- 1Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong SAR, China
- 2Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom
- 3Department of Epidemiology & Biostatistics, and Department of Respiratory Diseases of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Cancer Center, Zhejiang University, Hangzhou, China
Background: In addition to adiposity, lifestyle factors such as poor diet, low physical activity, alcohol intake and smoking are noted to be associated with the development of colorectal cancer (CRC). This study aims to investigate the association and dose-response relationship between adherence to a healthy lifestyle and CRC risk.
Methods: A systematic literature search was conducted in MEDLINE and EMBASE for studies examining multiple lifestyle factors with risk of CRC, incident colorectal adenoma (CRA), and CRC-specific mortality through June 2021 without restrictions on language or study design. Meta-analysis was performed to pool hazard ratios using random-effects model. Subgroup analyses were performed based upon study and sample characteristics. Random-effects dose-response analysis was also conducted for CRC risk to assess the effect of each additional healthy lifestyle factor.
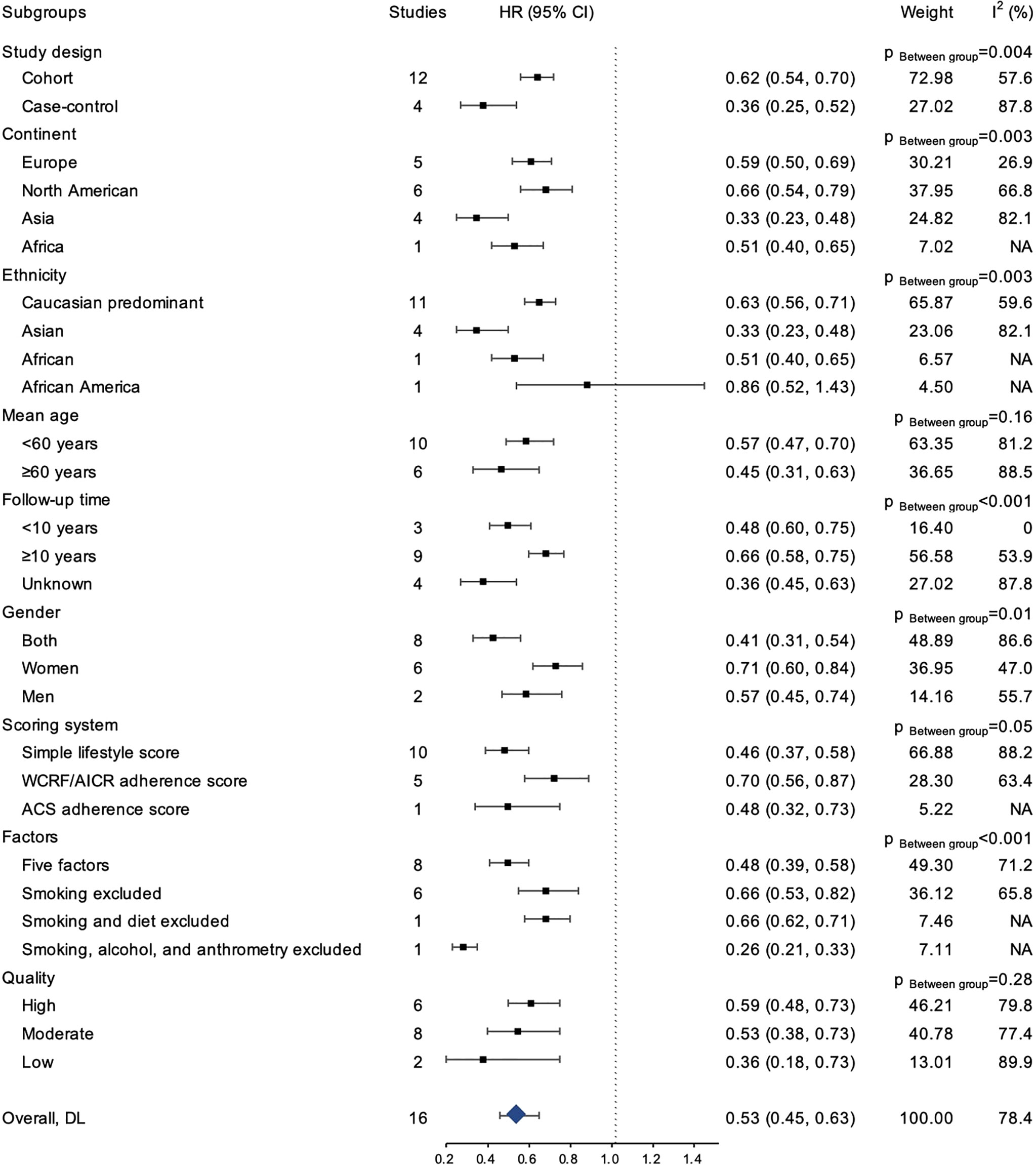
Results: A total of 28 studies (18 cohort studies, eight case-control studies, and two cross-sectional study) were included. When comparing subjects with the healthiest lifestyle to those with the least healthy lifestyle, the pooled HR was statistically significant for CRC (0.52, 95% CI 0.44-0.63), colon cancer (0.54, 95% CI 0.44-0.67), rectal cancer (0.51, 95% CI 0.37-0.70), CRA (0.39, 95% CI 0.29-0.53), and CRC-specific mortality (0.65, 95% CI 0.52-0.81). The pooled HR for CRC was 0.91 (95% CI: 0.88-0.94) for each increase in the number of healthy lifestyles. The inverse association between healthy lifestyle and CRC risk was consistently observed in all subgroups (HR ranging from 0.26 to 0.86).
Conclusions: Adoption of a higher number of healthy lifestyles is associated with lower risk of CRC, CRA, and CRC-specific mortality. Promoting healthy lifestyle could reduce the burden of CRC.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=231398, identifier CRD42021231398.
Introduction
Globally, colorectal cancer (CRC) ranks the third most commonly diagnosed malignancy and the second leading cause for cancer mortality (1). In 2020, CRC accounted for approximately 1.9 million new cases and more than 935,000 deaths worldwide (1). Its disease burden is projected to continue increasing globally, particularly in regions undergoing rapid industrialization (2). The increased risk of CRC and colorectal adenoma (CRA), one of its primary precancerous lesions (3), is closely linked to a variety of modifiable lifestyle risk factors, including excess adiposity (4–6), physical inactivity (7, 8), high intake of red meat and/or processed meat (9, 10), alcohol consumption (11, 12), and smoking (13); higher intake of dietary fiber, vegetables, and fruits are noted to be protective against CRC and CRA (14–17).
While the associations between CRC and single lifestyle factors have been extensively investigated in previous studies (18–21), far fewer studies have examined the effect of the adherence to a healthy lifestyle, defined as a combination of various modifiable factors. A latest meta-analysis that included 17 studies showed an overall inverse association between combined healthy lifestyle factors and CRC risk (22). However, it remains unclear whether the association differs by study settings or population characteristics and whether the association presents a dose-response relationship. In addition to CRC incidence, healthy lifestyle is also suggested to be related to CRC mortality in both CRC patients and general population (23–25). However, to the best of our knowledge, no systematic review and meta-analysis are available so far on the combined healthy lifestyle in relation to CRC-specific mortality.
Hence, this systematic review aims to investigate the association between adherence to a healthy lifestyle and the risk of CRC, CRA, and CRC-specific mortality, and to examine whether the association is dose-dependent and any potential effect modification by population characteristics.
Methods
This systematic review was registered on PROSPERO (CRD42021231398) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (26).
Data Sources and Search
We searched MEDLINE and EMBASE for relevant studies from their inceptions through June 2021. The search strategy combined three groupings of keywords with their derivatives and synonyms related to the following concepts: 1) combined or integrated effect; 2) lifestyle factors or health behaviors; and 3) colorectal cancer and adenoma. The search terms of these three concepts were combined using the Boolean operator “AND”. More details on search strategy is described in Supplementary Tables 1 and 2. The reference lists of eligible studies and relevant reviews were manually searched to identify additional publications. The search strategy did not impose any restriction on language, publication period, or publication status.
