- 1Laboratory of Brain Science, Innovation Research Institute of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Experimental Center, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3School of health, Shandong University of Traditional Chinese Medicine, Jinan, China
- 4Department of Anesthesiology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 5School of Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 6School of Acupuncture, Shandong University of Traditional Chinese Medicine, Jinan, China
- 7Innovation Research Institute of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Background and objective: Antidepressants are widely prescribed to treat depression and anxiety disorders that may become chronic conditions among women. Epidemiological studies have yielded inconsistent results on the correlation between antidepressant use and the incidence risk of female breast and gynecological cancer, along with uncertain dose–response relationship. Therefore, we performed a systematic review and dose–response meta-analysis to investigate the association.
Methods: Web of Science, Embase, PubMed, The Cochrane Library, and PsycINFO were systematically searched in January 2022, with no language limits. Random-effect models were used to calculate pooled effect sizes and 95% confidence intervals between studies. Linear and non-linear dose–response analyses were performed to evaluate the dose or duration of antidepressant use affecting the incidence risk of female breast and gynecological cancer. Further subgroup analyses were systematically performed by stratifying almost all study characteristics and important potential confounders, in order to further clarify and validate the important potential hypotheses regarding the biological mechanism underlying this association.
Results: Based on a systematic literature search, 34 eligible studies (27 case–control studies and 7 cohort studies) involving 160,727 female breast and gynecological cancer patients found that antidepressant use did not increase the incidence risk of female breast and gynecological cancer (pooled OR: 1.01; 95% CI: 0.97, 1.04, I² = 71.5%, p < 0.001), and even decreased the incidence risk of ovarian cancer (pooled OR: 0.91; 95% CI: 0.83, 1, I² = 17.4%, p = 0.293). There were a non-linear dose–response relationship (p non-linearity < 0.05) between the duration of antidepressant use and incidence risk of female breast cancer, and an inverse linear dose–response relationship between antidepressant use and the incidence risk of gynecological cancer, specifically with an increase of cumulative defined daily dose or duration to a high level, like 25,550 doses (OR: 0.91, 95% CI: 0.85–0.98, p linearity < 0.05) or 4,380 days (OR: 0.82; 95% CI: 0.7, 0.96, p linearity < 0.05), compared to never antidepressant users.
Conclusion: This systematic review and dose–response meta-analysis found that antidepressant use did not increase the incidence risk of female breast and gynecological cancer and even decreased the incidence risk of ovarian cancer, along with a non-linear or linear dose–response relationship.
Systematic Review Registration: PROSPERO https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=313364, identifier CRD42022313364.
Introduction
Female breast and gynecological cancers account for 18.7% of all reported new cancer cases in all cancer cases worldwide in 2020, representing 38.9% new incidence and approximately 27.9% mortality (1) in women. Current projections indicate that, by 2070, the worldwide number of new breast and cervix cases diagnosed reach 5.03 million annually (2).
Prevalence of antidepressant (AD) use has been rising for decades (3, 4), in parallel with increased diagnosis of mental disorders, expanding indications for use, and longer treatment duration (5–7). Furthermore, women use AD at a rate approximately twofold higher than men (6), as the incidence of depression is 70% higher among women (8). Approximately 23% of women have an episode of depression throughout life and high recurrences (9, 10).
Recently, preclinical in vivo studies from the US Food and Drug Administration (FDA) have found that 63.6% (7/11) of examined ADs were associated with carcinogenicity (11). Actually, since the early 1990s, several studies, in both tumor cell cultures and animal models, have raised a possible association between AD and cancer risk (12). Several potential hypotheses have been proposed regarding the biological mechanism underlying this association. First, the specific tricyclic ADs have been found to be genotoxic and carcinogenic by using somatic mutation and recombination test (SMART) in wing cells of Drosophila melanogaster because of the nitrogen atom at position 5 in the seven-membered ring of the tricyclic molecule (13). Second, fluoxetine, a selective serotonin reuptake inhibitor (SSRI), and amitriptyline, a tricyclic AD (TCA), have been shown to promote the growth of mammary tumors in animal models by binding to growth regulatory intracellular histamine receptors and perturbing cellular growth and differentiation (12, 14). Third, the more important finding is that the growth and normal function of these organs are controlled by gonadotropin and female sex hormones, and it has been postulated that these hormones have an important role in the development of cancer of these organs. Certain monoaminergic medications may play a role in breast and gynecological carcinogenesis by affecting the release of gonadotropins and prolactin (15–18), as the human cervix contains functional gonadotropin receptors as do other parts of female genital tract (19). Both estrogens and prolactin have been shown to increase the incidence of spontaneous and chemically induced mammary tumors in rodents (20, 21). Raised endogenous estrogen levels and exogenous estrogen use are associated with the risk of breast and endometrial cancer in humans (22, 23). In addition, higher plasma prolactin levels are associated with breast cancer risk in a prospective study (24). Furthermore, prolactin, as a tumor-promoter, has been shown to stimulate proliferative activity in the mammary gland, suppress apoptosis (normal process of cell self-destruction), and upregulate the BRCA1 (breast cancer 1) gene (25, 26). Furthermore, the results of subsequent epidemiologic studies suggest that AD prescriptions are associated with increased risk of hormone-related cancer, including breast, endometrial, ovarian cancer (relative risk [RR] : 1.8; 95% CI: 1.15–2.81) (27) and cervical cancer (odds ratio [OR]: 1.22, 95% CI : 1.03–1.43) (28). However, this relationship has not been validated subsequently by in vivo or in vitro studies (29, 30). Furthermore, the antiproliferative effects of AD use have been supported by subsequent studies (31–35). Moreover, the results of epidemiologic studies have been inconclusive and demonstrated high (27, 36–43), low (44–47), or no change in risk (28, 48–63). Thus, the association of breast and gynecological cancer risk with the use of AD is highly conflicted, leaving an open question that will impede clinical practice. Given the increasing and widespread usage of AD, even a small increase in cancer risk associated with their use could translate into a large number of cancer cases at the population level. Furthermore, ADs are commonly prescribed after the diagnosis of cancer, not only for the treatment of depression but also for pain management (64). On the other hand, if the inverse association between AD use and breast and gynecological cancer risk proves to be causal, this may have major implications for the indications and prescribing of ADs. Thus, the hypothesis that ADs could promote or inhibit breast and gynecological cancer growth has important implications in terms of the etiology and treatment of breast and gynecological cancer and certainly merits further investigation.
Although a few systemic reviews have been carried out to elaborate this association, they all have limitations in some aspects. Most of them have been published mostly before 2012 (65) and have no new evidence published recently (52, 66). Some of them have performed neither a formal meta-analysis (67–72) nor a non-linear or linear dose–response analysis (73), and some of them have either no subgroup analysis or only incomplete subgroup analysis (73). Some of them have only focused on single tumor sites, such as breast (73) or ovarian cancer, respectively (74). However, as the majority of gynecological cancer share morphological and molecular features and familial breast and ovarian cancers have common genetic predisposition (75), a systemic review is needed that puts all hormone-related cancers, such as endometrial (45, 49), corpus uteri, and cervical cancer (28), together as an integral part of hormone-related cancer in female patients.
Therefore, in this study, we performed a comprehensive systematic review and meta-analysis, and a comprehensive subgroup analysis stratified by almost all study characteristics and important potential confounders to investigate the association between AD use and the incidence risk of female breast and gynecological cancer. Moreover, we performed a dose–response meta-analysis to evaluate the dose or duration of AD use affecting the incidence risk of female breast and gynecological cancer, and further clarify and validate the several important potential hypotheses regarding the biological mechanism underlying this association.
Methods
This meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration No.: CRD42022313364: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=313364) and adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist (Supplementary Material Table 1) and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for all processes (76, 77).
Search strategy
We searched the following electronic databases for studies published from their inception until January 2022: Web of Science, Embase, MEDLINE (PubMed), The Cochrane Library (CENTRAL), and PsycINFO. The search strategy was implemented using combined index terms (Medical Subject Headings, and Emtree) and free-text keywords, including (e.g., neoplasms or cancer) AND (e.g., antidepressive agents or anti-depress*) AND (e.g., morbidity or incidence or risk or occurrence) AND (e.g., case–control studies or cohort studies). A full description of the initial and supplementary search strategies is available in Supplementary Material Table 2. We combined search results using a bibliographic management tool (EndNote, version X9).
Selection criteria
The included studies were limited to (1) observational studies that (2) explored the relationship between AD use and the incidence risk of female breast and gynecological cancer, and (3) provided maximum adjusted risk estimates [risk ratios (RRs), odds ratios (ORs), and hazard ratios (HRs) with 95% confidence intervals (CIs)] or data allowing the calculation of the risk estimates and 95% CIs, and (4) studies were conducted in humans. Exclusion criteria were as follows: studies assessing the relationship between AD use and recurrence and mortality of treated breast and gynecological cancer; studies lacking a control group; specified population (e.g., HIV-infected patient); non-peer-reviewed reports (e.g., oral presentations, posters, dissertations, and conference abstracts) and ongoing studies; and studies with insufficient information. Additionally, only the latest and/or complete one was used in the meta-analysis for duplicate articles.
