Abstract
Objective:
We conducted a phase 2 trial to compare the safety and efficacy of intravenous paclitaxel or intraperitoneal paclitaxel plus mFOLFOX6 vs. mFOLFOX6 in untreated advanced gastric cancer.
Methods:
Participants with untreated advanced gastric cancer were randomly assigned (1:1:1) to: intravenous paclitaxel 135 mg/m2 or intraperitoneal paclitaxel 80 mg/m2 plus mFOLFOX6 omitting bolus fluorouracil; or mFOLFOX6 (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, fluorouracil 400 mg/m2 bolus, fluorouracil 2,400 mg/m2 46-h continuous infusion). Treatment was every 14 days for up to 9 cycles followed by S-1 maintenance. The primary outcome was progression-free survival.
Results:
Of 90 enrolled participants, 30 in the intravenous paclitaxel group, 29 in the intraperitoneal paclitaxel group, and 30 in the mFOLFOX6 group were included in the analyses. The median progression-free survival was 6.52, 5.83, and 4.55 months, respectively, for the intravenous paclitaxel group, intraperitoneal paclitaxel group, and mFOLFOX6 group. The hazard ratios were 0.56 (95% CI: 0.33–0.94; p = 0.026) and 0.56 (95% CI: 0.33–0.96; p = 0.037), respectively, for the intravenous paclitaxel group and the intraperitoneal paclitaxel group vs. the mFOLFOX6 group. The most common grade 3/4 adverse events for the intravenous paclitaxel group, intraperitoneal paclitaxel group, and mFOLFOX6 group, respectively, were neutropenia (30.0%, 34.5%, 33.3%), diarrhea (13.3%, 20.7%, 13.3%), and leukopenia (10.0%, 13.8%, 10.0%). No treatment-related death occurred.
Conclusion:
The findings of this phase 2 trial suggest that adding intravenous paclitaxel or intraperitoneal paclitaxel to mFOLFOX6 for untreated advanced gastric cancer improved progression-free survival with manageable adverse events.
Introduction
China has one of the world’s highest incidence rates of gastric cancer (GC), accounting for 42.6% of global incidence (1–3). Although GC in China has decreased in recent years, it remains the second most common cancer among men and the third most common among women (2, 3). While surgery with or without pre- or postoperative chemotherapy is the only potential curative treatment for early-stage GC (3), more than 80% of patients present with advanced GC (AGC), particularly in rural areas (2, 3).
The safety and efficacy of doublet regimens including fluoropyrimidine combined with either oxaliplatin or cisplatin have been widely reported (4, 5) and are recommended for untreated AGC (uAGC) (3, 6, 7). However, for fit patients, triplet chemotherapy also has been recommended (3, 6, 7). The V325 phase 3 trial reported that docetaxel, cisplatin, and fluorouracil (DCF) significantly improved time to progression, overall survival (OS), and overall response rate (ORR) compared with cisplatin and fluorouracil (CF) in uAGC. However, DCF has not been accepted globally due to its severe myelosuppression and small survival advantage (8). Various modifications of the DCF regimen, including intravenous (IV) paclitaxel, oxaliplatin, fluorouracil, and leucovorin (ivPOF) demonstrated improved safety, which was validated in Chinese patients with AGC (9–11). However, a randomized phase 3 trial showed no significant difference in progression-free survival (PFS), OS, or ORR between doublet irinotecan, fluorouracil, and leucovorin (FOLFIRI) and triplet epirubicin, cisplatin, and fluorouracil (ECF), with less toxicity and better tolerance attributed to FOLFIRI (12). Nonetheless, some studies suggest that DCF is superior to ECF (13, 14,), and controversy remains concerning triplet vs. doublet therapy in uAGC.
Peritoneal involvement, the most frequent site of metastasis in AGC, confers a dismal prognosis (3, 6, 7). Compared with plasma clearance, peritoneal clearance of certain hydrophobic and high-molecular-weight agents, such as paclitaxel, is much slower (15, 16). Therefore, intraperitoneal (IP) paclitaxel is designed to target peritoneal tumor nodules while minimizing systemic toxicity (15–20). The phase 2 studies of IP paclitaxel with S-1 and IV paclitaxel showed promising results, with a median OS of 17.6–23.6 months and ORR of 56%–71% (17–19). However, dosages of IP paclitaxel (recommended: 20 mg/m2 to 80 mg/m2) are controversial (15, 16). We conducted a phase 1b study comparing IP paclitaxel 60 mg/m2 day 1 plus modified ivPOF (IV paclitaxel 100 mg/m2) with IP paclitaxel 80 mg/m2 day 1 plus mFOLFOX6 (oxaliplatin, fluorouracil, and leucovorin). Both dose levels of IP paclitaxel were well-tolerated (published at the 2017 meeting of the Chinese Society of Clinical Oncology). IP paclitaxel 80 mg/m2 day 1 plus mFOLFOX6 (ipPOF) was selected for the current trial with ivPOF to clearly delineate their safety and efficacy compared with mFOLFOX6 in participants with uAGC.
Materials and methods
Study design
SYLT/FNF-004 is a multicenter, randomized, parallel, open-label, phase 2 trial conducted at six oncology centers in China. Subjects were randomly assigned (1:1:1) to receive ivPOF, ipPOF, or mFOLFOX6 (Figure 1) after providing written informed consent. There were no stratification criteria for randomization. The protocol was approved by the central ethics committee of the Fujian Cancer Hospital and the local ethics committees of all participating hospitals and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All participants provided written informed consent.
Figure 1
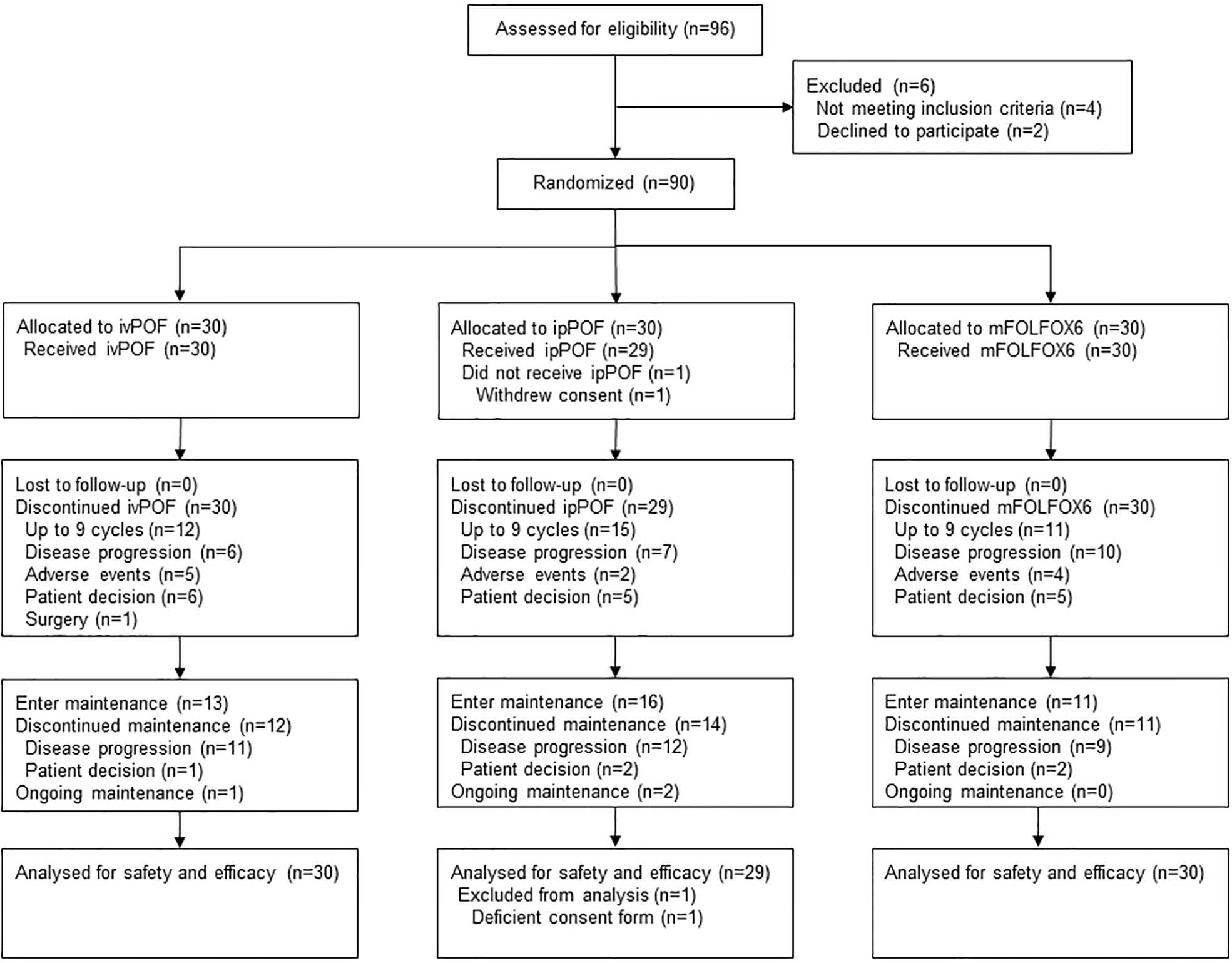
Trial profile. ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; ipPOF, intraperitoneal paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin.