Eligible Criteria and Study Selection
We included epidemiological studies that investigated the association between combined lifestyle factors and colorectal outcomes. The exposure was combination of lifestyle factors, including but not limited to diet, smoking, alcohol consumption, physical activity, overweight/obesity, sleep duration, and others. The primary outcomes were risk of CRC, colon cancer, and rectal cancer. The secondary outcomes included risk of incident CRA, advanced colorectal neoplasia, and CRC-specific mortality. We included cross-sectional studies, case-control studies, and cohort studies. For CRC-specific mortality, we included studies of healthy population or CRC patients, while for the other outcome, the study population should be free of the outcomes at baseline if the study design was prospective cohort.
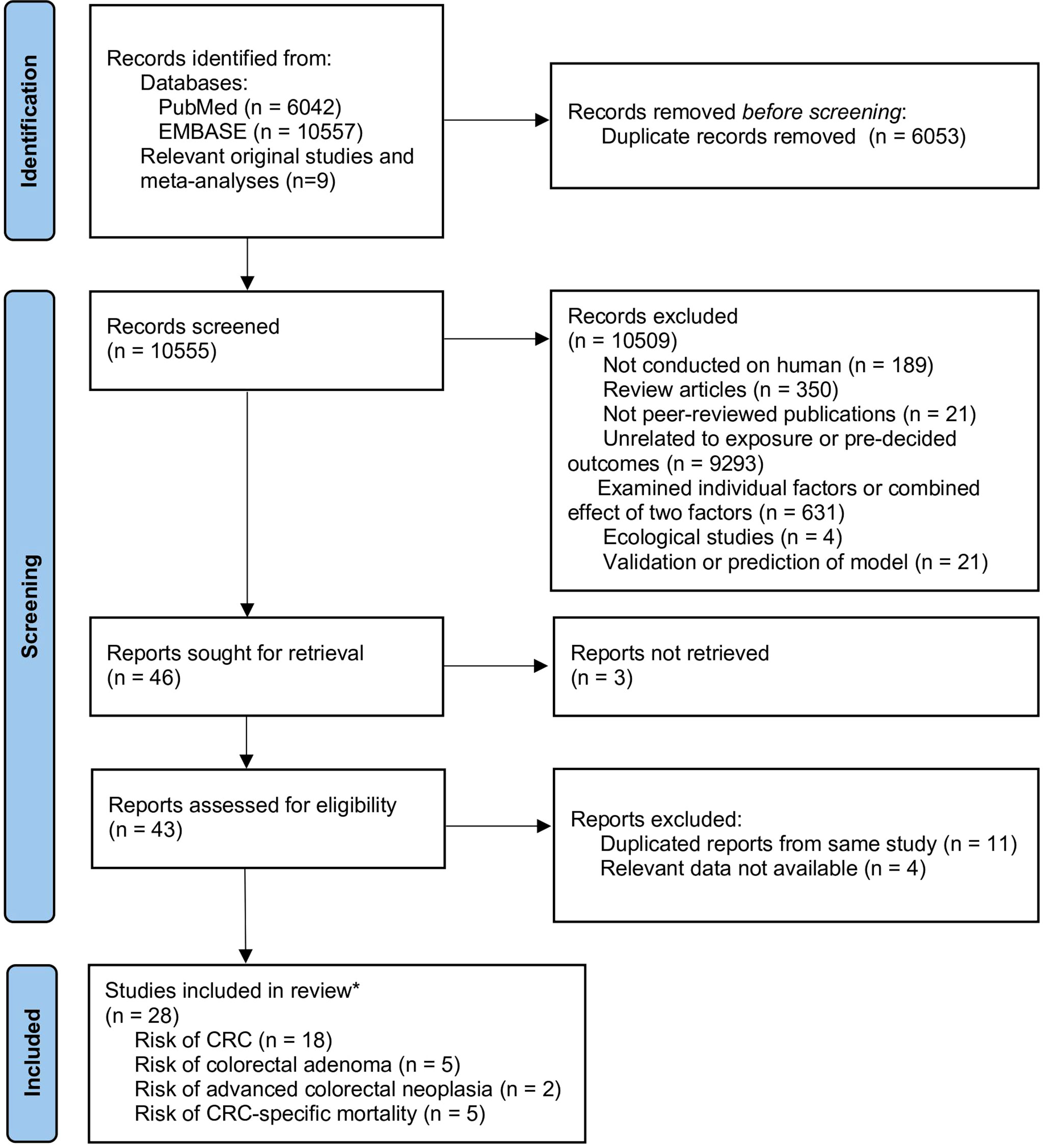
We excluded studies if they were (1) reviews, protocols, conference abstracts or not peer-reviewed publications, (2) focusing on a single lifestyle factor or a combination of less than three factors, (3) development or validation of prediction models, or (4) not reporting relevant data. For duplicate reports from the same cohort, we only included the report that had examined the largest number of lifestyle factors.
We used a two-step study selection procedure. The title and abstract of all electronically and manually identified records were screened first to identify potentially eligible studies. Second, full texts of the potentially eligible studies were examined for final eligibility. Two authors independently performed the selection process. All disagreements were resolved by discussion with a third reviewer until consensus was reached.
Data Extraction and Quality Assessment
Data were extracted by using an a priori designed form which collected the following information: (1) basic characteristics of study and subjects (e.g. first author, publication year, country, study period, sample size), (2) basic characteristics of participants (e.g. age, gender, ethnicity); (3) methodological characteristics, including study design, exposure definitions, outcome attainment, and follow-up period (for cohort studies) (4) effect estimates for the associations of interest, and (5) other information for quality assessment.
The methodological quality of cohort studies, case-control studies, and cross-sectional studies were assessed by the Newcastle-Ottawa Scale, which covers three domains: selection of participants, comparability of study groups, the ascertainment of exposure (for case-control studies) or outcomes (for cohort studies and cross-sectional studies) (27, 28). A star system, with a maximum of nine stars for cohort studies and case-control studies and ten stars for cross-sectional studies, was used to present the result of quality assessment, with more stars representing higher quality and lower risk of bias. We consider cohort studies and case-control studies high quality if they received more than seven stars, moderate quality if they received five or six stars, otherwise low quality. Cross-sectional studies were considered of high quality if they received more than eight stars, moderate quality if they received six or seven stars, otherwise low quality. Data extraction and quality assessment were performed independently by two authors. Any discrepancy was resolved by discussion until consensus was reached.
