- Department of Thoracic Surgery and Lung Transplantation, First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Third-generation tyrosine kinase inhibitors (TKIs), such as osimertinib, almonertinib and furmonertinib, overcome the mechanisms of resistance to first-generation inhibitors (such as gefitinib, erlotinib and icotinib) by incorporating an acrylamide group that alkylates the Cys797 of EGFR T790M. However, drug resistance is inevitable, even for third-generation TKIs. Screening for drug-resistant mutations by repeat biopsy and repeat gene sequencing is necessary after TKI treatment. Among various third-generation TKI-resistant mutations, secondary mutation of the L718 residue of EGFR exon 18 was found in approximately 8% of patients and is responsible for drug resistance in vitro and in vivo. Furthermore, there is limited clinical experience of targeted therapy for this mutation. Herein, we report for the first time that afatinib and cetuximab combination therapy can overcome such drug resistance.
Highlights
1. The EGFR L718Q mutation is responsible for resistance to third-generation TKIs in vitro and in vivo, and few targeted regimens can effectively overcome this resistance.
2. We demonstrated the first use of combination therapy with afatinib and cetuximab to achieve response in a patient with almonertinib-resistant lung adenocarcinoma bearing the L858R/L718Q mutation.
Patient history
A 72-year-old man with a smoking history of approximately 20-pack-years complained of abdominal distension and was admitted to our hospital on August 22, 2020. Thoracoabdominal computed tomography (CT) (Figure 1A) and a radionuclide bone scan showed a 2.8-cm right upper lung mass, hydrothorax, ascites and multiple bone metastases (skull, right clavicle, multiple vertebrae, and pelvis). Left cervical lymph node metastasis was diagnosed based on color Doppler ultrasonography. CT-guided biopsy showed that the mass was lung adenocarcinoma, and the patient was diagnosed with adenocarcinoma of the right upper lung with multiple metastases (cT1bN3M1c, stage IVB). Tissue from the biopsy was analyzed via next-generation sequencing (NGS) of a panel of 8 cancer-related genes (Geneseeq, Nanjing, China) on September 8, 2020. Mutations were detected in EGFR exon 21 (L858R) (abundance: 14.5%) and PIK3CA exon 8 (E474D) (abundance: 36.6%), as shown in Figure 2A. The patient received first-line therapy with the third-generation TKI almonertinib (110 mg/d) and bisphosphonates (zoledronic acid, 4 mg, q4w). Stable disease was achieved initially, but the patient ultimately experienced progressive disease (appearance of hydrothorax and ascites), with a progression-free survival (PFS) time of 12 months (Figures 1B, C). After disease progression, the patient was admitted to the local hospital and received 2 cycles of chemotherapy with cis-platinum (75 mg/m2, d1) and pemetrexed (500 mg/m2, d1). However, the hydrothorax and ascites were not controlled. Then, the patient was admitted to our hospital, and metastatic lung adenocarcinoma was diagnosed in the ascites sediment. A repeat examination for gene mutations was performed on the ascites sediment (Geneseeq, Nanjing, China), and mutations in EGFR exon 21 (L858R) (abundance: 1.7%), EGFR exon 18 (L718Q) (abundance: 0.8%) and TP53 (V157F) (abundance: 1.6%) were found (Figure 2B).
