- 1Digestive Disease Center, Changbing Show-Chwan Memorial Hospital, Lukang, Taiwan
- 2Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 3Department of Food Science and Technology, Hungkuang University, Taichung, Taiwan
- 4Institue of Population Health Sciences, National Health Research Institutes, Zhunan, Taiwan
- 5College of Public Health, China Medical University, Taichung, Taiwan
- 6Graduate Institute of Clinical Medical Science, China Medical University, Taichung, Taiwan
- 7Long Beach VAMC Hospital, University of Irvine Medical Center, Irvine, CA, United States
- 8Center for Biostatistics, Bioinformatics and Big Data, The Second Affiliated Hospital and School of Public Health, Zhejiang University School of Medicine, Hangzhou, China
- 9National Institute for Data Science in Health and Medicine, Zhejiang University, Hangzhou, China
- 10Department of Obstetrics and Gynecology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 11Taipei MJ Health Screening Center, Taipei, Taiwan
Although the link between sugar-sweetened beverages (SSB) and pancreatic cancer has been suggested for its insulin-stimulating connection, most epidemiological studies showed inconclusive relationship. Whether the result was limited by sample size is explored. This prospective study followed 491,929 adults, consisting of 235,427 men and 256,502 women (mean age: 39.9, standard deviation: 13.2), from a health surveillance program and there were 523 pancreatic cancer deaths between 1994 and 2017. The individual identification numbers of the cohort were matched with the National Death file for mortality, and Cox models were used to assess the risk. The amount of SSB intake was recorded based on the average consumption in the month before interview by a structured questionnaire. We classified the amount of SSB intake into 4 categories: 0–<0.5 serving/day, ≥0.5–<1 serving per day, ≥1–<2 servings per day, and ≥2 servings per day. One serving was defined as equivalent to 12 oz and contained 35 g added sugar. We used the age and the variables at cohort enrolment as the reported risks of pancreatic cancers. The cohort was divided into 3 age groups, 20–39, 40–59, and ≥60. We found young people (age <40) had higher prevalence and frequency of sugar-sweetened beverages than the elderly. Those consuming 2 servings/day had a 50% increase in pancreatic cancer mortality (HR = 1.55, 95% CI: 1.08–2.24) for the total cohort, but a 3-fold increase (HR: 3.09, 95% CI: 1.44–6.62) for the young. The risk started at 1 serving every other day, with a dose–response relationship. The association of SSB intake of ≥2 servings/day with pancreatic cancer mortality among the total cohort remained significant after excluding those who smoke or have diabetes (HR: 2.12, 97% CI: 1.26–3.57), are obese (HR: 1.57, 95% CI: 1.08–2.30), have hypertension (HR: 1.90, 95% CI: 1.20–3.00), or excluding who died within 3 years after enrollment (HR: 1.67, 95% CI: 1.15–2.45). Risks remained in the sensitivity analyses, implying its independent nature. We concluded that frequent drinking of SSB increased pancreatic cancer in adults, with highest risk among young people.
Introduction
The public health significance of pancreatic cancer and its increasing mortality have been largely ignored. Most incidence died within a few years and incidence correlated with mortality. In the United States, a 1.6 and 1.9% increase per year in incidence and mortality of pancreatic cancer, respectively, has been observed (1–4). A trend of rapid increase in incidence has also been noted in Taiwan, with a 3-fold increase in incidence in the recent 2 decades (Supplementary Figures 1–2) (5, 6). Pancreatic cancer is now the fourth leading cause of cancer deaths in all age groups and the third leading cause of cancer deaths among people aged ≥40 in the United States (1, 2). Most people are more familiar with colorectal cancer than with pancreatic cancer; in 2021, colorectal cancer cases (149,500) were 2–3 times higher than pancreatic cancer cases (60,430) in the United States, which may contribute to less attention being paid to pancreatic cancer (1). However, the number of pancreatic cancer deaths has been increasing faster than that of colorectal cancer, approximating that of colorectal cancer in 2021 (pancreatic cancer: 48,220 vs. colorectal cancer: 52,980). This implies that the seriousness of pancreatic cancer or the need for its prevention has been under-appreciated. The reduction of pancreatic cancer cases merits the same attention as that devoted to reducing colorectal cancer cases.
Only a few lifestyle risk factors, such as smoking and drinking, have been identified for pancreatic cancer, but the size of increase in incidence due to these lifestyle risks is minimal (7–9). Moreover, smoking and drinking have both been highly prevalent in the United States and Taiwan for decades before the recent concerns regarding the rapidly increasing pancreatic cancer cases. Furthermore, neither smoking nor drinking could account for the strong increasing trend observed in the last 20 years (3, 10).
The overall consumption of sugar-sweetened beverages (SSBs), the main source of added sugars in diets of people, has remained high since 1970s (11–14). Furthermore, a survey conducted between 2013 and 2016 revealed that approximately 83.6% of Taiwanese adults (age: 19–44 years) consumed more than 1 serving/week of SSB, with a mean of 7.8 servings/week (15). Sweetened beverages in Asia also include a popular drink among young people—bubble milk tea—which is a Taiwan specialty drink containing tapioca. The amount of sugar added to this drink can be excessive. Studies have suggested an association between SSBs and pancreatic cancer (16–19), most probably due to SSB-induced rapid increase in blood sugar, which stimulates insulin secretion and cancer cell proliferation (20, 21). A high sugar intake induces hyperinsulinemia, consequently enhancing carcinogenesis by inhibiting apoptosis and downregulating binding protein 1 for the insulin-like growth factor (22). Fructose syrup, commonly added to sweetened beverages, is rapidly absorbed by glucose transporter 5 and easily induces insulin resistance by hampering the insulin signaling pathway (22). Compared with glucose, fructose is more readily utilized by pancreatic cancer cells through the non-oxidative pentose phosphate pathway to increase ribonucleic acid synthesis (23). The endocrine function of the pancreas and the cancer-causing nature of insulin have made the sugar drinks hypothesis plausible; however, evidence from most epidemiological studies addressing this causal association has been inconclusive (24–37).