Data Synthesis and Analysis
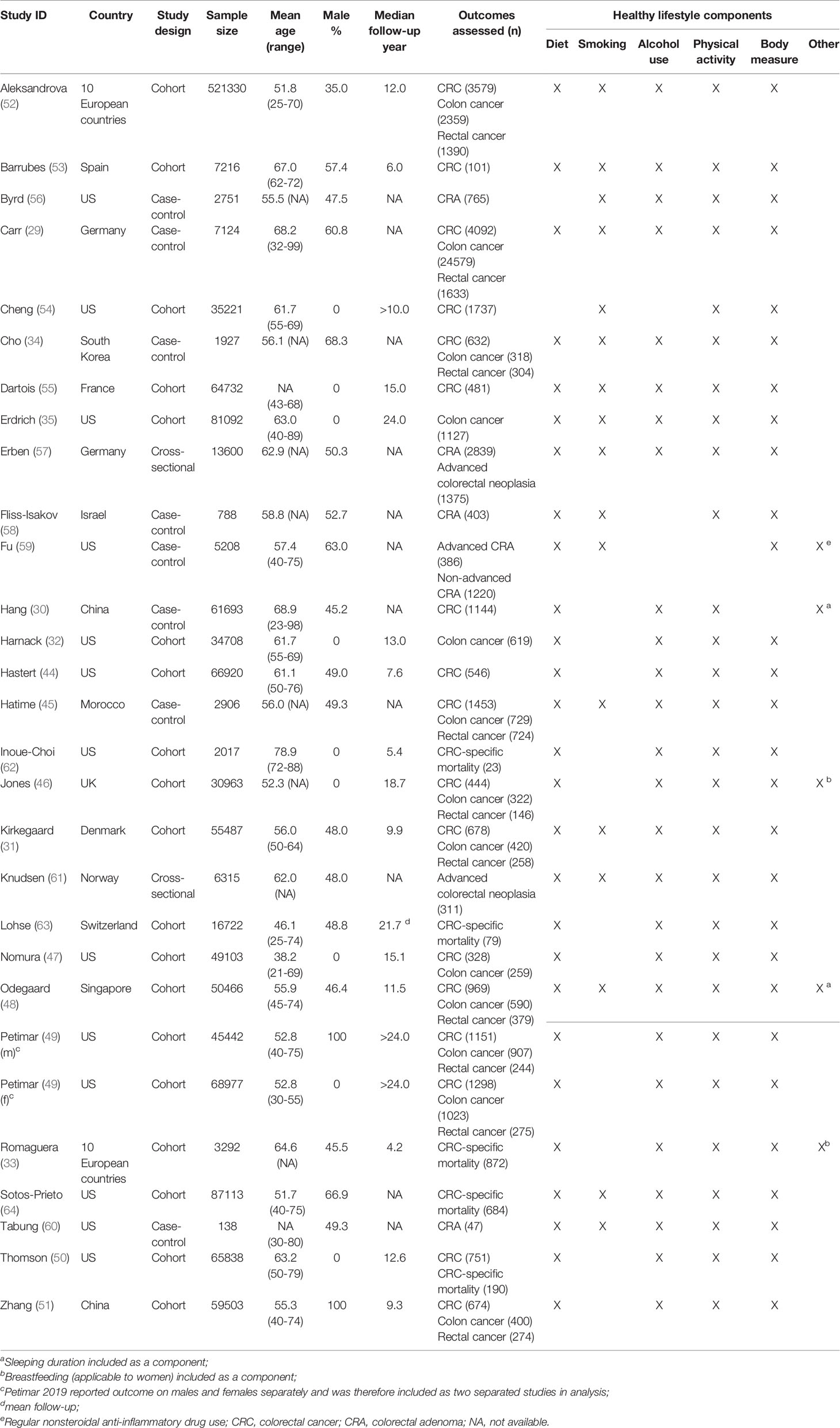
There has been no universal consensus on the quantification of combined lifestyle factors. Most studies constructed a simple unweighted lifestyle score, where one point was given to each of the present healthy lifestyles, although the exact lifestyle factors may vary across studies; for example, in Carr 2018 (29), Hang 2015 (30), and Kirkegaard 2010 (31). Some studies used weighted lifestyle score, in which the factors were weighted differently; for example, in Harnack 2002 (32) and Romaguera 2015 (33). However, some studies constructed risky lifestyle score that assign points to presence of unhealthy lifestyle habits; for example, in Cho 2019 (34) and Erdrich 2015 (35). In order to keep the directionality consistent with studies examining healthy lifestyle factors, we calculated a new score by deducting the original risky lifestyle score from the total number of the lifestyle factors for the studies that focus on unhealthy lifestyle habits (34, 35). The healthy lifestyle score was either used as a continuous variable (measuring the effect of per 1-unit increase in score) or transformed into a categorical variable (measuring the effect of adherence to healthiest lifestyle relative to the least healthy lifestyle) in original studies. In the originals studies, the five most commonly examined lifestyle factors were: diet, smoking status, alcohol consumption, physical activity level, and body measure. Most studies examined all five factors while others included some of them (see Supplementary Table 3).
Effect measures comparing the group with the healthiest lifestyle to the group with the least healthy lifestyle was pooled to present the associations of interest. Hazard Ratios (HR) with 95% confidence intervals (CIs) was the most commonly used as the measure of effect in original studies and was therefore used in this meta-analysis. Odds ratios, where applicable, were transformed into RR using the following formula: RR=OR/[(1-P0)+(P0*OR)], where P0 is the risk of an event in the non-exposed group (36). The transformed RRs and those extracted from some original studies were converted into HR using the formula: RR=(1-eHR ln(1-r))/r, where r is the rate of outcome in reference group (37).
Studies reporting the effect size for each unit increase in lifestyle score were included in a separate meta-analysis. Given the heterogeneity across studies in study population characteristics and healthy lifestyle scoring (the number, component, and weights of different lifestyle factors), all meta-analyses were conducted using random-effects model.
Pre-specified subgroup analyses were performed to detect potential effect modification, according to study design (cohort, case-control), study setting (Europe, North America, Asia, Africa), ethnicity of the predominant study population (Caucasian, Asian, African, African American), mean age (<60, ≥60 years), follow-up time (<10 years, ≥10 years, unknown), gender (women, men, both), scoring system [simple lifestyle score, WCRF/AICR (World Cancer Research Fund and the American Institute for Cancer Research) recommendation adherence score, ACS (American Cancer Society) guideline adherence score], examined factors (five factors, smoking excluded, smoking and diet excluded, smoking, alcohol, and body measure excluded), and study quality (high, moderate, low). Cochran’s Q test and I2 were used to assess the heterogeneity across studies, with p<0.05 and/or I2>50% indicating significant heterogeneity (38, 39). Potential publication bias was assessed by visual inspection of funnel plots as well as the Egger’s test when the number of included studies is more than 10 (40). P-value <0.05 in Egger’s test indicates presence of publication bias. Sensitivity analysis was performed to assess the robustness of the summary estimates by excluding studies of low quality and by including studies with relative comprehensive covariates only.
Random-effect dose-response analysis with one-stage method was used to generate the study slope lines (41). To minimize the impact of methodological heterogeneity on effect estimates in dose-response analysis, we only included studies using simple unweighted scoring. We further standardized the score scale so that each point represents adherence to one healthy lifestyle. For example, we modified the score scale in studies that assigned two points to each lifestyle factors by multiplying the original score by 0.5. We investigated potential non-linear relationship by using restricted cubic splines with three knots located at 10th, 50th, and 90th percentiles of the exposure category (42). These three knots accordingly represented 0.5, 2.5, and 4.5 points in the 5-point healthy lifestyle score scale. The curve segments before the first knot and after the last knot was assumed to be linear. Akaike information criteria (AIC) was used to compare the fitness of models, with the lower AIC indicating the better-fitting model (43). All quantitative data analyses were conducted by using Stata 14.0 (Stata Corp LP, College Station, TX, USA).
Results
Summary of Study Selection
A total of 10,555 unique records were identified from the literature search, 28 of which were considered eligible and were included. Among the eligible studies, 18 reported the risk of CRC (29–32, 34, 35, 44–55), five reported the risk of incident CRA (56–60), two reported the risk of advanced colorectal neoplasia (57, 61), and five reported CRC-specific mortality (33, 50, 62–64). The details of study selection are outlined in Figure 1. Among the five studies on CRC-specific mortality, two were conducted on CRC patients (50, 62) while the other three were conducted among healthy populations (33, 63, 64).
Characteristics of Included Studies
The characteristics of included studies are shown in Table 1 and Supplementary Table 3. Among the 28 studies included in the analyses, 18 were cohort studies, eight case-control studies, and two cross-sectional studies. The mean age at baseline ranged from 46.1 to 78.9 years. Eight studies were conducted among women (32, 35, 46, 47, 50, 54, 55, 62) while one was conducted among men (51); the other studies included both sexes, one of which (49) reported data separately for men and women. In terms of the study setting, 12 were conducted in the US, 10 in European countries, five in Asia, and one in Africa. The mean sample size was 51,735, with a range between 138 and 521,330. The median follow-up of cohort studies ranged from 3.1 years to >24 years.
Quality Assessment
Using the Newcastle-Ottawa Scale, the included cohort studies received ratings ranging from five to eight stars. Nine studies were rated as of high quality (31, 32, 35, 48, 49, 51, 54, 62, 63), nine studies of moderate quality (33, 44, 46, 47, 50, 52, 53, 55, 64). None of the studies got star for the ascertainment of exposure because the lifestyle habits were self-reported by participants. In some studies, the sample was not well representative of general population. The included case-control studies received three to seven stars. Two studies were rated as high quality (29, 60), two moderate quality (30, 59), and four low quality (34, 45, 56, 58). The common biases in low quality studies were introduced by poor selection of cases and controls and unclear outcome ascertainment. Of the two cross-sectional studies included, one was rated as high quality (61) while the other moderate quality (57). The assessment results of all the included studies are described in Supplementary Table 4.