Data extraction
Two reviewers independently extracted and cross-checked the data from the included studies. The following details were presented in this review: first author’s name, year of publication, country, study design, participant number, age, type of ADs, exposure definition, exposure assessment, statistical indicators, outcome with the maximum covariate-adjusted ORs, RRs, HRs and 95% CIs, and adjusted/matched factors. All of the selected articles were examined by two researchers independently in terms of quality according to a Newcastle–Ottawa Scale (NOS; range 1–9 with 1–3 indicating low quality, 4–6 indicating moderate quality, and 7–9 indicating high quality) (78). Any conflicts were handled by consensus with a senior author.
Statistical analysis
Given the lower than 10% incidence of cancer, we approximated RR and HR as OR when pooling the estimates across the studies (79). Summary ORs (along with their corresponding 95% CIs) were calculated by performing random-effect meta-analysis for the overall relationship between the AD use and incidence risk of female breast and gynecological cancer on comparisons of the ever AD use group versus the never AD use group, and forest plots were used to summarize the pooled estimates and effect sizes. The Cochrane Q test and I² statistic were employed to evaluate heterogeneity between studies, and I² > 50% was considered statistically significant. In order to examine its source, we performed subgroup analyses according to almost all relevant factors that may lead to significant heterogeneity.
Moreover, we used the G-L method (80) to carry out a dose–response meta-analysis using the levels of cumulative defined daily dose (81) (CDDD) or duration of AD use (days) and the adjusted natural log of the ORs with their SE. If the dose or duration was reported by range, we assigned the midpoint of the upper and lower boundaries in each category as the average duration or dose. If the highest category was open-ended, we considered the width of the category to be the same as that of the adjacent category. If the lowest category was open-ended, the lowest boundary was assumed to be zero. We included studies for this dose–response analysis only if they reported the distributions of cases and total persons or person-years, as well as the ORs and 95% CI with the variance estimates for at least three quantitative exposure categories. Step 1, we performed a non-linear dose–response meta-analysis by restricted cubic splines with three or four knots of the distribution, then based on the χ² and p-value calculated in step 1, we determined whether a linear (p > 0.05) or non-linear (p < 0.05) dose–response meta-analysis should be adopted.
The funnel plots and Egger’s test were used to detect potential publication bias (82). To assess the stability of the results, a leave-one-out sensitivity analysis was carried out. All statistical tests were two-sided using a significance level of p < 0.05. Analyses were performed with Stata 13.1 (StataCorp LP, College Station, TX, USA).
Results
Eligible studies and study characteristics
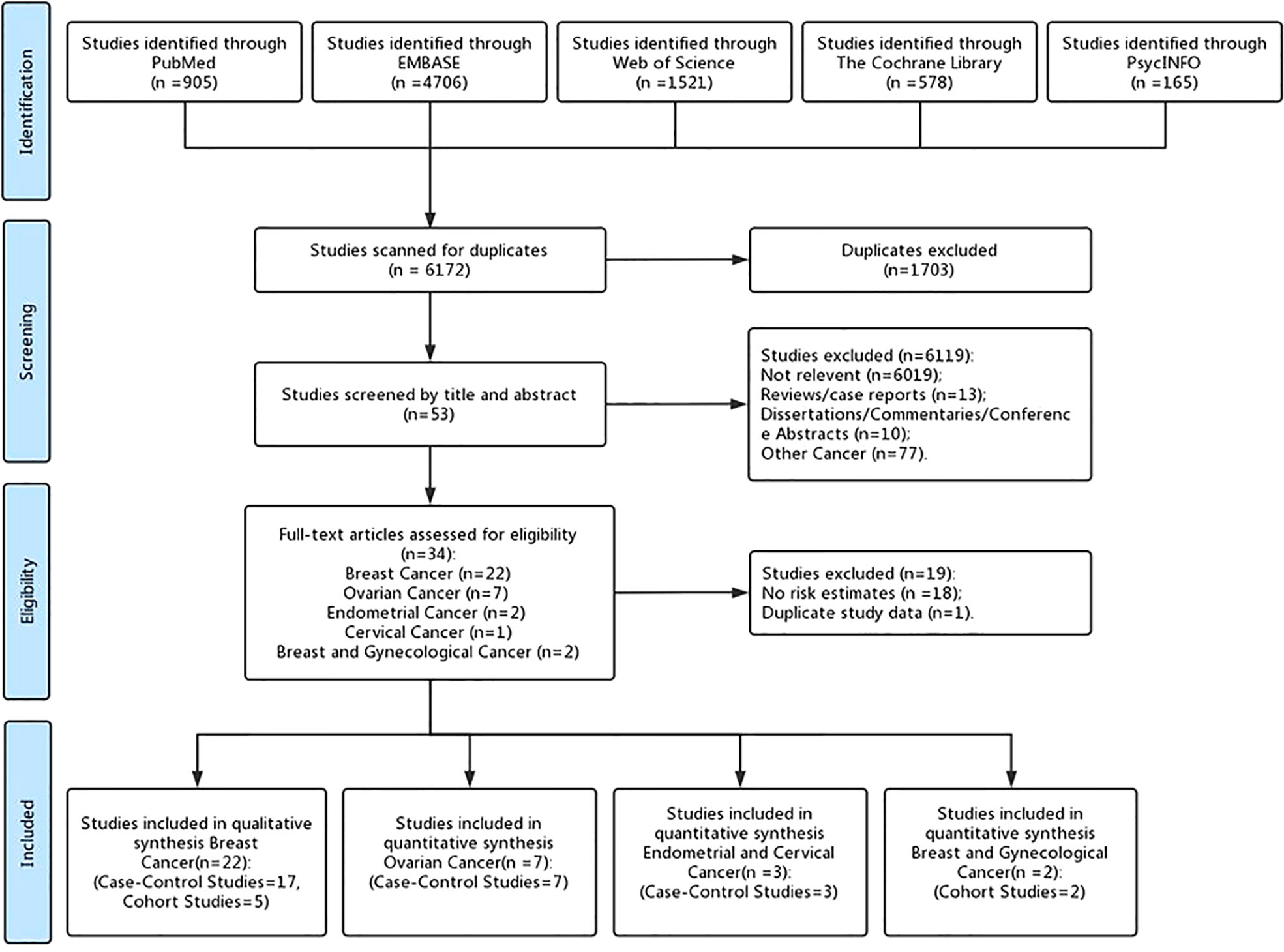
After identifying 7,875 references, 1,703 duplicate publications, 6,019 irrelevant studies, 13 reviews/case reports, 10 dissertations/commentaries/conference abstracts, and 77 other cancers were excluded after screening the titles and abstracts. The remaining 53 potentially studies were carefully read in full, and 18 of them were excluded for shorting the data of risk estimates and one was excluded for duplicate study data. Finally, 34 observational studies (27 case–control studies and 7 cohort studies) (27, 28, 36–57, 59–61, 63, 66, 83–87) were included in the present meta-analysis, including 22 female breast cancer studies, 7 ovarian cancer studies, 2 endometrial cancer studies, 1 cervical cancer study, and 2 female breast and gynecological cancer studies (Figure 1).
