- 1Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Institute of Clinical Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Department of Pathology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Research and Development, Foundation Medicine, Inc., Cambridge, MA, United States
BRAF fusions are rare driver oncogenes in non-small cell lung cancer (NSCLC). Similar with BRAF V600E mutation, it could also activate the MAPK signaling pathway. There are a few case reports which had indicated the potential response to BRAF inhibitors and its important role as de novo driver mutation. In addition, the co-occurring MET amplification has been defined as a poor prognostic factor in patients with epidermal growth factor receptor (EGFR) mutant NSCLC. Currently, there are ongoing clinical trials which investigate the MET amplification as a therapeutic target in patients with EGFR mutant NSCLC and acquired resistance to osimertinib, which imply that the MET amplification also had a therapeutic significance. However, the co-occurring MET amplification had not been studied in patients with BRAF fusion before. A 67-year-old man was diagnosed with metastatic poorly-differentiated adenocarcinoma. He received first-line therapy with the combination of pembrolizumab and chemotherapy because the genomic test revealed wild-type EGFR, and negativity of ALK and ROS1 by immunohistochemical stain. Upon disease progression, the next-generation sequencing revealed co-occurring KIAA1549-BRAF fusion and MET amplification. Subsequent dabrafenib, trametinib, and capmatinib combination therapy showed a remarkable treatment effect. The combination therapy targeting the co-occurring driver mutations is a potential effective treatment for NSCLC patients. Further prospective study is still warranted to investigate the role of co-occurring driver mutations and the relevant treatment strategy.
Introduction
BRAF fusions are mostly detected in melanoma, thyroid cancer, and astrocytoma and are rare driver oncogenes in non-small cell lung cancer (NSCLC) (1). They present in approximately 0.2% of NSCLC patients and have different activation mechanisms with BRAF mutations (2). The KIAA1549-BRAF fusion will cause constitutive activating kinase activity resulting from the loss of the BRAF autoregulatory N-terminal domain and retention of the C-terminal kinase domain (2). Currently, there is no prospective clinical trial regarding the treatment strategies for patients with BRAF fusion and no US Food and Drug Administration approved therapy. However, there are a few case reports have demonstrated the potential response to monotherapy with the BRAF inhibitor vemurafenib or the MEK inhibitor trametinib (3, 4), which indicate that the BRAF fusion is also an important targetable driver mutation.
In addition, the role of co-occurring MET amplification had been widely studied in patients with epidermal growth factor receptor (EGFR) mutant NSCLC and was defined as a poor prognostic factor (5). The combination of capmatinib and osimertinib could provide better progression-free survival than chemotherapy in patients with osimertinib-resistant EGFR mutant NSCLC and MET amplification (6). There are also ongoing clinical trials investigating the role of combination therapy targeting MET amplification in EGFR mutant NSCLC and acquired resistance to osimertinib (7, 8), which indicates the MET amplification had therapeutic role and combination of targeted therapy is a potential therapeutic strategy. However, the role of co-occurring MET amplification and the relevant treatment strategy has not been studied in patients with BRAF fusion. The profile of adverse events when using combination therapy was not assessed before. This case report presents co-occurring KIAA1549-BRAF fusion and MET amplification and showed a durable response and tolerant adverse events to combination therapy with dabrafenib, trametinib, and capmatinib.
Case Presentation
In December 2018, a 67-year-old man was diagnosed with stage IV pulmonary poorly differentiated adenocarcinoma with negativity of TTF-1 and P40 expression (Supplementary Figure 1A). The tumor involved the right middle lobe and had multiple satellite masses and pleural involvement. The EGFR mutation test yielded no sensitizing mutations, and the immunohistochemical (IHC) staining for anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 (ROS1) were both negative. The IHC staining of programmed death-ligand 1 was 3%. The next generation sequencing (NGS) could not be performed due to insufficient tissue. Thus, the combination of cisplatin, pemetrexed, and pembrolizumab was administered. After 12 months, the patient demonstrated disease progression with an enlarged right middle lung mass. The NGS was suggested in order to optimize subsequent therapy. To obtain sufficient tissue for NGS, video-assisted thoracoscopic surgery (VATS) biopsy was performed due to previous experience of insufficient tissue from computed tomography-guided biopsy.