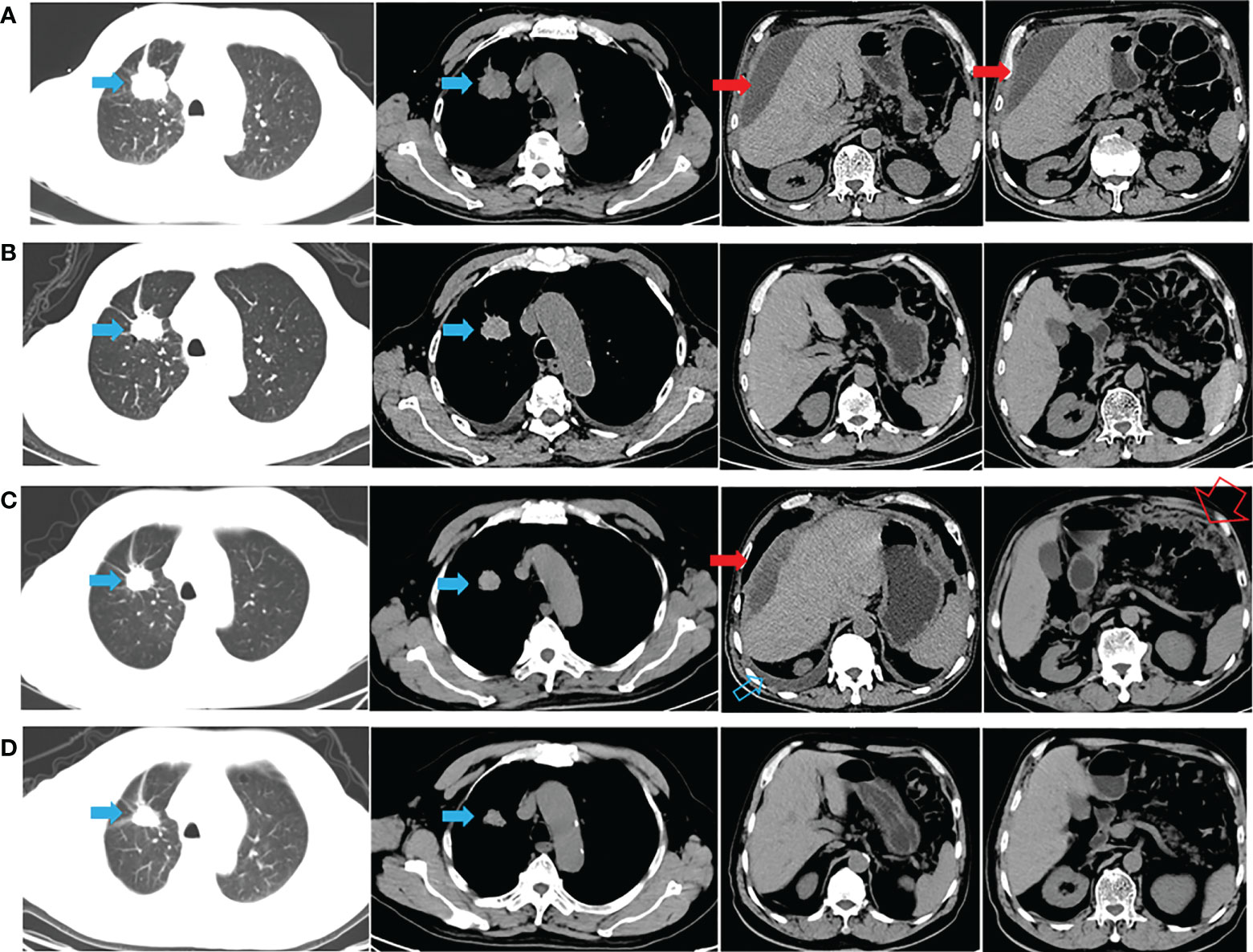
Figure 1 CT appearance across the therapeutic process. (A) Thoracoabdominal CT at diagnosis (after thoracoabdominal drainage); (B) 1 month after almonertinib treatment; (C) hydrothorax and ascites appeared after almonertinib resistance (after thoracoabdominal drainage); (D) hydrothorax and ascites disappeared 1 month after afatinib plus cetuximab treatment. The solid blue arrow indicates the primary lesion; the open blue arrow indicates the hydrothorax; the solid red arrow indicates the malignant ascites; the open red arrow indicates the greater omentum metastasis.

Figure 2 (A) Integrative Genomics Viewer snapshot of the L858R mutation in EGFR exon 21. (B) Integrative Genomics Viewer snapshot of the L718Q mutation in EGFR exon 18.
The patient’s case was discussed with a multidisciplinary team (MDT). Importantly, the patient was fully informed of the benefits and risks of the proposed therapy and signed an informed consent form. Studies have shown that afatinib combined with a lower dose of cetuximab (250 mg/m2) may have improved tolerability compared to a higher dose of cetuximab (500 mg/m2) (1, 2). Thus, combination therapy with afatinib (40 mg daily) and cetuximab (250 mg/m2, q2w) was started on November 16, 2021. CT performed after 1 month showed stable lung and metastatic lesions, and the hydrothorax and ascites were well controlled (Figure 1D). Then, follow-up was performed every 2-3 months, with the most recent follow-up on June 08, 2022 (Supplementary Image 1). A mild gastrointestinal reaction was the only adverse event (AE) observed. The patient is still receiving the combination regimen and has a satisfactory quality of life, with a PFS of nearly 7 months. The overall survival (OS) exceeded 21 months from the initial pathological diagnosis (Figure 1 and Supplementary Image 1).