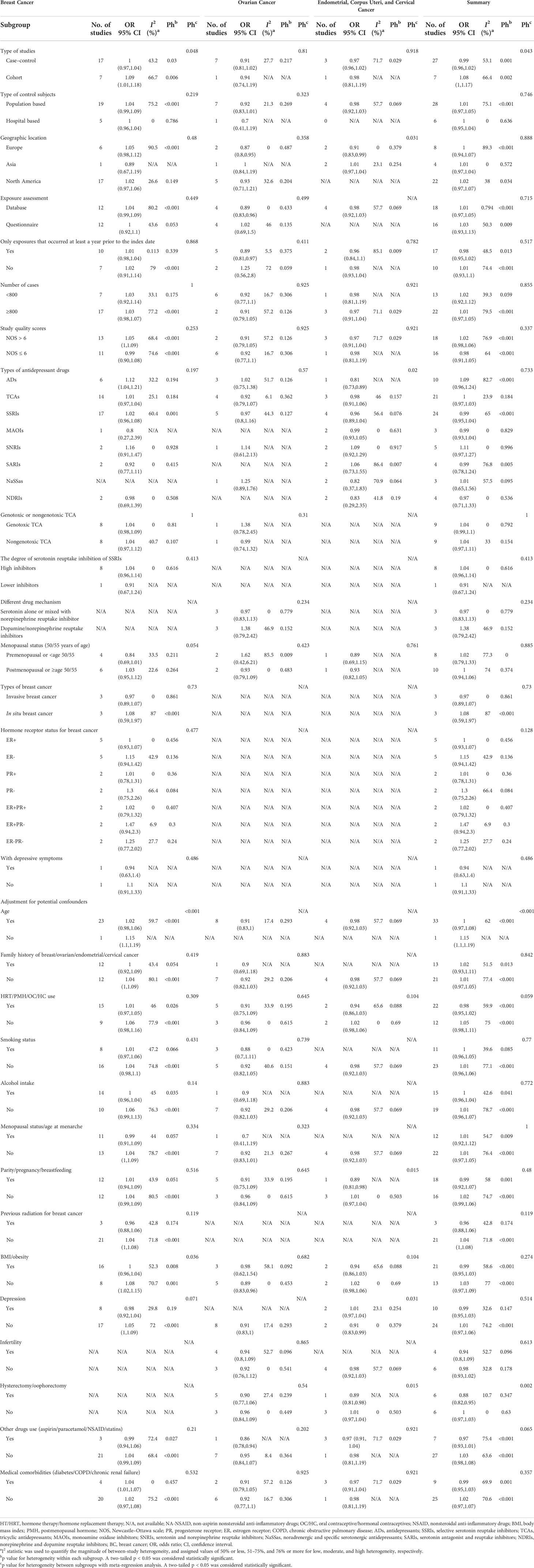
A total of 160,727 patients with female breast and gynecological cancer were involved, and the cases ranged in size from 20 to 45,147 participants. Overall, the studies were published between 1995 and 2021, involving participants from North America (n = 22), Europe (n = 8), and Asia (n = 4). Drug exposure was assessed and collected by questionnaires in 16 studies and databases in 18 studies, respectively. The quality of the included studies is assessed in Supplementary Material Table 3. The mean NOS score was 7 (median, 6.5; range, 5–8), indicating that the overall quality of articles was good. Additional characteristics of the included studies are shown in Table 1.

Table 1 Characteristics of observational studies of antidepressant drug use and breast and gynecological cancer risk.
Meta-analysis of ever vs. never antidepressant medication use and incidence risk of female breast and gynecological cancer
The meta-analysis results of 34 epidemiological studies showed that ever AD use was unrelated to overall incidence risk of breast and gynecological cancer (pooled OR: 1.01; 95% CI: 0.97, 1.04), with a high heterogeneity (I² = 71.5%, p < 0.001). Furthermore, subgroup analyses for specific cancer sites showed a slightly decreased incidence risk of ovarian cancer (pooled OR: 0.91; 95% CI: 0.83, 1, I² = 17.4%, p = 0.293); no significant relation with endometrial, corpus uteri, and cervical cancers (pooled OR: 0.98; 95% CI: 0.92, 1.03, I² = 57.7%, p = 0.069); and a weakly increased incidence risk of female breast cancer (pooled OR: 1.03; 95% CI: 0.99, 1.07), with a high heterogeneity (I² = 71.2%, p < 0.001), compared to never AD users (Figure 2).
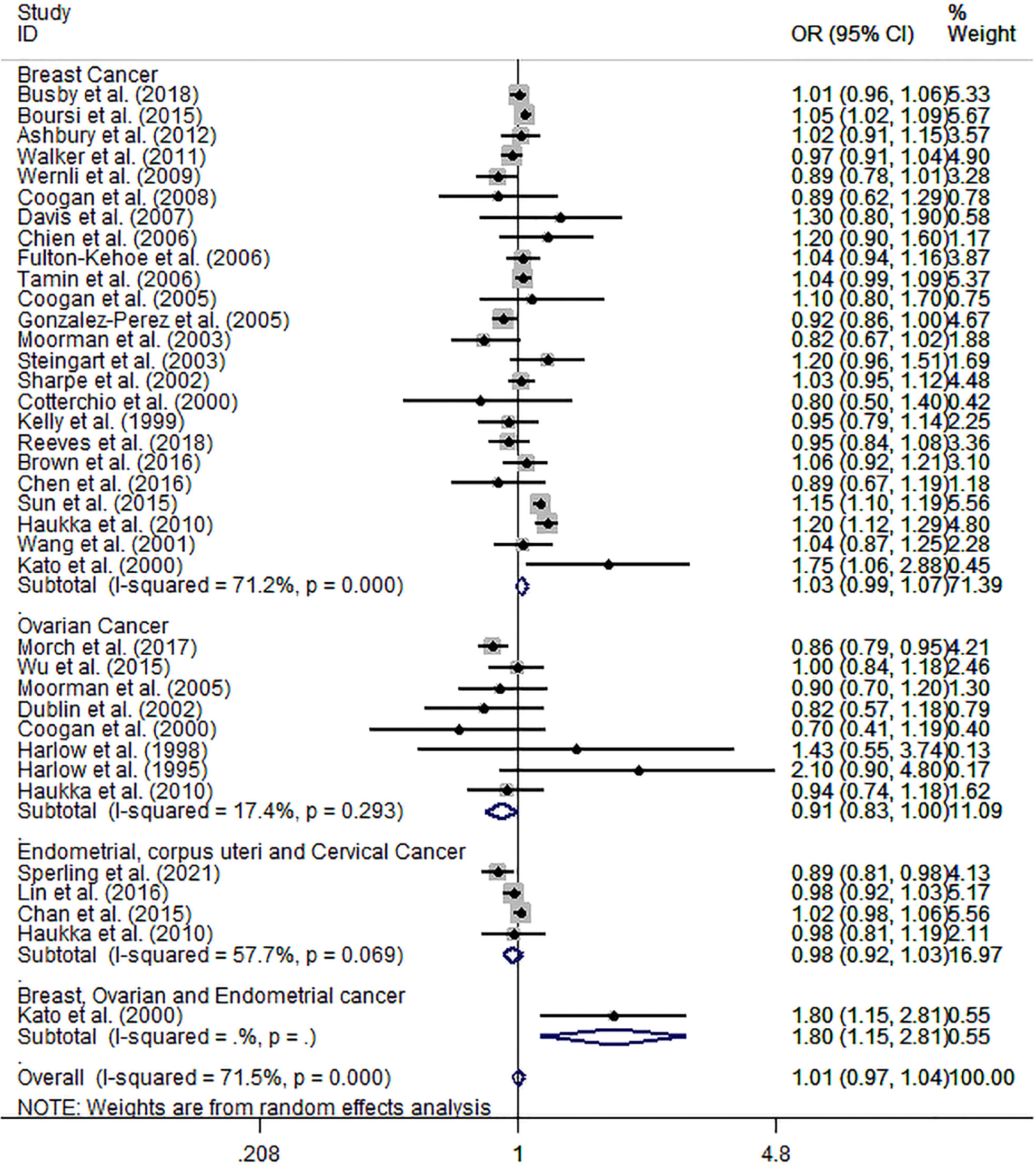
Figure 2 Forest plot of ever vs. never antidepressant use and incidence risk of breast and gynecological cancer.
In the light of the remarkable between-study heterogeneity, we investigated its potential sources by subgroup analyses of the study characteristics and confounders. The detailed results showed in Table 2. The results showed that the incidence risk of female breast and gynecological cancer was not statistically different across great majority strata, but the incidence risk appeared to be stronger in exposures that occurred less than 1 year prior to the index date (pooled OR: 1.25; 95% CI: 0.56, 2.8) than that in exposures that occurred at least 1 year prior to the index date (pooled OR: 0.89; 95% CI: 0.81, 0.97), and stronger in premenopausal female patients (pooled OR: 1.62; 95% CI: 0.42, 6.21) than that in postmenopausal female patients (pooled OR: 0.93; 95% CI: 0.79, 1.09) for ovarian cancer. It also appeared to be stronger in estrogen receptor (ER)+ progesterone receptor (PR)− (pooled OR: 1.47; 95% CI: 0.94, 2.3), PR− (pooled OR: 1.3; 95% CI: 0.75, 2.26), ER−PR− (pooled OR: 1.25; 95% CI: 0.77, 2.02), and ER− (pooled OR: 1.15; 95% CI: 0.94, 1.42) than that in ER+ (pooled OR: 1; 95% CI: 0.93, 1.07), PR+ (pooled OR: 1.01; 95% CI: 0.78, 1.31), and ER+PR+ (pooled OR: 1.02; 95% CI: 0.79, 1.32) for breast cancer.
