- 1Department of Oncology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel
- 2Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 3Thoracic Oncology Service, Institute of Oncology, Sheba Medical Center, Ramat Gan, Israel
- 4Thoracic Oncology Service, Cancer Institute, Soroka University Medical Center, Beer-Sheva, Israel
- 5Department of Oncology, Shaare Zedek Medical Center, Jerusalem, Israel
- 6Thoracic Cancer Service, Rambam Health Care Campus, Haifa, Israel
- 7Thoracic Cancer Service, Meir Medical Center, Kfar Sava, Israel
- 8Department of Oncology, Bnai Zion Medical Center, Haifa, Israel
- 9Faculty of Health Sciences, Ben Gurion University of Negev, Beer-Sheva, Israel
- 10Statistical Consulting Unit, Rabin Medical Center, Petah Tikva, Israel
- 11Thoracic Oncology Service, Assuta Medical Centers, Tel-Aviv, Israel
- 12Thoracic Oncology Service, Rabin Medical Center, Petah Tikva, Israel
Background: The use of CGP in guiding treatment decisions in aNSCLC with acquired resistance to ALK TKIs is questionable.
Methods: We prospectively assessed the impact of CGP on the decision-making process in ALK-rearranged aNSCLC patients following progression on 2nd/3rd-generation ALK TKIs. Physician’s choice of the most recommended next-line systemic treatment (NLST) was captured before and after receival of CGP results; the percentage of cases in which the NLST recommendation has changed was assessed along with the CGP turnaround time (TAT). Patients were divided into groups: patients in whom the NLST was initiated after (group 1) and before (group 2) receival of the CGP results. Time-to-treatment discontinuation (TTD) and overall survival (OS) with NLST were compared between the groups.
Results: In 20 eligible patients (median [m]age 63 years [range, 40-89], females 75%, adenocarcinoma 100%, failure of alectinib 90%, FoundationOne Liquid CDx 80%), CGP has altered NLST recommendation in 30% of cases. CGP findings were as follows: ALK mutations 30% (l1171X 10%, G1202R, L1196M, G1269A, G1202R+l1171N+E1210K 5% each), CDKN2A/B mutation/loss 10%, c-met amplification 5%. CGP mTAT was 2.9 weeks [IQR, 2.4-4.4]. mTTD was 11.3 months (95% CI, 2.1-not reached [NR]) and 5.4 months (95% CI, 2.0-NR) in groups 1 and 2, respectively (p-0.34). mOS was 13.2 months (95% CI, 2.9-NR) and 13.0 months (95% CI, 6.0-NR) in groups 1 and 2, respectively (p-0.86).
Conclusion: CGP has a significant impact on the decision-making process in ALK-rearranged aNSCLC following progression on 2nd/3rd-generation ALK TKIs.
Background
Approximately 3-5% of tumors in patients with advanced non-small cell lung cancer (aNSCLC) harbor rearrangements of the anaplastic kinase lymphoma gene (ALK) (1, 2). This is a unique aNSCLC subpopulation that mostly consists of young individuals, with no or limited history of smoking, and an adenocarcinoma histology. Although ALK fusion is a rare phenomenon, it shouldn’t be neglected considering the high prevalence of lung cancer overall, and the availability of several effective targeted treatment options (3–5).
The presence of ALK rearrangement results in tumor susceptibility to ALK tyrosine kinase inhibitors (ALK TKIs) (6). Crizotinib, a TKI of ALK, tyrosine-protein kinase Met (c-met), and ROS proto-oncogene 1 (ROS1) kinases (7), was the first ALK inhibitor to replace the standard chemotherapy in the 1st-line treatment of aNSCLC harboring an ALK fusion, providing the significant advantage of this therapy in terms of the progression-free survival - according to the results of the PROFILE 1014 trial (8). Since then, newer 2nd- and 3rd-generation ALK TKIs (e.g., alectinib, ceritinib, brigatinib, ensartinib and lorlatinib) were implemented into the management of ALK- rearranged aNSCLC - first in the post-progression setting (9–11), and later on - in the 1st- line setting - that based on the results of the ALEX trial (12), the ALTA-1L trial (13), the ASCEND-4 trial (14), and the CROWN trial (15).
The questions of ALK TKIs sequencing and optimal treatment strategy following the disease progression on specific ALK TKIs remain open, since these have never been evaluated in a randomized controlled clinical trial (16). Treatment decisions, however, can be guided by the acquired resistance mechanisms responsible for the disease progression during systemic treatment. The mechanisms of acquired resistance to ALK TKIs primarily include development of secondary resistant mutations in the ALK kinase domain occurring in 25-66% of patients (17–21). Of those, G1202R/del mutations predominate (42-53% of cases), while other ALK mutation types responsible for the development of secondary resistance to ALK TKIs are: L1196M, F1174X, G1269A, L1196M, and I1171X (18, 21). Moreover, sequential treatment with increasingly potent ALK TKIs may promote acquisition of treatment-refractory compound ALK mutations (21, 22). Off-target mechanisms of resistance to ALK TKIs involve up-regulation of bypass signaling pathways, such as epidermal growth factor receptor (EGFR), c-met, KIT proto-oncogene (KIT), insulin-like growth factor 1 receptor (IGF-1R), proto-oncogene SRC (SRC), MEK/ERK and others (17, 18, 23, 24). SCLC transformation has been described as a resistance mechanism to ALK TKIs as well (25, 26).
Since ALK resistance mutations appear to be the predominant mechanism of resistance to ALK TKIs, there is a clear rationale for its targeting. Moreover, it has been demonstrated that the presence of an ALK resistant mutation following the progression on 1st- and 2nd-generation ALK TKIs in ALK-rearranged aNSCLC is associated with better lorlatinib efficacy (19). However, different 2nd- and 3rd-generation ALK TKIs appear to have different in vitro activity against specific ALK resistant mutations, which, therefore, represents the rationale for identifying the underlying ALK resistant mutation subtype before making the decision regarding the next line of systemic treatment.
In our study, we prospectively assessed the impact of comprehensive genomic profiling (CGP) on the decision-making process in patients with ALK-rearranged aNSCLC following progression on 2nd- and 3rd-generation ALK TKIs.
