- 1Department of Radiation Oncology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 2BIH Charité Clinician Scientist Program, Berlin Institute of Health at Charité – Universitätsmedizin Berlin, BIH Biomedical Innovation Academy, Berlin, Germany
- 3Department of Nuclear Medicine, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 4PET Center, Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany
Background: PSMA PET is frequently used for staging of prostate cancer patients. Furthermore, there is increasing interest to use PET information for personalized local treatment approaches in surgery and radiotherapy, especially for focal treatment strategies. However, it is not well established which quantitative imaging parameters show highest correlation with clinical and histological tumor aggressiveness.
Methods: This is a retrospective analysis of 135 consecutive patients with non-metastatic prostate cancer and PSMA PET before any treatment. Clinical risk parameters (PSA values, Gleason score and D’Amico risk group) were correlated with quantitative PET parameters maximum standardized uptake value (SUVmax), mean SUV (SUVmean), tumor asphericity (ASP) and PSMA tumor volume (PSMA-TV).
Results: Most of the investigated imaging parameters were highly correlated with each other (correlation coefficients between 0.20 and 0.95). A low to moderate, however significant, correlation of imaging parameters with PSA values (0.19 to 0.45) and with Gleason scores (0.17 to 0.31) was observed for all parameters except ASP which did not show a significant correlation with Gleason score. Receiver operating characteristics for the detection of D’Amico high-risk patients showed poor to fair sensitivity and specificity for all investigated quantitative PSMA PET parameters (Areas under the curve (AUC) between 0.63 and 0.73). Comparison of AUC between quantitative PET parameters by DeLong test showed significant superiority of SUVmax compared to SUVmean for the detection of high-risk patients. None of the investigated imaging parameters significantly outperformed SUVmax.
Conclusion: Our data confirm prior publications with lower number of patients that reported moderate correlations of PSMA PET parameters with clinical risk factors. With the important limitation that Gleason scores were only biopsy-derived in this study, there is no indication that the investigated additional parameters deliver superior information compared to SUVmax.
Introduction
Various studies were able to show that Gallium-68-labelled prostate-specific membrane antigen (PSMA) positron emission tomography (PET) can improve nodal and distant staging of prostate cancer patients (1, 2). An additional benefit of PET imaging is that imaging parameters can be quantified, e.g., by the calculation of standardized uptake values (SUV), PSMA expressing tumor volume (PSMA-TV) and its derivatives. The maximum SUV (SUVmax) of tumor lesions has been shown to be prognostic for a plethora of diseases and tumor stages and various PET tracers, including the most commonly used tracer [18F]fluorodeoxyglucose (FDG) but also less frequently used tracers (3, 4). Recent studies reported that (semi-)quantitative PSMA parameters appear to be a promising prognostic parameter. These investigations were mainly performed in advanced metastatic disease with patients prior to PSMA radioligand treatment (5, 6). In these cohorts of patients, high PSMA uptake seems to be associated with adverse outcome. So far, no data is available for locally confined disease and primary staging of prostate cancer, probably due to the relatively short follow-up time with this novel radiotracer.
Regarding focal radiotherapy treatment escalation in non-metastatic primary prostate cancer patients, an important issue is the potential correlation between quantitative PSMA ligand uptake measures and tumor aggressiveness, e.g. its correlation with the histopathological defined Gleason score. Additional PET parameters could help in the decision for more personalized treatment options like focal radiation boost to tumors, which has shown promising results in magnetic resonance imaging (MRI) guided boost delineation and is currently investigated in PSMA based focal dose escalation trials (7–9). Only weak to moderate correlation has been observed between PSMA PET SUVmax during initial staging of prostate cancer and Gleason scores obtained by biopsy. Similar modest correlations were reported for serum PSA values and SUVmax (2, 10, 11). Most studies only investigated SUVmax and did not analyse further quantitative PET metrics. A novel quantitative PET parameter is tumor asphericity (ASP). ASP is a measure of tumor shape irregularity and has shown a strong association with patient outcome in various diseases and for different PET tracers (12–15). In a recent study with a relatively small number of patients, ASP from [68Ga]Ga-PSMA-11 PET was strongly associated with Gleason scores in patients with primary prostate cancer (16).
The aim of our study was to investigate the correlation between different quantitative PSMA parameters, including PSMA derived tumor volume (PSMA-TV) and ASP, with Gleason scores and PSA values and examine if one of these parameters outperforms SUVmax, especially regarding personalized treatment options of the primary tumour in patients without evidence of loco-regional or distant tumor lesions.
Patients and Methods
Patient Cohort
For this retrospective analysis, all patients that underwent [68Ga]Ga-PSMA-11 PET/CT imaging between January 2015 and December 2018 at a single tertiary hospital were screened for inclusion and exclusion criteria. Imaging findings and implications for staging of patients that were included until March 2018 have been previously published (17). For the current analysis, all additional consecutive patients with PSMA imaging until end of December 2018 were re-evaluated. Only treatment-naive patients without evidence for lymphonodal or distant metastases were included for further quantitative analyses. Since PSMA PET imaging is not part of the routine staging, referral for imaging was left at the discretion of the referring urologist or radiation oncologist. All except one patient had histologically confirmed prostate-cancer. The remaining patient had steadily rising PSA values during active surveillance, although repeated biopsies only revealed Gleason scores of 4. This patient was diagnosed with prostate cancer based on clinical findings (PSA increase, and characteristic findings in magnetic resonance imaging and PSMA PET/CT) and treated with radiotherapy.
Clinical Parameters
Clinical data were collected from patient files and electronic databases and included serological prostate-specific antigen (PSA) values, clinical T stage and Gleason scores obtained during biopsy prior to imaging. For a sub-group of patients that underwent surgery after PSMA PET imaging at the same institution, surgical Gleason scores were collected. Gleason scores were grouped following the recommendations of the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma (18). Patients were allocated to low, intermediate, or high-risk groups based on the established D’Amico risk classifier (19).