Discussion
The six broad resistance mechanisms against third-generation EGFR TKIs among patients harboring EGFR mutant non-small cell lung carcinoma (NSCLC) are as follows: loss of T790M, maintenance of T790M, EGFR mutations (C797S, G724S, L718Q), bypassing of pathway activation, SCLC transformation and enhancement of autophagy (3, 4). Targeted treatment is currently being developed for each resistance mechanism (5). For NSCLC with the C797S(+)/T790M(+) mutation, cetuximab combined with brigatinib or EAI045 is effective for the cis-C797S/T790M mutation, whereas a first-generation TKI combined with a third-generation TKI is effective for the trans-C797S/T790M mutation. For NSCLC with the C797S(+)/T790M(-) mutation, first- and second-generation EGFR TKIs are effective. For the other resistance mechanisms, promising targeted therapy combinations are emerging (third-generation EGFR TKIs combined with MEK inhibitors, MET inhibitors and HER2 inhibitors); other fourth-generation EGFR TKIs (BLU-945, TQB3804, etc.) are also being explored.
The EGFR T790M mutation is responsible for the reduction in or even lack of inhibitory potency of first-generation inhibitors (6). In silico protein structure modeling suggests that this effect may result from changes in the steric and lipophilic properties of the gatekeeper residue (7). Similarly, targeted therapy using third-generation inhibitors is rendered nonfunctional by the L718Q mutation due to a remarkable increase in spatial conflict and a decrease in local hydrophobicity (8).
Preclinical studies have shown that irreversible EGFR TKIs demonstrated activity against T790M (9, 10), but these findings failed to translate into clinically significant results (11). Similar to the case for the L718Q mutation, there are also inconsistencies between studies. A preclinical study showed that cells containing Del19/L718Q or L858R/L718Q retain sensitivity to first- and second-generation inhibitors, such as gefitinib and afatinib. However, strong