- 1Department of Nursing, Lishui People’s Hospital, Lishui, China
- 2Department of General Surgery, Lishui People’s Hospital, Lishui, China
- 3Department of Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objectives: The effect of laparoscopic gastrectomy (LG) for the treatment of advanced gastric cancer (AGC) is still controversial. The aim of this meta-analysis was to contrast the short- and long-term outcomes of laparoscopic versus conventional open gastrectomy (OG) for patients with AGC.
Methods: Databases including PubMed, Embase, Scopus, and Cochrane Library were systematically searched until December 2021 for randomized controlled trial-enrolled patients undergoing LG or OG for the treatment of AGC. Short-term outcomes were overall postoperative complications, anastomotic leakage, number of retrieved lymph node, surgical time, blood loss, length of hospital stay, and short-term mortality. Long-term outcomes were survival rates at 1, 3, and 5 years.
Results: A total of 12 trials involving 4,101 patients (2,059 in LG group, 2,042 in OG group) were included. No effect on overall postoperative complications (OR 0.84, 95% CI 0.67 to 1.05, p = 0.12, I2 = 34%) and anastomotic leakage (OR 1.26, 95% CI 0.82 to 1.95, p = 0.30, I2 = 0%) was found. Compared with the open approach, patients receiving LG had fewer blood loss (MD -54.38, 95% CI -78.09 to -30.67, p < 0.00001, I2 = 90%) and shorter length of hospital stay (MD -1.25, 95% CI -2.08 to -0.42, p = 0.003, I2 = 86%). However, the LG was associated with a lower number of retrieved lymph nodes (MD -1.02, 95% CI -1.77 to -0.27, p = 0.008, I2 = 0%) and longer surgical time (MD 40.87, 95% CI 20.37 to 54.44, p < 0.00001, I2 = 94%). Furthermore, there were no differences between LG and OG groups in short-term mortality and survival rate at 1, 3, and 5 years.
Conclusions: LG offers improved short-term outcomes including shorter hospital stays and fewer blood loss, with comparable postoperative complications, short-term mortality, and survival rate at 1, 3, and 5 years when compared to the open approach. Our results support the implementation of LG in patients with AGC.
Systematic Review Registration: PROSPERO (CRD 42021297141).
Introduction
Gastric cancer is one of the most common cancers and a main economic burden worldwide (1). According to the GLOBOCAN 2020 data, gastric cancer is the fifth most common malignancy and the fourth leading cause of cancer death, causing an estimated 768,793 deaths in 2020 globally (2). Surgical resection with lymphadenectomy is the cornerstone of multimodality curative treatment, and open gastrectomy (OG) has long been the gold standard worldwide (3). However, since Kitano et al. (4) reported the first laparoscopic gastrectomy (LG) for the treatment of early-stage distal gastric cancer in 1994, this minimally invasive technique has been rapidly developed and in the field of gastric cancer, especially for treatment of early gastric cancer (EGC) (5, 6).
Nowadays, the LG has gained growing popularity in the treatment of EGC since this minimally invasive technique has some definite benefits including lower postoperative complications, faster recovery, shortened postoperative length of stay, and better quality of life. Previously, several well-designed randomized controlled trials (RCTs) from China, Korea, and Japan demonstrated the beneficial short-term outcomes of laparoscopic distal gastrectomy (LDG) including less blood loss and postoperative pain, faster recovery, and shorter hospital stay, and similar oncologic safety to the open approach (7–10). However, despite the extensive use of laparoscopic surgery, whether this minimally invasive approach is beneficial for patients with advanced gastric cancer (AGC) remains controversial.
Recently, the CLASS-01 (11) and KLASS-02 trials (12) updated their results of long-term outcomes, indicating that locally AGC patients with LDG had similar long-term survival rates compared to open distal gastrectomy. Moreover, the LOGICA trial (13), which compared the LG with OG for treatment of AGC in the Western population, reported comparable outcomes including postoperative complications, length of hospital stay, R0 resection rate, lymph node yield, and 1-year overall survival (OS) rate. Therefore, in order to summarize the current high-quality evidence, we performed this meta-analysis of RCTs to compared the short- and long-term outcomes of LG versus OG for patients with AGC.
Methods
We conducted this meta-analysis according to the updated PRISRMA statement (14) (Supplementary Material 1) and registered the protocol on PROSPERO (CRD 42021297141). A literature search was performed in PubMed, Embase, Scopus, and Cochrane Library for eligible RCTs in English from inception through December 2021. The search used broad search terms containing “gastric cancer,” “gastric carcinoma,” “stomach cancer,” “laparoscopic,” “laparoscopy,” “open gastrectomy,” and “RCT” (complete search strategies in Supplementary Material 2).
Eligibility Criteria
The inclusion criteria were as follows: 1) population: adult patients (older than 18 years) with AGC; 2) intervention: laparoscopic surgery for gastrectomy; 3) comparison: open surgery for gastrectomy; 4) outcomes: short-term outcomes including postoperative complication, number of retrieved lymph nodes, surgical time, blood loss, length of hospital stay, and short-term mortality (including in-hospital mortality, or mortality within 30 days after operation). Long-term outcomes were survival rate at 1, 3, and 5 years, including OS rate and disease-free survival (DFS) rate; 5) design: RCT.
