- 1Department of Breast Surgery, General Surgery, Qilu Hospital of Shandong University, Jinan, China
- 2Breast Cancer Center, Jinan Central Hospital, Shandong First Medical University, Jinan, China
- 3Pathology Tissue Bank, Qilu Hospital of Shandong University, Jinan, China
- 4Research Institute of Breast Cancer, Shandong University, Jinan, China
Objective: Chemotherapy-induced amenorrhea (CIA) is one of the most common side effects in premenopausal patients with breast cancer, and several factors may contribute to the incidence of CIA. In this meta-analysis, we aimed to summarize clinical risk factors associated with CIA incidence and to evaluate their prognostic effects in patients with breast cancer.
Methods: Three electronic databases (Cochrane Library, EMBASE, and MEDLINE) were systematically searched for articles published up to October 2021. The articles included clinical trials that evaluated risk factors associated with CIA and their prognostic value in treatment. For the meta-analysis, pooled odds ratio estimates (ORs) and 95% confidence intervals (CIs) were calculated using the inverse variance-weighted approach, in addition to publication bias and the chi-square test.
Results: A total of 68 studies involving 26,585 patients with breast cancer were included in this meta-analysis, and 16,927 patients developed CIA. From the 68 studies, 7 risk factors were included such as age group, hormone receptor (HR) status, estrogen receptor (ER) status, progesterone receptor (PR) status, tamoxifen administration, chemotherapeutic regimen, and tumor stage. Based on our results, patients with age of ≤40, HR-negative status, ER-negative status, PR-negative status, no use of tamoxifen, and use of anthracycline-based regimen (A) compared with anthracycline-taxane-based regimen (A+T) were associated with less incidence of CIA in patients with breast cancer. Moreover, CIA was associated with favorable disease-free survival (OR = 0.595, 95% CI = 0.537 to 0.658, p < 0.001) and overall survival (OR = 0.547, 95% CI = 0.454–0.660, p < 0.001) in premenopausal patients with breast cancer.
Conclusion: Age, HR status, ER status, PR status, tamoxifen administration, and chemotherapeutic regimen can be considered independent factors to predict the occurrence of CIA. CIA is a favorable prognostic factor in premenopausal patients with breast cancer. CIA should be a trade-off in the clinical management of premenopausal patients with breast cancer, and further large cohort studies are necessary to confirm these results.
Introduction
Breast cancer has surpassed lung cancer to become the most frequently diagnosed cancer among women worldwide as per the latest data released by the International Agency for Research on Cancer of the World Health Organization in 2020 (1). With the development of the pharmaceutical field, the comprehensive treatment of breast cancer is constantly updated and the current systemic treatment includes surgery, chemotherapy, radiotherapy, endocrine therapy, and target therapy (2). Chemotherapy is still a predominant adjuvant therapy for the treatment of breast cancer, which could effectively prolong patient survival and reduce the recurrence rate of cancer. However, patients may develop various side effects including myelosuppression, cardiotoxicity, ovarian failure, nausea, and diarrhea, which affect the quality of life (3, 4). The early prevention and treatment of complications caused by chemotherapy have become an important supplement in the chemotherapy strategy for breast cancer.
Chemotherapy-induced amenorrhea (CIA) is a common complication observed in premenopausal women with breast cancer, and the incidence of CIA ranges from 15% to 94% (5) in patients with breast cancer after receiving chemotherapy. CIA is caused by suppression of ovarian function, which can lead to genitourinary dysfunctions, infertility, and peri-menopausal symptoms such as hot flushes and sweats. Furthermore, long-time hormone deprivation can increase osteoporosis and cardiovascular risk, thus causing both physical and psychological distress among patients (6–8). Moreover, published data indicate that the major concern for premenopausal women receiving chemotherapy for breast cancer is to preserve their future childbearing potential (9). Therefore, it is of great value to identify individuals who are vulnerable to CIA, to identify risk factors, and to determine their prognostic value for treatments of patients with breast cancer.
Although the definition of CIA varies among studies, risk factors identified for CIA include age (10), hormone receptor (HR) status (11, 12), tamoxifen administration, and chemotherapeutic regimens (12–15). However, these studies have some limitations such as small sample size and the inclusion of single or few potential risk factors. Importantly, some risk factors are debatable. Parulekar et al. reported that the HR status showed no significant association with the incidence of CIA, which was 73.3% in the receptor-positive group and 74.0% in the receptor-negative group (11). On the contrary, Yoo reported that the HR-positive status is one of the risk factors of CIA, with 64.4% incidence in the HR-positive group and 42.7% incidence in the HR-negative group (12). The use of tamoxifen as a risk factor of CIA is also debatable (13, 15). The incidence of CIA is closely related to the adjuvant chemotherapeutic regimen and dosage (16). The most common clinically used chemotherapeutic regimens are anthracycline-based (A) and anthracycline-taxane-based (A+T) (17). The addition of taxane to the anthracycline regimen could improve the overall survival (OS) rate of patients compared with anthracycline alone (18). However, there is no consensus on the effect of the chemotherapeutic regimen on CIA incidence. Some studies have reported that A+T could significantly increase the occurrence rate of CIA (14), whereas others reported that the incidence of CIA and the use of different regimens are not correlated (13, 19). As amenorrhea would impair the quality of life in premenopausal patients with breast cancer (20), studying risk factors for their prognostic effects on CIA incidence is necessary. Consistent findings are not available based on previous studies (21, 22). Walshe et al. reviewed 23 studies, and 10 of them demonstrated survival benefits of CIA (23). Thus, further confirmation of risk factors associated with CIA and their prognostic value are warranted.
In this study, we aimed to perform an updated meta-analysis to achieve more reliable and comprehensive data on specific risk factors associated with the CIA. Moreover, we aimed to determine their exact prognostic value for CIA among premenopausal women with breast cancer receiving adjuvant chemotherapy.
Methods
Search Strategy
Three electronic databases (Cochrane Library, EMBASE, and MEDLINE) were quarried with the inclusion dates between January 1900 and October 2021, and specific keywords and free-text searches were used in the following combinations: amenorrhea, breast cancer, breast neoplasm, chemotherapy, ovarian toxicity, and CIA. We also used the “related articles” function to broaden the search and manually searched the reference lists of the retrieved literature to identify the relevant literature. Copies of all eligible studies were collected and read. In case of overlap in the patient cohorts across more than one study, only data from the most recent publication were utilized. The studies and databases performed without language or region restrictions were included in our meta-analysis.
Inclusion and Exclusion Criteria
Papers included in our meta-analysis met all of the following inclusion criteria: (a) studies on breast cancer patients in the premenopausal age who received chemotherapy; (b) papers in which one or more factors associated with the incidence of CIA were discussed; (c) at least 20 patients were enrolled; (d) the study had to be published after 1990; and (e) in case of studies including patients both with and without the addition of GnRH analogues, we only extracted the data without the addition of GnRH analogues. The major exclusion criteria were as follows: (a) not meeting the inclusion criteria; (b) papers with insufficient data; and (c) the category of the paper was not an editorial, letter, review article, case report, or animal experimental study.
Data Abstraction and Quality Assessment
Based on the inclusion and exclusion criteria above, the following data parameters were extracted for each study: the name of the first author, year of publication, the total number of patients analyzed, patient characteristics, country of origin for the study, definition of CIA, the incidence of CIA based on different risk factors, the 5-year disease-free survival (DFS), and the overall survival (OS), if mentioned. Information was carefully and independently extracted from all eligible publications by two of the authors, and any disagreement between the researchers was resolved by discussions until reaching a consensus. In case of failing to reach a consensus, a third investigator (an experienced professional breast surgeon) was consulted to resolve the dispute. The quality of observational studies was assessed using the Newcastle–Ottawa quality assessment tool (24). The Cochrane Risk of Bias Tool was used to assess the quality of the randomized control trials (RCTs) (25). A score of 0–9 was allocated to each observational study. Observational studies achieving scoring 6–9 points were considered to be high quality, studies scoring 4–5 points were rated as moderate quality, and studies scoring 3 or fewer points were regarded as low quality.
Statistical Analysis
Stata V.12 software was utilized for all statistical analyses. The outcomes, OR, and 95% confidence intervals (CIs) were calculated, and the association between different risk factors and the incidence of CIA as well as its prognostic effect were assessed. Pooled ORs and subgroup analysis were performed, with the application of the Z-test to determine its statistical significance. Subgroup analyses were further conducted for the varied definitions of CIA across studies. Statistical heterogeneity was calculated by the chi-square test,and a fixed-effect-model was used for I2 <50%, with a random-effect model for I2 ≥50%, and further checked by sensitivity analyses. Publication bias was calculated using Begg’s test. For all tests, a probability level <0.05 was considered to indicate statistical significance. All statistical tests were two-sided.