Methods
Patient Selection, Study Design and Assessments
ALK-rearranged aNSCLC patients following failure of a 2nd/3rd-generation ALK TKI, regardless of prior crizotinib or platinum-based chemotherapy, treated in one of the participating Israeli oncological centres were selected for this prospective multicentre non-interventional clinical study. CGP [either in the form of FoundationOne CDx or FoundationOne Liquid CDx using algorithm as previously described in detail (27)] was performed, and the results were captured. A questionnaire was filled by the treating oncologist twice: before and after receival of the CGP results. The questionnaire (Supplementary Document S1) included the de-identified clinical patient data, the de-identified next-generation sequencing (NGS) results, and the physician’s choice of the most recommended next-line systemic treatment (NLST) captured before and after receival of CGP results. The percentage of cases in which the treatment recommendation has changed upon the receival of CGP results was assessed - reflecting the impact of the molecular testing on the decision-making process (Figure 1). We hypothesized that change in the treatment recommendation will occur in at least 30% of cases (the minimal clinically meaningful rate according to our perception, the cut-off was chosen arbitrarily).
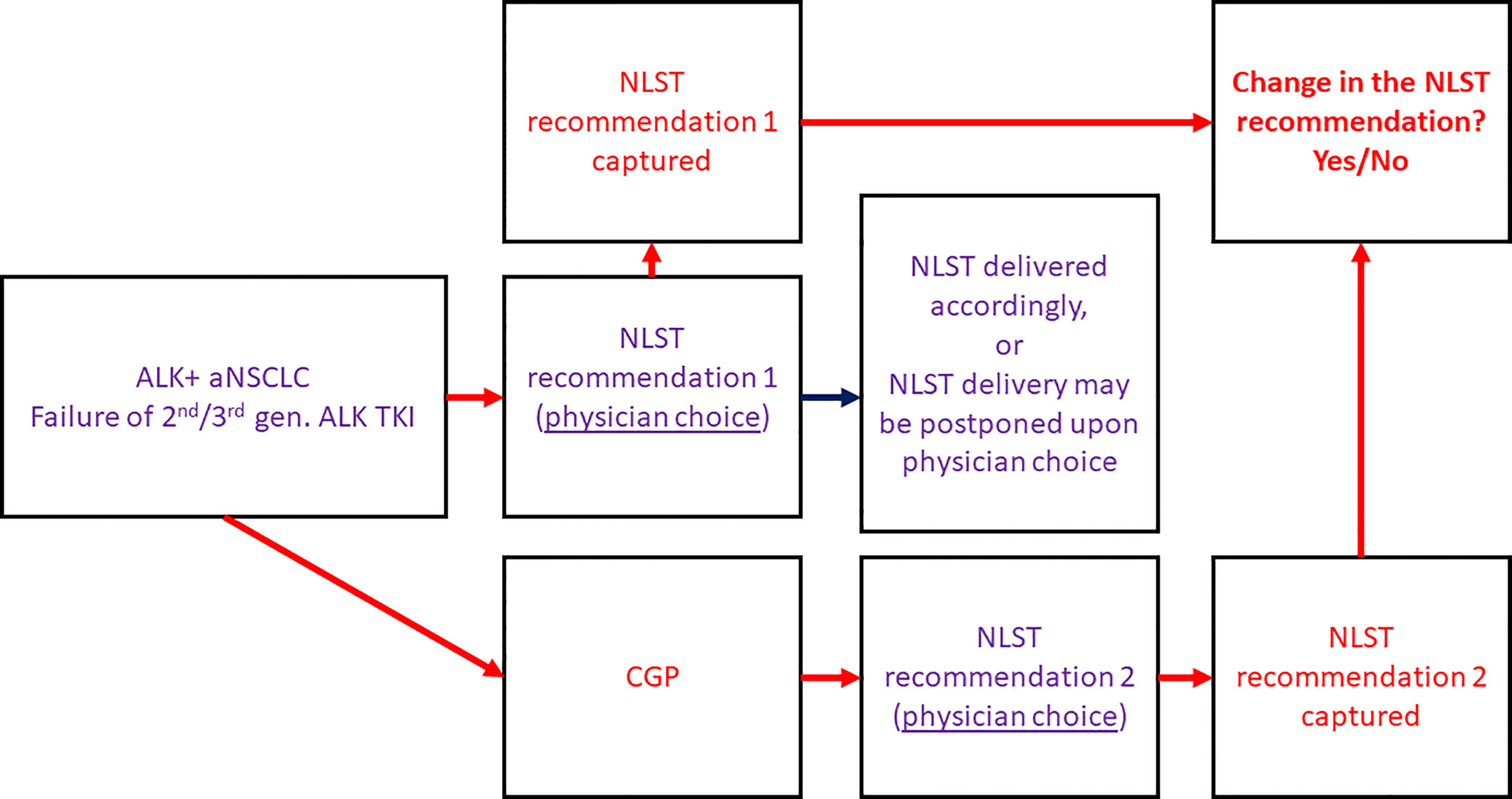
Figure 1 Study design. ALK, anaplastic lymphoma kinase; aNSCLC, advanced non-small cell lung cancer; CGP, comprehensive genomic profiling; gen., generation; NLST, next-line systemic treatment; TKI, tyrosine kinase inhibitor.
We prospectively gathered an information regarding the CGP turnaround time (TAT). The number of patients with adverse outcomes while waiting for the NGS results was collected as well. Additional demographic and clinical patient data were retrospectively retrieved from the patient medical records at each of the participating Israeli oncological centers.
The decision regarding the NLST type and initiation was done by the treating oncologist and was not specified by the protocol (Figure 1). Therefore, there were patients in whom the NLST was initiated after receival of the CGP results and in accordance with the NGS findings (group 1), and patients in whom the treatment was initiated before the CGP results became available (group 2). Time-to-treatment discontinuation (TTD) and overall survival (OS) with the NLST were retrospectively assessed and compared between the groups.
Next, we selected ALK-rearranged aNSCLC patients following failure of alectinib or ceritinib (the most commonly used 1st line ALK TKIs), regardless of prior platinum-based chemotherapy, and retrospectively assessed TTD with brigatinib and lorlatinib (the drugs typically used in this clinical scenario) in correlation with the NGS findings.
Statistical Analysis
The sample size was determined by the available patients meeting the inclusion criteria and referred for CGP. The statistical analysis was generated using SAS Software, version 9.4 (28). Categorical variables were presented by numbers and percentiles, medians and ranges were reported for continuous variables. TTD and OS were assessed by the Kaplan-Meier method, with the log-rank test for the comparison. Two-sided p values less than 0.05 were considered statistically significant.
Ethical Aspects
Institutional review board approval has been received before study initiation. No patient-identifying data was included in the central data collection.