Data Extraction and Quality Assessment
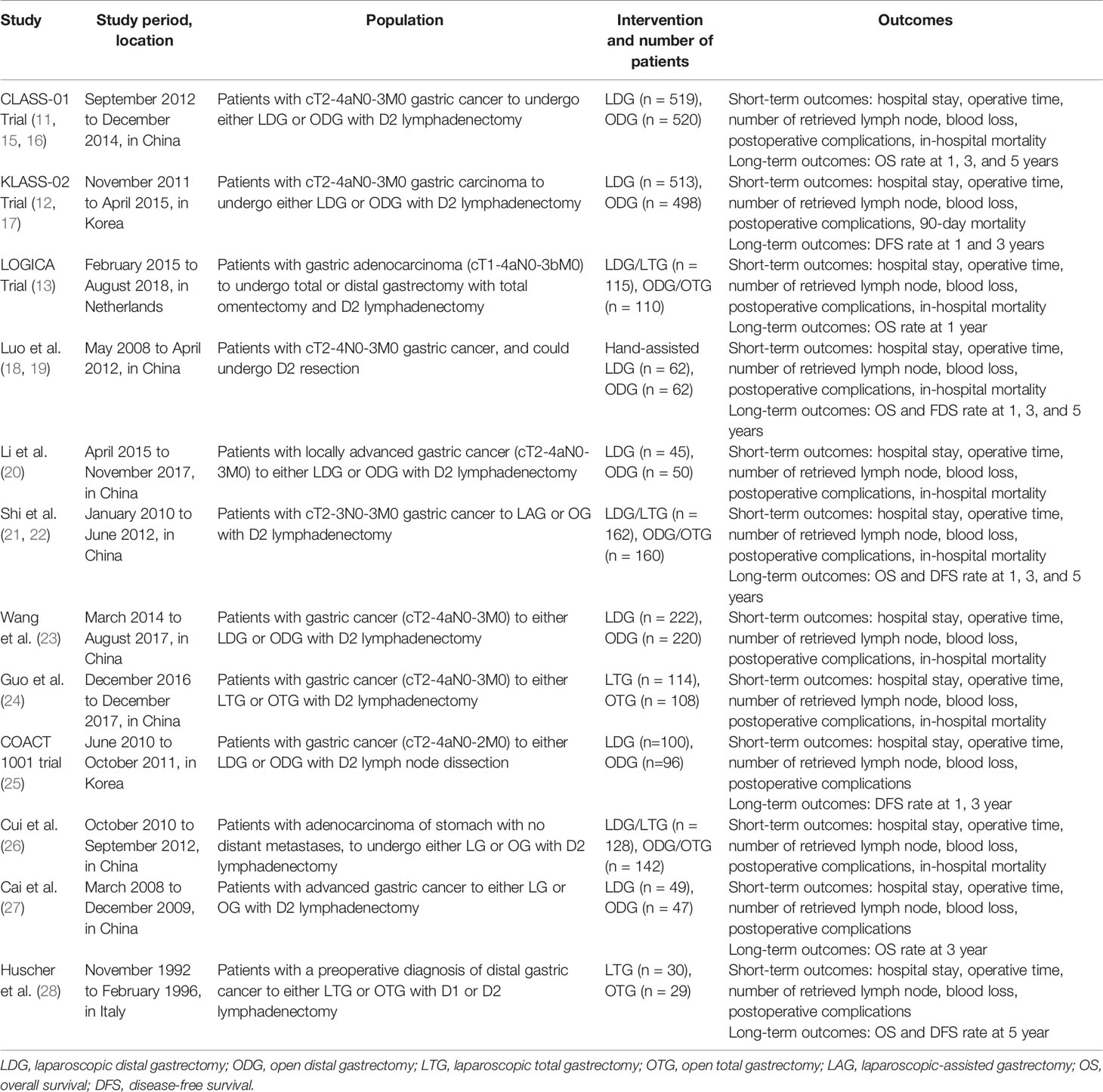
The data from included trials were independently extracted by two reviewers (JJ and SW). The characteristics of included studies (e.g., study, years of publication, study location, population, number of patients, intervention, outcomes) are recorded in Table 1.
For the methodological quality of including studies, two authors (JJ and SW) independently assessed the quality by using the Cochrane risk-of-bias tool (29).
Statistical Synthesis and Analysis
For short-term outcomes, we combined data from included studies to estimate the pooled odds ratio (OR) with 95% confidence interval (CI) for dichotomous outcomes, and continuous outcomes were pooled as mean difference (MD) with 95% CI. The meta-analysis of OS and DFS used the hazard ratio (HR) with 95% CI reported in the primary studies. If the primary studies did not provide the HR data, we obtained the HR data by digitizing the Kaplan–Meier survival curves (30). The heterogeneity between studies was tested by the chi-squared test with significance set at p value of 0.1, and quantitatively by inconsistency (I2) statistics (31). Substantial heterogeneity was identified when I2 value >30%, and we employed a random-effect model to perform the analysis; otherwise, a fixed-effect model would be used. In addition, we used the funnel plot and Egger’s regression test to assess the publication bias (32).
A predefined subgroup analysis was performed based on the extent of resection (partial versus total gastrectomy) and tumor stage (clinical stage II versus stage III). In addition, a sensitivity analysis by omitting each one trial at a time was performed to explore the effect of individual trials.
Results
Study Identification and Characteristics
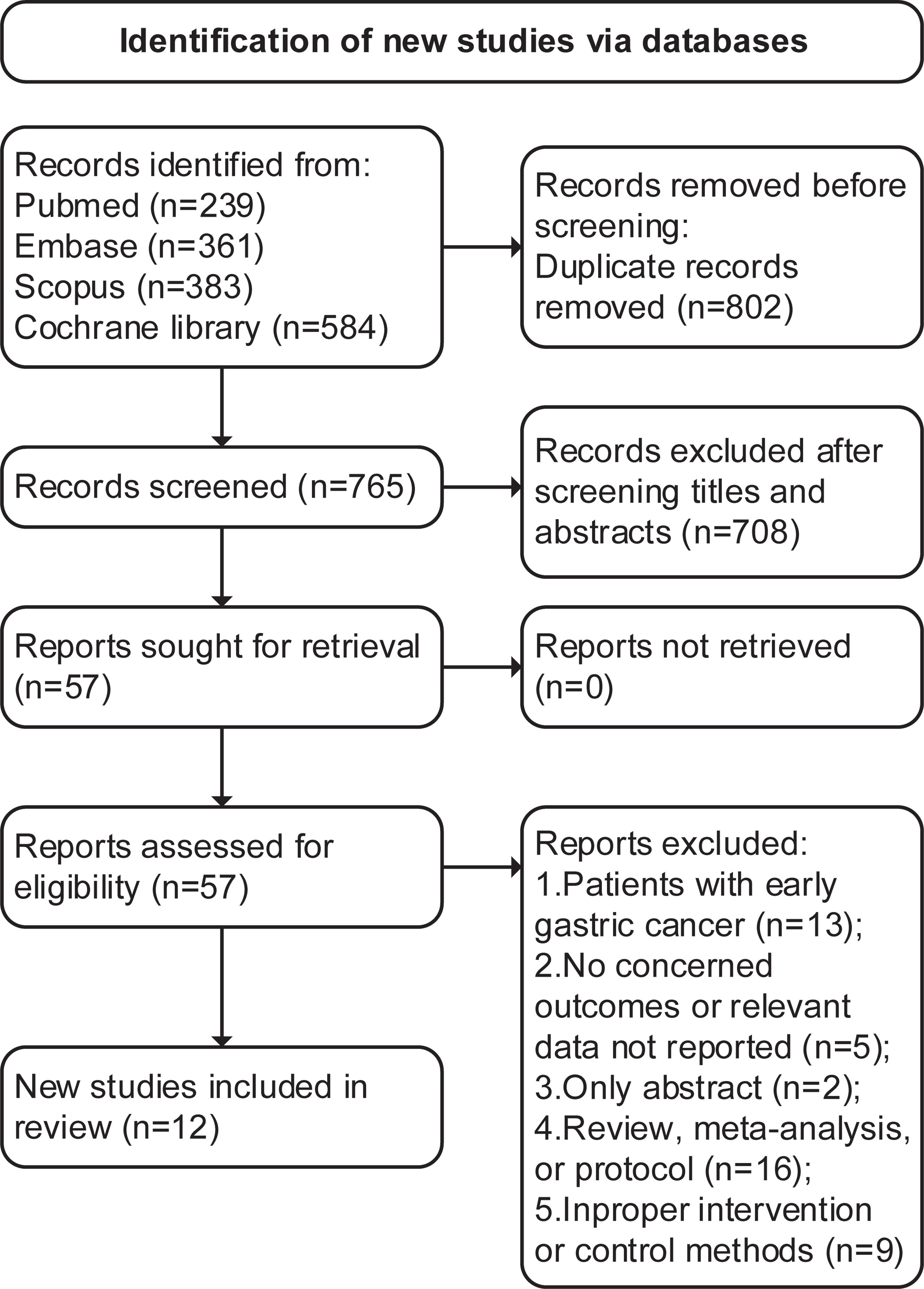
The initial search identified 1,567 articles (239 from PubMed, 361 from Embase, 383 from Scopus, and 584 from Cochrane Library), 802 were duplications, and 708 studies were excluded through title and abstract screening. In the full-text assessments, 45 studies were further excluded with reasons and a total of 12 trials with 17 articles (11–13, 15–28) were finally included (search process in Figure 1).
