739–44. doi: 10.1007/s10549-017-4657-y
48
KeshtgarMREatonDJReynoldsCPigottKDavidsonTGauter-FleckensteinBet al. Pacemaker and radiotherapy in breast cancer: is targeted intraoperative radiotherapy the answer in this setting? Radiat Oncol (2012) 7(1):128. doi: 10.1186/1748-717X-7-128
49
KolbergHCUhlVMassarutSHolmesDKolberg-LiedtkeCKellyEWet al. Targeted intraoperative radiotherapy during breast-conserving surgery for breast cancer in patients after implant augmentation. Anticancer Res (2019) 39(8):4215–18. doi: 10.21873/anticanres.13582
50
AlvaradoMOzanneEMohanAEssermanL. Cost-effectiveness of intraoperative radiation therapy for breast conservation. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29(Suppl):abstr 6081.
51
AlvaradoMDMohanAJEssermanLJParkCCHarrisonBLHoweRJet al. Cost-effectiveness analysis of intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol (2013) 20(9):2873–80. doi: 10.1245/s10434-013-2997-3
52
VaidyaJSWenzFBulsaraMTobiasJSJosephDSaundersCet al. An international randomised controlled trial to compare targeted intra-operative radiotherapy (TARGIT) with conventional post-operative radiotherapy after conservative breast surgery for women with early stage breast cancer (The TARGIT-a trial). Health Technol Assess (2016) 20(73):1–188. doi: 10.3310/hta20730
53
PatelRIvanovOVoigtJ. Lifetime cost-effectiveness analysis of intraoperative radiation therapy versus external beam radiation therapy for early stage breast cancer. Cost Eff Resour Alloc (2017) 15:22. doi: 10.1186/s12962-017-0084-5
54
VaidyaAVaidyaPBothBet al. Health economics of targeted intraoperative radiotherapy (TARGIT- IORT) for early breast cancer: a cost- effectiveness analysis in the united kingdom. BMJ Open (2017) 7:e014944. doi: 10.1136/bmjopen-2016-014944
55
GrobmyerSRLightseyJLBryantCMet al. Low-kilovoltage, single-dose intraoperative radiation therapy for breast cancer: results and impact on a multidisciplinary breast cancer program. J Am Coll Surg (2013) 216(4):617–23. doi: 10.1016/j.jamcollsurg.2012.12.038
56
Zioueche-MottetAHouvenaeghelGClasseJMet al. Eligibility criteria for intraoperative radiotherapy for breast cancer: study employing 12,025 patients treated in two cohorts. BMC Cancer (2014) 14:868. doi: 10.1186/1471-2407-14-868
57
MuñozGHHanyRPCossonAet al. Intraoperative radiation therapy (INTRABEAM) experience at the mastology unit leopoldo aguerrevere clinic. J Cancer Ther (2015) 06(10):932–42. doi: 10.4236/jct.2015.610101
58
AbbottAMDossettLALoftusLet al. Intraoperative radiotherapy for early breast cancer and age: clinical characteristics and outcomes. Am J Surg (2015) 210(4):624–8. doi: 10.1016/j.amjsurg.2015.05.012
59
ValenteSATendulkarRDCherianSet al. TARGIT-r (Retrospective): North American experience with intraoperative radiation using low-kilovoltage X-rays for breast cancer. Ann Surg Oncol (2016) 23(9):2809–15. doi: 10.1245/s10434-016-5240-1
60
ThomasTOSmallWJr.. Editorial: Intraoperative radiotherapy (IORT)-a new frontier for personalized medicine as adjuvant treatment and treatment of locally recurrent advanced malignancy. Front Oncol (2018) 8:234. doi: 10.3389/fonc.2018.00234
61
ObiETomMCManyamBVet al. Outcomes with intraoperative radiation therapy for early-stage breast cancer. Breast J (2020) 26(3):454–57. doi: 10.1111/tbj.13574
62
MoiniNAkbariMEMirzaeiHet al. Intraoperative boost radiotherapy with 50 kV X-rays versus external radiotherapy in breast cancer: Single-center experiences. Int J Cancer Manag (2020) 13(3):e98561. doi: 10.5812/ijcm.98561
63
TalletARacadotSBoherJMet al. The actual benefit of intraoperative radiation therapy using 50 kV x-rays in early breast cancer: A retrospective study of 676 patients. Breast J (2020) 26(11):2145–50. doi: 10.1111/tbj.13827
64
LemanskiCBourgierCDraghiciRet al. Intraoperative partial irradiation for highly selected patients with breast cancer: Results of the INTRAOBS prospective study. Cancer Radiother J Soc Fr Radiother Oncol (2020) 24(2):114–19. doi: 10.1016/j.canrad.2020.01.007
65
MiYLvPWangFLiLZhuMWangYet al. Targeted intraoperative radiotherapy is non-inferior to conventional external beam radiotherapy in Chinese patients with breast cancer: A propensity score matching study. Front Oncol (2020) 10:550327. doi: 10.3389/fonc.2020.550327
66
GoldhirschAWoodWCCoatesASGelberRDThurlimannBSennHJet al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol: Off J Eur Soc Med Oncol / ESMO (2011) 22(8):1736–47. doi: 10.1093/annonc/mdr304
67
BiganzoliLWildiersHOakmanCMarottiLLoiblSKunklerIet al. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and European society of breast cancer specialists (EUSOMA). Lancet Oncol (2012) 13(4):e148–60. doi: 10.1016/S1470-2045(11)70383-7
68
MarmotMAltmanDGCameronDADewarJAThompsonSGWilcoxM. Independent UK panel on breast cancer screening replies to Michael baum. BMJ (2013) 346:f873.