The pathologic report of VATS biopsy revealed also poorly-differentiated adenocarcinoma, and the tumor cells were also negative for TTF-1 and P40 (Supplementary Figure 1B). Furthermore, NGS by FoundationOne® CDx revealed KIAA1549-BRAF fusion (Figure 1) and MET amplification (copy number gain: 10; Table 1). The detailed report was summarized in Supplementary Figure 2A. The patient then received second-line chemotherapy with docetaxel and ramucirumab. However, after approximately 8 months, he experienced disease progression with increased right pleural effusion. With multidisciplinary team discussion, capmatinib was administered as the third-line therapy to target the MET amplification according to the GEOMETRY mono-1 study (9). Unfortunately, after 3 months of therapy with capmatinib, the right pleural effusion increased gradually concurrently with development of left pleural effusion. A pleuroscopic biopsy was performed on the left pleura and also revealed poorly-differentiated adenocarcinoma with negative TTF-1 and P40 expression on tumor cells (Supplementary Figure 1C). To optimize the subsequent treatment strategy, NGS was repeated and revealed KIAA1549-BRAF fusion and absence of MET amplification (Table 1). The detailed report was summarized in Supplementary Figure 2B. Based on the second NGS report and previous case report which indicate BRAF inhibitor could be a potential therapy for patient with BRAF fusion (4), we shifted the treatment to dabrafenib and trametinib.
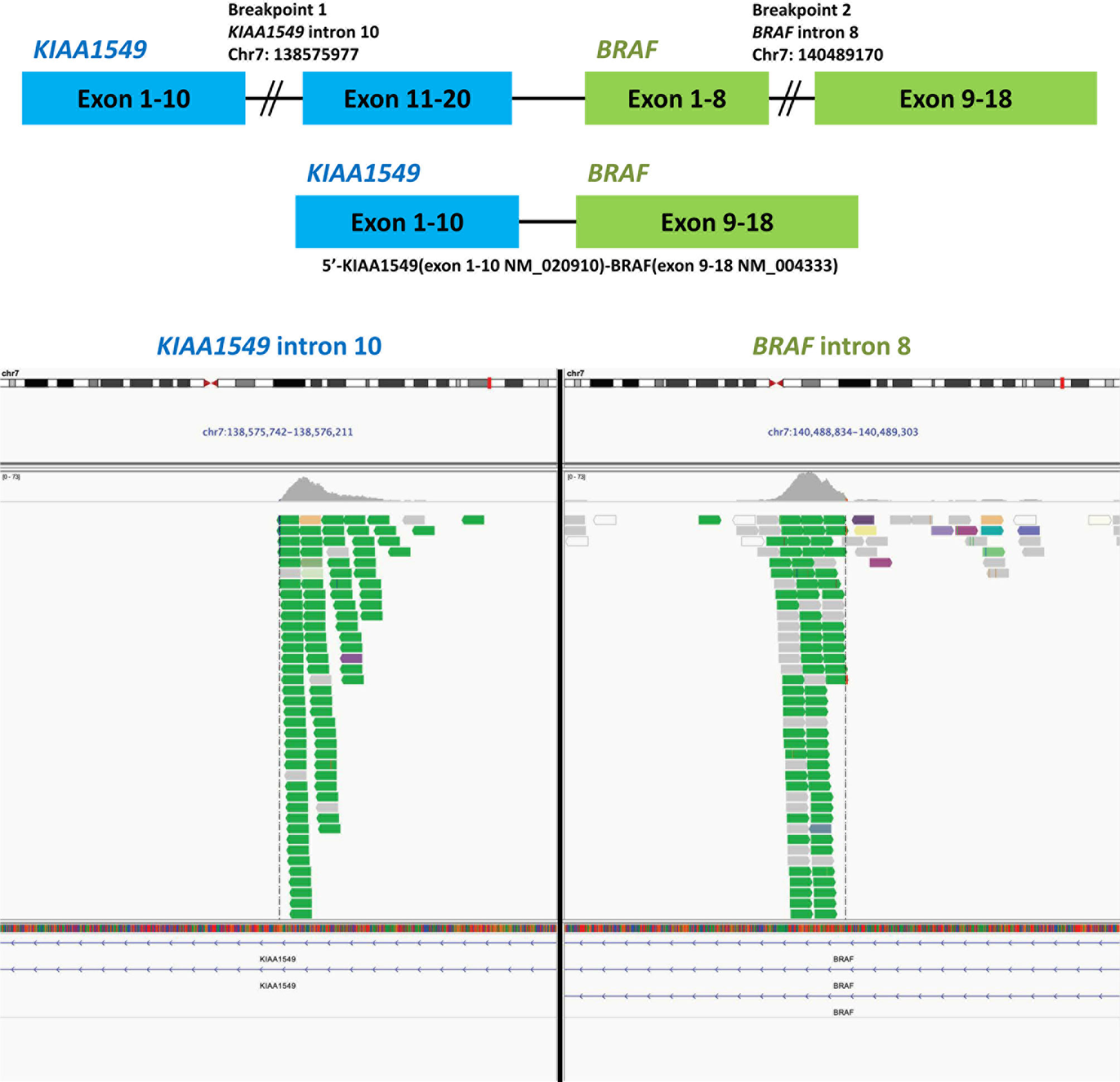
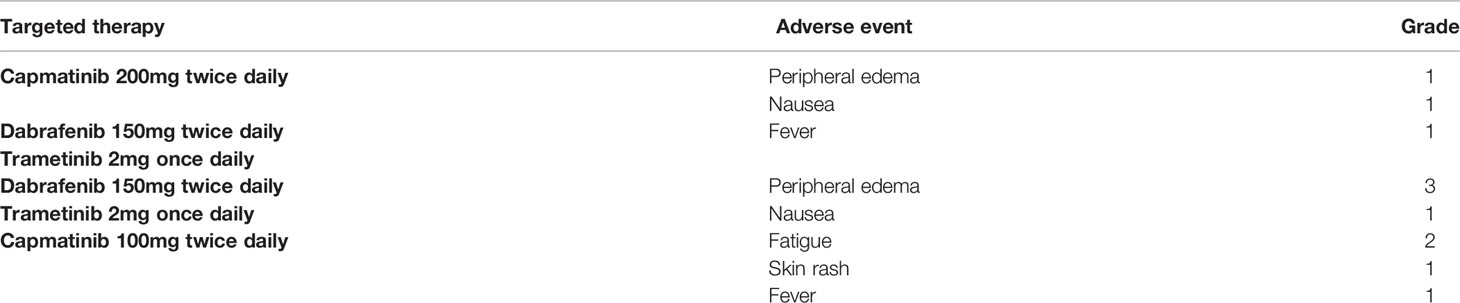
Figure 1 Next-generation sequencing of the tissue specimen from the video-assisted thoracic surgery biopsy of right middle lung cancer revealed a BRAF-KIAA1549 fusion. The data was provided by the Department of Research and Development, Foundation Medicine Inc.

Table 1 The detailed genomic alterations of the repeated biopsy specimen detected by FoundationOne CDx.