Table 1 presents the characteristics of including trials. A total of 4,101 patients with ACG were analyzed, 2,059 in the LG group and 2,042 in the OG group. The sample size of included trials ranged from a minimum of 59 up to 1,039. Among the 12 included trials, eight were done in China (11, 15, 16, 18–24, 26, 27), two in Korea (12, 17, 25), one in Netherlands (13), and one in Italy (28). In each included trial, the LG and OG groups were similar as regards age, gender, tumor size, clinical TNM stage, and the American Society of Anesthesiologist (ASA) score. The types of operation varied among each trial, seven trials (11, 12, 15–20, 23, 25, 27) performed partial gastrectomy, and two (24, 28) performed total gastrectomy; the operation in the rest of the three trials (13, 21, 22, 26) included total or partial gastrectomy.
In addition, the number of retrieved lymph nodes, length of hospital stay, and blood loss in two trials (13, 20) were expressed in the form of median and interquartile range (IQR). Thus, we used the methodology of Wan et al. (33) to convert these data into mean and standard deviation (SD).
Quality Assessment
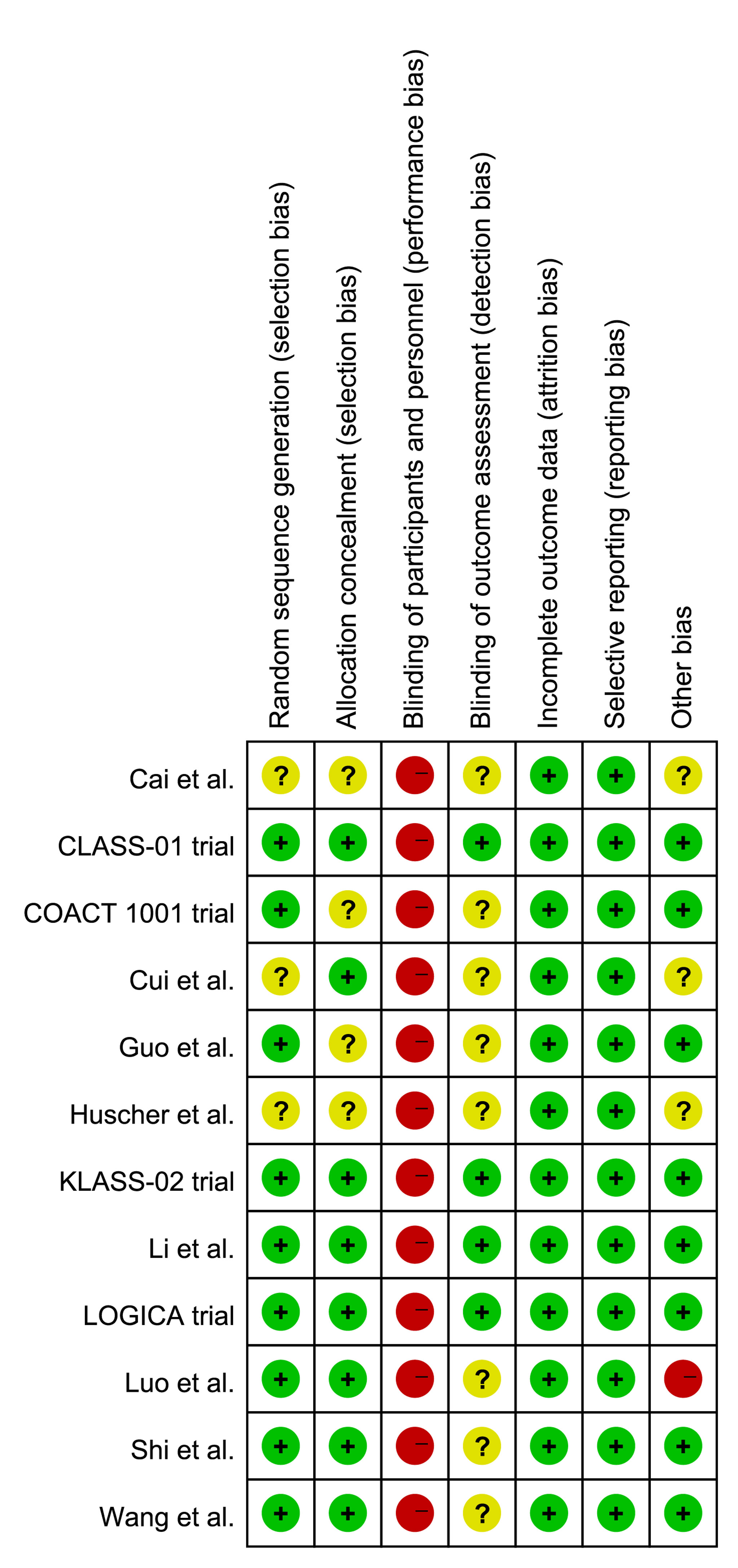
The quality assessment results are presented in Figure 2. Since all included trials were open-label study, they had a high risk of performance bias. Three trials (26–28) did not provide the detailed information of random sequence generation and four trials (24, 25, 27, 28) did not state the allocation concealment. The blinding method for outcome assessment was unclear in eight trials (19, 22–28). Moreover, Cai et al. (27), Cui et al. (26), and Huscher et al. (28) did not clarify the clinical TMN stage of included patients in the inclusion criteria, and Luo et al. performed a hand-assisted laparoscopic surgery, which was different from other trials.
The funnel plot and Egger’s test were used to evaluate the publication bias (Supplementary Material 3); the results showed that there was potential risk of publication bias for the blood loss and 5-year survival rate (Egger’s test, p < 0.10). Therefore, we used the trim-and-fill method to perform an additional analysis. The analysis after imputing continued to show that the LG group was associated with decreased blood loss (MD -37.13, 95% CI -62.20 to -12.06, p = 0.0037, I2 = 91%) and similar 5-year survival rate (OR 0.85, 95% CI 0.69 to 1.04, p = 0.12, I2 = 0%).
Short-Term Outcomes
All included trials reported the incidence of overall postoperative complications. The pooled analysis indicated no effect on the overall postoperative complications (OR 0.84, 95% CI 0.67 to 1.05, p = 0.12, I2 = 34%; Table 2, Figure 3A). Moreover, we compared the incidence of anastomotic leakage; the results indicated that there was no significant difference of anastomotic leakage rate between the two surgical options (OR 1.26, 95% CI 0.82 to 1.95, p = 0.30, I2 = 0%; Table 2, Figure 3B).
