Abstract
EGFR mutations are the most important drivers of gene alterations in lung adenocarcinomas and are sensitive to EGFR-TKIs. However, resistance to EGFR-TKIs is inevitable in the majority of EGFR-mutated lung cancer patients. Numerous resistant mechanisms have been revealed to date, and more are still under investigation. Owing to the selective pressure, intratumoral heterogeneity may exist after resistance, especially in patients after multiple lines of treatment. For those patients, it is important to choose therapies focused on the trunk/major clone of the tumor in order to achieve optimal clinical benefit. Here, we will report an EGFR-mutated lung adenocarcinoma patient with heterogeneity of resistant mechanisms including EGFR amplification, large fragment deletion of RB1, and histological transformations after targeted treatments. In our case, EGFR amplification seemed to be the major clone of the resistant mechanism according to the next-generation sequencing (NGS) results of both liquid biopsy monitoring and tissue biopsies. In consideration of the high EGFR amplification level, the patient was administered by combination treatment with EGFR-TKI plus nimotuzumab, an anti-EGFR monoclonal antibody (mAb), and achieved a certain degree of clinical benefit. Our case sheds light on the treatment of EGFR-mutant patients with EGFR amplification and indicates that a combination of EGFR-TKI with anti-EGFR mAb might be one of the possible treatment options based on genetic tests. Moreover, the decision on therapeutic approaches should focus on the major clone of the tumor and should make timely adjustments according to the dynamic changes of genetic characteristics during treatment.
Introduction
EGFR mutations are the most important driver of gene alterations in lung adenocarcinoma (LUAD) patients, especially in Asian non-smoking women (1). EGFR-TKIs are normally highly effective in patients with EGFR mutations. However, acquired resistance would eventually occur in the majority of patients. Among multiple mechanisms leading to EGFR-TKI resistance, the T790M mutation is most commonly found in subsequent first- and second-generation EGFR-TKIs (2, 3). Other resistant mechanisms, such as bypass activation and histological transformations, have also been reported (2, 4–6). Moreover, individual reports have also indicated that EGFR amplification contributes to acquired resistance to EGFR-TKIs (7–9). According to the AURA study, EGFR amplification occurred in 5.3% (1/19) of patients who developed resistance to first-line osimertinib (10). EGFR amplification was found in 42.9% (3/7) of T790M-positive tissues after resistance to third-generation tyrosine kinase inhibitor (TKI) (11).
Owing to the selective pressure, intratumoral heterogeneity may exist after resistance, especially in patients after multiple lines of treatment. Overcoming tumor heterogeneity is a major challenge for cancer treatment. Several studies have demonstrated that higher levels of heterogeneity in lung cancer predict inferior responses to anticancer therapies, including targeted therapy and immunotherapy (12, 13). For patients with intratumoral heterogeneity, it is important to choose therapies focused on the trunk/major clone in the tumor to achieve optimal clinical benefit. Herein, we report a LUAD patient with heterogeneity of resistant mechanisms to EGFR-TKIs, mainly based on EGFR amplification, who was successfully treated with a combination treatment containing nimotuzumab and EGFR-TKIs subsequently. We will present the following case in accordance with the CARE reporting checklist.
Case description
A 53-year-old Chinese man was found to have an occupying lesion in the upper lobe of the right lung during a routine checkup in March 2017. Positron emission tomography–computed tomography (PET-CT) showed multiple metastatic nodules in the hilum of the lung, lymph nodes, bone, and brain. The patient underwent tissue biopsy through a bronchoscope and was diagnosed with stage IV LUAD (Figure 1A). Next-generation sequencing (NGS) was performed using the tissue for driver gene alteration testing and revealed mutations of EGFR L858R and TP53 R280T. Physical examination in this patient suggested no significant abnormalities except that the Karnofsky performance status (KPS) was 70. Laboratory findings were within the normal range, except for the carcinoembryonic antigen (CEA) level of 10.87 ng/ml (normal range, 0 to 5 ng/ml) in the serum. Other exams showed no positive signs at diagnosis.
