- 1Department of Thoracic Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2The Medical Department, Jiangsu Simcere Diagnostics Co., Ltd., Nanjing, China
- 3Nanjing Simcere Medical Laboratory Science Co., Ltd., Nanjing, China
- 4The State Key Laboratory of Translational Medicine and Innovative Drug Development, Jiangsu Simcere Diagnostics Co., Ltd., Nanjing, China
Novel adjuvant strategies are needed to optimize outcomes after complete surgical resection in patients with early-stage non-small-cell lung cancer (NSCLC). The adjuvant treatment of ROS Proto-Oncogene 1 (ROS1) fusion-positive resected NSCLC is challenging because there is no curative confirmed randomized controlled trial. Next-generation sequencing (NGS) and immunohistochemistry (IHC) staining were performed on the biopsy sample. In this case, we identified a novel LDLR–ROS1 fusion in a resectable stage IIIA NSCLC patient. The patient received crizotinib as adjuvant treatment and achieved recurrence-free survival (RFS) for 29 months, without significant symptoms of toxicity. In this case, we report a novel LDLR–ROS1 fusion responding to crizotinib in a patient with lung adenocarcinoma, supporting the use of adjuvant treatment with the ROS1 inhibitor exerting clinical survival benefit in ROS1 fusion-positive resected NSCLC.
Introduction
Novel adjuvant strategies are needed to optimize outcomes after complete surgical resection in patients with early-stage non-small-cell lung cancer (NSCLC). The ADAURA trial and the IMpower010 trial have demonstrated significant clinical benefit in patients with resectable NSCLC who received targeted (1) and immune adjuvant therapy (2), respectively. Herein, we reported that the use of crizotinib, a ROS1 inhibitor, exerted favorable survival benefit (exceeded 29 months) in a resectable stage IIIA NSCLC patient with novel LDLR–ROS1 fusion.
Case Report