SSB consumption has been reported to be associated with increased risks of cardiovascular diseases or all-cause mortality, implying the systemic nature of this risk (25, 38). This study evaluated the pancreatic cancer risk in a cohort of approximately half a million individuals attending a self-paid medical screening program. Completed lifestyle questionnaires and blood test results were collected. The cohort was divided into three age groups (20–39, 40–59, and ≥60 years) at enrollment to examine the association between specific cancers and drinks, and the independent nature of the association was assessed by adjusting for, or excluding, all known confounders. Given the paucity of our knowledge in preventing this highly fatal cancer, the assessment and quantification of risks induced by consuming sweetened beverages could be a crucial public health contribution.
Materials and Methods
Study Population
This prospective cohort study enrolled 491,929 individuals aged ≥20 years without known cancer history from the four MJ clinics that have been conducting a self-paid medical screening program across Taiwan with well-administered medical records since 1994 (39, 40). The follow-up period ranged from 1994 to 2017 (median period: 15 years; interquartile range: 9–20 years). The participants paid to become members of the MJ Health Management Institution and to undergo physical check-ups. The battery of examinations was completed within a few hours in the morning, and the complete interpretation and multidisciplinary education for individualized counseling was provided before the participants left the clinic in the afternoon. Many participants were willing to undergo repeated examinations as a result of the efficiency and friendliness of MJ.
Measurements
The participants underwent sequential blood, urine, and pulmonary function tests and also electrocardiography. They also underwent physical examination and a review of medical history. Moreover, the participants completed a self-report structured questionnaire about lifestyle.
Questionnaire and Lab Data
Hypertension and Diabetes
Hypertension was defined as a self-reported history of hypertension, systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mmHg, or use of antihypertensive agents. Diabetes was defined as a self-reported history of diabetes, fasting blood glucose level of ≥126 mg/dl, or use of hypoglycemic agents.
Assessing SSBs
The definition of total SSB included sugar-added drinks such as caffeinated or decaffeinated cola, carbonated SSBs, and noncarbonated SSBs (27). In addition, bubble milk tea, a Taiwan handmade specialty drink with high sugar content, was included. Natural fruit juice without added sugar was not classified as an SSB. The questionnaire referred to SSB consumption in the most recent month with four choices of answer to quantify the amount: 0 to <0.5 serving/day, ≥0.5 to <1 serving per day, ≥1 to <2 servings per day, and ≥2 servings per day. One serving was defined as equivalent to 12 oz or 350 ml and contained 150 Kcal or 35 g added sugar (41).
Assessing Physical Activity by Exercise Volume
We classified the leisure time physical activity of each individual into five groups: inactive (<3.75 metabolic equivalent task (MET)-h/week or <5 min/day), low activity (3.75–7.49 MET-h/week or approximately 15 min/day), moderate activity (7.50–16.49 MET-h/week or approximately 30 min/day), high activity (16.50–25.49 MET-h/week or approximately 60 min/day), and very high activity (≥25.50 MET-h/week or approximately 90 min/day or more) (40).
Assessment of Outcome
The individual identification numbers of the cohort were matched with the National Death File for mortality and with the National Cancer Registry for cancer incidence. We coded mortality according to the International Classification of Diseases, Ninth and Tenth revisions, with pancreatic cancer coded as 157 and C25, respectively. We referred to age and variables at cohort enrollment in this study. Written informed consent was provided by each participant, and this study was approved by the Institutional Review Board of China Medical University in Taiwan. All data were encrypted and remained anonymous during the entire study process.
Statistical Analysis
The association of SSB with pancreatic cancer can be attributed to unhealthy behaviors accompanying SSB intake, such as smoking or alcohol, or to metabolic syndrome resulting from SSB intake (13–15, 22–24, 38, 42, 43). To support the causal relationship between SSB and pancreatic cancer, we performed several sensitivity analyses to validate our findings: 1) to assess the dose–response relationship, we measured the p-value for the trend between SSB consumption and the pancreatic cancer mortality risk; 2) to mitigate the comorbid effect on pancreatic cancer development, we excluded the subgroups with smoking, alcohol consumption, obesity, hypertension, or diabetes; and 3) to avoid participants with reverse causality or incipient cancer before the study, we excluded those who died within 3 years after enrollment. We calculated the hazard ratios (HRs) with 95% confidence intervals of incidence and mortality by using the Cox model.
Univariate analysis was used to assess the possible risk factors of pancreatic cancer. Variables with statistical significance in univariate analysis were considered for multivariate analysis to assess the association between SSB intake and pancreatic cancer risk. For the total cohort, the HR was adjusted for age, sex, education level, smoking status, drinking status, physical activity, body mass index (BMI), hypertension, and diabetes. In the sensitivity analysis, adjustments for potential confounders were unnecessary. For age-classified models, the HR was adjusted for sex, education level, smoking status, drinking status, physical activity, BMI, hypertension, and diabetes. No violation of the proportional hazard assumption was noted in our study. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC), and a two-tailed P <0.05 was considered significant.