Image Acquisition
Imaging was performed as previously described (17). Briefly, PSMA PET/CT was performed with the radiotracer [68Ga]Ga-PSMA-11-HBED-CC on a dedicated PET/CT scanner (Gemini TF 16; Philips, Netherlands) with Philips Astonish TF technology. [68Ga]Ga-PSMA-11-HBED-CC was injected intravenously (median activity: 153 MBq; range: 71-227 MBq). PET imaging was performed after a median time of 98 minutes after injection (range: 39-188 minutes). Patients were placed in supine position and scanned from base of skull to the proximal femora (scan duration: 90 to 180 s per bed position; 3D acquisition mode; bed overlap: 53.3%). Attenuation correction was based on non-enhanced low-dose CT (automatic tube current modulation; maximum tube current-time product: 50 mA; tube voltage: 120 kV; gantry rotation time: 0.5 s) reconstructed with a slice thickness of 5 mm (convolution kernel: B08). PET raw data was reconstructed using iterative reconstruction with TOF analysis (Philips Astonish TF technology; BLOB-OS-TF; iterations: 3; subsets: 33). The projection data was reconstructed with 4 mm slice thickness (voxel size: 4×4×4 mm3) (17).
Image Evaluation
In a first step, a large spheric mask was placed around the prostate and base of seminal vesicles. The PSMA expressing part of the primary tumor was delineated inside this mask based on a threshold of 41% SUVmax as suggested by a recent analysis (20). The resulting volumes of interest (VOI) were inspected visually by an experienced observer (SZ), and tracer uptake of surrounding normal tissue (bladder and/or rectum) was manually excluded. Patients who exhibited only low or diffuse tracer accumulation in the respective lesion were manually delineated by selecting the most intense single voxel, the volume in these patients was regarded 0.1 ml. This was the case in four patients.
For the obtained VOIs, ASP was computed according to the following formula, where V is the volume of the VOI and S is its surface.
ASP is equal to zero for spheres. For non-spherical shapes ASP is higher than 0 and is a quantitative measure of the degree of deviation from a spherical shape.
In addition, the PSMA based tumor volume (PSMA-TV), the maximum standardized uptake value (SUVmax) and average standardized uptake value (SUVmean) and SUVpeak were calculated. SUVs were computed using the patients body weight. All VOI definitions and image analyses were performed using the ROVER software, version 3.0.41 (ABX, Radeberg, Germany).