Results
Search Results
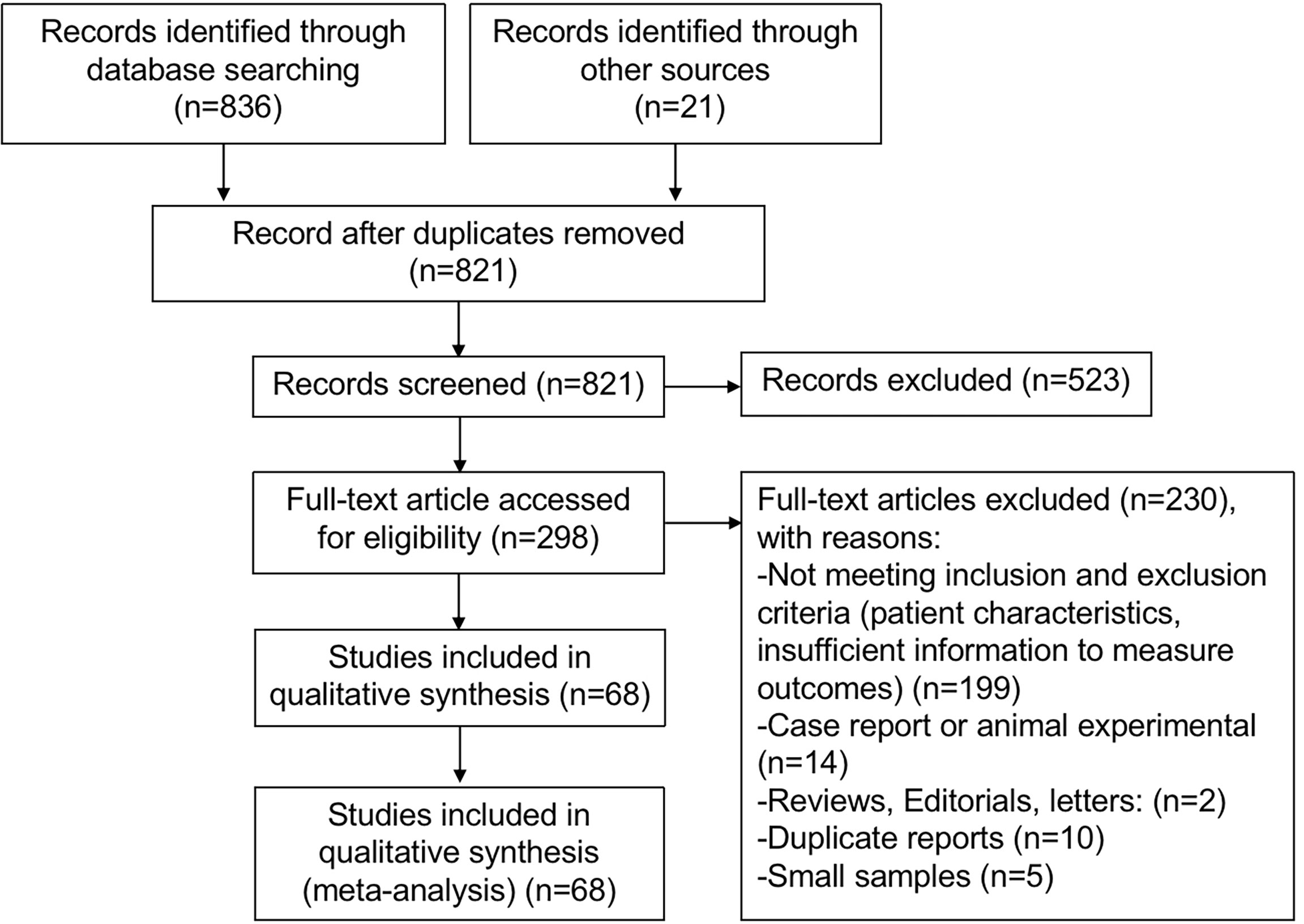
Systematic retrieval using electronic searches of the Cochrane Library, EMBASE, and MEDLINE gave a total of 836 studies, and 21 other articles were identified from other sources by reviewing citations in the reference lists. After removing duplicates and reviewing titles and abstracts, a total of 298 articles were potentially eligible for inclusion. The full text of the 298 studies was reviewed thoroughly, and 230 articles were excluded because of (1) not meeting inclusion and exclusion criteria (patient characteristics, insufficient information to measure outcomes) (n = 199); (2) case report or animal experiments (n = 14); (3) reviews, editorials, and letters (n = 2); (4) duplicate reports (n = 10); and (5) small samples (n = 5). Ultimately, 68 studies (6, 10–15, 19, 20, 22, 26–83) met all inclusion criteria and were included in this meta-analysis. The flowchart of the literature search is shown in Figure 1.
Characteristics of the Included Studies
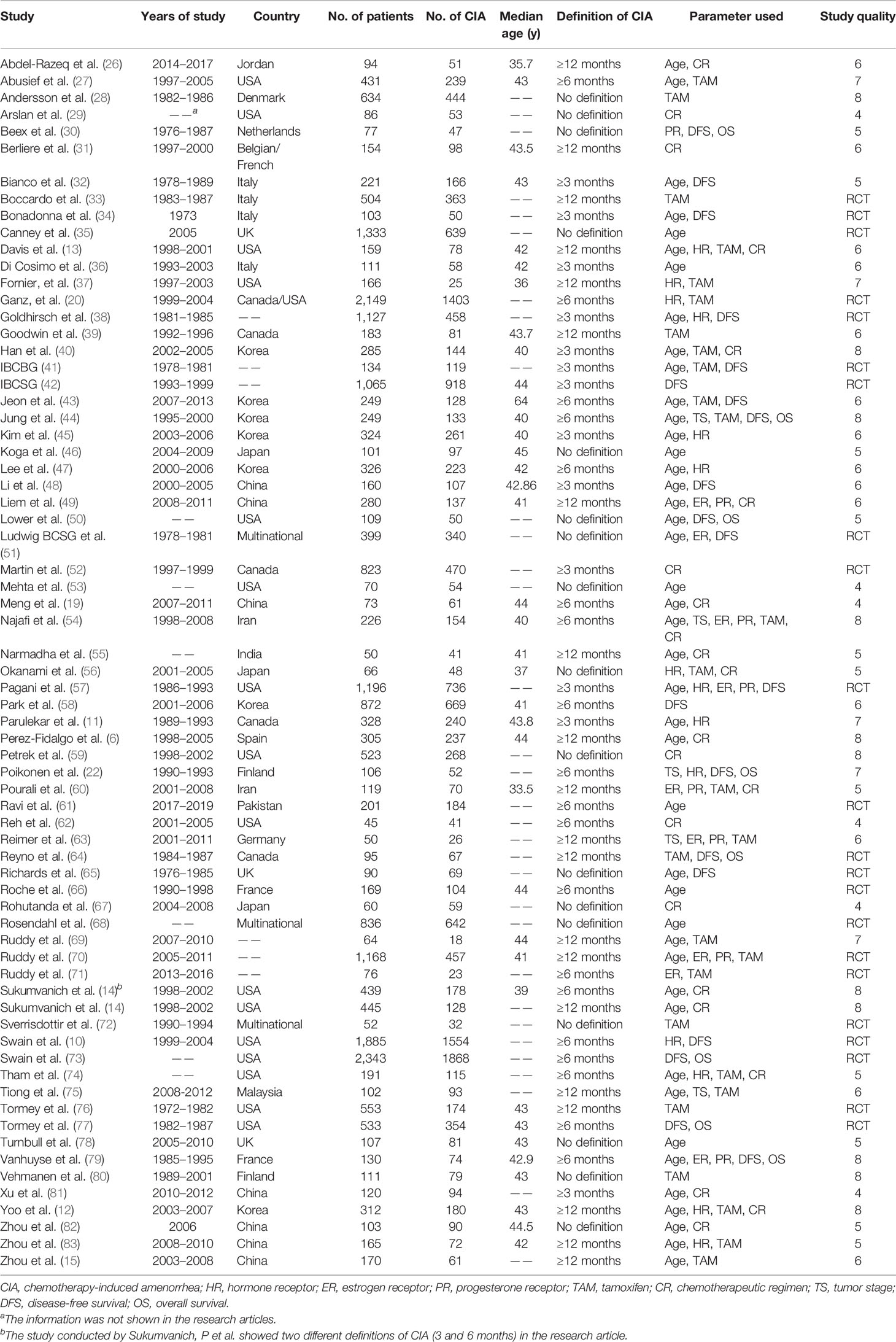
The 68 filtered trials were published between January 1990 and October 2021, and the patient age ranged from 18 to 59 years, with sample sizes ranging from 45 to 2343. The definitions of CIA varied among the studies, 13 studies used 3 months as the minimal lasting time of amenorrhea, 19 studies used 6 months, and 20 studies used 12 months. Moreover, 17 studies did not explicitly state the definition of CIA. Of the total of 26,585 premenopausal patients with breast cancer who had received adjuvant chemotherapy, 16,927 (63.67%) patients developed CIA, with the CIA rate ranging from 15.06% to 98.33% among studies.
To evaluate risk factors associated with the occurrence of CIA, factors that were correlated with CIA among studies were identified. Finally, age (≤40 vs. >40), HR status (negative vs. positive), estrogen receptor (ER) status (negative vs. positive), progesterone receptor (PR) status (negative vs. positive), usage of tamoxifen (with vs. without), and chemotherapy regimens (anthracycline-based vs. anthracycline + Taxol based) were selected and pooled, with 43, 14, 9, 8, 27, and 22 studies enrolled in each analysis. The basic characteristics of patients with CIA in the 68 clinical trials and the associated prognostic factors are listed in Table 1.
Quality of Included Studies
The risk of bias of each study included had been evaluated, and the risk of bias in all the studies was within acceptable limits. We used the Cochrane risk-of-bias tool to evaluate the risk of bias in the 22 published RCTs (Supplementary Figure S1). Few of the RCTs provided information regarding the blinding method. For the 46 observational studies, the risk of bias was evaluated with a modification of the Newcastle–Ottawa scale (Supplementary Table S1). 30 studies were considered to be of high quality.
Pooled Analyses of Risk Factors
Incidence of CIA in Patients With Age ≤40 and Age >40
More than half of the included studies have reported the effect of age on the occurrence of CIA, indicating that age might be an important predictor of CIA. To first investigate the role of age on the incidence of CIA in premenopausal patients with breast cancer, 43 of the 68 studies containing age information were extracted and the pooled ORs were assessed. The incidence of CIA was 35.53% in patients with an age of ≤40 and 72.72% in patients with an age of >40. The overall pooled ORs of CIA in patients with an age of ≤40 versus an age of >40 was 0.136 (95% CI = 0.104–0.177, p <0.001), indicating that younger patients were less likely to develop CIA. For the detected heterogeneity found among studies (I2 = 86.8%), we then divided the studies into 4 subgroups based on the definitions of CIA, and the pooled ORs of the subgroups were assessed (Figure 2). We found that the pooled ORs of each subgroup were significant and further found a remarkable decrease in the incidence of CIA for patients with age of ≤40. To explore the study heterogeneity, we investigated the influence of each individual study on the overall meta-analysis summary estimate and found that no study was suspected of excessive influence (Supplementary Figure S3A). Significant reporting bias was not detected among studies by Begg’s test (Begg’s p = 0.391, Supplementary Figure S2A).