Figure 1
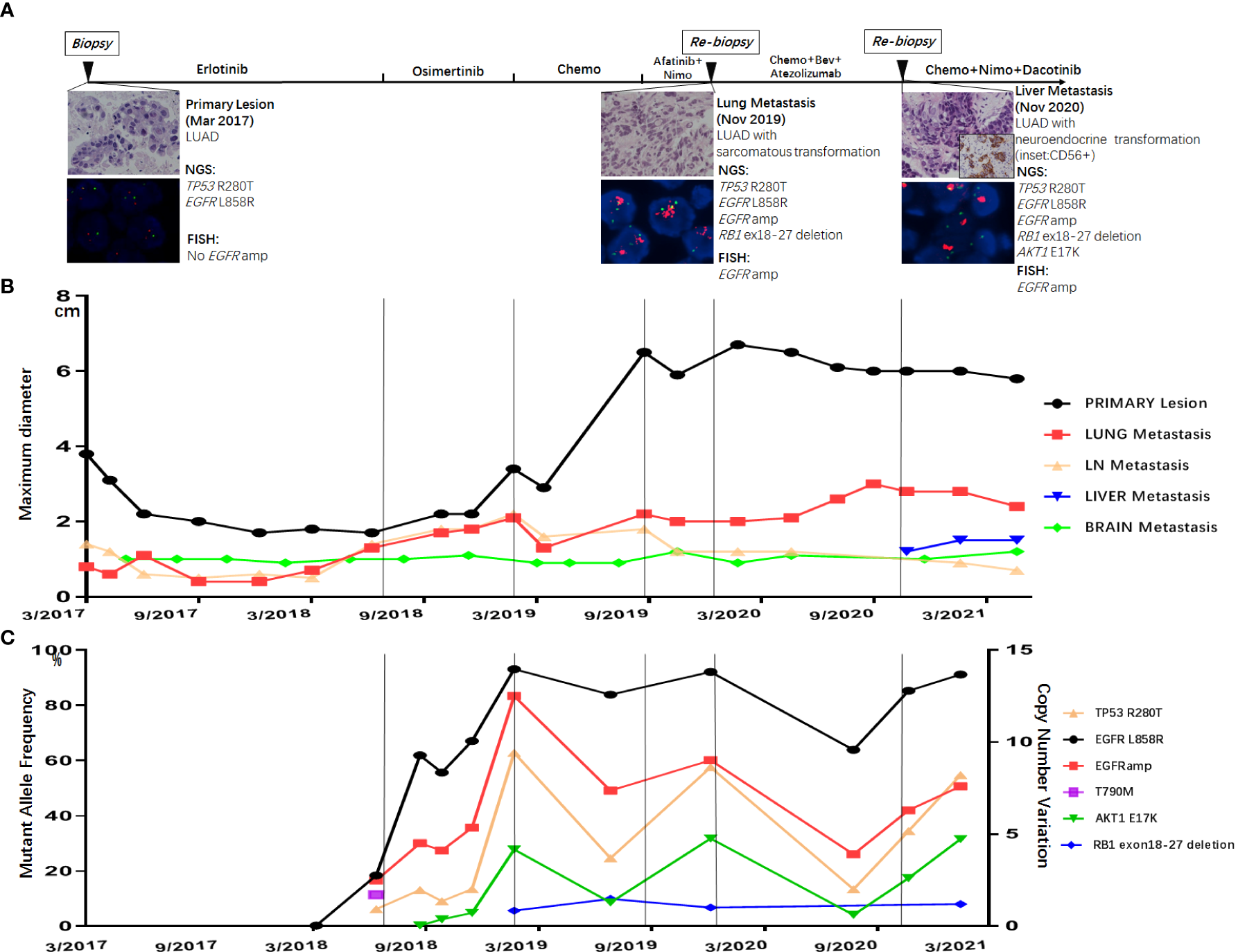
(A) Treatment timeline of the patient, histological morphology, and genetic results of three tissue biopsies (primary lesion in March 2017, lung metastasis in November 2019, and liver metastasis in November 2020). Dynamic monitoring of radiological results (B) and circulating tumor DNA using next-generation sequencing (NGS) (C).