Dose–response meta-analysis between the CDDD or duration of antidepressant use and incidence risk of female breast and gynecological cancer
There was a significant linear dose–response association between the CDDD of AD use and incidence risk of female breast cancer in two eligible studies (66, 86) involving 48,852 cases and 2,141,294 participants (p linearity < 0.05, Figure 3A). The OR kept a slightly increasing trend without breaking 1.2 until the CDDD increased to 1,520. By comparison, a non-linear dose–response meta-analysis with 13 eligible studies (37, 38, 42, 47, 48, 55–57, 60, 61, 66, 83, 84) involving 109,215 cases and 398,024 controls (p non-linearity < 0.05, Figure 3B) showed a very slight increase in incidence risk of female breast cancer along with the increase of duration of AD use until 1,460 days (OR: 1.05; 95% CI: 1.01, 1.09), then followed a subsequent statistically significant inverse trend, specifically when an increase of duration to 6,570 days (OR: 0.9, 95% CI:0.79–1.02), compared to never AD users.
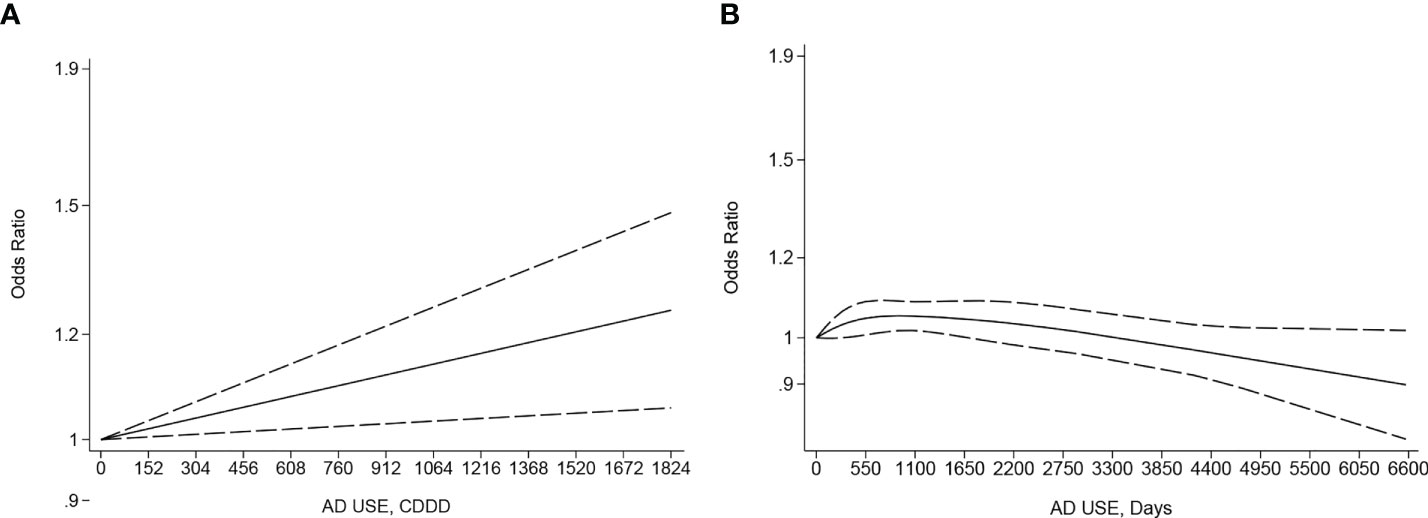
Figure 3 (A) Dose–response for cumulative defined daily dose (CDDD) of antidepressant (AD) use and incidence risk of breast cancer. (B) Dose–response for duration of antidepressant use and incidence risk of breast cancer. The black solid line and the black long dashed line represent the estimated odds ratios (ORs) with corresponding 95% confidence intervals (CIs) for the non-linearity or the linearity.
A negative linearity association existed between the CDDD or duration of AD use and the incidence risk of ovarian, endometrial, and cervical cancer, specifically with an increase of CDDD or duration to a high level, like 25,550 CDDD (OR: 0.91, 95% CI: 0.85–0.98, p linearity < 0.05, Figure 4A) or 4,380 days (OR: 0.82; 95% CI: 0.7, 0.96, p linearity < 0.05, Figure 4B) from four eligible studies (28, 45, 49, 86) involving 38,843 cases and 5,709,516 controls and eight eligible studies (36, 44–46, 49, 50, 59, 87) with 23,201 cases and 289,483 participants, respectively. Another negative linearity association also existed between the CDDD and the incidence risk of endometrial, corpus uteri, and cervical cancer, from four eligible studies (28, 45, 49, 86) with 38,370 cases and 3,658,516 participants, specifically with an increase of CDDD to a high level, like 25,550 CDDD (OR:0.91; 95% CI: 0.85, 0.98, p linearity < 0.05, Supplementary Material Figure 1), compared to never AD users.
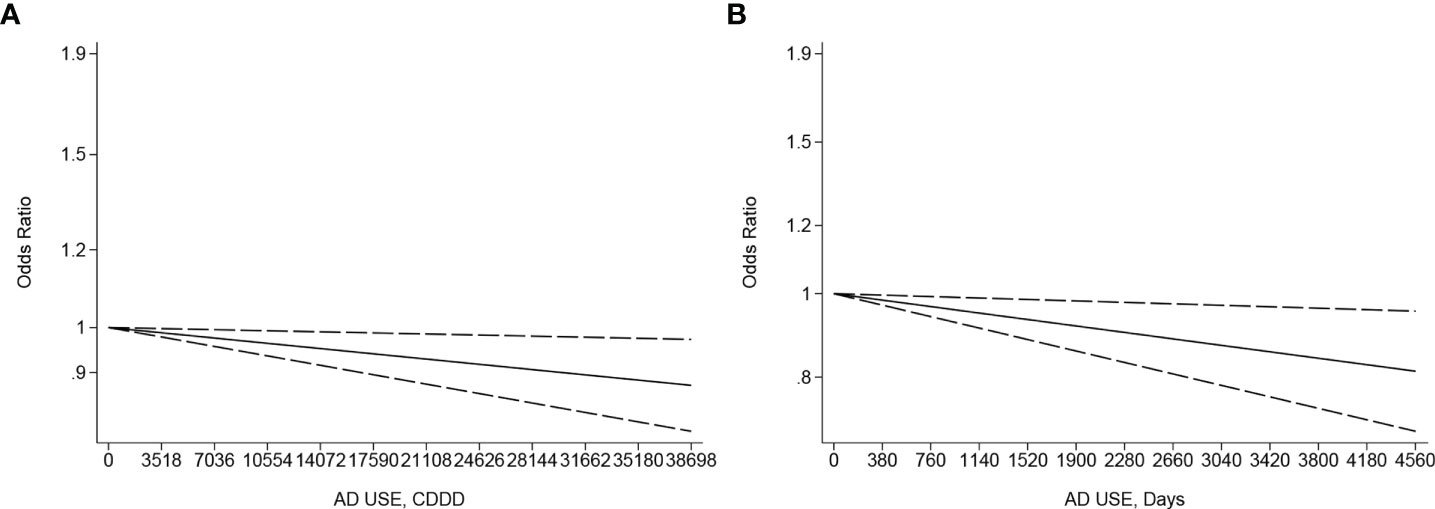
Figure 4 (A) Dose–response for CDDD of antidepressant use and incidence risk of ovarian, endometrial, and cervical cancer. (B) Dose–response for duration of antidepressant use and incidence risk of ovarian, endometrial, and cervical cancer. The black solid line and the black long dashed line represent the estimated odds ratios (ORs) with corresponding 95% confidence intervals (CIs) for the non-linearity or the linearity.
Sensitivity analysis and publication bias
Applying the leave-one-out sensitivity analysis showed that none of the eligible studies had considerable effect on the overall estimate (Supplementary Material Figure 2). No publication bias was observed based on funnel plot symmetry (Figure 5) and results of Egger’s test (p = 0.354).