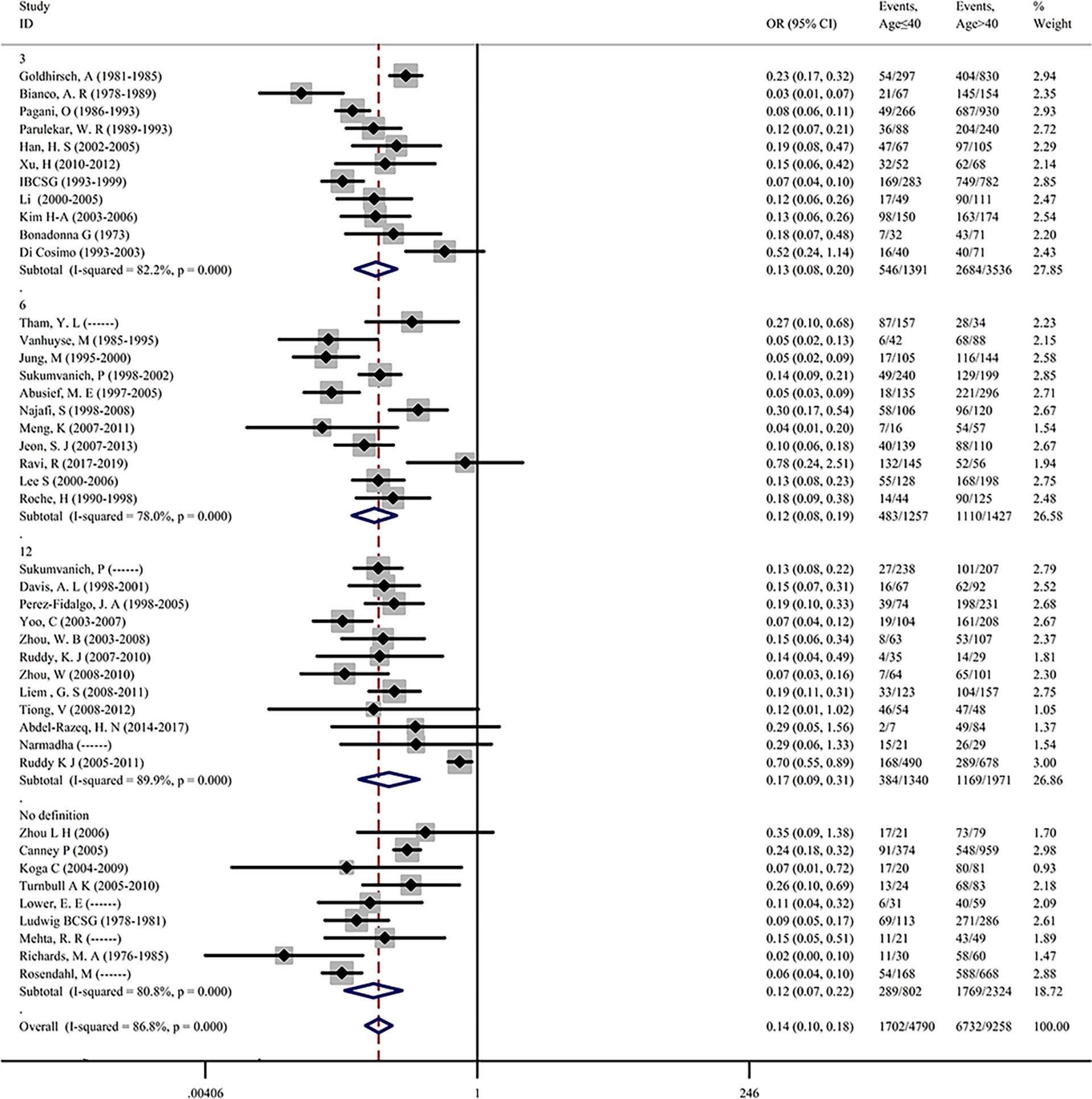
Figure 2 Premenopausal breast cancer patients age ≤40 years versus age >40 years in terms of the incidence of CIA.
Association Between HR Status and CIA Incidence
A total of 14 studies investigated the association between HR status and CIA incidence. The overall pooled OR was 0.611 (95% CI = 0.495–0.753, p < 0.001) in patients with HR-negative status versus patients with HR-positive status (Supplementary Figure S4A). For the detected heterogeneity among studies (I2 = 66.3), a random-effect model was used to assess the pooled ORs, and subgroup analysis was further performed. We found that the OR values were significant in the 3-, 6-, and 12-month groups, except for the “no definition” group including only one trial, suggesting that patients with an HR-negative status are less prone to CIA than patients with an HR-positive status. No publication bias was found during the analysis (Begg’s p = 0.661, Supplementary Figure S2B).
Our results showed that the HR status is correlated with the incidence of CIA. We further separately evaluated the effect of ER status and PR status based on information acquired from the studies. A total of 9 studies contained information about ER status and CIA incidence in premenopausal patients with breast cancer. As shown in Supplementary Figure S4B, the overall pooled OR was 0.683 (95% CI = 0.518–0.900, p = 0.007) in patients with an ER-negative status versus patients with an ER-positive status. After subgroup analysis, only 6- and 12-month groups contained more than one study, and the OR values were both significant, suggesting that patients with an ER-negative status may have a lower incidence of CIA. No publication bias was found during the analysis (Begg’s p = 0.602, Supplementary Figure S2C).
As observed for the group of the relationship between ER status and CIA incidence, we acquired information of PR status from 8 studies, and the pooled OR was assessed. We concluded that patients with a PR-negative status were less prone to CIA than patients with a PR-positive status, as the overall pooled OR was 0.690 (95%CI = 0.495–0.961, p = 0.028) in patients with a PR-negative status versus patients with a PR-positive status (Supplementary Figure S4C). No publication bias was found during the analysis (Begg’s p = 0.711, Supplementary Figure S2D). Since heterogeneity was found in all 3 analyses above, sensitivity analysis was further performed, and no studies that had a significant impact on the results were found (Supplementary Figures S3B–D).
Effect of Tamoxifen Use on CIA Incidence
As a postoperative treatment strategy for premenopausal breast cancer, hormone therapy by tamoxifen (TAM) following chemotherapy for patients with an HR-positive status is recommended (84). To evaluate the effect of tamoxifen on CIA in premenopausal patients with breast cancer, 27 studies were included in this meta-analysis. As shown in Figure 3, the overall pooled OR was 0.568 (95%CI = 0.461–0.701, p < 0.001), which showed that the use of tamoxifen can significantly increase the risk of CIA. Subgroup analysis also revealed that tamoxifen could increase the risk of CIA, regardless of the definition of CIA (p < 0.001). Significant reporting bias was not found in the meta-analysis on tamoxifen (Begg’s p = 0.243, Supplementary Figure S2E). For the detected heterogeneity found among studies (I2 = 68.9%), we then proceeded with the sensitivity analysis, and no study was found to have excessive influence (Supplementary Figure S3E).

Figure 3 Premenopausal breast cancer patients with or without the administration of tamoxifen on the incidence of CIA.
Association Between Chemotherapeutic Regimens and CIA Incidence
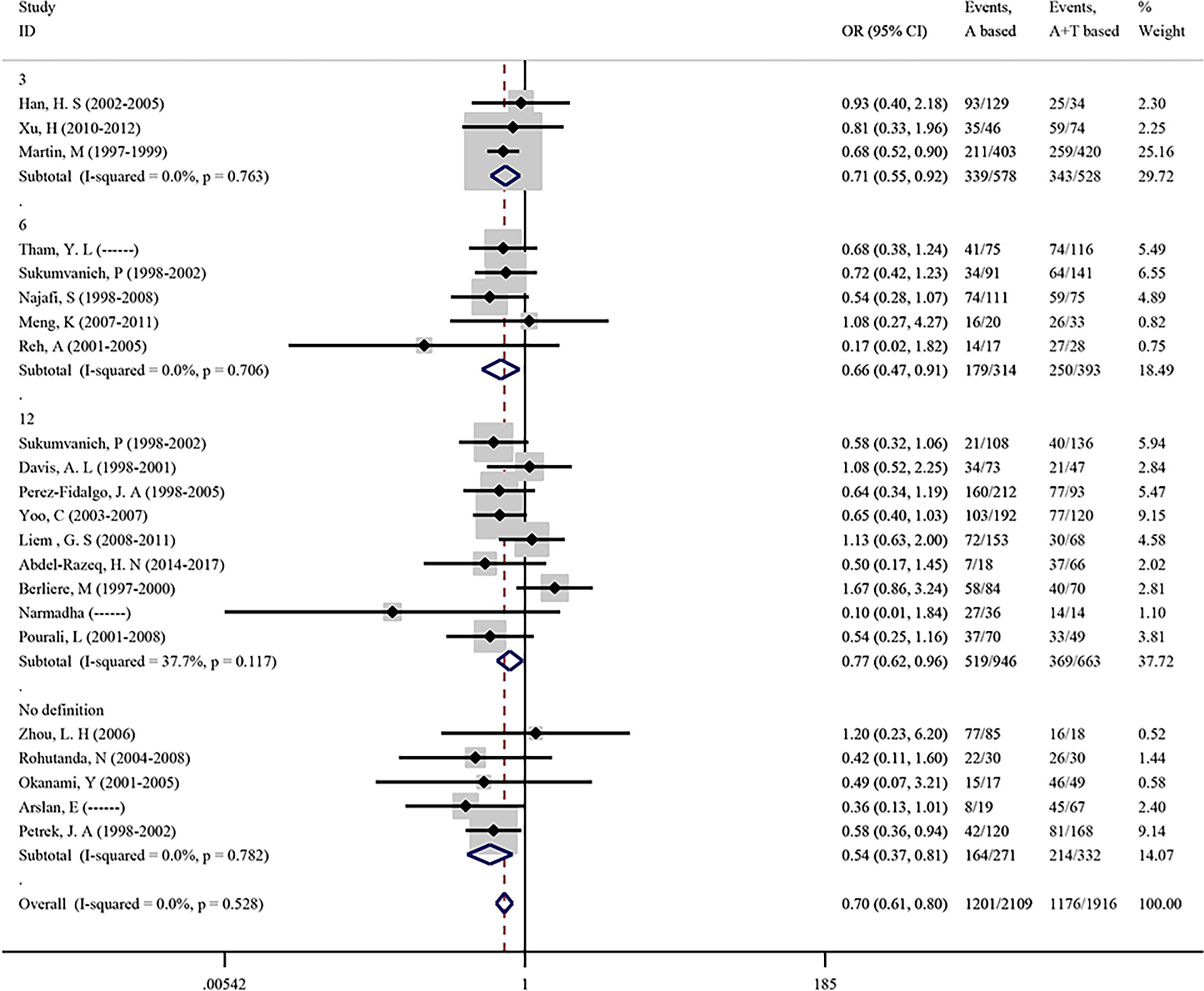
To evaluate the effect of the two most common chemotherapeutic regimens on CIA incidence, we conducted a meta-analysis focused on the chemotherapy drug which included anthracycline-based (A) and anthracycline-taxane-based (A+T) in 22 studies. As shown in Figure 4, the overall pooled OR in anthracycline-based (A) versus anthracycline-taxane-based (A+T) was 0.699 (95% CI = 0.608–0.803, p <0.001), suggesting that taxane can significantly increase the incidence of CIA. No heterogeneity (I2 = 0.0%) and publication bias (Begg’s p = 0.236, Supplementary Figure S2F) were found in the analysis of chemotherapeutic regimens.
Relationship Between Tumor Stage and CIA Incidence
We found that 5 studies involving 733 patients reported the relationship between tumor stage and CIA incidence, with 60.17% of patients developing CIA during stage I/II and 70.77% patients during the III/IV stage. After meta-analysis, the overall pooled OR of CIA in stages I and II versus III and IV was 0.765 (95% CI = 0.512–1.145, p = 0.193), suggesting that there was no correlation between the tumor stage and CIA incidence (Supplementary Figure S5). The detected heterogeneity of overall studies was low (I2 = 15.9%). The publication bias of this analysis was calculated using Begg’s test, and no significant publication bias was found (Begg’s p = 0.806, Supplementary Figure S2G).