Results
Patient Baseline and Treatment Characteristics
Twenty-two ALK-rearranged aNSCLC patients performed CGP within the study. One patient did not meet the eligibility criteria (failure of crizotinib and no administration of 2nd/3rd-generation ALK TKIs before enrolment), and the questionnaire was not filled by the treating oncologist in one additional patient – these two were excluded from the analysis. The baseline and treatment characteristics of the selected cohort (n=20) are presented in Table 1.
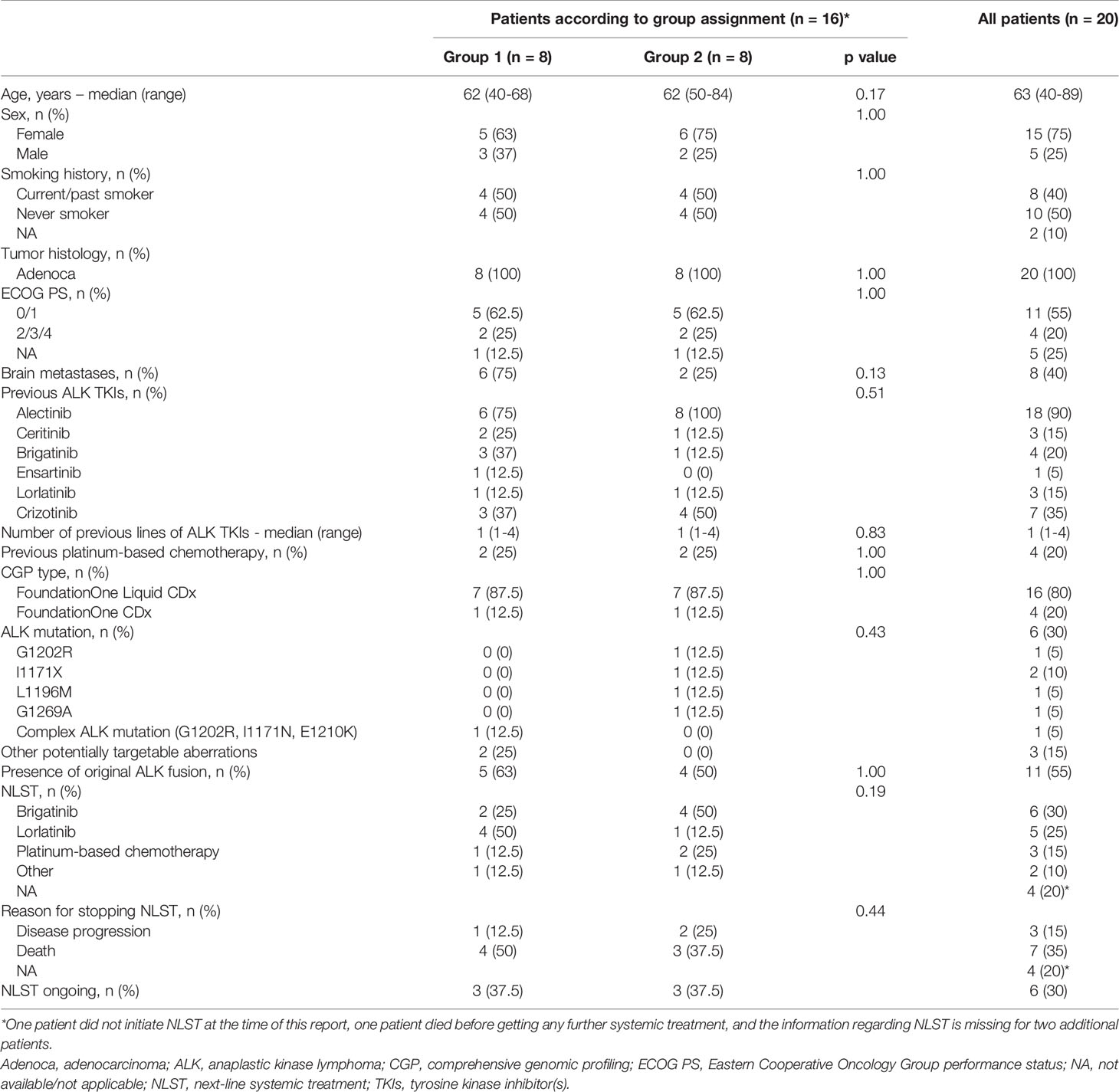
Table 1 Patient baseline and treatment characteristics in the whole study population and according to whether NLST was initiated before (group 1) or after (group 2) receival of CGP results.
The median age of the patients in the cohort was 63 (range, 40-89); females and never smoking patients with adenocarcinoma histology predominated - as expected for the enrolled population. The majority of patients received alectinib (with or without crizotinib) before enrolment; four, three, three, and one patient, respectively, were treated by brigatinib, ceritinib, lorlatinib and ensartinib; four patients received platinum-based chemotherapy before enrolment.
CGP Results and Change in Treatment Recommendation Upon Their Receival
FoundationOne Liquid CDx was the predominant CGP type performed. The molecular alterations diagnosed by NGS are presented in Table 1. ALK resistant mutations were present in 6 (30%) of cases (of those, G1202R in 1 case, l1171X in 2 cases, L1196M in 1 case, G1269A in 1 case, and a complex mutation in ALK gene combining G1202R, l1171N, and E1210K mutations in 1 case). With regards to another potentially targetable genomic aberrations, high level of c-met amplification was present in 1 case, and a cyclin dependent kinase inhibitor 2A/B (CDKN2A/B) mutation or loss was present in 2 additional cases. The original ALK fusion was presented in 11 (55%) of cases.
Overall, the change in NLST recommendation upon receival of the CGP results was registered in 6 patients (30% of the patients in the cohort). The initial physician’s choice of the most recommended NLST captured before receival of NGS results was as follows: brigatinib, n=9 (45%); lorlatinib, n=5 (25%); platinum-based chemotherapy, n=4 (20%), alectinib, n=1 (5%); pemetrexed, n=1 (5%) (Figure 2A). The physician’s choice of the most recommended NLST captured after receival of NGS results was as follows: brigatinib, n=5 (25%); lorlatinib, n=7 (35%); platinum-based chemotherapy, n=5 (25%), alectinib, n=1 (5%); crizotinib, n=2 (10%) (Figure 2A).