The patients started with erlotinib 150 mg once daily as first-line therapy. After 16 months of erlotinib treatment, CT scans showed progressed disease (PD) due to the enlargement of lung metastatic nodules (Figure 1B). Liquid biopsy using plasma detected T790M along with L858R mutation, as well as EGFR amplification (Figure 1C). The patient started osimertinib treatment at 80 mg once daily as second-line therapy, and liquid biopsy monitoring only detected L858R and EGFR amplification 2 months after osimertinib, with no T790M retained. Osimertinib treatment lasted for 7 months before the resistance occurred (Figure 1B). Liquid biopsy after osimertinib resistance showed L858R mutation with high EGFR amplification. Moreover, AKT1 E17K and a large fragment deletion of RB1 (exon18–27) were also detected (Figure 1C). The patient then received pemetrexed plus platinum for six cycles. CT scans showed rapid growth of the primary lesion in September 2019 (Figure 1B). Taking the high level of EGFR amplification into consideration, the patient was administered by combination treatment with afatinib plus nimotuzumab and had stable disease (SD) with a slight shrinking of all the tumor sites including the primary and metastatic lesions. The combination treatment of afatinib and nimotuzumab lasted for 3.7 months, and then the patient underwent a re-biopsy in November 2019. Tissue biopsy guided by CT of metastatic lung lesion showed LUAD with sarcomatoid differentiation (Figure 1A). Then the patient asked for chemotherapy plus immunotherapy (bevacizumab + Abraxane + atezolizumab), and the therapy lasted for 10.4 months. In November 2020, a new liver metastatic lesion was found and was confirmed to be adenocarcinoma with neuroendocrine differentiation via tissue biopsy (Figure 1A). The NGS test showed different results in the lung and liver metastatic lesions: only the liver lesion was detected with AKT1 E17K, while both harboring L858R, TP53 R280T, EGFR amplification, and a large fragment deletion of RB1 (exon18–27). Since then, the patient received a combination treatment of irinotecan, nimotuzumab, and dacomitinib with optimal efficacy of SD. The combination therapy has caused grade 3 hepatic disorders, represented as increased alanine aminotransferase (ALT), aspartate aminotransferase (ALP), and aspartate aminotransferase (AST) compared to baseline examination before the treatment, based on the Common Terminology Criteria for Adverse Events (CTCAE; version 5.0).
EGFR amplification was validated by fluorescence in situ hybridization (FISH) via tissue biopsies, including pre-treatment biopsies from primary tumors and post-treatment re-biopsies from lung and liver metastasis lesions. FISH signal accounts (copy number) were recorded for a total of 50 nuclei, and the tumor was considered EGFR amplification when EGFR/CEP7 ratio was greater than or equal to 2 in 15% of recorded cells (14). Figure 1A shows that FISH revealed EGFR amplification in the metastatic lesions of both lung and liver, while no EGFR amplification was observed in the primary lesion. Dynamic monitoring of radiological results and liquid biopsy NGS results are shown in Figures 1B, C. The study was approved by the institutional review board of the Cancer Hospital, Chinese Academy of Cancer Science (CAMS). Written informed consent was signed by the patient. The images of chest CT scans at different timepoints are shown in Figure 2.