However, the patient suffered from progressive exertional dyspnea after 3-month treatment of dabrafenib and trametinib. The plain film radiograph revealed resolved left pleural effusion but had a drastically increased right pleural effusion. Repeated pleuroscopic biopsy at right side pleura still revealed poorly-differentiated adenocarcinoma with negativity in TTF-1 and P40 expression (Supplementary Figure 1D). The pleurodesis was performed but invalid because of multiloculated pleural effusion. We had also tried to performed NGS analysis but failed due to low tumor purity. Nonetheless, the resolved left pleural effusion and drastic increase of right pleural effusion, which developed immediately after discontinuation of capmatinib, implied that the tumor in right side pleura still harbor co-occurring MET amplification and KIAA1549-BRAF fusion while the tumor in left side pleura harbor only KIAA1549-BRAF fusion. In addition, the performance status of the patient was poor and chemotherapy could not be administrated. Based on the successful combination strategy targeting MET amplification in EGFR mutant NSCLC (6) and the animal study demonstrated the potential benefit of combination therapy in BRAF mutation (10), the treatment strategy was shifted to combination therapy with dabrafenib, trametinib, and capmatinib after multidisciplinary team discussion. Finally, the right pleural effusion subsided and remained stable for more than 6 months. The treatment course is summarized in Figure 2. During combination therapy, higher grade of adverse events developed compared to monotherapy, including peripheral edema, nausea, fatigue, skin rash, and fever. The symptom got subsided partially after the reduction of capmatinib dosage to 100mg twice daily. The profile of adverse events and the doses of relevant targeted therapy was summarized in Table 2.