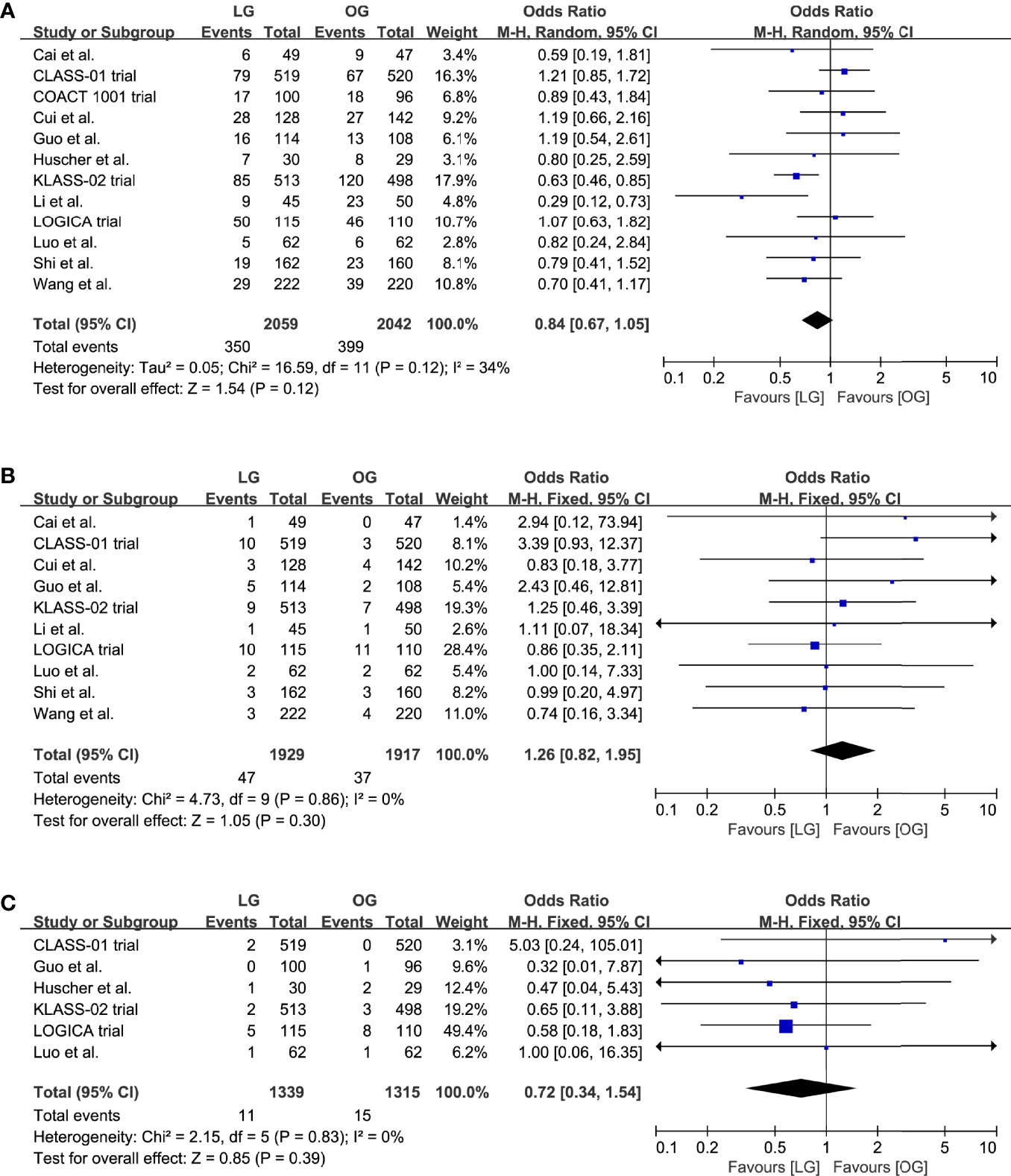
Figure 3 Pooled estimates of (A) overall postoperative complications, (B) incidence of anastomotic leakage, (C) short-term mortality.
Data on the short-term mortality were reported in ten trials, and in five trials the mortality was 0% for both groups. A meta-analysis of the remaining five trials revealed no differences in short-term mortality between the groups (OR 0.72, 95% CI 0.34 to 1.54, p = 0.39, I2 = 0%; Table 2, Figure 3C).
Eleven trials reported the data on length of hospital stay and blood loss; our results indicated that the LG was associated with a shorter length of hospital stay (MD -1.25, 95% CI -2.08 to -0.42, p = 0.003, I2 = 86%; Table 2, Figure 4A) and reduced blood loss (MD -54.38, 95% CI -78.09 to -30.67, p < 0.00001, I2 = 90%; Table 2, Figure 4B).
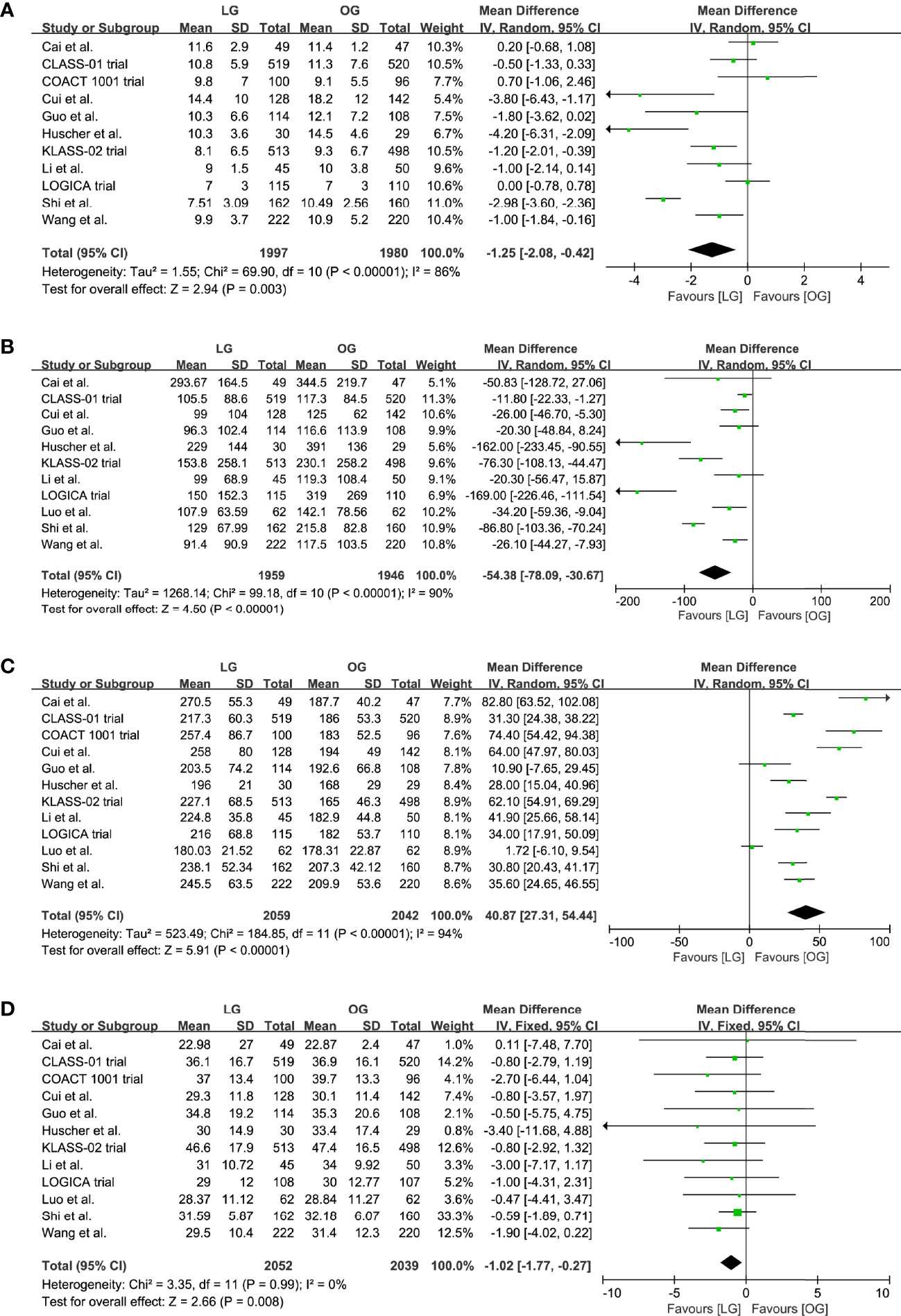
Figure 4 Pooled estimates of (A) length of hospital stay, (B) blood loss, (C) surgical time, (D) number of retrieved lymph nodes.