Meta-Analysis
Overall Risk for CRC
We included 15 studies (1,139,361 participants), 11 studies (953,541 participants) and 8 studies (788,038 participants) in the meta-analyses of CRC, colon cancer and rectal cancer, respectively (Figure 2). Compared with the least healthy lifestyle, the adherence of the healthiest lifestyle was associated with 48% (HR=0.52, 95% CI 0.44-0.63, I2= 86.2%), 46% (HR=0.54, 95% CI 0.44-0.67, I2= 80.2%), and 49% (HR=0.51, 95% CI 0.37-0.70, I2= 92.0%) lower risk of CRC, colon cancer and rectal cancer, respectively. After pooling the studies using continuous lifestyle scores, the results showed that the per 1-unit increase in healthy lifestyle score was associated with a pooled HR of 0.88 (95% CI 0.84-0.92) for CRC, 0.87 (95% CI 0.83-0.92) for colon cancer, and 0.86 (95% CI 0.79-0.90) for rectal cancer (Supplementary Figure 1).
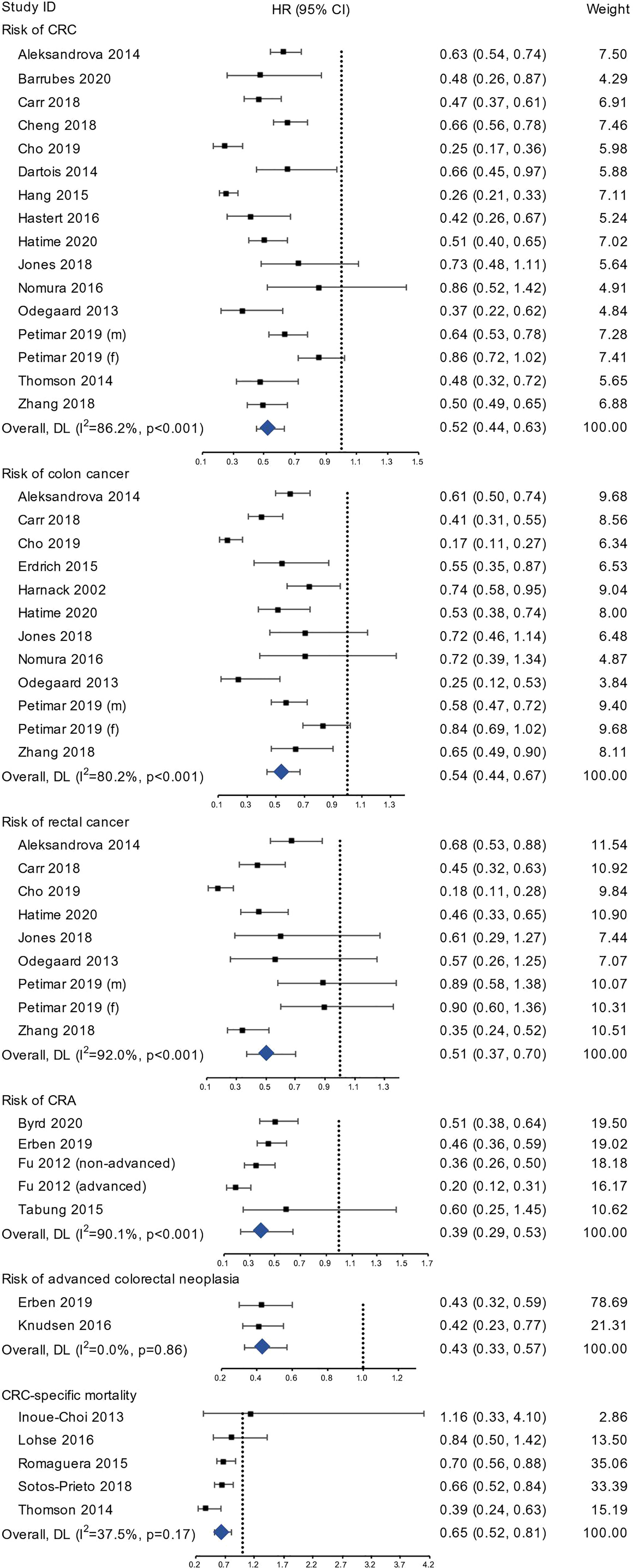
Figure 2 The forest plots of risk of CRC, colon cancer, rectal cancer, CRA, advanced colorectal neoplasia, and CRC-specific mortality.
Nine studies were included in the dose-response meta-analysis for risk of CRC (29, 30, 45, 49–52, 55, 65). The reported risk estimates for association between the number of present healthy lifestyles and risk of CRC from these studies generally showed an inverse linear relationship, as displayed in Figure 3A. The AIC was -54.7 for linear model (Figure 3B) and -44.1 for model using cubic splines (Figure 3C). Given the lower AIC, the linear model was considered better-fitting and was adopted for further analysis. Overall, the pooled HR for CRC was 0.91 (95% CI 0.88-0.94) per 1-unit increase in the number of healthy lifestyles, similar to the overall estimate in the meta-analysis of continuous lifestyle scores.
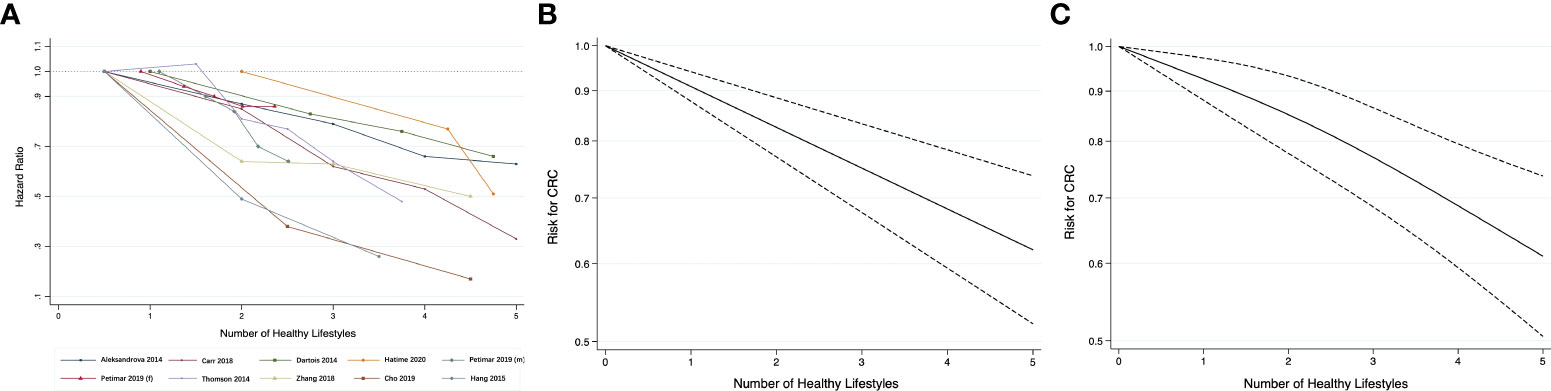
Figure 3 (A) Line graph of association between healthy lifestyles and risk for CRC; dose-response relationship between the number of healthy lifestyles and the risk for CRC: (B) Linear trend; (C) Restricted cubic splines.
The result of subgroup analyses was presented in Figure 4. Overall, the inverse association between healthy lifestyle and risk for CRC was consistently observed within each subgroup (HR ranging from 0.26 to 0.86), and the association was statistically significant for all subgroups except among African Americans. Similarly, the inverse association for colon cancer was statistically significant in all subgroups, except among African Americans (Supplementary Figure 2). The associations for rectal cancer similarly remained directionally consistent with the primary analysis, although statistical significance was not reached in some subgroups (Supplementary Figure 3).