A 72-year-old Chinese male patient was admitted to the first affiliated hospital of Anhui Medical University in June 2019, complaining of irregular cough, having smoked for 40 years. Computed tomography on the chest revealed a space-occupying lesion in the upper lobe of the right lung (Figures 1A, B). After the relevant preoperative inspection, right lobectomy and lymph node dissection were performed subsequently in June 2019. Postoperative pathological examination revealed complete surgical resection (R0), tumor involvement in the chest wall, and metastases in the positive hilar lymph node (3/4, tumor cells were detected in 3 of the 4 examined hilar lymph nodes), and there were no metastases in the distal lymph nodes. Ultimately, the disease was diagnosed as a stage IIIA lung adenocarcinoma with hilar node metastasis (T3N1M0, according to AJCC 8th edition). Considering the long history of smoking and tumor invasion, postoperative adjuvant therapy was proposed.
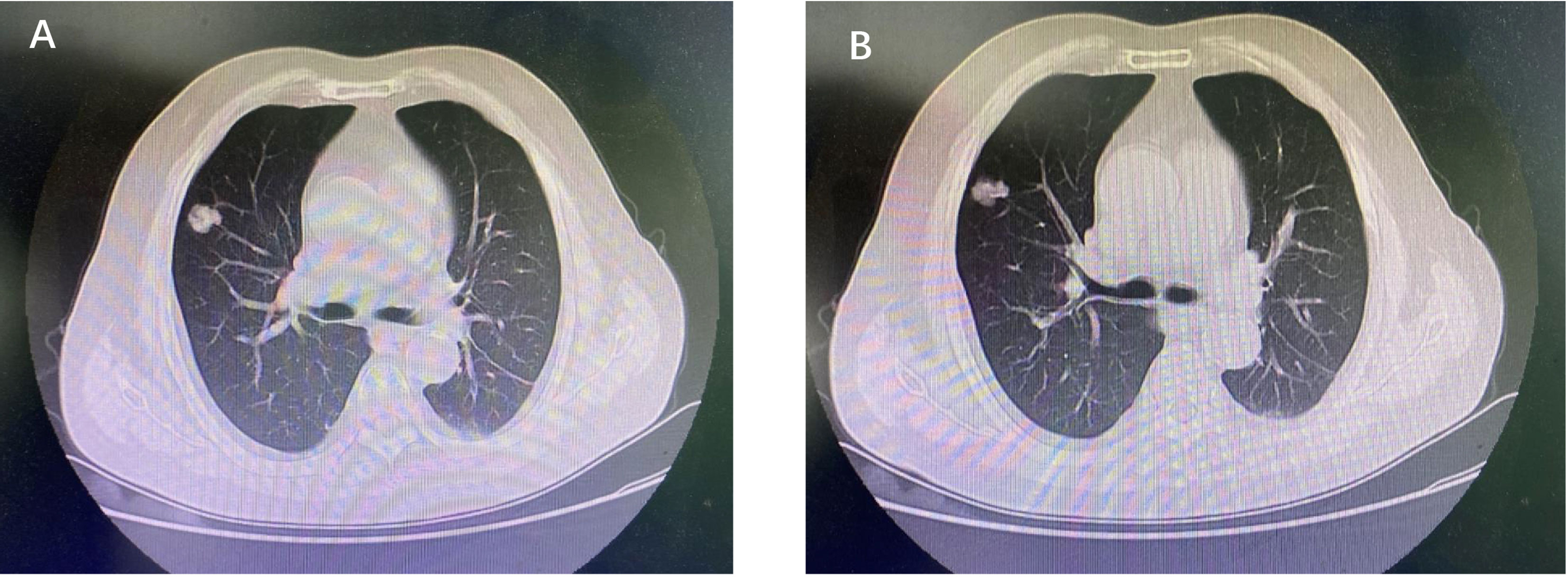
Figure 1 (A, B) Computed tomography of chest revealed a space-occupying lesion in the upper lobe of the right lung.
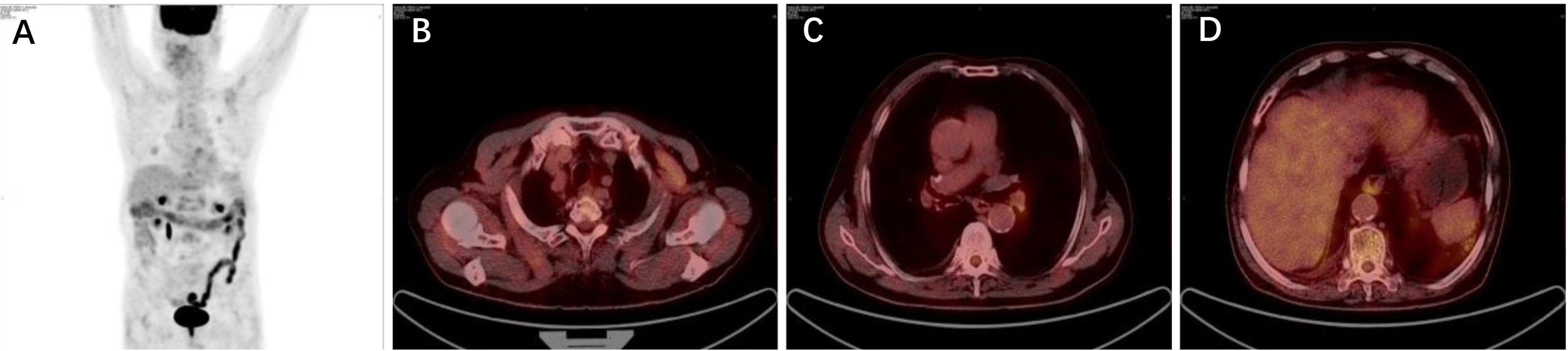
As the patient explicitly refused further chemotherapy and sought the possibility of adjuvant targeted therapy, next-generation sequencing analysis via DNA-based hybrid capture based on a pan-cancer 539-gene panel of the biopsy of primary foci sample was conducted in a CAP-certificated laboratory. A novel LDLR (Exon1–14)–ROS1 (Exon34–43) fusion variant (44.8% abundance) was detected and confirmed by immunohistochemistry (IHC) staining (Figures 2A–D). No other key driver gene mutations were detected. Crizotinib (250 mg orally twice daily) was immediately administered beginning in June 2019. Follow-up examinations were performed every 6 months after surgery, clinical and radiological follow-up showed no evidence of progression or recurrence, and the recurrence-free survival (RFS) had exceeded 29 months by the time of submission (December 2021) (Figures 3A–D), without significant symptoms of toxicity.