Statistical Analyses
The nonparametric Spearman correlation was used for calculation of correlations between imaging and clinical parameters to avoid bias due to existing outliers (as depicted in Figures 1, 2). Receiver operating characteristics (ROC) curves were plotted to show sensitivity and specificity of each quantitative PET parameter for detection of high-risk prostate cancer (as defined by D’Amico criteria). Area under the curve (AUC) comparison between quantitative PET parameters were calculated using the DeLong test (MedCalc version 19.3, MedCalc Software Lt, Ostend, Belgium). All other statistical calculations and figure plots were performed using SPSS version 24 (IBM Corporation, Armonk, NY, USA).
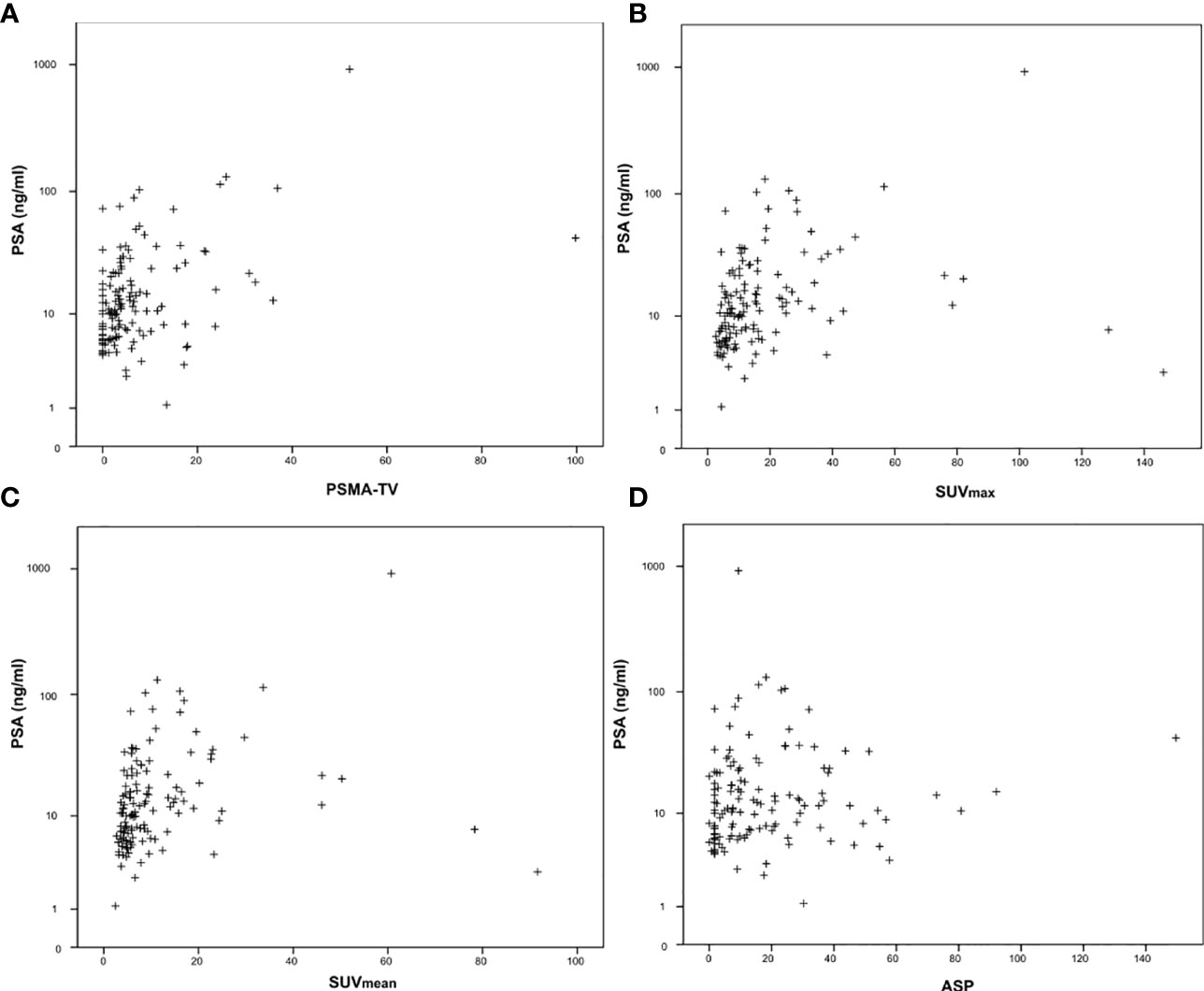
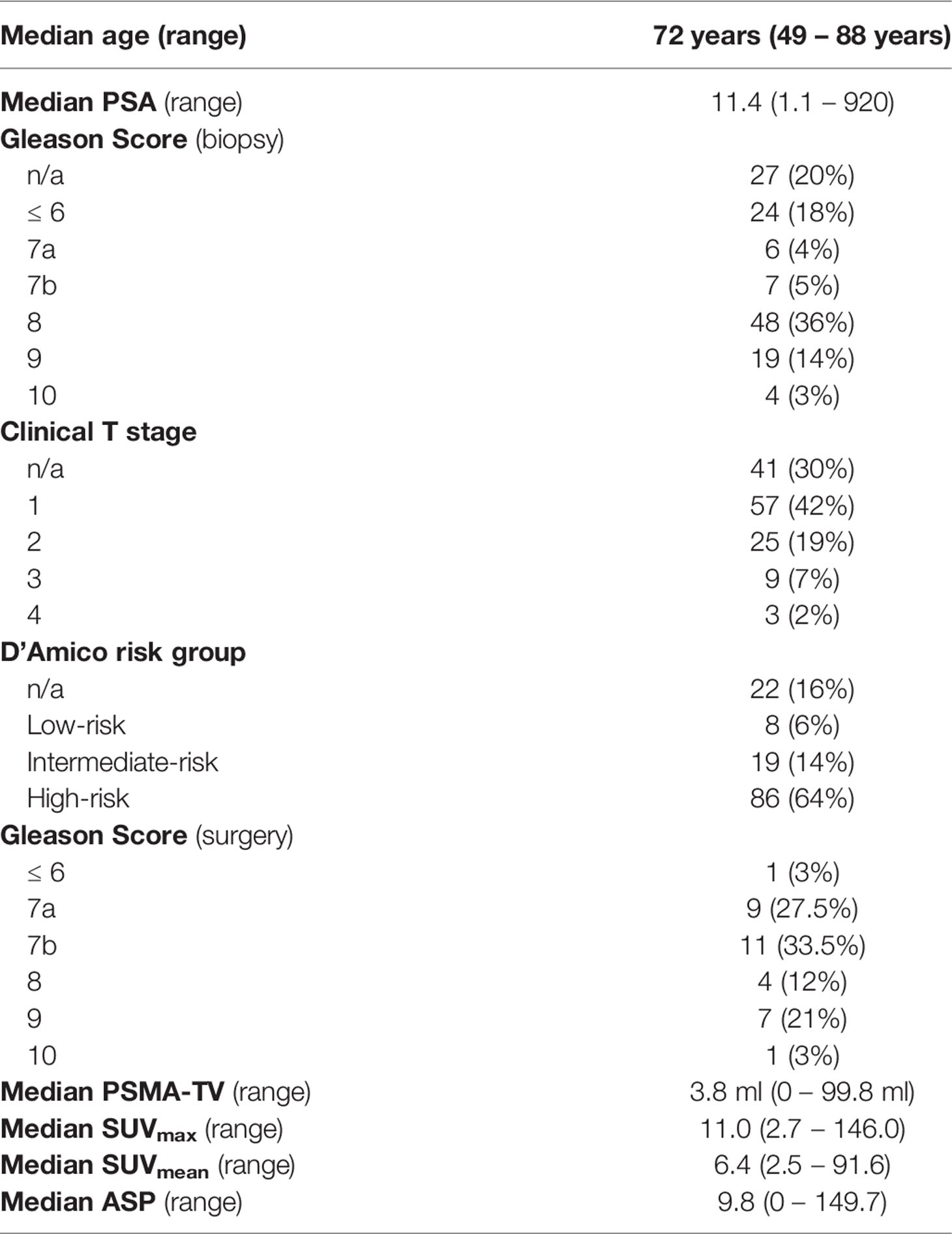
Figure 1 Correlation between serum prostate-specific antigen (PSA) values and quantitative PSMA-PET parameters. (A) PSMA-derived tumor volume (PSMA-TV), (B) Maximum standardized uptake value (SUVmax), (C) Mean standardized uptake value (SUVmean) and (D) Tumor asphericity (ASP). PSA values are plotted on a logarithmic scale.
Figure 2 Correlation between Gleason scores obtained by biopsy before imaging and quantitative PSMA-PET parameters. (A) PSMA-derived tumor volume (PSMA-TV), (B) Maximum standardized uptake value (SUVmax), (C) Mean standardized uptake value (SUVmean) and (D) Tumor asphericity (ASP).
Results
Most patients had high-risk prostate cancer. Table 1 summarizes clinical characteristics and quantitative imaging findings of the study cohort.
The investigated quantitative PSMA PET parameters were significantly inter-correlated with correlation coefficients between 0.20 and 0.95. The only exception was SUVmean and ASP, which were not significantly correlated (p = 0.79). Details are shown in Supplementary Table 1. Regarding correlation between quantitative parameters of the primary tumor and clinical parameters, a significant, however low to moderate correlation with initial serum PSA values (Spearman rho between 0.19 and 0.45, all p < 0.05; Table 2; Figure 1) was observed. Correlation with Gleason scores obtained by previous biopsy was slightly lower (Spearman rho between 0.17 and 0.31, all p < 0.05 except for ASP; Figure 2).
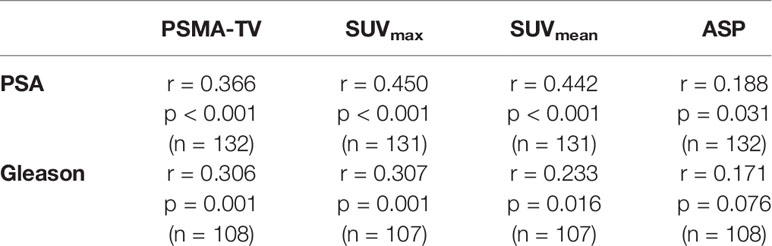
Table 2 Correlation between initial PSA values and biopsy-derived Gleason scores with quantitative PSMA-PET parameters.
Figure 3 shows the distribution of quantitative PET parameters for each Gleason score.