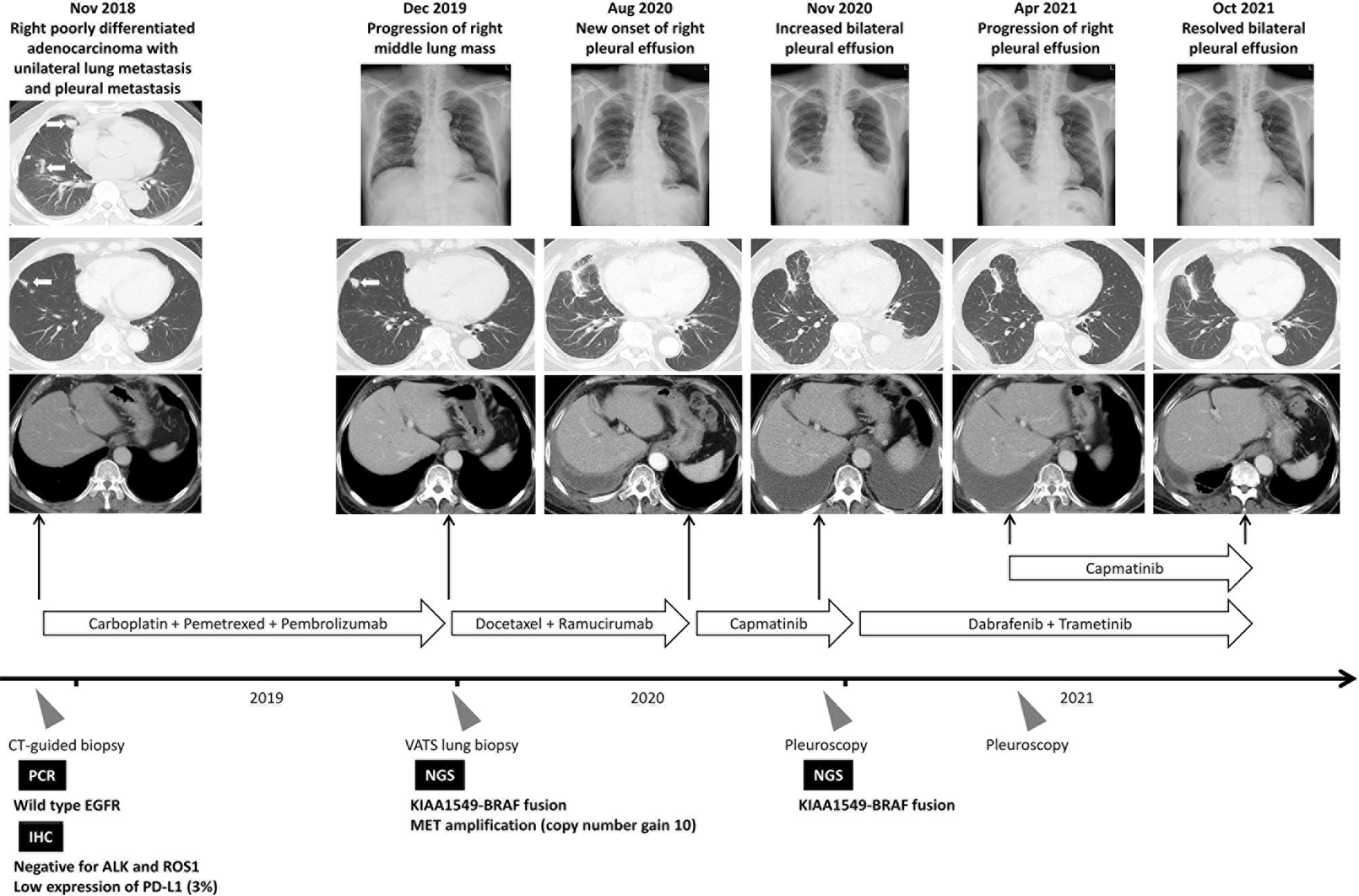
Figure 2 Summary of treatment courses mentioned in this case report. The white arrows indicate the target lesion in the serial image study. ALK, anaplastic lymphoma kinase; CT, computed tomography; EGFR, epidermal growth factor receptor; IHC, immunohistochemical; NGS, next-generation sequencing; PCR, polymerase chain reaction; ROS1, ROS proto-Oncogene 1; VATS, video-assisted thoracic surgery.