69
MooneyH. NICE gives go ahead to intrabeam radiotherapy for breast cancer. BMJ (2014) 349:g4863. doi: 10.1136/bmj.g4863
70
VaidyaJSBulsaraMWenzFCoombsNSingerJEbbsSet al. Reduced mortality with partial-breast irradiation for early breast cancer: A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys (2016) 96(2):259–65. doi: 10.1016/j.ijrobp.2016.05.008
71
Medical Services Advisory Committee A. 1189 - targeted intraoperative radiotherapy (IORT) for early breast cancer 2016 (2020). Available at: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1189-public (Accessed 23 Mar 2020).
72
WiseJ. NICE recommends controlled intrabeam use for breast cancer after three year delay. BMJ (2017) 356:j725. doi: 10.1136/bmj.j72510.1136/bmj.h2874
73
(NICE) NIfHaCE. Intrabeam radiotherapy system for adjuvant treatment of early breast cancer: Technology appraisal guidance [TA501] (2018). Available at: https://www.nice.org.uk/guidance/ta501 (Accessed 23 Mar 2020).
74
Surgeons ASoB. Consensus guideline on accelerated partial breast irradiation 2018. Available at: https://www.breastsurgeons.org/docs/statements/Consensus-Statement-for-Accelerated-Partial-Breast-Irradiation.pdf.
75
CardosoFKyriakidesSOhnoSPenault-LlorcaFPoortmansPRubioITet al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol Off J Eur Soc Med Oncol / ESMO (2019) 30(8):1194–220. doi: 10.1093/annonc/mdz173
76
SimcockRThomasTVEstesCFilippiARKatzMSPereiraIJet al. COVID-19: Global radiation oncology’s targeted response for pandemic preparedness. Clin Trans Radiat Oncol (2020) 22:55–68. doi: 10.1016/j.ctro.2020.03.009
77
ChanJJSimYOwSGWLimJSJKusumawidjajaGZhuangQet al. The impact of COVID-19 on and recommendations for breast cancer care: the Singapore experience. Endocr Relat Cancer (2020) 27(9):R307–R27. doi: 10.1530/ERC-20-0157
78
BattistiNMLMislangARCooperLO'DonovanAAudisioRACheungKLet al. Adapting care for older cancer patients during the COVID-19 pandemic: Recommendations from the international society of geriatric oncology (SIOG) COVID-19 working group. J Geriatr Oncol (2020) 11(8):1190–98. doi: 10.1016/j.jgo.2020.07.008
79
CombsSEBelkaCNiyaziMCorradiniSPigorschSWilkensJet al. First statement on preparation for the COVID-19 pandemic in large German speaking university-based radiation oncology departments. Radiat Oncol (2020) 15(1):1–12. doi: 10.1186/s13014-020-01527-1
Summary
Keywords
TARGIT IORT, breast cancer, radiation therapy, lumpectomy (breast conserving surgery), TARGIT, IORT, radiotherapy, partial breast irradiation
Citation
Vaidya JS, Vaidya UJ, Baum M, Bulsara MK, Joseph D and Tobias JS (2022) Global adoption of single-shot targeted intraoperative radiotherapy (TARGIT-IORT) for breast cancer—better for patients, better for healthcare systems. Front. Oncol. 12:786515. doi: 10.3389/fonc.2022.786515
Received
30 September 2021
Accepted
28 June 2022
Published
11 August 2022
Volume
12 - 2022
Edited by
William Small Jr., Loyola University Chicago, United States
Reviewed by
Anil Vaidya, Masimo Inc., United States; Emanuela Esposito, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Updates
Copyright
© 2022 Vaidya, Vaidya, Baum, Bulsara, Joseph and Tobias.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jayant Sharad Vaidya, jayant.vaidya@ucl.ac.uk; jayantvaidya@gmail.com
†The full list of authors along with their affiliations is given at the end of this document
This article was submitted to Radiation Oncology, a section of the journal Frontiers in Oncology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.