resistance to osimertinib was observed (8, 12). The EGFR tertiary mutations Del19/L718Q/T790M and L858R/L718Q/T790M were resistant to first-, second- and third-generation TKIs (8, 12) (Table 1). Interestingly, Yang (8) demonstrated that cells with the Del19/L718Q/T790M mutations showed slightly less resistance than those with the L858R/L718Q/T790M mutations (8). However, another study failed to replicate this result (12).
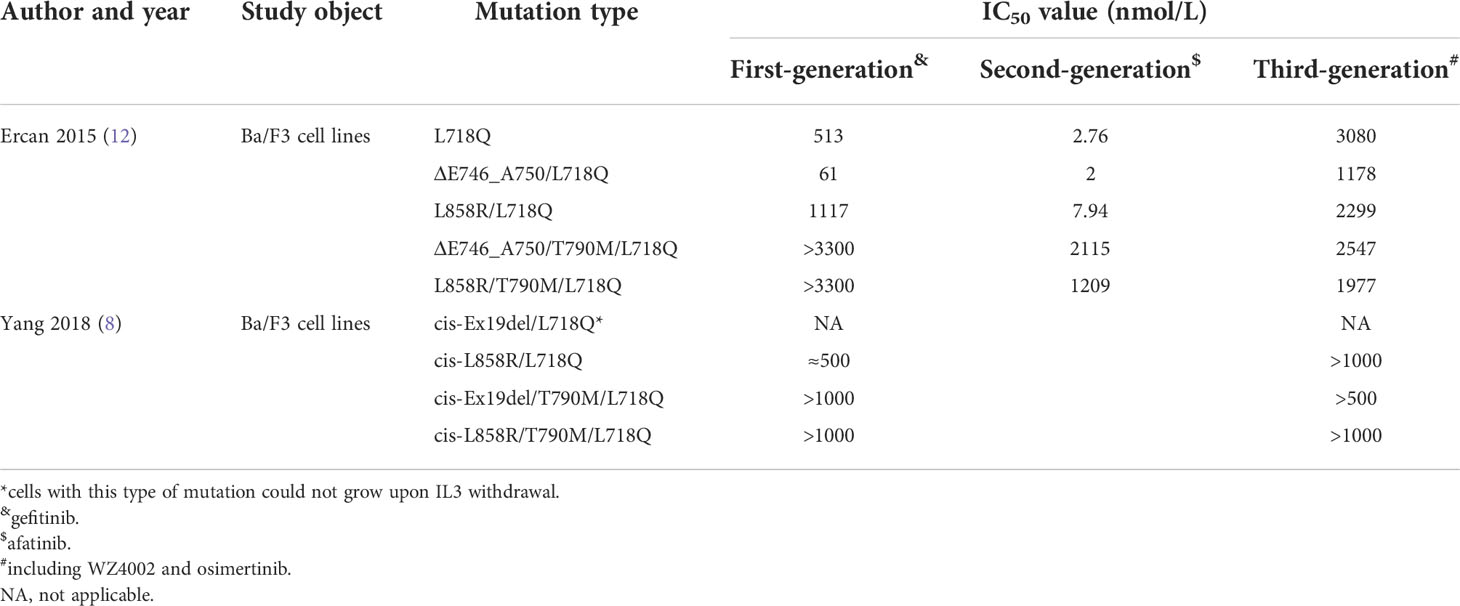
Table 1 Preclinical data evaluating the sensitivity of EGFR TKIs in cell models harboring the L718Q mutation.
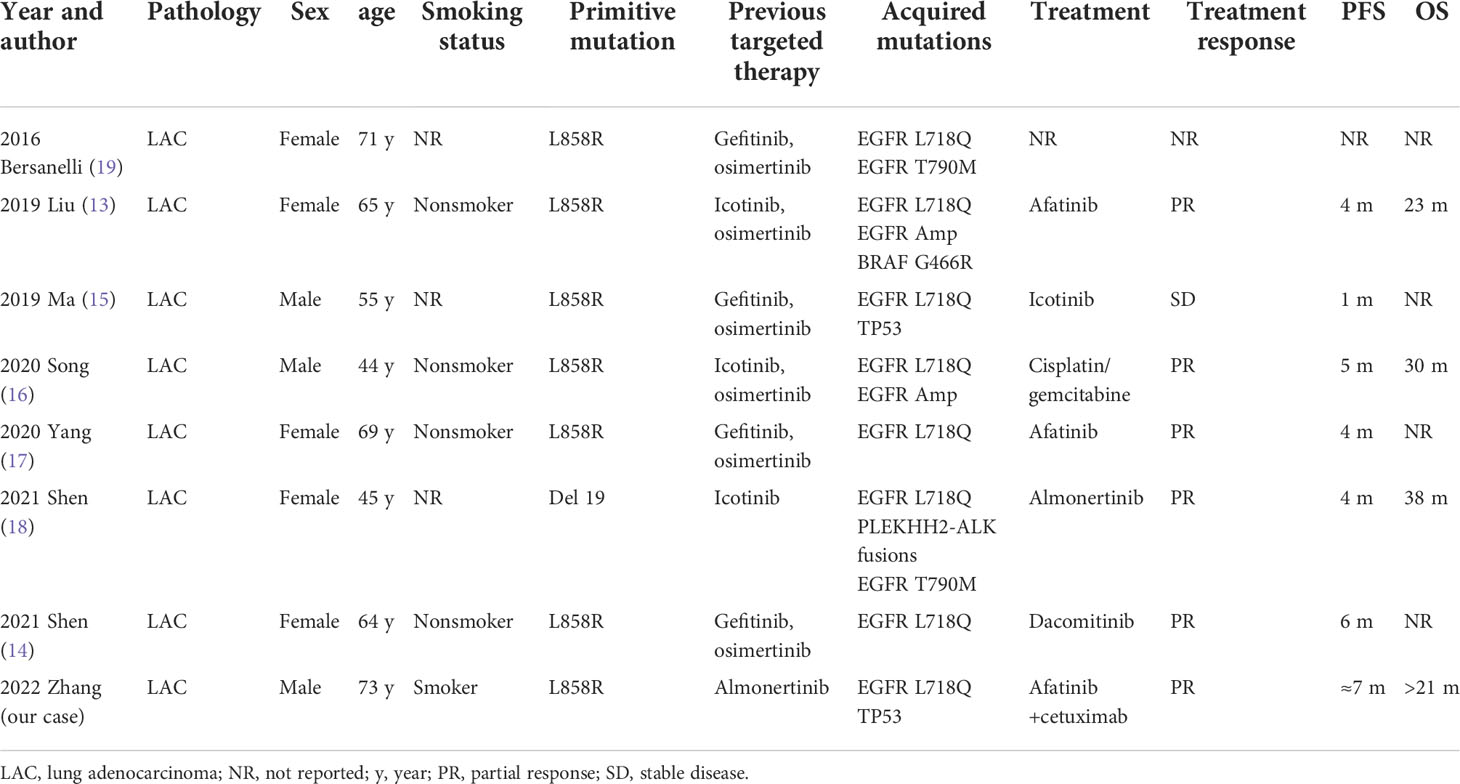
In terms of clinical studies, most of the mutations studied to date are EGFR L858R/L718Q (13–17) mutations, with only two EGFR tertiary mutations, Del19/L718Q/T790M (18) and L858R/L718Q/T790M (19), reported (Table 2). Studies suggest that patients with complex secondary L718Q mutations [EGFR L858R/L718Q (13–15, 17) or EGFR Del19/L718Q/T790M (18)] experience poor therapeutic response to targeted therapy, with PFS of 1 month for first-generation treatment and 4-6 months for second-generation treatment. Interestingly, Shen reported that a patient with an EGFR Del19/L718Q/T790M mutation who acquired resistance to osimertinib was sensitive to treatment with almonertinib (18). This was surprising because the third-generation TKIs almonertinib and osimertinib share a similar mechanism for EGFR inhibition and drug resistance. Upon careful reading of the study, we found that osimertinib was discontinued due to AEs, not drug resistance, as described in the title. In this study (18), almonertinib inhibited the growth of a lesion with an EGFR Del19/L718Q/T790M mutation, with a PFS of 4 months, which was not consistent with the results of preclinical studies (8, 12). The sensitivity of the EGFR Del19/L718Q/T790M mutation to almonertinib may reflect the complex responses of complex mutations; it is also possible that the L718Q mutation does not always confer resistance to almonertinib.