Results
Population Distribution of SSB Consumption
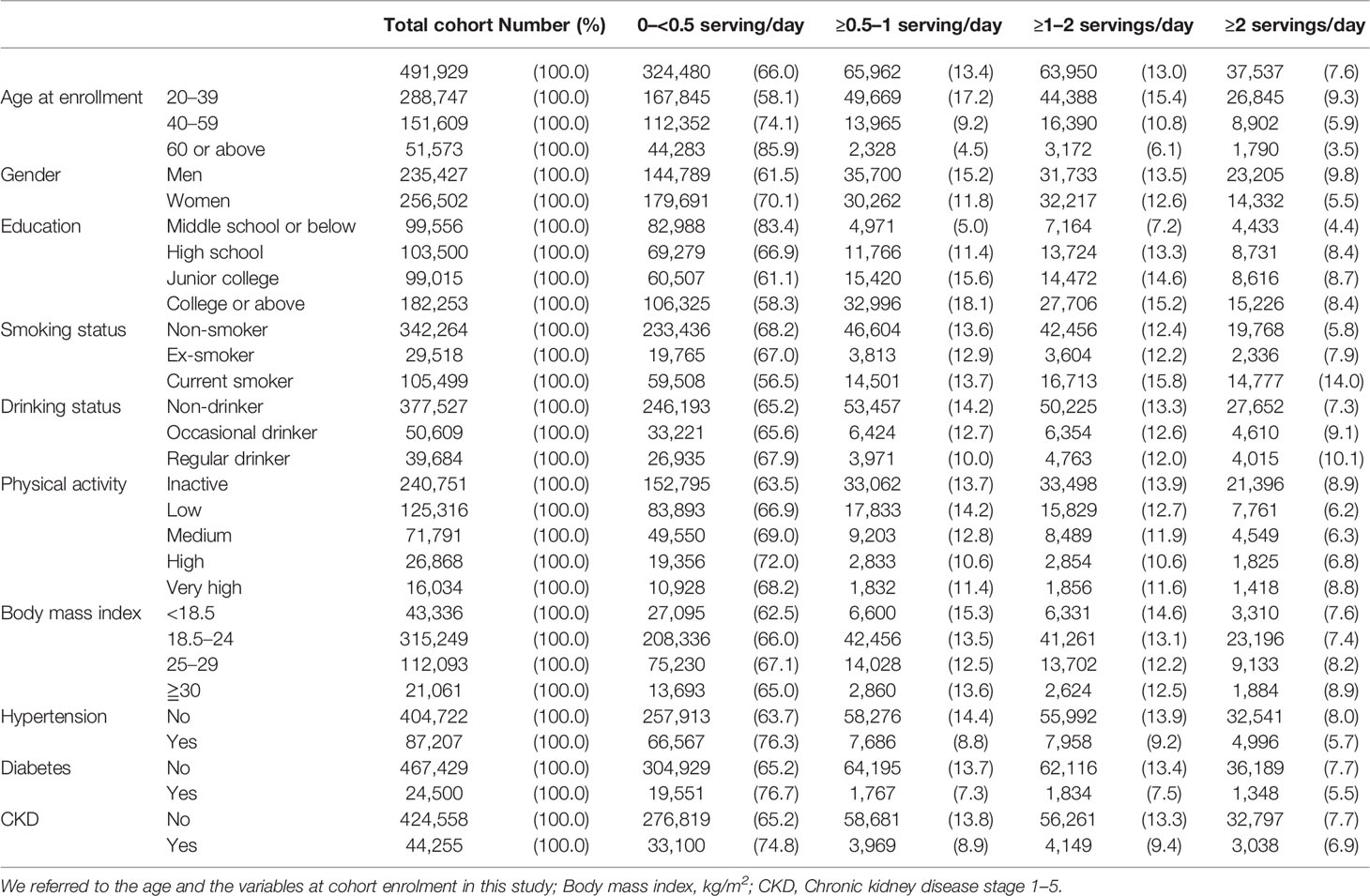
The study cohort included 491,929 participants, with 235,427 men and 256,502 women (mean age: 39.9 years, standard deviation: 13.2). The follow-up period was between 1994 and 2017 (median follow-up: 15 years). In total, 523 cases of pancreatic cancer deaths and 489 cases of incident pancreatic cancers were observed during a total of 89 million person-years during follow-up. Because the 5-year survival of pancreatic cancer is 10%, mortality data can be expected to be slightly less than incidence. However, mortality data were available for 2 additional years, censoring by 2017, compared with incidence data, censoring by 2015. Therefore, mortality registered in the National Death File might outnumber the incidence registered in the National Cancer Registry in our study. Table 1 indicates that the population distribution of SSB consumption from 0 to <0.5 serving/day to ≥2 servings/day tended to increase for those in the 20–39 years age group, men, current smokers, regular drinkers, and obese participants. One-fifth (20.6%) of the total cohort and one-fourth (24.7%) of the young population (age <40 years) reported SSB intake of ≥1 serving/day.
Risk Factors Associated With Pancreatic Cancer
Risk factors found from the Cox model for pancreatic cancer, either incidence or mortality, were age, male sex, SSB consumption, smoking, alcohol drinking, overweight or obesity (BMI ≥25 or 30 kg/m2), hypertension, and diabetes (Table 2).
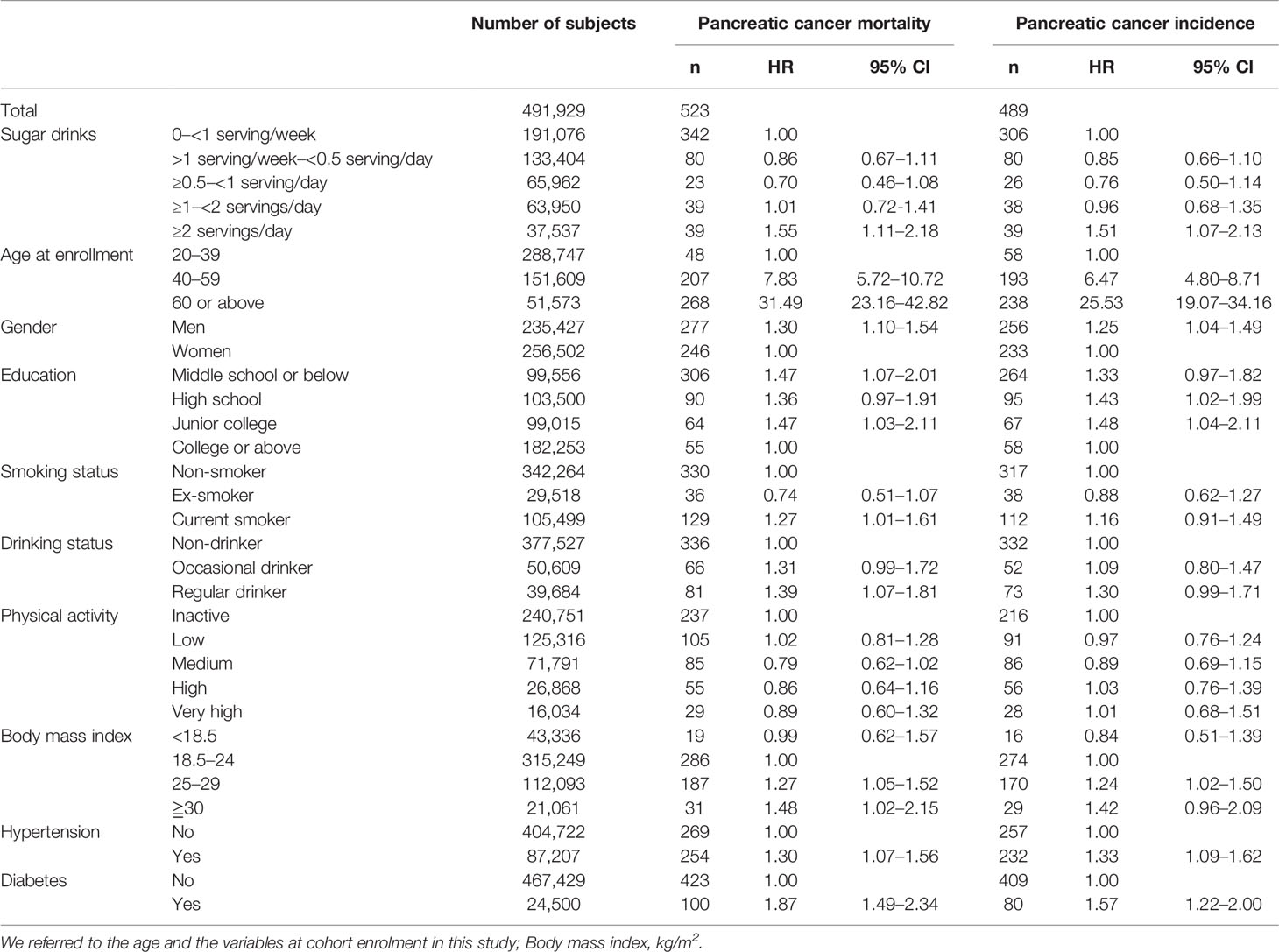
Table 2 Univariate analysis of the possible risk factors for pancreatic cancer mortality and incidence among the total cohort.