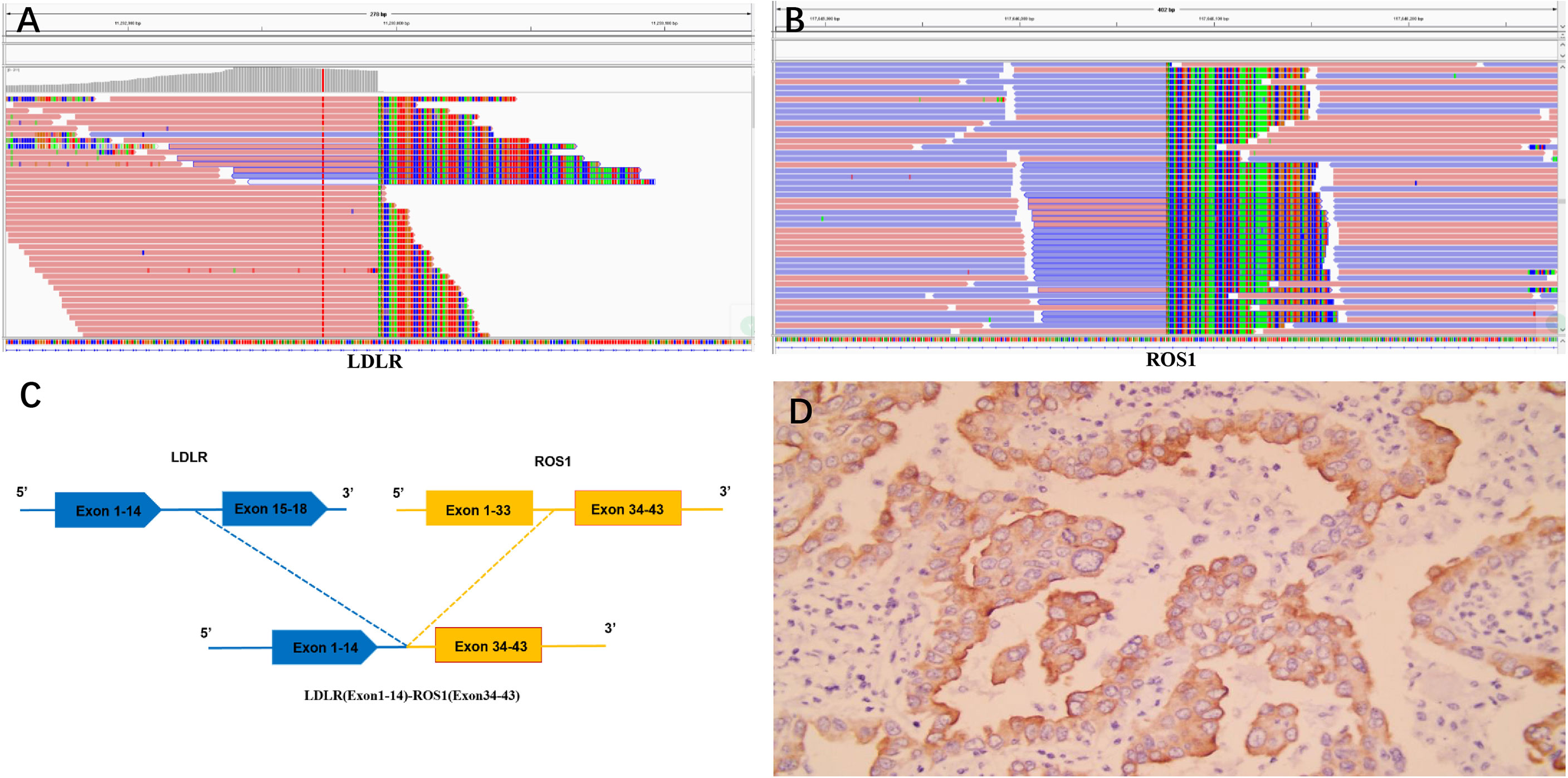
Figure 2 (A–C) The Integrative Genomics Viewer snapshot of LDLR–ROS1 fusion; LDLR–ROS1 included exons 1–14 of LDLR and exons 34–43 of ROS1. (D) Immunohistochemical staining results of ROS1 fusion.
Discussion
The adjuvant treatment of ROS1 fusion-positive resected NSCLC is challenging because no confirmed randomized controlled trial has been reported. In this case, we identified a novel LDLR–ROS1 fusion gene in a lung adenocarcinoma patient. Also, we considered that the patient was sensitive to treatment with the ROS1 inhibitor that the RFS had exceeded 29 months, supporting the use of adjuvant treatment with the ROS1 inhibitor exerting clinical survival benefit in ROS1 fusion-positive resected NSCLC.