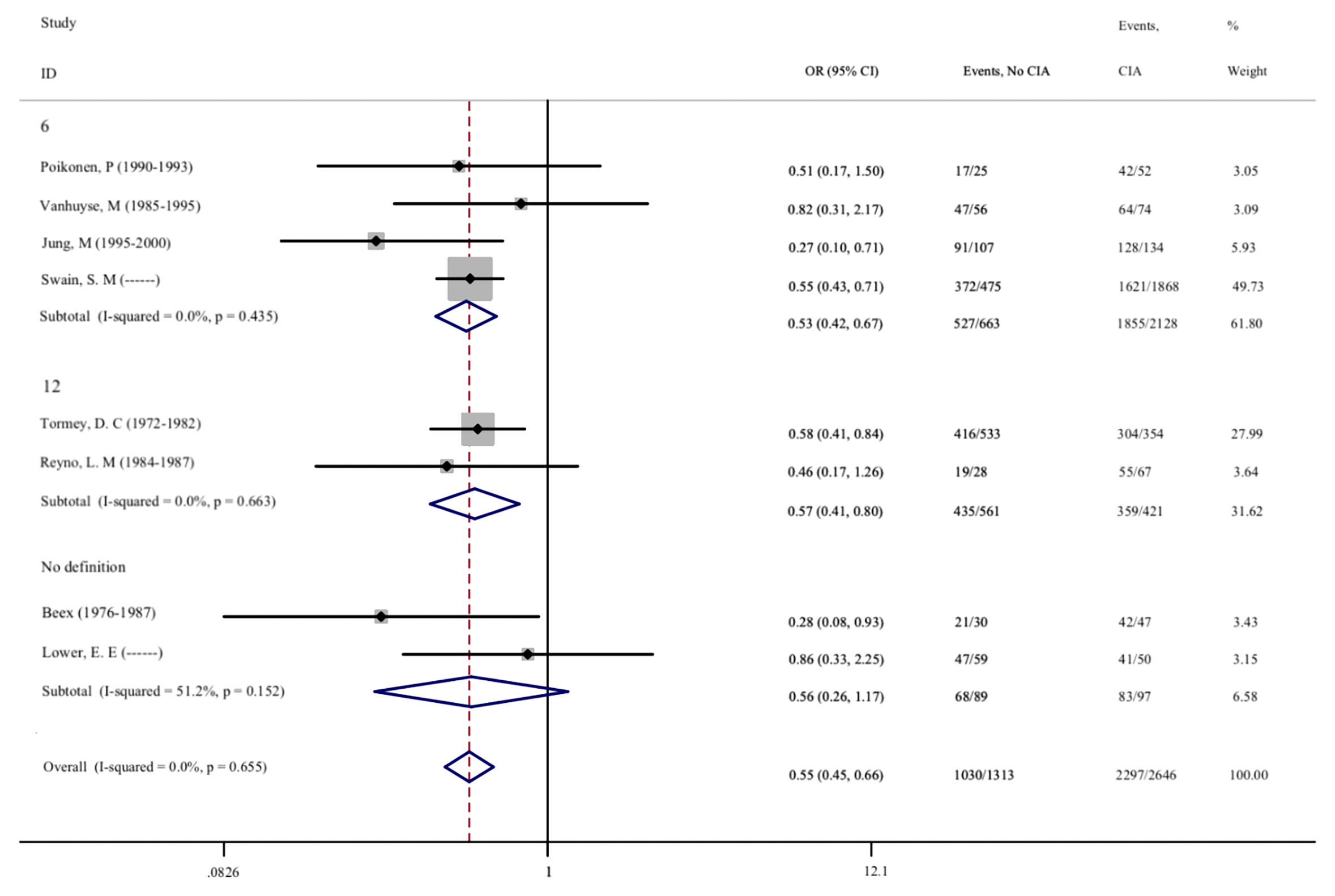
The Prognostic Effect of CIA in Premenopausal Patients With Breast Cancer
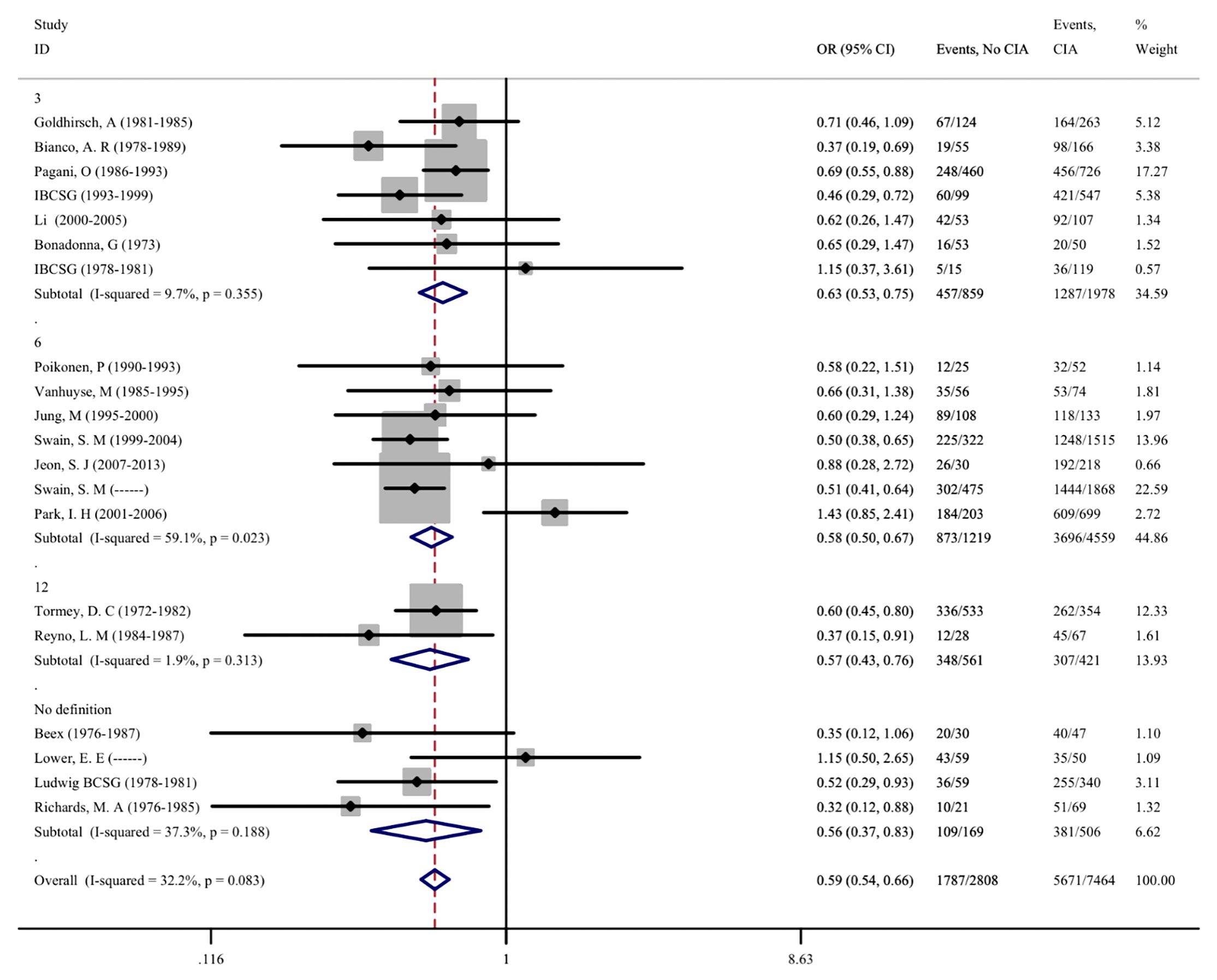
As CIA is one of the most common side effects of adjuvant chemotherapy, we further evaluated the correlation between CIA and disease prognosis in patients. A total of 20 studies involving 11,163 patients were included, and the effects of CIA on the 5-year DFS of patients were evaluated. Moreover, 8 studies that assessed the 5-year OS of patients were included. After meta-analysis, we found that the pooled OR of DFS for premenopausal patients with breast cancer and without CIA was 0.595 (95% CI = 0.537–0.658, p < 0.001) compared with patients with CIA, and the OR of OS was 0.547 (95%CI = 0.454–0.660, p <0.001). The same results were also found in different groups after subgroup analysis, indicating that patients who developed CIA after chemotherapy had a significantly better prognosis (Figures 5, 6). The heterogeneity in the analysis of DFS was not significant (I2 = 32.2) and no publication bias (Begg’s p = 0.581, Supplementary Figure S2H) was found in the analysis of the prognostic value of CIA. No heterogeneity (I2 = 0.0%) and publication bias (Begg’s p = 0.386, Supplementary Figure S2I) were found in the analysis of OS. We investigated the DFS and OS effect of each individual study on the result by sensitivity analysis to further explore the heterogeneity of the included studies and found that no study was suspected of having a noticeable effect (Supplementary Figures S3H, I).
Discussion
Our meta-analysis provides an updated, more reliable, and comprehensive conclusion on the risk and prognostic effect of CIA in premenopausal women with breast cancer. This meta-analysis will serve as a tool to help doctors in counseling patients on fertility issues. This meta-analysis consisted of 68 studies and 26,585 premenopausal patients with breast cancer, and all the patients are in early-stage breast cancer except 5 patients in 1 study published by Najafi (54). Based on the 68 studies included through inclusion and exclusion criteria, a total of 7 factors that may affect the incidence of CIA were assessed, among which age of patients, HR status, ER status, PR status, use of tamoxifen, and use of anthracycline-based (A)/anthracycline-taxane-based (A+T) regimens were found to be associated with CIA occurrence. No significant correlation was found between tumor stage and CIA incidence. Moreover, the results showed that CIA is associated with favorable disease-free survival (DFS) and overall survival (OS) in premenopausal patients with breast cancer.