Second-generation inhibitors, such as afatinib, may play an important role in the treatment of disease that is resistant to first/third-generation inhibitors, which implies that second-generation inhibitors have some unique properties. For example, afatinib is theoretically a multitarget inhibitor that can irreversibly bind to Cys797 of EGFR, Cys805 of HER2 and Cys803 of HER4 (20). Furthermore, the acrylamide warhead of afatinib alkylates Cys797, thus circumventing ATP competition and overcoming drug resistance caused by T790M or L718Q mutations (21). According to preclinical studies, Del19/L718Q and L858R/L718Q mutations exhibited good sensitivity to second-generation TKIs such as afatinib; this was further confirmed in clinical cases, as patients with EGFR L858R/L718Q mutations achieved 4- to 6-month PFS under these treatments.
EGFR TKIs and EGFR monoclonal antibodies (mAbs) have been combined to increase the intensity of EGFR inhibition because they can exert synergistic antitumor effects and because their combination permits a reduced dose of each drug to be used, allowing AEs to be controlled without impairing the therapeutic effect. In our previous study, we reviewed in detail the potential mechanisms (such as direct inhibition, receptor internalization, and immunological effects) by which cetuximab could increase the efficacy of EGFR TKIs (22). This combination regimen may be particularly effective for EGFR exon 20 ins mutations (1), C797S/T790M/sensitive mutations (23), and EGFR–intergenic region (IGR) (SEC61G) fusion/EGFR amplification mutations (24). We searched databases including PubMed, Embase, OvidSP, and Web of Science up to July 2022 and found no investigation focused on clinical combination therapy for the L718Q mutation; therefore, there is no clearly preferred therapeutic option. We predicted that the patient might benefit from a second-generation EGFR TKI and EGFR mAb combination because of the L858R/L718Q mutation.