SSB Consumption and Pancreatic Cancer Risks
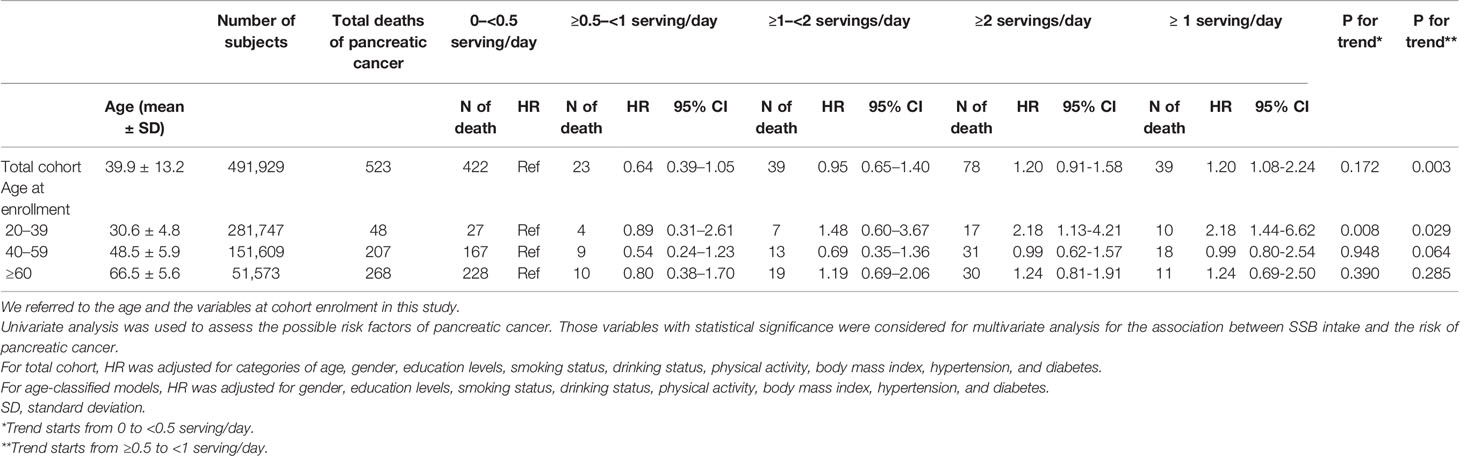
The total cohort exhibited a 50% increase in pancreatic cancer mortality (HR = 1.55, 95% CI: 1.08–2.24) and incidence (HR = 1.55, 95% CI: 1.08–2.23) for those consuming 2 servings/day of SSB, with a dose–response relationship observed when the intake was greater than 1 serving every other day (Table 3 and Supplementary Table 1).
Sensitivity Analysis for the Relation of SSB Consumption With Pancreatic Cancer
>Table 4 presents the results of sensitivity analysis for the relation of SSB consumption with pancreatic cancer mortality based on SSB consumption levels. The association of SSB intake of ≥2 servings/day with pancreatic cancer mortality among the total cohort was significant after excluding those who smoke or have diabetes (HR: 2.12, 97% CI: 1.26–3.57), are obese (HR: 1.57, 95% CI: 1.08–2.30), have hypertension (HR: 1.90, 95% CI: 1.20–3.00), and who died within 3 years after enrollment (HR: 1.67, 95% CI: 1.15–2.45). The sensitivity analysis results for the young group (aged 20–39) are presented in Supplementary Table 2. Because relatively few pancreatic cancer deaths were noted in the total cohort, age and variables at enrollment were adjusted for these models in Tables 3, 4 (44).
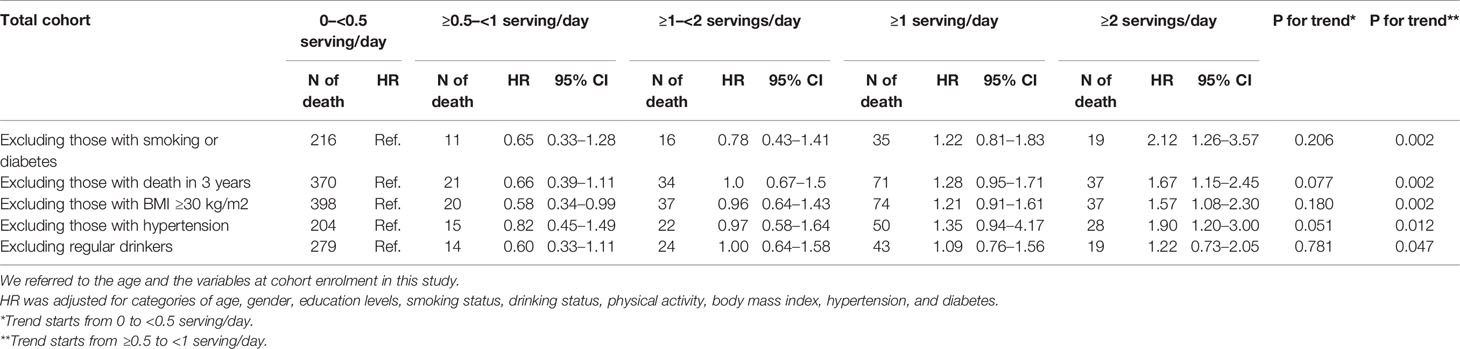
Table 4 Sensitivity analyses for the mortality risks of pancreatic cancer by levels of consumed sugar-sweetened beverages.