Breast cancer in women has now become the most commonly diagnosed cancer worldwide; the data released by the World Health Organization showed that 2.26 million new cases of breast cancer were diagnosed globally in 2020 (1). Adjuvant chemotherapy is necessary for most patients to reduce the risk of recurrence and metastasis, which also prolongs the survival interval (85, 86). As more patients with breast cancer benefit from the use of adjuvant chemotherapy, long-term side effects such as premature ovarian failure presented as CIA have become a major concern (9). Premature ovarian failure is characterized by the suppression of ovarian function, which leads to a menopause-like state in the premenopausal period (87). In previous reports, the quality of life in premenopausal patients with breast cancer and CIA was reported to be impaired because of symptoms associated with premature ovarian dysfunction as well as the other side effects of chemotherapy (20, 88). A significant number of women receive a cancer diagnosis before their age of natural menopause, the most frequent neoplasms included breast cancer, and most of these patients desire to preserve fertility (89); it is important to consider the assessment and management of CIA in the clinical treatment of premenopausal patients with breast cancer. In 2014, Zhao published a meta-analysis (5) that included 46 studies on the risk factors that affected CIA incidence and their prognostic effect. In 7 years, several trials have been reported but no meta-analysis has been reported on this. Although a meta-analysis was published by Zavos (90) in 2016, only 14 studies were included on risk factors associated with CIA, and there is no analysis estimating the prognostic effect of CIA. To further gain a more reliable and comprehensive conclusion, we conducted an updated meta-analysis.
Age was identified as a crucial factor associated with the incidence of CIA in previous studies. Older patients (>40 years) are more likely to develop CIA after adjuvant chemotherapy (68, 91). Moreover, the incidence of CIA is positively correlated with age in more detailed groups in some studies (6, 32). Perez-Fidalgo et al. reported that the risk of amenorrhea increased with the increase of age, and the incidence of CIA in groups with an age of ≤40, 41–45, and >45 was 52.0%, 70.8%, and 95.1%, respectively (6). Furthermore, studies reported that the occurrence time of CIA is negatively correlated with the age of the patients, suggestive of increasing sensitivity to the toxic effect of chemotherapeutic regimens in older women (32, 92). Consistent with previous studies, the present meta-analysis showed that age plays a dominant role in the incidence of CIA. The overall pooled OR for patients with an age of ≤40 versus age of >40 was 0.136, which suggested that CIA is associated with age, and older women are more prone to amenorrhea. Premenopausal patients older than 40 should be informed about the high risk of amenorrhea and its adverse effect on the quality of life.
The effect of HR status on the incidence of CIA has not been well documented in previous studies, and whether the HR status affects the incidence of CIA is debatable. Parulekar et al. reported no difference in CIA risk between two HR status groups, with 73.3% incidence in the receptor-positive group and 74.0% incidence for the receptor-negative group (11). Fornier reported that hormone-positive patients had a significantly increased risk of CIA (37). Results from our meta-analysis showed that patients with a positive hormone status were more prone to develop CIA. The overall OR was 0.611 for HR-negative patients compared with HR-positive patients, which suggested that the HR status can be a potential predictive factor of CIA incidence in premenopausal patients with breast cancer. In addition, our meta-analysis separately analyzed the relationship between the ER or PR status and CIA incidence, and the results were as follows: the overall ORs were 0.683 for ER-negative patients compared with ER-positive patients, and the overall ORs were 0.690 for PR-negative patients compared with PR-positive patients. This means that patients only with an ER- or PR-positive status also have a higher incidence of CIA. Whether there is a direct biological link between HR status and CIA is still unknown.
The role of tamoxifen in the incidence of CIA is debatable. The IBCSG trial 13-93 that involved 1,293 premenopausal patients with breast cancer showed no difference between the use of tamoxifen and CIA incidence (42). However, some other studies have reported that tamoxifen plays an obvious role in the occurrence of ovarian failure. NSABP B-30 consisting of 708 premenopausal patients showed that the use of tamoxifen could significantly increase the incidence of CIA (10). Our meta-analysis evaluated the effect of tamoxifen on CIA incidence and showed a significant increase in the incidence of CIA after the use of tamoxifen, with overall OR = 0.568, p <0.001 for therapy without tamoxifen versus with tamoxifen. Our meta-analysis further confirmed that the use of tamoxifen would increase the risk of CIA. Based on these results, premenopausal patients with breast cancer willing to preserve their fertility should be informed of the potential risks of amenorrhea while prescribing tamoxifen, and alternative treatments should be recommended.
There is no single worldwide standard adjuvant chemotherapeutic regimen in the treatment of breast cancer, and the preferred regimens are variable. Chemotherapy drugs used for the treatment of breast cancer include cyclophosphamide, epirubicin, fluorouracil, docetaxel, and paclitaxel. The most commonly used chemotherapeutic regimens include anthracycline-based (A) and anthracycline-taxane-based (A+T) regimens (17). Because taxanes are usually administered concomitantly or sequentially with A in anthracycline-taxane-based (A+T) regimens, verifying the true effect of taxanes on CIA is difficult. Based on previous studies, we learned that there are discordant results in the effect of the CIA. Some studies reported that the addition of taxane to anthracycline-based regimens would increase the incidence of CIA (52, 54). However, other studies indicated that taxane had no significant effect on the risk of CIA (15, 93).
Our meta-analysis showed that the incidence of CIA in the A+T group is significantly higher than that in the A group (OR = 0.699), which suggests that the addition of taxane to the anthracycline-based regimen could increase the incidence of CIA in premenopausal women with breast cancer. It indicated that taxane should be used cautiously in chemotherapy for premenopausal patients with breast cancer willing to preserve fertility, and ovarian protective medications should be appropriately administered when patients undergo chemotherapy.
To further evaluate the effect of CIA on the prognosis of patients with breast cancer, we performed an analysis to determine the role of CIA on 5-year DFS and OS. The prognostic effect of CIA has been reported by others, although this has not been a consistent conclusion. A 20-year follow-up of women with breast cancer showed no significant difference in both relapse-free and OS between women with CIA and without CIA (34). However, Park reported that CIA is associated with improved 5-year DFS and OS regardless of the treatment (58). Based on our findings, the incidence of CIA could significantly improve the prognosis of patients with breast cancer in their premenopausal age. Just as the SOFT & TEXT (94)demonstrated that GnRH analogues could improve the prognosis of premenopausal patients by inhibiting ovarian function, this may also explain why CIA could significantly improve the prognosis. Considering the effect of fertility issues on patients’ quality of life due to dysfunction of the ovary, more premenopausal patients use GnRH analogues to protect ovary function during chemotherapy; however, due to their effects on menopausal status, patients with the addition of GnRH analogues are excluded from our analysis. A 5-year follow-up of the S0230/POEMS study (95) demonstrated that the patient with goserelin could avoid premature menopause and preserve future fertility, and DFS and OS were not inferior to those used in the chemotherapy group alone. Lambertini et al. conducted a systemic review (96), observing that the use of GnRH analogues has no significant effect on survival and can significantly decrease the premature ovarian insufficiency (POI) rate. It is proved that adding GnRH analogues in premenopausal breast cancer patients could effectively prevent the occurrence of CIA and has no significant effect on prognosis. There are both positive and negative effects of CIA on prognosis and life quality; therefore, more individualized strategies should be carried out in clinical practice.
Our study has some limitations. First, although we tried to minimize heterogeneity by dividing included studies into 4 subgroups based on the definitions of CIA, the meta-analysis includes some results with statistical heterogeneity (I2>50%) because of high heterogeneity of size, design, method, and therapeutic regimen, which were not explained by our sensitivity analyses. However, in prevalence meta-analysis, heterogeneity was common, which may be because a large number of sample sizes of individual studies have accurate estimates that can lead to statistical heterogeneity (97). Second, although the quality assessment showed that most studies were of high quality, some studies nevertheless had a small sample size, leading to potential bias. Third, most eligible studies were retrospective, and confounding factors may have biased the results. Fourth, the duration of CIA was not available in most studies we included and was therefore not analyzed in the present study. Despite these limitations, this meta-analysis is robust enough to be considered effective and provides valuable and up-to-date information on the risk factors of CIA and the relationship between CIA and prognostic factors.
Conclusion
To summarize, this meta-analysis of premenopausal patients with breast cancer and CIA showed that age, HR-positive status, ER-positive status, PR-positive status, use of tamoxifen, and anthracycline-taxane-based (A+T) regimens are significantly associated with a higher incidence of CIA, whereas tumor stage showed no significant correlation. Although the occurrence of CIA might induce fertility dysfunction and other syndromes, CIA was found to be an indicator that correlated with a better prognosis of premenopausal patients with breast cancer. Large-scale prospective cohort studies are necessary to further verify the factors associated with CIA incidence and to confirm the effects of prognostic factors. Based on our study, the CIA is a double-edged sword between the quality of life and prognosis of premenopausal patients with breast cancer; both effects should be considered in clinical treatments to perform individualized treatments.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: Cochrane Library, EMBASE, MEDLINE.
Author Contributions
YL and QY designed and conceived this meta-analysis. YW and JL were engaged in the collection, extraction, and analysis of data. YW and YL were responsible for writing this article. YW and JL conducted the quality assessment and data analysis. YL was responsible for the English language editing. All authors made their own contributions to this paper and agreed to the final version of this paper for submission.
Funding
This work was supported by the National Key Research and Development Program (No. 2020YFA0712400), Special Foundation for Taishan Scholars (No. ts20190971), National Natural Science Foundation of China (No. 81874119; No. 82072912), Special Support Plan for National High Level Talents (Ten Thousand Talents Program W01020103), National Key Research and Development Program (No. 2018YFC0114705), Foundation from Clinical Research Center of Shandong University (No.2020SDUCRCA015), and Qilu Hospital Clinical New Technology Developing Foundation (No. 2018-7; No. 2019-3).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.859974/full#supplementary-material
Supplementary Figure 1 | The risk of bias in the randomized controlled studies.
Supplementary Figure 2 | The publication bias of the meta-analyses included in this study. (A) The publication bias of age ≤40 years versus age >40 years in terms of the incidence of CIA. (B) The publication bias of patients with HR negativity versus positivity in the incidence of CIA. (C) The publication bias of patients with ER negativity versus positivity in the incidence of CIA. (D) The publication bias of patients with PR negativity versus positivity in the incidence of CIA. (E) The publication bias of patients with or without the usage of tamoxifen on the incidence of CIA. (F) The publication bias of different chemotherapy regimens on the incidence of CIA. (G) The publication bias of patients with stage I/II verse stage III/IV in terms of the incidence of CIA. (H) The publication bias of CIA on DFS in premenopausal breast cancer patients. (I) The publication bias of CIA on OS in premenopausal breast cancer patients.
Supplementary Figure 3 | The sensitivity analysis of the subgroup of the meta-analysis. (A) The sensitivity analysis of age ≤ 40 years versus age >40 years in the terms of incidence of CIA. (B) The sensitivity analysis of patients with HR negativity versus positivity in the incidence of CIA. (C) The sensitivity analysis of patients with ER negativity versus positivity in the incidence of CIA. (D) The sensitivity analysis of patients with PR negativity versus positivity in the incidence of CIA. (E) The sensitivity analysis of patients with or without the usage of tamoxifen on the incidence of CIA. (F) The sensitivity analysis of different chemotherapy regimens on the incidence of CIA. (G) The sensitivity analysis of patients with stage I/II verse stage III/IV in the terms of incidence of CIA. (H) The sensitivity analysis of CIA on DFS in premenopausal breast cancer patients. (I) The sensitivity analysis of CIA on OS in premenopausal breast cancer patients.