Participants
Eligibility criteria included: histologically or cytologically confirmed metastatic or unresectable gastric or gastroesophageal junction adenocarcinoma with measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) (21), no prior therapy except neo- or adjuvant chemotherapy completed ≥6 months prior to relapse, age 18 to 75 years, Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1, and adequate organ function. Exclusion criteria included: ascites requiring frequent drainage, peripheral neuropathy grade ≥2 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03, brain or leptomeningeal involvement, concurrent cancer, or uncontrolled significant comorbidities. When the SYLT/FNF-004 trial was designed, trastuzumab was not yet covered by medical insurance and was not affordable to most Chinese patients; those who did not intend to use trastuzumab were allowed study entry regardless of human epidermal growth factor receptor 2 (HER2) status. Peritoneal metastasis (PM) diagnosed by ultrasound, computed tomography, magnetic resonance imaging, positron emission tomography, or ascites was designated as macroscopic PM (MAPM). MAPM was not a mandatory eligibility criterion.
Randomization and masking
Before the start of the study, a computer-generated sequence of random numbers was placed in a series of plain, sealed envelopes with patient numbers on them for the research nurse. These envelopes were created and kept at the School of Public Health of Fujian Medical University and only opened at the time of subject randomization, again by the research nurse. Individuals directly involved in the study had no access to these envelopes. Subjects were randomized into blocks of three. Subjects had an equal chance of being assigned to the groups. The statistician and research nurse are blinded to the recruitment procedure prior to the initiation of the trial. Because this was an open-label trial, the patients and physicians were not masked from the study groups. A site radiologist who assessed tumor radiographic responses, a central radiologist who verified them, and a statistician who statistically analyzed the data were blinded to the study groups.
Procedures
Participants in the mFOLFOX6 group received induction treatment consisting of a 2-h infusion of oxaliplatin at 85 mg/m2 plus leucovorin at 400 mg/m2, followed by a fluorouracil bolus of 400 mg/m2 and a 46-h infusion of fluorouracil at 2,400 mg/m2. Those assigned to ivPOF or ipPOF received a 3-h infusion of IV paclitaxel at 135 mg/m2 or IP paclitaxel at 80 mg/m2 followed by mFOLFOX6 (omitting the fluorouracil bolus). A central venous catheter was indwelled into the abdominal cavity before the administration of IP paclitaxel, which was diluted in normal saline to 500 ml. Normal saline perfusion of 500 ml was planned to be given before and after paclitaxel (total 1,000 ml) but was reduced for ascites, accordingly. The catheter was removed 2 days after treatment administration. Induction treatment was repeated every 14 days for up to 9 cycles. Thereafter, the investigator determined whether to use maintenance therapy (S-1 80 mg/m2 days 1–14, repeated every 3 weeks). Induction or maintenance therapy continued until progressive disease (PD), unacceptable toxicity, subject refusal, or investigator decision. Antiemetic prophylaxis was given according to local protocols; granulocyte colony-stimulating factor was not recommended as primary prophylaxis. Premedications (antihistamine, corticosteroid, and H2 receptor antagonist) were administered for prophylaxis of hypersensitivity reactions to IV or IP paclitaxel. Doses were modified in response to toxicities (Supplementary Material S1). Patients with dose interruptions for more than 4 weeks should permanently discontinue the treatment. Laboratory studies to monitor bone marrow, liver, and kidney function were done within 7 days of randomization and up to 2 days before each treatment after the first treatment cycle. Tumor assessment by computed tomography or magnetic resonance imaging was performed every 6 weeks until evidence of PD was detected. After PD, participants were contacted every 12 weeks to assess survival and obtain information on subsequent treatment. Adverse events (AEs), including serious adverse events, were monitored throughout the study period until resolved, returned to baseline, or deemed irreversible, or until lost to follow-up or study withdrawal by participant or investigator. HER2 status was assessed by immunohistochemistry or fluorescence in situ hybridization using biopsy or surgical specimens. HER2 positivity is defined as immunohistochemistry 3+ or as immunohistochemistry 2+ plus fluorescence in situ hybridization positive (HER2:CEP17 ratio ≥2). HER2 negativity is defined as immunohistochemistry 0 or 1+ or as immunohistochemistry 2+ plus fluorescence in situ hybridization negative (22, 23).
Outcomes
The primary outcome was PFS, defined as the time from treatment assignment to documented PD per RECIST version 1.1 according to investigator assessment, or death from any cause, whichever occurred first. Secondary outcomes included OS (time from treatment assignment to death from any cause), best overall tumor response from baseline per RECIST version 1.1, and AEs graded according to the NCI-CTCAE version 4.03.
Statistical analysis
On the basis of previous studies and our clinical practice (4, 5, 11, 17–19), we expected a median PFS of 7 months in either the ivPOF or ipPOF arm and 4 months for mFOLFOX6. The chosen sample size was calculated by a log-rank test based on the primary endpoint to verify the superiority of ivPOF or ipPOF vs. mFOLFOX6. A two-sided α of 0.05 was used, with 0.025 allocated to the hypothesis of ivPOF vs. mFOLFOX6 or ipPOF vs. mFOLFOX6, separately. We calculated that 54 subjects in each arm were needed, over 24 months of accrual and 24 months of follow-up, to achieve 80% statistical power for each hypothesis. Considering a dropout rate of 10%, the total number to be enrolled was 178. The full dataset for efficacy and safety analyses included all randomly assigned participants who received at least one dose of study medication. Categorical variables are presented as frequencies/proportions, and continuous variables as medians/interquartile ranges (IQRs). Between-group differences were analyzed with the χ2 test and Fishers’ exact test. We estimated PFS and OS using Kaplan–Meier with a p-value. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards model. p < 0.05 is considered statistically significant. The data were analyzed using R software, version 4.0.