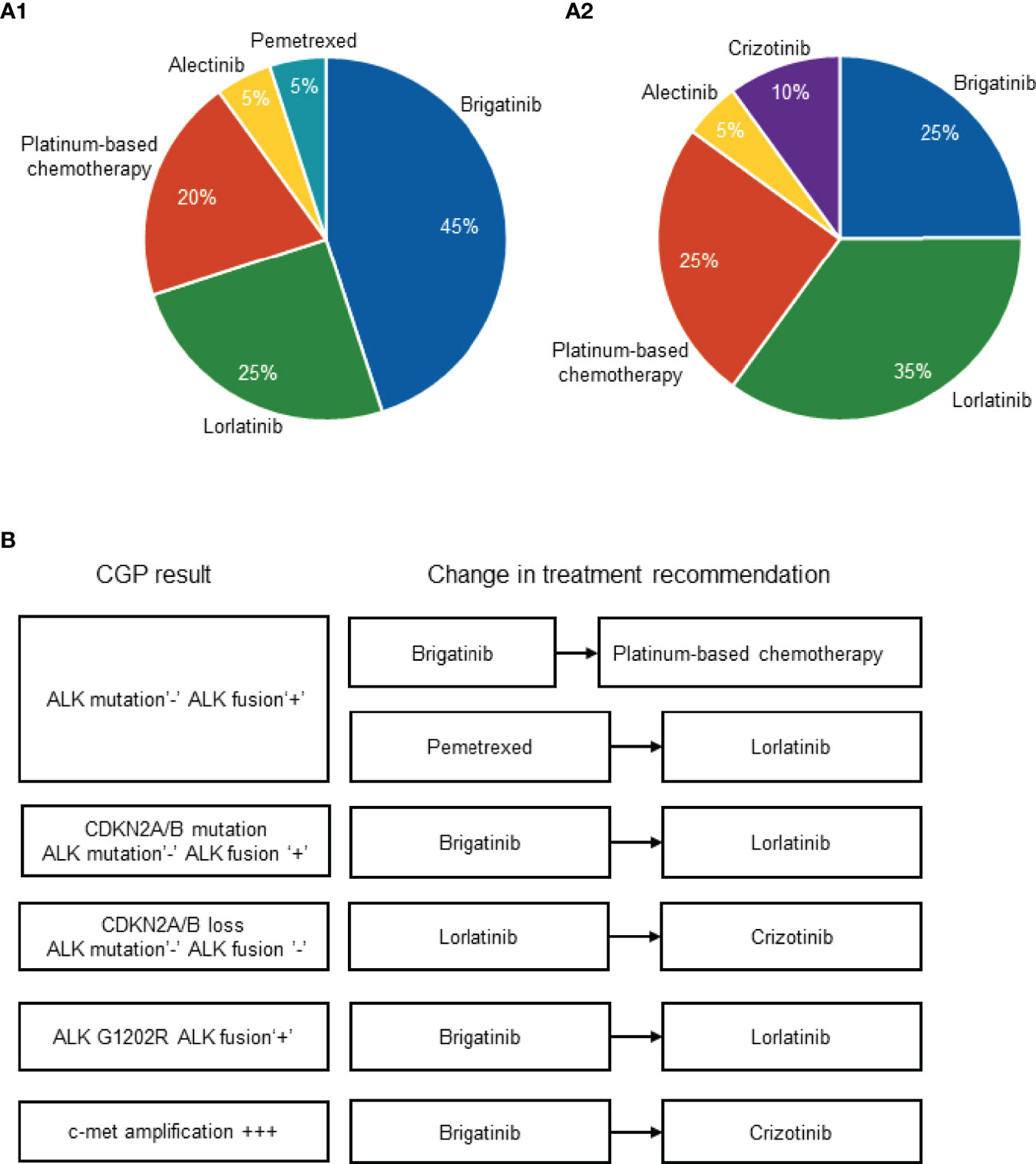
Figure 2 Physician’s choice of the most recommended NLST captured before (A1) and after (A2) the receival of CGP results. Change in treatment recommendation upon the receival of CGP results (B). ALK, anaplastic lymphoma kinase; CDKN2A/B, cyclin dependent kinase inhibitor 2A/B; CGP, comprehensive genomic profiling; c-met, tyrosine-protein kinase Met; NLST, next-line systemic treatment.
The change in the physician’s recommendation occurred upon the diagnosis of the following molecular alterations: absence of ALK resistant mutation and presence of original ALK fusion, n=2 (which drove the switch from brigatinib to platinum-based chemotherapy in once case and the switch from pemetrexed to lorlatinib in another case); CDKN2A/B mutation, absence of ALK resistant mutation and presence of original ALK fusion, n=1 (which drove the switch from brigatinib to lorlatinib); CDKN2A/B loss, absence of ALK resistant mutation or original ALK fusion, n=1 (which drove the switch from lorlatinib to crizotinib); presence of ALK G1202R and presence of original ALK fusion, n=1 (which drove the switch from brigatinib to lorlatinib); high level of c-met amplification, n=1 (which drove the switch from brigatinib to crizotinib) (Figure 2B).
CGP TAT
The median NGS testing TAT was 2.9 weeks [Interquartile range (IQR), 2.4-4.4]. One patient has died while waiting for the NGS results.
Time-To-Treatment Discontinuation and Overall Survival With the NLST
The NLST was initiated after receival of the CGP results and in accordance with the NGS findings in 8 patients (group 1), and included: brigatinib, n=2; lorlatinib, n=4; crizotinib, n=1; and platinum-based chemotherapy, n=1. The NLST was initiated before the NGS results became available in 8 patients (group 2), and included: brigatinib, n=4; lorlatinib, n=1; alectinib, n=1; and platinum-based chemotherapy, n=2 (Table 1). In addition, one patient did not initiate NLST at the time of this report, one patient died before getting any further systemic treatment (the same patient described in the previous section), and the information regarding the NLST is missing for two additional patients.
The baseline and treatment characteristics according to group assignment are presented in Table 1. Higher proportion of patients in group 1 had brain metastases and had previous exposure to novel 2nd-generation ALK TKIs (e.g., brigatinib and ensartinib). Patients in group 2 were less frequently approached with lorlatinib; higher proportion of patients in group 2 appeared to harbor an ALK resistant mutation. Those differences were not statistically significant.
Importantly, the change in NLST recommendation upon receival of NGS results was registered in 4 out of 8 patients included in group 1 (50%); these included cases #1, #2, #3, and #4 (Figure 2B). Case #5 was included in group 2, and the information regarding the NLST is missing in case #6 (Figure 2B).