In sensitivity analysis, we conducted separate meta-analysis for risk of CRC of (1) all studies after excluding the two studies of low quality (34, 45); and (2) the three studies that adjusted for a relative comprehensive list of covariates (socio-demographic factors, family history, and intake of nutritional supplement and nonsteroidal anti-inflammatory drugs at baseline) (45, 49, 50). The results of these analyses were consistent with main analysis (Supplementary Figure 4).
Overall Risk for Colorectal Adenoma and Advanced Colorectal Neoplasia
Four studies (21,697 participants) reporting risk for incident colorectal adenoma using categorical lifestyle variables were included in the analysis (Figure 2), and the pooled HR was 0.39 (95% CI 0.29-0.53, I2= 90.1%). Two studies reported the risk for advanced colorectal neoplasia and the pooled HR for the healthiest group was 0.43 (95% CI 0.33-0.57, I2= 0.0%).
Overall Risk for CRC-Specific Mortality
Five studies with 174,982 participants were included in the analysis of CRC-specific mortality. The group with the highest lifestyle score showed 35% lower risk (HR=0.65, 95% CI 0.52-0.81, I2= 37.5%) compared to the group with lowest score (Figure 2). Using continuous lifestyle score, 1-unit increase in healthy lifestyle score was associated with an HR of 0.84 (95% CI 0.77-0.91) (Supplementary Figure 1). Subgroup analyses showed largely consistent results of similar directions (Supplementary Figure 5).
Publication Bias
The result of Egger’s test suggested no evidence of significant publication bias (p=0.23 for CRC risk, p=0.09 for colon cancer risk). The funnel plots for these two outcomes with more than 10 studies showed overall asymmetrical pattern (Supplementary Figure 6).
Discussion
This systematic review and meta-analysis found that adopting multiple healthy lifestyles is associated with a considerably lower risk of multiple colorectal diseases. Compared with individuals with the least healthy lifestyle, those with the healthiest lifestyle had 48%, 46% and 49% lower risk of CRC, colon cancer, and rectal cancer, respectively. The associations were consistent across populations with different socio-demographic characteristics. A dose-response relationship between the number of healthy lifestyles and risk of CRC was identified, and adoption of each additional healthy lifestyle lowers the risk of CRC by 9% on average. We have also found that adherence to the healthiest lifestyle was associated with 61% lower risk of incident colorectal adenoma and 57% lower risk of advanced colorectal neoplasia. Among CRC survivors, those with the healthiest lifestyle had 31% lower risk of CRC-specific mortality.
The dose-response relationship between various individual lifestyle factors and CRC risk has been well established. It is reported that the relative risk for developing CRC is 0.90 for an increase of 10 g/day of dietary fibre (14), 1.24 for 120 g/day increase of red meat, 1.36 for 30 g/day increase of processed meat (66), 1.34 for one-point increase of Dietary Inflammatory Index (67), 1.38 for 50 g/day increase of alcohol intake (68), 1.07 for 2 kg/m2 increase in BMI, 1.04 for 2-cm increase in waist circumference (69), and 0.99 for 1 metabolic equivalent task (MET)-hour/week increase when the physical activity is over 10 MET-hour/week (70). In this study, we further revealed a dose-response association between the number of adopted healthy lifestyles and CRC risk, which further supports the significant difference in CRC risk between those with the healthiest lifestyle and those with the least. Previous studies have reported that healthy or unhealthy lifestyles tend to aggregate in individuals (71, 72), and the prevalence of adopting a healthiest lifestyle is generally low among general populations. For example, only 5.7% of the study population reported having all four healthy lifestyles (non-smoking, low alcohol consumption, sufficient fruit and vegetable consumption, regular physical activity) in England (73), while in Netherland, approximately 20% of the general population presented at least three of the five unhealthy lifestyles (smoking, low vegetable and fruit consumption, excessive alcohol intake, low physical activity) examined and all lifestyle factors showed significant clustering (72). It can, therefore, be expected that promotion of all healthy lifestyles among the populations could produce a synergistic effect on preventing CRC. A prospective study from the US estimated that 71% of colon cancer risk was attributable to a combination of unhealthy lifestyles, including being overweight, physical inactivity, alcohol consumption, smoking, and unhealthy diet (74). A prospective study in Denmark estimated that an overall 16% of the new CRC cases (22% for male and 11% for female) were attributable to lack of adherence to a combination of five healthy lifestyle factors (healthy weight, physical activity, non-smoking, limited alcohol consumption, healthy diet) (52).
The subgroup analyses showed that associations between multiple lifestyle factors and colorectal cancer risk were largely consistent across different age groups, sexes, geographic settings, and ethnicities. This suggests that the promoting healthy lifestyles could benefit populations universally regardless of their demographic characteristics. However, it should be noted that the association was found not statistically significant in the group of African American, but given that only one study was included in this group, future studies with bigger sample size are warranted to further explore the association among this ethnicity.
A previous meta-analysis concluded that adherence to at least four of the five healthy lifestyles examined (non-smoking, normal weight, healthy diet, moderate or lower alcohol consumption, and regular physical activity) could reduce all-cause mortality by 66% compared to those with no more than one healthy lifestyle (75). Our result suggested that adopting the healthiest lifestyle lowers CRC-specific mortality by 35%, and this protective effect was found significant among both CRC patients and healthy populations. This indicates that improving lifestyles could significantly benefit CRC survivors. Previous evidence has demonstrated that a variety of interventions are effective in improving awareness of CRC risk factors and facilitating adoption of healthy lifestyles among CRC patients after diagnosis, including telephone-delivered coaching (76), combined exercise and dietary advice (77), and education and behavioral change techniques (78). Such strategies could be considered as an integral part of CRC management to improve survival outcomes.
This study is the first systematic review and meta-analysis to reveal the dose-response relationship between the number of healthy lifestyles and CRC risk. Given the lack of large randomized controlled trials to examine the effect of adopting multiple healthy lifestyles on the risk of CRC and CRA as well as the survival outcomes of CRC patients, our study has provided high quality evidence by including a pooled sample of more than one million participants and generating results that are unlikely to be affected by publication bias. Our findings support the recommendations by the World Health Organization (79), American Cancer Society (80), and WCRF/AICF (81) on prevention and management of cancer. Adopting healthy lifestyles could not only prevent colorectal adenoma and CRC among the general population, but also improve clinical outcomes among CRC survivors. Nonetheless, international evidence has shown that population at risk of CRC generally demonstrated low awareness of lifestyle risk factors of CRC, particularly the effect of weight and physical activity (78, 82, 83). It would be strategic to provide information to increase awareness of lifestyle risk factors and promote interventions targeting behavior change among both healthy populations and CRC patients. Similar to our findings, previous meta-analyses have revealed that adopting multiple healthy lifestyles is associated with lower risk for cardiovascular disease (84), all-cause mortality (85), and type 2 diabetes (86), and such associations are generally found to be consistent among different populations. Hence, promoting healthy lifestyles could produce health benefits not only for CRC, but also for a variety of other health outcomes.
A few limitations should be noted when interpreting the study results. First, composition of healthy lifestyle and definitions of lifestyle factors varied considerably across studies, which may introduce heterogeneity to meta-analysis. We used random-effects model to minimize the effect of heterogeneity on the overall estimates. To explore the potential heterogeneity caused by this variation, we conducted subgroup analysis based on scoring system and factor composition. Although heterogeneity remained substantial within subgroups, the protective effect was still consistent within each group. For dose-response relationship, we only included studies using unweighted score system to exclude this attrition. Second, most original studies are from high-income, Western settings whose populations are comprised predominantly of Caucasians. Hence, more evidence from other populations, particularly Asian and African populations is needed. Third, only five studies have reported on CRC-specific mortality, which may restrict the power of performing stratified analyses. Fourth, socio-economic status is a key determinant for individual lifestyles (87–89), but few included studies have fully adjusted for all socio-economic factors. Other factors related to CRC risk, such as the use of certain pharmacological agents and nutritional supplements at baseline, were not collected and therefore not adjusted for in some studies. Despite this heterogeneity of covariate adjustment, the consistent finding from sensitivity analysis supports the robustness of the pooled estimate from the main analysis. Lastly, immortal time bias may exist in the original cohort studies assessing mortality given the possible time gap between study initiation and exposure assessment.