All trials reported a longer surgical time of the LG group, and our meta-analysis further confirmed this effect (MD 40.87, 95% CI 27.31 to 54.44, p < 0.00001, I2 = 94%; Table 2, Figure 4C). However, these results should be interpreted prudently because of the significant heterogeneity.
In addition, the LG group had a lower number of retrieved lymph nodes when compared with the OG group (MD -1.02, 95% CI -1.77 to -0.27, p = 0.008, I2 = 0%; Table 2, Figure 4D).
Long-Term Outcomes
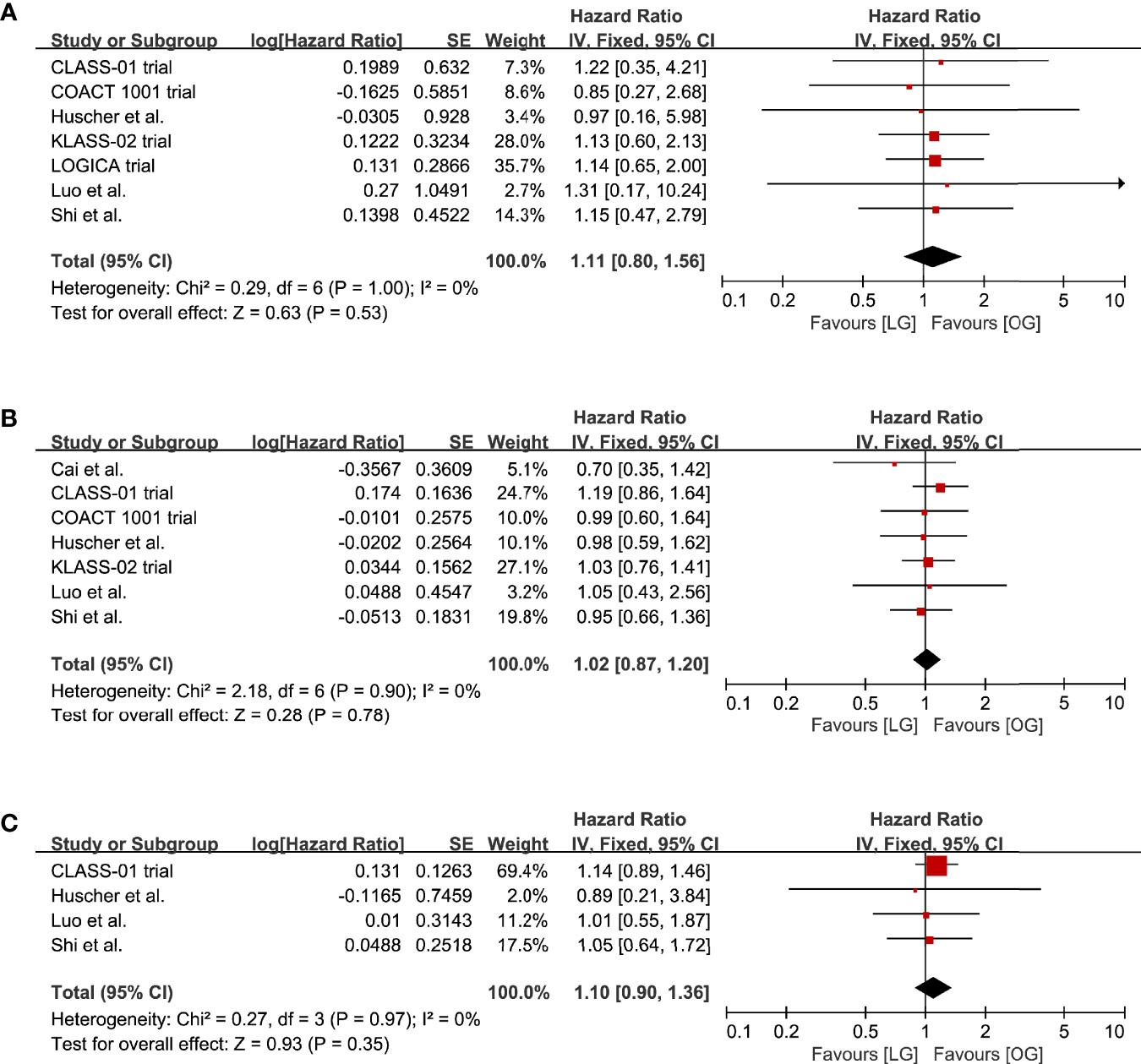
The survival rates at 1, 3, and 5 years were reported in eight studies. The LG group had survival rates of 91.6%, 64.7%, and 32.9% at 1, 3, and 5 years. The survival rates in the OG group were 89.0%, 59.1%, and 31.4%, respectively. The meta-analysis indicated that there was no significant difference in the survival rates at 1, 3, and 5 years between the LG and OG groups (1-year: HR 1.11, 95% CI 0.80 to 1.56, p = 0.53, I2 = 0%; 3-year: HR 1.02, 95% CI 0.87 to 1.20, p = 0.78, I2 = 0%; 5-year: HR 1.10, 95% CI 0.90 to 1.36, p = 0.35, I2 = 0%; Table 2, Figure 5).
Furthermore, we stratified survival data by OS or DFS rate; the pooled results showed no significant difference in the OS or DFS rate at 1, 3, and 5 years between the LG and OG groups, respectively (Supplementary Material 4).
Subgroup and Sensitivity Analyses
Predefined subgroup analyses stratified by the extent of resection (partial versus total gastrectomy) and tumor stage (clinical stage II versus stage III) were performed to explore the potential discrepant treatment effect of different subgroups (Table 2, Supplementary Material 5). In addition, based on the Clavien–Dindo classification (grades I to II as minor complications, grades III to V as major complications) (34), we divided the data of postoperative complications into minor and major complications.