Figure 2
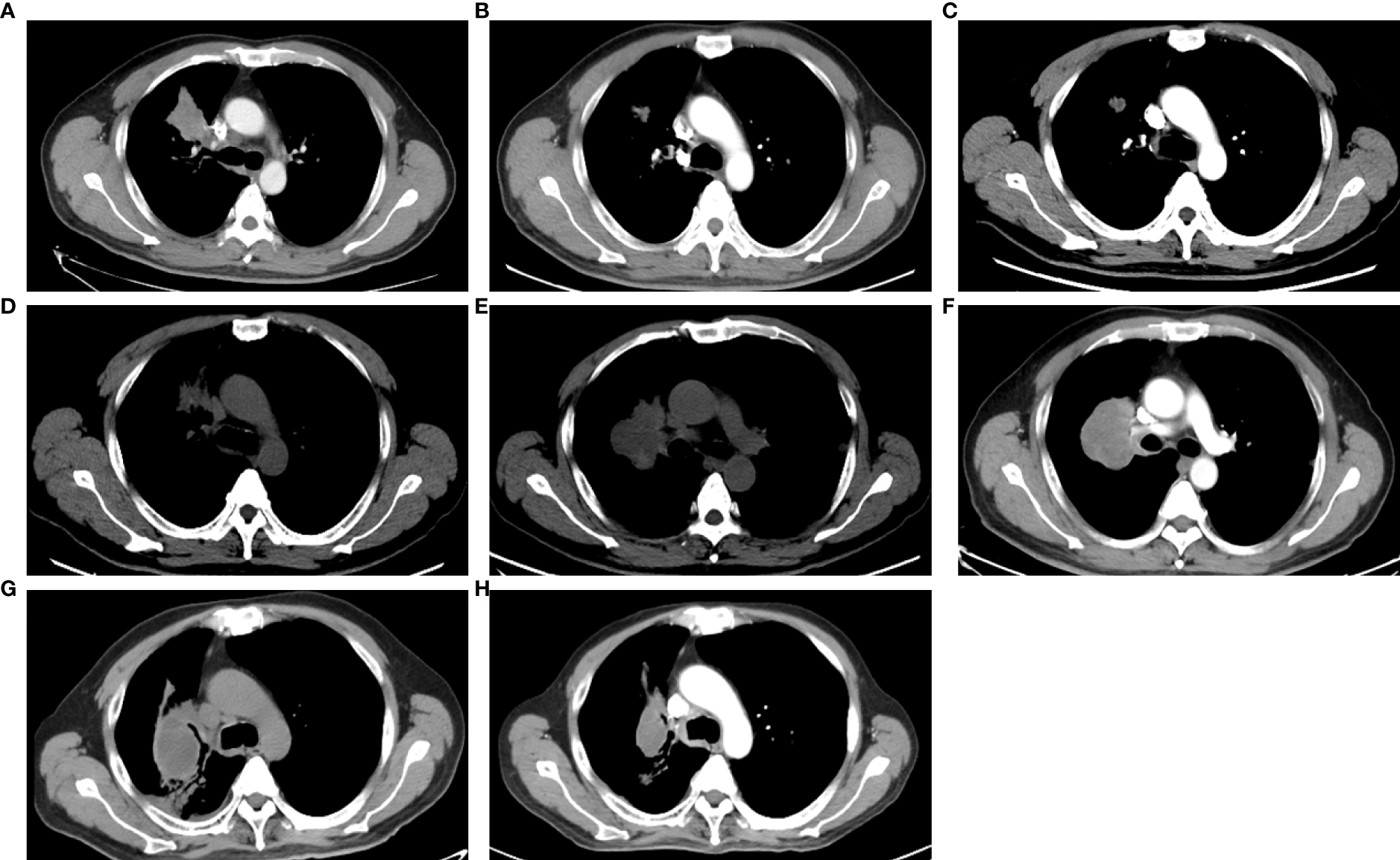
The images of CT scans with different treatments. (A) CT scan of basic examination before first-line erlotinib. (B) CT scan during the first-line erlotinib treatment. (C) CT scan at the progression of first-line erlotinib. (D) CT scan at progression of second-line osimertinib. (E) CT scan at the progression of chemotherapy. (F) CT scan at progression of afatinib plus nimotuzumab. (G) CT scan at the progression of immunotherapy. (H) CT scan during combination therapy of irinotecan, nimotuzumab, and dacomitinib.