Results
A total of 96 patients were screened and 90 (93.8%) were randomly assigned to receive ivPOF (n = 30), ipPOF (n = 30), or mFOLFOX6 (n = 30) at six oncology centers in China between 30 November 2015 and 21 May 2018 (Figure 1). The trial was closed due to poor accrual. One participant in the ipPOF group did not receive any study medication after randomization and was excluded from efficacy and safety analyses. The patient’s baseline characteristics were well-balanced (Table 1). Most patients (88.9%, 80/90) were HER2 negative. The median number of cycles administered/participant was 6 (IQR, 4 to 9) for ivPOF, 9 (IQR, 4 to 9) for ipPOF, and 4 (IQR, 3 to 9) for mFOLFOX6. Two participants in the ivPOF group and one in the mFOLFOX6 group received >9 cycles, considered protocol violations. Treatment was more often delayed in the ivPOF group (15.1%) compared with the ipPOF (8.2%; p = 0.034) or mFOLFOX6 (5.5%; p = 0.004). Doses were more frequently reduced in the ivPOF group (18.8%) compared with the ipPOF (9.2%; p = 0.007) or mFOLFOX6 (7.3%; p = 0.002). In the ivPOF group, average dose intensities of paclitaxel, oxaliplatin, and fluorouracil were 86.6%, 86.7%, and 87.4%, respectively, compared with 92.8%, 91.9%, and 92.3%, respectively, for ipPOF. In the mFOLFOX6 group, the average dose intensities of oxaliplatin and fluorouracil were 93.5% and 93.9%, respectively.
Table 1
| Characteristic | ivPOF | ipPOF | mFOLFOX6 |
|---|---|---|---|
| Participants (n) | 30 | 29 | 30 |
| Age (years) | |||
| Median (IQR) | 58.5 (40, 64) | 52 (44, 62) | 59.5 (46, 69) |
| Male [n (%)] | 22 (73.3) | 14 (48.3) | 18 (60.0) |
| ECOG PS [n (%)] | |||
| 0 | 14 (46.7) | 13 (44.8) | 10 (33.3) |
| 1 | 16 (53.3) | 16 (55.2) | 20 (66.7) |
| Histologic typea [n (%)] | |||
| Differentiated | 7 (23.3) | 4 (13.8) | 6 (20.0) |
| Undifferentiated | 19 (63.3) | 23 (79.3) | 18 (60.0) |
| Unknown | 4 (13.3) | 2 (6.9) | 6 (20.0) |
| Disease status [n (%)] | |||
| Initially metastatic | 21 (70.0) | 14 (48.3) | 18 (60.0) |
| Postgastrectomy | 9 (30.0) | 15 (51.7) | 12 (40.0) |
| Metastatic sites [n (%)] | |||
| 1 | 14 (46.7) | 15 (51.7) | 12 (40.0) |
| ≥2 | 16 (53.3) | 14 (48.3) | 18 (60.0) |
| Organs involved [n (%)] | |||
| Primary tumor site | 21 (70.0) | 15 (51.7) | 16 (53.3) |
| Peritoneum | 12 (40.0) | 17 (58.6) | 16 (53.3) |
| Abdominal cavity lymph nodes | 20 (66.7) | 12 (41.4) | 22 (73.3) |
| Lymph nodes | 21 (70.0) | 13 (44.8) | 23 (76.7) |
| Liver | 9 (30.0) | 8 (27.6) | 7 (23.3) |
| Lung | 4 (13.3) | 2 (6.9) | 0 |
| Ovary | 1 (3.3) | 3 (10.3) | 5 (16.7) |
| Bone | 4 (13.3) | 1 (3.4) | 3 (10.0) |
| Soft tissue | 1 (3.3) | 0 | 0 |
| Adrenal gland | 0 | 0 | 1 (3.3) |
| Prior chemotherapyb [n (%)] | 5 (16.7) | 3 (10.3) | 6 (20.0) |
| HER2 positivec [n (%)] | 3 (10.0) | 4 (13.8) | 3 (10.0) |
Patient demographics and baseline characteristics.
Differentiated = 1, papillary adenocarcinoma and tubular adenocarcinoma (well differentiated, moderately differentiated); undifferentiated = 2, poorly differentiated adenocarcinoma (solid type, nonsolid type), signet ring cell carcinoma, and mucinous adenocarcinoma.
Prior chemotherapy refers to recurrence or metastasis >6 months following neoadjuvant or adjuvant chemotherapy.
HER2 positivity is defined as immunohistochemistry 3+ or as immunohistochemistry 2+ plus fluorescence in-situ hybridization positive (HER2:CEP17 ratio≥2).
ECOG PS, Eastern Cooperative Oncology Group Performance Status; ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; ipPOF, intraperitoneal paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin; IQRs, interquartile ranges; HER2, human epidermal growth factor receptor 2.
The main reason for induction discontinuation for ivPOF, ipPOF, and mFOLFOX6 was the completion of 9 cycles: ivPOF, 12/30 (40.0%); ipPOF, 15/29 (51.7%); and mFOLFOX6, 11/30 (36.7%). Thirteen (43.3%) patients in the ivPOF group, 16 (51.2%) in the ipPOF group, and 11 (36.7%) in the mFOLFOX6 group received S-1 as maintenance (Figure 1). One patient in the ivPOF group began maintenance prior to completing 9 cycles due to an AE. The main reason for maintenance discontinuation for ivPOF, ipPOF, and mFOLFOX6, respectively, was PD: 11 patients (84.6%), 12 patients (75.0%), and 9 patients (81.8%).
As of 31 December 2020, data cutoff, median follow-up (months) was 41 (IQR, 37 to 43). Compared with ivPOF or ipPOF separately, mFOLFOX6 demonstrated positive results for PFS and OS. Median PFS (months) and OS (months) for ivPOF were 6.52 (95% CI: 4.13–10.27) and 9.83 (95% CI: 7.70–19.2); for ipPOF, they were 5.83 (95% CI: 4.43–10.93) and 11.03 (95% CI: 9.93–21.8); and for mFOLFOX6, they were 4.55 (95% CI: 2.73–6.87) and 6.87 (95% CI: 5.83–13.6). For PFS, ivPOF vs. mFOLFOX6 HR = 0.56 (95% CI: 0.33–0.94; p = 0.026; Figure 2A); ipPOF vs. mFOLFOX6 HR = 0.56 (95% CI: 0.33–0.96; P=0.037; Figure 2B). For OS, ivPOF vs. mFOLFOX6 HR = 0.59 (95% CI: 0.35–1.00; p = 0.043; Figure 2C); ipPOF vs. mFOLFOX6 HR = 0.54 (95% CI: 0.32–0.93; p = 0.029; Figure 2D). Response rates were 17/30 (56.7%; 95% CI: 38.9–74.4) for ivPOF and 11/29 (37.9%, 95% CI: 20.3–55.6) for ipPOF. Compared with mFOLFOX6, these were not significantly different (Table 2).