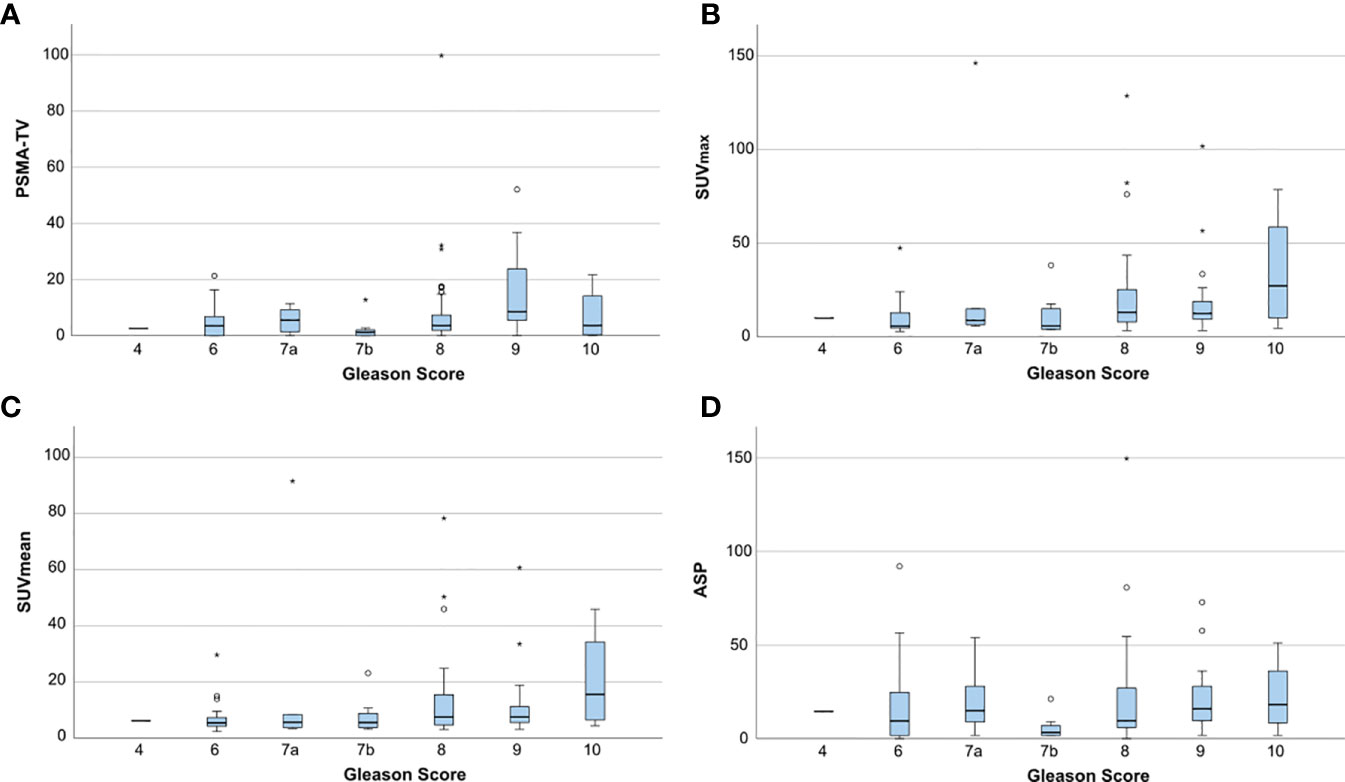
Figure 3 Boxplots showing the distribution of quantitative PET parameters for each Gleason score. (A) PSMA-derived tumor volume (PSMA-TV), (B) Maximum standardized uptake value (SUVmax), (C) Mean standardized uptake value (SUVmean) and (D) Tumor asphericity (ASP). Outliers are plotted as points (< 3 * interquartile range) or asterisks (> 3 * interquartile range).
AUC analysis regarding the differentiation of high-risk from low- or intermediate-risk prostate cancer patients revealed poor to fair sensitivity and specificity for all investigated imaging parameters. AUC plots are depicted in Figure 4 and the respective values are shown in Table 3. Comparison between AUC characteristics for different PET parameters showed that SUVmax is significantly better suited than SUVmean to predict high-risk prostate cancer (p = 0.035), no significant differences between other quantitative metrics could be observed as shown in Table 4. Additionally, SUVpeak was investigated in the whole cohort, SUVpeak showed a very high correlation with SUVmax (r = 0.99, p < 0.001) and similar results regarding all investigated endpoints as shown in Supplementary Figure 1.