The median follow-up was 11.3 mounts [IQR, 5.3-15.1] and 7.8 months [IQR, 6.4-11.9] in groups 1 and 2, respectively. Five (62.5%) patients in each group discontinued the NLST at the time of the last follow-up. Four (50%) patients in group 1, and 3 (37.5%) patients in group 2, respectively, have died (Table 1). Median TTD was 11.3 months (95% CI, 2.1-not reached [NR]) and 5.4 months (95% CI, 2.0-NR) in groups 1 and 2, respectively (p-0.34). Median OS was similar in both groups, and comprised 13.2 months (95% CI, 2.9-NR) and 13.0 months (95% CI, 6.0-NR) in groups 1 and 2, respectively (p-0.86). The Kaplan-Meyer curves for the TTD and OS with the next-line systemic treatment according to group assignment are presented in Figure 3.
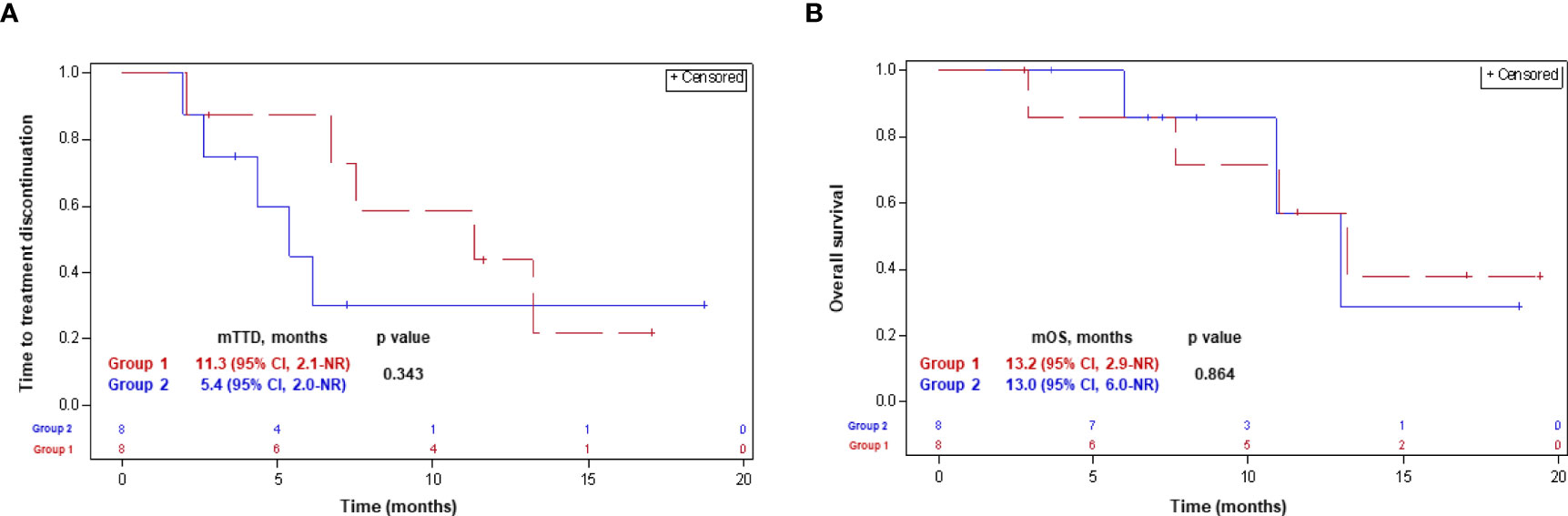
Figure 3 Time-to-treatment discontinuation (A) and overall survival (B) with the NLST in patients in whom the treatment decision was made before (group 2) and after (group 1) getting the CGP results. CGP, comprehensive genomic profiling; CI, confidence interval; NLST, next-line systemic treatment; NR, not reached; (m)OS, (median) overall survival; (m)TTD, (median) time-to-treatment discontinuation.
Time-To-Treatment Discontinuation and Overall Survival With Brigatinib and Lorlatinib in Correlation With the NGS Findings
In one patient diagnosed with a complex G1202R, l1171N, and E1210K ALK mutation, TTD and OS with lorlatinib were 13.0 months and 13.0 months, respectively. In one patient diagnosed with a G1202R ALK mutation, TTD and OS with brigatinib were 2.0 months and 6.0 months, respectively. In one patient diagnosed with an l1171T ALK mutation, TTD and OS with brigatinib were 4.5 months and 8.0 months, respectively. One patient diagnosed with an L1196M ALK mutation, continues lorlatinib at the time of the report for 8.0 month since treatment initiation.
Discussion
This is the first prospective study illustrating the value of CGP and molecular assessment of acquired resistance mechanisms in treatment decision-making process in ALK-rearranged aNSCLC patients.
According to our observation, CGP performed at the time of progression on 2nd- and 3rd-generation ALK TKIs, has altered treatment recommendation in one third of cases - which confirmed the initial hypothesis and, overall, appeared to be a clinically meaningful result. In cases the initiation of the NLST was postponed until getting NGS results, the proportion of patients in whom the treatment recommendation has changed was even higher (50%) - pointing to potentially larger effect of genomic assessment on the decision-making process.
Importantly, the median CGP TAT was only 2.9 weeks [IQR, 2.4-4.4] which seems acceptable considering the CGP impact on treatment decision. In our cohort, only one patient has died while waiting for the CGP results. This fact emphasizes the need to assess further the phenomenon of clinical deterioration attributable to rapid disease progression, and the potential adverse effect of the CGP in this association.
Looking into the specific treatment recommendation changes following the receival of the CGP results, we observed increase in the proportion of recommendations on lorlatinib, platinum-based chemotherapy, and crizotinib. There were three clinical scenarios which seemed to have a biologic rationale behind the treatment decision alteration. The 1st clinical scenario was switching to a different targeted treatment following the diagnosis of another potentially targetable molecular aberration, or a bypass pathway activation, such as switching from ALK TKI to c-met TKI following the diagnosis of c-met amplification. Indeed, c-met alterations represent one of the most common mechanisms responsible for acquired resistance to osimertinib in EGFR mutant aNSCLC (10-25%) (29), and to ALK TKIs in ALK-rearranged aNSCLC (15%) (24). Moreover, c-met inhibition has been associated with objective response rate of 30% and median duration of response of 7.9 months in c-met-amplified aNSCLC patients following progression on 3rd-generation EGFR TKIs (30). Additionally, there are several case reports suggesting that met-inhibition may overcome c-met-driven resistance in ALK-positive aNSCLC (24, 31, 32). The 2nd clinical scenario of treatment decision alteration in our cohort was switching from brigatinib to lorlatinib following the diagnosis of ALK G1202R mutation – which is justified by the high activity of lorlatinib in tumors harboring this ALK resistant mutation (19). The 3rd clinical scenario in our study included switching from ALK TKIs to platinum-based chemotherapy in the absence of ALK resistant mutation - which, again, seems reasonable considering modest next-generation ALK TKI activity in patients without ALK resistant mutations following progression on prior ALK TKIs (19). Specifically, a positive correlation between presence of ALK resistant mutations, their type, and outcomes with lorlatinib have been reported in ALK-rearranged aNSCLC patients following failure of a 2nd-generation ALK TKI. It remains unknown, however, whether similar correlation is true for brigatinib. Moreover, no comparative clinical studies have been done or planned to be done in order to explore the comparative efficacy of the two agents in correlation with the molecular biomarkers in this clinical setting.