Discussion
SSB consumption was independently associated with pancreatic cancer in this Asian cohort. The risk remained when confounders such as smoking, diabetes, obesity, and hypertension were adjusted or excluded. The risk started at ≥1 servings/day for age < 40 years and ≥2 servings/day for all age groups. Moreover, a dose–response relationship was observed for cancer starting at 1 serving every other day. The increased risks remained even when all confounders, such as smoking, drinking, obesity, hypertension, and diabetes, were excluded or when death within the first 3 years of enrollment was excluded, implying the independent nature of the association.
With a large sample size, this is the first study to report the increased cancer risk for the entire Asian cohort, particularly among the younger population. The finding that young people have the highest risk of this highly fatal disease is of great concern. First, the risk started at ≥1 serving/day and not ≥2 servings/day as was noted for the remainder of the group, highlighting the vulnerability of the young population. Second, young people exhibited a higher prevalence and frequency of SSB intake than older people did, and their habitual SSB consumption typically started during their teenage years, making its weaning extremely difficult. Third, the young group also developed a taste for handmade bubble milk tea, which has high sugar content. This specialty drink was first invented in Taiwan. With a continuous increase in its popularity, this drink now occupies a large market share among sweetened beverages, both in Asia and globally (45).
From the perspective of Hill’s criteria of causality (46), the relationship between the increase in pancreatic cancer risk and SSB consumption observed in this study may not only be a chance association among the younger generation. First, the risk coincided with the highest consumers among all age groups. As reported in the United States, younger participants consumed three times more SSB in quantity than older participants (47). In the present study, participants aged <40 years exhibited a three times higher prevalence for each of the three different servings (≥0.5 to 1 serving/day: 17.2% vs. 4.5%; ≥1 to 2 servings/day: 15.4% vs. 6.1%; ≥ 2 servings/day: 9.3 vs. 3.5%; Table 1), implying that each young person on average consumed three times more than an older person (Supplementary Figure 3). Second, a dose–response relationship was observed within this age group and within the total cohort, with the highest risks observed among those who consumed the most drinks (2 servings/day or more). Men consumed more drinks and exhibited higher risks. Third, the increases remained even after most of the known pancreatic cancer risks, such as smoking, diabetes, drinking, hypertension, and obesity, were excluded, implying the independent nature of the relationship. Fourth, with pancreatic cancer having a 5-year survival rate of <10%, exclusion of people who died within the first 3 years of enrollment would have eliminated most pre-existing conditions. Fifth, SSB consumption is related to insulin release, which is known to be associated with pancreatic cancer (20–23, 29, 42). These factors pointed more favorably toward its causal association (46).
Similar to the increase in pancreatic cancer during the past 2 decades in the United States (1–4), Taiwan has experienced an approximately three-fold increase in pancreatic cancer incidence (5, 6). Smoking and drinking cannot sufficiently explain the increasing pancreatic cancer incidence because these behaviors have been highly prevalent, both in the United States and Taiwan, for decades before the increase in pancreatic cancer cases became evident. Moreover, in Taiwan, the number of both alcohol drinkers and adult smokers has declined in the past 10–20 years (48, 49). Between 1955 and 2016, the prevalence of current adult smokers in the United States also declined markedly (50). By contrast, the increase in pancreatic cancer incidence over the past 3 years has been accompanied by persisent high SSB consumption (51). The amount of SSB consumed has tended to increase gradually or remain stable worldwide, although the intake amount in the United States, Canada, the United Kingdom, and Australia has declined in the most recent decade. However, Supplementary Table 3 showed that the temporal relationship between SSB intake and pancreatic cancer mortality was similar to the 10–20 years lagged effect of tobacco smoking or alcohol consumption on overall cancer mortality (52). Moreover, the sales figure of SSB in Taiwan has increased 8.9% annually from 2005 to 2019 (53). Bubble milk tea, an emerging popular drink, is worth mentioning as an addition to the existing sweetened beverages in the last 3–4 decades. The market has been growing since the launch of the product in the 1980s, and it is expected to double in the coming decade (45, 54). An aggravating factor is the reluctantance of Asians to use sugar substitutes, for soft drinks and for bubble milk tea, for fear of them being potential carcinogens. The extent to which bubble milk tea has exacerbated the cancer risk remains to be investigated.
The strengths of this study include its large sample size, a long follow-up period (median: 15 years), a cohort design rather than a case–control design, exclusion of participants who died within 3 years of enrollment, a series of sensitivity analyses conducted with risks found in both incidence and mortality, and high-quality cancer incidence data collected from the nationwide cancer registry (55, 56). A case–control study is subject to 1) recall bias because diseased individuals are reporting more exposure than their healthy counterparts, and 2) difficulty in replicating the results because the reference group is small and may not be sufficiently representative. The case–control case number ratio is often 2:1 or 4:1, but the ratio in our cohort study was approximately 500:500,000 or 1:1,000, with the reference group being much more representative.