Figure 2
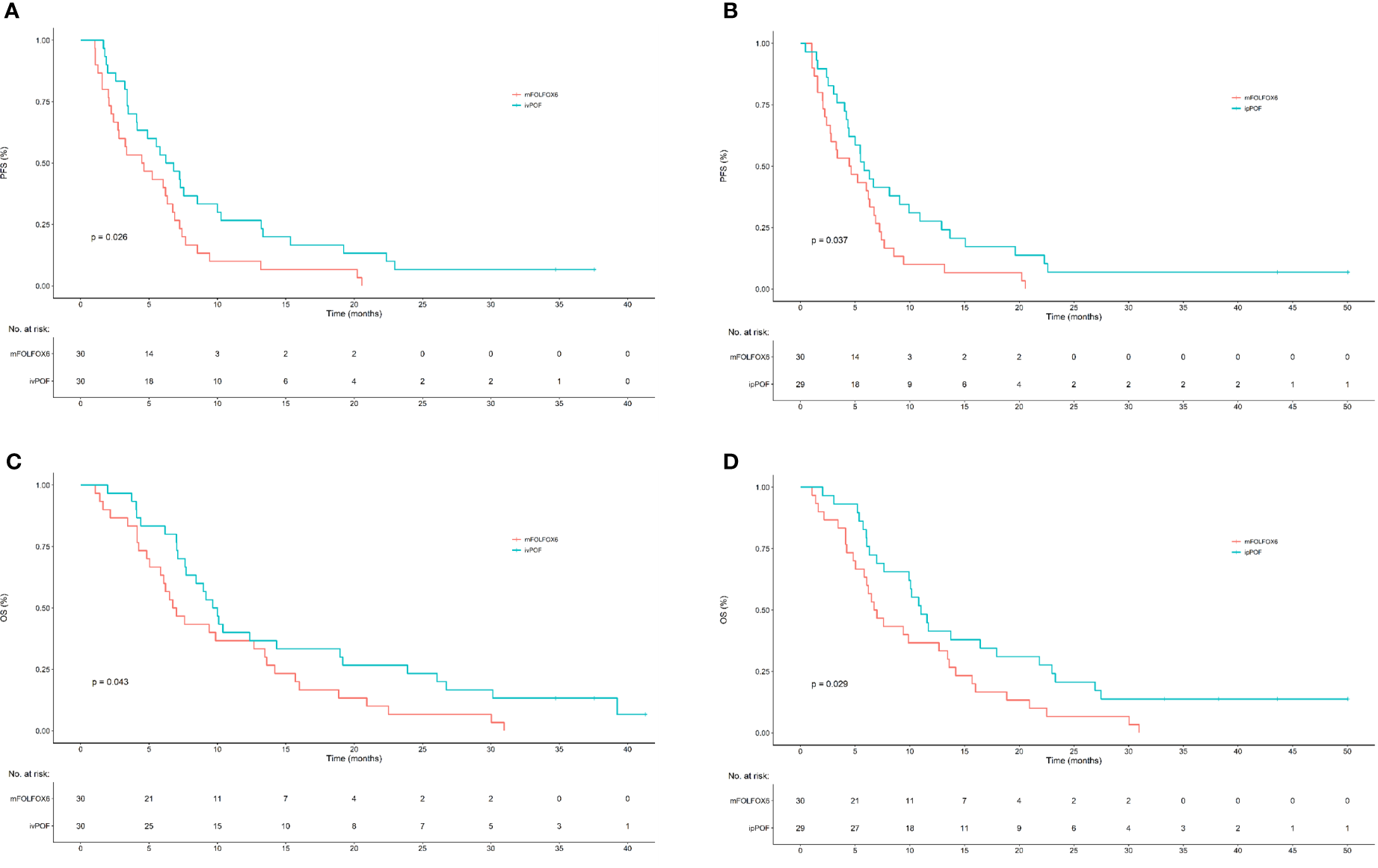
Kaplan–Meier estimates of PFS and OS for ivPOF or ipPOF vs. mFOLFOX6. (A) Median PFS (months) was 6.52 (95% CI: 4.13–10.27) in the ivPOF group and 4.55 (95% CI: 2.73–6.87) in the mFOLFOX6 group (HR, 0.56; 95% CI: 0.33–0.94; p = 0.026). (B) Median PFS (months) was 5.83 (95% CI: 4.43–10.93) in the ipPOF group and 4.55 (95% CI: 2.73–6.87) in the mFOLFOX6 group (HR, 0.56; 95% CI: 0.33–0.96; p = 0.037). (C) Median OS (months) was 9.83 (95% CI: 7.70–19.2) in the ivPOF group and 6.87 (95% CI: 5.83–13.6) in the mFOLFOX6 group (HR, 0.59; 95% CI: 0.35–1.00; p = 0.043). (D) Median OS (months) was 11.03 (95% CI: 9.93–21.8) in the ipPOF group and 6.87 (95% CI: 5.83–13.6) in the mFOLFOX6 group (HR, 0.54; 95% CI: 0.32–0.029). PFS, progression-free survival; OS, overall survival; ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin; CI, confidence interval; HR, hazard ratio.
Table 2
| Response (n (%) | ivPOF (n = 30) | ipPOF (n = 29) | mFOLFOX6 (n = 30) |
|---|---|---|---|
| Complete response | 4 (13.3%) | 2 (6.9%) | 2 (6.7%) |
| Partial response | 13 (43.3%) | 9 (31.0%) | 9 (30.0%) |
| Response rate | 17 (56.7%) | 11 (37.9%) | 11 (36.6%) |
| 95% CI | 38.9–74.4 | 20.3–55.6 | 38.9–74.4 |
| Stable disease | 9 (30.0%) | 12 (41.4%) | 12 (40%) |
| Progressive disease | 3 (10.0%) | 6 (20.7%) | 6 (20.0%) |
| Not evaluable | 1 (3.3%) | 0 | 1 (3.3%) |
Best overall response rate.
p = 0.121 for ivPOF vs. mFOLFOX6; p = 0.920 for ipPOF vs. mFOLFOX6; p = 0.150 for ivPOF vs. ipPOF.
ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; ipPOF, intraperitoneal paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin; CI, confidence interval.
The exploratory post-hoc subgroup analyses of PFS or OS according to baseline demographics and disease characteristics consistently favored ivPOF or ipPOF over mFOLFOX6 (Figure 3). Median PFS and OS is 4.83 months (95% CI: 3.19, 7.30) vs. 6.13 months (95% CI: 4.93, 7.56, p = 0.121) and 8.84 months (95% CI: 6.64, 11.41) vs. 11.54 months (95% CI: 8.32, 18.7, p = 0.251) in MAPM vs. non-MAPM, respectively. The therapeutic efficacy for HER2-positive subjects is displayed in Table 3. Patients 6 and 7 went on to a phase 2 trial of RC48-ADC (HER2-targeting antibody-drug conjugate).