Discussion
BRAF fusions are rare driver oncogene in patients with advanced NSCLC (2), which mostly be discovered as acquired resistance mechanism to EGFR-TKIs and rarely be a de novo mutation (1, 11). They lack the RAS-binding auto-inhibitory domain found in the N-terminal and the fusion partner often harbors a constitutive dimerization or oligomerization motif (2). Similar with BRAF V600E mutation, the BRAF fusion could also activate the mitogen-activated protein kinase (MAPK) signaling pathway and respond to MEK inhibitor in a case with melanoma (12). Similarly, there are also case reports which had demonstrated potential therapies in NSCLC patients with BRAF fusion. For example, a patient with advanced lung adenocarcinoma harboring the LIMD1-BRAF fusion showed a partial response and remained on treatment with trametinib for more than 7 months (3). Another case report on a patient harboring the TRIM24-BRAF fusion demonstrated a durable response to vemurafenib (4). The patient in this case study demonstrated significant decrease of the pleural effusion on the left side after receiving dabrafenib and trametinib, which aligns with the NGS report of the left side pleuroscopic biopsy (Table 1) and indicate the importance of BRAF fusion as a de novo driver mutation.
Combination therapy targeting co-occurring MET amplification has been studied in patients with EGFR mutations (13). In a xenograft study using osimertinib resistant EGFR mutant lung cancer cells with MET amplification, the knockdown MET signal pathway or the combination of MET inhibitor could induce tumor shrinkage, indicating that targeting MET amplification may reverse EGFR-TKI resistance (14). In the phase 2 INSIGHT study, which enrolled patients with EGFR mutant NSCLC who had MET amplification or MET overexpression after acquired resistance to EGFR-TKI, the median progression-free survival was 16.6 months among patients received tepotinib and gefitinib combination (6). In the exploratory analysis of phase 2 study regarding the treatment efficacy of combined erlotinib and emibetuzumab, MET inhibitor patients with a high level of MET expression (MET immunohistochemistry score of 3+ in at least 90% of tumor cells) had significantly long progression-free survival when receiving combination therapy with erlotinib and emibetuzumab (20.7 versus 5.4 months, hazard ratio 0.39 [0.17–0.91]) (5). Currently, there are also ongoing clinical trials investigating the role of combination with capmatinib therapy in osimertinib-resistant EGFR mutant NSCLC and MET amplification, including SAVANNAH (NCT03778229) (7), INSIGHT 2 (NCT03940703) (8), and GEOMETRY-E (NCT04816214). The studies above implied the clinical benefit of combination therapy to co-occurring MET amplification.
Similarly, in a cell line study using primary culture from patients harboring BRAF mutation and MET amplification, the inhibition of MEK expression induces dose-responsive MET activation (10). The combination of MEK inhibitor and MET monoclonal antibody provided significant tumor shrinkage in a patient-derived xenograft mouse model of cancer with co-occurring BRAF mutation and MET amplification (10), which is in line with our case report. This study highlights the importance of combination therapy. The underlying mechanism may result from the activation of downstream MAPK pathway induced by MET amplification, which enhance the kinase activity of BRAF fusion protein. The combination therapy which targets both MET amplification and BRAF fusion is the potential treatment strategy (Figure 3). Here, the patient with co-occurring KIAA1549-BRAF fusion and MET amplification suffered from progressively increased pleural effusion on the right side despite receiving combination therapy with dabrafenib and trametinib. The pleural effusion resolved gradually and remained stable for more than 6 months after adding capmatinib, a selective MET inhibitor. This case report highlights the importance of comprehensive genomic profiling to identify the druggable driver mutations and the combination therapy may be a potential strategy for patients with co-occurring mutations.