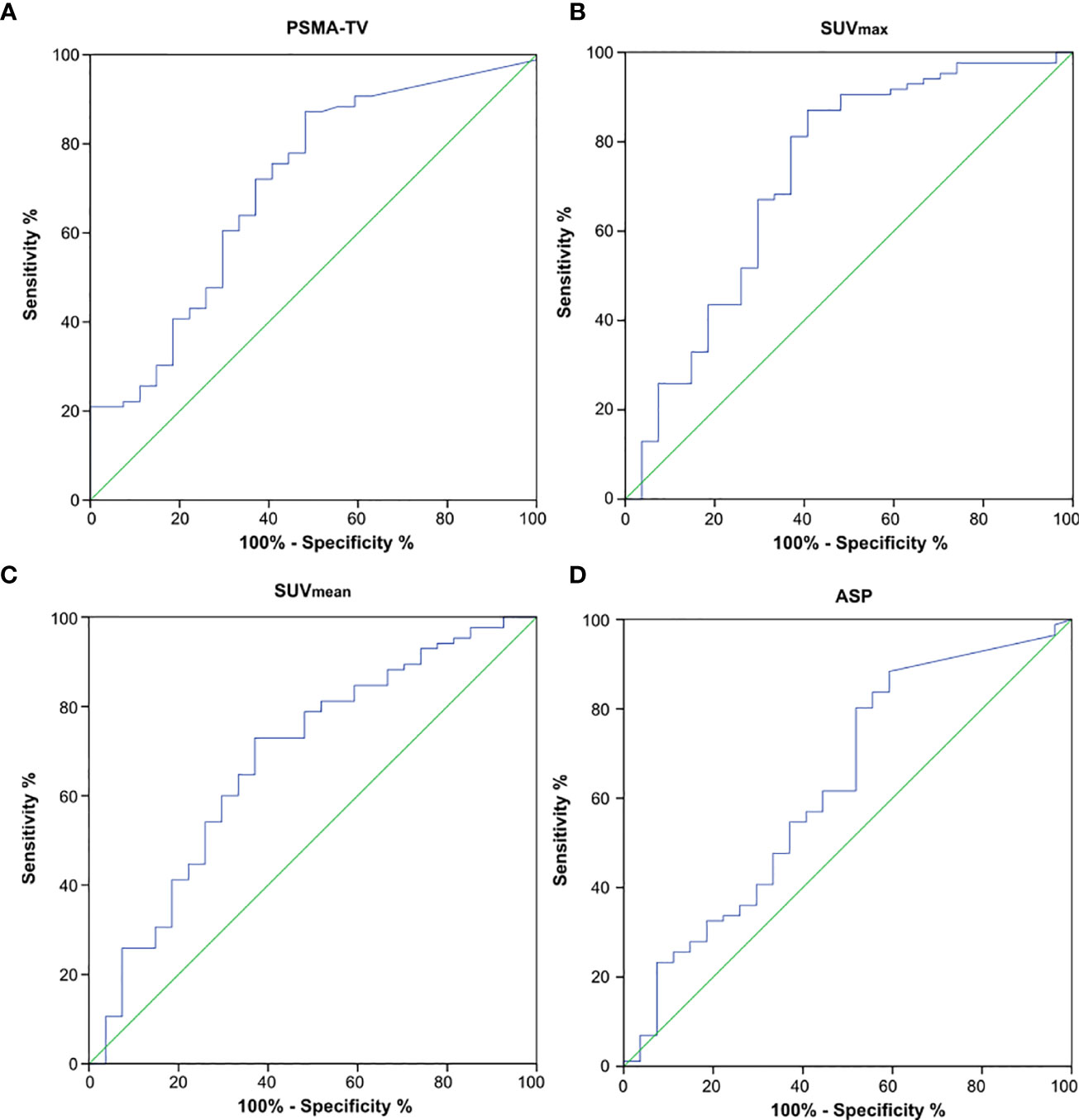
Figure 4 Receiver operating characteristics (ROC) curves to detect high-risk prostate cancer using quantitative PSMA-PET parameters. (A) PSMA-derived tumor volume (PSMA-TV), (B) Maximum standardized uptake value (SUVmax), (C) Mean standardized uptake value (SUVmean) and (D) Tumor asphericity (ASP).
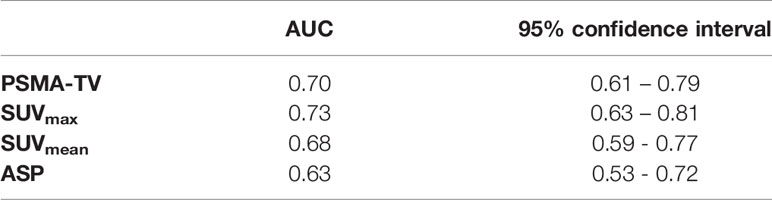
Table 3 Area under the curve (AUC) characteristics for the investigated quantitative PSMA-PET parameters to detect high-risk prostate cancer.
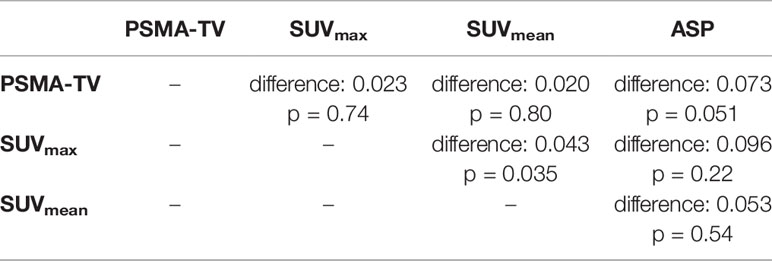
Table 4 Comparison of AUC characteristics to detect high-risk prostate cancer using the DeLong test.
Since Gleason scores obtained from biopsy might over- or underestimate surgically obtained Gleason scores of whole prostate specimens, a sub-group of 38 surgically treated patients was further evaluated. Similar correlation coefficients as in the main analysis (but with each p>0.05) were obtained between quantitative imaging parameters and surgical Gleason scores (Supplementary Table 2, Supplementary Figure 2).
Discussion
PSMA PET has shown great potential for focal treatment strategies. Bettermann and colleagues were able to show that PSMA PET-based tumor delineation is superior to MRI regarding the sensitivity to detect prostate cancer foci on whole mount histopathology specimens (21). Several studies are currently investigating focal treatment escalation by the implementation of PET imaging. Identification of the optimal imaging parameter as a surrogate for tumor aggressiveness is therefore an important need.
In this study, we examined the correlation between PET parameters and clinical risk factors in non-metastatic primary prostate cancer patients. We were able to validate prior publications that reported a moderate correlation between clinical risk parameters like Gleason score, PSA levels or D’Amico risk category and SUVmax of primary prostate tumors. Further analysis of additional quantitative PET parameters like ASP or PSMA-TV did not show superiority compared to SUVmax in this monocenter investigation. Only a moderate correlation of any investigated parameter with Gleason scores could be observed.
The reported correlation coefficients in our study are comparable with published data on correlations between SUVmax and Gleason scores that ranged between 0.096 and 0.5 and correlation coefficients between SUVmax and PSA values that ranged between 0.071 and 0.57 (2, 10, 11, 22–27). All but one of these studies reported lower numbers of patients, Supplementary Table 3 gives an overview of the published data on correlation coefficients.
Gleason scores of needle biopsies show discrepancies with surgical Gleason scores in up to 50% of cases, especially upgrading to higher Gleason scores is a frequent observation (28, 29). This can influence the observed correlations with quantitative PSMA metrics, probably underestimating the real Gleason score. Analysis of the patient sub-group that underwent surgery did not show any significant correlation between the investigated quantitative PET metrics and surgical Gleason grades. However, this is most likely due to the comparatively low number of patients in this sub-group, because correlation coefficients were similar to the correlation coefficients for biopsy-based Gleason scores.