Figure 3
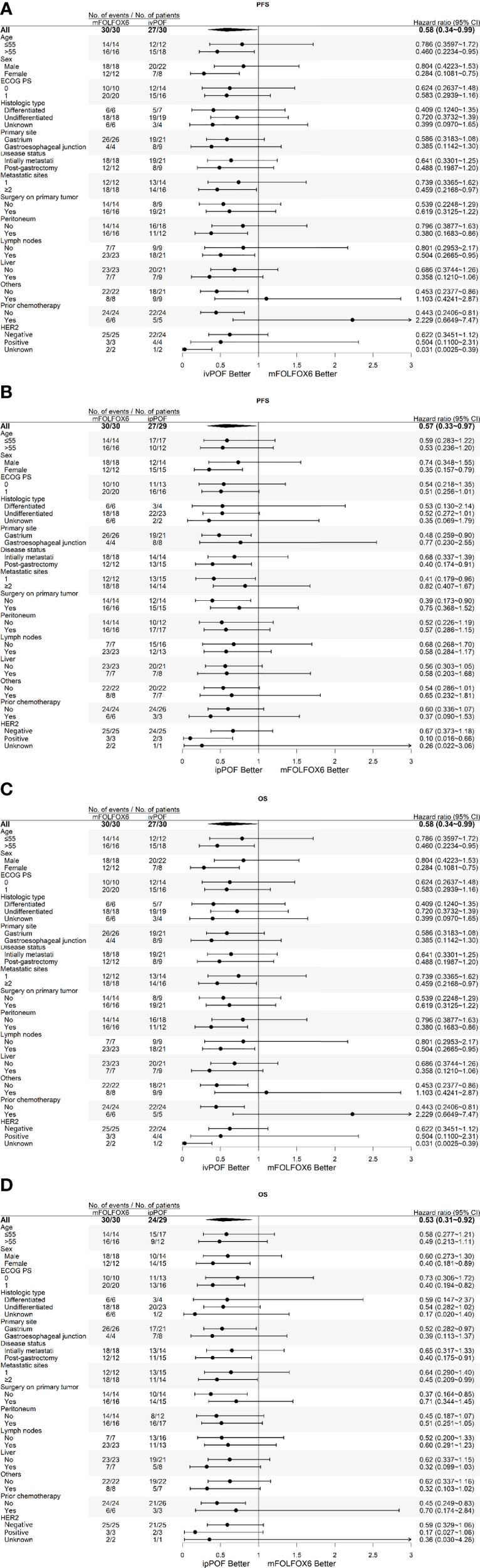
Subgroup analyses of PFS for (A) ivPOF vs. mFOLFOX6 and (B) ipPOF vs. mFOLFOX6 or OS for (C) ivPOF vs. mFOLFOX6 and (D) ipPOF vs. mFOLFOX6. HER2 positivity is defined as immunohistochemistry 3+ or as immunohistochemistry 2+ plus fluorescence in situ hybridization positive (HER2:CEP17 ratio ≥2), HER2 negativity is defined as immunohistochemistry 0 or 1+ or as immunohistochemistry 2+ plus fluorescence in situ hybridization negative. PFS, progression-free survival; OS, overall survival; ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; ipPOF, intraperitoneal paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin; HR, hazard ratio.
Table 3
| Subject | Group | Best response | PFS (months) | OS (months) |
|---|---|---|---|---|
| 1 | ivPOF | SD | 7.20 | 9.53 |
| 2 | ivPOF | SD | 2.53 | 6.90 |
| 3 | ivPOF | PR | 9.86 | 9.86 |
| 4 | ipPOF | CR | 43.00a | 43.00a |
| 5 | ipPOF | SD | 8.94 | 11.54 |
| 6 | ipPOF | PR | 8.02 | 37.71a |
| 7 | ipPOF | SD | 12.72 | 32.81a |
| 8 | mFOLFOX6 | SD | 2.37 | 4.18 |
| 9 | mFOLFOX6 | PD | 1.55 | 6.41 |
| 10 | mFOLFOX6 | SD | 4.41 | 6.90 |
Therapeutic efficacy in HER2-positive subjects.
No event occurred until the cutoff date.
PFS, progression-free survival; OS, overall survival; ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; ipPOF, intraperitoneal paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Reports of treatment-emergent AEs (any grade) were similar (ivPOF, 93.3%; ipPOF, 100%; and mFOLFOX6, 93.3%). The frequency of grade 3 or 4 AEs were also similar (ivPOF, 50.0%; ipPOF, 51.7%; and mFOLFOX6, 56.7%). The most common grade 3 or 4 AEs were neutropenia, diarrhea, leukopenia, fatigue, and sensory neuropathy (Table 4). There was no between-group difference in all-grade or grade 3 or 4 toxicity, except for increased all-grade alanine aminotransferase in ivPOF. One subject had subcutaneous tumor implantation with a poor response to chemotherapy in the ipPOF group. All five allergic reactions were attributed to oxaliplatin (ivPOF, 3; ipPOF, 1; mFOLFOX6, 1). Grade 1 or 2 abdominal pain occurred in six patients in the ipPOF group, induced by catheter stimulation, which was resolved by repositioning the catheter. No unexpected serious adverse event or protocol-related death occurred.
Table 4
| ivPOF (n = 30) | ipPOF (n = 29) | mFOLFOX6 (n = 30) | ||||
|---|---|---|---|---|---|---|
| Toxicity [n (%)] | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 |
| Any event | 28 (93.3) | 15 (50.0) | 29 (100) | 15 (51.7) | 28 (93.3) | 17 (56.7) |
| Leukopenia | 20 (66.7) | 3 (10.0) | 19 (65.5) | 4 (13.8) | 18 (60.0) | 3 (10.0) |
| Neutropenia | 18 (60.0) | 9 (30.0) | 17 (58.6) | 10 (34.5) | 17 (56.7) | 10 (33.3) |
| Febrile neutropenia | 1 (3.3) | 1 (3.3) | 1 (3.4) | 1 (3.4) | 0 | 0 |
| Anemia | 18 (60.0) | 1 (3.3) | 19 (65.5) | 1 (3.4) | 15 (50.0) | 0 |
| Thrombocytopenia | 7 (23.3) | 0 | 7 (24.1) | 0 | 4 (13.3) | 1 (3.3) |
| Hyperbilirubinemia | 2 (6.7) | 0 | 5 (17.2) | 0 | 4 (13.3) | 0 |
| Alanine aminotransferase increased | 13 (43.3) | 0 | 4 (13.8) | 0 | 6 (20.0) | 0 |
| Creatinine increased | 0 | 0 | 1 (3.4) | 0 | 1 (3.3) | 0 |
| Fatigue | 24 (80.0) | 3 (10.0) | 22 (75.9) | 2 (6.9) | 24 (80.0) | 1 (3.3) |
| Anorexia | 19 (63.3) | 2 (6.7) | 22 (75.9) | 1 (3.4) | 21 (70.0) | 1 (3.3) |
| Nausea | 12 (40.0) | 2 (6.7) | 16(55.2) | 0 | 19 (63.3) | 0 |
| Vomiting | 6 (20.0) | 1 (3.3) | 4 (13.8) | 0 | 7 (23.3) | 0 |
| Diarrhea | 11 (36.7) | 4 (13.3) | 11 (37.9) | 6 (20.7) | 9 (30.0) | 4 (13.3) |
| Sensory neuropathy | 18 (60.0) | 3 (10.0) | 13 (44.8) | 1 (3.4) | 15 (50.0) | 3(3.3) |
| Stomatitis | 6 (20.0) | 1 (3.3) | 7 (24.1) | 0 | 6 (20.0) | 1 (3.3) |
| Hand–foot syndrome | 3 (10.0) | 0 | 2 (6.9) | 0 | 4 (13.3) | 0 |
| Myalgia | 4 (13.3) | 0 | 4 (13.9) | 1 (3.4) | 2 (6.7) | 0 |
| Allergic reaction | 3 (10.0) | 0 | 1 (3.4) | 0 | 1 (3.3) | 0 |
| Abdominal pain induced by catheter | 0 | 0 | 6 (20.7) | 0 | 0 | 0 |
Adverse events.
ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; ipPOF, intraperitoneal paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin.
Twelve (40.0%), 19 (65.5%), and 19 (63.3%) patients in the ivPOF, ipPOF, and mFOLFOX6 arms, respectively, received tumor-related drug therapy after completing the study treatment. Details of late-line treatments are displayed in Table 5. Five participants underwent curative intervention (Table 6).