A major feature of this case was that the primary lesion remained stable throughout the treatment. Chest discomfort and abdominal distension were the chief complaints after the progression of disease. Correspondingly, hydrothorax and ascites were the major manifestations of almonertinib resistance, and the control of hydrothorax and ascites was evidence that this combination therapy works. In addition to the underlying theory presented above, the occurrence of TP53 mutation might have conferred almonertinib resistance in this specimen, and the loss of PIK3CA would be a potential mechanism for sensitization to afatinib plus cetuximab. On the other hand, the NGS results were obtained from ascites sediment; thus, it is possible that the primary lung tumor and hydrothorax/ascites had mixed responses to treatment due to tumor heterogeneity. Gene mutation examination for primary lung tumors, when necessary, may provide more information. Finally, other modes of combination therapy (such as TKI combined with antiangiogenic therapy and/or chemotherapy) and other drug delivery routes (such as intraluminal cetuximab perfusion combined with oral afatinib) after ascites and pleural effusion progression may also be interesting and worthy of further exploration.
Conclusion
Herein, we demonstrated the first use of combination therapy with afatinib and cetuximab for a patient with an L858R/L718Q mutation who achieved a response after almonertinib resistance. However, a limitation of our study is that our patient could have responded by chance because of limited data, even though a series of theoretical foundations for the response exists. In the future, we intend to pursue fundamental research on such rare mutations and explore their impacts on the response to combination therapy.
Data availability statement
The datasets presented in this article are not readily available because further studies are in progress about this case. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Zhengzhou University Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GZ, BY, and YG contributed to conception and design of the study. GZ wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the Key Scientific Research Projects of Institutions of Higher Learning in Henan Province (No. 21A320032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.995624/full#supplementary-material
Supplementary Image 1 | Timeline of patient events from admission until this report. AC, adenocarcinoma; OS, overall survival; PD, progression of disease; PFS, progression-free survival.
Abbreviations
AE, adverse event; EGFR, epidermal growth factor receptor; mAb, monoclonal antibody; MDT, multidisciplinary team; NGS, next-generation sequencing; OS, overall survival; PD, progression of disease; PFS, progression free survival; TKI, tyrosine kinase inhibitor.
References
1. van Veggel B, de Langen AJ, Hashemi SMS, Monkhorst K, Heideman DAM, Thunnissen E, et al. Afatinib and cetuximab in four patients with EGFR exon 20 insertion-positive advanced NSCLC. J Thorac Oncol Aug (2018) 13(8):1222–6. doi: 10.1016/j.jtho.2018.04.012
2. Goldberg SB, Redman MW, Lilenbaum R, Politi K, Stinchcombe TE, Horn L, et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for first-line treatment of EGFR-mutant non-Small-Cell lung cancer: Final results from SWOG S1403. J Clin Oncol (2020) 38(34):4076–85. doi: 10.1200/JCO.20.01149
3. Kwon Y, Kim M, Jung H, Kim Y, Jeoung D. Targeting autophagy for overcoming resistance to anti-EGFR treatments. Cancers (2019) 11(9): 1374. doi: 10.3390/cancers11091374
4. Lazzari C, Gregorc V, Karachaliou N, Rosell R, Santarpia M. Mechanisms of resistance to osimertinib. J Thorac Dis (2020) 12(5):2851–8. doi: 10.21037/jtd.2019.08.30
5. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd−generation EGFR−TKI resistance in advanced non−small cell lung cancer (Review). Int J Oncol (2021) 59(5): 90. doi: 10.3892/ijo.2021.5270
6. Skoulidis F, Papadimitrakopoulou VA. Targeting the gatekeeper: Osimertinib in EGFR T790M mutation-positive non-small cell lung cancer. Clin Cancer Res (2017) 23(3):618–22. doi: 10.1158/1078-0432.CCR-15-2815