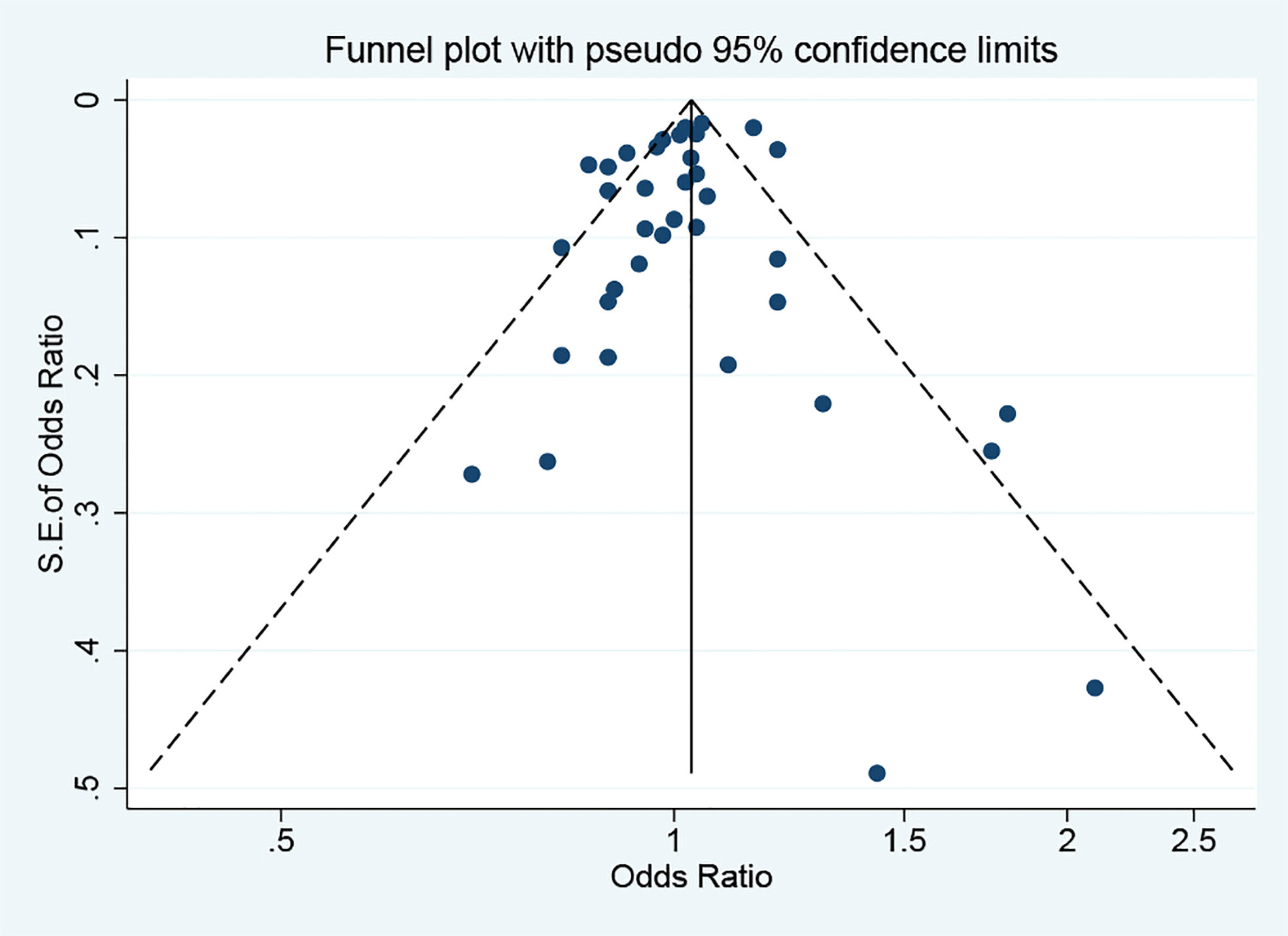
Figure 5 Funnel plot for identifying the publication bias. S.E., standard error. The circles alone are real studies. The vertical lines represent the summary effect estimates, and the dashed lines represent pseudo-95% confidence interval limits.
Discussion
The pooled results of this systematic review and meta-analysis found that AD use did not increase the incidence risk of female breast and gynecological cancer, either by tricyclic ADs (pooled OR: 1; 95% CI: 0.97, 1.03) or by selective serotonin reuptake inhibitors (pooled OR: 0.99; 95% CI: 0.95, 1.04), and even decreased the incidence risk of ovarian cancer. Further subgroup analyses of confounders found that AD use was associated with higher risk in almost all of subgroups of unadjusted confounders; thus, the incomplete adjustment for these potential confounders was likely to bias our results toward a positive relationship. Additionally, further subgroup analyses of study characteristics found that the incidence risk of female breast and gynecological cancer was not statistically different across great majority strata, but it appeared to be stronger in exposures that occurred less than 1 year prior to the index date (1.25 for less than 1 year vs. 0.89 for more than 1 year) for ovarian cancer, as protopathic bias and reverse causation might drive the estimates towards an increased risk (88). It was also stronger in questionnaire for exposure assessment (1.02 for questionnaire vs. 0.89 for database) for ovarian cancer due to recall bias and selection bias by questionnaire for collecting information (88).
However, several potential hypotheses have been proposed regarding the biological mechanism underlying this positive association and have been supported by subsequent epidemiologic studies. However, most of them are limited for some reason, such as exposure misclassifications (27, 43), reverse causation bias (27, 37–40, 42, 43), recall bias, selection bias (27, 38–41), and unadjusted confounders (27, 42, 43). Moreover, the weight of epidemiologic evidence does not support the hypothesis that AD use increases the overall risk of breast and gynecological cancer (28, 44–63), which are consistent with our finding. Although the incidence risk of ovarian cancer is stronger in genotoxic TCA (pooled OR: 1.38; 95% CI: 0.78, 2.45) than that in nongenotoxic TCA, and in dopamine/norepinephrine reuptake inhibitors (pooled OR: 1.38; 95% CI: 0.79, 2.42) than that in serotonin alone or mixed with norepinephrine reuptake inhibitors in our finding, the results are only based on one (44) or three studies (36, 46, 50). Nevertheless, exposure to either genotoxic or nongenotoxic TCAs is not associated with a significant increase in the incidence of female breast cancer. In addition, Ashbury et al. (48) grouped SSRIs as higher and lower inhibitors dependent on the dissociation constant (Kd) in order to accurately assess for levels of prolactin secreted by the secondary pituitary gland, and found that neither higher nor lower inhibitor of serotonin reuptake increased the risk for breast cancer, which was consistent with our result.
If AD medications work through changes in the secretion of gonadotropins and female sex hormones, the observed association may be more pronounced in premenopausal women who have functioning ovaries than that in postmenopausal women. Our results in this study support this hypothesis, but they are only based on limited two studies (40, 44) with possible bias for ovarian cancer. Conversely, prior prospective studies have linked prolactin with increased postmenopausal women breast cancer risk (89), but our results in this comprehensive study shows that these associations are weak or null as a whole. Moreover, different from several studies that have found that prolactin may encourage the development of estrogen receptor (ER)-positive tumors (89) and higher risk in in situ (52, 85), our study shows reverse results and/or weak associations, but they are based on limited studies and possible bias, respectively. Additionally, previous studies have shown a significant relationship between depression and the risk of cancer incidence (90). Our subgroup analyses in this study have also found that after adjusting the depressive symptoms, OR tends to slightly decrease for female breast cancer. It cannot be ruled out that depression itself may have an impact on cancer incidence. Thus, it is possible that the positive association observed between AD use and female breast cancer risk is due to depressive symptoms rather than AD use itself. Therefore, further studies are needed to figure it out.
Our stratified analyses for specific types of ADs indicated that the results were more pronounced in serotonin and norepinephrine reuptake inhibitors (SNRIs) for female breast, ovarian, endometrial, and cervical cancer, and stronger in noradrenergic and specific serotonergic antidepressants (NaSSas) for ovarian cancer. We also found a reduced risk of female breast cancer with the use of monoamine oxidase inhibitors (MAOIs) and serotonin antagonist and reuptake inhibitors (SARIs), and a reduced risk of endometrial, corpus uteri, and cervical cancer with the use of norepinephrine and dopamine reuptake inhibitors (NDRIs) and NaSSas. Amerio et al. reviewed the US Food and Drug Administration (FDA) preclinical in vivo evidence and found that 63.6% (7/11) of examined ADs were associated with carcinogenicity, including duloxetine (SNRIs), mirtazapine (NaSSas), and NDRI (bupropion), but the agents unassociated with carcinogenicity were trazodone (SARIs) and venlafaxine (SNRIs) (11). Meanwhile, several previous studies (91, 92) had reported the mechanisms on how mirtazapine (NaSSas) and MAOIs acted to inhibit tumor growth by enhancing immune function and causing neurotoxicity and repressing BHC110/LSD1, respectively. Conversely, a previous study has provided preliminary data of the possible association of trazodone (SARIs) and invasive cervical cancer (28). However, information pertaining to breast, uterine, and ovarian carcinogenesis clearly highlights that cervical cancer carcinogenesis is very different to the others, and is almost exclusively HPV driven and vastly different to that of the other organs analyzed, which is supported by the results of epidemiologic study that TCAs, MAOIs, and SSRIs are not associated with increased risk of invasive cervical cancer by Chan et al. (28). Furthermore, our results are based on limited one or two studies for each cancer site and short of adjusting the HPV infection as the critical potential confounders (28), and the incomplete adjustment for this potential confounder is likely to bias final results in an epidemiologic study. Thus, the above results are based on preliminary analysis and has so far proved inconclusive. Further large-scale prospective cohort studies adjusting for all possible confounding factors (including HPV infection) or animal and in vitro studies are needed to clarify the tumor-inhibiting or growth-promoting effect by different types of ADs and the biological mechanism underlying this association.