The study also has several limitations. First, the study might be subject to selection bias because only people who could afford the membership fee were likely enrolled in this self-paid screening program. Different from other self-paid medical screening programs, the MJ clinics emphasize family-centered screening, with incentives to recruit more family members, namely, members of the extended family such as uncles, cousins, or grandparents, paid for by the head of the household. Therefore, MJ participants were from almost all levels of social classes, and selection bias, as commonly perceived, was minimized. With half a million participants, constituting nearly 3% of the Taiwan population, the socioeconomic status effect was further mitigated with the internal comparison study design analyzing relative risks in this study. Furthermore, our cohort exhibited a prevalence of risk factors, incidence, and mortality of cancer that is consistent with the values for the general Taiwan population (36, 39). Second, only data from the self-reported questionnaires from initial visits were used, and the dietary habits were subject to individual bias and changed with time. We also examined questionnaires from those who returned for a second visit and noted that the reported amount of SSB consumption was highly similar between the two visits. When the caloric contribution from sweetened beverages nears 15% of daily energy consumption, with 280 Kcal from 2 servings in an individual consuming 2,000 Kcal, some dietary replacement or modification may be adopted, leading to considerable nutritional implications for some individuals. However, we observed the increased pancreatic cancer risk, regardless of the variable amount of dietary modification. Nevertheless, randomized trials are required to ascertain the causal relationship between SSB and pancreatic cancer (16–38, 42, 43). Third, all sweetened drinks consumed in this cohort were assumed to contain real sugar and not sugar substitutes. As mentioned, sugar substitutes are viewed in Taiwan as potential carcinogens, and nearly all soft drinks consumed and 100% of handmade bubble milk tea do not contain these substitutes (47, 57). Regardless of the amount of substitutes, the drinks were associated with cancer risks. Fourth, although we adjusted for numerous variables in calculating the Cox model, some residual confounding factors could have been overlooked. Based on the literature, we believe that none of the residual risks would have a risk sufficiently high to affect our conclusion. Fifth, we studied the Asian population and results may not apply to non-Asians. Similar studies have been conducted worldwide, with some positive and negative results. With an increase in the pancreatic cancer rate in the United States, Taiwan, and elsewhere, future research could verify our results with a larger adult sample.
Our results indicate that SSB intake was independently associated with pancreatic cancer in this Asian cohort, with highest risks among young people (age <40 years). Starting from one drink a day, a dose–response relationship was observed between the amount of SSB intake and pancreatic cancer risk. The risk was compounded by the increasing popularity of bubble milk tea. Considerable effort should be devoted to encourage modification of SSB consumption behavior.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the China Medical University & Hospital Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study concept and design: CPW and CHC. Analysis and interpretation of data: MKT, CHL, and CW. Drafting of the manuscript: CPW and CHC. Critical revision of the manuscript for important intellectual content: CPW, CHC, RTL, CW, MKT, and CHL. Technical or material support; study supervision: CPW, CHC, CYH, XW, and TWDC. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), the MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), the China Medical University Hospital (DMR-109-231), and the Tseng-Lien Lin Foundation, Taichung, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.835901/full#supplementary-material
Abbreviations
SSB, sugar-sweetened beverages; MET, metabolic equivalent task; HR, hazard ration; CI, confidence interval; BMI, body mass index; SES, socioeconomic status.
References
1. Howlader N, Noone AM, Krapcho M. SEER Cancer Statistics Review, 1975–2018 Posted to the SEER Website. Rockville: National Cancer Institute (2021). Available at: https://www.cancer.gov/types/common-cancers.
2. Henrikson NB, Aiello Bowles EJ, Blasi PR, Morrison CC, Nguyen M, Pillarisetty VG, et al. Screening for Pancreatic Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA (2019) 322(5):445–54. doi: 10.1001/jama.2019.6190
3. Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, et al. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology (2021) 160(3):744–54. doi: 10.1053/j.gastro.2020.10.007
4. da Costa WL Jr., Oluyomi AO, Thrift AP, pkaa033. Trends in the Incidence of Pancreatic Adenocarcinoma in All 50 United States Examined Through an Age-Period-Cohort Analysis. JNCI Cancer Spectr (2020) 4(4):pkaa033. doi: 10.1093/jncics/pkaa033
5. Chang JS, Chen LT, Shan YS, Chu PY, Tsai CR, Tsai HJ. The Incidence and Survival of Pancreatic Cancer by Histology, Including Rare Subtypes: A Nation-Wide Cancer Registry-Based Study From Taiwan. Cancer Med (2018) 7(11):5775–88. doi: 10.1002/cam4.1795
6. Health Promotion Administration. Taiwan Cancer Registry. Welfare MoHa, Editor (2020). Available at: https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=1061&pid=6069.
7. Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the Risk of Pancreatic Cancer: A Review and Meta-Analysis. Langenbeck Arch Surg (2008) 393(4):535–45. doi: 10.1007/s00423-007-0266-2
8. Park W, Chawla A, O’Reilly EM. Pancreatic Cancer: A Review. JAMA (2021) 326(9):851–62. doi: 10.1001/jama.2021.13027
9. Tramacere I, Scotti L, Jenab M, Bagnardi V, Bellocco R, Rota M, et al. Alcohol Drinking and Pancreatic Cancer Risk: A Meta-Analysis of the Dose-Risk Relation. Int J Cancer (2010) 126(6):1474–86. doi: 10.1002/ijc.24936
10. Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global Alcohol Exposure Between 1990 and 2017 and Forecasts Until 2030: A Modelling Study. Lancet (2019) 393(10190):2493–502. doi: 10.1016/S0140-6736(18)32744-2
11. Centers for Disease Control and Prevention. Get the Facts: Sugar-Sweetened Beverages and Consumption. Services USDoHH, Editor (2021). Available at: https://www.cdc.gov/nutrition/data-statistics/sugar-sweetened-beverages-intake.html.
12. Malik VS, Willett WC, Hu FB. Global Obesity: Trends, Risk Factors and Policy Implications. Nat Rev Endocrinol (2013) 9(1):13–27. doi: 10.1038/nrendo.2012.199
13. Powell ES, Smith-Taillie LP, Popkin BM. Added Sugars Intake Across the Distribution of US Children and Adult Consumers: 1977-2012. J Acad Nutr Diet (2016) 116(10):1543–1550 e1. doi: 10.1016/j.jand.2016.06.003
14. Rosinger A, Herrick K, Gahche J, Park S. Sugar-Sweetened Beverage Consumption Among U.S. Youth, 2011-2014. NCHS Data Brief (2017) 271):1–8.
15. Health Promotion Administration. Nutrition and Health Survey in Taiwan. Taipei: NAHSIT (2021). Available at: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=3998.