In conclusion, the number of healthy lifestyle attributes is inversely correlated with the risk of colorectal adenoma, cancer, and CRC-specific mortality. Lifestyle interventions could effectively reduce incidence of CRC. Future research may explore the effect of complex interventions targeting multiple lifestyle factors on prevention and management of CRC; randomized controlled trial is needed to provide high-quality evidence on the combined effect of healthy lifestyles and CRC risk.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
JY, QF, and YZ designed the research. JY and QF conducted literature search and performed data extraction and meta-analysis. JHK and YZ reviewed studies for inclusion. JY, QF, JHK, and YZ contributed to the interpretation of data. JY drafted the paper. QF, JHK, and YZ made substantial contribution to the critical revision and editing of the manuscript. All authors contributed to the article and approved the submission version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.827019/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–249. doi: 10.3322/caac.21660
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Fearon ER, Vogelstein B. A Genetic Model for Colorectal Tumorigenesis. Cell (1990) 61(5):759–67. doi: 10.1016/0092-8674(90)90186-I
4. Song M, Hu FB, Spiegelman D, Chan AT, Wu K, Ogino S, et al. Long-Term Status and Change of Body Fat Distribution, and Risk of Colorectal Cancer: A Prospective Cohort Study. Int J Epidemiol (2016) 45(3):871. doi: 10.1093/ije/dyv177
5. Dong Y, Zhou J, Zhu Y, Luo L, He T, Hu H, et al. Abdominal Obesity and Colorectal Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Biosci Rep (2017) 37(6):20170945. doi: 10.1042/BSR20170945
6. Hong S, Cai Q, Chen D, Zhu W, Huang W, Li Z. Abdominal Obesity and the Risk of Colorectal Adenoma: A Meta-Analysis of Observational Studies. Eur J Cancer Prev (2012) 21(6):523–31. doi: 10.1097/CEJ.0b013e328351c775
7. Wolin KY, Yan Y, Colditz GA. Physical Activity and Risk of Colon Adenoma: A Meta-Analysis. Br J Cancer (2011) 104(5):882–5. doi: 10.1038/sj.bjc.6606045
8. Schmid D, Leitzmann MF. Television Viewing and Time Spent Sedentary in Relation to Cancer Risk: A Meta-Analysis. JNCI J Natl Cancer Inst (2014) 106(7):dju098. doi: 10.1093/jnci/dju098
9. Vieira A, Abar L, Chan D, Vingeliene S, Polemiti E, Stevens C, et al. Foods and Beverages and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies, an Update of the Evidence of the WCRF-AICR Continuous Update Project. Ann Oncol Off J Eur Soc Med Oncol (2017) 28(8):1788–802. doi: 10.1093/annonc/mdx171
10. Xu X, Yu E, Gao X, Song N, Liu L, Wei X, et al. Red and Processed Meat Intake and Risk of Colorectal Adenomas: A Meta-Analysis of Observational Studies. Int J Cancer (2013) 132(2):437–48. doi: 10.1002/ijc.27625
11. International Agency for Research on Cancer. Agents Classified by the IARC Monographs, Vol. 1-123. (2018). Lyon: IARC
12. Zhu J-Z, Wang Y-M, Zhou Q-Y, Zhu K-F, Yu C-H, Li Y-M. Systematic Review With Meta-Analysis: Alcohol Consumption and the Risk of Colorectal Adenoma. Aliment Pharmacol Ther (2014) 40(4):325–37. doi: 10.1111/apt.12841
13. Giovannucci E, Marti ME. Tobacco, Colorectal Cancer, and Adenomas: A Review of the Evidence. J Natl Cancer Institute J Natl Cancer Inst (1996) 88:1717–30. doi: 10.1093/jnci/88.23.1717
14. Aune D, Chan DSM, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary Fibre, Whole Grains, and Risk of Colorectal Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMJ (2011) 343(7833):1082. doi: 10.1136/bmj.d6617
15. Dahm C, Keogh R, Spencer E, Greenwood D, Key T, Fentiman I, et al. Dietary Fiber and Colorectal Cancer Risk: A Nested Case-Control Study Using Food Diaries. J Natl Cancer Inst (2010) 102(9):614–26. doi: 10.1093/jnci/djq092
16. Millen AE, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, et al. Fruit and Vegetable Intake and Prevalence of Colorectal Adenoma in a Cancer Screening Trial. Am J Clin Nutr (2007) 86(6):1754–64. doi: 10.1093/ajcn/86.5.1754
17. Nucci D, Fatigoni C, Salvatori T, Nardi M, Realdon S, Gianfredi V. Association Between Dietary Fibre Intake and Colorectal Adenoma: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health (2021) 18(8):4168. doi: 10.3390/ijerph18084168
18. Harriss DJ, Atkinson G, George K, Tim Cable N, Reilly T, Haboubi N, et al. Lifestyle Factors and Colorectal Cancer Risk (1): Systematic Review and Meta-Analysis of Associations With Body Mass Index. Color Dis (2009) 11(6):547–63. doi: 10.1111/j.1463-1318.2009.01766.x
19. Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, et al. Lifestyle Factors and Colorectal Cancer Risk (2): A Systematic Review and Meta-Analysis of Associations With Leisure-Time Physical Activity. Color Dis (2009) 11(7):689–701. doi: 10.1111/j.1463-1318.2009.01767.x
20. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and Colorectal Cancer: A Meta-Analysis. JAMA (2008) 300(23):2765–78. doi: 10.1001/jama.2008.839
21. Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The Impact of Dietary and Lifestyle Risk Factors on Risk of Colorectal Cancer: A Quantitative Overview of the Epidemiological Evidence. Int J Cancer (2009) 125(1):171–80. doi: 10.1002/ijc.24343
22. Zhang Y-B, Pan X-F, Chen J, Cao A, Zhang Y-G, Xia L, et al. Combined Lifestyle Factors, Incident Cancer, and Cancer Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Br J Cancer (2020) 122(7):1085–93. doi: 10.1038/s41416-020-0741-x
23. Van Zutphen M, Kampman E, Giovannucci EL, Van Duijnhoven FJB. Lifestyle After Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr Colorectal Cancer Rep (2017) 13(5):370–401. doi: 10.1007/s11888-017-0386-1
24. Jayasekara H, English DR, Haydon A, Hodge AM, Lynch BM, Rosty C, et al. Associations of Alcohol Intake, Smoking, Physical Activity and Obesity With Survival Following Colorectal Cancer Diagnosis by Stage, Anatomic Site and Tumor Molecular Subtype. Int J Cancer (2018) 142(2):238–50. doi: 10.1002/ijc.31049
25. Haydon AMM, MacInnis RJ, English DR, Giles GG. Effect of Physical Activity and Body Size on Survival After Diagnosis With Colorectal Cancer. Gut (2006) 55(1):62. doi: 10.1136/gut.2005.068189
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
27. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 9):25. doi: 10.1007/s10654-010-9491-z
28. Amedeo Modesti P, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis Working Group on CV Risk in Low Resource Settings. PLoS ONE (2016) 11(1): e0147601. doi: 10.1371/journal.pone.0147601
29. Carr PR, Weigl K, Jansen L, Walter V, Erben V, Chang-Claude J, et al. Healthy Lifestyle Factors Associated With Lower Risk of Colorectal Cancer Irrespective of Genetic Risk. Gastroenterology (2018) 155(6):1805-1815.e5.