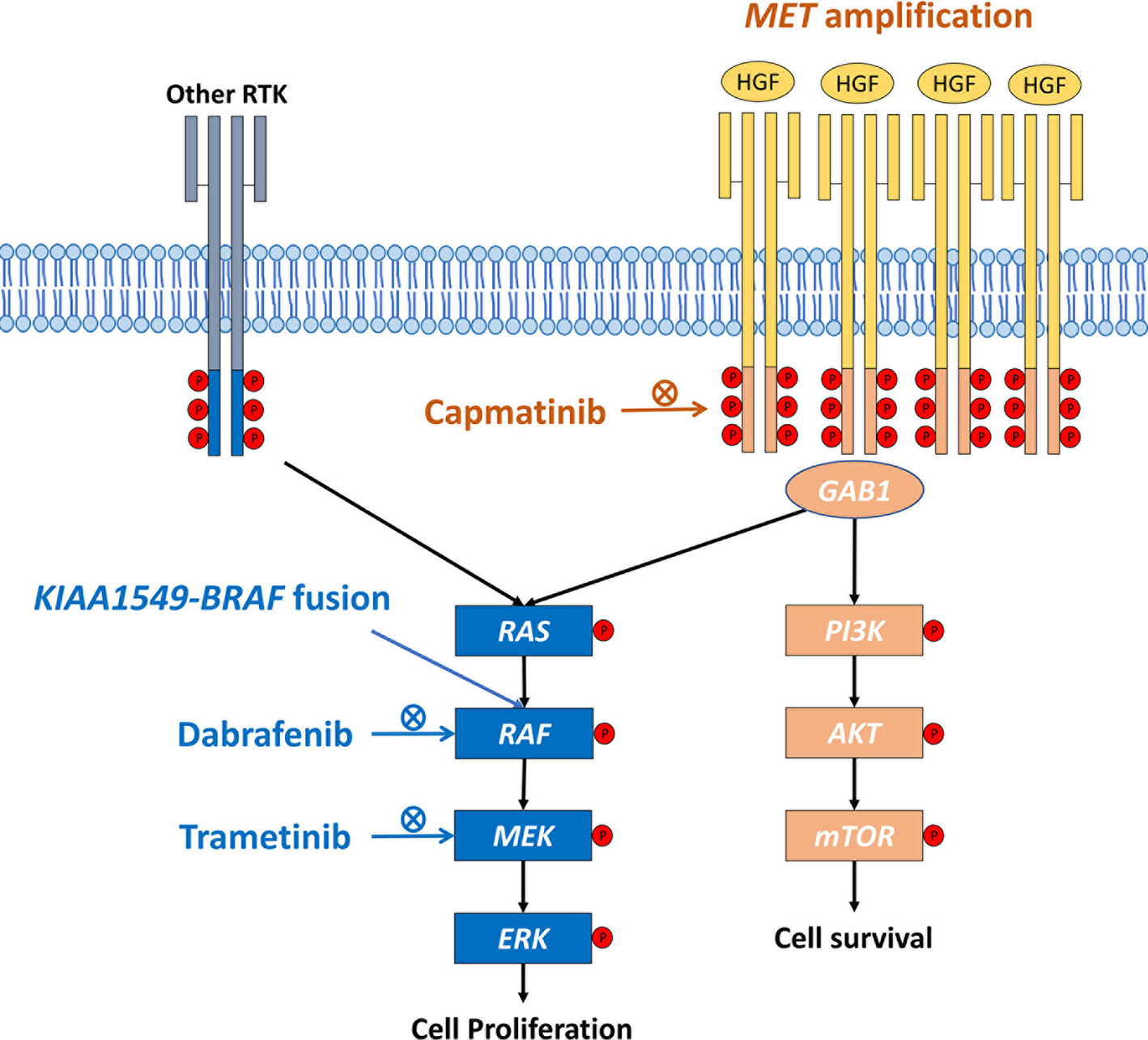
Figure 3 Schematic diagram of the cross-reactivity of BRAF fusion and MET amplification. HGF, hepatocyte growth factor; RTK, receptor tyrosine kinase.