Furthermore, there is a non-linear dose–response relationship between the duration of AD use and incidence risk of female breast cancer, in which the bi-phasic phenomenon is characterized by “low-dose stimulation and high-dose inhibition” (12, 93) of malignant cell proliferation. This bi-phasic phenomenon shows that short-term use and/or low-dose AD may increase the risk of breast cancer in women or exacerbate cancer cell growth in women in the early stages of breast cancer, and long-term use and/or high dose inhibit tumor growth, which may help explain the high between-study heterogeneity for female breast cancer based on distinct dose or duration from different studies. Nevertheless, there is a positive linearity association between the CDDD of AD use and incidence risk of female breast cancer based on limited studies with possible bias (86). Additionally, an inverse linear dose–response association exists between the CDDD or duration of AD use and incidence risk of gynecological cancer, as well as between the CDDD and the incidence risk of endometrial, corpus uteri, and cervical cancer. Furthermore, the antiproliferative effects of AD use have been supported by previous studies (34, 44, 45) with a possible biological mechanism (31–33). However, this negative linearity association does not exist in individual cancer sites partly due to the limited numbers of studies and the strong non-linearity or linearity phenomenon that only happens with an increase of CDDD or duration to a high level. Thus, further large-scale prospective cohort studies specifying dose or duration are needed to accurately assess and clarify the protective or bi-phasic effect and biological mechanism.
Strength and limitation
Notably, our meta-analysis has the following advantages. First, to the best of our knowledge, this is the first study to systematically perform a qualitative dose–response meta-analysis of the relationship between AD use and the incidence of female breast and gynecological cancer as a whole of hormone-related cancer in female patients, along with comprehensive subgroup analyses stratified by almost all study characteristics and important potential confounders. Furthermore, this is the first study to demonstrate shapes of non-linear or linear association between the CDDD or duration of AD use and female breast or gynecological cancer, and further clarify and validate the several important potential hypotheses regarding the biological mechanism underlying this association.
Inevitably, this study also has some limitations. First, moderate or high heterogeneity among studies was observed when pooling estimates for female breast cancer. Furthermore, a small portion of subgroup analyses of study characteristics and important potential confounders was based on a limited number of existing studies. Second, a linearity association was not observed in the relation between the CDDD or duration of AD use and the incidence risk of ovarian, endometrial, corpus uteri, and cervical cancer, respectively, partly due to the limited number of existing studies for each cancer site. Finally, the interpretation criteria for exposure were inconsistent, and misclassification bias might affect the results.
Conclusion
Overall, the results of the present updated meta-analysis involving the largest sample size to date and mostly included comprehensive observational studies show that AD use does not increase the incidence risk of female breast and gynecological cancer, either by TCA or by SSRI, and even decreases the incidence risk of ovarian cancer, compared to never AD users. There is a non-linear dose–response relationship between the duration of AD use and incidence risk of female breast cancers, with a very slight increase in incidence risk of female breast cancer on short-term usage. An inverse linearity association exists between the CDDD or duration of AD use and incidence risk of gynecological cancer, and also between the CDDD of AD use and incidence risk of endometrial, corpus uteri, and cervical cancer. More future studies specifying dose or duration are needed in order to accurately assess and clarify this protective or bi-phasic effect and biological mechanism.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HS, YYL, and YZ conceived and designed the study. HS, YYL, and YZ selected the studies and collected the data. YYL and YZ analyzed data. All authors interpreted the results. YYL and YZ drafted the paper. All authors revised the draft paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Joint Funds for the National Key Research and Development Program of China (Grant No. 2021ZD0202900), the National Natural Science Foundation of China (Grant Nos. 62027812, 81771470, and 82101608), and the Natural Science Foundation of Shandong Province (Grant No. ZR2020ZD17). The funders of this study had no role in study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.939636/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Soerjomataram I, Bray F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol (2021) 18(10):663–72. doi: 10.1038/s41571-021-00514-z
3. Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United states, 2011-2014. nchs data brief. number 283. Natl Center Health Stat (2017) 283:1–8. doi: 10.1016/j.jad.2019.01.039
4. Gomez-Lumbreras A, Ferrer P, Ballarín E, Sabaté M, Vidal X, Andretta M, et al. Study of antidepressant use in 5 European settings. could economic, sociodemographic and cultural determinants be related to their use? J Affect Disord (2019) 249:278–85. doi: 10.1016/j.jad.2019.01.039
5. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: Results from the us national health and nutrition examination survey. J Clin Psychiatry (2013) 74(2):12452. doi: 10.4088/JCP.13m08443
6. Luo Y, Kataoka Y, Ostinelli EG, Cipriani A, Furukawa TA. National prescription patterns of antidepressants in the treatment of adults with major depression in the us between 1996 and 2015: A population representative survey based analysis. Front Psychiatry (2020) 11:35. doi: 10.3389/fpsyt.2020.00035
7. Jannini TB, Lorenzo GD, Bianciardi E, Niolu C, Toscano M, Ciocca G, et al. Off-label uses of selective serotonin reuptake inhibitors (Ssris). Curr Neuropharmacol (2022) 20(4):693–712. doi: 10.2174/1570159X19666210517150418
8. Stute P, Spyropoulou A, Karageorgiou V, Cano A, Bitzer J, Ceausu I, et al. Management of depressive symptoms in peri-and postmenopausal women: Emas position statement. Maturitas (2020) 131:91–101. doi: 10.1016/j.maturitas.2019.11.002
9. Monroe SM, Slavich GM, Gotlib IH. Life stress and family history for depression: The moderating role of past depressive episodes. J Psychiatr Res (2014) 49:90–5. doi: 10.1016/j.jpsychires.2013.11.005
10. Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: Results from the national comorbidity survey replication (Ncs-r). psychol Med (2010) 40(2):225–37. doi: 10.1017/S0033291709990213
11. Amerio A, Gálvez JF, Odone A, Dalley SA, Ghaemi SN. Carcinogenicity of psychotropic drugs: A systematic review of us food and drug administration-required preclinical in vivo studies. Aust N Z J Psychiatry (2015) 49(8):686–96. doi: 10.1177/0004867415582231
12. Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res (1992) 52(13):3796–800.
13. Brambilla G, Mattioli F, Martelli A. Genotoxic and carcinogenic effects of antipsychotics and antidepressants. Toxicology (2009) 261(3):77–88. doi: 10.1016/j.tox.2009.04.056
14. Brandes LJ, Macdonald LM, Bogdanovic RP. Evidence that the antiestrogen binding site is a histamine or histamine-like receptor. Biochem Biophys Res Commun (1985) 126(2):905–10. doi: 10.1016/0006-291X(85)90271-2
15. Sloley BD, Kah O, Trudeau VL, Dulka JG, Peter RE. Amino acid neurotransmitters and dopamine in brain and pituitary of the goldfish: Involvement in the regulation of gonadotropin secretion. J Neurochem (1992) 58(6):2254–62. doi: 10.1111/j.1471-4159.1992.tb10971.x
16. Bedran-de-Castro JC, Petrovic SL, McCann SM. Involvement of beta-adrenergic receptors in the differential release of gonadotropins in acutely orchidectomized rats. Braz J Med Biol Res (1990) 23(10):1025–7.
17. Donnelly PJ, Dailey RA. Effects of dopamine, norepinephrine and serotonin on secretion of luteinizing hormone, follicle-stimulating hormone and prolactin in ovariectomized, pituitary stalk-transected ewes. Domest Anim Endocrinol (1991) 8(1):87–98. doi: 10.1016/0739-7240(91)90043-j
18. Aguilar E, Ranchal A, Aguilar R, Pinilla L. Gonadotropin and prolactin secretion in prepubertal female rats treated with 8-Hydroxy-2-(Di-N-Propylamino) tetralin. J Neural Transm Gen Sect (1993) 94(3):165–73. doi: 10.1007/bf01277022
19. Lin PC, Li X, Lei ZM, Rao Ch V. Human cervix contains functional luteinizing Hormone/Human chorionic gonadotropin receptors. J Clin Endocrinol Metab (2003) 88(7):3409–14. doi: 10.1210/jc.2002-021966