Supplementary Figure 4 | (A) Premenopausal breast cancer patients with HR negativity versus positivity in terms of the incidence of CIA. (B) Premenopausal breast cancer patients with ER negativity versus positivity in terms of the incidence of CIA. (C) Premenopausal breast cancer patients with PR negativity versus positivity in terms of the incidence of CIA.
Supplementary Figure 5 | Premenopausal breast cancer patients with stage I/II verse stage III/IV in terms of the incidence of CIA.
References
1. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Bray F. Cancer Statistics for the Year 2020: An Overview. Int J Cancer (2021) 149(4):778–89. doi: 10.1002/ijc.33588
2. Pondé NF, Zardavas D, Piccart M. Progress in Adjuvant Systemic Therapy for Breast Cancer. Nat Rev Clin Oncol (2019) 16(1):27–44. doi: 10.1038/s41571-018-0089-9
3. Rossi L, Stevens D, Pierga JY, Lerebours F, Reyal F, Robain M, et al. Impact of Adjuvant Chemotherapy on Breast Cancer Survival: A Real-World Population. PloS One (2015) 10(7):e0132853. doi: 10.1371/journal.pone.0132853
4. Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-Releasing Hormone Agonists for Prevention of Chemotherapy-Induced Ovarian Damage: Prospective Randomized Study. Fertil Steril (2009) 91(3):694–7. doi: 10.1016/j.fertnstert.2007.12.044
5. Zhao J, Liu J, Chen K, Li S, Wang Y, Yang Y, et al. What Lies Behind Chemotherapy-Induced Amenorrhea for Breast Cancer Patients: A Meta-Analysis. Breast Cancer Res Treat (2014) 145(1):113–28. doi: 10.1007/s10549-014-2914-x
6. Pérez-Fidalgo JA, Roselló S, García-Garré E, Jordá E, Martín-Martorell P, Bermejo B, et al. Incidence of Chemotherapy-Induced Amenorrhea in Hormone-Sensitive Breast Cancer Patients: The Impact of Addition of Taxanes to Anthracycline-Based Regimens. Breast Cancer Res Treat (2010) 120(1):245–51. doi: 10.1007/s10549-009-0426-x
7. Molina JR, Barton DL, Loprinzi CL. Chemotherapy-Induced Ovarian Failure: Manifestations and Management. Drug Saf (2005) 28(5):401–16. doi: 10.2165/00002018-200528050-00004
8. Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of Fertility and Ovarian Function and Minimizing Chemotherapy-Induced Gonadotoxicity in Young Women. J Soc Gynecol Investig (1999) 6(5):229–39. doi: 10.1016/s1071-5576(99)00028-3
9. Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-Based Survey of Fertility Issues in Young Women With Breast Cancer. J Clin Oncol (2004) 22(20):4174–83. doi: 10.1200/JCO.2004.01.159
10. Swain SM, Land SR, Ritter MW, Costantino JP, Cecchini RS, Mamounas EP, et al. Amenorrhea in Premenopausal Women on the Doxorubicin-And-Cyclophosphamide-Followed-By-Docetaxel Arm of Nsabp B-30 Trial. Breast Cancer Res Treat (2009) 113(2):315–20. doi: 10.1007/s10549-008-9937-0
11. Parulekar WR, Day AG, Ottaway JA, Shepherd LE, Trudeau ME, Bramwell V, et al. Incidence and Prognostic Impact of Amenorrhea During Adjuvant Therapy in High-Risk Premenopausal Breast Cancer: Analysis of a National Cancer Institute of Canada Clinical Trials Group Study–Ncic Ctg Ma.5. J Clin Oncol (2005) 23(25):6002–8. doi: 10.1200/jco.2005.07.096
12. Yoo C, Yun MR, Ahn JH, Jung KH, Kim HJ, Kim JE, et al. Chemotherapy-Induced Amenorrhea, Menopause-Specific Quality of Life, and Endocrine Profiles in Premenopausal Women With Breast Cancer Who Received Adjuvant Anthracycline-Based Chemotherapy: A Prospective Cohort Study. Cancer Chemother Pharmacol (2013) 72(3):565–75. doi: 10.1007/s00280-013-2227-5
13. Davis AL, Klitus M, Mintzer DM. Chemotherapy-Induced Amenorrhea From Adjuvant Breast Cancer Treatment: The Effect of the Addition of Taxanes. Clin Breast Cancer (2005) 6(5):421–4. doi: 10.3816/CBC.2005.n.046
14. Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, et al. Incidence and Time Course of Bleeding After Long-Term Amenorrhea After Breast Cancer Treatment: A Prospective Study. Cancer (2010) 116(13):3102–11. doi: 10.1002/cncr.25106
15. Zhou WB, Yin H, Liu XA, Zha XM, Chen L, Dai JC, et al. Incidence of Chemotherapy-Induced Amenorrhea Associated With Epirubicin, Docetaxel and Navelbine in Younger Breast Cancer Patients. BMC Cancer (2010) 10:281. doi: 10.1186/1471-2407-10-281
16. Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel After Doxorubicin Plus Cyclophosphamide as Adjuvant Chemotherapy for Node-Positive Breast Cancer: Results From Nsabp B-28. J Clin Oncol (2005) 23(16):3686–96. doi: 10.1200/jco.2005.10.517
17. Najafi S, Mehrdad N, Djavid GE, Rajaii E. Taxane Based Regimen as a Risk Factor for Chemotherapy Induced Amenorrhea (Cia). Ejc Supplements (2010) 8(3):166–7. doi: 10.1016/s1359-6349(10)70401-8
18. Bedard PL, Di Leo A, Piccart-Gebhart MJ. Taxanes: Optimizing Adjuvant Chemotherapy for Early-Stage Breast Cancer. Nat Rev Clin Oncol (2010) 7(1):22–36. doi: 10.1038/nrclinonc.2009.186
19. Meng K, Tian W, Zhou M, Chen H, Deng Y. Impact of Chemotherapy-Induced Amenorrhea in Breast Cancer Patients: The Evaluation of Ovarian Function by Menstrual History and Hormonal Levels. World J Surg Oncol (2013) 11:101. doi: 10.1186/1477-7819-11-101
20. Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life After Breast Cancer: Understanding Women’s Health-Related Quality of Life and Sexual Functioning. J Clin Oncol (1998) 16(2):501–14. doi: 10.1200/jco.1998.16.2.501
21. Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and Dose Intensity as Determinants of Outcome in the Adjuvant Treatment of Breast Cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst (1998) 90(16):1205–11. doi: 10.1093/jnci/90.16.1205
22. Poikonen P, Saarto T, Elomaa I, Joensuu H, Blomqvist C. Prognostic Effect of Amenorrhoea and Elevated Serum Gonadotropin Levels Induced by Adjuvant Chemotherapy in Premenopausal Node-Positive Breast Cancer Patients. Eur J Cancer (2000) 36(1):43–8. doi: 10.1016/s0959-8049(99)00225-7
23. Walshe JM, Denduluri N, Swain SM. Amenorrhea in Premenopausal Women After Adjuvant Chemotherapy for Breast Cancer. J Clin Oncol (2006) 24(36):5769–79. doi: 10.1200/jco.2006.07.2793
24. Wells G, Shea B, O’Connell D, Peterson J, Welch V. The Newcastle-Ottawa Scale (Nos) for Assessing the Quality of Case-Control Studies in Meta-Analyses. Eur J Epidemiol (2011) 25:603–5. doi: 10.2307/632432
25. Nasser M. Cochrane Handbook for Systematic Reviews of Interventions. Am J Public Health (2020) 110(6):753–4. doi: 10.2105/ajph.2020.305609
26. Abdel-Razeq HN, Mansour RA, Ammar KS, Abdel-Razeq RH, Zureigat HY, Yousef LM, et al. Amenorrhea, Fertility Preservation, and Counseling Among Young Women Treated With Anthracyclines and Taxanes for Early-Stage Breast Cancer, a Retrospective Study. Med (Baltimore) (2020) 99(11):e19566. doi: 10.1097/md.0000000000019566
27. Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. The Effects of Paclitaxel, Dose Density, and Trastuzumab on Treatment-Related Amenorrhea in Premenopausal Women With Breast Cancer. Cancer (2010) 116(4):791–8. doi: 10.1002/cncr.24835
28. Andersson M, Kamby C, Jensen MB, Mouridsen H, Ejlertsen B, Dombernowsky P, et al. Tamoxifen in High-Risk Premenopausal Women With Primary Breast Cancer Receiving Adjuvant Chemotherapy. Report From the Danish Breast Cancer Co-Operative Group Dbcg 82b Trial. Eur J Cancer (1999) 35(12):1659–66. doi: 10.1016/s0959-8049(99)00141-0
29. Arslan E, Karsy M, Moy F, Oktay KH. The Effect of Taxanes on Menstruation and Ovarian Reserve in Women With Breast Cancer. Fertil Steril (2011) 96(3):S77–S. doi: 10.1016/j.fertnstert.2011.07.298
30. Beex LV, Mackenzie MA, Raemaekers JM, Smals AG, Benraad TJ, Kloppenborg PW. Adjuvant Chemotherapy in Premenopausal Patients With Primary Breast Cancer; Relation to Drug-Induced Amenorrhoea, Age and the Progesterone Receptor Status of the Tumour. Eur J Cancer Clin Oncol (1988) 24(4):719–21. doi: 10.1016/0277-5379(88)90304-5