In three additional clinical scenarios which prompted treatment decision changes in our study, it was hard to explain the physician’s decision from the biologic perspective. For instance, presence of CDKN2A/B loss or mutation was anticipated to alter the decision towards cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, however, it was not the case. Having said that, it should be emphasized that CDKN2A/B alterations are only rarely seen following progression on 2nd/3rd-generation ALK TKIs (17, 23, 33), and the majority of CDK4/6 inhibitors did not demonstrate a significant antitumor activity in aNSCLC (34–39). Although CDK4/6 inhibitors have demonstrated a myelo-preserving effect in conjunction with chemotherapy in advanced small-cell lung cancer, it did not appear to improve tumor control (40).
The prevalence of ALK resistant mutations (30%) and their distribution in our study were in line with the previously reported data in ALK-positive aNSCLC following treatment with 2nd/3rd-generation ALK TKIs (19, 23). Only one case of high-level c-met amplification was present in our cohort, while higher prevalence of c-met alterations (12-22%) has been reported in the literature (24). This discrepancy might be attributable to the lower proportion of patients progressing on lorlatinib in our cohort, and less so - to the technical limitations of the liquid biopsy assay. For instance, Dagogo-Jack et al. reported on higher prevalence of c-met amplification following treatment with 3rd-generation ALK TKI, on one hand, and on the other hand - on high overall accuracy of liquid NGS as compared to tissue genotyping (24). The detection of CDKN2A/B alterations was not unique to our cohort either (33). Another interesting observation in our study was tissue versus liquid biopsy referral patterns. For instance, liquid biopsy was the preferred method of assessment - probably due to its simplicity and high patient advocacy, which reflected real-world physician and patient preferences. This pattern was also in line with the recently updated International Association for the Study of Lung Cancer (IASLC) guideline on liquid biopsy to discover molecular resistance mechanisms (41).
CGP performed at the time of progression on 2nd/3rd-generation ALK TKIs demonstrated a positive impact on NLST duration but did not affect the OS. Several factors might have attributed to that. First, some of the most expedient alterations in treatment recommendations were not implemented. Second, OS was the subject for the lead-time bias: i.e., those patients in whom the NLST was initiated following the receival of the CGP results, initiated the treatment later as opposed to patients in whom the NLST was started before the CGP results became available. Finally, higher proportion of patients in whom the treatment was initiated before receival of the CGP results appeared to harbor an ALK resistant mutation, which might have an impact on outcomes as well. The lack of the ability to demonstrate an impact of CGP on oncological outcomes remains the most significant limitation of our study, along with the small sample size. Another important study limitation is its non-randomized design allowing patient selection for immediate versus postponed treatment initiation based on the tempo of the disease.
Overall, the study has demonstrated the feasibility and the significant impact of the CGP on the decision-making process in ALK-rearranged aNSCLC following failure of 2nd/3rd-generation ALK TKIs. It remains to be seen whether such strategy affects oncological outcomes.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Helsinky ethics committee of the Rabin Medical Center (RMC), approval number: 0561-19-RMC. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Credit statement conceptualization: ED, AR, and AO. Methodology: ED, AR, and AO. Validation: ED. Formal analysis: TS. Investigation: ED. Resources: ED, AR, AO, LH, JD, DU, WK, AC, MM, AZ, JB, NR, SG, CO, AA, NP, and TS. Data Curation: ED. Writing - original draft: ED, AR, AO, and LH. Writing - review and editing: ED, AR, AO, LH, JD, DU, WK, AC, MM, AZ, JB, NR, SG, CO, AA, NP, and TS. Visualization: ED and LH. Supervision: ED. Project administration: ED. All authors contributed to the article and approved the submitted version.
Conflict of Interest
Author TS was employed by the company Statistical Consulting Unit. Disclosure (all outside of the submitted work): AR reported personal fees from Roche, Astra Zeneca, Merck Sharpe & Dohme, Novartis, Takeda, Elli Lilly, support for attending meetings from Bristol Myers Squibb, Roche, Boehringer Ingelheim. AO reported advisory fees from Merck Sharpe & Dohme, Bristol Myers Squibb, Roche, Astra Zeneca, Novartis, Boehringer Ingelheim. Damien Urban reported personal and consulting fees from Roche, Merck Sharpe & Dohme, Takeda, Astra Zeneca, Rhenium Oncotest, Bristol Myers Squibb. MM reported consulting fees from Boehringer Ingelheim, Roche, Astra Zeneca, MSD, BMS, Abbvie, Takeda, Pomicell. AZ reported grants from Bristol Myers Squibb, personal fees from Roche, Merck Sharpe & Dohme, Bristol Myers Squibb, Astra Zeneca, Takeda. JB reported grants and personal fees from Merck Sharpe & Dohme, Bristol Myers Squibb, Astra Zeneca, Roche, Abbvie, Takeda, OncoHost, ImmuneAI, Bayer, Novartis. AA reported research funding from Bristol Myers Squibb, personal and consulting fees from Bristol Myers Squibb, Roche, Pfizer, Astra Zeneca, Takeda, Novartis. NP reported research funding from Bristol-Myers Squibb, Eli Lilly, Foundation Medicine, Gaurdant360, Merk, MSD, Novartis, NovellusDx, Pfizer, Roche, Takeda, IP: Volatile Organic Compounds For Detecting Cell Dysplasia And Genetic Alterations Associated With Lung Cancer, WO2012023138; Breath Analysis of Pulmonary Nodules, US20130150261 A1; Apparatus for treating a target site of a body, WO/2015/059646 - all outside of the submitted work. ED reported grants from Astra Zeneca, Boehringer Ingelheim, personal fees from Boehringer Ingelheim, Roche, Astra Zeneca, Pfizer, Merck Sharpe & Dohme, Bristol Myers Squibb, Novartis, Takeda, Sanofi, Merck Serono, Medison Pharma, Janssen Israel- all outside of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
FoundationOne CDx and FoundationOne Liquid CDx were provided by Roche Pharmaceuticals within the compassionate use program.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.874712/full#supplementary-material
Abbreviations
Adenoca, adenocarcinoma; ALK, anaplastic kinase lymphoma; (a)NSCLC, advanced non-small cell lung cancer; CDK4/6, cyclin-dependent kinase 4/6; CDKN2A/B, cyclin dependent kinase inhibitor 2A/B; CGP, comprehensive genomic profiling; CI, confidence interval; c-met, tyrosine-protein kinase Met; ECOG PS - Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; gen., generation; IASLC, International Association for the Study of Lung Cancer; IGF-1R, insulin-like growth factor 1 receptor; IQR, Interquartile range; KIT, KIT proto-oncogene; (m)OS, (median) overall survival; (m)TTD, (median) time-to-treatment discontinuation; NA, not available/not applicable; NGS, next-generation sequencing, NLST, next-line systemic treatment; NR, not reached; ROS1, ROS proto-oncogene 1; SRC, proto-oncogene SRC; TAT, turnaround time; TKI, tyrosine kinase inhibitor.