20. Welsch CW, Nagasawa H. Prolactin and murine mammary tumorigenesis: A review. Cancer Res (1977) 37(4):951–63.
21. Curtis JR. The role of 7, 12 dmba and three hormones in 7, 12 dmba-induced rat mammary cancer: Three hypotheses. Med Hypotheses (1982) 9(5):489–507. doi: 10.1016/0306-9877(82)90018-4
22. Schottenfeld D. Epidemiology of endometrial neoplasia. J Cell Biochem Suppl (1995) 23:151–9. doi: 10.1002/jcb.240590920
23. Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst (1995) 87(3):190–7. doi: 10.1093/jnci/87.3.190
24. Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Longcope C, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst (1999) 91(7):629–34. doi: 10.1093/jnci/91.7.629
25. Vonderhaar BK. Prolactin involvement in breast cancer. Endocr Relat Cancer (1999) 6(3):389–404. doi: 10.1677/erc.0.0060389
26. Harvey PW. Human relevance of rodent prolactin-induced non-genotoxic mammary carcinogenesis: Prolactin involvement in human breast cancer and significance for toxicology risk assessments. J Appl Toxicol (2005) 25(3):179–83. doi: 10.1002/jat.1063
27. Kato I, Zeleniuch-Jacquotte A, Toniolo PG, Akhmedkhanov A, Koenig K, Shore RE. Psychotropic medication use and risk of hormone-related cancers: The new York university women’s health study. J Public Health Med (2000) 22(2):155–60. doi: 10.1093/pubmed/22.2.155
28. Chan HL, Hsieh YH, Lin CF, Liang HY, Huang KY, Chiu WC, et al. Invasive cervical cancer and antidepressants: A nationwide population-based study. Med (Baltimore) (2015) 94(42):e1866. doi: 10.1097/md.0000000000001866
29. Volpe DA, Ellison CD, Parchment RE, Grieshaber CK, Faustino PJ. Effects of amitriptyline and fluoxetine upon the in vitro proliferation of tumor cell lines. J Exp Ther Oncol (2003) 3(4):169–84. doi: 10.1046/j.1359-4117.2003.01091.x
30. Bendele RA, Adams ER, Hoffman WP, Gries CL, Morton DM. Carcinogenicity studies of fluoxetine hydrochloride in rats and mice. Cancer Res (1992) 52(24):6931–5.
31. Lin CJ, Robert F, Sukarieh R, Michnick S, Pelletier J. The antidepressant sertraline inhibits translation initiation by curtailing mammalian target of rapamycin signaling. Cancer Res (2010) 70(8):3199–208. doi: 10.1158/0008-5472.CAN-09-4072
32. Lee CS, Kim YJ, Jang ER, Kim W, Myung SC. Fluoxetine induces apoptosis in ovarian carcinoma cell line ovcar-3 through reactive oxygen species-dependent activation of nuclear factor-Kb. Basic Clin Pharmacol Toxicol (2010) 106(6):446–53. doi: 10.1111/j.1742-7843.2009.00509.x
33. Krishnan A, Hariharan R, Nair SA, Pillai MR. Fluoxetine mediates G0/G1 arrest by inducing functional inhibition of cyclin dependent kinase subunit (Cks) 1. Biochem Pharmacol (2008) 75(10):1924–34. doi: 10.1016/j.bcp.2008.02.013
34. Cloonan SM, Drozgowska A, Fayne D, Williams DC. The antidepressants maprotiline and fluoxetine have potent selective antiproliferative effects against burkitt lymphoma independently of the norepinephrine and serotonin transporters. Leukemia lymphoma (2010) 51(3):523–39. doi: 10.3109/10428190903552112
35. Cordero MD, Sánchez-Alcázar JA, Bautista-Ferrufino MR, Carmona-López MI, Illanes M, Ríos MJ, et al. Acute oxidant damage promoted on cancer cells by amitriptyline in comparison with some common chemotherapeutic drugs. Anticancer Drugs (2010) 21(10):932–44. doi: 10.1097/CAD.0b013e32833ed5f7
36. Harlow BL, Cramer DW, Baron JA, Titus-Ernstoff L, Greenberg ER. Psychotropic medication use and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev (1998) 7(8):697–702.
37. Boursi B, Lurie I, Mamtani R, Haynes K, Yang YX. Anti-depressant therapy and cancer risk: A nested case-control study. Eur Neuropsychopharmacol (2015) 25(8):1147–57. doi: 10.1016/j.euroneuro.2015.04.010
38. Chien C, Li CI, Heckbert SR, Malone KE, Boudreau DM, Daling JR. Antidepressant use and breast cancer risk. Breast Cancer Res Treat (2006) 95(2):131–40. doi: 10.1007/s10549-005-9056-0
39. Cotterchio M, Kreiger N, Darlington G, Steingart A. Antidepressant medication use and breast cancer risk. Am J Epidemiol (2000) 151(10):951–7. doi: 10.1093/oxfordjournals.aje.a010138
40. Harlow BL, Cramer DW. Self-reported use of antidepressants or benzodiazepine tranquilizers and risk of epithelial ovarian cancer: Evidence from two combined case-control studies (Massachusetts, united states). Cancer Causes Control (1995) 6(2):130–4. doi: 10.1007/BF00052773
41. Kelly JP, Rosenberg L, Palmer JR, Rao RS, Strom BL, Stolley PD, et al. Risk of breast cancer according to use of antidepressants, phenothiazines, and antihistamines. Am J Epidemiol (1999) 150(8):861–8. doi: 10.1093/oxfordjournals.aje.a010091
42. Sharpe CR, Collet JP, Belzile E, Hanley JA, Boivin JF. The effects of tricyclic antidepressants on breast cancer risk. Br J Cancer (2002) 86(1):92–7. doi: 10.1038/sj.bjc.6600013
43. Sun YL, Vedsted P, Fenger-Gron M, Sen Wu C, Bech BH, Olsen J, et al. Cancer mortality in people treated with antidepressants before cancer diagnosis: A population based cohort study. PloS One (2015) 10(9):e0138134. doi: 10.1371/journal.pone.0138134
44. Mørch LS, Dehlendorff C, Baandrup L, Friis S, Kjær SK. Use of antidepressants and risk of epithelial ovarian cancer. Int J Cancer (2017) 141(11):2197–203. doi: 10.1002/ijc.30919
45. Sperling CD, Aalborg GL, Dehlendorff C, Friis S, Mørch LS, Kjaer SK. Use of antidepressants and endometrial-cancer risk: A nationwide nested case-control study. Int J Epidemiol (2021) 51(3):799–806. doi: 10.1093/ije/dyab200
46. Dublin S, Rossing MA, Heckbert SR, Goff BA, Weiss NS. Risk of epithelial ovarian cancer in relation to use of antidepressants, benzodiazepines, and other centrally acting medications. Cancer Causes Control (2002) 13(1):35–45. doi: 10.1023/A:1013969611593
47. Wernli KJ, Hampton JM, Trentham-Dietz A, Newcomb PA. Antidepressant medication usexxx and breast cancer risk. Pharmacoepidemiology and Drug Safety (2009) 18(4):284–90. doi: 10.1002/pds.1719.