31. Berliere M, Dalenc F, Malingret N, Vindevogel A, Piette P, Roche H, et al. Incidence of Reversible Amenorrhea in Women With Breast Cancer Undergoing Adjuvant Anthracycline-Based Chemotherapy With or Without Docetaxel. BMC Cancer (2008) 8:56. doi: 10.1186/1471-2407-8-56
32. Bianco AR, Del Mastro L, Gallo C, Perrone F, Matano E, Pagliarulo C, et al. Prognostic Role of Amenorrhea Induced by Adjuvant Chemotherapy in Premenopausal Patients With Early Breast Cancer. Br J Cancer (1991) 63(5):799–803. doi: 10.1038/bjc.1991.177
33. Boccardo F, Rubagotti A, Bruzzi P, Cappellini M, Isola G, Nenci I, et al. Chemotherapy Versus Tamoxifen Versus Chemotherapy Plus Tamoxifen in Node-Positive, Estrogen Receptor-Positive Breast Cancer Patients: Results of a Multicentric Italian Study. Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative Group. J Clin Oncol (1990) 8(8):1310–20. doi: 10.1200/jco.1990.8.8.1310
34. Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant Cyclophosphamide, Methotrexate, and Fluorouracil in Node-Positive Breast Cancer: The Results of 20 Years of Follow-Up. N Engl J Med (1995) 332(14):901–6. doi: 10.1056/nejm199504063321401
35. Canney P, Coleman R, Morden J, Barrett-Lee P, Banerji J, Wardley A, et al. 200 Tact2 Trial in Early Breast Cancer (Ebc): Differential Rates of Amenorrhoea in Premenopausal Women Following Adjuvant Epirubicin (E) or Accelerated Epirubicin (Ae) Followed by Capecitabine (X) or Cmf (Cruk/05/019). Eur J Cancer (2012) 48:S102–S. doi: 10.1016/s0959-8049(12)70268-x
36. Di Cosimo S, Alimonti A, Ferretti G, Sperduti I, Carlini P, Papaldo P, et al. Incidence of Chemotherapy-Induced Amenorrhea Depending on the Timing of Treatment by Menstrual Cycle Phase in Women With Early Breast Cancer. Ann Oncol (2004) 15(7):1065–71. doi: 10.1093/annonc/mdh266
37. Fornier MN, Modi S, Panageas KS, Norton L, Hudis C. Incidence of Chemotherapy-Induced, Long-Term Amenorrhea in Patients With Breast Carcinoma Age 40 Years and Younger After Adjuvant Anthracycline and Taxane. Cancer (2005) 104(8):1575–9. doi: 10.1002/cncr.21385
38. Goldhirsch A, Gelber RD, Castiglione M. The Magnitude of Endocrine Effects of Adjuvant Chemotherapy for Premenopausal Breast Cancer Patients. The International Breast Cancer Study Group. Ann Oncol (1990) 1(3):183–8. doi: 10.1093/oxfordjournals.annonc.a057718
39. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of Menopause During the First Year After Breast Cancer Diagnosis. J Clin Oncol (1999) 17(8):2365–70. doi: 10.1200/jco.1999.17.8.2365
40. Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH, et al. Analysis of Chemotherapy-Induced Amenorrhea Rates by Three Different Anthracycline and Taxane Containing Regimens for Early Breast Cancer. Breast Cancer Res Treat (2009) 115(2):335–42. doi: 10.1007/s10549-008-0071-9
41. The International Breast Cancer Study Group. Late Effects of Adjuvant Oophorectomy and Chemotherapy Upon Premenopausal Breast-Cancer Patients. Ann Oncol (1990) 1(1):30–5. doi: 10.1097/00000421-199012001-00010
42. Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M, Price KN, et al. Tamoxifen After Adjuvant Chemotherapy for Premenopausal Women With Lymph Node-Positive Breast Cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol (2006) 24(9):1332–41. doi: 10.1200/jco.2005.03.0783
43. Jeon SJ, Lee JI, Jeon MJ, Lee M. Prognostic Effects of Adjuvant Chemotherapy-Induced Amenorrhea and Subsequent Resumption of Menstruation for Premenopausal Breast Cancer Patients. Med (Baltimore) (2016) 95(14):e3301. doi: 10.1097/md.0000000000003301
44. Jung M, Shin HJ, Rha SY, Jeung HC, Hong S, Moon YW, et al. The Clinical Outcome of Chemotherapy-Induced Amenorrhea in Premenopausal Young Patients With Breast Cancer With Long-Term Follow-Up. Ann Surg Oncol (2010) 17(12):3259–68. doi: 10.1245/s10434-010-1172-3
45. Kim H-A, Shin D-S, Moon N-M, Paik N-S, Noh W-C. The Incidence of Chemotherapy-Induced Amenorrhea and Recovery in Young (<45-Year-Old) Breast Cancer Patients. J Breast Cancer (2009) 12(1):20–6. doi: 10.4048/jbc.2009.12.1.20
46. Koga C, Akiyoshi S, Ishida M, Nakamura Y, Ohno S, Tokunaga E. Chemotherapy-Induced Amenorrhea and the Resumption of Menstruation in Premenopausal Women With Hormone Receptor-Positive Early Breast Cancer. Breast Cancer (2017) 24(5):714–9. doi: 10.1007/s12282-017-0764-1
47. Lee S, Kil WJ, Chun M, Jung YS, Kang SY, Kang SH, et al. Chemotherapy-Related Amenorrhea in Premenopausal Women With Breast Cancer. Menopause (2009) 16(1):98–103. doi: 10.1097/gme.0b013e3181844877
48. Li HP, Ma LW, Zhang SL, Jia TZ, Deng HJ, Zhang ZH, et al. Observation and Clinical Significance of Adjuvant Chemotherapy-Induced Amenorrhea in Premenopausal Breast Cancer Patients. Zhonghua Zhong Liu Za Zhi (2006) 28(11):848–51. doi: 10.1007/s11769-006-0026-1
49. Liem GS, Mo FK, Pang E, Suen JJ, Tang NL, Lee KM, et al. Chemotherapy-Related Amenorrhea and Menopause in Young Chinese Breast Cancer Patients: Analysis on Incidence, Risk Factors and Serum Hormone Profiles. PloS One (2015) 10(10):e0140842. doi: 10.1371/journal.pone.0140842
50. Lower EE, Blau R, Gazder P, Tummala R. The Risk of Premature Menopause Induced by Chemotherapy for Early Breast Cancer. J Womens Health Gend Based Med (1999) 8(7):949–54. doi: 10.1089/jwh.1.1999.8.949
51. Ludwig Breast Cancer Study Group. A Randomized Trial of Adjuvant Combination Chemotherapy With or Without Prednisone in Premenopausal Breast Cancer Patients With Metastases in One to Three Axillary Lymph Nodes. Cancer Res (1985) 45(9):4454–9. doi: 2926702/cr0450094454
52. Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant Docetaxel for Node-Positive Breast Cancer. N Engl J Med (2005) 352(22):2302–13. doi: 10.1056/NEJMoa043681
53. Mehta RR, Beattie CW, Das Gupta TK. Endocrine Profile in Breast Cancer Patients Receiving Chemotherapy. Breast Cancer Res Treat (1992) 20(2):125–32. doi: 10.1007/bf01834642
54. Najafi S, Djavid GE, Mehrdad N, Rajaii E, Alavi N, Olfatbakhsh A, et al. Taxane-Based Regimens as a Risk Factor for Chemotherapy-Induced Amenorrhea. Menopause (2011) 18(2):208–12. doi: 10.1097/gme.0b013e3181f3e6e7
55. Narmadha MP, Veena M, Rajendran NN. Assessment of Chemotherapy Induced Amenorrhea (Cia) in Hormone Receptor Positive Premenopausal Women With Breast Cancer. Res J Pharm Biol Chem Sci (2012) 3(4):97–106.
56. Okanami Y, Ito Y, Watanabe C, Iijima K, Iwase T, Tokudome N, et al. Incidence of Chemotherapy-Induced Amenorrhea in Premenopausal Patients With Breast Cancer Following Adjuvant Anthracycline and Taxane. Breast Cancer (2011) 18(3):182–8. doi: 10.1007/s12282-011-0256-7
57. Pagani O, O’Neill A, Castiglione M, Gelber RD, Goldhirsch A, Rudenstam CM, et al. Prognostic Impact of Amenorrhoea After Adjuvant Chemotherapy in Premenopausal Breast Cancer Patients With Axillary Node Involvement: Results of the International Breast Cancer Study Group (Ibcsg) Trial Vi. Eur J Cancer (1998) 34(5):632–40. doi: 10.1016/s0959-8049(97)10036-3
58. Park IH, Han HS, Lee H, Lee KS, Kang HS, Lee S, et al. Resumption or Persistence of Menstruation After Cytotoxic Chemotherapy Is a Prognostic Factor for Poor Disease-Free Survival in Premenopausal Patients With Early Breast Cancer. Ann Oncol (2012) 23(9):2283–9. doi: 10.1093/annonc/mds006
59. Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, et al. Incidence, Time Course, and Determinants of Menstrual Bleeding After Breast Cancer Treatment: A Prospective Study. J Clin Oncol (2006) 24(7):1045–51. doi: 10.1200/JCO.2005.03.3969
60. Pourali L, Taghizadeh Kermani A, Ghavamnasiri MR, Khoshroo F, Hosseini S, Asadi M, et al. Incidence of Chemotherapy-Induced Amenorrhea After Adjuvant Chemotherapy With Taxane and Anthracyclines in Young Patients With Breast Cancer. Iran J Cancer Prev (2013) 6(3):147–50. doi: 4654
61. Ravi R, Haider G, Ahmed K, Sami A, Zahoor S, Lata R. Amenorrhea After Chemotherapy in Breast Cancer Patient. J Ayub Med Coll Abbottabad (2020) 32(1):73–7.