Table 5
| Treatment regimen | ivPOF (n = 30) | ipPOF (n = 29) | mFOLFOX6 (n = 30) |
|---|---|---|---|
| Second-line (n) | 12 | 19 | 19 |
| APA+PAC+OXA+FU/LV | 2 | 0 | 0 |
| PAC/DOC+OXA+FU/LV | 4 | 2 | 1 |
| DOC+S-1+APA | 0 | 0 | 1 |
| FOLFOX+ipPAC | 0 | 3 | 0 |
| FOLFOX+APA | 0 | 0 | 1 |
| FOLFOX | 0 | 0 | 1 |
| FOLFIRI | 2 | 5 | 1 |
| DOC/PAC+S-1/FU/LV | 0 | 8 | 11 |
| APA+S-1 | 1 | 0 | 0 |
| APA+DOC | 0 | 0 | 2 |
| APA | 1 | 0 | 0 |
| S-1 | 1 | 0 | 1 |
| IRI | 1 | 0 | 0 |
| RC48-ADC | 0 | 1 | 0 |
| Third-line (n) | 5 | 12 | 8 |
| APA+PAC+OXA+FU/LV | 1 | 1 | 1 |
| APA+FOLFIRI+ipPAC | 0 | 0 | 1 |
| PAC+OXA+FU/LV | 1 | 2 | 0 |
| FOLFIRI+ipPAC | 1 | 0 | 0 |
| PAC+CAP+APA | 0 | 1 | 0 |
| FOLFIRI | 0 | 1 | 1 |
| DOC+RAL | 0 | 1 | 0 |
| APA+S-1 | 1 | 0 | 0 |
| ANA+SIN | 1 | 0 | 0 |
| IRI+RAL | 0 | 2 | 0 |
| DOC+S-1 | 0 | 2 | 2 |
| S-1 | 0 | 0 | 1 |
| CAP | 0 | 0 | 1 |
| APA | 0 | 1 | 1 |
| RC48-ADC | 0 | 1 | 0 |
| Fourth-line (n) | 3 | 3 | 2 |
| APA+PAC+OXA+FU/LV | 0 | 0 | 1 |
| PAC+RAL+APA+SIN | 0 | 1 | 0 |
| FOLFIRI+SIN | 1 | 0 | 0 |
| Nab-PAC+OXA+SIN | 1 | 0 | 0 |
| FOLFIRI | 0 | 1 | 1 |
| DOC+SIN | 0 | 1 | 0 |
| Nab-PAC+OXA | 1 | 0 | 0 |
| Fifth-line (n) | 0 | 1 | 0 |
| DOC+RAL+APA+SIN | 0 | 1 | 0 |
Subsequent chemotherapy.
APA, apatinib; PAC, paclitaxel; OXA, oxaliplatin; FU/LV, fluorouracil/leucovorin; FOLFIRI, fluorouracil/leucovorin/irinotecan; DOC, docetaxel; IRI, irinotecan; SIN, sintilimab; ipPAC, intraperitoneal paclitaxel; ANA, anlotinib; FOLFOX, fluorouracil/leucovorin/oxaliplatin; RAL, raltitrexed; CAP, capecitabine; RC48-ADC, a novel, investigational, HER2-targeting antibody-drug conjugate.
Table 6
| Subject | Group | Curative intervention | Best response before intervention | PFS (months) | OS (months) |
|---|---|---|---|---|---|
| 1 | ivPOF | SBRT for liver metastases | PR | 15.12 | 40.70a |
| 2 | ivPOF | RFA for liver metastases | PR | 6.15 | 29.72 |
| 3 | ivPOF | Total gastrectomy with D2 lymphadenectomy | PR | 34.26a | 34.26a |
| 4 | ivPOF | EBRT for retroperitoneal lymph nodes | PR | 18.97 | 25.71 |
| 5 | mFOLFOX6 | Total gastrectomy with D2 lymphadenectomy | PR | 6.12 | 12.49 |
Therapeutic efficacy in subjects who underwent curative intervention.
No event occurred until the cutoff date.
SBRT, stereotactic body radiotherapy; RFA: radiofrequency ablation; EBRT, external beam radiotherapy; PFS, progression-free survival; OS, overall survival; PR, partial response; ivPOF, intravenous paclitaxel, oxaliplatin, fluorouracil, and leucovorin; mFOLFOX6, modified oxaliplatin, fluorouracil, and leucovorin.
Discussion
Our study shows that, compared with mFOLFOX6, IV or IP paclitaxel plus mFOLFOX6 significantly improves PFS and OS, with acceptable toxicity, for patients with uAGC.
Following the V325 trial, a multicenter phase III study (Chinese V325) utilizing reduced-dose DCF vs. CF for 243 patients with uAGC was conducted in China (9). Compared with CF, DCF significantly improved PFS (7.2 vs. 4.9 months, HR = 0.58), OS (10.2 vs. 8.5 months, HR = 0.71), and ORR (48.7% vs. 33.9%). In our study, comparing ivPOF with mFOLFOX6, PFS, OS, and ORR were 6.52 vs. 4.55 months (HR=0.56), 9.83 vs. 6.87 months (HR = 0.59), and 56.7% vs. 36.6%, respectively. Survival, which was numerically lower than in Chinese V325, may be explained by poorer PS among participants in our study, based on ECOG PS 0 or 1, respectively (ivPOF, 46.7% and 53.3%; mFOLFOX6, 33.3% and 66.7%). In the Chinese V325 study, PS was based on the Karnofsky Scale, such that scores ≥80 vs. <80, respectively, were as follows: DCF at 96.6% vs. 3.4% and CF at 93.9% vs. 6.1%. However, survival in the mFOLFOX6 group in our study was consistent with fluoropyrimidine plus oxaliplatin regimens in other Chinese investigator-initiated trials (5, 24). In terms of HR, PFS in our study is consistent with Chinese V325.
A randomized phase 3 trial with a triplet regimen of docetaxel, cisplatin, and S-1 did not improve survival compared to cisplatin and S-1 in previously untreated AGC. The reason may be that oral S-1 (which should be administered over 14 days every 21 days) replaced fluorouracil (which should be administered over 48 h). A longer medication-free interval benefits recovery in a strong triplet regimen, improving tolerance and preparing patients for the next treatment cycle. In this trial, dose intensity in the triplet group was relatively lower, which, consequently, may have impacted efficacy (25). In a randomized phase 2 study of docetaxel and oxaliplatin plus either capecitabine or fluorouracil, the capecitabine arm was worse for survival and response rate (26). Therefore, triplet regimens may benefit from continuous infusion fluorouracil with a shorter duration vs. oral fluoropyrimidine with a longer duration.
In the PHOENIX-GC study, IP paclitaxel failed to show a statistically significant improvement in survival; however, the IP paclitaxel dose is relatively low (20 mg/m2 days 1 and 8, every 3 weeks) compared with our study (80 mg/m2 day 1, every 2 weeks). The higher concentration gradient results in a higher rate of drug diffusion and anticancer effect (27). A recent prospective phase 2 study with oral capecitabine and IV oxaliplatin plus IP paclitaxel (40 mg/m2 days 1 and 8, every 3 weeks) showed improved survival compared with historical control (28). Therefore, we speculate that IP paclitaxel should improve survival, consistent with the PHOENIX-GC study, which showed a survival benefit in sensitivity analysis adjusted for baseline ascites (20), an effect that may be greater at higher doses.
Consistent with the V325 and Chinese V325 trials (8, 9), ORR was numerically higher for ivPOF vs. mFOLFOX6, such that more patients in the ivPOF group underwent curative treatment. A depth of only 1 µm in tumor nodules could be reached by IP paclitaxel (29). The diameter of target lesions to evaluate response was much larger than 1 µm according to RECIST criteria, precluding IP paclitaxel from taking effect. Therefore, it is understandable that the ORR for ipPOF is similar to that for mFOLFOX6. Additionally, in some patients, target lesions are outside the peritoneal cavity. Nonetheless, patients with relatively lower response rates may experience a survival benefit resulting from control of PM, which commonly appears as unmeasurable lesions, a main cause of death (3, 6, 7).