Previous study regarding the adverse events in the combination therapy of different targeted therapies remains limited. According to the phase 2 study investigating the treatment efficacy of dabrafenib and trametinib in patients with BRAF V600E positive NSCLC, the most common grade 3 or 4 adverse events were pyrexia (11%), elevated liver enzyme (11%), hypertension (11%), and vomiting (8%) (15). Meanwhile, patients received capmatinib had adverse events of peripheral edema (51%) and nausea (45%), but these events were mostly of grade 1 or 2 (9). In this case report, patient suffered from higher grade of adverse events after combination therapy. The symptom subsided partially after the reduction of capmatinib dosage and the clinical condition remains stable. It implies that the combination therapy may aggravate the adverse event of each targeted therapy, and dose reduction instead of interruption might be a better choice.
Conclusion
We report a case of advanced NSCLC harboring co-occurring KIAA1549-BRAF fusion and MET amplification in a patient with a durable response and tolerant adverse event to combination therapy with dabrafenib, trametinib, and capmatinib. Future prospective studies are warranted to validate the efficacy of combination therapy in patients with multiple driver oncogenes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Review Board and Ethics Committee of National Cheng Kung University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-TC and P-LS had full access to data in this case report and takes responsibility for the integrity and accuracy of data analysis. Y-TC and P-LS contributed to pleuroscopic examination. C-CL, DP, and P-LS contributed to the scientific review and final approval of this manuscript. All authors read and approved the final manuscript.
Funding
The present study was funded by grant no. MOST 110-2314-B-006-102 from the Ministry of Science and Technology, Taiwan.
Conflict of Interest
DP is an employee of Foundation Medicine Inc. (FMI) and has equity interest in F. Hoffmann-La Roche AG, of which FMI is a wholly owned subsidiary.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The patient involved in this case report gave her informed consent authorizing use and disclosure of his health information.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.838798/full#supplementary-material
References
1. Schrock AB, Zhu VW, Hsieh WS, Madison R, Creelan B, Silberberg J, et al. Receptor Tyrosine Kinase Fusions and BRAF Kinase Fusions Are Rare But Actionable Resistance Mechanisms to EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol (2018) 13:1312–23. doi: 10.1016/j.jtho.2018.05.027
2. Sheikine Y, Pavlick D, Klempner SJ, Trabucco SE, Chung JH, Rosenzweig M, et al. BRAF in Lung Cancers: Analysis of Patient Cases Reveals Recurrent BRAF Mutations, Fusions, Kinase Duplications, and Concurrent Alterations. JCO Precis Oncol (2018) 2:PO.17.00172. doi: 10.1200/PO.17.00172
3. Wang CY, Hsia JY, Li CH, Ho CC, Chao WR, Wu MF. Lung Adenocarcinoma With Primary LIMD1-BRAF Fusion Treated With MEK Inhibitor: A Case Report. Clin Lung Cancer (2021) 22:e878–80. doi: 10.1016/j.cllc.2021.05.003
4. Zhu YC, Wang WX, Xu CW, Zhuang W, Du KQ, Chen G, et al. A Patient With Lung Adenocarcinoma With BRAF Gene Fusion and Response to Vemurafenib. Clin Lung Cancer (2019) 20:e224–8. doi: 10.1016/j.cllc.2019.02.020