The extent of resection had no effect on the overall postoperative complications, anastomotic leakage, short-term mortality, and long-term outcomes. Similarly, there was no significant difference in minor and major complications between the LG and OG groups. The beneficial effect of LG in reducing the length of hospital stay and blood loss was more significant after total gastrectomy than partial gastrectomy. Moreover, compared with total gastrectomy, patients receiving partial gastrectomy by a laparoscopic route was associated with more significantly longer surgical time and lower number of retrieved lymph nodes than open surgery.
Regarding tumor stage, five trials (11–13, 21, 25) provided the long-term survival data of clinical stage II and III gastric cancer, respectively. The results of subgroup analyses showed no significant difference for survival rates at 1, 3, and 5 years between LG and OG groups in both clinical stage II and III gastric cancer population.
In the sensitivity analysis, the LG was relevant to the obvious decrease in postoperative complications (OR 0.77, 95% CI 0.63 to 0.95) after omitting the CLASS-01 trial, indicating the poor robustness. Furthermore, other short- and long-term outcomes showed no significant differences with primary results (Supplementary Material 6).
Discussion
There is growing high-quality RCTs supporting the feasibility of LG for AGC, and its safety is confirmed in our study. In this up-to-date meta-analysis, we reviewed 12 RCTs with 4,101 patients to compare the short- and long-term outcomes of LG versus OG. The result shows that the LG significantly reduces the length of hospital stay and blood loss, whereas the number of retrieved lymph nodes was lower and surgical time was longer in the LG group. Furthermore, there were no differences between LG and OG groups in terms of postoperative complications, short-term mortality, and survival rate at 1, 3, and 5 years. The finding provides further evidence for the safety and efficacy of LG for the treatment of ACG.
To the best of our knowledge, this study is not the first meta-analysis of RCTs to compare the LG with OG for the treatment of gastric cancer. Recently, Vasavada and Patel (35) performed an updated meta-analysis of 11 RCTs (6 RCTs for EGC and 5 RCTs for AGC); the results demonstrated that the LG was associated with lesser wound-related complications without decreasing the length of hospital stay. However, for the patients with AGC, most trials analyzed in previous meta-analyses were non-randomized, which may increase the risk of potential selection and publication bias. Therefore, the current meta-analysis provides the most comprehensive and accurate analysis, since it sums up the up-to-date and high-quality RCTs in terms of comparing LG to OG for patients with AGC. Also, compared with previous meta-analyses, more RCTs with a long-term follow-up were included in it. In general, our results are in compliance with previous meta-analyses (36–40), showing that when compared to the open approach, the LG provides improved short-term outcomes and similar long-term outcomes in patients with AGC.
When choosing the clinical outcomes of our study, we compared the LG with OG on different levels in terms of safety (postoperative morbidity), difficulty (operative time, blood loss, number of retrieved lymph nodes), efficiency (length of hospital stay), and its long-term oncologic results (OS and DFS rates). The results of our meta-analysis indicate that the short-term outcomes consisting of blood loss and length of hospital stay are in favor of a laparoscopic approach, especially for total gastrectomy. Since the advanced laparoscopic approach provides a magnified surgical view while minimizing the length of the incision, a more delicate surgical manipulation of the organs, vessels, and nerves could be achieved during operation (23). In addition, the reduction of hospital stay may be a combined result of fewer blood loss during operation, faster postsurgical recovery of bowel function, and lighter postoperative pain (36).
The overall postoperative complications, including minor and major complications, were similar between the two surgical procedures in our study. However, a recent meta-analysis of data from 6 RCTs and 18 non-randomized trials found that LG was associated with a lower postoperative complication rate, with a significantly lower incidence of medical and minor surgical complications (36). The difference may result from several newly published RCTs, especially the CLASS-01 (16) and LOGICA trials (13). In the sensitivity analysis, we found a significant difference for the postoperative complications in the LG group after omitting the CLASS-01 trial (41). Considering the high risk of imprecision bias, more evidence about the effect of LG on postoperative complications is compellingly needed in the future. Furthermore, anastomotic leakage, the major postoperative complication of gastric surgery, was comparable between two groups. Notably, although the observed difference was meaningless from the statistical perspective, anastomotic leakage seemed to have a higher possibility to occur after laparoscopic surgery. It highlights that this potential risk should be put more emphasis on. There are studies that propose that the application of mini-laparotomy for extracorporeal anastomosis in laparoscopic surgery for AGC may result in increased surgical difficulty, which may increase the likelihood of anastomotic drawbacks on the other hand (41).
Based on the updated studies, LG requires a longer surgical time, which is in line with the results of all included trials. Compared with open approaches, laparoscopic techniques are more complex and less flexible. Frequently cleaning cameras and changing instruments during operation can also extend the surgical time (42, 43). In addition, it is a challenge to perform the dissection of enlarged or suprapancreatic lymph nodes through the laparoscopic approach, as it is difficult to follow the no-touch principle for laparoscopic lymphadenectomy at a deep lymph node station. Moreover, due to the restriction of the visibility and the narrowness of the peritoneal cavity, total omentectomy in LG is also hard to achieve compared with that in OG.
Although the result of our meta-analysis indicates that the number of retrieved lymph nodes was lower in the LG group, the mean number of retrieved lymph nodes in the LG group was 32.45 (95% CI 29.01 to 35.89). Based on the American Joint Committee on Cancer, an adequate dissection should include at least 15 lymph nodes for patients with gastric cancer to ensure accurate and robust N staging (44). A recent study demonstrated that an examined lymph node threshold of more than 30 was shown to be beneficial of survival for patients with gastric cancer and should be considered a clinical benchmark for practice (45). It is in accordance with the most crucial results of our study in terms of the long-term outcomes. The 1-, 3, and 5-year survival rates were similar among the two groups. When the surgical margins fulfilled the R0 resection criteria and adequate lymph node dissection could be achieved, long-term survival largely depended on the intrinsic biological characteristic of the cancer rather than the surgical approach (46, 47).
Furthermore, the learning curve was proved to have significant effects on most of the important surgical and short-term recovery outcome parameters (48). Yoo et al. performed a prospective study to estimate the learning curve of LG for EGC, indicating that surgeons who performed at least 50 cases of LG could achieve lower postoperative complications, more resected lymph nodes, shorter surgical time, and postoperative hospital stay (49). Thus, the LG should be restricted to specialist centers where adequate training and supervision could be provided during the learning curve.
However, some limitations of our meta-analysis must be acknowledged. First of all, the sample size of some included trials was relatively small, which may decrease the credibility of the results in our study or lead to small study effect bias (50). Secondly, our study did not consider the differences and potential impact of surgeons’ experience, perioperative care protocols, and surgical technique between studies, despite their application having been shown to be beneficial in many studies (51–53). Thirdly, most of all included trials were conducted in East Asia (eight in China, two in Korea), except one in Italy and one in Netherlands. Therefore, the generalizability of the findings to Western countries may be limited. Moreover, since the Western population has a comparatively low incidence of gastric cancer, higher body mass index, and more comorbidities (54), the results may not necessarily apply in the Western population.
Last but not least, there was significant heterogeneity in some pooled estimates, which might be explained by differences in sample sizes, surgeons’ experience, perioperative care protocols, surgical technique, pre- and postoperative chemotherapy, and other factors. Variations in sample size among studies were large, and some studies enrolled patients during a wide study interval, which may have introduced biases due to a progression in mastering the surgical skills and improvements in surgical instruments.