Conventional imaging technology to diagnose PM is the most common approach in daily clinical practice. However, staging laparoscopy detected more than 50% of cases with microscopic PM (including positive peritoneal cytology) for a locally advanced disease that was not detected by conventional imaging (30, 31). Systemic advanced disease may have a higher rate of microscopic PM. That may explain why patients who received IP triplet therapy had better PFS with or without MAPM (HR: 0.52 and 0.57, respectively) in forest plots. This phenomenon supports our observation that many patients without MAPM can benefit from IP paclitaxel. This finding emphasizes the need to develop more sensitive techniques to diagnose microscopic PM for IP treatment (32), because it is infeasible to perform staging laparoscopy for every patient.
From our clinical experience, we anticipated that both IV and IP paclitaxel would produce a good response in subjects with PM. PFS and OS with IV and IP paclitaxel in combination with mFOLFOX6 are numerically close, although our study is not powered to detect the difference. A study showed that paclitaxel concentration in ascites and plasma is similar 24 h after IV administration (33), which might support our hypothesis. Regarding the selection of ivPOF or ipPOF for uAGC, we suggest that ipPOF be considered when the predominant metastatic feature is a small mass in the peritoneum. Contrarily, for patients with a large mass or metastasis outside the abdominal cavity, ivPOF is preferable. Moreover, as an indwelling catheter may increase financial and provider burden, patient preference is an important consideration.
It is understandable that the frequency of AEs in the ipPOF arm is similar to that of mFOLFOX6, since IP administration reduces systemic toxicity (27). The frequency of AEs in ivPOF is also similar to that of mFOLFOX6, suggesting that higher response rates may relate to better performance status, hence patients’ ability to tolerate treatment. In a previous study, AEs were numerically lower in patients with docetaxel, oxaliplatin, and 5-FU (ORR: 46.6%) compared with docetaxel and oxaliplatin (23.1%) (26). Hematologic toxicity was the most common AE in the V325 and Chinese V325 studies, respectively: grade 3 or 4 neutropenia, 82% and 60.5%; febrile neutropenia, 29% and 13.4% in the DCF group. In our study, grade 3 or 4 neutropenia/febrile neutropenia rates were lower (30.0% and 3.3%, respectively, for ivPOF; 34.5% and 3.4%, respectively, for ipPOF), consistent with previous reports (4, 5, 10, 11, 15–20, 28). There was one subject in the ipPOF group who responded poorly to chemotherapy and experienced subcutaneous tumor implantation related to the indwelling catheter. It is unknown whether this would have occurred with better treatment response.
This study was terminated early due to slow accrual. The reasons for poor accrual were multiple and require further evaluation if this important patient-centered question is to be answered. Although the small sample size is a major limitation of this trial, given the prospective randomized design and planned patient treatment and follow-up, in addition to CIs on median OS and PFS and on the OS and PFS hazard ratios provided, this assumption should be reasonable. Further studies are warranted to confirm the superiority of ivPOF or ipPOF to mFOLFOX6 in a phase 3 setting with larger sample size. In our study, HER2-positive patients who might benefit from anti-HER2 treatment were not excluded. However, this cohort without anti-HER2 treatment did not reduce chemotherapeutic benefits. In addition, another limitation of this study is that the PD-L1 status is not available because this test was not a standard at the time of the trial.
Although contemporary treatment increasingly incorporates targeted or immunotherapy, the mainstay therapy for AGC continues to be chemotherapy combined with other modalities (23, 34, 35). Therefore, it is vitally important to evaluate and identify the most effective chemotherapeutic regimens. Adding IV or IP paclitaxel to mFOLFOX6 produced significantly improved PFS and OS with good tolerability as the first-line treatment of AGC. Although docetaxel-containing triplets are well-studied, paclitaxel and docetaxel are not identical in terms of structure, mechanism of action, or synergy with other agents; in particular, IV paclitaxel-based regimens are not more toxic compared with IV docetaxel (36). Moreover, a higher dose of IP paclitaxel appears to confer more clinical benefit, as shown in our study. Thus, further study of IV paclitaxel and high-dose IP paclitaxel-containing triplets is justified. To our knowledge, ours is the first randomized controlled multicenter study to evaluate an IV paclitaxel-containing triplet and is also the first to demonstrate the positive survival effect of an IP paclitaxel-containing regimen in previously untreated AGC.
Funding
This study was funded by the 2021 China Anti-Cancer Association-HengRui Anti-angiogenesis Targeted Tumor Research Fund (Grant No. 7).
Acknowledgments
The authors thank the study participants, their families, investigators, site staff, and research teams who contributed to this study. The authors wish to acknowledge Caron Modeas, Evolved Editing, LLC, for editorial assistance.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the central ethics committee of the Fujian Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
RL and SZ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors accessed and verified the data. All authors approved the final version of the manuscript. Study concept and design: RL, SZ, YC, NF, and XL. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: RL, SZ, and LS. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: RL, PL, WC, and WF. Obtained funding: RL and SZ. Administrative, technical, or material support: all authors. Study supervision: RL and SZ. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.850242/full#supplementary-material
Supplementary Material S1References
1
Torre LA Bray F Siegel RL Ferlay J Lortet-Tieulent J Jemal A . Global cancer statistics, 2012. CA Cancer J Clin (2015) 65:87–108. doi: 10.3322/caac.21262
2
Chen W Zheng R Baade PD Zhang S Zeng H Bray F et al . Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3
Wang FH Shen L Li J Zhou ZW Liang H Zhang XT et al . The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). (2019) 39:10. doi: 10.1186/s40880-019-0349-9
4
Al-Batran SE Hartmann JT Probst S Schmalenberg H Hollerbach S Hofheinz R et al . Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol (2008) 26:1435–42. doi: 10.1200/JCO.2007.13.9378
5
Li Q Wen F Zhou C Qiu M Liu J Chen J et al . Prospective randomized phase II study of FOLFIRI versus FOLFOX7 in advanced gastric adenocarcinoma: A Chinese Western cooperative gastrointestinal oncology group study. Oncotarget (2017) 8:97890–9. doi: 10.18632/oncotarget.18426
6
Smyth EC Verheij M Allum W Cunningham D Cervantes A Arnold D et al . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27:v38–49. doi: 10.1093/annonc/mdw350
7
National comprehensive cancer network . NCCN clinical practice guidelines in oncology (NCCN guidelines), in: Gastric cancer, version 2.2021 . Available at: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (Accessed June 20, 2021).