30. Hang J, Cai B, Xue P, Wang L, Hu H, Zhou Y, et al. The Joint Effects of Lifestyle Factors and Comorbidities on the Risk of Colorectal Cancer: A Large Chinese Retrospective Case-Control Study. PloS One (2015) 10(12):e0143696. doi: 10.1371/journal.pone.0143696
31. Kirkegaard H, Johnsen NF, Christensen J, Frederiksen K, Overvad K, Tjønneland A. Association of Adherence to Lifestyle Recommendations and Risk of Colorectal Cancer: A Prospective Danish Cohort Study. BMJ (2010) 341(7780):978. doi: 10.1136/bmj.c5504
32. Harnack L, Nicodemus K, Jacobs DR, Folsom AR. An Evaluation of the Dietary Guidelines for Americans in Relation to Cancer Occurrence. Am J Clin Nutr (2002) 76(4):889–96. doi: 10.1093/ajcn/76.4.889
33. Romaguera D, Ward H, Wark PA, Vergnaud AC, Peeters PH, van Gils CH, et al. Pre-Diagnostic Concordance With the WCRF/AICR Guidelines and Survival in European Colorectal Cancer Patients: A Cohort Study. BMC Med (2015) 13(1):45. doi: 10.1186/s12916-015-0332-5
34. Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, et al. Genetic Risk Score, Combined Lifestyle Factors and Risk of Colorectal Cancer. Cancer Res Treat (2019) 51(3):1033–40. doi: 10.4143/crt.2018.447
35. Erdrich J, Zhang X, Giovannucci E, Willett W. Proportion of Colon Cancer Attributable to Lifestyle in a Cohort of US Women. Cancer Causes Control (2015) 26(9):1271–9. doi: 10.1007/s10552-015-0619-z
36. Zhang J, Yu KF. What’s the Relative Risk? A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. J Am Med Assoc (1998) 280(19):1690–1. doi: 10.1001/jama.280.19.1690
37. Shor E, Roelfs D, Vang ZM. The “Hispanic Mortality Paradox” Revisited: Meta-Analysis and Meta-Regression of Life-Course Differentials in Latin American and Caribbean Immigrants’ Mortality. Soc Sci Med (2017) 186:20–33. doi: 10.1016/j.socscimed.2017.05.049
38. Higgins JPT, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
39. Ju SY, Lee JY, Kim DH. Low 25-Hydroxyvitamin D Levels and the Risk of Frailty Syndrome: A Systematic Review and Dose-Response Meta-Analysis. BMC Geriatr (2018) 4;18(1):206. doi: 10.1186/s12877-018-0904-2
40. Egger M, Smith GD, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Br Med J (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
41. Orsini N, Bellocco R, Greenland S. Generalized Least Squares for Trend Estimation of Summarized Dose-Response Data. Stata J DPC Nederland (2006) 6:40–57. doi: 10.1177/1536867X0600600103
42. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. SecondNew York: Springer (2015).
43. Akaike H. A New Look at the Statistical Model Identification. IEEE Trans Automat Contr (1974) 19(6):716–23. doi: 10.1109/TAC.1974.1100705
44. Hastert TA, White E. Association Between Meeting the WCRF/AICR Cancer Prevention Recommendations and Colorectal Cancer Incidence: Results From the VITAL Cohort. Cancer Causes Control (2016) 27(11):1347–59. doi: 10.1007/s10552-016-0814-6
45. Hatime Z, El Kinany K, Huybrechts I, Gunter MJ, Khalis M, Deoula M, et al. Extended Healthy Lifestyle Index and Colorectal Cancer Risk in the Moroccan Population. Eur J Nutr (2021) 60:1013–22. doi: 10.1007/s00394-020-02311-3
46. Jones P, Cade JE, Evans CEL, Hancock N, Greenwood DC. Does Adherence to the World Cancer Research Fund/American Institute of Cancer Research Cancer Prevention Guidelines Reduce Risk of Colorectal Cancer in the UK Women’s Cohort Study? Br J Nutr (2018) 119(3):340–8. doi: 10.1017/S0007114517003622
47. Nomura SJO, Dash C, Rosenberg L, Yu J, Palmer JR, Adams-Campbell LL. Is Adherence to Diet, Physical Activity, and Body Weight Cancer Prevention Recommendations Associated With Colorectal Cancer Incidence in African American Women? Cancer Causes Control (2016) 27(7):869–79. doi: 10.1007/s10552-016-0760-3
48. Odegaard AO, Koh WP, Yuan JM. Combined Lifestyle Factors and Risk of Incident Colorectal Cancer in a Chinese Population. Cancer Prev Res (2013) 6(4):360–7. doi: 10.1158/1940-6207
49. Petimar J, Smith-Warner SA, Rosner B, Chan AT, Giovannucci EL, Tabung FK. Adherence to the World Cancer Research Fund/American Institute for Cancer Research 2018 Recommendations for Cancer Prevention and Risk of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev (2019) 28(9):1469–79. doi: 10.1158/1055-9965.EPI-19-0165
50. Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, et al. Nutrition and Physical Activity Cancer Prevention Guidelines, Cancer Risk, and Mortality in the Women’s Health Initiative. Cancer Prev Res (2014) 7(1):42–53. doi: 10.1158/1940-6207.CAPR-13-0258
51. Zhang QL, Zhao LG, Li HL, Gao J, Yang G, Wang J, et al. The Joint Effects of Major Lifestyle Factors on Colorectal Cancer Risk Among Chinese Men: A Prospective Cohort Study. Int J Cancer (2018) 142(6):1093–101. doi: 10.1002/ijc.31126
52. Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T, et al. Combined Impact of Healthy Lifestyle Factors on Colorectal Cancer: A Large European Cohort Study. BMC Med (2014) 12(1):1–15. doi: 10.1186/s12916-014-0168-4
53. Barrubés L, Babio N, Hernández-Alonso P, Toledo E, Ramírez Sabio JB, Estruch R, et al. Association Between the 2018 WCRF/AICR and the Low-Risk Lifestyle Scores With Colorectal Cancer Risk in the Predimed Study. J Clin Med (2020) 9(4):1215. doi: 10.3390/jcm9041215
54. Cheng E, Um CY, Prizment AE, Lazovich DA, Bostick RM. Evolutionary-Concordance Lifestyle and Diet and Mediterranean Diet Pattern Scores and Risk of Incident Colorectal Cancer in Iowa Women. Cancer Epidemiol Biomarkers Prev (2018) 27(10):1195–202. doi: 10.1158/1055-9965.EPI-17-1184
55. Dartois L, Fagherazzi G, Boutron-Ruault MC, Mesrine S, Clavel-Chapelon F. Association Between Five Lifestyle Habits and Cancer Risk: Results From the E3N Cohort. Cancer Prev Res (2014) 7(5):516–25. doi: 10.1158/1940-6207.CAPR-13-0325
56. Byrd DA, Judd S, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Associations of Novel Dietary and Lifestyle Inflammation Scores With Incident, Sporadic Colorectal Adenoma. Cancer Epidemiol Biomarkers Prev (2020) 29(11):2300–8. doi: 10.1158/1055-9965.EPI-20-0568
57. Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Strong Associations of a Healthy Lifestyle With All Stages of Colorectal Carcinogenesis: Results From a Large Cohort of Participants of Screening Colonoscopy. Int J Cancer (2019) 144(9):2135–43. doi: 10.1002/ijc.32011
58. Fliss-Isakov N, Kariv R, Webb M, Ivancovsky-Wajcman D, Zaslavsky O, Margalit D, et al. A Healthy Lifestyle Pattern has a Protective Association With Colorectal Polyps. Eur J Clin Nutr (2020) 74(2):328–37. doi: 10.1038/s41430-019-0481-2
59. Fu Z, Shrubsole MJ, Smalley WE, Wu H, Chen Z, Shyr Y, et al. Lifestyle Factors and Their Combined Impact on the Risk of Colorectal Polyps. Am J Epidemiol (2012) 176(9):766–76. doi: 10.1093/aje/kws157
60. Tabung FK, Steck SE, Burch JB, Chen CF, Zhang H, Hurley TG, et al. A Healthy Lifestyle Index Is Associated With Reduced Risk of Colorectal Adenomatous Polyps Among Non-Users of Non-Steroidal Anti-Inflammatory Drugs. J Prim Prev (2015) 36(1):21–31. doi: 10.1007/s10935-014-0372-1
61. Knudsen MD, De Lange T, Botteri E, Nguyen DH, Evensen H, Steen CB, et al. Favorable Lifestyle Before Diagnosis Associated With Lower Risk of Screen-Detected Advanced Colorectal Neoplasia. World J Gastroenterol (2016) 22(27):6276–86. doi: 10.3748/wjg.v22.i27.6276
62. Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR Guidelines for Cancer Prevention is Associated With Lower Mortality Among Older Female Cancer Survivors. Cancer Epidemiol Biomarkers Prev (2013) 22(5):792–802. doi: 10.1158/1055-9965.EPI-13-0054
63. Lohse T, Faeh D, Bopp M, Rohrmann S. Adherence to the Cancer Prevention Recommendations of the World Cancer Research Fund/American Institute for Cancer Research and Mortality: A Census-Linked Cohort. Am J Clin Nutr (2016) 104(3):678–85. doi: 10.3945/ajcn.116.135020
64. Sotos-Prieto M, Mattei J, Cook NR, Hu FB, Willett WC, Chiuve SE, et al. Association Between a 20-Year Cardiovascular Disease Risk Score Based on Modifiable Lifestyles and Total and Cause-Specific Mortality Among US Men and Women. J Am Heart Assoc (2018) 7(21):e010052. doi: 10.1161/JAHA.118.010052
65. GBD 2016 Alcohol Collaborators MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol Use and Burden for 195 Countries and Territories, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet (London England) (2018) 392(10152):1015–35. doi: 10.1016/S0140-6736(18)31310-2
66. Norat T, Lukanova A, Ferrari P, Riboli E. Meat Consumption and Colorectal Cancer Risk: Dose-Response Meta-Analysis of Epidemiological Studies. Int J Cancer (2002) 98(2):241–56. doi: 10.1002/ijc.10126
67. Syed Soffian SS, Mohammed Nawi A, Hod R, Ja’afar MH, Isa ZM, Chan HK, et al. Meta-Analysis of the Association Between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutr (2022) 14(8):1555.
68. Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol Drinking and Colorectal Cancer Risk: An Overall and Dose–Response Meta-Analysis of Published Studies. Ann Oncol (2011) 22(9):1958–72. doi: 10.1093/annonc/mdq653
69. Moghaddam AA, Woodward M, Huxley R. Obesity and Risk of Colorectal Cancer: A Meta-Analysis of 31 Studies With 70,000 Events. Cancer Epidemiol Prev Biomarkers (2007) 16(12):2533–47. doi: 10.1158/1055-9965.EPI-07-0708
70. Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, et al. The Dose-Response Effect of Physical Activity on Cancer Mortality: Findings From 71 Prospective Cohort Studies. Br J Sports Med (2016) 50(6):339–45. doi: 10.1136/bjsports-2015-094927
71. Morris LJ, D’Este C, Sargent-Cox K, Anstey KJ. Concurrent Lifestyle Risk Factors: Clusters and Determinants in an Australian Sample. Prev Med (Baltim) (2016) 84:1–5. doi: 10.1016/j.ypmed.2015.12.009
72. Schuit AJ, Van Loon AJM, Tijhuis M, Ocké MC. Clustering of Lifestyle Risk Factors in a General Adult Population. Prev Med (Baltim) (2002) 35(3):219–24. doi: 10.1006/pmed.2002.1064
73. Poortinga W. The Prevalence and Clustering of Four Major Lifestyle Risk Factors in an English Adult Population. Prev Med (Baltim) (2007) 44(2):124–8. doi: 10.1016/j.ypmed.2006.10.006
74. Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of Colon Cancer Risk That Might be Preventable in a Cohort of Middle-Aged US Men. Cancer Causes Control (2000) 11(7):579–88. doi: 10.1023/A:1008999232442
75. Loef M, Walach H. The Combined Effects of Healthy Lifestyle Behaviors on All Cause Mortality: A Systematic Review and Meta-Analysis. Prev Med (2012) 55p:163–70. doi: 10.1016/j.ypmed.2012.06.017
76. Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, et al. Effects of a Telephone-Delivered Multiple Health Behavior Change Intervention (CanChange) on Health and Behavioral Outcomes in Survivors of Colorectal Cancer: A Randomized Controlled Trial. J Clin Oncol (2013) 31(18):2313–21. doi: 10.1200/JCO.2012.45.5873
77. Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, et al. Pragmatic Lifestyle Intervention in Patients Recovering From Colon Cancer: A Randomized Controlled Pilot Study. Arch Phys Med Rehabil (2011) 92(5):749–55. doi: 10.1016/j.apmr.2010.12.020
78. Anderson AS, Caswell S, Macleod M, Craigie AM, Stead M, Steele RJC, et al. Awareness of Lifestyle and Colorectal Cancer Risk: Findings From the BeWEL Study. BioMed Res Int (2015) 2015:871613. doi: 10.1155/2015/871613
79. World Health Organization. Tackling NCDs: 'best buys' and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases. Geneva: World Health Organization (2017).
80. Kushi L, Doyle C, McCullough M, CRock C, Demark-Wahnefried W, Bandera E, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention: Reducing the Risk of Cancer With Healthy Food Choices and Physical Activity. CA Cancer J Clin (2012) 62(1):30–67. doi: 10.3322/caac.20140
81. World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective A Summary of the Third Expert Report. Washington DC: AICR (2018).
82. Keighley MRB, O’Morain C, Giacosa A, Ashorn M, Burroughs A, Crespi M, et al. Public Awareness of Risk Factors and Screening for Colorectal Cancer in Europe. Eur J Cancer Prev (2004) 13(4):257–62. doi: 10.1097/01.cej.0000136575.01493.9b
83. Lynes K, Kazmi SA, Robery JD, Wong S, Gilbert D, Thaha MA. Public Appreciation of Lifestyle Risk Factors for Colorectal Cancer and Awareness of Bowel Cancer Screening: A Cross-Sectional Study. Int J Surg (2016) 36:312–8. doi: 10.1016/j.ijsu.2016.11.002
84. Tsai M-C, Lee C-C, Liu S-C, Tseng P-J, Chien K-L. Combined Healthy Lifestyle Factors are More Beneficial in Reducing Cardiovascular Disease in Younger Adults: A Meta-Analysis of Prospective Cohort Studies. Sci Rep (2020) 10:18165. doi: 10.1038/s41598-020-75314-z
85. Zhang Y-B, Pan X-F, Chen J, Cao A, Xia L, Zhang Y, et al. Combined Lifestyle Factors, All-Cause Mortality and Cardiovascular Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J Epidemiol Community Heal (2021) 75(1):92–9. doi: 10.1136/jech-2020-214050
86. Zhang Y, Pan XF, Chen J, Xia L, Cao A, Zhang Y, et al. Combined Lifestyle Factors and Risk of Incident Type 2 Diabetes and Prognosis Among Individuals With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Diabetol Diabetol (2020) 63:21–33. doi: 10.1007/s00125-019-04985-9
87. Roos E, Prättälä R, Lahelma E, Kleemola P, Pietinen P. Modern and Healthy?: Socioeconomic Differences in the Quality of Diet. Eur J Clin Nutr (1996) 50(11):753–60.
88. Ball K, Crawford D. Socioeconomic Status and Weight Change in Adults: A Review. Soc Sci Med (2005) 60(9):1987–2010. doi: 10.1016/j.socscimed.2004.08.056
Keywords: colorectal, lifestyle, index, incident, dose-response, prevention
Citation: Yu J, Feng Q, Kim JH and Zhu Y (2022) Combined Effect of Healthy Lifestyle Factors and Risks of Colorectal Adenoma, Colorectal Cancer, and Colorectal Cancer Mortality: Systematic Review and Meta-Analysis. Front. Oncol. 12:827019. doi: 10.3389/fonc.2022.827019
Received: 01 December 2021; Accepted: 20 June 2022;
Published: 22 July 2022.
Edited by:
Yawei Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Azam Majidi, QIMR Berghofer Medical Research Institute, AustraliaDaniele Nucci, Veneto Institute of Oncology (IRCCS), Italy
Copyright © 2022 Yu, Feng, Kim and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Zhu, emh1eW1Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jiazhou Yu
Jiazhou Yu Qi Feng
Qi Feng Jean H. Kim
Jean H. Kim Yimin Zhu
Yimin Zhu