The combination treatment of irinotecan, nimotuzumab, and dacomitinib remained for 8.6 months with continued disease control before the patient died in July 2021 due to tumor progression and complications. The PFS of different treatments is listed in Table S1.
Discussion
Resistance to EGFR-TKIs is inevitable, and numerous mechanisms have been discovered to date. Previously studies have described heterogeneity of resistant mechanisms after EGFR-TKI treatment. Roper et al. reported that 73% of osimertinib relapsed patients harbored at least two co-existing resistant mechanisms (15). Chabon et al. reported that intra-patient heterogeneity was observed in 46% of patients after first-line EGFR-TKI and 33% of patients after osimertinib treatment, according to circulating tumor DNA analysis (12). Our case emphasized the tumor spatial and temporal heterogeneity (Figure 3) by adequate investigation of tissue biopsies and liquid biopsy monitoring. Although the resistant mechanisms showed heterogeneity in our patients, EGFR amplification seemed to be the major clone of the resistant mechanism. Since EGFR amplification was observed early in liquid biopsy monitoring and all tissue biopsies after resistance harbored EGFR amplification, we speculated that other resistant mechanisms, such as large fragment deletion of RB1 and histological transformation, were all divergent propagation of subclones from EGFR amplification. To achieve the optimal clinical benefit, it is important to choose therapies focused on EGFR amplification for this patient. Combination approaches that target heterogeneous tumor clones have been proved to be successful in pre-clinical studies (16). Nimotuzumab, an anti-EGFR monoclonal antibody (mAb), was used to treat EGFR overexpression cancers including glioma (17), squamous cell carcinomas of the head and neck (18), non-small cell lung cancer (NSCLC) (19), and other tumors of epithelial origin (20). It was also reported to have superior antitumor activity when combined with EGFR-TKI such as afatinib (21) in EGFR-mutant lung cancer. In our case, combination treatment of EGFR-TKI (afatinib or dacomitinib) plus nimotuzumab showed efficacy to some extent and significantly inhibited the rapid growth of