5. Scagliotti G, Moro-Sibilot D, Kollmeier J, Favaretto A, Cho EK, Grosch H, et al. A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination With Erlotinib as First-Line Treatment for EGFR Mutation-Positive NSCLC Patients. J Thorac Oncol (2020) 15:80–90. doi: 10.1016/j.jtho.2019.10.003
6. Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib Plus Gefitinib in Patients With EGFR-Mutant Non-Small-Cell Lung Cancer With MET Overexpression or MET Amplification and Acquired Resistance to Previous EGFR Inhibitor (INSIGHT Study): An Open-Label, Phase 1b/2, Multicentre, Randomised Trial. Lancet Respir Med (2020) 8:1132–43. doi: 10.1016/S2213-2600(20)30154-5
7. Oxnard GR, Cantarini M, Frewer P, Hawkins G, Peters J, Howarth P, et al. SAVANNAH: A Phase II Trial of Osimertinib Plus Savolitinib for Patients (Pts) With EGFR-Mutant, MET-Driven (MET+), Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC), Following Disease Progression on Osimertinib. J Clin Oncol (2019) 37:TPS9119. doi: 10.1200/JCO.2019.37.15_suppl.TPS9119
8. Smit EF, Dooms C, Raskin J, Nadal E, Tho LM, Le X, et al. INSIGHT 2: A Phase II Study of Tepotinib Plus Osimertinib in MET-Amplified NSCLC and First-Line Osimertinib Resistance. Future Oncol (2021). doi: 10.2217/fon-2021-1406
9. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med (2020) 383:944–57. doi: 10.1056/NEJMoa2002787
10. Virzì AR, Gentile A, Benvenuti S, Comoglio PM. Reviving Oncogenic Addiction to MET Bypassed by BRAF (G469A) Mutation. Proc Natl Acad Sci USA (2018) 115:10058–63. doi: 10.1073/pnas.1721147115
11. Vojnic M, Kubota D, Kurzatkowski C, Offin M, Suzawa K, Benayed R, et al. Acquired BRAF Rearrangements Induce Secondary Resistance to EGFR Therapy in EGFR-Mutated Lung Cancers. J Thorac Oncol (2019) 14:802–15. doi: 10.1016/j.jtho.2018.12.038
12. McEvoy CR, Xu H, Smith K, Etemadmoghadam D, Leong HS, Choong DY, et al. Profound MEK Inhibitor Response in a Cutaneous Melanoma Harboring a GOLGA4-RAF1 Fusion. J Clin Invest (2019) 129:1940–5. doi: 10.1172/JCI123089
13. Clement MS, Ebert EBF, Meldgaard P, Sorensen BS. Co-Occurring MET Amplification Predicts Inferior Clinical Response to First-Line Erlotinib in Advanced Stage EGFR-Mutated NSCLC Patients. Clin Lung Cancer (2021) 22:e870–7. doi: 10.1016/j.cllc.2021.05.002
14. Nishiyama A, Takeuchi S, Adachi Y, Otani S, Tanimoto A, Sasaki M, et al. MET Amplification Results in Heterogeneous Responses to Osimertinib in EGFR-Mutant Lung Cancer Treated With Erlotinib. Cancer Sci (2020) 111:3813–23. doi: 10.1111/cas.14593
Keywords: BRAF fusion, MET amplification, case report, double driver mutation, combination therapy
Citation: Chou Y-T, Lin C-C, Lee C-T, Pavlick DC and Su P-L (2022) Durable Response of Dabrafenib, Trametinib, and Capmatinib in an NSCLC Patient With Co-Existing BRAF-KIAA1549 Fusion and MET Amplification: A Case Report. Front. Oncol. 12:838798. doi: 10.3389/fonc.2022.838798
Received: 18 December 2021; Accepted: 21 February 2022;
Published: 18 March 2022.
Edited by:
Yuki Sato, Kobe City Medical Center General Hospital, JapanReviewed by:
Kei Kunimasa, Osaka International Cancer Institute, JapanPierre Jean Souquet, Hospices Civils de Lyon, France
Copyright © 2022 Chou, Lin, Lee, Pavlick and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Lan Su, cG9sYW4uNzUwMzE3QGdtYWlsLmNvbQ==
 Yun-Tse Chou
Yun-Tse Chou Chien-Chung Lin
Chien-Chung Lin Chung-Ta Lee
Chung-Ta Lee Dean C. Pavlick
Dean C. Pavlick Po-Lan Su
Po-Lan Su