Data on the correlation between quantitative PSMA PET metrics other than SUVmax and clinical risk factors are sparse. Meißner and colleagues reported a strong correlation between ASP and Gleason scores (rho 0.88) and a moderate correlation between tumor volume and Gleason scores (rho 0.51) in a small cohort of 37 patients (16). However, patients with lymphatic or distant metastases were not excluded in their analysis, the exact number of patients with extraprostatic lesions was unfortunately not reported. Hoberück et al. evaluated various quantitative PSMA PET metrics including SUVmax, SUVmean and PSMA-TV. In a small cohort of 21 patients with consecutive PSMA scans before and during androgen deprivation therapy, they observed a strong correlation between the investigated PET parameters and no superiority of a specific parameter (30). The same quantitative parameters were investigated by Schmidkonz et al. in patients with bone metastases. They reported that all quantitative metrics were higher for Gleason scores > 7, but did not provide further comparative details (31).
Our study has several limitations. First, the retrospective nature of the investigation with its known limitations. Second, no spatial correlation analyses with whole-mount histology was performed in surgically resected patients. Current analyses in this regard showed an excellent correlation of PET parameters with intraprostatic tumor foci (32, 33). Third, the used radiotracer might not be the best modality for local tumor assessment. The high urinary clearance of [68Ga]Ga-PSMA-11 hampers automatic delineation in close vicinity to the bladder. The necessary manual modifications are observer-dependent and might complicate independent reproducibility. Furthermore, high bladder uptake can potentially affect quantitative PET metrics of the prostate, e.g. by halo artifacts (34). The F-18-labeled PSMA-1007 radiotracer might be superior for evaluation of primary prostate cancer due to its favorable biodistribution, in particular lower bladder activity (35). Furthermore, SUVmax in primary prostate cancer lesions are systematically higher with [18F]F-PSMA-1007 compared to [68Ga]Ga-PSMA-11 (36). Nonetheless, a current meta-analysis was not able to show clear superiority of one of the specific PSMA radioligands in the recurrent situation (37). If prolonged uptake times are encountered in routine clinical care, [18F]F-PSMA-1007 could be advantageous over [68Ga]Ga-PSMA-11 by providing beneficial count statistics due to its longer physical half-life. Additionally, the higher positron range of Gallium-68 compared to Fluor-18 results in decreased spatial resolution, although Soderlund et al. observed only marginal differences using clinical PET scanners (38). The range of uptake times in the current analysis was relatively high, which might hamper inter-patient comparability of SUV. Lesion uptake of [68Ga]Ga-PSMA-11 increases over time after injection and has been described as approximately irreversible (39). However, the average increase in lesion SUV between 1h and 3h post injection has been reported to be moderate (25%) (40). The same PET scanner was used in all patients, which benefits comparability of PET parameters between patients. However, strictly speaking, applicability to other scanner models with different image properties and reconstruction methods would require dedicated analyses.
An important strength of our analysis is the restriction to patients without evidence of metastases by imaging including PSMA PET. Inclusion of metastatic patients might partly explain the high heterogeneity between previous publications, especially regarding correlation coefficients with PSA values (which is highly correlated with the total tumor volume). Additionally image evaluation was performed in a standardized fashion and with the observer being blinded to clinical risk parameters.
Overall, the observed association of the investigated quantitative imaging parameters with clinical risk factors is only fair. Novel methods like radiomics might be more suitable to detect high-risk sub-volumes within the prostate (41, 42).
In summary, this comprehensive analysis of quantitative PSMA PET metrics confirms prior studies that showed a moderate correlation with clinical risk factors. All investigated quantitative PET metrics intercorrelated and showed similar association with Gleason score, PSA values or D’Amico risk groups. The widely used reporting of SUVmax only seems therefore reasonable for personalized treatment options like focal boost in primary prostate cancer. Further prospective studies in a large cohort are needed to confirm our results, especially regarding the outcome after PET-guided personalized treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethikkomission der Charité Universitätsmedizin Berlin, Germany. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
Study conception and design: SZ and KH. Drafting of manuscript: SZ and SA. Image processing and analysis: SZ, FH, and JR. Study Investigators: SZ, SA, HA, CF, JR, MB, FH, and KH. Interpretation of data: all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
HA declares research grants, travel grants, and lecture fees from Sirtex Medical Europe; HA confirms that none of the above funding sources were involved in the preparation of this paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.879089/full#supplementary-material
References
1. Petersen LJ, Zacho HD. PSMA PET for Primary Lymph Node Staging of Intermediate and High-Risk Prostate Cancer: An Expedited Systematic Review. Cancer Imaging (2020) 20:10. doi: 10.1186/s40644-020-0290-9
2. Cytawa W, Seitz AK, Kircher S, Fukushima K, Tran-Gia J, Schirbel A, et al. 68Ga-PSMA I&T PET/CT for Primary Staging of Prostate Cancer. Eur J Nucl Med Mol Imaging (2020) 47:168–77. doi: 10.1007/s00259-019-04524-z