48. Ashbury J, Lévesque L, Beck P, Aronson K. Selective serotonin reuptake inhibitor (Ssri) antidepressants, prolactin and breast cancer. Front Oncol (2012) 2:177. doi: 10.3389/fonc.2012.00177
49. Lin CF, Chan HL, Hsieh YH, Liang HY, Chiu WC, Huang KY, et al. Endometrial cancer and antidepressants a nationwide population-based study. Med (United States) (2016) 95(29):e4178. doi: 10.1097/MD.0000000000004178
50. Wu C-S, Lu M-L, Liao Y-T, Lee CT-C, Chen VC-H. Ovarian cancer and antidepressants. Psycho-Oncology (2015) 2015:579–84. doi: 10.1002/pon.3700
51. Chen VCH, Liao YT, Yeh DC, Tseng HC, Stewart R, Lee CTC. Relationship between antidepressant prescription and breast cancer: A population based study in Taiwan. Psycho-Oncology (2016) 25(7):803–7. doi: 10.1002/pon.3929
52. Reeves KW, Okereke OI, Qian J, Tamimi RM, Eliassen AH, Hankinson SE. Depression, antidepressant use, and breast cancer risk in pre- and postmenopausal women: A prospective cohort study. Cancer Epidemiol Biomarkers Prev (2018) 27(3):306–14. doi: 10.1158/1055-9965.EPI-17-0707
53. Walker AJ, Card T, Bates TE, Muir K. Tricyclic antidepressants and the incidence of certain cancers: A study using the gprd. Br J Cancer (2011) 104(1):193–7. doi: 10.1038/sj.bjc.6605996
54. Coogan PF, Strom BL, Rosenberg L. Ssri use and breast cancer risk by hormone receptor status. Breast Cancer Res Treat (2008) 109(3):527–31. doi: 10.1007/s10549-007-9664-y
55. Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of selective serotonin reuptake inhibitors and the risk of breast cancer. Am J Epidemiol (2005) 162(9):835–8. doi: 10.1093/aje/kwi301
56. Fulton-Kehoe D, Rossing MA, Rutter C, Mandelson MT, Weiss NS. Use of antidepressant medications in relation to the incidence of breast cancer. Br J Cancer (2006) 94(7):1071–8. doi: 10.1038/sj.bjc.6603017
57. González-Pérez A, García Rodríguez LA. Breast cancer risk among users of antidepressant medications. Epidemiology (2005) 16(1):101–5. doi: 10.1097/01.ede.0000147103.92638.c0
58. Haque R, Enger SM, Chen W, Petitti DB. Breast cancer risk in a Large cohort of female antidepressant medication users. Cancer Lett (2005) 221(1):61–5. doi: 10.1016/j.canlet.2004.11.003
59. Moorman PG, Berchuck A, Calingaert B, Halabi S, Schildkraut JM. Antidepressant medication use for and risk of ovarian cancer. Ob Gynecol (2005) 105(4):725–30. doi: 10.1097/01.AOG.0000157113.98061.eb
60. Steingart A, Cotterchio M, Kreiger N, Sloan M. Antidepressant medication use and breast cancer risk: A case-control study. Int J Epidemiol (2003) 32(6):961–6. doi: 10.1093/ije/dyg155
61. Tamim H, Boivin JF, Hanley J, Stang M, Collet JP. Risk of breast cancer in association with exposure to two different groups of tricyclic antidepressants. Pharmacoepidemiol Drug Saf (2006) 15(10):689–97. doi: 10.1002/pds.1233
62. Wallace RB, Sherman BM, Bean JA. A case-control study of breast cancer and psychotropic drug use. Oncology (1982) 39(5):279–83. doi: 10.1159/000225651
63. Wang PS, Walker AM, Tsuang MT, Orav EJ, Levin R, Avorn J. Antidepressant use and the risk of breast cancer: A non-association. J Clin Epidemiol (2001) 54(7):728–34. doi: 10.1016/S0895-4356(00)00354-1
64. Lucas LK, Lipman AG. Recent advances in pharmacotherapy for cancer pain management. Cancer Pract (2002) 10 Suppl 1:S14–20. doi: 10.1046/j.1523-5394.10.s.1.6.x
65. Cosgrove L, Shi L, Creasey DE, Anaya-McKivergan M, Myers JA, Huybrechts KF. Antidepressants and breast and ovarian cancer risk: A review of the literature and researchers’ financial associations with industry. PloS One (2011) 6(4):e18210. doi: 10.1371/journal.pone.0018210
66. Busby J, Murray L, Mills K, Zhang SD, Liberante F, Cardwell CR. A combined connectivity mapping and pharmacoepidemiology approach to identify existing medications with breast cancer causing or preventing properties. Pharmacoepidemiol Drug Saf. (2018) 27(1):78–86. doi: 10.1002/pds.4345
67. Coogan PF. Review of the epidemiological literature on antidepressant use and breast cancer risk. Expert Rev Neurother (2006) 6(9):1363–74. doi: 10.1586/14737175.6.9.1363
68. Lawlor DA, Jüni P, Ebrahim S, Egger M. Systematic review of the epidemiologic and trial evidence of an association between antidepressant medication and breast cancer. J Clin Epidemiol (2003) 56(2):155–63. doi: 10.1016/S0895-4356(02)00568-1
69. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer–epidemiology, risk factors, classification, prognostic markers, and current treatment strategies–an updated review. Cancers (2021) 13(17):4287. doi: 10.3390/cancers13174287
70. Moysich KB, Beehler GP, Zirpoli G, Choi JY, Baker JA. Use of common medications and breast cancer risk. Cancer Epidemiol Biomarkers Prev (2008) 17(7):1564–95. doi: 10.1158/1055-9965.EPI-07-2828
71. Szelei A, Dome P. Cancer and depression: A concise review. Orvosi Hetilap (2020) 161(22):908–16. doi: 10.1556/650.2020.31759
72. Theoharides TC, Konstantinidou A. Antidepressants and risk of cancer: A case of misguided associations and priorities. J Clin Psychopharmacol (2003) 23(1):1–4. doi: 10.1097/00004714-200302000-00001
73. Li R, Li X, Yan P, Bing Z, Cao L, Hui X, et al. Relationship between antidepressive agents and incidence risk of breast cancer: Systematic review and meta-analysis. Future Oncol (2020) 17(9):1105–24. doi: 10.2217/fon-2020-0822
74. Huo YL, Qiao JM, Gao S. Association between antidepressant medication use and epithelial ovarian cancer risk: A systematic review and meta-analysis of observational studies. Br J Clin Pharmacol (2018) 84(4):649–58. doi: 10.1111/bcp.13498
75. Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of Brca1 and Brca2 among ashkenazi jews. New Engl J Med (1997) 336(20):1401–8. doi: 10.1056/NEJM199705153362001
76. Brooke BS, Schwartz TA, Pawlik TM. Moose reporting guidelines for meta-analyses of observational studies. JAMA Surg (2021) 156(8):787–8. doi: 10.1001/jamasurg.2021.0522
77. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement (Chinese edition). J Chin Integr Med (2009) 7(9):889–96. doi: 10.3736/jcim20090918
78. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (Nos) for assessing the quality of nonrandomised studies in meta-analyses. Oxford (2000). Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
79. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev (1987) 9(1):1–30. doi: 10.1093/oxfordjournals.epirev.a036298
80. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol (1992) 135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237
81. Organization WH. Anatomical therapeutic chemical (Atc)/Ddd index 2020. WHO Collab Centre Drugs Stat Method (2020). Available from: https://www.whocc.no/atc_ddd_index/?code=N06A.
82. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
83. Davis S, Mirick DK. Medication use and the risk of breast cancer. Eur J Epidemiol (2007) 22(5):319–25. doi: 10.1007/s10654-007-9135-0
84. Moorman PG, Grubber JM, Millikan RC, Newman B. Antidepressant medications and their association with invasive breast cancer and carcinoma in situ of the breast. Epidemiology (2003) 14(3):307–14. doi: 10.1097/01.EDE.0000050695.99660.D1
85. Brown SB, Hankinson SE, Arcaro KF, Qian J, Reeves KW. Depression, antidepressant use, and postmenopausal breast cancer risk. Cancer Epidemiol Prev Biomarkers (2016) 25(1):158–64. doi: 10.1158/1055-9965.EPI-15-1063
86. Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, et al. Incidence of cancer and antidepressant medication: Record linkage study. Int J Cancer (2010) 126(1):285–96. doi: 10.1002/ijc.24537
87. Coogan PF, Rosenberg L, Palmer JR, Strom BL, Stolley PD, Zauber AG, et al. Risk of ovarian cancer according to use of antidepressants, phenothiazines, and benzodiazepines (United states). Cancer Causes Control (2000) 11(9):839–45. doi: 10.1023/A:1008982417022
88. Csizmadi I, Collet JP, Boivin JF. Bias and confounding in pharmacoepidemiology. In: Strom BL, ed. Pharmacoepidemiology. New York: Wiley; (2006), 791–809. doi: 10.1002/9780470059876.ch47
89. Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res (2013) 73(15):4810–9. doi: 10.1158/0008-5472.Can-13-0665
90. Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol Psychiatry (2020) 25(7):1487–99. doi: 10.1038/s41380-019-0595-x
91. Fang CK, Chen HW, Chiang IT, Chen CC, Liao JF, Su TP, et al. Mirtazapine inhibits tumor growth Via immune response and serotonergic system. PloS One (2012) 7(7):e38886. doi: 10.1371/journal.pone.0038886
92. Huang J, Liu F, Tang H, Wu H, Li L, Wu R, et al. Tranylcypromine causes neurotoxicity and represses Bhc110/Lsd1 in human-induced pluripotent stem cell-derived cerebral organoids model. Front Neurol (2017) 8:626. doi: 10.3389/fneur.2017.00626
Keywords: antidepressant, depression, breast cancer, gynecological cancer, incidence, meta-analysis, systematic review, dose–response analysis
Citation: Zhuang Y, Pang X, Qi Y, Zhang T, Cao G, Xue H, Xu Y, Xie S, Liu Y, Wang Y, Li Y, Xiong Y, Li Y and Shen H (2022) The incidence risk of breast and gynecological cancer by antidepressant use: A systematic review and dose–response meta-analysis of epidemiological studies involving 160,727 patients. Front. Oncol. 12:939636. doi: 10.3389/fonc.2022.939636
Received: 09 May 2022; Accepted: 23 September 2022;
Published: 14 October 2022.
Edited by:
Azin Nahvijou, Tehran University of Medical Science, IranReviewed by:
Franco Guidozzi, University of the Witwatersrand, South AfricaIon-George Anghelescu, Clinic Pacelliallee, Germany
Copyright © 2022 Zhuang, Pang, Qi, Zhang, Cao, Xue, Xu, Xie, Liu, Wang, Li, Xiong, Li and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Shen, aHVpc2hlbmxqQGhvdG1haWwuY29t; Yuanyuan Li, bHl5MDVfMjAxMkAxNjMuY29t
 Yanjia Zhuang
Yanjia Zhuang Xiaogang Pang1,2
Xiaogang Pang1,2 Yinuo Wang
Yinuo Wang Yuanyuan Li
Yuanyuan Li Hui Shen
Hui Shen