16. Bao Y, Stolzenberg-Solomon R, Jiao L, Silverman DT, Subar AF, Park Y, et al. Added Sugar and Sugar-Sweetened Foods and Beverages and the Risk of Pancreatic Cancer in the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr (2008) 88(2):431–40. doi: 10.1093/ajcn/88.2.431
17. Everhart J, Wright D. Diabetes-Mellitus as a Risk Factor for Pancreatic-Cancer - A Metaanalysis. Jama-J Am Med Assoc (1995) 273(20):1605–9. doi: 10.1001/jama.273.20.1605
18. Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical Activity, Obesity, Height, and the Risk of Pancreatic Cancer. JAMA (2001) 286(8):921–9. doi: 10.1001/jama.286.8.921
19. Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, et al. Diabetes Mellitus, Other Medical Conditions and Familial History of Cancer as Risk Factors for Pancreatic Cancer. Brit J Cancer (1999) 80(11):1830–7. doi: 10.1038/sj.bjc.6690607
20. Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal Glucose Metabolism and Pancreatic Cancer Mortality. JAMA (2000) 283(19):2552–8. doi: 10.1001/jama.283.19.2552
21. Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, et al. Insulin, Glucose, Insulin Resistance, and Pancreatic Cancer in Male Smokers. JAMA (2005) 294(22):2872–8. doi: 10.1001/jama.294.22.2872
22. Hart AR, Kennedy H, Harvey I. Pancreatic Cancer: A Review of the Evidence on Causation. Clin Gastroenterol Hepatol (2008) 6:275–82. doi: 10.1016/j.cgh.2007.12.041
23. Zanini S, Renzi S, Limongi AR, Bellavite P, Giovinazzo F, Bermano G. A Review of Lifestyle and Environment Risk Factors for Pancreatic Cancer. Eur J Cancer (2021) 145:53–70. doi: 10.1016/j.ejca.2020.11.040
24. Malik VS, Li YP, Pan A, De Koning L, Schernhammer E, Willett WC, et al. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation (2019) 139(18):2113–25. doi: 10.1161/Circulationaha.118.037401
25. Mullee A, Romaguera D, Pearson-Stuttard J. Association Between Soft Drink Consumption and Mortality in 10 European Countries (Vol 179, Pg 1479, 2019). JAMA Intern Med (2019) 179(11):1607–7. doi: 10.1001/jamainternmed.2019.5327
26. Navarrete-Munoz EM, Wark PA, Romaguera D, Bhoo-Pathy N, Michaud D, Molina-Montes E, et al. Sweet-Beverage Consumption and Risk of Pancreatic Cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Am J Clin Nutr (2016) 104(3):760–8. doi: 10.3945/ajcn.116.130963
27. Schernhammer ES, Hu FB, Giovannucci E, Michaud DS, Colditz GA, Stampfer MJ, et al. Sugar-Sweetened Soft Drink Consumption and Risk of Pancreatic Cancer in Two Prospective Cohorts. Cancer Epidem Biomar (2005) 14(9):2098–105. doi: 10.1158/1055-9965.Epi-05-0059
28. World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective (2018). Available at: https://www.wcrf.org/wp-content/uploads/2021/02/pancreatic-cancer-report.pdf.
29. Larsson SC, Bergkvist L, Wolk A. Consumption of Sugar and Sugar-Sweetened Foods and the Risk of Pancreatic Cancer in a Prospective Study. Am J Clin Nutr (2006) 84(5):1171–6. doi: 10.1093/ajcn/84.5.1171
30. Aune D, Chan DSM, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Dietary Fructose, Carbohydrates, Glycemic Indices and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies. Ann Oncol (2012) 23(10):2536–46. doi: 10.1093/annonc/mds076
31. Bartrina JA, Rodrigo CP. Association Between Sucrose Intake and Cancer: A Review of the Evidence. Nutr Hosp (2013) 28:95–105. doi: 10.3305/nh.2013.28.sup4.6802
32. Chan JM, Wang F, Holly EA. Sweets, Sweetened Beverages, and Risk of Pancreatic Cancer in a Large Population-Based Case-Control Study. Cancer Causes Control (2009) 20(6):835–46. doi: 10.1007/s10552-009-9323-1
33. Li Y, Guo L, He K, Huang C, Tang S. Consumption of Sugar-Sweetened Beverages and Fruit Juice and Human Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. J Cancer (2021) 12(10):3077–88. doi: 10.7150/jca.51322
34. Llaha F, Gil-Lespinard M, Unal P, de Villasante I, Castaneda J, Zamora-Ros R. Consumption of Sweet Beverages and Cancer Risk. A Systematic Review and Meta-Analysis of Observational Studies. Nutrients (2021) 13(2):516. doi: 10.3390/nu13020516
35. Makarem N, Bandera EV, Nicholson JM, Parekh N. Consumption of Sugars, Sugary Foods, and Sugary Beverages in Relation to Cancer Risk: A Systematic Review of Longitudinal Studies. Annu Rev Nutr (2018) 38:17–39. doi: 10.1146/annurev-nutr-082117-051805
36. Milajerdi A, Larijani B, Esmaillzadeh A. Sweetened Beverages Consumption and Pancreatic Cancer: A Meta-Analysis. Nutr Cancer (2019) 71(3):375–84. doi: 10.1080/01635581.2019.1578390
37. Contreras Garcia E, Zaragoza-Marti A. Influence of Food or Food Groups Intake on the Occurrence and/or Protection of Different Types of Cancer: Systematic Review. Nutr Hosp (2020) 37(1):169–92. doi: 10.20960/nh.02588
38. Genkinger JM, Li R, Spiegelman D, Anderson KE, Albanes D, Bergkvist L, et al. Coffee, Tea, and Sugar-Sweetened Carbonated Soft Drink Intake and Pancreatic Cancer Risk: A Pooled Analysis of 14 Cohort Studies. Cancer Epidemiol Biomarkers Prev (2012) 21(2):305–18. doi: 10.1158/1055-9965.EPI-11-0945-T
39. Wu X, Tsai SP, Tsao CK, Chiu ML, Tsai MK, Lu PJ, et al. Cohort Profile: The Taiwan MJ Cohort: Half a Million Chinese With Repeated Health Surveillance Data. Int J Epidemiol (2017) 46(6):1744–1744g. doi: 10.1093/ije/dyw282
40. Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M-C, et al. Minimum Amount of Physical Activity for Reduced Mortality and Extended Life Expectancy: A Prospective Cohort Study. Lancet (2011) 378(9798):1244–53. doi: 10.1016/s0140-6736(11)60749-6