3. Ambrosini V, Campana D, Polverari G, Peterle C, Diodato S, Ricci C, et al. Prognostic Value of 68Ga-DOTANOC PET/CT SUVmax in Patients With Neuroendocrine Tumors of the Pancreas. J Nucl Med (2015) 56:1843–8. doi: 10.2967/jnumed.115.162719
4. Huang K, Schatka I, Rogasch JMM, Lindquist RL, De Santis M, Erber B, et al. Explorative Analysis of a Score Predicting the Therapy Response of Patients With Metastatic, Castration Resistant Prostate Cancer Undergoing Radioligand Therapy With 177Lu-Labeled Prostate-Specific Membrane Antigen. Ann Nucl Med (2021) 35:314–20. doi: 10.1007/s12149-020-01567-3
5. Komek H, Can C, Yilmaz U, Altindag S. Prognostic Value of 68 Ga PSMA I&T PET/CT SUV Parameters on Survival Outcome in Advanced Prostat Cancer. Ann Nucl Med (2018) 32:542–52. doi: 10.1007/s12149-018-1277-5
6. Vlachostergios PJ, Niaz MJ, Sun M, Mosallaie SA, Thomas C, Christos PJ, et al. Prostate-Specific Membrane Antigen Uptake and Survival in Metastatic Castration-Resistant Prostate Cancer. Front Oncol (2021) 11:630589. doi: 10.3389/fonc.2021.630589
7. Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol (2021) 39:787–96. doi: 10.1200/JCO.20.02873
8. Nicholls L, Suh Y-E, Chapman E, Henderson D, Jones C, Morrison K, et al. Stereotactic Radiotherapy With Focal Boost for Intermediate and High-Risk Prostate Cancer: Initial Results of the SPARC Trial. Clin Transl Radiat Oncol (2020) 25:88–93. doi: 10.1016/j.ctro.2020.10.004
9. Zamboglou C, Spohn SKB, Adebahr S, Huber M, Kirste S, Sprave T, et al. PSMA-PET/MRI-Based Focal Dose Escalation in Patients With Primary Prostate Cancer Treated With Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial. Cancers (Basel) (2021) 13:5795. doi: 10.3390/cancers13225795
10. Hong J-J, Liu B, Wang Z-Q, Tang K, Ji X-W, Yin W-W, et al. The Value of 18F-PSMA-1007 PET/CT in Identifying Non-Metastatic High-Risk Prostate Cancer. EJNMMI Res (2020) 10:138. doi: 10.1186/s13550-020-00730-1
11. Demirci E, Kabasakal L, Şahin OE, Akgün E, Gültekin MH, Doğanca T, et al. Can SUVmax Values of Ga-68-PSMA PET/CT Scan Predict the Clinically Significant Prostate Cancer? Nucl Med Commun (2019) 40:86–91. doi: 10.1097/MNM.0000000000000942
12. Apostolova I, Steffen IG, Wedel F, Lougovski A, Marnitz S, Derlin T, et al. Asphericity of Pretherapeutic Tumour FDG Uptake Provides Independent Prognostic Value in Head-and-Neck Cancer. Eur Radiol (2014) 24:2077–87. doi: 10.1007/s00330-014-3269-8
13. Hofheinz F, Lougovski A, Zöphel K, Hentschel M, Steffen IG, Apostolova I, et al. Increased Evidence for the Prognostic Value of Primary Tumor Asphericity in Pretherapeutic FDG PET for Risk Stratification in Patients With Head and Neck Cancer. Eur J Nucl Med Mol Imaging (2015) 42:429–37. doi: 10.1007/s00259-014-2953-x
14. Zschaeck S, Li Y, Lin Q, Beck M, Amthauer H, Bauersachs L, et al. Prognostic Value of Baseline [18F]-Fluorodeoxyglucose Positron Emission Tomography Parameters MTV, TLG and Asphericity in an International Multicenter Cohort of Nasopharyngeal Carcinoma Patients. PloS One (2020) 15:e0236841. doi: 10.1371/journal.pone.0236841
15. Wetz C, Rogasch J, Genseke P, Schatka I, Furth C, Kreissl M, et al. Asphericity of Somatostatin Receptor Expression in Neuroendocrine Tumors: An Innovative Predictor of Outcome in Everolimus Treatment? Diagnostic (Basel) (2020) 10:E732. doi: 10.3390/diagnostics10090732
16. Meißner S, Janssen J-C, Prasad V, Brenner W, Diederichs G, Hamm B, et al. Potential of Asphericity as a Novel Diagnostic Parameter in the Evaluation of Patients With 68Ga-PSMA-HBED-CC PET-Positive Prostate Cancer Lesions. EJNMMI Res (2017) 7:85. doi: 10.1186/s13550-017-0333-9
17. Rogasch JM, Cash H, Zschaeck S, Elezkurtaj S, Brenner W, Hamm B, et al. Ga-68-PSMA PET/CT in Treatment-Naïve Patients With Prostate Cancer: Which Clinical Parameters and Risk Stratification Systems Best Predict PSMA-Positive Metastases? Prostate (2018) 78(14):1103–10. doi: 10.1002/pros.23685
18. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol (2016) 40:244–52. doi: 10.1097/PAS.0000000000000530
19. D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA (1998) 280:969–74. doi: 10.1001/jama.280.11.969
20. Draulans C, De Roover R, van der Heide UA, Kerkmeijer L, Smeenk RJ, Pos F, et al. Optimal 68ga-PSMA and 18F-PSMA PET Window Levelling for Gross Tumour Volume Delineation in Primary Prostate Cancer. Eur J Nucl Med Mol Imaging (2021) 48:1211–8. doi: 10.1007/s00259-020-05059-4
21. Bettermann AS, Zamboglou C, Kiefer S, Jilg CA, Spohn S, Kranz-Rudolph J, et al. [68ga-]PSMA-11 PET/CT and Multiparametric MRI for Gross Tumor Volume Delineation in a Slice by Slice Analysis With Whole Mount Histopathology as a Reference Standard - Implications for Focal Radiotherapy Planning in Primary Prostate Cancer. Radiother Oncol (2019) 141:214–9. doi: 10.1016/j.radonc.2019.07.005
22. Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in Primary Staging of Prostate Cancer: PSA and Gleason Score Predict the Intensity of Tracer Accumulation in the Primary Tumour. Eur J Nucl Med Mol Imaging (2017) 44:941–9. doi: 10.1007/s00259-017-3631-6
23. Liu C, Liu T, Zhang N, Liu Y, Li N, Du P, et al. 68Ga-PSMA-617 PET/CT: A Promising New Technique for Predicting Risk Stratification and Metastatic Risk of Prostate Cancer Patients. Eur J Nucl Med Mol Imaging (2018) 45:1852–61. doi: 10.1007/s00259-018-4037-9
24. Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol (2016) 70:829–36. doi: 10.1016/j.eururo.2015.12.053
25. Jena A, Taneja R, Taneja S, Singh A, Kumar V, Agarwal A, et al. Improving Diagnosis of Primary Prostate Cancer With Combined 68ga-Prostate-Specific Membrane Antigen-HBED-CC Simultaneous PET and Multiparametric MRI and Clinical Parameters. AJR Am J Roentgenol (2018) 211:1246–53. doi: 10.2214/AJR.18.19585
26. Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA-11 Dynamic PET/CT Imaging in Primary Prostate Cancer. Clin Nucl Med (2016) 41:e473–9. doi: 10.1097/RLU.0000000000001349
27. Donato P, Roberts MJ, Morton A, Kyle S, Coughlin G, Esler R, et al. Improved Specificity With 68Ga PSMA PET/CT to Detect Clinically Significant Lesions “Invisible” on Multiparametric MRI of the Prostate: A Single Institution Comparative Analysis With Radical Prostatectomy Histology. Eur J Nucl Med Mol Imaging (2019) 46:20–30. doi: 10.1007/s00259-018-4160-7
28. Kvåle R, Møller B, Wahlqvist R, Fosså SD, Berner A, Busch C, et al. Concordance Between Gleason Scores of Needle Biopsies and Radical Prostatectomy Specimens: A Population-Based Study. BJU Int (2009) 103:1647–54. doi: 10.1111/j.1464-410X.2008.08255.x
29. Mian BM, Lehr DJ, Moore CK, Fisher HAG, Kaufman RP, Ross JS, et al. Role of Prostate Biopsy Schemes in Accurate Prediction of Gleason Scores. Urology (2006) 67:379–83. doi: 10.1016/j.urology.2005.08.018
30. Hoberück S, Löck S, Winzer R, Zöphel K, Froehner M, Fedders D, et al. [68ga]Ga-PSMA-11 PET Before and After Initial Long-Term Androgen Deprivation in Patients With Newly Diagnosed Prostate Cancer: A Retrospective Single-Center Study. EJNMMI Res (2020) 10:135. doi: 10.1186/s13550-020-00723-0
31. Schmidkonz C, Cordes M, Goetz TI, Prante O, Kuwert T, Ritt P, et al. 68Ga-PSMA-11 PET/CT Derived Quantitative Volumetric Tumor Parameters for Classification and Evaluation of Therapeutic Response of Bone Metastases in Prostate Cancer Patients. Ann Nucl Med (2019) 33:766–75. doi: 10.1007/s12149-019-01387-0
32. Zamboglou C, Kramer M, Kiefer S, Bronsert P, Ceci L, Sigle A, et al. The Impact of the Co-Registration Technique and Analysis Methodology in Comparison Studies Between Advanced Imaging Modalities and Whole-Mount-Histology Reference in Primary Prostate Cancer. Sci Rep (2021) 11:5836. doi: 10.1038/s41598-021-85028-5
33. Spohn SKB, Kramer M, Kiefer S, Bronsert P, Sigle A, Schultze-Seemann W, et al. Comparison of Manual and Semi-Automatic [18f]PSMA-1007 PET Based Contouring Techniques for Intraprostatic Tumor Delineation in Patients With Primary Prostate Cancer and Validation With Histopathology as Standard of Reference. Front Oncol (2020) 10:600690. doi: 10.3389/fonc.2020.600690
34. Heußer T, Mann P, Rank CM, Schäfer M, Dimitrakopoulou-Strauss A, Schlemmer H-P, et al. Investigation of the Halo-Artifact in 68Ga-PSMA-11-PET/MRI. PloS One (2017) 12:e0183329. doi: 10.1371/journal.pone.0183329
35. Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 Labelled PSMA-1007: Biodistribution, Radiation Dosimetry and Histopathological Validation of Tumor Lesions in Prostate Cancer Patients. Eur J Nucl Med Mol Imaging (2017) 44:678–88. doi: 10.1007/s00259-016-3573-4
36. Kuten J, Fahoum I, Savin Z, Shamni O, Gitstein G, Hershkovitz D, et al. Head-To-Head Comparison of 68Ga-PSMA-11 With 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J Nucl Med (2020) 61:527–32. doi: 10.2967/jnumed.119.234187
37. Alberts IL, Seide SE, Mingels C, Bohn KP, Shi K, Zacho HD, et al. Comparing the Diagnostic Performance of Radiotracers in Recurrent Prostate Cancer: A Systematic Review and Network Meta-Analysis. Eur J Nucl Med Mol Imaging (2021) 48:2978–89. doi: 10.1007/s00259-021-05210-9
38. Soderlund AT, Chaal J, Tjio G, Totman JJ, Conti M, Townsend DW. Beyond 18f-FDG: Characterization of PET/CT and PET/MR Scanners for a Comprehensive Set of Positron Emitters of Growing Application–18F, 11C, 89Zr, 124I, 68Ga, and 90Y. J Nucl Med (2015) 56:1285–91. doi: 10.2967/jnumed.115.156711
39. Ringheim A, Campos Neto G de C, Anazodo U, Cui L, da Cunha ML, Vitor T, et al. Kinetic Modeling of 68Ga-PSMA-11 and Validation of Simplified Methods for Quantification in Primary Prostate Cancer Patients. EJNMMI Res (2020) 10:12. doi: 10.1186/s13550-020-0594-6
40. Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET Imaging With a [68Ga]Gallium-Labelled PSMA Ligand for the Diagnosis of Prostate Cancer: Biodistribution in Humans and First Evaluation of Tumour Lesions. Eur J Nucl Med Mol Imaging (2013) 40:486–95. doi: 10.1007/s00259-012-2298-2
41. Zamboglou C, Carles M, Fechter T, Kiefer S, Reichel K, Fassbender TF, et al. Radiomic Features From PSMA PET for Non-Invasive Intraprostatic Tumor Discrimination and Characterization in Patients With Intermediate- and High-Risk Prostate Cancer - A Comparison Study With Histology Reference. Theranostics (2019) 9:2595–605. doi: 10.7150/thno.32376
Keywords: PSMA, prostate specific membrane antigen, positron emission tomography, primary prostate cancer, quantitative PET parameters
Citation: Zschaeck S, Andela SB, Amthauer H, Furth C, Rogasch JM, Beck M, Hofheinz F and Huang K (2022) Correlation Between Quantitative PSMA PET Parameters and Clinical Risk Factors in Non-Metastatic Primary Prostate Cancer Patients. Front. Oncol. 12:879089. doi: 10.3389/fonc.2022.879089
Received: 18 February 2022; Accepted: 28 March 2022;
Published: 22 April 2022.
Edited by:
Constantinos Zamboglou, University of Freiburg Medical Center, GermanyReviewed by:
Kerstin Michalski, University of Freiburg, GermanySebastian Hoberück, University Hospital Carl Gustav Carus, Germany
Copyright © 2022 Zschaeck, Andela, Amthauer, Furth, Rogasch, Beck, Hofheinz and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Zschaeck, U2ViYXN0aWFuLlpzY2hhZWNrQGNoYXJpdGUuZGU=; orcid.org/0000-0003-3109-0662
†These authors have contributed equally to this work
 Sebastian Zschaeck
Sebastian Zschaeck Stephanie Bela Andela1†
Stephanie Bela Andela1† Christian Furth
Christian Furth Julian M. Rogasch
Julian M. Rogasch Frank Hofheinz
Frank Hofheinz Kai Huang
Kai Huang