ROS1 has proven to be a valuable therapeutic target in patients with NSCLC; the prevalence of ROS1 rearrangements is estimated to be 1%–2% of NSCLC patients with at least 20 fusion partners already reported (3–6). It has been established that genomic instability is considered an early step of tumorigenesis, and many fusions are caused by genetic instability. The fusion of LDLR–ROS1 included exons 1–14 of LDLR and exons 34–43 of ROS1; this novel ROS1 fusion retained the complete kinase domain of ROS1 and might lead to abnormal ROS1 activation. Immunohistochemistry results also confirmed the presence of ROS1 fusion.
Both the ADAURA trial and the IMpower010 trial have demonstrated significant clinical benefit in patients with resectable NSCLC who received targeted and immune adjuvant therapy. In the ADAURA trial, the benefit of favoring osimertinib with respect to RFS was observed consistently across all predefined subgroups (1). Moreover, IMpower010 also showed an RFS benefit with atezolizumab versus best supportive care after adjuvant chemotherapy in patients with resected stage II–IIIA NSCLC (2). In our case, postoperative targeted therapy demonstrated a good curative effect, the patient had clinical and radiological follow-up highlighting no evidence of progression or recurrence, and the RFS had exceeded 29 months, which exceeded the median RFS of resectable stage IIIA NSCLC in the placebo group in the ADAURA trial (less than 12 months).
There are also some limitations in our present study. Firstly, this is only a one-patient case report, and more cases are needed to analyze the correlation survival benefit of resectable NSCLC patients with ROS1 fusion. Secondly, the biological function of LDLR–ROS1 should be further investigated. All in all, our case report expands the spectrum of ROS1 fusion types and may recommend a promising option to resectable NSCLC patients with ROS1 fusion intending to target therapy.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
Written consent for publication was obtained from the patient.
Author Contributions
We were all involved in the clinical care and management of the patient, collection of the data, and drafting of the manuscript. D-SC, SL, L-LZ and M-ZX collected the data and wrote the first draft of the manuscript. A-GC supervised the work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
Authors D-SC, SL, L-LZ and M-ZX were employed by company Simcere Diagnostics Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Chuang Qi and Dr. Yang-yang Yu from Jiangsu Simcere Diagnostics for his kind assistance.
References
1. Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
2. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. IMpower010 Investigators, Adjuvant Atezolizumab After Adjuvant Chemotherapy in Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5
3. Zhang Y, Yu M, Yuan M, Chen R, Huang M-J. Identification of a Novel RBPMS-ROS1 Fusion in an Adolescent Patient With Microsatellite-Instable Advanced Lung Adenocarcinoma Sensitive to Crizotinib: A Case Report. Clin Lung Cancer (2020) 21:e78–83. doi: 10.1016/j.cllc.2019.09.003
4. Xu S, Wang W, Xu C, Li X, Ye J, Zhu Y, et al. ROS1-ADGRG6: A Case Report of a Novel ROS1 Oncogenic Fusion Variant in Lung Adenocarcinoma and the Response to Crizotinib. BMC Cancer (2019) 19:769. doi: 10.1186/s12885-019-5948-y
5. Stacher G, Gaupmann G, Mittelbach G, Schneider C, Steinringer H, Langer B. Effects of Oral Cisapride on Interdigestive Jejunal Motor Activity, Psychomotor Function, and Side-Effect Profile in Healthy Man. Dig Dis Sci (1987) 32:1223–30. doi: 10.1007/BF01296370
Keywords: ROS1 fusion, crizotinib, adjuvant treatment, NSCLC, case report
Citation: Chen A-g, Chen D-s, Li S, Zhao L-l and Xiao M-z (2022) Case Report: Adjuvant Crizotinib Therapy Exerted Favorable Survival Benefit in a Resectable Stage IIIA NSCLC Patient With Novel LDLR–ROS1 Fusion. Front. Oncol. 12:837219. doi: 10.3389/fonc.2022.837219
Received: 16 December 2021; Accepted: 25 January 2022;
Published: 01 March 2022.
Edited by:
Dharmendra Kumar Yafav, Gachon University, South KoreaReviewed by:
Geeta G. Sharma, City of Hope National Medical Center, United StatesLuca Porcu, Mario Negri Pharmacological Research Institute (IRCCS), Italy
Copyright © 2022 Chen, Chen, Li, Zhao and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: An-guo Chen, Y2hlbmFnMTE5QDEyNi5jb20=
 An-guo Chen
An-guo Chen Dong-sheng Chen2,3,4
Dong-sheng Chen2,3,4