both primary and metastasis lesions (Figure 1B). Other EGFR mAbs have been also evaluated in lung cancer for a long time, and EGFR amplification may be a predictive factor of EGFR mAb such as cetuximab and necitumumab plus chemotherapy (22, 23). In gastrointestinal cancers such as colorectal cancer, panitumumab, another EGFR mAb, has also been proved to be effective for patients with EGFR amplification (24, 25). However, evidence of the combination treatment of EGFR mAb plus EGFR-TKI for patients with both EGFR mutation and amplification has been scarce.
Figure 3
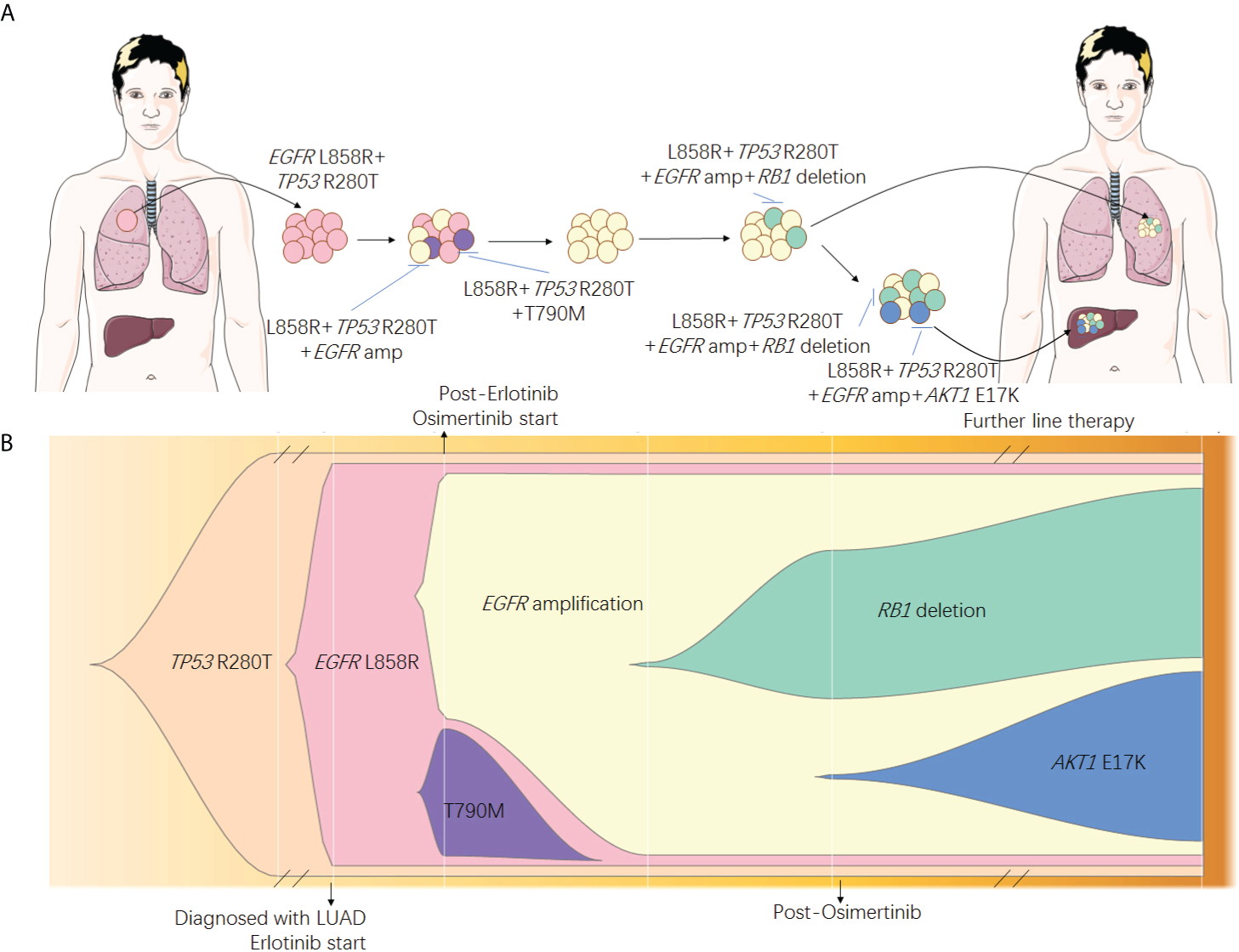
Speculated spatial heterogeneity (A) and evolutionary spectrum (B) of the patient.
It is noticed that patients underwent chemotherapy plus immunotherapy (bevacizumab + Abraxane + atezolizumab) for 10.4 months subsequent to standard treatment and received stable disease. The relationship between immunotherapy and driver mutations has long been a research hotspot. A previous meta-analysis study showed that immunotherapy could not enhance overall survival (OS) or progression-free survival (PFS) in EGFR-mutant patients (26). At the same time, IMpower 150 reported a prolonged OS in EGFR-mutated patients with atezolizumab plus chemotherapy and bevacizumab (27). In our study, the EGFR-mutant patient did benefit from immunotherapy after multiple lines of treatments. Thus, further studies are needed to effectively evaluate the efficacy of immunotherapy for NSCLC individuals with EGFR mutations.
Our case sheds light on the treatment of EGFR-mutant patients with EGFR amplification and indicates that a combination of EGFR-TKI with anti-EGFR mAb might be one of the possible treatment options. Moreover, as there are more and more treatment options for LUAD, patients could survive with tumors for a longer time. It brings up another problem that intratumoral heterogeneity might be more complicated after multi-line treatments. Thus, importance should be attached to therapeutic approaches to the major clone of the tumor, and these approaches should be multidimensional and dynamic with timely adjustments according to the patients’ genetic characteristics.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (NSFC No. 81802294).