7. Kannan S, Fox SJ, Verma CS. Exploring gatekeeper mutations in EGFR through computer simulations. J Chem Inf Modeling (2019) 59(6):2850–8. doi: 10.1021/acs.jcim.9b00361
8. Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res (2018) 24(13):3097–107. doi: 10.1158/1078-0432.CCR-17-2310
9. Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res (2007) 67(24):11924–32. doi: 10.1158/0008-5472.CAN-07-1885
10. Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U.S.A. (2005) 102(21):7665–70. doi: 10.1073/pnas.0502860102
11. Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol (2013) 31(31):3987–96. doi: 10.1200/JCO.2012.45.2029
12. Ercan D, Choi HG, Yun CH, Capelletti M, Xie T, Eck MJ, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res (2015) 21(17):3913–23. doi: 10.1158/1078-0432.CCR-14-2789
13. Liu J, Jin B, Su H, Qu X, Liu Y. Afatinib helped overcome subsequent resistance to osimertinib in a patient with NSCLC having leptomeningeal metastasis baring acquired EGFR L718Q mutation: A case report. BMC Cancer (2019) 19(1):702. doi: 10.1186/s12885-019-5915-7
14. Shen Q, Qu J, Chen Z, Zhou J. Case report: Dacomitinib overcomes osimertinib resistance in NSCLC patient harboring L718Q mutation: A case report. Front Oncol (2021) 11:760097. doi: 10.3389/fonc.2021.760097
15. Ma L, Chen R, Wang F, Ma LL, Yuan MM, Chen RR, et al. EGFR L718Q mutation occurs without T790M mutation in a lung adenocarcinoma patient with acquired resistance to osimertinib. Ann Trans Med (2019) 7(9):207. doi: 10.21037/atm.2019.04.37
16. Song Y, Jia Z, Wang Y, Wang Y, Liu P, Zhang S, et al. Potential treatment strategy for the rare osimertinib resistant mutation EGFR L718Q. J Thorac Dis (2020) 12(5):2771–80. doi: 10.21037/jtd.2020.03.29
17. Yang X, Huang C, Chen R, Zhao J. Resolving resistance to osimertinib therapy with afatinib in an NSCLC patient with EGFR L718Q mutation. Clin Lung Cancer (2020) 21(4):e258–60. doi: 10.1016/j.cllc.2019.12.002
18. Shen G, Shi L, Tian X, Huang D, Chen H, Gao C, et al. Case report: Response to almonertinib in a patient with metastatic NSCLC resistant to osimertinib due to acquired EGFR L718Q mutation. Front Pharmacol (2021) 12:731895. doi: 10.3389/fphar.2021.731895
19. Bersanelli M, Minari R, Bordi P, Gnetti L, Bozzetti C, Squadrilli A, et al. L718Q mutation as new mechanism of acquired resistance to AZD9291 in EGFR-mutated NSCLC. J Thorac Oncol (2016) 11(10):e121–123. doi: 10.1016/j.jtho.2016.05.019
20. Wecker H, Waller CF. Afatinib. Recent results in cancer research. (2018) 211:199–215. doi: 10.1007/978-3-319-91442-8_14.
21. de Antonellis P. Afatinib, a lung cancer inhibitor of ErbB family. Naunyn-Schmiedeberg’s Arch Pharmacol (2014) 387(6):503–4. doi: 10.1007/s00210-014-0980-6
22. Zhang G, Yan B, Guo Y, Yang H, Li J. “Sandwich” strategy to intensify EGFR blockade by concurrent tyrosine kinase inhibitor and monoclonal antibody treatment in highly selected patients. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.952939
23. Wang X, Zhou L, Yin JC, Wu X, Shao YW, Gao B. Lung adenocarcinoma harboring EGFR 19del/C797S/T790M triple mutations responds to brigatinib and anti-EGFR antibody combination therapy. J Thorac Oncol (2019) 14(5):e85–8. doi: 10.1016/j.jtho.2019.01.015
Keywords: lung adenocarcinoma, EGFR, drug resistance, L718Q, afatinib, cetuximab
Citation: Zhang G, Yan B, Guo Y, Yang H, Li X and Li J (2022) Case Report: A patient with the rare third-generation TKI-resistant mutation EGFR L718Q who responded to afatinib plus cetuximab combination therapy. Front. Oncol. 12:995624. doi: 10.3389/fonc.2022.995624
Received: 16 July 2022; Accepted: 13 October 2022;
Published: 31 October 2022.
Edited by:
Alessia Ligresti, Department of Chemical Sciences and Materials Technologies, (CNR), ItalyReviewed by:
Jing Cai, Second Affiliated Hospital of Nanchang University, ChinaSheng-Kai Liang, National Taiwan University Cancer Center, Taiwan
Hanxiao Chen, Beijing Cancer Hospital, China
Copyright © 2022 Zhang, Yan, Guo, Yang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jindong Li, MTM1OTg4MjA1ODlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Guoqing Zhang
Guoqing Zhang Beibei Yan
Beibei Yan Yanan Guo
Yanan Guo Hang Yang
Hang Yang Xiangnan Li
Xiangnan Li Jindong Li
Jindong Li