41. Calories.info. Soda & Soft Drinks Calories (2021). Available at: https://www.calories.info/food/soda-soft-drinks.
42. Mueller NT, Odegaard A, Anderson K, Yuan JM, Gross M, Koh WP, et al. Soft Drink and Juice Consumption and Risk of Pancreatic Cancer: The Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev (2010) 19(2):447–55. doi: 10.1158/1055-9965.EPI-09-0862
43. Gallus S, Turati F, Tavani A, Polesel J, Talamini R, Franceschi S, et al. Soft Drinks, Sweetened Beverages and Risk of Pancreatic Cancer. Cancer Causes Control (2011) 22(1):33–9. doi: 10.1007/s10552-010-9665-8
44. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and Cause-Specific Mortality in a Prospective Cohort of One Million U.S. Adults. Diabetes Care (2012) 35:1835–44. doi: 10.2337/dc12-0002
45. Sangwai V, Deshmukh R. Bubble Tea Market by Base Ingredient, Flavor and Component: Global Opportunity Analysis and Industry Forecast, 2020-2027. Portland, United States: Allied Market Research (2020). p. 261, (Report MR, editor.).
46. Shimonovich M, Pearce A, Thomson H, Keyes K, Katikireddi SV. Assessing Causality in Epidemiology: Revisiting Bradford Hill to Incorporate Developments in Causal Thinking. Eur J Epidemiol (2020) p. 873–87. doi: 10.1007/s10654-020-00703-7
47. Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, et al. Consumption of Sugar Sweetened Beverages, Artificially Sweetened Beverages, and Fruit Juice and Incidence of Type 2 Diabetes: Systematic Review, Meta-Analysis, and Estimation of Population Attributable Fraction. BMJ (2015) 351:h3576. doi: 10.1136/bmj.h3576
48. Huang YC, Wu SC, Hsiao PC, Chen LY, Ting TT, Chen CY, et al. Mne’s Decrease and Women Incraese in Harmful Alcohol Use From the 2014 to 2018 National Surveys in Tiawan: A Harbinger for an Emerging National Trend in Esat Asia? Int J Drug Policy (2022) 99:103441. doi: 10.1016/j.drugpo.2021.103441
49. Sanna M, Gao W, Chiu YW, Chiou HY, Chen YH, Wen CP, et al. Tobacco Control Within and Beyond WHO MPOWER: Outvcomes From Taiwan SimSmoke. Tob Control (2020) 29:36–42. doi: 10.1136/tobaccocontrol-2018-054544
50. Agaku IT, Odani S, Okuyemi KS, Armour B. Disparities in Current Cigarette Smoking Among US Adults, 2002-2016. Tob Control (2020) 29:269–76. doi: 10.1136/tobaccocontrol-2019-054948
51. Della Corte K, Fife J, Gardner A, Murphy BL, Kleis L, Della Corte D, et al. World Trends in Sugar-Sweetened Beverage and Dietary Sugar Intakes in Children and Adolescents: A Systematic Review. Nutr Rev (2021) 79(3):274–88. doi: 10.1093/nutrit/nuaa070
52. Jiang H, Livingston M, Room R, Chenhall R, English DR. Temporal Associations of Alcohol and Tobacco Consumption With Cancer Mortality. JAMA Netw Open (2018) 1(3):e180713. doi: 10.1001/jamanetworkopen.2018.0713
53. Department of Statistics. Ministry of Economic Affairs. Industrial Production Indexes (In Chinese) (2021). Available at: https://www.moea.gov.tw/Mns/dos/bulletin/Bulletin.aspx?kind=9&html=1&menu_id=18808&bull_id=6099.
54. Ong AKS, Prasetyo YT, Libiran MADC, Lontoc YMA, Lunaria JAV, Manalo AM, et al. Consumer Preference Analysis on Attributes of Milk Tea: A Conjoint Analysis Approach. Foods (2021) 10(6):1382. doi: 10.3390/foods10061382
55. Kao WH, Hong JH, See LC, Yu HP, Hsu JT, Chou IJ, et al. Validity of Cancer Diagnosis in the National Health Insurance Database Compared With the Linked National Cancer Registry in Taiwan. Pharmacoepidem Dr S (2018) 27(10):1060–6. doi: 10.1002/pds.4267
56. Lu TH, Lee MC, Chou MC. Accuracy of Cause-of-Death Coding in Taiwan: Types of Miscoding and Effects on Mortality Statistics. Int J Epidemiol (2000) 29(2):336–43. doi: 10.1093/ije/29.2.336
Keywords: sugar-sweetened beverages, pancreatic cancer, mortality, incidence, cohort
Citation: Chen CH, Tsai MK, Lee JH, Lin R-T, Hsu CY, Wen C, Wu X, Chu T-W and Wen CP (2022) “Sugar-Sweetened Beverages” Is an Independent Risk From Pancreatic Cancer: Based on Half a Million Asian Cohort Followed for 25 Years. Front. Oncol. 12:835901. doi: 10.3389/fonc.2022.835901
Received: 15 December 2021; Accepted: 07 March 2022;
Published: 07 April 2022.
Edited by:
Junchao Guo, Peking Union Medical College Hospital CAMS, ChinaReviewed by:
Jerry Polesel, Aviano Oncology Reference Center Institute di Ricovero e Cura a Carattere Scientifico (IRCCS), ItalyAzam Majidi, QIMR Berghofer Medical Research Institute, Australia
Copyright © 2022 Chen, Tsai, Lee, Lin, Hsu, Wen, Wu, Chu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi Pang Wen, Y3dlbmdvb2RAbmhyaS5vcmcudHc=
 Chien Hua Chen
Chien Hua Chen Min Kuang Tsai4
Min Kuang Tsai4 Ta-Wei Chu
Ta-Wei Chu Chi Pang Wen
Chi Pang Wen