Acknowledgments
We also would like to thank the patient and his family for consenting to the present publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by institutional review board of the Cancer Hospital, CAMS. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JL was the patient’s physician, proposed the conception, collected the data for case presentation. YL conducted the literature search, performed the NGS tests for tissues and liquid biospies, and prepared the first draft of the manuscript. JY contributed to the conceptualization and contributed with final manuscript drafting. ZX conducted the follow-up and the revision of manuscript. TX was responsible for data analysis and figure drawing. PX contributed with final manuscript drafting. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.937282/full#supplementary-material
References
1
Rosell R Moran T Queralt C Porta R Cardenal F Camps C et al . Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med (2009) 361:958–67. doi: 10.1056/NEJMoa0904554
2
Sequist LV Waltman BA Dias-Santagata D Digumarthy S Turke AB Fidias P et al . Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med (2011) 3:75ra26. doi: 10.1126/scitranslmed.3002003
3
Yu HA Arcila ME Rekhtman N Sima CS Zakowski MF Pao W et al . Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res (2013) 19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246
4
Takezawa K Pirazzoli V Arcila ME Nebhan CA Song X de Stanchina E et al . HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discovery (2012) 2:922–33. doi: 10.1158/2159-8290.CD-12-0108
5
Engelman JA Zejnullahu K Mitsudomi T Song Y Hyland C Park JO et al . MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (2007) 316:1039–43. doi: 10.1126/science.1141478
6
Lee JK Lee J Kim S Kim S Youk J Park S et al . Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol (2017) 35:3065–74. doi: 10.1200/JCO.2016.71.9096
7
Ercan D Zejnullahu K Yonesaka K Xiao Y Capelletti M Rogers A et al . Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene (2010) 29:2346–56. doi: 10.1038/onc.2009.526
8
Nukaga S Yasuda H Tsuchihara K Hamamoto J Masuzawa K Kawada I et al . Amplification of EGFR wild-type alleles in non-small cell lung cancer cells confers acquired resistance to mutation-selective EGFR tyrosine kinase inhibitors. Cancer Res (2017) 77:2078–89. doi: 10.1158/0008-5472.CAN-16-2359
9
Knebel FH Bettoni F Shimada AK Cruz M Alessi JV Negrao MV et al . Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer (2017) 108:238–41. doi: 10.1016/j.lungcan.2017.04.004
10
Ramalingam SS Yang JC Lee CK Kurata T Kim DW John T et al . Osimertinib as first-line treatment of EGFR mutation-positive advanced non-Small-Cell lung cancer. J Clin Oncol (2018) 36:841–9. doi: 10.1200/JCO.2017.74.7576
11
Piotrowska Z Niederst MJ Karlovich CA Wakelee HA Neal JW Mino-Kenudson M et al . Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discovery (2015) 5:713–22. doi: 10.1158/2159-8290.CD-15-0399
12
Chabon JJ Simmons AD Lovejoy AF Esfahani MS Newman AM Haringsma HJ et al . Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun (2016) 7:11815. doi: 10.1038/ncomms11815
13
Fang W Jin H Zhou H Hong S Ma Y Zhang Y et al . Intratumoral heterogeneity as a predictive biomarker in anti-PD-(L)1 therapies for non-small cell lung cancer. Mol Cancer (2021) 20:37. doi: 10.1186/s12943-021-01331-9
14
French PJ Eoli M Sepulveda JM de Heer I Kros JM Walenkamp A et al . Defining EGFR amplification status for clinical trial inclusion. Neuro Oncol (2019) 21:1263–72. doi: 10.1093/neuonc/noz096
15
Roper N Brown AL Wei JS Pack S Trindade C Kim C et al . Clonal evolution and heterogeneity of osimertinib acquired resistance mechanisms in EGFR mutant lung cancer. Cell Rep Med (2020) 1. doi: 10.1016/j.xcrm.2020.100007