References
1. Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H , et al. Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell (2007) 131:1190–203. doi: 10.1016/j.cell.2007.11.025
2. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the Transforming EML4-ALK Fusion Gene in non-Small-Cell Lung Cancer. Nature (2007) 448:561–6. doi: 10.1038/nature05945
3. Blackhall FH, Peters S, Bubendorf L, Dafni U, Kerr KM, Soltermann HHA, et al. Prevalence and Clinical Outcomes for Patients With ALK-Positive Resected Stage I-III Adenocarcinoma: Results From the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol (2014) 32:2780–7. doi: 10.1200/JCO.2013.54.5921
4. Camidge DR, Doebele RC. Treating ALK-Positive Lung Cancer — Early Successes and Future Challenges. Nat Rev Clin Oncol (2012) 9:268–77. doi: 10.1038/nrclinonc.2012.43
5. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Solomon RSHB, et al. Clinical Features and Outcome of Patients With Non-Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol (2009) 27:4247–53. doi: 10.1200/JCO.2009.22.6993
6. Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, et al. Cytoreductive Antitumor Activity of PF-2341066, A Novel Inhibitor of Anaplastic Lymphoma Kinase and C-Met, in Experimental Models of Anaplastic Large-Cell Lymphoma. Mol Cancer Ther (2007) 6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365
7. McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, et al. Genomic Alterations of Anaplastic Lymphoma Kinase may Sensitize Tumors to Anaplastic Lymphoma Kinase Inhibitors. Cancer Res (2008) 68(9):3389–95. doi: 10.1158/0008-5472.CAN-07-6186
8. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-Line Crizotinib Versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
9. Yang JC, Ou SI, De Petris L, Gadgeel S, Gandhi L, Kim DW, et al. Pooled Systemic Efficacy and Safety Data From the Pivotal Phase II Studies (NP28673 and NP28761) of Alectinib in ALK-Positive Non-Small Cell Lung Cancer. J Thorac Oncol (2017) 12(10):1552–60. doi: 10.1016/j.jtho.2017.06.070
10. Shaw AT, Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, et al. Ceritinib in ALK-Rearranged non-Small-Cell Lung Cancer. N Engl J Med (2014) 370(13):1189–97. doi: 10.1056/NEJMoa1311107
11. Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol (2017) 35(22):2490–8. doi: 10.1200/JCO.2016.71.5904
12. Solange P, Camidge R, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib Versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med (2017) 377:829–38. doi: 10.1056/NEJMoa1704795
13. Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Lee JS, et al. Brigatinib Versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med (2018) 379:2027–39. doi: 10.1056/NEJMoa1810171
14. Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-Line Ceritinib Versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet (2017) 389(10072):917–29. doi: 10.1016/S0140-6736(17)30123-X
15. Shaw AT, Bauer TM, De Marinis F, Felip E, Goto Y, Liu G, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med (2020) 383:2018–29. doi: 10.1056/NEJMoa2027187
16. Gristina V, La Mantia M, Iacono F, Galvano A, Russo A, Bazan V, et al. The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals (Basel) (2020) 13(12):474. doi: 10.3390/ph13120474
17. Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, et al. Role and Targeting of Anaplastic Lymphoma Kinase in Cancer. Mol Cancer (2018) 17(30):1–9. doi: 10.1186/s12943-018-0776-2
18. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK Mutations in Lung Cancer That Confer Resistance to ALK Inhibitors. N Engl J Med (2010) 363:1734–9. doi: 10.1056/NEJMoa1007478
19. Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol (2019) 37(16):1370–9. doi: 10.1200/JCO.18.02236
20. Noé J, Lovejoy A, Ou SI, Klass DM, Cummings CA, Shaw AT, et al. ALK Mutation Status Before and After Alectinib Treatment in Locally Advanced or Metastatic ALK-Positive NSCLC: Pooled Analysis of Two Prospective Trials. J Thorac Oncol (2020) 15(4):601–8. doi: 10.1016/j.jtho.2019.10.015
21. Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment With Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res (2019) 25(22):6662–70. doi: 10.1158/1078-0432.CCR-19-1436
22. Yoda S, Lin JJ, Lawrence MS, Burke BJ, Friboulet L, Langenbucher A, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discovery (2018) 8(6):714–29. doi: 10.1158/2159-8290.CD-17-1256
23. Gainor J, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discovery (2016) 6(10):1118–33. doi: 10.1158/2159-8290.CD-16-0596
24. Dagogo-Jack I, Yoda S, Lennerz JK, Langenbucher A, Lin JJ, Rooney MM, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res (2020) 26(11):2535–45. doi: 10.1158/1078-0432.CCR-19-3906
25. Fujita S, Masago K, Katakami N, Yatabe Y. Transformation to SCLC After Treatment With the ALK Inhibitor Alectinib. J Thorac Oncol (2016) 11(6):e67-72. doi: 10.1016/j.jtho.2015.12.105
26. Cha YJ, Cho BC, Kim HR, Lee HJ, Shim HS. A Case of ALK-Rearranged Adenocarcinoma With Small Cell Carcinoma-Like Transformation and Resistance to Crizotinib. J Thorac Oncol (2016) 11(5):e55–8. doi: 10.1016/j.jtho.2015.12.097
27. Chalmers ZA, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med (2017) 9:34. doi: 10.1186/s13073-017-0424-2
28. SAS®. 9.4 Statistical Software. Available at: https://www.sas.com/en_us/software/sas9.html (Accessed August 2, 2021).
29. Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review. J Clin Oncol (2020) 38(25):1903123. J Clin Oncol. doi: 10.1200/JCO.19.03123
30. Oxnard GR, Yang JCH, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: A Multi-Arm, Phase Ib Trial of Osimertinib Combined With Selumetinib, Savolitinib, or Durvalumab in EGFR-Mutant Lung Cancer. Ann Oncol (2020) 31:507–16. doi: 10.1016/j.annonc.2020.01.013
31. Gouji T, Takashi S, Mitsuhiro T, Yukito I. Crizotinib Can Overcome Acquired Resistance to CH5424802: Is Amplification of the MET Gene a Key Factor? J Thorac Oncol (2014) 9(3):e27–28. doi: 10.1097/JTO.0000000000000113
32. Sakakibara-Konishi J, Kitai H, Ikezawa Y, Hatanaka , Sasaki T, Yoshida R, et al. Response to Crizotinib Re-Administration After Progression on Lorlatinib in a Patient With ALK-Rearranged Non-Small-Cell Lung Cancer. Clin Lung Cancer (2019) 20(5):e555–9. doi: 10.1016/j.cllc.2019.06.021
33. Dagogo-Jack I, Brannon AR, Ferris LA, Campbell CD, Lin JJ, Schultz KR, et al. Tracking the Evolution of Resistance to ALK Tyrosine Kinase Inhibitors Through Longitudinal Analysis of Circulating Tumor DNA. JCO Precis Oncol (2018) 2018:PO.17.00160. doi: 10.1200/PO.17.00160
34. Stephenson JJ, Nemunaitis J, Joy AA, Martin JC, Ying-Ming J, Zhang D, et al. Randomized Phase 2 Study of the Cyclin-Dependent Kinase Inhibitor Dinaciclib (MK-7965) Versus Erlotinib in Patients With non-Small Cell Lung Cancer. Lung Cancer (2014) 83:219–23. doi: 10.1016/j.lungcan.2013.11.020
35. Gopalan PK, Pinder MC, Chiappori A, Ivey AM, Villegas AG, Kaye FJ, et al. A Phase II Clinical Trial of the CDK 4/6 Inhibi-Tor Palbociclib (PD 0332991) in Previously Treated, Advanced Non-Small Cell Lung Cancer (NSCLC) Patients With Inactivated CDKN2A. J Clin Oncol (2014) 32:8077–7. doi: 10.1200/jco.2014.32.15_suppl.8077
36. Edelman MJ, Redman MW, Albain KS, McGary , Noman M, Rafique NM, Petro D, et al. SWOG S1400C (NCT02154490)-A Phase II Study of Palbociclib for Previously Treated Cell Cycle Gene Alter-Ation-Positive Patients With Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J Thorac Oncol (2019) 14:1853–9. doi: 10.1016/j.jtho.2019.06.027
37. Goldman JW, Mazieres J, Barlesi F, Dragnev KH, Koczywas M, Göskel T, et al. A Randomized Phase III Study of Abemaciclib Ver-Sus Erlotinib in Patients With Stage IV non-Small Cell Lung Cancer With a Detectable KRAS Mutation Who Failed Prior Platinum-Based Therapy: JUNIPER. Front Oncol (2020) 10:1. sdoi: 10.3389/fonc.2020.578756
38. Scagliotti GV, Govindan R, Hurt K, Chiang A. A Randomized Phase 2 Study of Abemaciclib VerSus Docetaxel in Patients With Stage IV Squamous Non-Small Cell Lung Cancer (SqCLC) Previously Treated With Platinum-Based Chemotherapy. J Clin Oncol (2016) 34(15_suppl). doi: 10.1200/JCO.2016.34.15_suppl.TPS9101
39. Santoro A, Su WC, Navarro A, Simonelli M, Yang J.C, Ardizzoni A, et al. Dose-Determination Results From a Phase Ib/II Study of Ceritinib (CER) + Ribociclib (RIB) in ALK-Positive (ALK+) Non-Small Cell Lung Cancer (NSCLC). Ann Oncol (2018) 29:viii501–2. doi: 10.1093/annonc/mdy292.015
40. Weiss J, Goldschmidt J, Zoran A, Dragnev KH, Pritchett Y, Morris SR, et al. Myelopreservation and Reduced Use of Supportive Care With Trilaciclib in Patients With Small Cell Lung Cancer. J Clin Oncol (2020) 38:12096–6. doi: 10.1200/JCO.2020.38.15_suppl.12096
Keywords: comprehensive genomic profiling, next-generation sequencing, ALK, failure of ALK TKI, acquired resistance, decision impact
Citation: Raphael A, Onn A, Holtzman L, Dudnik J, Urban D, Kian W, Cohen AY, Moskovitz M, Zer A, Bar J, Rabinovich NM, Grynberg S, Oedegaard C, Agbarya A, Peled N, Shochat T and Dudnik E (2022) The Impact of Comprehensive Genomic Profiling (CGP) on the Decision-Making Process in the Treatment of ALK-Rearranged Advanced Non-Small Cell Lung Cancer (aNSCLC) After Failure of 2nd/3rd-Generation ALK Tyrosine Kinase Inhibitors (TKIs). Front. Oncol. 12:874712. doi: 10.3389/fonc.2022.874712
Received: 12 February 2022; Accepted: 11 April 2022;
Published: 13 May 2022.
Edited by:
Pasquale Pisapia, University of Naples Federico II, ItalyReviewed by:
Valerio Gristina, University of Palermo, ItalyIlaria Attili, European Institute of Oncology (IEO), Italy
Alessandro Russo, A.O. Papardo, Italy
Copyright © 2022 Raphael, Onn, Holtzman, Dudnik, Urban, Kian, Cohen, Moskovitz, Zer, Bar, Rabinovich, Grynberg, Oedegaard, Agbarya, Peled, Shochat and Dudnik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth Dudnik, ZWxpemFiZXRoLmR1ZG5pazE2MDNAZ21haWwuY29t
†These authors have contributed equally to this work
 Ari Raphael
Ari Raphael Amir Onn2,3†
Amir Onn2,3† Liran Holtzman
Liran Holtzman Damien Urban
Damien Urban Waleed Kian
Waleed Kian Jair Bar
Jair Bar Shirly Grynberg
Shirly Grynberg Abed Agbarya
Abed Agbarya Nir Peled
Nir Peled Elizabeth Dudnik
Elizabeth Dudnik