62. Reh A, Oktem O, Oktay K. Impact of Breast Cancer Chemotherapy on Ovarian Reserve: A Prospective Observational Analysis by Menstrual History and Ovarian Reserve Markers. Fertil Steril (2008) 90(5):1635–9. doi: 10.1016/j.fertnstert.2007.09.048
63. Reimer T, Kempert S, Gerber B, Thiesen HJ, Hartmann S, Koczan D. Slco1b1*5 Polymorphism (Rs4149056) Is Associated With Chemotherapy-Induced Amenorrhea in Premenopausal Women With Breast Cancer: A Prospective Cohort Study. BMC Cancer (2016) 16(1):8. doi: 10.1186/s12885-016-2373-3
64. Reyno LM, Levine MN, Skingley P, Arnold A, Abu Zahra H. Chemotherapy Induced Amenorrhoea in a Randomised Trial of Adjuvant Chemotherapy Duration in Breast Cancer. Eur J Cancer (1992) 29a(1):21–3. doi: 10.1016/0959-8049(93)90569-2
65. Richards MA, O’Reilly SM, Howell A, George WD, Fentiman IS, Chaudary MA, et al. Adjuvant Cyclophosphamide, Methotrexate, and Fluorouracil in Patients With Axillary Node-Positive Breast Cancer: An Update of the Guy’s/Manchester Trial. J Clin Oncol (1990) 8(12):2032–9. doi: 10.1200/jco.1990.8.12.2032
66. Roche H, Kerbrat P, Bonneterre J, Fargeot P, Fumoleau P, Monnier A, et al. Complete Hormonal Blockade Versus Epirubicin-Based Chemotherapy in Premenopausal, One to Three Node-Positive, and Hormone-Receptor Positive, Early Breast Cancer Patients: 7-Year Follow-Up Results of French Adjuvant Study Group 06 Randomised Trial. Ann Oncol (2006) 17(8):1221–7. doi: 10.1093/annonc/mdl107
67. Rokutan Da N, Horiguchi J, Koibuchi Y, Kikuchi M, Takeyoshi I. 53 Chemotherapy-Induced Amenorrhea and Adjuvant Endocrine Therapy for Premenopausal Women With Early Breast Cancer. Ejc Suppl (2010) 8(3):74–. doi: 10.1016/S1359-6349(10)70084-7
68. Rosendahl M, Ahlgren J, Andersen J, Bergh J, Blomquist C, Lidbrink E, et al. The Risk of Amenorrhoea After Adjuvant Chemotherapy for Early Stage Breast Cancer Is Related to Inter-Individual Variations in Chemotherapy-Induced Leukocyte Nadir in Young Patients: Data From the Randomised Sbg 2000-1 Study. Eur J Cancer (2009) 45(18):3198–204. doi: 10.1016/j.ejca.2009.09.019
69. Ruddy KJ, Guo H, Barry W, Dang CT, Yardley DA, Moy B, et al. Chemotherapy-Related Amenorrhea After Adjuvant Paclitaxel-Trastuzumab (Apt Trial). Breast Cancer Res Treat (2015) 151(3):589–96. doi: 10.1007/s10549-015-3426-z
70. Ruddy KJ, Schaid DJ, Partridge AH, Larson NB, Batzler A, Häberle L, et al. Genetic Predictors of Chemotherapy-Related Amenorrhea in Women With Breast Cancer. Fertil Steril (2019) 112(4):731–9.e1. doi: 10.1016/j.fertnstert.2019.05.018
71. Ruddy KJ, Zheng Y, Tayob N, Hu J, Dang CT, Yardley DA, et al. Chemotherapy-Related Amenorrhea (Cra) After Adjuvant Ado-Trastuzumab Emtansine (T-Dm1) Compared to Paclitaxel in Combination With Trastuzumab (Th) (Tbcrc033: Atempt Trial). Breast Cancer Res Treat (2021) 189(1):103–10. doi: 10.1007/s10549-021-06267-8
72. Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant Goserelin and Ovarian Preservation in Chemotherapy Treated Patients With Early Breast Cancer: Results From a Randomized Trial. Breast Cancer Res Treat (2009) 117(3):561–7. doi: 10.1007/s10549-009-0313-5
73. Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, et al. Longer Therapy, Iatrogenic Amenorrhea, and Survival in Early Breast Cancer. N Engl J Med (2010) 362(22):2053–65. doi: 10.1056/NEJMoa0909638
74. Tham YL, Sexton K, Weiss H, Elledge R, Friedman LC, Kramer R. The Rates of Chemotherapy-Induced Amenorrhea in Patients Treated With Adjuvant Doxorubicin and Cyclophosphamide Followed by a Taxane. Am J Clin Oncol (2007) 30(2):126–32. doi: 10.1097/01.coc.0000251398.57630.4f
75. Tiong V, Rozita AM, Taib NA, Yip CH, Ng CH. Incidence of Chemotherapy-Induced Ovarian Failure in Premenopausal Women Undergoing Chemotherapy for Breast Cancer. World J Surg (2014) 38(9):2288–96. doi: 10.1007/s00268-014-2542-y
76. Tormey DC, Gray R, Gilchrist K, Grage T, Carbone PP, Wolter J, et al. Adjuvant Chemohormonal Therapy With Cyclophosphamide, Methotrexate, 5-Fluorouracil, and Prednisone (Cmfp) or Cmfp Plus Tamoxifen Compared With Cmf for Premenopausal Breast Cancer Patients. An Eastern Cooperative Oncology Group Trial. Cancer (1990) 65(2):200–6. doi: 10.1002/1097-0142(19900115)65:2<200::aid-cncr2820650203>3.0.co;2-q
77. Tormey DC, Gray R, Abeloff MD, Roseman DL, Gilchrist KW, Barylak EJ, et al. Adjuvant Therapy With a Doxorubicin Regimen and Long-Term Tamoxifen in Premenopausal Breast Cancer Patients: An Eastern Cooperative Oncology Group Trial. J Clin Oncol (1992) 10(12):1848–56. doi: 10.1200/jco.1992.10.12.1848
78. Turnbull AK, Patel S, Martinez-Perez C, Rigg A, Oikonomidou O. Risk of Chemotherapy-Related Amenorrhoea (Cra) in Premenopausal Women Undergoing Chemotherapy for Early Stage Breast Cancer. Breast Cancer Res Treat (2021) 186(1):237–45. doi: 10.1007/s10549-020-05951-5
79. Vanhuyse M, Fournier C, Bonneterre J. Chemotherapy-Induced Amenorrhea: Influence on Disease-Free Survival and Overall Survival in Receptor-Positive Premenopausal Early Breast Cancer Patients. Ann Oncol (2005) 16(8):1283–8. doi: 10.1093/annonc/mdi241
80. Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen Treatment After Adjuvant Chemotherapy Has Opposite Effects on Bone Mineral Density in Premenopausal Patients Depending on Menstrual Status. J Clin Oncol (2006) 24(4):675–80. doi: 10.1200/JCO.2005.02.3515
81. Xu H, Zhang F, Kou D, Wang J. The Occurrence of Chemotherapy-Induced Amenorrhea in Postoperative and Premenopausal Patients With Breast Cancer and Its Influencing Factors. Cancer Res Clinic (2016) 28(1):32–5. doi: 10.3760/cma.j.issn.1006-9801.2016.01.007
82. Zhou LH, Yin WJ, Lu JS, Di GH, Wu J, Shen KW, et al. The Association of Menstruation of Breast Cancer Patients With Chemotherapy Regimen and Aging Period. Tumor (2007) 27(12):999–1002. doi: 1000-7431(2007)12-0999-04
83. Zhou W, Ding Q, Liang X, He Z, Zha X, Liu X, et al. The Risk of Amenorrhea Is Related to Chemotherapy-Induced Leucopenia in Breast Cancer Patients Receiving Epirubicin and Taxane Based Chemotherapy. PloS One (2012) 7(5):e37249. doi: 10.1371/journal.pone.0037249
84. Davidson NE, O’Neill AM, Vukov AM, Osborne CK, Martino S, White DR, et al. Chemoendocrine Therapy for Premenopausal Women With Axillary Lymph Node-Positive, Steroid Hormone Receptor-Positive Breast Cancer: Results From Int 0101 (E5188). J Clin Oncol (2005) 23(25):5973–82. doi: 10.1200/jco.2005.05.551
85. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet (2005) 365(9472):1687–717. doi: 10.1016/s0140-6736(05)66544-0
86. Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Nomura Y, et al. Polychemotherapy for Early Breast Cancer: An Overview of the Randomised Trials. Lancet (1998) 352(9132):930–42. doi: 10.1016/S0140-6736(01)05483-6
87. Zhang Y, Xiao Z, Wang Y, Luo S, Li X, Li S. Gonadotropin-Releasing Hormone for Preservation of Ovarian Function During Chemotherapy in Lymphoma Patients of Reproductive Age: A Summary Based on 434 Patients. PloS One (2013) 8(11):e80444. doi: 10.1371/journal.pone.0080444
88. Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of Menopausal Symptoms Among Women With a History of Breast Cancer and Attitudes Toward Estrogen Replacement Therapy. J Clin Oncol (1995) 13(11):2737–44. doi: 10.1200/jco.1995.13.11.2737
89. Arecco L, Ruelle T, Martelli V, Boutros A, Latocca MM, Spinaci S, et al. How to Protect Ovarian Function Before and During Chemotherapy? J Clin Med (2021) 10(18):18. doi: 10.3390/jcm10184192
90. Zavos A, Valachis A. Risk of Chemotherapy-Induced Amenorrhea in Patients With Breast Cancer: A Systematic Review and Meta-Analysis. Acta Oncol (2016) 55(6):664–70. doi: 10.3109/0284186X.2016.1155738
91. Bines J, Oleske DM, Cobleigh MA. Ovarian Function in Premenopausal Women Treated With Adjuvant Chemotherapy for Breast Cancer. J Clin Oncol (1996) 14(5):1718–29. doi: 10.1200/jco.1996.14.5.1718
92. Rose DP, Davis TE. Effects of Adjuvant Chemohormonal Therapy on the Ovarian and Adrenal Function of Breast Cancer Patients. Cancer Res (1980) 40(11):4043–7. doi: 10.1016/0304-3835(80)90154-8
93. Iwamoto T, Hara F, Uemura Y, Mukai H, Watanabe T, Ohashi Y. Nsas-Bc02 Substudy of Chemotherapy-Induced Amenorrhea (Cia) in Premenopausal Patients Who Received Either Taxane Alone or Doxorubicin(a) Cyclophosphamide(C) Followed by Taxane as Postoperative Chemotherapy. Breast Cancer Res Treat (2020) 182(2):325–32. doi: 10.1007/s10549-020-05692-5
94. Zickl L, Francis P, Fleming G, Pagani O, Walley B, Price KN, et al. Soft and Text: Trials of Tamoxifen and Exemestane With and Without Ovarian Function Suppression for Premenopausal Women With Hormone Receptor-Positive Early Breast Cancer. Cancer Res (2012) 72(24a):92–3. doi: 10.1158/0008-5472.SABCS12-OT2-2-01
95. Moore HCF, Unger JM, Phillips KA, Boyle F, Hitre E, Moseley A, et al. Final Analysis of the Prevention of Early Menopause Study (Poems)/Swog Intergroup S0230. J Natl Cancer Inst (2019) 111(2):210–3. doi: 10.1093/jnci/djy185
96. Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, et al. Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient-Level Data. J Clin Oncol (2018) 36(19):1981–90. doi: 10.1200/jco.2018.78.0858
Keywords: breast cancer, premenopausal, chemotherapy-induced amenorrhea, prognosis, meta-analysis
Citation: Wang Y, Li Y, Liang J, Zhang N and Yang Q (2022) Chemotherapy-Induced Amenorrhea and Its Prognostic Significance in Premenopausal Women With Breast Cancer: An Updated Meta-Analysis. Front. Oncol. 12:859974. doi: 10.3389/fonc.2022.859974
Received: 25 January 2022; Accepted: 04 March 2022;
Published: 05 April 2022.
Edited by:
Fabio Puglisi, University of Udine, ItalyReviewed by:
Filippo Montemurro, IRCCS Candiolo Cancer Institute, ItalyGrazia Arpino, University of Naples Federico II, Italy
Eva Blondeaux, University of Genoa, Italy
Copyright © 2022 Wang, Li, Liang, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qifeng Yang, cWlmZW5neV9zZHVAMTYzLmNvbQ==
 Yifei Wang
Yifei Wang Yaming Li
Yaming Li Jingshu Liang1
Jingshu Liang1 Qifeng Yang
Qifeng Yang