16
Dagogo-Jack I Shaw AT . Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol (2018) 15:81–94. doi: 10.1038/nrclinonc.2017.166
17
Ramos TC Figueredo J Catala M Gonzalez S Selva JC Cruz TM et al . Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther (2006) 5:375–9. doi: 10.4161/cbt.5.4.2522
18
Crombet T Osorio M Cruz T del Castillo R Mon R et al . Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol (2004) 22:1646–54. doi: 10.1200/JCO.2004.03.089
19
Takeda M Okamoto I Nishimura Y Nakagawa K . Nimotuzumab, a novel monoclonal antibody to the epidermal growth factor receptor, in the treatment of non-small cell lung cancer. Lung Cancer (Auckl) (2011) 2:59–67. doi: 10.2147/LCTT.S16440
20
Ramakrishnan MS Eswaraiah A Crombet T Piedra P Saurez G Iyer H et al . Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs (2009) 1:41–8. doi: 10.4161/mabs.1.1.7509
21
Lee JY Sun JM Lim SH Kim HS Yoo KH Jung KS et al . A phase Ib/II study of afatinib in combination with nimotuzumab in non-small cell lung cancer patients with acquired resistance to gefitinib or erlotinib. Clin Cancer Res (2016) 22:2139–45. doi: 10.1158/1078-0432.CCR-15-1653
22
Paz-Ares L Socinski MA Shahidi J Hozak RR Soldatenkova V Kurek R et al . Correlation of EGFR-expression with safety and efficacy outcomes in SQUIRE: a randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin plus necitumumab versus gemcitabine-cisplatin alone in the first-line treatment of patients with stage IV squamous non-small-cell lung cancer. Ann Oncol (2016) 27:1573–9. doi: 10.1093/annonc/mdw214
23
Douillard JY Pirker R O'Byrne KJ Kerr KM Storkel S von Heydebreck A et al . Relationship between EGFR expression, EGFR mutation status, and the efficacy of chemotherapy plus cetuximab in FLEX study patients with advanced non-small-cell lung cancer. J Thorac Oncol (2014) 9:717–24. doi: 10.1097/JTO.0000000000000141
24
Jiang Z Li C Li F Wang X . EGFR gene copy number as a prognostic marker in colorectal cancer patients treated with cetuximab or panitumumab: a systematic review and meta analysis. PLoS One (2013) 8:e56205. doi: 10.1371/journal.pone.0056205
25
Peraldo-Neia C Cavalloni G Fenocchio E Cagnazzo C Gammaitoni L Cereda S et al . Prognostic and predictive role of EGFR pathway alterations in biliary cancer patients treated with chemotherapy and anti-EGFR. PLoS One (2018) 13:e0191593. doi: 10.1371/journal.pone.0191593
26
Liu W Huo G Chen P . Efficacy of atezolizumab for advanced non-small cell lung cancer based on clinical and molecular features: A meta-analysis. Front Immunol (2022) 13:909027. doi: 10.3389/fimmu.2022.909027
27
Peraldo-Neia C Cavalloni G Fenocchio E Cagnazzo C Gammaitoni L Cereda S et al . Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0
Summary
Keywords
case report, resistance, heterogeneity, EGFR amplification, nimotuzumab
Citation
Li Y, Xu Z, Xie T, Xing P, Ying J and Li J (2022) Heterogeneity of resistant mechanisms in an EGFR-TKI relapsed patient with EGFR amplification and response to nimotuzumab: A case report. Front. Oncol. 12:937282. doi: 10.3389/fonc.2022.937282
Received
06 May 2022
Accepted
18 July 2022
Published
11 August 2022
Volume
12 - 2022
Edited by
Claudia De Vitis, Faculty of Medicine and Psychology, Sapienza University of Rome, Italy
Reviewed by
Andrea Botticelli, Sapienza University of Rome, Italy; Jen-Chung Ko, National Taiwan University Hospital, Taiwan
Updates
Copyright
© 2022 Li, Xu, Xie, Xing, Ying and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junling Li, lijunling@cicams.ac.cn
†These authors have contributed equally to this work
This article was submitted to Molecular and Cellular Oncology, a section of the journal Frontiers in Oncology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.