Conclusion
Our findings, which are contingent on rigorous meta-analyses of high-quality RCTs, suggest that LG offers improved short-term outcomes including shorter hospital stays and fewer blood loss, with comparable postoperative complications, short-term mortality, and long-term survival rates when compared to the open approach. However, considering the significant heterogeneity, more RCTs are needed to further evaluate the clinical outcomes of LG versus OG for patients with AGC. Furthermore, this updated meta-analysis could be the basis of future meta-analyses, as the inclusion criteria, statistical analysis, and short- and long-term outcomes were clearly defined and meticulously analyzed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
JJ and SW conceived the idea, performed the analysis, and drafted the initial draft writing of this paper. GY, JW, and XX contributed to the collection and interpretation of data. KZ helped to frame the idea of the study and provided technical support. SW contributed to the revision of this paper and the final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.844803/full#supplementary-material
References
1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed With One of 18 Cancers From 322 Population-Based Registries in 71 Countries. Lancet (2018) 391(10125):1023–75. doi: 10.1016/s0140-6736(17)33326-3
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Terashima M. The 140 Years' Journey of Gastric Cancer Surgery: From the Two Hands of Billroth to the Multiple Hands of the Robot. Ann Gastroenterol Surg (2021) 5(3):270–7. doi: 10.1002/ags3.12442
4. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-Assisted Billroth I Gastrectomy. Surg Laparosc Endosc (1994) 4(2):146–8.
5. Hatta W, Gotoda T, Koike T, Masamune A. History and Future Perspectives in Japanese Guidelines for Endoscopic Resection of Early Gastric Cancer. Dig Endosc (2020) 32(2):180–90. doi: 10.1111/den.13531
6. Wong J, Jackson P. Gastric Cancer Surgery: An American Perspective on the Current Options and Standards. Curr Treat Options Oncol (2011) 12(1):72–84. doi: 10.1007/s11864-010-0136-y
7. Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-Term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg (2016) 263(1):28–35. doi: 10.1097/sla.0000000000001346
8. Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, et al. Short-Term Surgical Outcomes From a Phase III Study of Laparoscopy-Assisted Versus Open Distal Gastrectomy With Nodal Dissection for Clinical Stage IA/IB Gastric Cancer: Japan Clinical Oncology Group Study Jcog0912. Gastric Cancer (2017) 20(4):699–708. doi: 10.1007/s10120-016-0646-9
9. Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Watanabe M, et al. Laparoscopy Versus Open Distal Gastrectomy by Expert Surgeons for Early Gastric Cancer in Japanese Patients: Short-Term Clinical Outcomes of a Randomized Clinical Trial. Surg Endosc (2013) 27(5):1695–705. doi: 10.1007/s00464-012-2658-9
10. Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, et al. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol (2020) 6(10):1590–7. doi: 10.1001/jamaoncol.2020.3152
11. Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg (2021) 157(1):9–17. doi: 10.1001/jamasurg.2021.5104
12. Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, et al. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol (2020) 38(28):3304–13. doi: 10.1200/jco.20.01210
13. van der Veen A, Brenkman HJF, Seesing MFJ, Haverkamp L, Luyer MDP, Nieuwenhuijzen GAP, et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J Clin Oncol (2021) 39(9):978–89. doi: 10.1200/jco.20.01540
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
15. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA (2019) 321(20):1983–92. doi: 10.1001/jama.2019.5359
16. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol (2016) 34(12):1350–7. doi: 10.1200/jco.2015.63.7215
17. Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, et al. Short-Term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg (2019) 270(6):983–91. doi: 10.1097/sla.0000000000003217
18. Luo G, Wang X, Li Y, Chen G, Cao Y, Gong J, et al. Hand-Assisted Laparoscopic Versus Open Surgery for Radical Gastrectomy in the Treatment of Advanced Distal Gastric Cancer: Long-Term Overall and Disease-Free Survival (Final Results of a Single-Center Study). J Int Med Res (2021) 49(9):3000605211047700. doi: 10.1177/03000605211047700
19. Luo G, Cao Y-K, Gong J, Wang X, Wang B, Zhou J, et al. Hand-Assisted Laparoscopic Versus Open Surgery Radical Gastrectomy for Advanced Distal Gastric Cancer: A Prospective Randomized Study. Int J Clin Exp Med (2017) 10:5001–10.
20. Li Z, Shan F, Ying X, Zhang Y, JY E, Wang Y, et al. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg (2019) 154(12):1093–101. doi: 10.1001/jamasurg.2019.3473
21. Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, et al. Long-Term Oncologic Outcomes of a Randomized Controlled Trial Comparing Laparoscopic Versus Open Gastrectomy With D2 Lymph Node Dissection for Advanced Gastric Cancer. Surgery (2019) 165(6):1211–6. doi: 10.1016/j.surg.2019.01.003
22. Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, et al. Short-Term Surgical Outcomes of a Randomized Controlled Trial Comparing Laparoscopic Versus Open Gastrectomy With D2 Lymph Node Dissection for Advanced Gastric Cancer. Surg Endosc (2018) 32(5):2427–33. doi: 10.1007/s00464-017-5942-x
23. Wang Z, Xing J, Cai J, Zhang Z, Li F, Zhang N, et al. Short-Term Surgical Outcomes of Laparoscopy-Assisted Versus Open D2 Distal Gastrectomy for Locally Advanced Gastric Cancer in North China: A Multicenter Randomized Controlled Trial. Surg Endosc (2019) 33(1):33–45. doi: 10.1007/s00464-018-6391-x
24. Guo X, Peng Z, Lv X, Cui J, Zhang K, Li J, et al. Randomized Controlled Trial Comparing Short-Term Outcomes of Laparoscopic and Open Spleen-Preserving Splenic Hilar Lymphadenectomy for Advanced Proximal Gastric Cancer: An Interim Report. J Surg Oncol (2018) 118(8):1264–70. doi: 10.1002/jso.25262
25. Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, et al. Laparoscopy-Assisted Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg (2018) 267(4):638–45. doi: 10.1097/sla.0000000000002168
26. Cui M, Li Z, Xing J, Yao Z, Liu M, Chen L, et al. A Prospective Randomized Clinical Trial Comparing D2 Dissection in Laparoscopic and Open Gastrectomy for Gastric Cancer. Med Oncol (2015) 32(10):241. doi: 10.1007/s12032-015-0680-1
27. Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A Prospective Randomized Study Comparing Open Versus Laparoscopy-Assisted D2 Radical Gastrectomy in Advanced Gastric Cancer. Dig Surg (2011) 28(5-6):331–7. doi: 10.1159/000330782
28. Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic Versus Open Subtotal Gastrectomy for Distal Gastric Cancer: Five-Year Results of a Randomized Prospective Trial. Ann Surg (2005) 241(2):232–7. doi: 10.1097/01.sla.0000151892.35922.f2
29. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
30. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
33. Wan X, Wang W, Liu J, Tong T. Estimating the Sample Mean and Standard Deviation From the Sample Size, Median, Range and/or Interquartile Range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
34. Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
35. Vasavada B, Patel H. Laparoscopic vs Open Gastrectomy: An Updated Meta-Analysis of Randomized Control Trials for Short-Term Outcomes. Indian J Surg Oncol (2021) 12(3):587–97. doi: 10.1007/s13193-021-01396-4
36. Chen X, Feng X, Wang M, Yao X. Laparoscopic Versus Open Distal Gastrectomy for Advanced Gastric Cancer: A Meta-Analysis of Randomized Controlled Trials and High-Quality Nonrandomized Comparative Studies. Eur J Surg Oncol (2020) 46(11):1998–2010. doi: 10.1016/j.ejso.2020.06.046
37. Quan Y, Huang A, Ye M, Xu M, Zhuang B, Zhang P, et al. Comparison of Laparoscopic Versus Open Gastrectomy for Advanced Gastric Cancer: An Updated Meta-Analysis. Gastric Cancer (2016) 19(3):939–50. doi: 10.1007/s10120-015-0516-x
38. Zhu Z, Li L, Xu J, Ye W, Zeng J, Chen B, et al. Laparoscopic Versus Open Approach in Gastrectomy for Advanced Gastric Cancer: A Systematic Review. World J Surg Oncol (2020) 18(1):126. doi: 10.1186/s12957-020-01888-7
39. Zou ZH, Zhao LY, Mou TY, Hu YF, Yu J, Liu H, et al. Laparoscopic vs Open D2 Gastrectomy for Locally Advanced Gastric Cancer: A Meta-Analysis. World J Gastroenterol (2014) 20(44):16750–64. doi: 10.3748/wjg.v20.i44.16750
40. Liao XL, Liang XW, Pang HY, Yang K, Chen XZ, Chen XL, et al. Safety and Efficacy of Laparoscopic Versus Open Gastrectomy in Patients With Advanced Gastric Cancer Following Neoadjuvant Chemotherapy: A Meta-Analysis. Front Oncol (2021) 11:704244. doi: 10.3389/fonc.2021.704244
41. Jeong O, Jung MR, Kang JH, Ryu SY. Reduced Anastomotic Complications With Intracorporeal Esophagojejunostomy Using Endoscopic Linear Staplers (Overlap Method) in Laparoscopic Total Gastrectomy for Gastric Carcinoma. Surg Endosc (2020) 34(5):2313–20. doi: 10.1007/s00464-019-07362-0
42. Wottawa CR, Cohen JR, Fan RE, Bisley JW, Culjat MO, Grundfest WS, et al. The Role of Tactile Feedback in Grip Force During Laparoscopic Training Tasks. Surg Endosc (2013) 27(4):1111–8. doi: 10.1007/s00464-012-2612-x
43. Zhang CD, Yamashita H, Zhang S, Seto Y. Reevaluation of Laparoscopic Versus Open Distal Gastrectomy for Early Gastric Cancer in Asia: A Meta-Analysis of Randomized Controlled Trials. Int J Surg (2018) 56:31–43. doi: 10.1016/j.ijsu.2018.05.733
44. Yuan SQ, Chen YT, Huang ZP. Equipping the 8th Edition American Joint Committee on Cancer Staging for Gastric Cancer With the 15-Node Minimum: A Population-Based Study Using Recursive Partitioning Analysis. J Gastrointest Surg (2017) 21(10):1591–8. doi: 10.1007/s11605-017-3504-0
45. Erstad DJ, Blum M, Estrella JS, Das P, Minsky BD, Ajani JA, et al. Navigating Nodal Metrics for Node-Positive Gastric Cancer in the United States: An NCDB-Based Study and Validation of AJCC Guidelines. J Natl Compr Canc Netw (2021) 22:1–12. doi: 10.6004/jnccn.2021.7038
46. Chen ZD, Zhang PF, Xi HQ, Wei B, Chen L, Tang Y. Recent Advances in the Diagnosis, Staging, Treatment, and Prognosis of Advanced Gastric Cancer: A Literature Review. Front Med (Lausanne) (2021) 8:744839. doi: 10.3389/fmed.2021.744839
47. Lordick F, Janjigian YY. Clinical Impact of Tumour Biology in the Management of Gastroesophageal Cancer. Nat Rev Clin Oncol (2016) 13(6):348–60. doi: 10.1038/nrclinonc.2016.15
48. Zhou D, Quan Z, Wang J, Zhao M, Yang Y. Laparoscopic-Assisted Versus Open Distal Gastrectomy With D2 Lymph Node Resection for Advanced Gastric Cancer: Effect of Learning Curve on Short-Term Outcomes. A Meta-Analysis. J Laparoendosc Adv Surg Tech A (2014) 24(3):139–50. doi: 10.1089/lap.2013.0481
49. Yoo CH, Kim HO, Hwang SI, Son BH, Shin JH, Kim H. Short-Term Outcomes of Laparoscopic-Assisted Distal Gastrectomy for Gastric Cancer During a Surgeon's Learning Curve Period. Surg Endosc (2009) 23(10):2250–7. doi: 10.1007/s00464-008-0315-0
50. Zhang Z, Xu X, Ni H. Small Studies may Overestimate the Effect Sizes in Critical Care Meta-Analyses: A Meta-Epidemiological Study. Crit Care (2013) 17(1):R2. doi: 10.1186/cc11919
51. Trencheva K, Morrissey KP, Wells M, Mancuso CA, Lee SW, Sonoda T, et al. Identifying Important Predictors for Anastomotic Leak After Colon and Rectal Resection: Prospective Study on 616 Patients. Ann Surg (2013) 257(1):108–13. doi: 10.1097/SLA.0b013e318262a6cd
52. Portinari M, Ascanelli S, Targa S, Dos Santos Valgode EM, Bonvento B, Vagnoni E, et al. Impact of a Colorectal Enhanced Recovery Program Implementation on Clinical Outcomes and Institutional Costs: A Prospective Cohort Study With Retrospective Control. Int J Surg (2018) 53:206–13. doi: 10.1016/j.ijsu.2018.03.005
53. Giuliante F, Ardito F, Vellone M, Ranucci G, Federico B, Giovannini I, et al. Role of the Surgeon as a Variable in Long-Term Survival After Liver Resection for Colorectal Metastases. J Surg Oncol (2009) 100(7):538–45. doi: 10.1002/jso.21393
Keywords: laparoscopic gastrectomy (LG), open gastrectomy (OG), advanced gastric cancer (AGC), postoperative complication, survival rate (SR), meta-analysis, randomized controlled trial
Citation: Jiang J, Ye G, Wang J, Xu X, Zhang K and Wang S (2022) The Comparison of Short- and Long-Term Outcomes for Laparoscopic Versus Open Gastrectomy for Patients With Advanced Gastric Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 12:844803. doi: 10.3389/fonc.2022.844803
Received: 28 December 2021; Accepted: 14 March 2022;
Published: 05 April 2022.
Edited by:
Annamaria Auricchio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Bhavin Vasavada, Shalby Hospitals, IndiaSusumu Shibasaki, Fujita Health University, Japan
Copyright © 2022 Jiang, Ye, Wang, Xu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Wang, MTg5NTcwOTI2NjdAMTYzLmNvbQ==
 Jinyan Jiang1
Jinyan Jiang1 Kai Zhang
Kai Zhang