8
Van Cutsem E Moiseyenko VM Tjulandin S Majlis A Constenla M Boni C et al . Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol (2006) 24:4991–7. doi: 10.1200/JCO.2006.06.8429
9
Wang J Xu R Li J Bai Y Liu T Jiao S et al . Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. (2016) 19:234–44. doi: 10.1007/s10120-015-0457-4
10
Lin R Fan N Wu G Chen Y Guo Z Wang X et al . A phase 2 study of fluorouracil/leucovorin in combination with paclitaxel and oxaliplatin as a salvage treatment in patients with refractory or relapsed advanced gastric cancer. J Chemother (2015) 27:52–6. doi: 10.1179/1973947814Y.0000000198
11
Lin RB Fan NF Guo ZQ Wang XJ Liu J Chen L . A phase II study of 5-fluorouracil/leucovorin in combination with paclitaxel and oxaliplatin as first-line treatment for patients with advanced gastric cancer. J Chemother (2008) 20:744–8. doi: 10.1179/joc.2008.20.6.744
12
Guimbaud R Louvet C Ries P Ychou M Maillard E Andre T et al . Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: A French intergroup (Federation francophone de cancerologie digestive, federation nationale des centres de lutte contre le cancer, and groupe cooperateur multidisciplinaire en oncologie) study. J Clin Oncol (2014) 32:3520–6. doi: 10.1200/JCO.2013.54.1011
13
Li B Chen L Luo HL Yi FM Wei YP Zhang WX . Docetaxel, cisplatin, and 5-fluorouracil compared with epirubicin, cisplatin, and 5-fluorouracil regimen for advanced gastric cancer: A systematic review and meta-analysis. World J Clin Cases. (2019) 7:600–15. doi: 10.12998/wjcc.v7.i5.600
14
Babu KG Chaudhuri T Lakshmaiah KC Dasappa L Jacob LA Babu M et al . Efficacy and safety of first-line systemic chemotherapy with epirubicin, cisplatin plus 5-fluorouracil and docetaxel, cisplatin plus 5-fluorouracil regimens in locally advanced inoperable or metastatic gastric or gastroesophageal junction adenocarcinoma: A prospective phase II study from south India. Indian J Cancer. (2017) 54:47–51. doi: 10.4103/ijc.IJC_168_17
15
Ishigami H Kitayama J Otani K Kamei T Soma D Miyato H et al . Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with s-1 for advanced gastric cancer. Oncology (2009) 76:311–4. doi: 10.1159/000209277
16
Kurita N Shimada M Iwata T Nishioka M Morimoto S Yoshikawa K et al . Intraperitoneal infusion of paclitaxel with s-1 for peritoneal metastasis of advanced gastric cancer: Phase I study. J Med Invest. (2011) 58:134–9. doi: 10.2152/jmi.58.134
17
Ishigami H Kitayama J Kaisaki S Hidemura A Kato M Otani K et al . Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with s-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol (2010) 21:67–70. doi: 10.1093/annonc/mdp260
18
Yamaguchi H Kitayama J Ishigami H Emoto S Yamashita H Watanabe T . A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with s-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer (2013) 119:3354–8. doi: 10.1002/cncr.28204
19
Kitayama J Ishigami H Yamaguchi H Yamashita H Emoto S Kaisaki S . S-1 plus intravenous and intraperitoneal paclitaxel for gastric cancer with peritoneal metastasis. Gastrointest Cancer Res (2012) 5:S10–3.
20
Ishigami H Fujiwara Y Fukushima R Nashimoto A Yabusaki H Imano M et al . Phase III trial comparing intraperitoneal and intravenous paclitaxel plus s-1 versus cisplatin plus s-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol (2018) 36:1922–9. doi: 10.1200/JCO.2018.77.8613
21
Eisenhauer EA Therasse P Bogaerts J Schwartz LH Sargent D Ford R et al . New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
22
Shan L Ying J Lu N . HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population. Diagn Pathol (2013) 8:76. doi: 10.1186/1746-1596-8-76
23
Bang YJ Van Cutsem E Feyereislova A Chung HC Shen L Sawaki A et al . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
24
Guo W Zhu X Huang M Wang Y Chen Z Feng W et al . Phase III trial comparing XELOX regimen (oxaliplatin plus capecitabine) versus EOX regimen (epirubicin, oxaliplatin and capecitabine) as first-line treatment for advanced gastric cancer: EXELOX trial. J Clin Oncol (2021) 39:4014–40. doi: 10.2139/ssrn.3875437
25
Yamada Y Boku N Mizusawa J Iwasa S Kadowaki S Nakayama N et al . Docetaxel plus cisplatin and s-1 versus cisplatin and s-1 in patients with advanced gastric cancer (JCOG1013): An open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol (2019) 4:501–10. doi: 10.1016/S2468-1253(19)30083-4
26
Van Cutsem E Boni C Tabernero J Massuti B Middleton G Dane F et al . Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: A randomized phase II study. Ann Oncol (2015) 26:149–56. doi: 10.1093/annonc/mdu496
27
Ceelen W Braet H van Ramshorst G Willaert W Remaut K . Intraperitoneal chemotherapy for peritoneal metastases: an expert opinion. Expert Opin Drug Deliv. (2020) 17:511–22. doi: 10.1080/17425247.2020.1736551
28
Chia D Sundar R Kim GW Ang J Lum J Nga ME et al . Outcomes of a phase II study of intraperitoneal paclitaxel plus systemic capecitabine and oxaliplatin (XELOX) for gastric cancer with peritoneal metastases. J Clin Oncol (2021) 39:165. doi: 10.1200/JCO.2021.39.3_suppl.165
29
Kamei T Kitayama J Yamaguchi H Soma D Emoto S Konno T et al . Spatial distribution of intraperitoneally administrated paclitaxel nanoparticles solubilized with poly (2-methacryloxyethyl phosphorylcholine-co n-butyl methacrylate) in peritoneal metastatic nodules. Cancer Sci (2011) 102:200–5. doi: 10.1111/j.1349-7006.2010.01747.x
30
Miki Y Tokunaga M Tanizawa Y Bando E Kawamura T Terashima M . Staging laparoscopy for patients with cM0, type 4, and Large type 3 gastric cancer. World J Surg (2015) 39:2742–7. doi: 10.1007/s00268-015-3144-z
31
Nakagawa S Nashimoto A Yabusaki H . Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. (2007) 10:29–34. doi: 10.1007/s10120-006-0406-3
32
Jiang Y Liang X Wang W Chen C Yuan Q Zhang X et al . Noninvasive prediction of occult peritoneal metastasis in gastric cancer using deep learning. JAMA Netw Open (2021) 4:e2032269. doi: 10.1001/jamanetworkopen.2020.32269
33
Kobayashi M Sakamoto J Namikawa T Okamoto K Okabayashi T Ichikawa K et al . Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World J Gastroenterol (2006) 12:1412–5. doi: 10.3748/wjg.v12.i9.1412
34
Janjigian YY Shitara K Moehler M Garrido M Salman P Shen L et al . First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet (2021), 398(10294): 27–40. doi: 10.1016/S0140-6736(21)00797-2
35
Boku N Ryu MH Oh D-Y Oh SC Chung HC Lee K-W et al . Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann Oncol (2020) 31(suppl 4): S1192, SEPTEMBER 01. doi: 10.1016/j.annonc.2020.08.2297
36
Park SH Lee WK Chung M Lee Y Han SH Bang SM et al . Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs (2006) 17:225–9. doi: 10.1097/00001813-200602000-00015
Summary
Keywords
advanced, gastric cancer, intraperitoneal, intravenous, paclitaxel
Citation
Zhao S, Su L, Chen Y, Li X, Lin P, Chen W, Fang W, Zhu J, Li H, Ren L, Liu J, Hong Y, Lin S, Fan N and Lin R (2022) Phase 2 randomized controlled trial of intravenous or intraperitoneal paclitaxel plus mFOLFOX6 vs. mFOLFOX6 as first-line treatment of advanced gastric cancer. Front. Oncol. 12:850242. doi: 10.3389/fonc.2022.850242
Received
07 January 2022
Accepted
09 August 2022
Published
07 September 2022
Volume
12 - 2022
Edited by
Hiroki Tanabe, Asahikawa Medical University, Japan
Reviewed by
Takahiro Kogawa, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Japan; Haiping Jiang, Zhejiang University, China
Updates
Copyright
© 2022 Zhao, Su, Chen, Li, Lin, Chen, Fang, Zhu, Li, Ren, Liu, Hong, Lin, Fan and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanfeng Fan, 563393717@qq.com; Rongbo Lin, linrongbo@fjzlhospital
†These authors have contributed equally to this work and share first authorship
This article was submitted to Gastrointestinal Cancers: Gastric and Esophageal Cancers, a section of the journal Frontiers in Oncology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.